Abstract
BACKGROUND:
Pulsed field ablation uses electrical fields to cause nonthermal cell death over several hours. Polarization-sensitive optical coherence reflectometry is an optical imaging technique that can detect changes in the tissue ultrastructure in real time, which occurs when muscular tissue is damaged. The objective of this study was to evaluate the ability of a polarization-sensitive optical coherence reflectometry system to predict the development of chronic lesions based on acute changes in tissue birefringence during pulsed field ablation.
METHODS:
Superior vena cava isolation was performed in 30 swine using a biphasic, bipolar pulsed field ablation system delivered with a nonirrigated focal tip catheter. Acute changes in tissue birefringence and voltage abatement were analyzed for each individual lesion. A high-resolution electroanatomical map was performed at baseline and 4 to 12 weeks after ablation to locate electrical gaps in the ablated area.
RESULTS:
A total of 141 lesions were delivered and included in the analysis. Acute electrical isolation based on the electroanatomical map was achieved in 96% of the animals, but chronic isolation was only seen in 14 animals (46%). The mean voltage abatement of lesions that showed recovery was 82.8%±14.6% versus 84.4%±17.4% for those that showed fibrosis (P=0.7). The mean acute reduction in tissue birefringence in points demonstrating fibrosis was 63.8%±11.3% versus 9.1%±0.1% in the points that resulted in electrical gaps. A threshold of acute reduction of birefringence of ≥20% could predict chronic lesion formation with a sensitivity of 96% and a specificity of 83%.
CONCLUSIONS:
Acute tissue birefringence changes assessed with polarization-sensitive optical coherence reflectometry during pulsed field ablation can predict chronic lesion formation and guide the ablation procedure although limited by the tissue thickness.
Keywords: atrial fibrillation; birefringence; myocytes, cardiac; phrenic nerve; pulmonary veins
WHAT IS KNOWN?
Pulsed field ablation using cardiac electroporation can eliminate bipolar electrograms even with subtherapeutic dosing.
Knowing when tissue has been ablated to the point of causing permanent fibrosis is empirical.
There is a need for an objective method to determine the occurrence of permanent fibrosis postpulsed field ablation.
WHAT THE STUDY ADDS
Pulsed field ablation causes acute changes in tissue optical characteristics.
Loss of tissue optical birefringence can be measured using a technology called polarization-sensitive optical coherence reflectometry is seen after pulsed field ablation.
An acute decrease in tissue birefringence can predict chronic fibrotic lesions at 4 to 8 weeks after ablation.
The goal of catheter ablation of atrial fibrillation is to eliminate the triggers for atrial fibrillation by creating transmural lesions, which will result in durable pulmonary vein isolation.1 Our inability to create continuous and transmural lesions with thermal energy is one reason why the success rates of ablation are still limited to 55% to 70%.2–4 Pulsed field ablation (PFA) is a novel energy that uses electrical fields to cause cardiac cell death. PFA causes a supraphysiological transmembrane voltage gradient in the cardiomyocyte cells that damage voltage-dependent membranes and intracellular proteins, leading to cellular dysfunction and nonthermal cardiac cell death over hours to days.5,6 PFA promises a safer procedure because noncardiac tissues have higher thresholds for PFA damage, minimizing adverse events such as esophageal fistula or phrenic nerve palsy.
See Editorial by Mehta and Haines
However, the typical lesion depth of bipolar PFA systems is limited to 2 to 4 mm7 due to the distribution of the electric field between the ablation electrodes. Furthermore, monitoring for the disappearance of bipolar signals in tissue is not reliable with PFA because even subtherapeutic pulses cause signal abatement because of cellular stunning.7 Therefore, assessing for the completion of transmural PFA lesions is challenging, and operators are left with performing an empirical number of applications in hopes that a complete lesion is achieved.
