Abstract
The contractile elements in skeletal muscle fibers operate in series with elastic elements, tendons and potentially aponeuroses, in muscle–tendon units (MTUs). Elastic strain energy (ESE), arising from either work done by muscle fibers or the energy of the body, can be stored in these series elastic elements (SEEs). MTUs vary considerably in their design in terms of the relative lengths and stiffnesses of the muscle fibers and SEEs, and the force and work generating capacities of the muscle fibers. However, within an MTU it is thought that contractile and series elastic elements can be matched or tuned to maximize ESE storage. The use of ESE is thought to improve locomotor performance by enhancing contractile element power during activities such as jumping, attenuating contractile element power during activities such as landing, and reducing the metabolic cost of movement during steady-state activities such as walking and running. The effectiveness of MTUs in these potential roles is contingent on factors such as the source of mechanical energy, the control of the flow of energy, and characteristics of SEE recoil. Hence, we suggest that MTUs specialized for ESE storage may vary considerably in the structural, mechanical, and physiological properties of their components depending on their functional role and required versatility.
Keywords: Muscle–tendon unit morphology, Elasticity, Locomotion, Muscle energetics, Power amplification
1. Introduction
In skeletal muscle, interactions between contractile proteins consume metabolic energy and generate the force, work, and power required for movement. Regular arrangements of these contractile proteins, sarcomeres, are organized into muscle fibers. It has long been recognized that these muscle fibers operate alongside connective tissue structures, tendons and aponeuroses, in muscle–tendon units (MTUs) (Stensen, 1667; Elliott, 1965). Materials testing of these connective tissue structures has demonstrated that they are elastic, and can stretch (Benedict, 1968; Rack and Westbury, 1984) and recoil, storing and returning elastic strain energy (ESE) (Ker, 1981; Bennett et al., 1986; Pollock and Shadwick, 1994a; Azizi et al., 2009). Analysis of limb and joint mechanics in a jumping dog (Alexander, 1974) and pacing camels (Alexander et al., 1982), recording of muscle spindle afferents in anesthetized cats (Rack and Westbury, 1984), and direct measurements of muscle fiber length in a walking cat (Griffiths 1991) showed that this stretch and recoil of elastic elements also occurred in vivo, and could decouple joint rotation and changes in the energy of the body from muscle fiber length changes. The implications of this decoupling for organismal performance were quickly recognized with proposals that tendon elasticity could limit proprioceptive feedback (Rack and Westbury, 1984) and positional control of joints (Pollock and Shadwick, 1994b). While others argued that the interaction of contractile and elastic elements may improve locomotor performance by enhancing (Hill, 1950; Bobbert et al., 1986; Marsh and John-Alder, 1994; Peplowski and Marsh, 1997; Aerts, 1998) and attenuating (Griffith, 1991; Reeves and Narici, 2003; Roberts and Azizi, 2010; Konow et al., 2012) contractile element velocity and power, and reducing metabolic costs (Cavagna et al., 1964; Alexander and Vernon, 1975; Heglund et al., 1982; Alexander and Bennet-Clark, 1977).
Considerable variation has been observed in the structural and mechanical properties of contractile and series elastic elements (SEEs) across MTUs (Hill, 1950; Zajac, 1989; Pollock and Shadwick, 1994b; Loren and Lieber, 1995; Ettema, 1996; Biewener and Roberts, 2000; Kargo and Rome, 2002; Ward et al., 2005; Williams et al., 2007; Wilson and Lichtwark, 2011), and in how these components interact to store ESE (Anderson and Pandy, 1993; Roberts and Azizi, 2011; Fig. 1). SEEs can be stretched by muscle fiber shortening (i.e., muscle fibers do work directly on SEEs; Azizi and Roberts, 2010; Fig. 1B), or upon landing and braking with ESE arising from the energy of the body (i.e., external work is done on the MTU; Rack and Westbury, 1984; Roberts et al., 1997; Fukunaga et al., 2001; Fig. 1C).
Fig. 1.
Schematic representations of energy storage in MTUs. A – inactive pennate muscle fibers (pink) are shown organized in series with an elastic element (grey) with both ends of the MTU fixed in place. B – muscle fibers are activated (red) and generate force (black arrow). Both ends of the MTU are fixed in place and the active muscle fibers shorten and stretch the series elastic element. In this case, stored ESE arises from muscle work. C – muscle fibers are activated (red) and the whole MTU is stretched by an external load (black arrow). The active force generated by muscle fibers is sufficient to resist the external force, however, the stiffness of the tendon is not. The tendon is stretched and stored ESE arises from the external work done. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In this review, we explore the variation in the contractile and elastic components of the MTU in relation to the diversity of potential locomotor benefits of ESE storage in terrestrial vertebrates. We review historical and contemporary literature from human and comparative fields on: 1) the structural and mechanical properties of the contractile and elastic components of MTUs; 2) the design and tuning of MTUs for ESE storage; 3) the benefits of ESE storage for locomotor performance; and 4) the potential for MTUs to be specialized for enhancing specific features of locomotor performance.
2. Muscle-tendon units
Muscle-tendon units contain contractile and elastic elements. Actin (Hanson and Lowy 1963), myosin (Huxley 1963), and potentially the molecular spring titin (Maruyama, 1976), are the contractile components of MTUs (Huxley and Niedergerke, 1954; Huxley and Hanson, 1954; Kellermayer and Granzier, 1996). These contractile proteins are organized into sarcomeres which are arranged, in series and parallel, into muscle fibers. Muscle fibers are arranged either parallel, or at some angle (the pennation angle), to the line of action of the muscle (Gans, 1982; Otten, 1988; Wilson and Lichtwark, 2011), and are surrounded by the extracellular matrix (ECM) (Borg and Caulfield, 1980; Purslow and Trotter, 1994; Kjaer, 2004; Sleboda et al., 2020). The ECM inserts onto tendinous sheets, or aponeuroses (Lieber et al., 1991; Scott and Loeb, 1995; Azizi et al., 2009), which narrow to tendons (Elliott, 1965; Alexander, 2002). Titin, ECM, aponeuroses, and tendons all have some degree of elasticity (Roberts, 2016; Williams and Holt, 2018; Holt, 2019), with tendons and aponeuroses considered to be the primary elastic elements in MTUs (Alexander and Bennet-Clark, 1977; Huijing, 1991; Alexander, 2002; Roberts, 2016). Titin and the ECM are generally considered to be in parallel with contractile elements, and to define the passive stiffness of the muscle fibers (Meyer and Lieber, 2011, 2018). Tendons are in series with muscle fibers (Hill, 1950); they are stretched by active muscle fiber shortening (Fig. 1B) and by externally applied loads (Fig. 1C). Aponeuroses can also stretch upon the development of muscle force (Maganaris and Paul, 2000; Roberts et al., 1997; Azizi and Roberts, 2009). However, their relationship between force and length is more complex than for tendon (Zuurbier et al., 1994; Raiteri et al., 2018; Herzog, 2019; Bossuyt et al., 2023).
2.1. Structure, function, and diversity in contractile elements
Upon muscle activation myosin heads can bind to actin, cyclically forming crossbridges, and titin stiffness increases (Kellermayer and Granzier, 1996; Labeit et al., 2003; Nishikawa et al. 2012; Dutta et al., 2018). Both these changes increase muscle fiber stiffness (Labeit et al., 2003; Powers et al., 2014). However, crossbridge cycling also acts to shorten the sarcomere and muscle fiber (Huxley 1953; Huxley and Hanson 1954; Huxley and Niedergerke 1954; Huxley 1957, 1969). The length change that muscle fibers undergo while producing force is key to their mechanical function. External forces lower than, equal to, or greater than muscle force will result in shortening contractions, isometric contractions, and lengthening contractions, respectively (Griffiths, 1991; Roberts et al., 1997; Biewener et al., 1998; Fukunaga et al., 2001; Carr et al., 2011). Metabolic energy is consumed as adenosine triphosphate (ATP) is hydrolysed by myosin heads during crossbridge cycling (Engelhardt and Ljubimowa 1939; He et al., 2000).
The capacity of the contractile elements to generate force, do work, produce power, and absorb energy depends on contractile conditions under which they operate, and muscle morphology and physiology. In a given muscle, the active force that can be produced is highly dependent on sarcomere length and rate of length change, as described by the force–length and force–velocity relationships. Isometric force is maximal at an intermediate sarcomere length corresponding to optimal actin-myosin overlap (Gordon et al., 1966; Herzog et al., 1992). Muscle force declines with increasing shortening speed (Hill, 1938; Alcazar et al., 2019), and increases, up to a point, with increasing lengthening speed (Abbott et al 1952; Nishikawa 2016). When muscle fibers shorten, they do mechanical work equal to the dot product of their force and displacement vectors, and generate instantaneous mechanical power equal to the dot product of their force and velocity vectors. Work and power are limited by force–length and force–velocity relationships, with work being maximized by slow length changes across the plateau of the force–length relationship (Peplowski and Marsh, 1997; Azizi and Roberts, 2010) and power being maximized at intermediate velocities (Barclay et al., 1993; Bottinelli et al., 1996).
Across MTUs, force can be increased by increasing the number of sarcomeres in parallel, or physiological cross-sectional area (XSAphys), often by increasing pennation angle (Powell et al., 1984; Wilson and Lichtwark 2011). The capacity of a muscle to change length without sacrificing significant force due to force–length and force–velocity effects, so enhancing work and power, can be increased by increasing the number of sarcomeres in series, or muscle fiber length (Bodine et al., 1982; Powell et al 1984; Wilson and Lichtwark, 2011). Maximum shortening velocity and power can be increased further by increasing the proportion of faster muscle fiber types containing faster myosin isoforms (Rome et al., 1988; Bottinelli et al., 1996; Schiaffino and Reggiani, 2011).
Contractile conditions and muscle morphology and physiology affect not only muscle force, work, and power, but also the metabolic cost per unit force and efficiency. The cost per unit force is higher during shortening than isometric contractions, due to an increased rate of crossbridge cycling (Fenn, 1923; Rall, 1982; Barclay, 2022) and force–velocity effects (Roberts et al., 1997; Fletcher and MacIntosh, 2017; Bohm et al., 2019), and lower during lengthening contractions (Abbott et al., 1952; Curtin and Davies, 1973; Beltman et al., 2004; Holt et al., 2014), possibly due to the increased force contributions of titin (Nishikawa, 2016). Muscle efficiency varies with muscle shortening velocity and is typically maximal at ~ 20% of maximum shortening velocity (Barclay et al., 1993). However, muscle energetics may be complicated by the activation state of the muscle (Lou et al., 1998) and its contractile history (Joumaa and Herzog, 2013; Holt et al., 2014; Curtin et al., 2019).
Across different MTUs, energy consumption is determined by the rate of crossbridge cycling and force generation (Barclay et al., 1993; Roberts et al., 1998a; He et al., 2000; Beck et al., 2020), and the volume of muscle active (Alexander, 1991; Roberts et al., 1998b; Biewener and Roberts, 2000). MTUs with larger contractile elements and faster muscle fiber types consume more metabolic energy. However, variation in metabolic energy consumption is best considered alongside variation in mechanical output. For example, the dependency of force on XSAphys, and metabolic cost on volume, means that reduced muscle fiber length will decrease the cost per unit of force. And faster contractile elements will increase the cost of force production, but may allow relatively high efficiencies to be sustained at higher absolute shortening velocities (Barclay et al., 1993).
