Abstract
Enhancement of glucose-stimulated insulin secretion (GSIS) in exogenously delivered pancreatic β-cells is desirable, for example, to overcome the insulin resistance manifested in type 2 diabetes or to reduce the number of β-cells for supporting homeostasis of blood sugar in type 1 diabetes. Optogenetically engineered cells can potentiate their function with exposure to light. Given that cyclic adenosine monophosphate (cAMP) mediates GSIS, we surmised that optoamplification of GSIS is feasible in human β-cells carrying a photoactivatable adenylyl cyclase (PAC). To this end, human EndoC-βH3 cells were engineered to express a blue-light-activated PAC, and a workflow was established combining the scalable manufacturing of pseudoislets (PIs) with efficient adenoviral transduction, resulting in over 80% of cells carrying PAC. Changes in intracellular cAMP and GSIS were determined with the photoactivation of PAC in vitro as well as after encapsulation and implantation in mice with streptozotocin-induced diabetes. cAMP rapidly rose in β-cells expressing PAC with illumination and quickly declined upon its termination. Light-induced amplification in cAMP was concomitant with a greater than 2-fold GSIS vs β-cells without PAC in elevated glucose. The enhanced GSIS retained its biphasic pattern, and the rate of oxygen consumption remained unchanged. Diabetic mice receiving the engineered β-cell PIs exhibited improved glucose tolerance upon illumination compared to those kept in the dark or not receiving cells. The findings support the use of optogenetics for molecular customization of the β-cells toward better treatments for diabetes without the adverse effects of pharmacological approaches.
Keywords: human beta cells, cyclic adenosine monophosphate, diabetes, insulin secretion, optogenetics, pancreas
Introduction
The release of insulin by pancreatic β-cells is critical for the proper homeostasis of blood glucose (BG). Glucose-stimulated insulin secretion (GSIS) entails the uptake of glucose by the β-cells and the production of adenosine triphosphate (ATP), closing ATP-sensitive potassium (K+ATP) channels and altering the cell membrane potential. The ensuing influx of calcium ions (Ca2+) induces exocytosis of insulin. The hormonal response of the β-cells is further tuned by moieties in the underlying molecular circuits including cyclic adenosine monophosphate (cAMP) and its downstream effectors, protein kinase A and exchange protein directly activated by cAMP.1,2 Altered activity of these cascades, which is noted in metabolic syndrome and type 2 diabetes characterized by hyperglycemia, is linked to suppression of the GSIS which is further exacerbated by the insulin resistance of adipose, liver, and muscle cells. Secretagogues such as sulfonylureas and glucagon-like peptide 1 (GLP-1) agonists are characterized by low specificity, diffusion limitations, and clearance rates, which are incompatible with the physiological kinetics of GSIS and entail a higher risk for hypoglycemic episodes.
Technologies for control of cellular function using light afford unparalleled spatial and temporal resolution, reversible induction, and fewer, if any, off-target effects. To this end, photoactivatable adenylyl cyclases (PACs), which are naturally expressed in microorganisms including the Euglena gracilis,3Oscillatoria acuminata,4 and Beggiatoa (bPAC)5,6 or engineered synthetically,7 have been adapted for use in animal cells for modulation of intracellular cAMP [cAMP]i. Given the role of this secondary messenger in GSIS, PACs may provide a handle for amplifying β-cell insulin secretion with light. Indeed, we reported that rodent β-cells and islets expressing bPAC display a 2- to 3-fold increase in GSIS during illumination with blue light.8,9 As a result, a lower number of insulin-producing cells is adequate to ameliorate diabetes upon transplantation in mice treated with streptozotocin (STZ).
These findings illustrate the potential of optogenetically engineered β-cells in the development of novel strategies for diabetes treatment and therapy. While cAMP is a mediator of GSIS in rodent and human β-cells, whether PAC-mediated modulation of GSIS is feasible and renders a similar hormonal amplification in the latter remain unclear. Indeed, there are significant interspecies differences in GSIS-related features,10,11 glucose transporters, composition, and regulation of the K+ATP- and Ca2+-channels, which result in varied responses to glucose and incretins. Additionally, when compared to rodent β-cells, human β-cells have significantly higher resting [cAMP]i.12 Given that GSIS in human islets is 2–3 times lower in comparison to that in murine islets,13 further investigation is motivated for employing optogenetics to amplify the hormone released by human β-cells.
In this study, we engineered human EndoC-βH3 β-cells,14 which retain β-cell-specific features such as native GSIS, to express bPAC. The temporal augmentation of [cAMP]i with illumination, and the associated off-kinetics were determined along with the resulting GSIS at different glucose concentrations. Moreover, the scalable cultivation of EndoC-βH3 β-cells as clusters termed pseudoislets (PIs) in stirred-tank (spinner flask) cultures was developed in conjunction with efficient adenoviral transfer of the bPAC gene. The extent of GSIS amplification with blue light was assessed for bPAC+ PIs in culture and after their transplantation in STZ-treated diabetic mice. Molecular customization of human β-cells with optogenetic moieties allied to scalable manufacturing of the engineered islet cells is an appealing avenue toward the development of cell replacement therapies for diabetes.
Results
Light-Modulated [cAMP]i and GSIS in Monolayers of EndoC-βH3 β-Cells Expressing bPAC
We set out to investigate the changes in [cAMP]i and insulin response of human β-cells expressing bPAC with blue light illumination (Figure 1A). For this purpose, EndoC-βH3 cells routinely maintained as monolayers were transduced with an adenoviral vector, AdbPAC, for coexpression of bPAC (tagged with cMyc) and the mCherry via an internal ribosomal entry site (IRES) under the cytomegalovirus (CMV) promoter.8 The extent of bPAC (mCherry) expression was assessed by microscopy (Figure 1B) and flow cytometry (Figure 1C) at different multiplicities of infection (MOIs). The fraction of mCherry+ cells leveled off at ∼80% for MOI values greater than 100, while viability was maintained at 90%. Subsequent experiments were carried out with cells transduced at an MOI of 200. The presence of the bPAC protein in these cells was confirmed by Western blotting (Figure 1D).
Figure 1.
Expression of bPAC by human β-cells and modulation of cAMP with light. (A) Schematic of the blue-light-stimulated increase of cAMP in β-cells expressing bPAC. (B) Fluorescence and brightfield micrographs of EndoC-βH3 β-cells transduced with AdbPAC (MOI: 100). (C) Cell viability and the fraction of mCherry-positive cells following infection with AdbPAC at different MOI values. (D) Western blotting for the expression of bPAC. 1: NT cells, 2: cells transduced with AdeGFP, 3: cells transduced with AdbPAC, L: marker ladder. (E) cAMP concentration was determined for cells exposed to blue light or maintained in dark for different glucose concentrations as indicated. *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant. (F) On-kinetics of [cAMP]i for cells under continuous illumination (starting at 0 min) and (G) off-kinetics at 15 mM glucose. Time is after termination of a 60 min exposure to blue light. The results are mean ± standard error of the mean (SEM) values from at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant.