Optical imaging to directly visualize the tissue may help determine the depth and durability of a delivered lesion during ablation using polarization-sensitive optical coherence reflectometry (PS-OCR) based on the birefringence tissue properties.8,9 PS-OCR has been shown to predict lesion depth and durability with radiofrequency ablation.10–12 However, it is unknown whether PS-OCR could be useful in monitoring lesion formation with PFA. The purpose of this preclinical trial is to evaluate the ability of PS-OCR to assess for durability of PFA cardiac lesions.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal Preparation
The study was performed on healthy Yorkshire swine (n=30; average weight, 48±15 kg; 66% female). The experiments were conducted in 2 different locations: the Sunnybrook Research Institute (Toronto, ON, Canada) and the Centro de Cirugía de Mínima Invasión Jesús Usón (Cáceres, Spain) after approval of the Institutional Animal Care and Use Committee at the Sunnybrook Research Institute and the Comité de Ética en Experimentación Animal del Centro de Cirugía de Mínima Invasión Jesús Usón, respectively.
On the day of the procedure, the animals were sedated with a combination of diazepam (0.4 mg/Kg) and ketamine (15 mg/Kg) or ketamine alone (33 mg/Kg). Once intubated, they were ventilated and maintained on either isoflurane or sevoflurane at a dosage ranging from 1.5% to 3.5%. An intramuscular injection of tramadol (1 mg/Kg) was administered for pain amelioration, followed by continuous infusion. The use of PFA can cause the contraction of skeletal muscle, so all animals received rocuronium at a dose of 1.2 mg/Kg before ablation. Intravenous amiodarone (150–300 mg) was also given at the beginning of the procedure to decrease the risk of ventricular arrhythmias during ablation and catheter manipulation.
PS-OCR PFA Ablation System
Optical coherence reflectometry is a noninvasive, high-resolution optical imaging system analogous to ultrasound with 20× greater accuracy for reconstructing tissue structure based on back-reflected light as a function of depth. In addition, PS-OCR measures depth-resolved tissue birefringence. Birefringence is an optical property of tissues, whereby the refractive index depends on the polarization and direction of light propagation. Healthy organized muscle tissue is known to exhibit basic birefringent properties because it is an anisotropic medium and the light is propagated at different speeds depending on its orientation with respect to the muscular fibers. If the tissue is exposed to an injury such as ablation, the affected area loses its ultrastructure having a reduction in the birefringence because the light propagates at the same speed in either direction (isotropic medium).8,10 The PS-OCR system has been described in detail in a previous publication.11
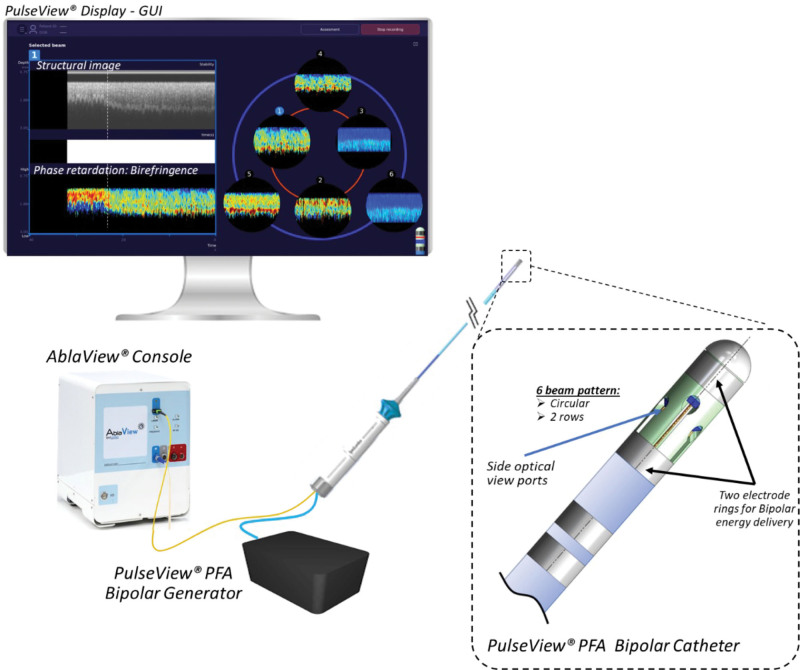
The PulseView Bipolar PFA System (MedLumics S.L., Tres Cantos, Spain) was developed to measure the birefringent properties of the tissue and is made up of 4 components (Figure 1): (1) PulseView PFA bipolar catheter, (2) PulseView PFA bipolar generator, (3) Ablaview console, and (4) PulseView graphic user interface.