2.2. Structure, function, and diversity in elastic elements
Tendons and aponeurosis are the primary elastic elements, potentially in series with contractile elements, in MTUs. Tendons are composed of highly-organised collagen fibrils aligned parallel to the long axis of the tendon (Elliott, 1965; Jozsa et al., 1991). They deform in a characteristic and somewhat reproducible manner when loaded both by external forces and muscle fibers (Bennett et al., 1986; Pollock and Shadwick, 1994a; Cui et al 2009), indicating a mechanically in-series operation with contractile elements (Herzog, 2019). Tendon loading gives a J-shaped stress–strain response whereby modulus is initially low, representing a compliant “toe” region, and gradually increases to a constant, higher value at large loads (Bennett et al., 1986; Pollock and Shadwick, 1994a). Tendon hysteresis is relatively low, with <10% of the energy stored typically lost on recoil (Bennett et al., 1986; Shadwick, 1990; Pollock and Shadwick, 1994b; Wilson and Goodship, 1994).
Aponeuroses can constitute a significant proportion of total tendinous tissue length within an MTU (Lieber et al., 1991; Trestik and Lieber, 1993; Zuurbier et al., 1994; Maganaris and Paul, 2000). They have a similar pattern of collagen orientation to tendons, a superficially similar stress–strain response (Lieber et al., 1991; Zuurbier et al., 1994; Scott and Loeb, 1995; Maganaris and Paul, 2000; Muramatsu et al., 2001; Monti et al., 2003; Stafilidis et al., 2005), and have been reported to undergo similar (Trestik and Lieber, 1993; Scott and Loeb, 1995; Muramatsu et al., 2001), or greater (Huijing and Ettema 1988; Lieber et al 1991; Maganaris and Paul, 2000; Monti et al., 2003) strains. Hence, aponeuroses could be important sites of ESE storage within MTUs. However, significantly lower strain in aponeuroses than tendons (Magnusson et al., 2003; Iwanuma et al., 2011), and considerable variation in strain along their length (Zuurbier et al., 1994; Maganaris and Paul, 2000), have also been reported. More importantly, aponeuroses may not exhibit characteristic and reproducible extensions in response to force, and so may not function as simple in-series elastic elements (Ettema and Huijing, 1989; Zuurbier et al., 1994; Scott and Loeb, 1995; Lieber et al. 2000; Azizi and Roberts, 2009; Arellano et al., 2016; Raiteri et al., 2018; Arellano et al 2019; Herzog, 2019; Bossuyt et al., 2023). For example, the turkey lateral gastrocnemius aponeurosis is 50% stiffer when loaded actively compared to passively (Azizi and Roberts, 2009), likely due to the width-wise expansion that results from muscle fiber bulging during active shortening (Zuurbier et al., 1994; Scott and Loeb, 1995; van Donkelaar et al., 1999; Azizi and Roberts, 2009; Iwanuma et al., 2011; Raiteri et al., 2016; Raiteri et al., 2018; Roberts, 2019). This variable stiffness is likely to affect aponeurosis capacity for ESE storage and return (Arellano et al., 2019; Herzog, 2019), and it has been suggested that aponeuroses function simply to transmit force, while the tendon stores ESE (Zuurbier et al., 1994; Magnusson et al., 2003). However, ESE storage and return has been clearly demonstrated in MTUs in which the majority of the elasticity resides in the aponeurosis rather than the tendon (Roberts et al., 1997; Konow et al., 2012). Hence, while aponeuroses can, at least in some cases, store and return ESE, the mechanics of this remain poorly understood.
SEE stiffness, determined by modulus and geometry, is a major determinant of ESE storage capacity (Alexander and Bennet-Clark, 1977; Fukashiro et al., 1995). Across many mammalian species and muscles, the stress–strain relationship of tendon, and therefore its modulus, is largely invariant. The linear region of this relationship is attained at stresses of approximately 25–30 MPa, and has a modulus of 1.24–1.5 GPa (Bennett et al., 1986; Pollock and Shadwick, 1994b). Subsequent studies have showed some variation in modulus across functionally diverse muscles (Shadwick, 1990; Matson et al., 2012; Javidi et al., 2019), and with inactivity (Almeida-Silveira et al., 2000; Reeves et al., 2005; Couppé et al., 2012). However, it appears that a consistent modulus may be attained in all tendons with sufficient, long-term, loading (Woo et al., 1980, 1981; Buchanan and Marsh, 2001; Seynnes et al., 2009; Heinemeier et al., 2012; Geremia et al., 2018; Massey et al., 2018a; Massey et al., 2018b; Waugh et al., 2018; Centner et al., 2019; Charcharis et al., 2019; Walker et al., 2020; Quinlan et al., 2021). In contrast to tendon, there are relatively few measurements of aponeurosis modulus. Studies on isolated aponeuroses from turkey and human gastrocnemius muscles report values of 0.75 (Azizi and Roberts, 2009) and 0.2–0.3 GPa (Shan et al., 2019), respectively. However, no extensive comparative studies exist.
Despite their relatively consistent material properties, tendons can vary broadly in their stiffness as a result of variation in their geometry and operating stresses; increased length, reduced XSA, and reduced operating stress are all known to reduce the effective stiffness of tendon (Zajac, 1989; Hoy et al., 1990; Pollock and Shadwick, 1994b; Loren and Lieber, 1995; Ker et al., 1988 Kargo and Rome, 2002). Similar variability presumably exists in aponeuroses, although, as with modulus, aponeurosis morphology and effective stiffness are relatively unstudied in a comparative context. In order to understand the functional consequences of variation in tendon and aponeurosis properties, they must be considered in the context of variation in contractile tissue.
3. Design and tuning of muscle–tendon units
The relationship between contractile and elastic elements in MTUs has been considered in two major ways. Historically, variation in MTU design has been described based on the structural and functional properties of the contractile and elastic elements. More recently, the relationship between contractile and series elastic elements has been described in terms of “tuning” of the MTU (Galantis and Woledge, 2003; Lichtwark and Wilson, 2008; Fletcher et al., 2010; Ilton et al., 2018; Mendoza and Azizi, 2021).
3.1. MTU design
MTU structural design is often described by the ratio of tendon to muscle fiber length. This ratio tends to increase distally across the limb and varies considerably in homologous muscles across species (Hoy et al., 1990; Zajac, 1989; Williams et al., 2007; Ettema, 1996). Human gluteal, quadriceps, and triceps surae muscle groups have tendon-to-fiber length ratios of ~ 0.25, ~4, and ~ 10, respectively (Hoy et al., 1990), and values of 2.2, 3.7, and 7.0 have been reported for the gastrocnemius muscles of rats, hopping mice, and wallabies respectively (Ettema, 1996). Relatively longer tendons will decrease tendon stiffness and increase ESE storage for a given load (Ettema, 1996; Hoy et al., 1990; Kargo and Rome, 2002; Loren and Lieber, 1995; Pollock and Shadwick, 1994b; Zajac, 1989). However, relatively short muscle fibers may not be able to perform sufficient work on a long compliant tendon. In which case, external work may be required to store significant ESE (Ker et al., 1988; Pollock and Shadwick, 1994b).
MTU functional design has been described by tendon operating stresses, fiber length factors, and fixed-end compliance. Maximal tendon operating stresses have been directly measured (Lieber et al., 1991; Maganaris and Paul, 2000; Reeves et al., 2003; Cui et al., 2009; Stenroth et al., 2012) or estimated from measurements of muscle and tendon XSA (Ker et al., 1988; Pollock and Shadwick, 1994b; Wilson and Lichtwark, 2011), and compared to measured or assumed tendon stress–strain curves to determine potential strain, operating region on the stress–strain curve, and ESE storage. MTUs specialized for ESE are assumed to operate in the linear region of their stress–strain curves (Pollock and Shadwick, 1994b), and operating stress typically varies with body size due to the presence of relatively thicker tendons in smaller species (Pollock and Shadwick, 1994b). For example, estimated maximum operating stress for the gastrocnemius tendon in the hopping mouse is 19 MPa, compared to 62 MPa in the red-bellied Pademelon (Ettema, 1996).
Fiber length factors and fixed-end compliance expand on operating stresses by estimating or measuring tendon elongation, and expressing this relative to muscle fiber length (Ker et al, 1988; Griffiths, 1991; Pollock and Shadwick, 1994b; Williams et al., 2007; Wilson and Lichtwark, 2011, Biewener and Roberts, 2000). Fiber length factors use predictions of muscle force and tendon stiffness to estimate tendon deformation, which is then normalized to muscle fiber length (Pollock and Shadwick, 1994b). These metrics have been used to estimate the degree of specialization of an MTU for ESE, and ability of the muscle to stretch the tendon and so control joint position. Assuming a maximum physiologically realistic muscle fiber shortening of 25% (Dimery, 1985; Ker et al., 1988), fiber length factors < 4 were interpreted as muscle fibers no longer being able to fully stretch the tendon and so unable to control joint position, and fiber length factors of < 2 were assumed to indicate complete specialization for ESE storage rather than joint positioning (Pollock and Shadwick, 1994b).
Fixed-end compliance has been determined by direct measurement of muscle fiber shortening against series elasticity, normalized to muscle fiber length (Griffiths, 1991; Scott and Loeb, 1995; Kawakami and Lieber, 2000; Buchanan and Marsh, 2001; Roberts, 2002; Karamanidis et al., 2005; Sawicki et al., 2015; Ahn et al., 2018; Moo et al., 2020; Mendoza and Azizi, 2021). This direct measurement is beneficial as it accounts for all elasticity, including the often ignored but likely important aponeurosis, and incorporates the effects of force–length and force–velocity relationships on the capacity of contractile elements to generate force and stretch SEEs. Values ranging from ~ 10% in the mouse tibialis anterior (Moo et al., 2020) to ~ 28% in the rat gastrocnemius (Ahn et al., 2018), and ~ 45% in the the Cuban tree frog (Mendoza and Azizi, 2021) have been reported. It should be noted that only the latter MTU even approaches a fiber length factor value suggested to indicate specialization for ESE storage according to this metric (1/0.45 = 2.22; Ker et al., 1988; Pollock and Shadwick 1994b).
3.2. MTU tuning
In contrast to structural and functional descriptions of MTU design, which provide means of describing differences between MTUs, MTU tuning instead describes the matching of contractile and SEE properties for ESE storage (Fig. 2; Rosario et al., 2016; Ilton et al., 2018; Mendoza and Azizi, 2021). If SEEs are too stiff relative to the force capacity of the contractile element, the SEE cannot be stretched by the muscle, and the contractile element will instead stretch instead of the SEE when the MTU is loaded by external forces. If the SEE is too compliant relative to the force and length-change capacity of the muscle fibers, muscle fibers will undergo significant shortening strains to stretch elastic elements enough to store significant ESE. This will reduce muscle work capacity, due to force–length and force–velocity effects, and so ESE storage (Fig. 2A; Rosario et al., 2016).
Fig. 2.
Schematic representation of the effects of MTU tuning on ESE storage. Muscle active and passive force–length curves are shown in solid lines and dashed lines respectively. Upon muscle activation, muscle fibers shorten along the trajectory indicated by the arrows with the slope of the line determined by the stiffness of the SEE. The shaded triangles indicate the energy stored in the SEE by muscle fiber shortening during a fixed-end contraction. A - The effect of SEE stiffness on muscle fiber shortening and ESE storage. Fibers shorten (and so SEEs stretch) most with a low stiffness (light grey), and least with a high (dark grey) stiffness. The most ESE is stored with intermediate stiffness (mid-grey). B – The effect of muscle passive stiffness and muscle fiber operating lengths on ESE. Lower passive muscle stiffness (dark grey) allows for increased muscle fiber length at the start of the contraction and so increases ESE storage compared to the stiffer muscle (light grey). Adapted from Azizi and Roberts, 2010; Cox et al., 2021; Mendoza and Azizi, 2021
Consideration of MTU tuning has also highlighted the potential importance of the passive stiffness of muscle fibers for ESE storage. Passive stiffness is likely to affect the operating range of muscle fibers, with lower passive stiffnesses allowing fibers to be stretched to longer lengths (Fig. 2B). This would allow muscle fibers to shorten over the plateau of the force–length relationship as they stretch elastic elements, and so incur a reduced force penalty compared to muscle starting at the plateau of the force–length relationship (Fig. 2B; Azizi and Roberts, 2010; Cox et al., 2021).