Changes in [cAMP]i induced by the photoactivation of bPAC were examined next. In addition to bPAC+ cells, nontransduced (NT) β-cells were included as well as NT cells exposed to 50 μM 3-isobutyl-1-methylxanthine (IBMX), a phosphodiesterase (PDE) inhibitor raising [cAMP]i. The cells were exposed to 0.5, 2.8, or 15 mM of glucose and were either illuminated or kept in the dark for 40 min (Figure 1E). The NT group showed an increase from 2.7 ± 1.8 at 0.5 mM and 1.6 ± 1.0 at 2.8 mM to 9.2 ± 4.5 ng cAMP/mg total protein at 15 mM of glucose and further to 16.5 ± 2.7 ng cAMP/mg total protein (p = 9 × 10–3, n = 3) with IBMX. As expected, exposure to blue light had no effect on the cAMP of the NT cells. Similarly, bPAC-expressing β-cells kept in dark exhibited 9.6 ± 6.4 ng cAMP/mg total protein at 15 mM. Yet, when these cells were irradiated with blue light, [cAMP]i was higher at all glucose concentrations tested: 15.0 ± 3.4, 55.5 ± 11.6, and 99.0 ± 16.7 ng cAMP/mg total protein at 0.5, 2.8, and 15 mM glucose, respectively. These findings prove that human β-cells with bPAC raise their [cAMP]i with blue light.
We also probed the [cAMP]i on- and off-kinetics with light-induced modulation at 15 mM of glucose. Cells were exposed to light continuously for 2.5, 5, 10, 15, 20, 40, and 60 min (Figure 1F). A marked [cAMP]i increase was observed within 2.5 min (64.0 ± 7.6 ng cAMP/mg total protein or about 10-fold vs NT cells), and a steady state was reached by 15 min (109.6 ± 19.1 ng cAMP/mg total protein) and was sustained for up to 60 min. Conversely, a rapid 60% reduction from peak [cAMP]i (45.1 ± 20.1 ng cAMP/mg total protein) was noted at 2.5 min postillumination (Figure 1G), with [cAMP]i returning to baseline within 15 min and no further fluctuations observed in comparison to that in the NT group. Thus, the on-demand regulation of [cAMP]i with light in human β-cells expressing bPAC affords rapid on/off-kinetics.
Next, we addressed how bPAC-mediated [cAMP]i regulation translated to the GSIS (Figure 2A). Insulin release was measured at 2.8 and 15 mM glucose (Figure 2B). As expected, the NT and bPAC+ β-cells without photoinduction displayed the same hormonal secretion at the corresponding glucose level. When the bPAC β-cells were exposed to blue light at 15 mM glucose, they exhibited a 2-fold greater insulin secretion rate versus that of the NT β-cells (200.0 ± 21.6 and 100.0 ± 9.4 ng insulin/mg total protein/h, respectively; p = 6 × 10–3, n = 3) and bPAC+ or NT β-cells kept in dark. This is also higher compared to that in NT β-cells treated with IBMX (143.2 ± 8.6 ng insulin/mg total protein/h; p = 3 × 10–3, n = 3). Similarly, the insulin release in bPAC+ cell cultures at 2.8 mM glucose under blue light was 2.7 times higher compared to that in the NT cells (131.0 ± 17.5 and 48.3 ± 3.7 ng insulin/mg total protein/h, respectively; p = 10–3, n = 3). It is important to note that the increase in insulin release occurred only when the bPAC+ β-cells were exposed to blue light. In parallel, EndoC-βH3 expressing enhanced green fluorescent protein (eGFP) after adenoviral transduction (AdeGFP)15 did not show any difference in GSIS from bPAC+ and NT β-cells in dark at both glucose concentrations, suggesting that transduction did not affect the hormonal response (Figure 2B).
Figure 2.
Profile of insulin release by β-cells expressing bPAC. (A) Schematic representation of GSIS amplification with blue light in β-cells expressing bPAC. (B) Insulin secretion of bPAC-expressing (bPAC), AdeGFP-transduced cells (eGFP), NT cells, as well as NT cells treated with 50 μM IBMX under light or dark conditions and 2.8 or 15 mM of glucose. (C) Secreted insulin vs time of illumination and (D) corresponding rate of insulin secretion for β-cell monolayers expressing bPAC with or without light. Results for the NT group are also shown. The results are mean ± SEM values from at least three independent experiments. #p < 0.02, *p < 0.03, **p < 0.001, ns: not significant.
The secreted insulin was also measured at different times over 60 min and 15 mM glucose in NT and bPAC+ EndoC-βH3 β-cell monolayers with or without light (Figure 2C,D). For all groups, a sharp increase in the release rate was noted first followed by a slower release, recapitulating the biphasic GSIS profile of native β-cells. Hence, the expression and activation of bPAC did not alter the physiological GSIS pattern of human β-cells. The initial production rate was similar among the three groups most likely because the greater surge in cAMP observed early on in bPAC+ cells under blue light (Figure 1F) requires time to boost the GSIS (Figure 2C). However, the rate of insulin production during the second phase was higher (p < 0.03) for bPAC-expressing cells under illumination than that without light and for NT cells (Figure 2D). Thus, light-mediated amplification of [cAMP]i and biphasic GSIS are realized with physiologically relevant kinetics in human β-cells expressing bPAC and exposed to blue light.
Scalable Generation of EndoC-βH3 β-Cell PIs Expressing bPAC
Dispersed primary β-cells and β-cell lines tend to form islet-like clusters or PIs, which display extensive homotypic interactions and enhanced GSIS16,17 in comparison to β-cell monolayers. Hence, we explored the formation of EndoC-βH3 PIs in multiwell plates on an orbital shaker and in scalable stirred-suspension (spinner flask) cultures (Figure 3A). Formation of these PIs is a promising way of amplifying the GSIS in addition to the bPAC-mediated elevation.
Figure 3.
Production of human EndoC-βH3 PIs in stirred-suspension culture modalities. (A) Culture of β-cells as monolayers or PIs in suspension cultures. (B) Growth (fold change over seeding density) of EndoC-βH3 cells in monolayer, orbital shaker, or spinner flask cultures in 8 days. (C,D) Average diameter over time of PIs grown in (C) orbital shaker and (D) spinner flask cultures (n ≥ 50 aggregates per time point). (E) Viable cell density and viability (%) within PIs cultivated in spinner flasks. (F) Secreted insulin of EndoC-βH3 monolayers and PIs from spinner flasks. **p < 0.01, ns: not significant. Results are shown as mean ± standard deviation (SD).
EndoC-βH3 cells in monolayers grew almost 2-fold in 8 days (Figure 3B) but 20% more (2.4-fold; p = 0.02) as PIs on an orbital shaker. Similarly, spinner flask PI cultures had a 17% (2.3-fold; p = 0.013) higher growth vs that of monolayers. There was no significant difference between the spinner flasks and shaker plate cultures. Within 24 h of seeding, the aggregates emerged with an average size of 91 ± 16 μm (Figures 3C and S1), which became 122 ± 20 μm by day 8. For PIs generated in the spinner flasks, their average diameter changed from 63 ± 14 μm on day 1 to 117 ± 25 μm at the end of day 8 (Figure 3D), and viability was over 90% for the duration of the culture (Figure 3E). PIs for subsequent experiments were produced in spinner flasks, given the scalability of this culture modality. The GSIS of these PIs was measured and compared to that of EndoC-βH3 cell monolayers (Figure 3F). Cells in PIs exhibited higher insulin secretion at 2.8 and 15 mM of glucose. For instance, PIs and monolayers at high glucose secreted 357.6 ± 39.6 and 100.0 ± 8.44 ng insulin/mg protein/h (p = 6 × 10–3), respectively. In contrast, there was no difference in the GSIS of PIs and monolayers at 0.5 mM of glucose. The results show that the EndoC-βH3 cells cultured in stirred suspension form PIs producing higher amounts of insulin in response to glucose.
