Abstract
Introduction:
Multiple factors and comorbidities have been implicated in the ability of arteriovenous fistulas (AVF) to mature, including vessel anatomy, advanced age, and the presence of coronary artery disease or peripheral vascular disease. However, little is known about the role of uremia on AVF primary failure. In this study, we attempt to evaluate the effect of uremia on AVF maturation by comparing AVF outcomes between pre-dialysis chronic kidney disease (CKD) stage five patients and those who had their AVF created after hemodialysis (HD) initiation.
Methods:
We included 612 patients who underwent AVF creation between 2003 and 2015 at the University of Miami Hospital and Jackson Memorial Hospital. Effects of uremia on primary failure were evaluated using univariate statistical comparisons and multivariate logistic regression analyses.
Results:
Primary failure occurred in 28.1% and 26.3% of patients with an AVF created prior to or after HD initiation, respectively (p = 0.73). The time of HD initiation was not associated with AVF maturation in multivariate logistic regression analysis (p = 0.57). In addition, pre-operative blood urea nitrogen (p = 0.78), estimated glomerular filtration rate (p = 0.66), and serum creatinine levels (p = 0.14) were not associated with AVF primary failure in pre-dialysis patients.
Conclusions:
Our results show that clearance of uremia with regular HD treatments prior to AVF creation does not improve the frequency of vascular access maturation.
Keywords: Arteriovenous fistula, Catheter, Hemodialysis, Maturation, Uremia
Introduction
Hemodialysis (HD) initiation with a tunneled central venous catheter is still a common occurrence in chronic kidney disease (CKD) patients with planned arteriovenous fistula (AVF) creation surgeries or during the time of AVF maturation. Tunneled catheters (TC) are associated with increased morbidity and mortality due to the high frequency of dysfunction and infections (1). The CHOICE study reported a 50% higher mortality rate in patients who initiate HD with a TC compared to an AVF (2). Based on these recognized complications, since 2006 the National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF KDOQI) recommends that at least 50% of patients have a functional AVF at HD initiation (3). In addition, since 2013 the Centers for Medicare and Medicaid Services (CMS) advocate a 68% of prevalent AVF use in HD patients (4).
After the implementation of the Fistula First Initiative (FFI) in 2003, AVF use in the USA has increased significantly from 32.2% in 2003 to 62.2% in 2013 (5–7). However, compared to other developed countries, the USA continues to have a lower incidence of AVF maturity prior to HD initiation (8). An estimated 71% of patients initiate HD sub-optimally using a temporary TC or arteriovenous graft (AVG), even with early nephrology referral of longer than 12 months (6). In addition, more than 80% of patients do not have a mature AVF at the start of HD, requiring placement of a TC, thus increasing morbidity, mortality and health-care costs in this population (9).
There is reason to believe that inflammation is one of the factors that can negatively affect the AVF maturation process (10–12). Both the underlying uremic milieu in CKD and the initiation of HD sessions have been suggested as potential sources of systemic inflammation (13–15). Uremia has been associated with endothelial dysfunction as a result of reduced vascular bioavailability of nitric oxide (NO) (16–18). In addition, uremia induces vasoconstriction to a greater extent than hypertension by increasing the intima-media thickness and reducing luminal diameter (19). A seminal study indicated that the duration of uremia correlates with the severity of intimal thickness and calcification in patients’ arteries (20). On the other hand, despite the ability of HD sessions to efficiently clear the circulating uremic milieu, HD treatments are also thought to accelerate vascular calcification, even in the pediatric CKD population (21). Therefore, whether early initiation of HD improves the survival or clinical outcomes of CKD patients is still debatable (22, 23). As these studies illustrate, there is no clear information on when is the appropriate time for AVF creation and HD initiation to improve both vascular access and overall clinical outcomes (24). However, the NKF KDOQI guidelines recommend having a fistula created at least 6 months before the expected start of HD in order to allow sufficient time for access maturation and revision (if needed), and prevent possible complications (3).
In this study, we aim to evaluate the effect of uremia and uremia markers on AVF maturation outcomes.