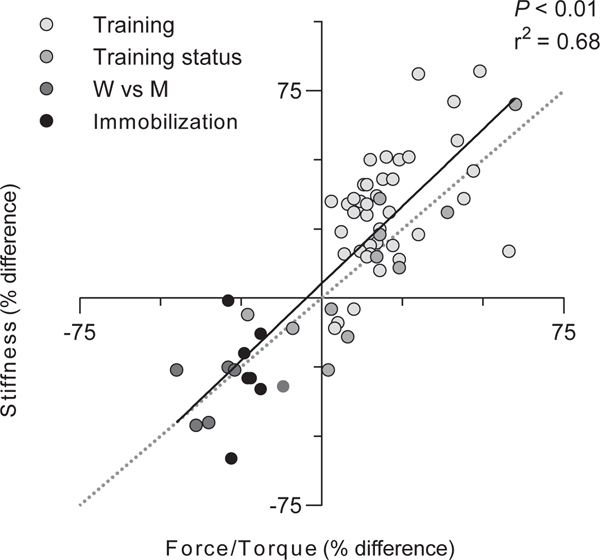
Evidence for the importance of this tuning in MTUs is thought to be provided by the coordinated changes in contractile and SEE properties (Elliott, 1965) across activity levels in an individual (Fig. 3), across individuals in a population (Fig. 3), and across species (Mendoza and Azizi, 2021). Increased loading of MTUs increases muscle strength and tendon stiffness in rough proportion to one another (Arampatzis et al., 2007a; Geremia et al., 2018; Waugh et al., 2018). Differences in Achilles tendon stiffness between men and women, and endurance runners and sprinters, are related to differences in muscle strength (Arampatzis et al., 2007b; Morrison et al., 2015; Muraoka et al., 2005). And Cuban tree frogs show elevated muscle strength and elastic element stiffness compared to cane toads and bullfrogs (Mendoza and Azizi, 2021).
Fig. 3.
Relationship between changes (i.e., following an intervention) or differences (i.e., between groups) in metrics of muscle strength and changes or differences in tendon or tendinous tissue (tendon and aponeurosis) stiffness. Data are fitted with a linear regression (black line). Dotted line (grey) represents unity. Data values and sources can be found in supplemental table 1. W, women. M, men.
Variation in MTU design and tuning has been suggested to explain variation in ESE storage and locomotor performance. Differences in tendon-to-fiber length ratios have been suggested to explain the 4-fold greater capacity for ESE storage in wallabies compared to rats (Ettema, 1996), and to contribute to the greater jump performance in Cuban tree frogs compared to cane toads (Roberts et al., 2011). Parallel increases in muscle strength and tendon stiffness and have been suggested to improve running economy in humans (Bohm et al., 2021a) and jump performance in frogs (Mendoza and Azizi, 2021). And lower muscle fiber passive stiffness and/ or longer operating lengths have been suggested to have the potential to increase muscle work and ESE storage in jumping frogs (Azizi and Roberts, 2010; Azizi, 2014) and guinea fowl (Cox et al., 2021). Understanding the relationships between contractile and SEEs, both in terms of MTU design and tuning, is clearly key for understanding ESE storage capacity and locomotor performance. However, what becomes apparent from the consideration of various metrics of MTU design, and a new appreciation of MTU tuning, is that optimal MTU properties may vary depending on the source of mechanical energy responsible for ESE in SEEs, and the feature of locomotor performance that is to be enhanced.
4. Enhancement of locomotor performance by elastic strain energy storage in series elastic elements
The storage of significant ESE in SEEs has been demonstrated to improve locomotor performance (Roberts and Azizi, 2011) by enhancing (Hill, 1950; Bobbert et al., 1986; Peplowski and Marsh, 1997; Aerts, 1998) and attenuating muscle power (Griffith, 1991; Reeves and Narici, 2003; Konow et al., 2012), and by reducing muscle metabolic energy consumption (Cavagna et al., 1964; Alexander and Vernon, 1975; Roberts et al., 1997).
4.1. Power enhancement and amplification
The recoil of SEE following ESE storage allows MTU velocity, and therefore the instantaneous power, to exceed that of the contractile element during MTU shortening. This discrepancy has been termed power enhancement or, in the case where MTU power exceeds maximum contractile element power, power amplification (Richards and Sawicki, 2012). Power enhancement and amplification have been studied extensively in jumping vertebrates (Bobbert et al., 1986; Marsh, 1994; Marsh and John-Alder, 1994; Peplowski and Marsh, 1997; Aerts, 1998; Bobbert, 2001; Kurokawa et al., 2001; Roberts and Marsh, 2003; Henry et al., 2005; Azizi and Roberts, 2010; Astley and Roberts, 2012; Longo et al., 2019; Mendoza and Azizi, 2021). Contractile elements are typically considered do work slowly on SEEs prior to take-off, with a latching mechanism thought to be required to prevent movement of the body during this time (Galantis and Woledge, 2003; Roberts and Marsh, 2003; Astley and Roberts, 2014; Sawicki et al., 2015; Ilton et al., 2018; Longo et al., 2019; Olberding et al., 2019; Roberts, 2019; Divi et al., 2020). SEEs then recoil rapidly during take-off enhancing or amplifying muscle power. This slow loading of SEEs is thought to reduce force–velocity penalties, so increasing contractile element work, while still allowing for rapid take-off as the SEE recoils (Peplowski and Marsh, 1997; Roberts and Marsh, 2003; Robertson et al., 2018; Marsh, 2022). A sevenfold amplification of power has been reported in jumping Cuban tree frogs (Peplowski and Marsh, 1997; Roberts and Marsh, 2003); and MTU power and velocity have been estimated to be 2–3 times that of muscle fibers in jumping humans (Bobbert, 2001; Kurokawa et al., 2001).
In jumping frogs, the plantaris muscle is activated and shortens, so doing work and stretching SEEs, prior to take-off (Azizi and Roberts, 2010; Astley and Roberts, 2012). Latching has been suggested to be achieved through an increasing mechanical advantage at the ankle (Roberts and Marsh, 2003; Astley and Roberts, 2014). This strategy of contractile element work being stored as ESE in SEEs, rather than done directly on the environment, is thought to only be beneficial at small body sizes (Marsh and John-Alder, 1994; Sutton et al., 2019; Mendoza et al., 2020) where take-off time is very limited, as contractile elements can only store a fraction of their total possible work in a SEE (Sutton et al., 2019). However, at least in frog jumping, muscle fibers contribute to shortening throughout take-off (Azizi and Roberts, 2010), and so also do work directly on the environment. This suggests the possibility of an optimal distribution of muscle work stored in SEEs versus done directly on the environment (Robertson et al., 2018), that may vary with body size (Sutton et al., 2019) and jump distance (Marsh, 2022).
Some degree of power amplification has been reported in larger animals including humans, guinea fowl, and dogs (Alexander, 1974; Bobbert, 2001; Kurokawa et al., 2001; Henry et al., 2005), despite the apparent size-based limitations. For humans, 17–50% of jump energy has been shown to arise from elastic recoil (Bosco et al 1982; Bobbert et al 1986; Anderson and Pandy, 1993; Fukashiro et al 1995). However, at least in humans, ESE often comes from the downward movement of the body in the preparatory phase of the jump, rather than directly from contractile element shortening (Anderson and Pandy, 1993; Henry et al., 2005; Farris et al., 2016; Bishop et al., 2021). This energy flow does not require latching, and the MTU is unloaded by the proximal-to-distal extensions of joints across the limb during take-off (Bobbert and van Ingen Schenau, 1988; Pandy and Zajac, 1991; Bobbert, 2001). Hence, while power enhancement has been observed extensively in both jumping frogs and humans, the source of mechanical energy and the control of the flow of energy through the system appear to be fundamentally different, in ways that may be related to body size and degree of specialization for jumping (Alexander, 1995; Sutton et al., 2019; Bishop et al., 2021).
4.2. Power attenuation
The storage of ESE in SEEs can reduce the lengthening velocity of contractile elements, and so attenuate negative muscle power, during energy dissipating activities such as landing, descent, or braking. The low hysteresis of SEEs (Bennett et al., 1986; Pollock and Shadwick, 1994a; Wilson and Goodship, 1994) makes them unlikely to dissipate significant energy during such activities (Roberts and Konow, 2013). However, their ability to decouple contractile element length changes from MTU stretch (Griffiths, 1991; Reeves and Narici, 2003; Spanjaard et al 2007; Hoffman et al., 2014; Smith et al., 2022) may improve locomotor performance by minimizing muscle damage (Konow et al., 2012; Roberts and Konow, 2013). During both in situ tests and drop landings in turkeys, energy was initially rapidly transferred to the SEE, and then to muscle fibers at a much lower rate. This reduced contractile element lengthening velocity and forces, and so could minimize muscle damage (Roberts and Azizi, 2010; Konow et al., 2012; Roberts and Konow, 2013). SEEs may also allow for safer muscle operating lengths during unexpected perturbations. Muscle fibers in the human distal limb shortened against SEEs prior to ground contact during an unexpected drop perturbation, resulting in shorter muscle fibers that were less likely to be stretched to damaging lengths during energy absorption (Dick et al., 2021).
4.3. Reducing metabolic cost of locomotion
The storage of ESE in SEEs has been suggested to reduce the metabolic cost of steady-state locomotion by reducing the need for contractile element work (Cavagna et al., 1968; Alexander, 1991; Voigt et al 1995; Roberts et al., 1998b; Alexander, 1974; Holt et al., 2014; Fletcher and MacIntosh, 2015; Labonte and Holt, 2022). In bouncing gaits such as running and hopping, MTUs must generate force to support bodyweight and accommodate the sequential decrease and increase in kinetic and potential energy that occurs during stance (Cavagna et al., 1964, 1976; Holt et al., 2014). In the absence of SEEs this would be done by active muscle stretch and shortening. However, in MTUs with significant SEEs, contractile elements can generate force isometrically with energy fluctuations accommodated by SEE stretch and recoil. Muscle fibers in distal MTUs often operate quasi-isometrically, doing little work (Biewener and Baudinette, 1995; Roberts et al., 1997; Biewener, 1998; Biewener and Roberts, 2000; Lichtwark et al., 2007; Dean and Kuo, 2011; Eng et al., 2019; Biewener, 1998; McBride, 2021); minimal length changes are seen in the wallaby plantaris (Biewener et al., 1998), and the return of ESE accounts for 16–60% of positive MTU work in hopping humans (Thys et al., 1975; Fukashiro et al 1995; Voigt et al., 1995; Lichtwark and Wilson, 2005; Dean and Kuo, 2011; McBride, 2021). The metabolic benefits of this strategy are supported by the dependence of the metabolic cost of running on force generation (Taylor et al 1980; Kram and Taylor, 1990; Roberts et al., 1998a; Roberts et al., 1998b; Heglund et al., 1982; Beck et al., 2020), the maximization of ESE storage and minimization of metabolic cost at resonant hopping frequencies (Dean and Kuo, 2011; Robertson and Sawicki., 2015), and decreased cost of running with changes to tendon stiffness (Arampatzis et al., 2006; Fletcher et al., 2010).