Transduction of EndoC-βH3 Cell PIs and Insulin Secretion
Having established the conditions for the formation and culture of human β-cell PIs in stirred suspension, a method was then developed for efficient adenoviral delivery of the bPAC gene to the clusters. When PIs were directly infected, only about 50% of the cells showed evidence of transgene expression (Figure 4A) with the transduced cells located mainly at the exterior of the aggregates. To increase the fraction of bPAC+ cells, the β-cells were exposed to virus in monolayers and harvested 48 h later for seeding in spinner flasks. As a result, the expression of mCherry was apparent throughout each PI in 82% of the cells (Figure 4A,B). This transduction protocol had no adverse effect on the cell viability.
Figure 4.
Adenoviral delivery of the bPAC gene and GSIS of PIs formed in stirred-suspension. (A) Schematic of adenoviral transduction of EndoC-βH3 β-cells. Infection of cells prior to PI formation yielded significantly more mCherry+ cells vs direct infection of PIs. Images and flow cytometry graphs are shown for PIs infected at an MOI of 200. (B) Confocal microscopy images of PIs formed after transduction at an MOI of 200: 4′,6-diamidino-2-phenylindole (blue), Calcein AM (green), bPAC-mCherry (red), and merged images. Scale bar: 100 μm. (C) Insulin secretion results for PIs expressing bPAC or eGFP or NT and either illuminated with blue light or kept in dark. *p < 0.05, **p < 0.01. (D) Insulin secreted over time at 15 mM glucose. *p < 0.05, **p < 0.01: comparison between bPAC-expressing PIs and NT PIs, both exposed to light. Results are shown as mean ± SD.
The GSIS of the PIs was determined at different glucose levels with or without illumination (Figure 4C). At 15 mM glucose, bPAC-expressing PIs irradiated with blue light released significantly more insulin than that by the NT PIs, 731.1 ± 140.0 and 357.6 ± 39.6 ng insulin/mg total protein/h, respectively, i.e., a greater than 2-fold increase. A similar difference was recorded between bPAC+ PIs with light stimulation and those kept in dark. Upon shining light, the GSIS increased by 1.8-fold at 2.8 mM glucose compared to that of NT β-cells. When the bPAC+ PIs were kept in the dark, there was no difference in insulin secretion with the NT β-cell PIs, and PIs transduced with an adenovirus carrying the eGFP gene, indicating that the dark state of bPAC and the adenoviral transduction had no detectable effects on GSIS. Insulin secretion was also low for all groups at 0.5 mM of glucose. Additionally, release of the hormone was monitored over time for EndoC-βH3 β-cell PIs expressing bPAC, eGFP, or without transgene and with or without light over 1 h at 15 mM of glucose (Figure 4D). A significant elevation in insulin release was observed in bPAC-expressing PIs with light versus all other groups.
In summary, the efficient generation of PIs of human β-cells transduced to express bPAC was established in a scalable cultivation modality. Cells in these PIs displayed high viability, enhanced β-cell specific function, and pronounced transgene expression. Activation with blue light led to a >2-fold amplification of GSIS compared to that of PIs of bPAC+ β-cells in dark and NT β-cells.
Improvement of Glucose Tolerance in a Mouse Model of Diabetes with bPAC-Expressing EndoC-βH3 PIs
The findings prompted us to assess the function of the engineered EndoC-βH3 β-cell bPAC-expressing PIs in vivo in a murine model of diabetes. In preparation for delivery to animals, the PIs were encapsulated in alginate for immunoprotection. The PIs were processed via electrostatic-field-assisted encapsulation, and the light-mediated amplification of their GSIS was evaluated. Beads with a diameter of 400–500 μm were generated, and most contained between one and three PIs (Figure 5A). The PIs remained intact during the encapsulation process maintaining mCherry expression and high viability (>90%; Figure S2). Moreover, the GSIS of encapsulated and naked PIs was the same (Figure 5B), underlining that encapsulation does not have notable adverse effects on the cells.
Figure 5.
Assessment of GSIS and OCR for encapsulated EndoC-βH3 PIs expressing bPAC. (A) PIs are shown at 24 h after encapsulation with prominent mCherry expression. Microscopic images are brightfield (right), epifluorescence (mCherry; middle), and merged (left). Scale bar: 200 μm. (B) GSIS response of EndoC-βH3 cell monolayers, spinner-flask cultured naked PIs, and PIs encapsulated in alginate beads. *p < 0.1, **p < 0.01, ns: not significant, n = 3 (Student t test). (C) OCR of NT PIs and those expressing bPAC, with or without light. The OCR of encapsulated bPAC+ PIs under illumination is also shown. Blue bars: blue light; open bar: no light. Results are shown as the mean ± SEM.
When the encapsulated bPAC+ PIs were exposed to light, their O2 consumption rate (OCR) remained unchanged compared to that of NT β-cells and bPAC+ β-cell PIs without encapsulation (Figure 5C). Thus, increased GSIS due to bPAC activation does not alter the OCR, as we reported for rodent β-cell PIs with bPAC,9 and the alginate beads permit adequate diffusion of O2. These findings show that human β-cells featuring bPAC are appealing as a GSIS system, easing the need for higher β-cell numbers and the associated greater demand for oxygenation for sufficient production of insulin to regulate BG.
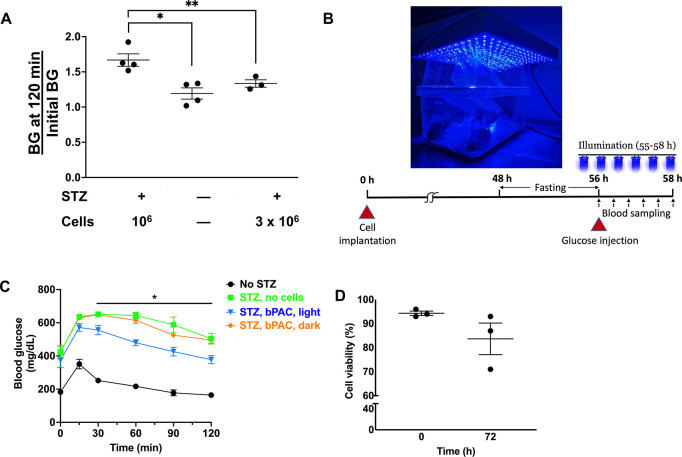
Given that EndoC-βH3 cells natively secrete insulin, the transplantation of a large number of these cells may improve diabetes in STZ-treated mice, perplexing our investigation of the efficacy of light-triggered amplification of GSIS for reducing hyperglycemia. To this end, we carried out a study to estimate the minimum number of EndoC-βH3 cells that is sufficient to improve hyperglycemia in mice with STZ-induced diabetes. Animals transplanted with encapsulated EndoC-βH3 cell PIs (without bPAC expression) underwent a glucose tolerance test (GTT) (Figure 6A) following overnight fasting. In control mice without diabetes, BG returned to baseline within 120 min. A similar pattern was observed in diabetic animals receiving 3 × 106 cells, proving that this number of cells is sufficient to improve diabetes without additional amplification of GSIS. Considering that bPAC-expressing PIs display a greater than 2-fold GSIS, we tested the glucose tolerance in STZ-treated mice upon subcutaneous delivery of 106 cells. The BG failed to decrease to the level prior to the GTT after 2 h, indicating that the implanted β-cell mass did not generate enough insulin to normalize blood sugar. Hence, we determined that diabetic mice receiving 106 EndoC-βH3 β-cells would not experience relief from hyperglycemia.
Figure 6.