Figure 1.
Components of the PulseView system. GUI indicates graphic user interface; and PFA, pulsed field ablation.
The PulseView PFA bipolar catheter is an 8F steerable, nonirrigated catheter designed to deliver PFA between the 2 distal electrodes and detect tissue birefringence simultaneously. It has 2 electric rings (platinum iridium; width, 1.5 mm) for bipolar energy delivery, separated by 4 mm, and 6 optical viewports located in a radial distribution in 2 levels. These 6 multifiber optical sensors incorporate PS-OCR technology that is interpreted by the Ablaview console (Figure 1). When the light penetrates the myocardium, the system detects in real time the contrast between healthy tissue and ablated tissue based on the tissue properties of birefringence and determines catheter stability and tissue contact. The PulseView graphic user interface displays the real-time birefringence in all 6 ports to guide the physician during the procedure (Figure 1).
The PulseView generator delivers bipolar, biphasic pulses between the 2 electric rings of the catheter. Every application consists of 3 packets (20 pulses/packet) of microseconds of duration pulses at ±1200 V. The protocol and waveform were optimized before this study in 10 acute swine models to minimize the thermal effect, assess bubble and lesion formation, and optimize birefringence change detection. The average lesion dimensions of the PFA lesions generated with this system had been determined in previous preclinical studies to be 9.1±1.6 mm in width and 4.2±1.8 mm in depth (Supplemental Methods).
Study Protocols
The study took place in 2 phases. Phase 1 consisted of ablation and acute electrical and optical assessment of lesion formation. Phase 2 was performed 4 to 12 weeks after phase 1 and consisted of remapping to assess for electrical and optical gaps and to perform histological assessment of the chronic tissue.
During phase 1, all animals underwent superior vena cava (SVC) isolation. A high-density electroanatomical map of the right atrium was created at the beginning of every procedure using the Ensite Precision Mapping System (Abbott, Plymouth, MN) and a multipolar mapping catheter (HD-Grid, Abbott). The focal ablation catheter was placed in the target line of ablation, located just above the right atrial appendage and below the ostium of the SVC. The SVC was chosen as the target to ensure a parallel alignment between the catheter and the tissue because lesions were delivered bipolar between the proximal and distal ablation electrodes. The SVC was divided into 12 anatomic segments to better localize each individual lesion and correlate with the chronic map in phase 2. An 8-mm-diameter lesion tag was placed immediately before energy delivery based on the lesion size reported in the preclinical study described previously. The ablation line was completed using a point-by-point overlapping technique circumferentially around the SVC. The acute end point was assessed by demonstrating a bipolar voltage ≤0.1 mV above the line, with no visual anatomic gaps on the map.
Tissue birefringence was assessed in real time during each ablation and after every lesion. The PulseView catheter was left in position for at least 1 minute after each ablation to document late changes or any possible recovery of tissue birefringence. If there was a recovery of tissue birefringence or no change was detected, no further energy was delivered to avoid lesion overlapping and perform a single lesion analysis. A significant change in tissue birefringence was defined as a ≥20% reduction from the baseline value based on prior data showing that a birefringence change after PFA of 20% in cardiomyocytes results in >50% cell viability loss9 (Video S1).