The metabolic benefit from this avoidance of costly muscle shortening (Fenn, 1923; Holroyd et al., 1996; Roberts et al., 1997; Roberts, 2002; Beltman et al., 2004; Farris and Sawicki, 2012; Fletcher and MacIntosh, 2017; Bohm et al 2019; Barclay, 2022) has however been questioned by the finding that under some, but not all (Van der Zee et al., 2019), conditions, force can be produced as cheaply during active stretch-shorten contractions as during isometric ones (Curtin et al., 2019; Holt et al., 2014). This has been attributed (Holt et al., 2014) to the low cost of force during and following active stretch (Abbott et al., 1952; Beltman et al., 2004; Joumaa and Herzog, 2013), and muscle work being done during relaxation (Lou et al., 1998). This finding requires a more nuanced consideration of muscle energetics, but does not necessarily negate the energetic benefits of SEEs. Reduced need for contractile element work may have allowed for a reduction in muscle volume, which reduces the cost of force and limb inertia (Alexander, 1991; Biewener and Roberts, 2000; Holt et al., 2014; Labonte and Holt, 2022). It also seems likely that the low cost of force in stretch-shorten cycles depends on a specific timing of activation that may not give the required pattern of force to support bodyweight, and requires stretch and shortening to occur in the same muscle rather than being distributed across synergistic muscles (Holt et al., 2014; Curtin et al., 2019; Van der Zee et al., 2019; Bohm et al., 2019, 2021a). Hence, the replacement of contractile element work with SEE stretch and recoil may allow for reduced metabolic cost under a more realistic range of contractile conditions.
The reduction of muscle fiber shortening velocity relative to MTU velocity enabled by ESE storage has also been proposed to reduce the metabolic cost of walking and running (Lichtwark and Wilson, 2006; Gabaldon et al., 2008; Lichtwark and Wilson, 2008; Lichtwark and ´ Barclay, 2010; Bohm et al., 2019; Bohm et al., 2021a; Bohm et al., 2021b; Uchida et al., 2016; Swinnen et al., 2023). For example, in running humans, soleus fibers shorten throughout stance, first stretching the tendon and then continuing to shorten as the tendon recoils. Contractile element shortening velocity is close to that giving maximal efficiency throughout stance, but the MTU shortens more rapidly during the latter half of stance (Bohm et al., 2021b). This reduction in fiber shortening velocity may also reduce cost by allowing for the use of slower, more economical muscle fiber types (Rome et al., 1988; Barclay et al., 1993; Schiaffino and Reggiani, 2011; Labonte and Holt, 2022). Hence, SEEs may save energy not only by recycling the energy of the body, but also by being loaded by muscle work and then recoiling to improve muscle efficiency for a given MTU velocity.
The storage of ESE in SEEs clearly has major and diverse benefits for locomotor performance. These benefits have been usefully characterized as: 1) storing contractile element work in SEEs and then rapidly delivering it to the body to enhance muscle positive power; 2) storing the energy of the body in SEEs and slowly delivering it to the contractile element to attenuate negative muscle power; and 3) storing and returning the energy of the body to reduce muscle work and save metabolic energy (Roberts and Azizi, 2011). However, what has become apparent from the extensive study of these phenomena is the intermediate conditions. For example, the ESE used to enhance power in jumping may arise from the energy of the body rather than from muscle work, the storage and return of ESE can save metabolic energy by allowing for the use of more efficient shortening velocities rather than by eliminating muscle work, and using both the energy of the body and muscle work to store ESE has been suggested to save metabolic energy and enhance power during walking (Fukunaga et al., 2001; Ishikawa et al., 2005). Hence, it may be beneficial to consider both the benefits of stored ESE and the flow of energy, particularly when considering whether MTU design and tuning might be optimized for specific aspects of locomotor performance.
5. Task-specific MTU design and tuning
Historically, MTUs have been designated as specialized for ESE storage based on their morphological or functional design (Pollock and Shadwick, 1994b). However, this may be an inadequate distinction given the diversity of ways in which MTUs can store and benefit from ESE. For example, in MTUs where ESE arises from muscle work, and the goal is power enhancement, we might expect to see increased contractile element length to allow for greater active shortening, increased contractile element XSAphys to increase force, stiff SEEs which store more ESE for a given amount of muscle fiber shortening, more compliant muscle fibers which would allow for longer operating lengths and, and tendons that can recoil rapidly (Fig. 4A). While in MTUs where ESE arises predominately from the energy of the body and the goal is power attenuation, we might expect to see long contractile elements that could stretch and dissipate energy, compliant SEEs that would be preferentially stretched as the MTU is actively stretched, stiff muscle fibers that would resist stretch, and SEEs with a high hysteresis that would reduce the energy dissipation required of contractile elements (Fig. 4B). And in MTUs where ESE arises primarily from the energy of the body and the goal is metabolic savings, we might expect to see shorter muscle fibers that would have a lower cost per unit force, stiffer muscle fibers that would resist contractile element stretch, and compliant SEEs that would be preferentially stretched (Fig. 4C). However, we must note that many of these changes are not mutually exclusive and may have detrimental effects if not properly tuned. For example, longer contractile elements will require shorter (and therefore stiffer) SEEs if MTU length is constant, and increased SEE stiffness will limit ESE unless combined with increased contractile element XSAphys. Hence, even in the most specialized MTUs we would likely only see some of these effects, and the potential for MTU task-specific design and tuning may be obscured by the need for MTUs to fulfil multiple functions.
Fig. 4.
Schematic showing proposed locomotor benefits of ESE storage (adapted from Roberts and Azizi, 2011). The flow of energy between the body, contractile elements, and elastic elements is highlighted in darker colors, and potentially beneficial MTU properties are shown. In power enhancement (A) contractile elements slowly do work on elastic elements, and stored ESE is subsequently delivered to the body rapidly during take-off. In power attenuation (B) the energy of the body stretches the tendon rapidly upon landing and contractile elements generate force to resist stretch. This energy is then delivered to the muscle fibers more slowly and they stretch and dissipate energy. In metabolic energy saving (C), the energy of the body is transferred to SEEs and contractile elements generate force to resist stretch in the first half of stance. This energy is then returned to the body in the second half of stance.
There has been little explicit consideration of contractile and elastic element properties in the context of the diversity of ways in which MTUs can store and benefit from ESE. However, we do tend to see the highest fiber length factors in large cursorial mammals thought to be specialized for reduced metabolic cost (Pollock and Shadwick, 1994a), and even the MTUs of highly specialized jumpers do not exhibit fiber length factors low enough to be considered specialized for ESE storage (Ker et al., 1988; Mendoza and Azizi, 2021). This suggests a pattern of relatively longer contractile elements in MTUs specialized for power amplification. We tend to see increased muscle XSAphys, increased SEE stiffness (Mendoza and Azizi, 2021) and more compliant muscle fibers (Azizi et al., 2014) in MTUs highly specialized for power amplification, and stiffer muscle fibers in MTUs used in braking (Azizi et al., 2014). It has been speculated that variation in SEE properties (Ilton et al., 2018), such as speed of recoil or hysteresis, might be beneficial. However, no evidence of such adaptation has been observed.
6. Conclusions and future directions
Extensive study of contractile and SEEs, the design and tuning of MTUs, and organismal performance has given us great insight into the potential for ESE to be stored in MTUs, and the locomotor benefits of this. However, open questions remain about the role of aponeuroses as SEEs and their variation across MTUs, the metabolic cost of contractile element force and work under the dynamic conditions relevant to locomotion, the more detailed mechanisms of power enhancement and attenuation and metabolic savings, and the potential for MTUs to be specialized for different aspects of locomotor performance rather than simply ESE storage. We suggest that comparative study of aponeurosis as has been undertaken in tendons, detailed muscle energetics studies as have been done for muscle mechanics, and study of MTU design and tuning that explicitly accounts for the source of mechanical energy and aspect of locomotor performance to be enhanced would greatly improve our understanding of the role of SEEs in enhancing muscle and locomotor performance in a comparative context.
Supplementary Material
Acknowledgements
The authors would like to thank D. Labonte, E. Azizi and 3 anonymous reviewers for helpful comments, and funding sources HFSP grant RGY0073/2020 to D.L., N.C.H. and M.B. and NIH/NIAMS R01AR080711 to N.C.H.
Footnotes
CRediT authorship contribution statement
N.C. Holt: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Visualization, Supervision. D.L. Mayfield: Data curation, Writing – original draft, Writing – review & editing, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbiomech.2023.111585.