Amelioration of glucose tolerance with encapsulated bPAC-expressing PIs. (A) BG at 120 min over the initial BG for mice rendered diabetic with STZ and receiving 106 or 3 × 106 EndoC-βH3 cells. Results (mean ± SEM) for control mice are included (without STZ treatment and cells). *p < 0.01, **p < 0.05. (B) Animals housed under a blue light LED array for photoinduction of the bPAC of transplanted cells. A schematic of the regimen for transplantation, illumination, and the GTT assay is shown. (C) BG levels are shown during a GTT. Results are mean ± SEM. *p < 0.05 for diabetic mice with bPAC+ cells and light (blue curve) vs no light (orange curve). Animals treated with STZ but not receiving cells (green curve) and untreated controls (black curve) are also shown. n = 5–6 animals per group. Comparative analysis based on Mann–Whitney and post hoc Holm–Šídák tests. (D) Viability (%) of PIs before transplantation and after retrieval at 72 h post-implantation.
To this end, PIs totaling 106 β-cells encapsulated in alginate were implanted subcutaneously in each animal that was housed under a blue light-emitting diode (LED) array (Figure 6B). BG in diabetic animals receiving bPAC+ β-cell PIs returned to their initial glucose level during the GTT with illumination (Figure 6C). This pattern was akin to that of nondiabetic mice (control) without transplanted cells. In contrast, STZ-treated mice with bPAC+ PIs but no exposure to blue light failed to reduce their BG within 120 min as did diabetic animals without cells.
72 h after transplantation, the encapsulated PIs were retrieved, and their viability was assessed (Figure 6D). We observed a reduction from 94.3 ± 1.53 to 83.7 ± 11.37%, potentially because the subcutaneous site is not ideal for long-term support of the transplanted cells. Overall, these findings are aligned with the notion that bPAC-carrying human β-cells display a GSIS that can be modulated by blue light, improving glucose tolerance in diabetic animals.
Discussion
The introduction of synthetic molecular pathways entailing photoactivatable moieties to cells has paved new avenues for the nonpharmacological regulation of diverse physiological functions, thereby offering exciting alternatives for cellular therapies in regenerative medicine. Optical modulation of cAMP and GSIS is possible with the introduction of PAC to rodent islets and β-cells.8,9 Here, we show for the first time that this is also feasible in human β-cells. Traditional approaches of GSIS amplification relying on drugs are burdened by side effects (e.g., hypoglycemia), low specificity (e.g., sulfonylureas target K+ATP channels, which are not expressed solely in β-cells), and overriding of the physiological dependence of insulin exocytosis on extracellular glucose levels. Our findings show that diabetic mice display improved glucose tolerance following the delivery of human β-cells with bPAC and illumination, and encourage the use of optogenetic strategies in order to engineer cells for insulin replacement therapies for diabetes. To this end, the expression of PACs can be engineered in insulin-secreting cells generated from human pluripotent stem cells (hPSCs).18
Optogenetic approaches for glucose homeostasis have been reported using pancreatic and extrapancreatic cells. In one such example, melanopsin, which is a Gq-linked G-protein-coupled receptor capable of activating the phospholipase C path, was employed and led to increases in cytosolic Ca2+, triggering the release of insulin.19 A cell line was created using 1.1 × 107 cells—derived by electrofusion of primary human β-cells with PANC1 pancreatic cancer cells and lacking glucose sensing—expressing melanopsin and a luciferase/insulin construct. The cells released insulin within 15 min of exposure to 475 nm light or a smartphone flashlight and improved the hyperglycemia of STZ-treated mice following encapsulation in alginate/poly-l-lysine/alginate beads and implantation. In another study, HEK293 cells were engineered to coexpress melanopsin and a short human GLP-1 (shGLP-1) under a nuclear factor of activated T cells (NFAT)-targeted promoter.20 Activation of melanopsin by blue light elevates [Ca2+]i and mobilizes calcineurin to initiate transcription of NFAT-mediated shGLP-1. Exposure of cultured murine βTC-6 β-cells to the supernatant from blue light-treated engineered HEK293 cells causes the secretion of insulin. In transplantation experiments, db/db type 2 diabetic mice showed both circulating shGLP1 and insulin levels increasing after 48 h of illumination along with faster reduction in BG compared to that in animals without photostimulation. Along the same vein, a far-red light (FRL)-inducible system was introduced to HEK293 cells employing an engineered bacterial photoreceptor (BphS), catalyzing the conversion of GTP into cyclic diguanylate (c-di-GMP).21 The production of c-di-GMP activated a c-di-GMP-responsive promoter, driving the expression of shGLP-1 and mouse insulin. When 2 × 106 HEK293 cells carrying this system were encapsulated in alginate and subcutaneously delivered to diabetic mice, the BG levels were improved reaching comparable levels to those of wild-type animals for approximately 13 days under FRL illumination. Of note, light was provided through a system interfacing a smartphone with glucose sensors and cell-harboring hydrogels with an embedded LED. Application of the FRL-induced system was also demonstrated in human mesenchymal stem cells, which upon implantation to STZ-treated cells ameliorated hyperglycemia for 40 days.22
Similar to these reports, the work presented here showcases the potential of optogenetic engineering of cells to control blood sugar in a diabetic setting. Yet, direct modulation of cAMP via bPAC activation to augment GSIS rather than employing more complex circuitries enables physiologically relevant response kinetics and thus efficient control of BG. Moreover, the use of human EndoC-βH3 β-cells is clinically relevant, and given their native capacity for glucose sensing and insulin secretion,14 it is advantageous compared to that of nonpancreatic cells and fusion-derived cells. Recently, the secretion of insulin was reported in pancreatic islet-like organoids of hPSCs carrying a variant of opto-stromal interaction molecule 1 that allows the modulation of intracellular Ca2+ with light.23 It should be noted that the elevation of Ca2+ causes the release of hormone in β-cells irrespective of the concentration of extracellular glucose.
EndoC-βH3 cells in PIs exhibited higher GSIS than that in monolayers backing findings by us and others using dispersed and clustered islet cells and β-cells.8,9,16,17,24,25 The enhanced function of PIs is most likely due to their ultrastructural similarities to pancreatic islets featuring extensive cell–cell juxtaposition and communication. As with rodent β-cells,17 the culture of EndoC-βH3 cells in stirred-suspension spinner flasks allowed for the scalable formation of PIs with a relatively uniform size and preserved viability, even after adenoviral transduction. Cell growth was only slightly higher in suspension vs monolayer cultures, possibly due to the better mass transfer and mixing characteristics of stirred suspension. The number of cells only doubled during 8 days of culture, precluding significant “dilution” of the adenovirus-delivered transgenes. Stirred-tank cultivation affords straightforward control of the size distribution of PIs via adjustments in the cell seeding density and agitation rate, although this dependence was not investigated exhaustively here. Along with the efficient adenoviral transduction for the delivery of transgenes encoding proteins with optical activity, the generation of human β-cell clusters in stirred-tank cultures points to a workflow for the scalable manufacturing of optogenetically engineered β-cells in clinically relevant quantities.17,25 If necessary, the growth of EndoC-βH3 cells can be curtailed through treatment with tamoxifen since the cells express CRE-ERT2, which upon induction excises the immortalizing transgene (large T antigen of the SV40), decreases proliferation, and elevates β-cell-specific features.14
This was not deemed necessary here, particularly for PIs intended for implantation because of the encapsulation, which confines cell clusters and limits their growth. The encapsulated PIs retained their ability for GSIS akin to that of naked PIs. Alginate has been traditionally used26 for implantation of islet cells in animals, given its excellent biocompatibility, minimal cytotoxicity, and permeability favoring the exchange of nutrients and waste between the cells and the peri-implantation area but disallowing immune cell infiltration. With the optical induction of cells in mind, the transparency of alginate can be increased when combined with d-glucono-δ-lactone, as shown for disc-shaped scaffolds.1,27 Previously, alginate hydrogels were employed to combine optogenetically engineered cells with remotely controlled red-light LEDs.21 Such designs offer maximum proximity of the cells to the light source but increase significantly the device size and require further engineering to address issues pertinent to the powering of the implanted LED and potential effects from heat generation particularly as scaling up of the scaffold is pondered for use in larger animals and humans. Compared to blue light, however, the use of red light for activation is advantageous due to its greater depth of tissue penetration. This may reduce the delivery device size by enabling extracorporeal photoinduction. To this end, engineered near-infrared window adenylyl cyclases (ACs)7,28 may be used with β-cells for delivery to sites such as the vasculature-rich omentum24,29 for long-term support of cell function.