Materials and methods
Study participants
This retrospective study includes 612 patients over 17 years of age who underwent either one-stage or two-stage AVF creation at Jackson Memorial Hospital or at the University of Miami hospital from May 2003 to August 2015. Patients’ medical records were reviewed to collect information on demographics, comorbidities, drug treatment at the time of AVF creation, prior vascular access history, and AVF characteristics such as type of anastomosis, location and outcomes. Glomerular filtration rate (eGFR), blood urea nitrogen (BUN), serum creatinine (SCr) levels were collected for pre-dialysis patients. Patients were excluded from the study if they were lost to follow-up or if the patient underwent a mid-arm fistula (brachial-brachial) because of the necessity of a graft extension. A single vascular surgeon (M.T.) performed all surgical procedures in 591 of the 612 patients (97%) included in the study. Patients on HD treatment prior to AVF creation were receiving three regular sessions per week using various vascular accesses. In addition, in order to discern the effect of HD initiation time (prior to or after AVF creation) on AVF maturation, patients who first started HD treatments with a TC during the AVF maturation period were excluded from the study. All sections of the study were performed according to the ethical principles of the Declaration of Helsinki and regulatory requirements at both institutions. The ethics committee and Institutional Review Board at the University of Miami approved the study.
Definitions
Anatomic maturation failure was defined as an AVF that was created but never reached a diameter greater than 6 mm, length greater than 6 cm (evaluated during post-operative follow-up using vascular ultrasound and physical exam), or that required an intervention (intravascular or surgical) within the first three months of AVF cannulation. One-stage AVF accounts for radio-cephalic and brachio-cephalic anastomoses, while two-stage AVF refers to brachio-basilic anastomoses with lateral superficialization surgeries.
Statistical analyses
Statistical analyses were performed using XLSTAT (New York, NY) and GraphPad Prism (La Jolla, CA). Results were considered significant when p<0.05. Patients were divided into those in whom an AVF was created prior to HD initiation and those who were already receiving HD treatments using a TC. Normally distributed data were compared using the Student’s t-test and expressed as mean ± standard deviation. Comparisons between group frequencies were performed using Fisher’s exact test or Chi-square tests. Statistical associations between baseline covariates and primary failure were assessed using multivariate logistic regressions (Logit algorithm) adjusted for age, sex, ethnicity, comorbidities, medications, AVF type, and time of HD initiation. Logistic regressions of primary failure in smaller patient subsets were minimally adjusted for baseline covariates as indicated in the respective analyses.
Results
Demographics, clinical and AVF characteristics of the study population
A total of 612 patients were included in the study, 18.6% with advanced CKD but not yet receiving HD sessions and 81.4% who had already initiated HD. The average age in both groups is 54.4 and 51.1 years, respectively (p = 0.026). The study population is formed by 46.5% and 39.2% females in the HD-naïve and -experienced groups, respectively, with 35%–44% Hispanics, 54%–61% non-Hispanic blacks and 3%–4% Caucasians (Tab. I). Over 95% of patients have hypertension in both subgroups, while approximately 50% have diagnosis of diabetes, 15% of CAD, and 6%–8% of congestive heart failure (CHF). Almost one-fifth of the population was on anti-platelet therapy (aspirin and/or clopidogrel) in both the HD-naïve and -experienced groups; 51.8% and 41.0% were statin users, respectively (p = 0.046), and 43%–46% were on either an angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB). The frequency of one-stage versus two-stage AVF was approximately the same (~50%) in both patient cohorts. Primary failure occurred in 28.1% and 26.3% of patients with an AVF created prior to or after HD initiation, respectively (p = 0.73; Tab. I).
TABLE I.
Baseline characteristics and maturation outcomes in patients with an arteriovenous fistula created before or after hemodialysis initiation
| Before HD (n = 114) | After HD (n = 498) | p Value | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age y, mean ± SD | 54.4 ± 15 | 51.1 ± 14 | 0.026 |
| Females no. (%) | 53 (46.5) | 195 (39.2) | 0.17 |
| Ethnicity no. (%) | |||
| Hispanic | 50 (43.9) | 173 (34.7) | |
| Non-Hispanic black | 61 (53.5) | 306 (61.4) | 0.18 |
| Caucasian | 3 (2.6) | 19 (3.8) | |
| Comorbidities no. (%) | |||
| Hypertension | 109 (95.6) | 488 (98.0) | 0.17 |
| Diabetes | 60 (52.6) | 257 (51.6) | 0.92 |
| CAD | 17 (14.9) | 76 (15.3) | 1.00 |
| CHF | 7 (6.1) | 42 (8.4) | 0.57 |
| Medications no. (%) | |||
| Antiplatelet agents | 21 (18.4) | 98 (19.7) | 0.90 |
| Statins | 59 (51.8) | 204 (41.0) | 0.046 |
| ACE-I/ARB | 49 (43.0) | 229 (46.0) | 0.60 |
| AVF characteristics | |||
| AVF type no. (%) | |||
| One-stage | 53 (46.5) | 240 (48.2) | 0.76 |
| Two-stage | 61 (53.5) | 258 (51.8) | |
| Maturation no. (%) | 82 (71.9) | 367 (73.7) | 0.73 |
HD = hemodialysis; SD = standard deviation; CAD = coronary artery disease; CHF = congestive heart failure; ACE-I = angiotensin I converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AVF = arteriovenous fistula.