References
- Abbott BC, Bigland B, Ritchie JM, 1952. The physiological cost of negative work. J. Physiol. 117, 380–390. 10.1113/jphysiol.1952.sp004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts P, 1998. Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Philos. Trans. R Soc. London. Ser. B Biol. Sci. 353, 1607–1620. 10.1098/rstb.1998.0313. [DOI] [Google Scholar]
- Ahn AN, Konow N, Tijs C, Biewener AA, 2018. Different segments within vertebrate muscles can operate on different regions of their force–length relationships. Integr. Comp. Biol. 58, 219–231. 10.1093/icb/icy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albracht K, Arampatzis A, 2013. Exercise-induced changes in triceps surae tendon stiffness and muscle strength affect running economy in humans. Eur. J. Appl. Physiol. 113, 1605–1615. 10.1007/s00421-012-2585-4. [DOI] [PubMed] [Google Scholar]
- Alcazar J, Csapo R, Ara I, Alegre LM, 2019. On the shape of the force-velocity relationship in skeletal muscles: the linear, the hyperbolic, and the double-hyperbolic. Front. Physiol. 10, 769. 10.3389/fphys.2019.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RM, 1974. The mechanics of jumping by a dog (Canis familiaris). J. Zool. 173, 549–573. 10.1111/j.1469-7998.1974.tb04134.x. [DOI] [Google Scholar]
- Alexander RM, 1982. The role of tendon elasticty in the locomotion of the camel (Camelus dromedarius). J. Zool. Lond. 198, 293–313. [Google Scholar]
- Alexander RM, 1991. Energy-saving mechansisms in walking and running. J. Exp. Biol. 160, 55–69. [DOI] [PubMed] [Google Scholar]
- Alexander RM, 1995. Leg design and jumping technique for humans, other vertebrates and insects. Phil. Trans. R Soc. Lond. 347, 235–248. [DOI] [PubMed] [Google Scholar]
- Alexander RM, 2002. Tendon elasticity and muscle function. Comp. Biochem Physiol. A. Mol. Integr. Physiol. 133, 1001–1011. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Bennet-Clark HC, 1977. Storage of elastic strain energy in muscle and other tissues. Nature. 265, 114–117. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Vernon A, 1975. The mechanics of hopping by kangaroos (Macropodidae). J. Zool. 177, 265–303. 10.1111/j.1469-7998.1975.tb05983.x. [DOI] [Google Scholar]
- Almeida-Silveira MI, Lambertz D, Pérot C, Goubel F, 2000. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur. J. Appl. Physiol. Occup. Physiol. 81, 252–257. [DOI] [PubMed] [Google Scholar]
- Anderson FC, Pandy MG, 1993. Storage and utilization of elastic strain energy during jumping. J. Biomech. 26, 1413–1427. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, De Monte G, Karamanidis K, Morey-Klapsing G, Stafilidis S, Bruggemann GP, 2006. Influence of the muscle-tendon unit’s mechanical and morphological properties on running economy. J. Exp. Biol. 209, 3345–3357. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Albracht K, 2007a. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J. Exp. Biol. 210, 2743–2753. 10.1242/jeb.003814. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Morey-Klapsing G, De Monte G, Stafilidis S, 2007b. Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. J. Biomech. 40, 1946–1952. [DOI] [PubMed] [Google Scholar]
- Arellano CJ, Gidmark NJ, Konow N, Azizi E, Roberts TJ, 2016. Determinants of aponeurosis shape change during muscle contraction. J. Biomech. 49, 1812–1817. 10.1016/j.jbiomech.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano CJ, Konow N, Gidmark NJ, Roberts TJ, 2019. Evidence of a tunable biological spring: elastic energy storage in aponeuroses varies with transverse strain in vivo. Proc. R. Soc. B 286, 3–8. 10.1098/rspb.2018.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley HC, Roberts TJ, 2012. Evidence for a vertebrate catapult: Elastic energy storage in the plantaris tendon during frog jumping. Biol. Lett. 8, 386–389. 10.1098/rsbl.2011.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley HC, Roberts TJ, 2014. The mechanics of elastic loading and recoil in anuran jumping. J. Exp. Biol. 217, 4372–4378. 10.1242/jeb.110296. [DOI] [PubMed] [Google Scholar]
- Azizi E, 2014. Locomotor fucntion shapes the passive mechanical properties and operating lengths of muscle. 281, 20132914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Halenda GM, Roberts TJ, 2009. Mechanical properties of the gastrocnemius aponeurosis in wild turkeys. Integr. Comp. Biol. 49, 51–58. 10.1093/icb/icp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Roberts TJ, 2009. Biaxial strain and variable stiffness in aponeuroses. J. Physiol. 587, 4309–4318. 10.1113/jphysiol.2009.173690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Roberts TJ, 2010. Muscle performance during frog jumping: Influence of elasticity on muscle operating lengths. Proc. R. Soc. B 277, 1523–1530. 10.1098/rspb.2009.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay CJ, 2022. A century of exercise physiology: key concepts in muscle energetics. Eur. J. Appl. Physiol. 10.1007/s00421-022-05070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay CJ, Constable JK, Gibbs CL, 1993. Energetics of fast- and slow-twitch muscle of the mouse. J. Physiol. 472, 61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck ON, Gosyne J, Franz JR, Sawicki GS, 2020. Cyclically producing the same average muscle-tendon force with a smaller duty increases metabolic rate. Proc. Roy. Soc. B 287, 20200431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltman JGM, Van Der Vliet MR, Sargeant AJ, De Haan A, 2004. Metabolic cost of lengthening, isometric and shortening contractions in maximally stimulated rat skeletal muscle. Acta Physiol. Scand. 182, 179–187. 10.1111/j.1365-201X.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- Benedict JV, Walker LB, Harris EH, 1968. Stress-strin characteristics and tensile strength of unembalmed human tendon. J. Biomech. 1, 53–63. [DOI] [PubMed] [Google Scholar]
- Bennett MB, Ker RF, Imery NJ, Alexander RMN, 1986. Mechanical properties of various mammalian tendons. J. Zool. 209, 537–548. 10.1111/j.1469-7998.1986.tb03609.x. [DOI] [Google Scholar]
- Biewener AA, 1998. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 120, 73–87. 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Baudinette RV, 1995. In vivo muscle force and elastic energy storage during steady-speed hopping of tammar wallabies (Macropus eugenii). J. Exp. Biol. 198, 1829–1841. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Konieczynski DD, Baudinette RV, 1998. In vivo muscle force-length behavior during steady-speed hopping in tammar wallabies. J. Exp. Biol. 201, 1681–1694. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Roberts TJ, 2000. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc. Sport Sci. Rev. 28, 99–107. [PubMed] [Google Scholar]
- Bishop PJ, Falisse A, De Groot F, Hutchinson JR, 2021. Predictive simualtions of musculoskeletal fucntion and jumping performance in a generalized bird. IOB 3, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbert MF, 2001. Dependence of human squat jump performance on the series elastic compliance of the triceps surae: a simulation study. J. Exp. Biol. 204, 533–542. 10.1242/jeb.204.3.533. [DOI] [PubMed] [Google Scholar]
- Bobbert MF, Huijing PA, Ingen Schenau GJ, 1986. An estimation of muscle power output and work done by the human triceps surare muscle-tendon complex in running. J. Biomech. 19, 899–906. [DOI] [PubMed] [Google Scholar]
- Bobbert MF, van Ingen Schenau GJ, 1988. Coordiantion in vertical jumping. J. Biomech. 21, 249–262. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Roy RR, Meadows DA, Zernicke RF, Sacks RD, Fournier M, Edgerton VR, 1982. Architectural, histochemical and contractile characteristics of a unique biarticular muscle: the cat semintendinosus. J. Neurophys. 48, 192–201. [DOI] [PubMed] [Google Scholar]
- Boesen AP, Dideriksen K, Couppe C, Magnusson SP, Schjerling P, Boesen M, ´ Aagaard P, Kjaer M, Langberg H, 2013. Effect of growth hormone on aging connective tissue in muscle and tendon: gene expression, morphology, and function following immobilization and rehabilitation. J. Appl. Physiol. 116, 192–203. 10.1152/japplphysiol.01077.2013. [DOI] [PubMed] [Google Scholar]
- Bohm S, Mersmann F, Santuz A, Arampatzis A, 2019. The force-length-velocity potential of the human soleus muscle is related to the energetic cost of running. Proceedings. Biol. Sci. 286, 20192560. 10.1098/rspb.2019.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm S, Mersmann F, Santuz A, Arampatzis A, 2021a. Enthalpy efficiency of the soleus muscle contributes to improvements in running economy. Proc. R. Soc. B 288, 20202784. 10.1098/rspb.2020.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm S, Mersmann F, Santuz A, Schroll A, Arampatzis A, 2021b. Muscle-specific economy of force generation and efficiency of work production during human running. Elife 10, e67182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg TK, Caulfield JB, 1980. Morphology of connective tissue in skeletal muscle. Tissue and Cell 12, 197–207. [DOI] [PubMed] [Google Scholar]
- Bosco C, Tarkka I, Komi PV, 1982. Effect of elastic energy and myoelectrical potentiation of triceps surae during stretch-shortening cycle exercise. Int. J. Sports Med. 3, 137–140. [DOI] [PubMed] [Google Scholar]
- Bossuyt FM, Abramovic S, Leonard T, Sawatsky A, Smith CR, Taylor WR, Scott WM, Herzog W, 2023. The non-intuitive, in vivo behavior of an aponeurosis in a unipennate muscle. J. Biomech. 147, 111430. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, 1996. Force-velocity properties of human skeletal muscle fibers: myosin heavy chain isoform and temperature dependence. J. Physiol. 495, 573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL, 2001. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J. Appl. Physiol. 90, 164–171. [DOI] [PubMed] [Google Scholar]
- Carr JA, Ellerby DJ, Marsh RL, 2011. Differential segmental strain during active lengthening in a large biarticular thigh muscle during running. J. Exp. Biol. 214, 3386–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA, 2008. Influence of aging on the in vivo properties of human patellar tendon. J. Appl. Physiol. 105, 1907–1915. 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Saibene FP, Margaria R, 1964. Mechanical work in running. J. Appl. Physiol. 19, 249–256. 10.1152/jappl.1964.19.2.249. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Dusman B, Margaria R, 1968. Positive work done by a previously stretched muscle. J. Appl. Physiol. 24, 21–32. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Thys H, Zamboni A, 1976. The sources of external work in level walking and running. J. Physiol. 262, 639–657. 10.1113/jphysiol.1976.sp011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centner C, Lauber B, Seynnes OR, Jerger S, Sohnius T, Gollhofer A, Konig D, ¨ 2019. Low-load blood flow restriction training induces similar morphological and mechanical Achilles tendon adaptations compared with high-load resistance training. J. Appl. Physiol. 127, 1660–1667. 10.1152/japplphysiol.00602.2019. [DOI] [PubMed] [Google Scholar]
- Charcharis G, Mersmann F, Bohm S, Arampatzis A, 2019. Morphological and mechanical properties of the quadriceps femoris muscle-tendon unit from adolescence to adulthood: effects of age and athletic training. Front. Physiol. 10 10.3389/fphys.2019.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppé C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP, 2008. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J. Appl. Physiol. 105, 805–810. 10.1152/japplphysiol.90361.2008. [DOI] [PubMed] [Google Scholar]
- Couppé C, Suetta C, Kongsgaard M, Justesen L, Hvid LGG, Aagaard P, Kjær M, Magnusson SPP, 2012. The effects of immobilization on the mechanical properties of the patellar tendon in younger and older men. Clin. Biomech. 27, 949–954. 10.1016/j.clinbiomech.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Couppé C, Svensson RB, Grosset J-F, Kovanen V, Nielsen RH, Olsen MR, Larsen JO, Praet SFE, Skovgaard D, Hansen M, Aagaard P, Kjaer M, Magnusson SP, 2014. Life-long endurance running is associated with reduced glycation and mechanical stress in connective tissue. Age (Dordr). 36, 9665. 10.1007/s11357-014-9665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, DeBoef A, Salzano MQ, Katugam K, Piazza SJ, Rubenson J, 2021. Plasticity of the gastrocnemius elastic system in repsonse to decreased work and power demand during growth. J. Exp. Biol. 224, jeb242694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Maas H, Perreault EJ, Sandercock TG, 2009. In situ estimation of tendon material properties: differences between muscles of the feline hindlimb. J. Biomech. 42, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N, Davies R, 1973. Chemical and mechanical changes during stretching of activated frog muscle. Cold Spring Harbor Symp. Quant. Biol. 37, 619–626. [Google Scholar]