Nevertheless, the subcutaneous delivery of bPAC+ EndoC-βH3 cell PIs to diabetic mice resulted in a GTT response upon illumination that was similar to that of nondiabetic animals. Complete reversion of diabetes would not be expected in this setting given that the viability of the delivered cells drops significantly at 72 h after implantation in agreement with previous findings.9 Notably, potential lysis of PI cells and release of intracellular insulin were ruled out as contributing to the observed decrease of blood sugar since a similar reduction was not observed in STZ-treated mice receiving encapsulated bPAC+ β-cell PIs without light. Phototoxicity was also excluded as a cause of cell death since bPAC-expressing cells under blue light in culture exhibit similar viability compared to that of the same cells kept in dark.8 Our findings warrant studies on alternate sites for delivery along with the design of scaffolds with superior mass transfer properties promoting vascularization30 for the maintenance and function of the implanted cells.
The suitability of the implantation site is also dependent on the metabolic requirements of the cells. The exchange of nutrients, particularly O2, between the cells and the host is a major hurdle in the design of β-cell replacement systems, which call for a substantial number of metabolically very active cells for sufficient insulin response. As with rodent β-cells expressing bPAC, light-induced augmentation of GSIS in bPAC+ human β-cells does not change their OCR compared with that in their counterparts without photoinduction or bPAC expression. The findings are also aligned with another study31 reporting no changes in O2 consumption of mouse and rat islets cultured in high glucose and the cAMP-elevating agents exendin-4, forskolin, or GLP-1, despite the marked potentiation of GSIS. Interestingly, GLP-1 raises ATP levels in the cytosol and mitochondria, which may reduce the need for additional O2 consumption.32 Thus, engineered β-cells featuring optogenetic moieties (e.g., JellyOp14,33,34 and PaaC35) for the regulation of cAMP with light such as the bPAC-based system described here may serve as tools to research the molecular dependencies between GSIS amplification and metabolic demands of human β-cells.
The human EndoC-βH3 cells expressing bPAC can also be employed to further unravel the functional roles of cAMP in normal and diabetic islets. For instance, the photoactivation of bPAC together with genomic and transcriptomic analyses will help to gain insights about the gene networks influenced by cAMP in human β-cells36 without the nonspecific effects of pertinent chemical inducers. The bPAC activation can also be combined with real-time monitoring of [cAMP]i dynamics using a cAMP biosensor.37 Changes in baseline [cAMP]i levels have also been reported in human and mouse islets or β-cells maintained in supraphysiological concentrations of glucose over long periods38 or following short exposure to saturated fatty acids.39 To this end, reduced glucose-induced production of [cAMP]i and PDE expression are reported in animal models of diabetes.40,41 The underlying mechanisms remain to be elucidated, creating opportunities for optogenetically engineered β-cells to expand our knowledge of islet cell biology and aid in devising improved strategies for treating diabetes.
Materials and Methods
EndoC-βH3 Cell Monolayer Culture
EndoC-βH3 cells14 were grown at 37 °C and 5% CO2 on tissue culture plates, which were precoated with Matrigel (1:50 v/v) and 10 μg/mL fibronectin at 37 °C for 1 h. Cells were passaged at a ratio of 1:3 every 9 days after 90% confluence. The culture medium consisted of Dulbecco’s modified Eagle’s medium (DMEM) with 1 g/L glucose and supplemented with 2% bovine serum albumin (BSA; fraction V), 50 μM 2-mercaptoethanol, 10 mM nicotinamide, 5.5 μg/mL transferrin, 6.7 ng/mL sodium selenite, penicillin (100 IU/mL)/streptomycin (100 μg/mL), and 2 μg/mL puromycin. Cell counts and viability were determined by trypan blue dye (ThermoFisher) exclusion using a hemocytometer or TC20 counter (Bio-Rad, Hercules, CA). Blue light stimulation of cells cultured in 6- and 12-well plates was performed using an Arduino-controlled LED array.9
PI Culture
Monolayers of EndoC-βH3 cells were treated with a Rho-associated kinase (ROCK) inhibitor (10 μM Y-27632) for 1 h before being washed with phosphate-buffered saline (PBS) and incubated with TrypLE Express Enzyme (Thermo Fisher) for 10 min at 37 °C. Upon neutralization with 10% fetal bovine serum (FBS) in low-glucose (1 g/L) DMEM, the cell suspension was centrifuged at 200×g for 5 min, and the pellet was resuspended in the culture medium with 10 μM Y-27632. For stirred suspension culture, 107 EndoC-βH3 cells were suspended in 50 mL of medium supplemented with 10 μM Y-27632 and Pluronic F-68 (1×) and transferred to 125 mL ProCulture spinner flasks (Corning, NY) for culture at 70–80 rpm. The culture medium was replaced every 3 days without the ROCK inhibitor. Alternatively, PIs were formed in an orbital shaker by seeding 1–2 × 106 EndoC-βH3 cells in 5.5 mL of medium/well of 6-well suspension plates (Greiner Bio-One, Monroe, NC) and culturing at 100 rpm at 5% CO2 and 37 °C. For cell counting and assessment of viability, the PIs were dissociated by incubation in TrypLE enzyme for 15 min at 37 °C and gentle pipetting. Upon neutralization with low-glucose DMEM and 10% FBS, the cells were stained with the trypan blue dye and counted using a hemocytometer or the TC20 counter.
Encapsulation of EndoC-βH3 Cell PIs
PIs were encapsulated as we reported.9 Briefly, PIs were collected after 1 h of treatment with 10 μM Y-27632 and subjected to a single wash with a 0.9% (w/v) NaCl aqueous solution. The buffers and alginate solution were sterilized by being autoclaved and filtered through a 0.2 μm filter, respectively. The PIs were then resuspended in a sterile solution of 0.8% (w/v) sodium alginate (FMC BioPolymer, Philadelphia, PA). Subsequently, the suspension was carefully transferred into a 5 mL syringe (Becton Dickinson, Franklin Lakes, NJ) equipped with a 23 G gauge blunt needle and connected to a syringe pump (Hamilton, Reno, NV). Alginate bead generation was carried out at a rate of 250 μL alginate suspension/min in an electrostatic field (5 kV) applied through a voltage power generator (American 3B Scientific, Tucker, GA) by dispensing alginate/cells droplets into a bath of 1.1% (w/v) CaCl2 solution. The formed alginate beads were collected and transferred to microcentrifuge tubes for further washing with NaCl solution to remove CaCl2. Then, a coating step was performed with 0.05% (w/v) poly-l-lysine (MilliporeSigma, Burlington, MA) solution for 10 min followed by another wash with the NaCl solution. The cell-loaded beads were transferred to suspension plates in culture medium with 10 μM Y-27632 and maintained in the orbital shaker at 100 rpm for 24 h prior to implantation to condition the capsules and enhance the survival of the cells. The viability of the encapsulated PIs was assessed as described above after solubilizing the beads with 0.2 M sodium citrate buffer (MilliporeSigma) and dissociating the PIs with TrypLE enzyme for 15 min at 37 °C.