Pre-operative BUN, eGFR and SCr were obtained from 86 patients in the pre-dialysis subset. Demographics, comorbidities, concurrent medications, and AVF characteristics were equivalent in this subgroup as in the original group of 114 patients. The frequency of primary failure was 29.1%. BUN ranged from 24–120 mg/dL (average 66.5 ± 21.6 mg/dL), eGFR from 4–30 mL/min/1.73 m2 (10.6 ± 4.6 mL/min/1.73 m2), and SCr from 2–18 mg/dL (6.1 ± 2.7 mg/dL).
Associations between baseline covariates and primary failure
Table II shows the demographics, clinical factors and AVF characteristics of patients with primary failure or successful maturation in both the HD-naïve and -experienced cohorts. There are no statistical differences across any of above baseline characteristics between failed and mature AVF, with the exception of demographics for patients already receiving HD sessions. In this latter group, a lower percentage of Hispanics (26.0% vs. 37.9%) and higher proportion of non-Hispanic blacks (69.5% vs. 58.6%) had primary failure compared to successful maturation, while the proportion of Caucasians for both outcomes was approximately the same (4.6% vs. 3.5%; p = 0.048 for the comparison of the three ethnic groups; Tab. II).
TABLE II.
Comparisons between patients with mature and failed arteriovenous fistulas that were created before or after hemodialysis initiation
| Before HD | After HD | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mature (n = 82) | Failure (n = 32) | p | Mature (n = 367) | Failure (n = 131) | p | |
|
| ||||||
| Demographics | ||||||
| Age y, mean ± SD | 53.8 ± 16 | 55.8 ± 13 | 0.53 | 50.5 ± 14 | 52.6 ± 15 | 0.15 |
| Females no. (%) | 36 (43.9) | 17 (53.1) | 0.41 | 137 (37.3) | 58 (44.3) | 0.18 |
| Ethnicity no. (%) | ||||||
| Hispanic | 36 (43.9) | 14 (43.8) | 139 (37.9) | 34 (26.0) | ||
| Non-Hispanic black | 43 (52.4) | 18 (56.3) | 0.54 | 215 (58.6) | 91 (69.5) | 0.048 |
| Caucasian | 3 (3.7) | 0 (0.0) | 13 (3.5) | 6 (4.6) | ||
| Comorbidities no. (%) | ||||||
| Hypertension | 77 (93.9) | 32 (100.0) | 0.32 | 360 (98.1) | 128 (97.7) | 0.73 |
| Diabetes | 42 (51.2) | 18 (56.3) | 0.68 | 184 (50.1) | 73 (55.7) | 0.31 |
| CAD | 12 (14.6) | 5 (15.6) | 1.00 | 52 (14.2) | 24 (18.3) | 0.26 |
| CHF | 3 (3.7) | 4 (12.5) | 0.096 | 30 (8.2) | 12 (9.2) | 0.72 |
| Medications no. (%) | ||||||
| Antiplatelet agents | 18 (22.0) | 3 (9.4) | 0.18 | 70 (19.1) | 28 (21.4) | 0.61 |
| Statins | 43 (52.4) | 16 (50.0) | 0.84 | 159 (43.3) | 45 (34.4) | 0.079 |
| ACE-I/ARB | 37 (45.1) | 12 (37.5) | 0.53 | 172 (46.9) | 57 (43.5) | 0.54 |
| AVF characteristics | ||||||
| AVF type no. (%) | ||||||
| One-stage | 37 (45.1) | 16 (50.0) | 0.68 | 173 (47.1) | 67 (51.1) | 0.48 |
| Two-stage | 45 (54.9) | 16 (50.0) | 194 (52.8) | 64 (48.8) | ||
HD = hemodialysis; SD = standard deviation; CAD = coronary artery disease; CHF = congestive heart failure; ACE-I = angiotensin I converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AVF = arteriovenous fistula.