- Curtin NA, Woledge RC, West TG, Goodwin D, Piercy RJ, Wilson AM, 2019. Energy turnover in mammalian skeletal muscle in contractions mimicking locomotion: effects of stimulus pattern on work, impulse and energetic cost and efficiency. J. Exp. Biol. 222, jeb203877. 10.1242/jeb.203877. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV, 2007. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J. Physiol. 583 (1079–1), 091. 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JC, Kuo AD, 2011. Energetic costs of producing muscle work and force in a cyclical human bouncing task. J. Appl. Physiol. 110, 873–880. 10.1152/japplphysiol.00505.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TJM, Clemente CJ, Punith LK, Sawicki GS, 2021. Series elasticity facilitates safe plantar flexor muscle-tendon shock absorption during perturbed human hopping. Proc. Roy. Soc. B. 288, 20210201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimery NJ, 1985. Muscle and sarcomere lengths in the hindlimb of the rabbit (Orytolagus cuniculus) during a galloping stride. J. Zool. 205, 373–383. [Google Scholar]
- Divi S, Ma X, Ilton M, St. Pierre R, Eslami B, Patek SN, Bergbreiter S, 2020. Latch-based control of energy output in spring actuated systems. J. R. Soc. Interface 17, 20200070. 10.1098/rsif.2020.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Tsiros C, Sundaer SL, Athar HA, Moore J, Nelson B, Gage MJ, Nishikawa K, 2018. Calcium increases titin N2A binding to F-actin and regulated thin filaments. Nat. Sci. Rep. 8, 13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DH, 1965. Structure and function of mammalian tendon. Biol. Rev. 40, 392–421. 10.1111/j.1469-185X.1965.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Eng CM, Konow N, Tijs C, Holt NC, Biewener AA, 2019. In vivo force-length and activation dynamics of two distal rat hindlimb muscles in relation to gait and grade. J. Exp. Biol. 222 10.1242/jeb.205559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt WA, Ljubimowa MN, 1939. Myosin and adenosinetriphosphate. Nature 144, 668–669. [Google Scholar]
- Ettema GJC, 1996. Elastic and length-force characteristics of the gastrocnemius of the hopping mouse (Notomys alexis) and the rat (Rattus norvegicus). J. Exp. Biol. 199, 1277–1285. 10.1242/jeb.199.6.1277. [DOI] [PubMed] [Google Scholar]
- Ettema GJC, Huijing PA, 1989. Properties of the tendinous structures and series elastic component of EDL muscle-tendon complex of the rat. J. Biomech. 22, 1209–1215. 10.1016/0021-9290(89)90223-6. [DOI] [PubMed] [Google Scholar]
- Farris DJ, Lichtwark GA, Brown NAT, Cresswell AG, 2016. The role of human ankle plantar flexor muscle-tendon interaction and architecture in maximal vertical jumping examined in vivo. J. Exp. Biol. 219, 528–534. 10.1242/jeb.126854. [DOI] [PubMed] [Google Scholar]
- Farris DJ, Sawicki GS, 2012. Linking the mechanics and energetics of hopping with elastic ankle exoskeletons. J. Appl. Physiol. 113, 1862–1872. [DOI] [PubMed] [Google Scholar]
- Fenn WO, 1923. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J. Physiol. 58, 175–203. 10.1113/jphysiol.1923.sp002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JR, Esau SP, MacIntosh BR, 2010. Changes in tendon stiffness and running economy in highly trained distance runners. Eur. J. Appl. Physiol. 110, 1037–1046. 10.1007/s00421-010-1582-8. [DOI] [PubMed] [Google Scholar]
- Fletcher JR, MacIntosh BR, 2015. Achilles tendon strain energy in distance running: consider the muscle energy cost. J. Appl. Physiol. 118, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JR, MacIntosh BR, 2017. Running economy from a muscle energetics perspective. Front. Physiol. 8, 433. 10.3389/fphys.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foure A, Nordez A, Cornu C, 2010. Plyometric training effects on Achilles tendon ´ stiffness and dissipative properties. J. Appl. Physiol. 109, 849–854. 10.1152/japplphysiol.01150.2009. [DOI] [PubMed] [Google Scholar]
- Fukashiro S, Komi PV, Jarvinen M, 1995. In vivo achilles tendon loading during jumping in humans. Eur. J. Appl. Physiol. 71, 453–458. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN, 2001. In vivo behaviour of human muscle tendon during walking. Proc. R. Soc. B 268, 229–233. 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldón AM, Nelson FE, Roberts TJ, 2008. Relative shortening velocity in locomotor muscles: turkey ankle extensors operate at low V/Vmax. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R200–R210. 10.1152/ajpregu.00473.2007. [DOI] [PubMed] [Google Scholar]
- Galantis A, Woledge RC, 2003. The theoretical limits to the power output of a muscle-tendon complex with inertial and gravitational loads. Proc. Roy. Soc. Lond. B. 270, 1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans C, 1982. Fiber architecture and muscle function. Exerc. Sports Sci. Rev. 10, 160–207. [PubMed] [Google Scholar]
- Geremia JM, Baroni BM, Bobbert MF, Bini RR, Lanferdini FJ, Vaz MA, 2018. Effects of high loading by eccentric triceps surae training on Achilles tendon properties in humans. Eur. J. Appl. Physiol. 118, 1725–1736. 10.1007/s00421-018-3904-1. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ, 1966. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184, 170–192. 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RI, 1991. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J. Physiol. 436, 219–236. 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Lowy J, 1963. The structure of F-actin and of actin filaments isolated from muscle. J. Mol. Biol 6, 46–60. [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C, 2000. ATP consumption and efficiency of human single muscle fibers with different myosin isoform compositions. Biophys. J. 79, 945–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heglund NC, Fedak MA, Taylor CR, Cavagna GA, 1982. Energetics and mechanics of terrestrial locomotion IV. Total mechanical energy changes as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 57–66. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Skovgaard D, Bayer ML, Qvortrup K, Kjaer A, Kjaer M, Magnusson SP, Kongsgaard M, 2012. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J. Appl. Physiol. 113, 827–836. 10.1152/japplphysiol.00401.2012. [DOI] [PubMed] [Google Scholar]
- Henry HT, Ellerby DJ, Marsh RL, 2005. Performance of guinea fowl Numida meleagris during jumping requires storage and release of elastic energy. J. Exp. Biol. 208, 3293–3302. 10.1242/jeb.01764. [DOI] [PubMed] [Google Scholar]
- Herzog W, 2019. The problem with skeletal muscle series elasticity. BMC Biomed. Eng. 1, 28. 10.1186/s42490-019-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Kamal S, Clarke HD, 1992. Myofilament lengths of cat skeletal muscle: theoretical considerations and functional implications. J. Biomech. 25, 945–948. 10.1016/0021-9290(92)90235-S. [DOI] [PubMed] [Google Scholar]
- Hill AV, 1938. The heat of shortening and the dynamic constants of muscle. Proc. Roy. Soc. B. 126, 136–195. 10.1098/rspb.1938.0050. [DOI] [Google Scholar]
- Hill AV, 1950. The series elastic component of muscle. Proc. R. Soc. London. Ser. B. Biol. Sci. 137, 273–280. 10.1098/rspb.1950.0035. [DOI] [PubMed] [Google Scholar]
- Hirayama K, Iwanuma S, Ikeda N, Yoshikawa A, Ema R, Kawakami Y, 2017. Plyometric training favors optimizing muscle-tendon behavior during depth jumping. Front. Physiol. 25, 16. 10.3389/fphys.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BW, Cresswell AG, Carroll TJ, Lichtwark GA, 2014. Muscle fascicle strains in human gastrocnemius during backward downhill walking. J. Appl. Physiol. 116, 1455–1462. [DOI] [PubMed] [Google Scholar]
- Holroyd SM, Gibbs CL, Luff AR, 1996. Shortening heat in slow- and fast-twitch muscles of the rat. Am. J. Physiol. 270, C293–C297. [DOI] [PubMed] [Google Scholar]
- Holt NC, 2019. Beyond bouncy gaits: the role of multiscale compliance in skeletal muscle performance. J. Exp. Zool. Part A Ecol. Integr. Physiol. 1–10. 10.1002/jez.2261. [DOI] [PubMed] [Google Scholar]
- Holt NC, Roberts TJ, Askew GN, 2014. The energetic benefits of tendon springs in running: Is the reduction of muscle work important? J. Exp. Biol. 217, 4365–4437. 10.1242/jeb.112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy MG, Zajac FE, Gordon ME, 1990. A musculoskeletal model of the human lower extremity: The effect of muscle, tendon, and moment arm on the moment-angle relationship of musculotendon actuators at the hip, knee, and ankle. J. Biomech. 23, 157–169. 10.1016/0021-9290(90)90349-8. [DOI] [PubMed] [Google Scholar]
- Huijing PA, 1991. Elastic potential of muscle. In: Komi PV (Ed.), Strength and Power in Sport. Blackwell, Oxford, pp. 151–168. [Google Scholar]
- Huijing PA, Ettema GJ, 1988. Length-force characteristics of aponeurosis in passive muscle and during isometric and slow dynamic contractions of rat gastrocnemius muscle. Acta. Morphol. Neerl. Scand. 26, 51–62. [PubMed] [Google Scholar]
- Huxley HE, 1953. Electron microscope studies of the organization of the filaments in striated muscle. Biochem. Biophys. Acta. 12, 387–394. [DOI] [PubMed] [Google Scholar]
- Huxley AF, 1957. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255–318. [PubMed] [Google Scholar]
- Huxley HE, 1963. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J. Mol. Biol. 7, 281–308. [DOI] [PubMed] [Google Scholar]
- Huxley HE, 1969. The mechanisms of muscle contraction. Science 164, 1356–1366. [PubMed] [Google Scholar]
- Huxley H, Hanson J, 1954. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173, 973–977. 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Niedergerke R, 1954. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 173, 971–973. [DOI] [PubMed] [Google Scholar]
- Ilton M, Bhamla MS, Ma X, Cox SM, Fitchett LL, Kim Y, Koh J, Krishnamurthy D, Kuo C-Y, Temel FZ, Crosby AJ, Prakash M, Sutton GP, Wood RJ, Azizi E, Bergbreiter S, Patek SN, 2018. The principles of cascading power limits in small, fast biological and engineered systems. Science. 360, eaao1082. 10.1126/science.aao1082. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Pakaslahti J, Komi P, 2005. Medial gastrocnemius muscle behavior during human running and walking. Gait Posture. 25, 380–384. [DOI] [PubMed] [Google Scholar]
- Iwanuma S, Akagi R, Kurihara T, Ikegawa S, Kanehisa H, Fukunaga T, Kawakami Y, 2011. Longitudinal and transverse deformation of human Achilles tendon induced by isometric plantar flexion at different intensities. J. Appl. Physiol. 110, 1615–1621. 10.1152/japplphysiol.00776.2010. [DOI] [PubMed] [Google Scholar]
- Javidi M, McGowan CP, Schiele NR, Lin DC, 2019. Tendons from kangaroo rats are exceptionally strong and tough. Sci. Rep. 9, 8196. 10.1038/s41598-019-44671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joumaa V, Herzog W, 2013. Energy cost of force production is reduced after active stretch in skinned muscle fibres. J. Biomech. 46, 1135–1139. 10.1016/j.jbiomech.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Balint JB, Reffy A, 1991. Three-dimensional ultrastructure of human tendons. Acta Anat. (Basel) 142, 306–312. 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A, 2006. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J. Biomech. 39, 406–417. 10.1016/j.jbiomech.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Stafilidis S, DeMonte G, Morey-Klapsing G, Brüggemann GP, Arampatzis A, 2005. Inevitable joint angular rotation affects muscle architecture during isometric contraction. J. Electromyogr. Kinesiol. 15, 608–616. 10.1016/j.jelekin.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Rome LC, 2002. Functional morphology of proximal hindlimb muscles in the frog Rana pipiens. J. Exp. Biol. 205, 1987–2004. 10.1242/jeb.205.14.1987. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Lieber RL, 2000. Interaction between series compliance and sarcomere kinetics determines internal sarcomere shortening during fixed-end contraction. J. Biomech. 33, 1249–1255. 10.1016/S0021-9290(00)00095-6. [DOI] [PubMed] [Google Scholar]
- Kellermayer MS, Granzier HL, 1996. Calcium-dependent inhibition of in vitro thin-filament motility by native titin. FEBS Letters. 380, 281–286. [DOI] [PubMed] [Google Scholar]
- Ker RF, 1981. Dynamic tensile properties of the plantaris tendon of sheep (Ovis nries). J. exp. Biol. 93, 283–302. [DOI] [PubMed] [Google Scholar]
- Ker RF, Alexander RMCN, Bennett MB, 1988. Why are mammalian tendons so thick? J. Zool. 216, 309–324. 10.1111/j.1469-7998.1988.tb02432.x. [DOI] [Google Scholar]