Recombinant Adenovirus Generation and Cell Transduction
The human-codon optimized bPAC gene construct used in this study contains a cMyc-derived epitope, an IRES sequence, and the gene encoding the reporter mCherry (bPAC-cMyc-IRES-mCherry) as described.8 An adenovirus stock (AdbPAC) carrying the transgene sequence under a CMV promoter was generated in 293AD cells (Cell Biolabs, San Diego, CA) with the AdEasy system (Agilent Technologies, Santa Clara, CA). An adenovirus (AdeGFP) with a CMV promoter and eGFP cassette (Vector Development Core, Baylor College of Medicine, Houston, TX) was used as a control. Adenoviral vectors were propagated in 293AD cells by transduction at 50% confluence and harvested when the cytopathic effect was complete. Purification and titer determination of the adenoviral particles were carried out using the Adeno-X Maxi Purification Kit (Takara Bio USA, San Jose, CA) and QuickTiter Adenovirus Titer ELISA Kit (Cell Biolabs), respectively. After initial screening studies, EndoC monolayers were transduced at 200 MOI with 6 × 106 cells/10 cm plate. For PI studies, the transduced monolayer plates were cultured for 2 days prior to seeding in suspension plates, as described above. PIs were grown for 2–3 days before testing.
Measurements of Secreted Insulin and Intracellular cAMP
Cells or PIs were incubated overnight in culture medium at 2.8 mM glucose followed by culture for 1 h in Krebs–Ringer bicarbonate buffer (KRB; 129 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 5 mM NaHCO3, 10 mM N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid, pH 7.2) containing 0.1% BSA (Thermo Fisher) and 0.5 mM glucose. Then, the buffer was exchanged with fresh KRB and 0.5, 2.8, or 15 mM of glucose with or without blue light illumination for a period as stated. Supernatant was harvested, and secreted insulin was measured via enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). For quantification of [cAMP]i, the cells were lysed in 0.1 M HCl on ice for 20 min and centrifuged at 14,000×g and 4 °C for 15 min. The samples were tested by ELISA (Cayman Chemical Co., Ann Arbor, MI). Encapsulated PIs were treated with a 0.2 M sodium citrate buffer to solubilize the alginate matrix prior to cell lysis. Insulin and cAMP concentrations were normalized by the lysate total protein content measured with the Bradford method (Thermo Fisher, Waltham, MA). The insulin secretion rate was calculated by dividing the secreted insulin/total protein values with the time elapsed since the start of the assay.
Flow Cytometry
Harvested EndoC-βH3 cells and PIs were treated with TrypLE enzyme for 10 and 15 min at 37 °C, respectively, and were centrifuged at 500×g for 5 min. Staining with the fluorescent Calcein-AM dye (Invitrogen) was carried out according to the manufacturer’s instructions. For this purpose, the pellet was resuspended in PBS and washed once before incubation with the dye for 20 min at room temperature. Cells were washed three times with PBS for flow cytometry analysis using an Attune NxT system (Thermo Fisher) to measure the fraction of cells taking up Calcein-AM or expressing mCherry. The results were analyzed with the FCS Express software (v 7, De Novo Software, Los Angeles, CA).
Imaging and PI Sizing
Brightfield and epifluorescence images of cultured cells and PIs were obtained with a tissue culture microscope (Leica DMIL LED, Leica, Deerfield, IL) equipped with a camera (DFC3000 G, Leica) and software (LAS X, Leica). For PI sizing, the clusters were transferred to 6-well plates, and images were obtained, which were processed using Fiji ImageJ42 to obtain the average diameter of each aggregate.17 Samples were also visualized on a Leica TCS SPE confocal microscope. The PIs were incubated with the Hoechst dye (Thermo Fisher) in PBS for 30 min at room temperature and Calcein-AM (Invitrogen) according to the manufacturer’s instructions. After three washes with PBS, the PIs were placed in a microscopic slide with a concave cavity (Electron Microscopy Sciences, Hatfield, PA) for imaging.
Western Blotting
Cells were lysed in a lysis buffer (Cell Signaling Technology, Danvers, MA) supplemented with a protease inhibitor cocktail (Thermo Fisher), and total protein was determined by the Bradford method (Thermo Fisher). Cell lysates were boiled for 5 min at 95 °C, loaded to a polyacrylamide gel (30 μg of total protein/lane), and after gel electrophoresis and protein transfer to polyvinylidene difluoride membranes (Millipore), the membranes were blocked with 5% milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 h at room temperature. The membranes were incubated with primary antibodies against cMyc-tag (Cell Signaling Technology) at 4 °C overnight or β-actin (ABclonal, Woburn, MA) for 1 h at room temperature. Following three more washes with TBST, secondary horseradish peroxidase-conjugated antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were added to the solution for 1 h at room temperature. Membranes were washed three times, incubated with an enhanced chemiluminescence ECL substrate (Clarity Max Western ECL Substrate, Bio-Rad, Hercules, CA), and detected using a C-DiGit scanner (Li-Cor BioSciences, Lincoln, NE).
GTT and Illumination Regimen
Animal procedures9 were approved by the Institutional Animal Care and Use Committee at Tufts University and were performed under the supervision of the Tufts Comparative Medicine Services. Six- to ten week old C57BL/6J male and female mice (Charles River Laboratories, Wilmington, MA) were rendered diabetic with daily administration of STZ (75 mg/g body weight prepared fresh in PBS) for 5 consecutive days. Diabetic BG levels were manifested 10–12 days later. Mice were anesthetized with 1% isoflurane, and their dorsal area was shaved and swiped with Betadine and isopropyl alcohol. Empty alginate beads (control) or with PIs (1× or 3 × 106 cells/animal as stated) were injected subcutaneously with an 18-G needle (Becton Dickinson). 48 h after transplantation, the mice were fasted overnight in preparation for a GTT. A 1 ft × 1 ft LED array was used to shine blue light9 for 1 h prior to the GTT and during the assay. A 20% glucose solution (2 g/kg of body weight) was injected intraperitoneally. Lateral tail vein blood samples (2–3 μL) were collected at 0, 15, 30, 60, 90, and 120 min postinjection, and BG was measured with a glucometer (Clarity Diagnostics, Boca Raton, FL). To retrieve the implanted cells, mice were sacrificed, and an incision was made to expose the beads, which were removed carefully and transferred to a 50 mL tube with culture medium. Upon being washed with fresh medium, the cells were transferred to tissue culture dishes and placed in the incubator for further analysis.
Oxygen Consumption
The O2 consumption of β-cell and PI suspensions of known cell concentration was determined using a NeoFox optical O2 sensor (Ocean Optics, Largo, FL), as we reported.9 Briefly, the probe was calibrated at 100% air saturation (vigorous gassing with air; 0.22 mM O2) and at 0% (bubbling of N2 gas). The cells were placed with the culture medium in a cuvette, which was sealed to eliminate gas exchange during measurement. The concentration of dissolved O2 (DO) was recorded for up to 30 min under blue light. Data were exported to MATLAB (Mathworks, Natick, MA), and the rate of O2 consumption was calculated by a 5-point, fourth-order approximation of the slope of the temporal change in DO and normalized to the number of cells in the cuvette.