Operators could assess catheter stability and tissue contact in real time to optimize catheter position before energy delivery. Change in tissue birefringence could be visualized for every individual lesion, but the change was quantified postprocedure.
After SVC isolation, a dwell time of at least 20 min was established to confirm the success of the procedure based on confirmation of the electrical vein isolation by remapping. Once the procedure was completed, the puncture sites were closed, and the animal was recovered and kept in the facilities of the center for 4 to 12 weeks.
Phase 2 followed the same animal preparation as phase 1. A new voltage map of the right atrium was obtained to look for any possible electrical gaps. All the prior lesion locations were interrogated by the PulseView catheter by placing the catheter above the line in the SVC and then dragging it down over the line and then to the level of the body of the right atrium. This was done in at least 6 locations circumferentially around the SVC to determine tissue birefringence changes between healthy and ablated tissues and to detect any lesion gaps. In the absence of electrical isolation, the area with the electrical gap was identified and optically characterized.
Birefringence Loss Calculation
In healthy cardiac muscle, there is a large dynamic range of periodic variation of birefringence between orthogonal polarization states showing high birefringence. After the first few seconds of PFA energy delivery, an abrupt change is observed, which reduces the dynamic range to the background noise level (ablated tissue). A typical image of birefringence after PFA is represented in Figure 2A. This birefringence change is approximately monotonic under optimal contact conditions. The PulseView ablation system is capable of measuring changes in birefringence with a speed of 50 ms. The birefringence value was calculated as the relative organization between muscle layers for every 50 µm depth. The absolute birefringence value was subsequently calculated as the mean value of birefringence at the depth of view: maximum, 1.5 mm (Figure 2B). For the calculation of birefringence loss, time averaging was performed every 10 measurements to obtain an average value of the birefringence value every 0.5 s. This allowed a smoothing of the time evolution, which was then fitted by local polynomial regression fitting, with a span of 0.2. This polynomial regression curve was scaled between 0 and 1, with 1 being the initial value of the birefringence of the tissue and 0 being the background noise of the system instead of using the absolute birefringence values. Tissue birefringence is known to significantly differ among individuals, tissues, and locations.
Figure 2.
Change of tissue birefringence during ablation. A, Dotted white line shows the time of ablation. Birefringence loss during the first few seconds of ablation. B, Graph showing the change in tissue birefringence during ablation. C, Polynomial regression fitting with normalized birefringence. Red circle shows the 20% loss. BiR indicates birefringence; and PFA, pulsed field ablation.
Based on the initial acute preclinical phase and the published study,9 a simple threshold of 20% reduction from the baseline tissue birefringence was set to consider that the ablation was effective, as represented in Figure 2C.
Tissue Processing
After completion of phase 2, the animals received a heparin bolus (100 UI/Kg), and they were euthanized with a dose of supersaturated potassium chloride solution. The heart was then excised, fixed with formalin for a minimum of 2 days, and sliced for histology. Histology consisted of staining tissue using hematoxylin and eosin and Masson trichrome stains.
Statistical Analysis
Outcomes are presented as means with standard deviations or proportions where applicable. Comparisons were performed using 1-way ANOVA or a 2-sample t test for continuous variables and χ2 test for categorical variables. A mixed model was used to account for potential correlation between measurements performed in the same animal. A P value of <0.05 was considered significant. The analysis was conducted with R, version 4.3.0.
RESULTS
SVC Isolation—Phase 1
Acute SVC electrical isolation was achieved in 29 of 30 animals. In one animal, a gap was left intentionally as a control for suboptimal ablations. The number of lesions required to achieve acute isolation was 5±1. A total of 141 lesions were included for final analysis. Lesions were excluded if they were delivered in the same anatomic position showing no tissue birefringence before ablation (n=6) or when there was catheter movement during ablation not allowing for proper delivery of the energy (n=2).