- Kjaer M, 2004. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84, 649–698. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP, 2007. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol. (Oxf) 191, 111–121. 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- Konow N, Azizi E, Roberts TJ, 2012. Muscle power attenuation by tendon during energy dissipation. Proc. Roy. Soc. B. 279, 1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R, Taylor CR, 1990. Energetics of running: a new perspective. Nature 346, 265–267. [DOI] [PubMed] [Google Scholar]
- Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T, 2000. Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. Eur. J. Appl. Physiol. 83, 463–468. 10.1007/s004210000309. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Ito M, Fukunaga T, 2001. Effects of isometric training on the elasticity of human tendon structures in vivo. J. Appl. Physiol. 91, 26–32. 10.1152/jappl.2001.91.1.26. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T, 2002. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J. Physiol. 538, 219–226. 10.1113/jphysiol.2001.012703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T, 2003. Gender differences in the viscoelastic properties of tendon structures. Eur. J. Appl. Physiol. 88, 520–526. 10.1007/s00421-002-0744-8. [DOI] [PubMed] [Google Scholar]
- Kubo K, Akima H, Ushiyama J, Tabata I, Fukuoka H, Kanehisa H, Fukunaga T, 2004. Effects of 20 days of bed rest on the viscoelastic properties of tendon structures in lower limb muscles. Br. J. Sports Med. 38, 324–330. 10.1136/bjsm.2003.005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Ohgo K, Takeishi R, Yoshinaga K, Tsunoda N, Kanehisa H, Fukunaga T, 2006. Effects of isometric training at different knee angles on the muscle–tendon complex in vivo. Scand. J. Med. Sci. Sports 16, 159–167. 10.1111/j.1600-0838.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Morimoto M, Komuro T, Yata H, Tsunoda N, Kanehisa H, Fukunaga T, 2007. Effects of plyometric and weight training on muscle-tendon complex and jump performance. Med. Sci. Sport, Exerc, p. 39. [DOI] [PubMed] [Google Scholar]
- Kubo K, Ikebukuro T, Yaeshima K, Yata H, Tsunoda N, Kanehisa H, 2009. Effects of static and dynamic training on the stiffness and blood volume of tendon in vivo. J. Appl. Physiol. 106, 412–417. 10.1152/japplphysiol.91381.2008. [DOI] [PubMed] [Google Scholar]
- Kubo K, Tabata T, Ikebukuro T, Igarashi K, Yata H, Tsunoda N, 2010. Effects of mechanical properties of muscle and tendon on performance in long distance runners. Eur. J. Appl. Physiol. 110, 507–514. 10.1007/s00421-010-1528-1. [DOI] [PubMed] [Google Scholar]
- Kubo K, Ikebukuro T, Maki A, Yata H, Tsunoda N, 2012. Time course of changes in the human Achilles tendon properties and metabolism during training and detraining in vivo. Eur. J. Appl. Physiol. 112, 2679–2691. 10.1007/s00421-011-2248-x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Ishigaki T, Ikebukuro T, 2017. Effects of plyometric and isometric training on muscle and tendon stiffness in vivo. Physiol. Rep. 5 10.14814/phy2.13374e13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Ikebukuro T, Yata H, 2021. Effects of plyometric training on muscle-tendon mechanical properties and behavior of fascicles during jumping. Physiol. Rep. 9, e15073. 10.14814/phy2.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa S, Fukunaga T, Fukashiro S, 2001. Behavior of fascicles and tendinous structures of human gastrocnemius during vertical jumping. J. Appl. Physiol. 90, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H, 2003. Calcium-dependent molecular spring elements in the giant protein titin. PNAS 100, 13716–13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte D, Holt NC, 2022. Elastic energy storage and the efficiency of movement. Curr. Biol. 32, R589–R683. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Barclay CJ, 2010. The influence of tendon compliance on muscle power output and efficiency during cyclic contractions. J. Exp. Biol. 213, 707–714. 10.1242/jeb.038026. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Bougoulias K, Wilson AM, 2007. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J. Biomech. 40, 157–164. 10.1016/j.jbiomech.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM, 2005. A modified Hill muscle model that predicts muscle power output and efficiency during sinusoidal length changes. J. Exp. Biol. 208, 2831–2843. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM, 2006. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J. Exp. Biol. 209, 4379–4388. 10.1242/jeb.02434. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM, 2008. Optimal muscle fascicle length and tendon stiffness for maximizing gastrocnemius efficiency during human walking an running. J. Theor. Biol. 252, 662–673. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Leonard ME, Brown CG, Trestik CL, 1991. Frog semitendinosis tendon load-strain and stress-strain properties during passive loading. Am. J. Physiol. Cell Physiol. 261, C86–C92. 10.1152/ajpcell.1991.261.1.C86. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Leonard ME, Brown-Maupin CG, 2000. Effects of muscle contraction on the load-strain properties of frog aponeurosis and tendon. Cells Tissues Organs 166, 48–54. 10.1159/000016708. [DOI] [PubMed] [Google Scholar]
- Longo SJ, Cox SM, Azizi E, Ilton M, Olberding JP, St Pierre R, Patek SN, 2019. Beyond power amplification: latch-mediated spring actuation is an emerging framework for the study of diverse elastic systems. J. Exp. Biol. 222 10.1242/jeb.197889. [DOI] [PubMed] [Google Scholar]
- Loren GJ, Lieber RL, 1995. Tendon biomechanical properties enhance human wrist muscle specialization. J. Biomech. 28, 791–799. 10.1016/0021-9290(94)00137-S. [DOI] [PubMed] [Google Scholar]
- Lou F, Curtin NA, Woledge RC, 1998. Contraction with shortening during stimulation or during relaxation: how do the energetic costs compare? J. Muscle Res. Cell Motil. 19, 797–802. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP, 2000. Load-elongation characteristics of in vivo human tendon and aponeurosis. J. Exp. Biol. 203, 751–756. 10.1242/jeb.203.4.751. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, Brond J, Dyre-Poulsen P, Bojsen-Moller J, Kjaer M, 2003. Differential strain patterns of the human gastronenous aponeurosis and free tendon, in vivo. Acta Physiol. Scand. 177, 185–195. [DOI] [PubMed] [Google Scholar]
- Malliaras P, Kamal B, Nowell A, Farley T, Dhamu H, Simpson V, Morrissey D, Langberg H, Maffulli N, Reeves ND, 2013. Patellar tendon adaptation in relation to load-intensity and contraction type. J. Biomech. 46, 1893–1899. 10.1016/j.jbiomech.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Marsh RL, 1994. Jumping ability of anuran amphibians. Adv. Vet. Sci. Como. Med. 38B, 51–111. [PubMed] [Google Scholar]
- Marsh RL, 2022. Muscle preactivation and the limits of muscle power output during jumping in the Cuban tree frog Osteopilus septentrionalis. J. Exp. Biol. 225, jeb244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh RL, John-Alder HB, 1994. Jumping performance of hylid frogs measured with high-speed cine film. J. Exp. Biol. 188, 131–141. 10.1242/jeb.188.1.131. [DOI] [PubMed] [Google Scholar]
- Maruyama K, 1976. Connectin, an elastic protein from myofibrils. J. Biomech. 80, 406–407. [DOI] [PubMed] [Google Scholar]
- Massey GJ, Balshaw TG, Maden-Wilkinson TM, Folland JP, 2018a. Tendinous tissue properties after short- and long-term functional overload: differences between controls, 12 weeks and 4 years of resistance training. Acta Physiol. 222 10.1111/apha.13019e13019. [DOI] [PubMed] [Google Scholar]
- Massey GJ, Balshaw TG, Maden-Wilkinson TM, Tillin NA, Folland JP, 2018b. Tendinous tissue adaptation to explosive-vs. sustained-contraction strength training. Front. Physiol. 9, 1170. 10.3389/fphys.2018.01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson A, Konow N, Miller S, Konow PP, Roberts TJ, 2012. Tendon material properties vary and are interdependent among turkey hindlimb muscles. J. Exp. Biol. 215, 3552–3558. 10.1242/jeb.072728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JM, 2021. Muscle actuators, not springs, drive maximal effort human locomotor performance. J. Sports Sci. Med. 20, 766–777. 10.52082/jssm.2021.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon GE, Morse CI, Burden A, Winwood K, Onambélé-Pearson GL, 2013. The manipulation of strain, when stress is controlled, modulates in vivo tendon mechanical properties but not systemic TGF-β1 levels. Physiol. Rep. 1, e00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G, Morse CI, Winwood K, Burden A, Onambélé GL, 2018. Gender associated muscle-tendon adaptations to resistance training. PLoS One 13, e0197852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza E, Azizi E, 2021. Tuned muscle and spring properties increase elastic energy storage. J. Exp. Biol. 224, jeb243180. 10.1242/jeb.243180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza E, Azizi E, Moen DS, 2020. What explains vast differences in jumping power within a clade? Diversity, ecology and evolution of anuran jumping power. Funct. Ecol. 34, 1053–1063. 10.1111/1365-2435.13545. [DOI] [Google Scholar]
- Meyer GA, Lieber RL, 2011. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J. Biomech. 44, 771–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer GA, Lieber RL, 2018. Frog muscle fibers bear a larger fraction of passive muscle tension than mouse. J. Exp. Biol. 221, jeb.182089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti RJ, Roy RR, Zhong H, Edgerton VR, 2003. Mechanical properties of rat soleus aponeurosis and tendon during variable recruitment in situ. J. Exp. Biol. 206, 3437–3445. 10.1242/jeb.00550. [DOI] [PubMed] [Google Scholar]
- Moo EK, Leonard TR, Herzog W, 2020. The sarcomere force–length relationship in an intact muscle–tendon unit. J. Exp. Biol. 223, jeb215020. 10.1242/jeb.215020. [DOI] [PubMed] [Google Scholar]
- Morrison SM, Dick TJM, Wakeling JM, 2015. Structural and mechanical properties of the human Achilles tendon: sex and strength effects. J. Biomech. 48, 3530–3533. 10.1016/j.jbiomech.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T, Muraka T, Takeshita D, Kawakami Y, Hirano Y, Fukunaga T, 2001. Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J. Appl. Physiol. 90, 1671–1678. [DOI] [PubMed] [Google Scholar]
- Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H, 2005. Elastic properties of human Achilles tendon are correlated to muscle strength. J. Appl. Physiol. 99, 665–669. 10.1152/japplphysiol.00624.2004. [DOI] [PubMed] [Google Scholar]
- Nishikawa KC, 2016. Eccentric contraction: Unraveling mechanisms of force enhancement and energy conservation. J. Exp. Biol. 219, 189–196. [DOI] [PubMed] [Google Scholar]
- Nishikawa KC, Monroy JA, Uyeno TE, Yeo SH, Pai DK, Lindstedt SL, 2012. Is titin a “winding filament”? A new twist on muscle contraction. Proc. R. Soc. B. 279, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olberding JP, Deban SM, Rosario MV, Azizi E, 2019. Modeling the determinants of mechanical advantage during jumping: consequences for spring- and muscle-driven movement. Integr. Comp. Biol. 59, 1515–1524. 10.1093/icb/icz139. [DOI] [PubMed] [Google Scholar]
- Onambélé GNL, Burgess K, Pearson SJ, 2007. Gender-specific in vivo measurement of the structural and mechanical properties of the human patellar tendon. J. Orthop. Res. 25, 1635–1642. 10.1002/jor.20404. [DOI] [PubMed] [Google Scholar]
- Otten E, 1988. Concepts and models of functional architecture in skeletal muscle. In: Pandolf KB (Ed.), Exercise and Sports Sciences Reviews, vol. 16. Macmillan, New York, pp. 89–137. [PubMed] [Google Scholar]
- Pandy MG, Zajac FE, 1991. Optimal muscular coordination strategies for jumping. J. Biomech. 24, 1–10. 10.1016/0021-9290(91)90321-d. [DOI] [PubMed] [Google Scholar]
- Peplowski MM, Marsh RL, 1997. Work and power output in the hindlimb muscles of Cuban tree frogs Osteopilus septentrionalis during jumping. J. Exp. Biol. 200, 2861–2870. [DOI] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE, 1994a. Relationship between body mass and biomechanical properties of limb tendons in adult mammals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 266, R1016–R1021. [DOI] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE, 1994b. Allometry of muscle, tendon, and elastic energy storage capacity in mammals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 266, R1022–R1031. [DOI] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR, 1984. Predictability of skeletal-muscle tension from architectural determinations in guinea-pig hindlimbs. J. Appl. Physiol. 57, 1715–1721. [DOI] [PubMed] [Google Scholar]