Statistical Analysis
Results are expressed as mean ± SD, unless stated otherwise, from at least three independent experiments with each experimental point analyzed in triplicates. Groups were compared using Student’s t test or one-way analysis of variance. Comparative analysis of the GTT results was carried out using the Mann–Whitney and post hoc Holm–Šídák tests in Prism (v. 7, GraphPad Software, Inc., La Jolla, CA). Values of p < 0.05 were considered as significant.
Acknowledgments
The authors would like to thank members of the Tzanakakis group for critical discussions during all stages of manuscript preparation. Funding support has been provided by the National Science Foundation (NSF; CBET-1951104, CBET-2015849, CBET-2326510) to E.S.T.
Glossary
Abbreviations
- AC
adenylyl cyclase
- ATP
adenosine triphosphate
- BG
blood glucose
- bPAC
Beggiatoa photoactivatable adenylyl cyclase
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphate
- c-di-GMP
cyclic diguanylate
- CMV
cytomegalovirus
- DO
dissolved oxygen
- eGFP
enhanced green fluorescent protein
- EPAC
exchange protein directly activated by cAMP
- EuPAC
Euglena gracilis photoactivatable adenylyl cyclase
- GDL
d-glucono-δ-lactone
- GLP-1
glucagon-like peptide 1
- GPCR
G-protein-coupled receptor
- GSIS
glucose-stimulated insulin secretion
- GTP
guanosine-5′-triphosphate
- GTT
glucose tolerance test
- hPSCs
human pluripotent stem cells
- IBMX
3-isobutyl-1-methylxanthine
- IRES
internal ribosomal entry site
- K+ATP
ATP-sensitive K+ channel
- LED
light-emitting diode
- MOI
multiplicity of infection
- NFAT
nuclear factor of activated T cells
- NT
nontransduced
- OaPAC
Oscillatoria acuminata photoactivatable adenylyl cyclase
- OCR
oxygen consumption rate
- PAC
photoactivatable adenylyl cyclase
- PBS
phosphate-buffered saline
- PDE
phosphodiesterase
- PI
pseudoislet
- PKA
protein kinase A
- pLL
poly-l-lysine
- ROCK
Rho-associated kinase
- SD
standard deviation
- SEM
standard error of the mean
- shGLP-1
short human GLP-1
- STZ
streptozotocin
- TBST
tris-buffered saline with 0.1% Tween-20
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssynbio.3c00653.
Generation of PIs in stirred-suspension cultures and the PI viability before and after encapsulation (PDF)
Author Contributions
ZC and DMS performed the experiments; EST designed research; ZC, DMS, and EST analyzed the data; ZC and EST wrote the article.
The authors declare no competing financial interest.
Supplementary Material
References
- Chen Z.; Truskinovsky L.; Tzanakakis E. S. Emerging molecular technologies for light-mediated modulation of pancreatic beta-cell function. Mol. Metab. 2022, 64, 101552. 10.1016/j.molmet.2022.101552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stozer A.; Paradiz Leitgeb E.; Pohorec V.; Dolensek J.; Krizancic Bombek L.; Gosak M.; Skelin Klemen M. The Role of cAMP in Beta Cell Stimulus-Secretion and Intercellular Coupling. Cells 2021, 10 (7), 1658. 10.3390/cells10071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki M.; Matsunaga S.; Murakami A.; Ohno K.; Shiga K.; Yoshida K.; Sugai M.; Takahashi T.; Hori T.; Watanabe M. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 2002, 415 (6875), 1047–1051. 10.1038/4151047a. [DOI] [PubMed] [Google Scholar]
- Ohki M.; Sugiyama K.; Kawai F.; Tanaka H.; Nihei Y.; Unzai S.; Takebe M.; Matsunaga S.; Adachi S.; Shibayama N.; et al. Structural insight into photoactivation of an adenylate cyclase from a photosynthetic cyanobacterium. Proc. Natl. Acad. Sci. U.S.A. 2016, 113 (24), 6659–6664. 10.1073/pnas.1517520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu M. H.; Moskvin O. V.; Siltberg-Liberles J.; Gomelsky M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J. Biol. Chem. 2010, 285 (53), 41501–41508. 10.1074/jbc.M110.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierl M.; Stumpf P.; Udwari D.; Gueta R.; Hagedorn R.; Losi A.; Gartner W.; Petereit L.; Efetova M.; Schwarzel M.; et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 2011, 286 (2), 1181–1188. 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu M. H.; Kang I. H.; Nelson M. D.; Jensen T. M.; Lyuksyutova A. I.; Siltberg-Liberles J.; Raizen D. M.; Gomelsky M. Engineering adenylate cyclases regulated by near-infrared window light. Proc. Natl. Acad. Sci. U.S.A. 2014, 111 (28), 10167–10172. 10.1073/pnas.1324301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Tzanakakis E. S. Optogenetic regulation of insulin secretion in pancreatic β-cells. Sci. Rep. 2017, 7 (1), 9357. 10.1038/s41598-017-09937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Tzanakakis E. S. Amelioration of Diabetes in a Murine Model upon Transplantation of Pancreatic β-Cells with Optogenetic Control of Cyclic Adenosine Monophosphate. ACS Synth. Biol. 2019, 8 (10), 2248–2255. 10.1021/acssynbio.9b00262. [DOI] [PubMed] [Google Scholar]
- Rorsman P.; Ashcroft F. M. Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol. Rev. 2018, 98 (1), 117–214. 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelin Klemen M.; Dolensek J.; Slak Rupnik M.; Stozer A. The triggering pathway to insulin secretion: Functional similarities and differences between the human and the mouse β cells and their translational relevance. Islets 2017, 9 (6), 109–139. 10.1080/19382014.2017.1342022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt E. P. S.; Harvey K. E.; Salyer A. E.; Hockerman G. H. Regulation of cAMP accumulation and activity by distinct phosphodiesterase subtypes in INS-1 cells and human pancreatic β-cells. PLoS One 2019, 14 (8), e0215188 10.1371/journal.pone.0215188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald P.; Tamayo-Garcia A.; Manzoli V.; Tomei A. A.; Stabler C. L. Glucose-stimulated insulin release: Parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets. Biotechnol. Bioeng. 2018, 115 (1), 232–245. 10.1002/bit.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazra M.; Lecomte M. J.; Colace C.; Muller A.; Machado C.; Pechberty S.; Bricout-Neveu E.; Grenier-Godard M.; Solimena M.; Scharfmann R.; et al. A human beta cell line with drug inducible excision of immortalizing transgenes. Mol. Metab. 2015, 4 (12), 916–925. 10.1016/j.molmet.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing D.; Kehoe D. E.; Tzanakakis E. S. Expression of Reg Family Proteins in Embryonic Stem Cells and Its Modulation by Wnt/β-Catenin Signaling. Stem Cells Dev. 2010, 19 (9), 1307–1319. 10.1089/scd.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A.; Powers S. L.; George K. L.; Bonner-Weir S. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes 1987, 36 (7), 783–790. 10.2337/diab.36.7.783. [DOI] [PubMed] [Google Scholar]