The logistic regression analysis for the overall patient population demonstrates that primary failure is positively associated with female sex (odds ratio [OR] 1.46, p = 0.049) and non-Hispanic black ethnicity (OR 1.61, p = 0.021), while negatively associated with statin use (OR 0.59, p = 0.013) (Tab. III). Further analyses of the HD-naïve and -experienced patient subsets indicate that none of the above factors predict non-maturation in patients with AVF placement prior to HD initiation, but do affect outcomes in patients already receiving HD. In this latter group, the non-Hispanic black demographics are associated with primary failure (OR 1.68, p = 0.025), while statin treatment improves maturation outcomes (OR 0.60, p = 0.023) (Tab. III).
TABLE III.
Associations between baseline clinical factors and maturation outcomes on multivariate logistic regression analysis
| Odds ratio | Confidence interval | p value | |
|---|---|---|---|
|
| |||
| All patients (n = 612) | |||
| Age | 1.01 | 1.00–1.02 | 0.13 |
| Female sex | 1.46 | 1.001–2.14 | 0.049 |
| Ethnicity | |||
| Non-Hispanic black | 1.61 | 1.07–2.40 | 0.021 |
| Caucasian | 1.32 | 0.47–3.68 | 0.60 |
| Hypertension | 1.47 | 0.40–5.40 | 0.56 |
| Diabetes | 1.28 | 0.88–1.88 | 0.20 |
| CAD | 1.37 | 0.79–2.39 | 0.26 |
| CHF | 1.55 | 0.80–3.03 | 0.20 |
| Antiplatelet agents | 0.89 | 0.53–1.50 | 0.66 |
| Statins | 0.59 | 0.39–0.90 | 0.013 |
| ACE-I/ARB | 0.85 | 0.58–1.24 | 0.39 |
| One-stage AVF | 1.25 | 0.86–1.81 | 0.24 |
| HD initiated before AVF | 0.87 | 0.54–1.39 | 0.57 |
| AVF created before HD initiation (n = 114)* | |||
| Age | 1.01 | 0.98–1.04 | 0.48 |
| Female sex | 1.46 | 0.64–3.31 | 0.37 |
| Ethnicity | |||
| Non-Hispanic black | 1.07 | 0.47–2.45 | 0.88 |
| Caucasian | 0.32 | 0.01–10.39 | 0.52 |
| Statins | 0.78 | 0.33–1.81 | 0.56 |
| AVF created after HD initiation (n = 498)* | |||
| Age | 1.01 | 1.00–1.03 | 0.083 |
| Female sex | 1.36 | 0.90–2.06 | 0.15 |
| Ethnicity | |||
| Non-Hispanic black | 1.68 | 1.07–2.64 | 0.025 |
| Caucasian | 1.74 | 0.60–5.03 | 0.31 |
| Statins | 0.60 | 0.39–0.93 | 0.023 |
Reference status: Male sex, Hispanic, negative status for comorbidities and medications, two-stage AVF, and HD initiated after AVF creation.
Logistic regression models were minimally adjusted using selected variables from the all-patients model.
CAD = coronary artery disease; CHF = congestive heart failure; ACE-I = angiotensin I converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AVF = arteriovenous fistula; HD = hemodialysis.
In the group of pre-dialysis patients, there was no statistically significant association between BUN, eGFR or SCr and AVF primary failure (Fig. 1). There was a trend for increased frequency of non-maturation with higher SCr values, but the analysis did not reach statistical significance (p = 0.14).
Fig. 1.