- Powers K, Schappacher-Tilp G, Jinha A, Leonard T, Nishikawa K, Herzog W, 2014. Titin force is enhanced in actively stretched skeletal muscle. J. Exp. Biol. 217, 3629–3636. [DOI] [PubMed] [Google Scholar]
- Purslow PP, Trotter JA, 1994. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J. Muscle. Res. Cell Motil. 15, 299–308. [DOI] [PubMed] [Google Scholar]
- Quinlan JI, Franchi MV, Gharahdaghi N, Badiali F, Francis S, Hale A, Phillips BE, Szewczyk N, Greenhaff PL, Smith K, Maganaris C, Atherton PJ, Narici MV, 2021. Muscle and tendon adaptations to moderate load eccentric vs. concentric resistance exercise in young and older males. GeroScience 43, 1567–1584. 10.1007/s11357-021-00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack PM, Westbury DR, 1984. Elastic properties of the cat soleus tendon and their functional importance. J. Physiol. 347, 479–495. 10.1113/jphysiol.1984.sp015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri BJ, Cresswell AG, Lichtwark GA, 2016. Three-dimensional geometrical changes of the human tibialis anterior muscle and its central aponeurosis measured with three-dimensional ultrasound during isometric contractions. PeerJ. 10.7717/peerj.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri BJ, Cresswell AG, Lichtwark GA, 2018. Muscle-tendon length and force affect human tibialis anterior central aponeurosis stiffness in vivo. Proc. Natl. Acad. Sci. U.S.A. 115, E3097–E3105. 10.1073/pnas.1712697115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JA, 1982. Sense and nonsense about the Fenn effect. Am. J. Physiol. 242, H1–H6. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Ferretti G, Narici MV, 2005. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J. Appl. Physiol. 98, 2278–2286. 10.1152/japplphysiol.01266.2004. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV, 2003. Behavior of human muscle fascicles during shortening and lengthening contractions in vivo. J. Appl. Physiol. 95, 1090–1096. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV, 2003. Effect of strength training on human patella mechanical properties of older individuals. J. Physiol. 548, 971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CT, Sawicki GS, 2012. Elastic recoil can either amplify or attenuate muscle-tendon power, depending on intertial vs fluid dynamic loading. J. Theor. Biol. 313, 68–78. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, 2002. The integrated function of muscles and tendons during locomotion. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 133, 1087–1099. 10.1016/S1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, 2016. Contribution of elastic tissues to the mechanics and energetics of muscle function during movement. J. Exp. Biol. 219, 266–275. 10.1242/jeb.124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, 2019. Some challenges of playing with power: does complex energy flows constrain neuromuscular performance. ICB. 59, 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Abbott EM, Azizi E, 2011. The weak link: do muscle properties determine locomotor performance in frogs? Philos. Trans. R. Soc. B Biol. Sci. 366, 1488–1495. 10.1098/rstb.2010.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Azizi E, 2010. The series-elastic shock absorber: Tendons attenuate muscle power during eccentric actions. J. Appl. Physiol. 214, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Azizi E, 2011. Flexible mechanisms: the diverse roles of biological springs in vertebrate movement. J. Exp. Biol. 214, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Konow N, 2013. How tendons buffer energy dissiaption by muscle. Exerc. Sports Sci. Rev. 41, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR, 1997. Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113–1115. 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Kram R, Weyand PG, Taylor CR, 1998a. Energetics of bipedal running: I. metabolic cost of generating force. J. Exp. Biol. 201, 2745–2751. 10.1242/jeb.201.19.2745. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, 2003. Probing the limits to muscle-powered accelerations: Lessons from jumping bullfrogs. J. Exp. Biol. 206, 2567–2580. 10.1242/jeb.00452. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Chen MS, Taylor CR, 1998b. Energetics of bipedal running II. Limb design and running mechanics. J. Exp. Biol. 201, 2753–2762. [DOI] [PubMed] [Google Scholar]
- Robertson BD, Sawicki GS, 2015. Unconstrained muscle-tendon workloops indicate resonance tuning as a mechanism for elastic behavior during terrestrial locomotion. PNAS. E5891–E5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JW, Struthers CN, Syme DA, 2018. Enhancement of muscle and locomotor performance by a series compliance: a mechanistic simulation study. PLoS One 13, e0191828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome LC, Funke RP, Alexander RM, Lutz G, Aldridge H, Scott F, Freadman M, 1988. Why animals have different muscle fiber types. Nature 335, 824–827. [DOI] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP, 2002. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand. J. Med. Sci. Sports 12, 90–98. 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Rosario MV, Sutton GP, Patek SN, Sawicki GS, 2016. Muscle-spring dynamics in time-limited elastic movements. Proc. Roy. Soc. B. 283, 20161561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki GS, Sheppard P, Roberts TJ, 2015. Power amplification in an isolated muscle-tendon unit is load dependent. J. Exp. Biol. 218, 3700–3709. 10.1242/jeb.126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C, 2011. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531. [DOI] [PubMed] [Google Scholar]
- Scott SH, Loeb GE, 1995. Mechanical properties of aponeurosis and tendon of the cat soleus muscle during whole-muscle isometric contractions. J. Morphol. 224, 73–86. 10.1002/jmor.1052240109. [DOI] [PubMed] [Google Scholar]
- Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF, Narici MV, 2009. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J. Appl. Physiol. 107, 523–530. 10.1152/japplphysiol.00213.2009. [DOI] [PubMed] [Google Scholar]
- Shadwick RE, 1990. Elastic energy storage in tendons: Mechanical differences related to function and age. J. Appl. Physiol. 68, 1033–1040. [DOI] [PubMed] [Google Scholar]
- Shan X, Otsuka S, Yakura T, Naito M, Nakano T, Kawakami Y, 2019. Morphological and mechanical proerpties from human triceps surae aponeuroses taken from elderly cadavers: Implications for muscle-tendon interactions. PloS ONE 14, e0211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleboda DA, Stover KK, Roberts TJ, 2020. Diversity of extracellular matrix morphology in vertebrate skeletal muscle. J. Morph. 281, 160–169. 10.1002/jmor.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Lichtwark GL, Kelly LA, 2022. Flexor digitorum brevis utilizes elastic strain energy storage to contribute to both work generation and energy absorption at the foot. J. Exp. Biol. 225, jeb243792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanjaard M, van Dieen JH, Baltzopoulos V, Maganaris CN, 2007. Gastrocnemius muscle fascicle behavior during stair negotiation in humans. J. Appl. Physiol. 102, 1618–1623. [DOI] [PubMed] [Google Scholar]
- Stafilidis S, Karamanidis K, Morey-Klapsing G, Demonte G, Bruggemann GP, Arampatsiz A, 2005. Strain and elongation of the vastus lateralis aponeurosis and tendon in vivo during maximal isometric contraction. Eur. J. Appl. Physiol. 94, 317–322. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, Sipilä S, Finni T, 2012. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J. Appl. Physiol. 113, 1537–1544. 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- Stensen N, 1667. Elementorum Myologiae Specimen, seu musculi descriptio geometrica. Florence. [Google Scholar]
- Sutton GP, Mendoza E, Azizi E, Longo SJ, Olberding JP, Ilton M, Patek SN, 2019. Why do large animals never actuate their jumps with latch-mediated springs? Because they can jump higher without them. Integr. Comp. Biol. 59, 1609–1618. 10.1093/icb/icz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen W, Hoogkamer W, De Groote F, Vanwanseele B, 2023. Faster triceps surae muscle contractions alter muscle activity and whole body metabolic rate. J. Appl. Physiol. 134, 395–404. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Heglund NC, McMahin TA, Looney TR, 1980. Energetic cost of generating muscular force during running. A comparison of large and small animals. J. Exp. Biol. 86, 9–18. [Google Scholar]
- Thys H, Cavagna GA, Margaria R, 1975. The role played by elasticity in an exercise involving movements of small amplitude. Pflϋger Arch. 354, 281–286. [DOI] [PubMed] [Google Scholar]
- Trestik CL, Lieber RL, 1993. Relationship between Achilles tendon mechanical properties and gastrocnemius muscle function. J. Biomech. Eng. 115, 225–230. 10.1115/1.2895479. [DOI] [PubMed] [Google Scholar]
- Uchida TK, Hicks JL, Dembia CL, Delp SL, 2016. Stretching your energetic budget: How tendon compliance affects the metabolic cost of running. PLoS One 11, e0150378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee TJ, Lemaire KK, van Soest AJ, 2019. The metabolic cost of in vivo constant force prodcution at zero net mechanical work. J. Exp. Biol. 222, jeb199158. [DOI] [PubMed] [Google Scholar]
- van Donkelaar CC, Willems PJB, Muijtjens AMM, Drost MR, 1999. Skeletal muscle transverse strain during isometric contraction at different lengths. J. Biomech. 32, 755–762. 10.1016/S0021-9290(99)00073-1. [DOI] [PubMed] [Google Scholar]
- Voigt M, Bojsen-Moller F, Simonsen EB, Dyhre-Poulsen P, 1995. The influence of tendon Youngs modulus, dimensions and instantaneous moment arms on the efficiency of human movement. J. Biomech. 28, 281–291. [DOI] [PubMed] [Google Scholar]
- Walker S, Trezise J, Haff GG, Newton RU, Häkkinen K, Blazevich AJ, 2020. Increased fascicle length but not patellar tendon stiffness after accentuated eccentric-load strength training in already-trained men. Eur. J. Appl. Physiol. 120, 2371–2382. 10.1007/s00421-020-04462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SR, Loren GJ, Lundber S, Lieber LL, 2005. High stiffness of human digital flexor tendons is suited for precise finger positional control. J. Neurophysiol. 96, 2815–2818. [DOI] [PubMed] [Google Scholar]
- Waugh CM, Alktebi T, de Sa A, Scott A, 2018. Impact of rest duration on Achilles tendon structure and function following isometric training. Scand. J. Med. Sci. Sports 28, 436–445. 10.1111/sms.12930. [DOI] [PubMed] [Google Scholar]
- Werkhausen A, Albracht K, Cronin NJ, Paulsen G, Bojsen-Møller J, Seynnes OR, 2018. Effect of training-induced changes in Achilles tendon stiffness on muscle-tendon behavior during landing. Front. Physiol. 9, 794. 10.3389/fphys.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Holt NC, 2018. Spatial scale and structural heterogeneity in skeletal muscle performance. Integr. Comp. Biol. 58, 163–173. 10.1093/icb/icy057. [DOI] [PubMed] [Google Scholar]
- Williams SB, Payne RC, Wilson AM, 2007. Functional specialization of the pelvic limb of the hare (Lepus europeus). J. Anat. 210, 472–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, Goodship AE, 1994. Exercise-induced hyperthermia as a possible mechanism for tendon degeneration. J. Biomech. 7, 899–905. [DOI] [PubMed] [Google Scholar]
- Wilson A, Lichtwark G, 2011. The anatomical arrangement of muscle and tendon enhances limb versatility and locomotor performance. Philos. Trans. R. Soc. B Biol. Sci. 366, 1540–1553. 10.1098/rstb.2010.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SLY, Gomez MA, Amiel D, Ritter MA, Gelberman RH, Akeson WH, Bitter MA, Gelberman RH, Akeson WH, 1981. The effects of exercise on the biomechanical and biochemical properties of swine digital flexor tendons. J. Biomech. Eng. 103, 51–56. 10.1115/1.3138246. [DOI] [PubMed] [Google Scholar]
- Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH, 1980. The biomechanical and biochemical properties of swine tendons–long term effects of exercise on the digital extensors. Connect. Tissue Res. 7, 177–183. [DOI] [PubMed] [Google Scholar]
- Zajac FE, 1989. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit. Rev. Biomed. Eng. 17, 359–411. [PubMed] [Google Scholar]
- Zuurbier CJ, Everard AJ, van der Wees P, Huijing PA, 1994. Length-force characteristics of the aponeurosis in the passive and active muscle condition and in the isolated condition. J. Biomech. 27, 445–453. 10.1016/0021-9290(94)90020-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.