- Lock L. T.; Laychock S. G.; Tzanakakis E. S. Pseudoislets in stirred-suspension culture exhibit enhanced cell survival, propagation and insulin secretion. J. Biotechnol. 2011, 151 (3), 278–286. 10.1016/j.jbiotec.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Nair G. G.; Liu J. S.; Russ H. A.; Tran S.; Saxton M. S.; Chen R.; Juang C.; Li M. L.; Nguyen V. Q.; Giacometti S.; et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 2019, 21 (2), 263–274. 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M.; Xue S.; Hussherr M. D.; Strittmatter T.; Camenisch G.; Fussenegger M. Smartphone-Flashlight-Mediated Remote Control of Rapid Insulin Secretion Restores Glucose Homeostasis in Experimental Type-1 Diabetes. Small 2021, 17 (35), e2101939 10.1002/smll.202101939. [DOI] [PubMed] [Google Scholar]
- Ye H.; Baba M. D. E.; Peng R. W.; Fussenegger M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 2011, 332 (6037), 1565–1568. 10.1126/science.1203535. [DOI] [PubMed] [Google Scholar]
- Shao J.; Xue S.; Yu G.; Yu Y.; Yang X.; Bai Y.; Zhu S.; Yang L.; Yin J.; Wang Y.; et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Sci. Transl. Med. 2017, 9 (387), eaal2298 10.1126/scitranslmed.aal2298. [DOI] [PubMed] [Google Scholar]
- Yu G.; Zhang M.; Gao L.; Zhou Y.; Qiao L.; Yin J.; Wang Y.; Zhou J.; Ye H. Far-red light-activated human islet-like designer cells enable sustained fine-tuned secretion of insulin for glucose control. Mol. Ther. 2022, 30 (1), 341–354. 10.1016/j.ymthe.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.; Shin E.; Lee J.; Devarasou S.; Kim D.; Shin J. H.; Choi J. H.; Heo W. D.; Han Y. M. Light-stimulated insulin secretion from pancreatic islet-like organoids derived from human pluripotent stem cells. Mol. Ther. 2023, 31 (5), 1480–1495. 10.1016/j.ymthe.2023.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelios M. G.; Afinowicz L. A.; Tipon R. C.; Akirav E. M. Human EndoC-betaH1 beta-cells form pseudoislets with improved glucose sensitivity and enhanced GLP-1 signaling in the presence of islet-derived endothelial cells. Am. J. Physiol.: Endocrinol. Metab. 2018, 314 (5), E512–E521. 10.1152/ajpendo.00272.2017. [DOI] [PubMed] [Google Scholar]
- Lecomte M. J.; Pechberty S.; Machado C.; Da Barroca S.; Ravassard P.; Scharfmann R.; Czernichow P.; Duvillie B. Aggregation of Engineered Human β-Cells into Pseudoislets: Insulin Secretion and Gene Expression Profile in Normoxic and Hypoxic Milieu. Cell Med. 2016, 8 (3), 99–112. 10.3727/215517916X692843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. M.; O’Shea G. M.; Goosen M. F. Injectable microencapsulated islet cells as a bioartificial pancreas. Appl. Biochem. Biotechnol. 1984, 10, 87–99. 10.1007/BF02783739. [DOI] [PubMed] [Google Scholar]
- Kuo C. K.; Ma P. X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22 (6), 511–521. 10.1016/S0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- Fomicheva A.; Zhou C.; Sun Q. Q.; Gomelsky M. Engineering Adenylate Cyclase Activated by Near-Infrared Window Light for Mammalian Optogenetic Applications. ACS Synth. Biol. 2019, 8 (6), 1314–1324. 10.1021/acssynbio.8b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merani S.; Toso C.; Emamaullee J.; Shapiro A. M. Optimal implantation site for pancreatic islet transplantation. Br. J. Surg. 2008, 95 (12), 1449–1461. 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- Vlahos A. E.; Cober N.; Sefton M. V. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc. Natl. Acad. Sci. U.S.A. 2017, 114 (35), 9337–9342. 10.1073/pnas.1619216114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyot M. L.; Gray J. P.; Lamontagne J.; Smith P. J.; Holz G. G.; Madiraju S. R.; Prentki M.; Heart E. Glucagon-Like Peptide-1 Induced Signaling and Insulin Secretion Do Not Drive Fuel and Energy Metabolism in Primary Rodent Pancreatic β-Cells. PLoS One 2009, 4 (7), e6221 10.1371/journal.pone.0006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T.; Silva Xavier G. d.; Holz G. G.; Jouaville L. S.; Thomas A. P.; Rutter G. A. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem. J. 2003, 369 (2), 287–299. 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M.; Takano K.; Tsukamoto H.; Ohtsu K.; Tokunaga F.; Terakita A. Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc. Natl. Acad. Sci. U.S.A. 2008, 105 (40), 15576–15580. 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowka P.; Bruegmann T.; Dusend V.; Malan D.; Beiert T.; Hesse M.; Fleischmann B. K.; Sasse P. Optogenetic stimulation of Gs-signaling in the heart with high spatio-temporal precision. Nat. Commun. 2019, 10 (1), 1281. 10.1038/s41467-019-09322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzl S.; Lindner R.; Nelson M. D.; Winkler A. Structure-guided design and functional characterization of an artificial red light-regulated guanylate/adenylate cyclase for optogenetic applications. J. Biol. Chem. 2018, 293 (23), 9078–9089. 10.1074/jbc.RA118.003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Odom D. T.; Koo S. H.; Conkright M. D.; Canettieri G.; Best J.; Chen H.; Jenner R.; Herbolsheimer E.; Jacobsen E.; et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. U.S.A. 2005, 102 (12), 4459–4464. 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D.; Cooper D. M. Live-cell imaging of cAMP dynamics. Nat. Methods 2008, 5 (1), 29–36. 10.1038/nmeth1135. [DOI] [PubMed] [Google Scholar]
- Roger B.; Papin J.; Vacher P.; Raoux M.; Mulot A.; Dubois M.; Kerr-Conte J.; Voy B. H.; Pattou F.; Charpentier G.; et al. Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia 2011, 54 (2), 390–402. 10.1007/s00125-010-1955-x. [DOI] [PubMed] [Google Scholar]
- Tian G.; Sol E. R.; Xu Y.; Shuai H.; Tengholm A. Impaired cAMP generation contributes to defective glucose-stimulated insulin secretion after long-term exposure to palmitate. Diabetes 2015, 64 (3), 904–915. 10.2337/db14-1036. [DOI] [PubMed] [Google Scholar]
- Dachicourt N.; Serradas P.; Giroix M. H.; Gangnerau M. N.; Portha B. Decreased glucose-induced cAMP and insulin release in islets of diabetic rats: reversal by IBMX, glucagon, GIP. Am. J. Physiol. 1996, 271 (4), E725–E732. 10.1152/ajpendo.1996.271.4.e725. [DOI] [PubMed] [Google Scholar]
- Dolz M.; Movassat J.; Bailbé D.; Le Stunff H.; Giroix M.-H.; Fradet M.; Kergoat M.; Portha B. cAMP-secretion coupling is impaired in diabetic GK/Par rat β-cells: a defect counteracted by GLP-1. Am. J. Physiol.: Endocrinol. Metab. 2011, 301 (5), E797–E806. 10.1152/ajpendo.00652.2010. [DOI] [PubMed] [Google Scholar]
- Schindelin J.; Arganda-Carreras I.; Frise E.; Kaynig V.; Longair M.; Pietzsch T.; Preibisch S.; Rueden C.; Saalfeld S.; Schmid B.; et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 2012, 9 (7), 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.