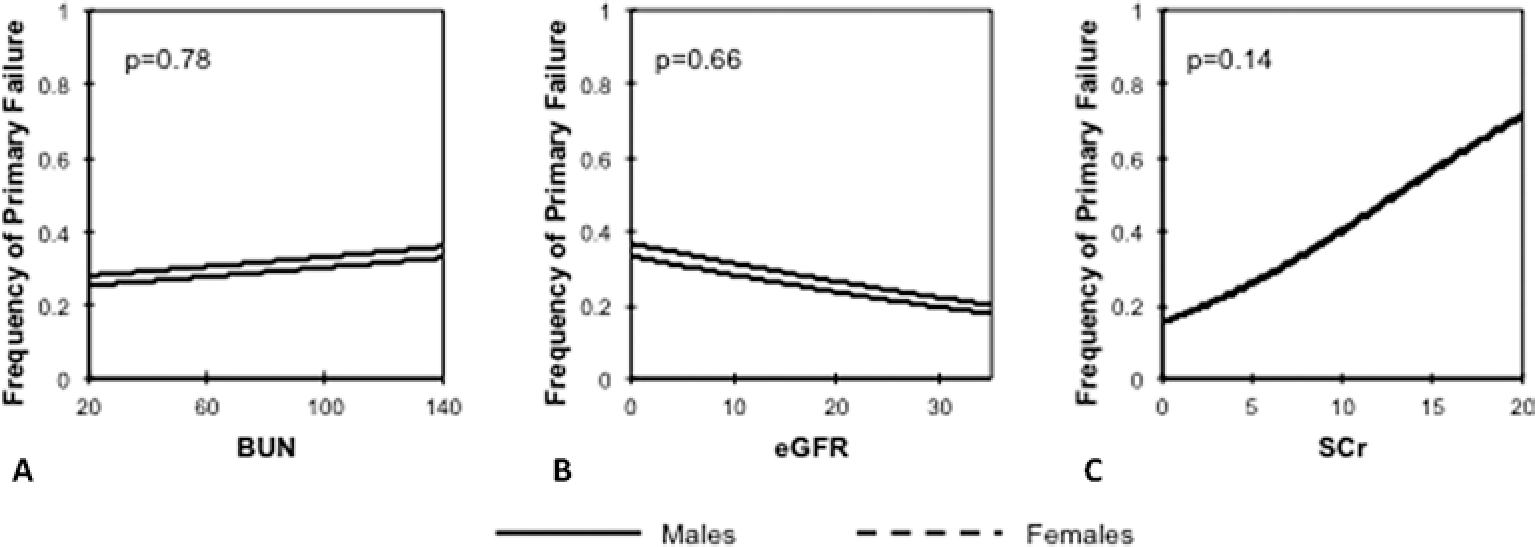
Logistic regression of primary failure as determined by pre-operative blood urea nitrogen (BUN); (A), estimated glomerular filtration rate (eGFR); (B) and serum creatinine (SCr); (C) in arteriovenous fistulas created prior to hemodialysis initiation. All analyses were adjusted for sex. The male and female curves in 1C are superimposed.
Discussion
The notion that there is an ideal time frame for AVF creation and HD initiation in advanced CKD patients, and that this may influence vascular access outcomes, remains a topic of heated debate. Uremic toxins are known to increase oxidative stress, endothelial dysfunction, intima-media thickness and calcification in the vasculature (15–20). However, it is not clear whether the potential benefits of uremia clearance with regular HD sessions to the vasculature offset the risks of infection and central venous stenosis associated with TC use. In this study, we demonstrate that patients with sustained uremia and those receiving regular HD prior to AVF creation have the same rate of AVF maturation. In addition, we reveal that pre-operative BUN, eGFR or SCr levels are not associated with primary failure of AVF created prior to HD initiation.
Current guidelines recommend that an AVF be created at least 6 months before the anticipated start of HD to allow sufficient time for its maturation (3). This is considered to be the best strategy to prevent medical complications related to TC and AVG use, and to decrease health-care costs in this patient population (25). Despite the rate of AVF creation in the USA nearly doubling from 32.2% in 2003 to 62.2% in 2013 (7), a large proportion of the advanced CKD population still initiates HD sub-optimally, either with a temporary TC or AVG (6). The main reasons for a sub-optimal start have been acute-on-CKD (31.3%) and patient-related delays (31.3%), followed by surgical delays (16.4%) and late decision making by nephrology (8.6%) (26).
Despite the deleterious effects of uremia to the vasculature, our results suggest that removing uremia with a TC solely with the purpose of improving AVF outcomes is not clinically justified. In our cohort, the time of HD initiation did not affect the frequency of primary failure. One explanation is that uremia does not impede proper AVF remodeling. Alternatively, we speculate that the damage done by uremic toxins to the vasculature is permanent, and cannot be reversed by regular HD sessions prior to AVF creation. Another factor to take into consideration is that the chronic inflammation associated with HD treatments (14–27) may offset the benefits of removing uremic toxins from the circulatory system. Several factors inherent to the HD technique are thought to initiate or promote inflammatory responses in the body. These include the retention or no elimination of pro-inflammatory molecules normally eliminated by the kidneys, exacerbation of oxidative stress, and stimulation of immune cells and activation of complement by dialysis membranes and pyrogenic substances (27). Of note, we show that statin therapy is associated with a lower risk of AVF primary failure in patients receiving HD treatments, but not in the HD-naïve cohort, possibly as a result of a heightened inflammatory state in the former. The potential benefit of statins in AVF maturation contradicts a previous retrospective study by Pisoni et al (28), but is supported by a recent publication by our group that suggests that not all statins are equally efficacious at reducing primary failure (29).
Our results are in agreement with a recent study of 569 patients, which reported that pre-dialysis patients and those receiving HD treatments had the same frequency of early AVF failure (30). In addition, the authors showed no association between eGFR and AVF outcomes, but did find a statistically significant relationship between serum urea and primary failure. In contrast with our cohort, the patient population in the above study was mostly of Caucasian origin, the average serum urea level was half the one in our patient cohort, and no brachio-basilic AVFs were included. Interestingly, among patients already on HD, Aitken et al (30) demonstrated that those who dialyzed on the day prior to AVF creation had an almost two-times higher frequency of failure than patients who dialyzed on the same day of surgery. Further investigations are needed to elucidate whether this improvement is due to the removal of one-day worth accumulation of uremic toxins or because the inflammatory responses triggered by the HD treatment had peaked in patients who had dialyzed the day prior to surgery.
The limitations of this study include the lack of vessel histology information, the smaller number of pre-dialysis patients compared to the HD-experienced cohort, and the possibility that additional clinical differences between both groups and not assessed in the present study play a major role in AVF maturation outcomes.
Conclusion
Despite the above limitations, our results indicate that timing of AVF creation (before or after HD initiation) is not statistically associated with vascular access outcomes. Our results also show that statins improve AVF maturation outcomes among patients already on HD. This important finding needs to be confirmed in a randomized control trial. If such a benefit is confirmed, it may lead to the reduction of morbidity, mortality, and HD access-related costs in ESRD patients.
Financial support:
The National Institutes of Health grant R01-DK-098511 to R.I.V-P and L.H.S supported this study.
Footnotes
Disclosures
Conflict of interest: All authors declare that they have no competing financial interests that might have influenced the present study or the preparation of the manuscript.
References
- 1.Lee T, Lok C, Vazquez M, Moist L, Maya I, Mokrzycki M. Minimizing hemodialysis catheter dysfunction: an ounce of prevention. Int J Nephrol. 2012;2012:170857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J; CHOICE Study. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol. 2005;16(5):1449–1455. [DOI] [PubMed] [Google Scholar]
- 3.Vascular Access Work G; Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–S247. [DOI] [PubMed] [Google Scholar]
- 4.The Fistula First Catheter Last Initiative. Available at http://fistulafirst.esrdncc.org/ffcl/for-ffcl-professionals/. Accessed Dec 26, 2016. [Google Scholar]
- 5.Malas MB, Canner JK, Hicks CW, et al. Trends in incident hemodialysis access and mortality. JAMA Surg. 2015;150(5):441–448. [DOI] [PubMed] [Google Scholar]
- 6.Lilly MP, Lynch JR, Wish JB, et al. Prevalence of arteriovenous fistulas in incident hemodialysis patients: correlation with patient factors that may be associated with maturation failure. Am J Kidney Dis. 2012;59(4):541–549. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. End Stage Renal Disease Network Organization Program 2013 Summary Annual Report. Baltimore, MD: CMS; 2015. Available from http://fistulafirst.org/wp-content/uploads/2014/10/SAR_2013_Report_. Accessed Dec 26, 2016. [Google Scholar]
- 8.Rayner HC, Pisoni RL, Gillespie BW, et al. ; Dialysis Outcomes and Practice Patterns Study. Creation, cannulation and survival of arteriovenous fistulae: data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;63(1):323–330. [DOI] [PubMed] [Google Scholar]
- 9.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23(10):3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hruby Z, Stanek-Piotrowska M, Turek J, et al. The clinicopathological determinants of native arteriovenous fistula failure in patients on maintenance hemodialysis. Adv Clin Exp Med. 2013;22(4):495–500. [PubMed] [Google Scholar]
- 11.Lee T, Roy-Chaudhury P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis. 2009;16(5):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JK, Jeong JH, Song YR, et al. Obesity-related decrease in intraoperative blood flow is associated with maturation failure of radiocephalic arteriovenous fistula. J Vasc Surg. 2015;62(4):1010–1017.e1. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Rodriguez S, Caballo C, Gutierrez G, et al. TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia. Eur J Clin Invest. 2015;45(2):160–169. [DOI] [PubMed] [Google Scholar]
- 14.Caglar K, Peng Y, Pupim LB, et al. Inflammatory signals associated with hemodialysis. Kidney Int. 2002;62(4):1408–1416. [DOI] [PubMed] [Google Scholar]
- 15.Rossi M, Campbell KL, Johnson DW, et al. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 34 chronic kidney disease. Arch Med Res. 2014;45(4):309–317. [DOI] [PubMed] [Google Scholar]
- 16.Baylis C Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294(1):F1–F9. [DOI] [PubMed] [Google Scholar]
- 17.Passauer J, Pistrosch F, Büssemaker E, Lässig G, Herbrig K, Gross P. Reduced agonist-induced endothelium-dependent vasodilation in uremia is attributable to an impairment of vascular nitric oxide. J Am Soc Nephrol. 2005;16(4):959–965. [DOI] [PubMed] [Google Scholar]
- 18.Wever R, Boer P, Hijmering M, et al. Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 1999;19(5):1168–1172. [DOI] [PubMed] [Google Scholar]
- 19.Ku YM, Kim YO, Kim JI, et al. Ultrasonographic measurement of intima-media thickness of radial artery in pre-dialysis uraemic patients: comparison with histological examination. Nephrol Dial Transplant. 2006;21(3):715–720. [DOI] [PubMed] [Google Scholar]
- 20.Ibels LS, Alfrey AC, Huffer WE, Craswell PW, Anderson JT, Weil R III. Arterial calcification and pathology in uremic patients undergoing dialysis. Am J Med. 1979;66(5):790–796. [DOI] [PubMed] [Google Scholar]
- 21.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118(17):1748–1757. [DOI] [PubMed] [Google Scholar]
- 22.Cooper BA, Branley P, Bulfone L, et al. ; IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. [DOI] [PubMed] [Google Scholar]
- 23.Tang SC, Ho YW, Tang AW, et al. ; Hong Kong Peritoneal Dialysis Study Group. Delaying initiation of dialysis till symptomatic uraemiais it too late? Nephrol Dial Transplant. 2007;22(7):1926–1932. [DOI] [PubMed] [Google Scholar]
- 24.Stehman-Breen CO, Sherrard DJ, Gillen D, Caps M. Determinants of type and timing of initial permanent hemodialysis vascular access. Kidney Int. 2000;57(2):639–645. [DOI] [PubMed] [Google Scholar]
- 25.Kimball TA, Barz K, Dimond KR, Edwards JM, Nehler MR. Efficiency of the kidney disease outcomes quality initiative guidelines for preemptive vascular access in an academic setting. J Vasc Surg. 2011;54(3):760–765, discussion 765–766. [DOI] [PubMed] [Google Scholar]
- 26.Hughes SA, Mendelssohn JG, Tobe SW, McFarlane PA, Mendelssohn DC. Factors associated with suboptimal initiation of dialysis despite early nephrologist referral. Nephrol Dial Transplant. 2013;28(2):392–397. [DOI] [PubMed] [Google Scholar]
- 27.Jofré R, Rodriguez-Benitez P, López-Gómez JM, Pérez-Garcia R. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol. 2006;17(12)(Suppl 3):S274–S280. [DOI] [PubMed] [Google Scholar]
- 28.Pisoni R, Barker-Finkel J, Allo M. Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol. 2010;5(8):1447–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez L, Duque JC, Escobar LA, et al. Distinct impact of three different statins on arteriovenous fistula outcomes: a retrospective analysis. J Vasc Access. 2016;17(6):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aitken E, Jackson A, Kong C, Coats P, Kingsmore D. Renal function, uraemia and early arteriovenous fistula failure. BMC Nephrol. 2014;15(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]