Voltage abatement occurred right after PFA delivery. Voltage preablation and postablation was available in 123 lesions. The overall average voltage preablation was 3.1±2.2 mV and postablation was 0.3±0.2 mV with a mean abatement of 84.1±17.4% per lesion. An example of typical voltage preablation and postablation is shown in Figure 3. The overall mean reduction in tissue birefringence from preablation to postablation was 74.3%±14.5% per lesion at the end of the ablation. An acute loss of tissue birefringence with >20% from the initial birefringence was achieved in 81.5% of the lesions delivered. The absence of a change in tissue birefringence was most seen in the septal position closest to the right atrial appendage. The time to achieve a 20% reduction of the initial birefringence was 7.7±11.6 s (Video S1).
Figure 3.
Acute changes in electrogram amplitude and tissue birefringence. Electrogram amplitude pre-pulsed field ablation (PFA) (A and D) and post-PFA (B and E). (Top) An example of acute lesion that resulted in chronic recovery and no fibrosis despite the presence of an acute voltage abatement of 95.1%. C, Tissue birefringence showed no loss post ablation. (Bottom) A similar change in electrogram amplitude with abatement of 96.1%. However, this lesion did create fibrosis. There is a loss in tissue birefringence seen in F. Note the ECG showed transient ST elevation post ablation, which is discussed in the text. OCR indicates optical coherence reflrectometry.
The mean voltage abatement of lesions that showed recovery was 82.8%±14.6% versus 84.4%±17.4% for those that showed fibrosis (P=0.55). A representative example of ablation with electrical reconnection in the chronic phase is shown in Figure 3A and 3C and with fibrosis in Figure 3D and 3F. Although voltage abatement at the time of ablation was detected in both lesions (Figure 3B and 3E), there was no loss of birefringence in the area identified as a gap in phase 2 of the study (Figure 3C). On the other hand, the chronic lesion represented in Figure 3F did display acute loss of birefringence, which was confirmed with the finding of fibrotic scar in the chronic phase.
Tissue birefringence did not significantly change when the catheter was held in the same position for at least 1 minute during the ablation.
Minor muscle contractions were observed when neuromuscular blocking agents were not given. Four of the animals had transient ST elevation, but this was only observed with septal or posteroseptal lesions, probably related to the close proximity of the right coronary artery. None of these events was related to hemodynamic instability, triggered arrhythmias, or required any specific treatment. There were no cases of acute phrenic nerve palsy after ablation. There were no ventricular arrhythmias, heart block, or sinus pauses because of energy delivery.
Remap of SVC—Phase 2
The average time from the first to second study was 43±14 days, ranging from 28 to 76 days.
Although SVC isolation was acutely achieved in 29 animals, only 14 animals demonstrated chronic isolation (46%). Figure 4A demonstrates fibrosis by identifying the birefringence loss on the ablation line by dragging the catheter from the vein to the right atrium. From the total 141 lesions analyzed, 24 lesions showed recovery (17%) and were confirmed by the absence of any loss in birefringence (Figure 4B). Of the 16 animals that did not have chronic isolation, 7 (44%) demonstrated one electrical gap, 5 (31%) demonstrated 2 gaps, and 4 (25%) demonstrated more than 2 gaps. Each gap was defined as a diameter of ≤8 mm demonstrating healthy voltage (>1 mV). The most frequent location for electrical gaps was anterior (42%) followed by septal (33%), posterior (12.5%), and lateral (12.5%) positions.
Figure 4.
Electroanatomical map of the right atrium 4 wk after ablation with chronic evaluation of lesions by dragging. A, Electroanatomical map (EAM) shows chronic isolation of the superior vena cava (SVC). The yellow dotted arrow shows the trajectory of dragging the catheter postablation to evaluate tissue birefringence. There is a clear loss of tissue birefringence in the line of ablation (area between the white dotted lines) that recovers when the catheter moves to the body of the right atrium. B, EAM shows chronic reconnection of the SVC with posterior and anterior electrical gaps. There is persistent tissue birefringence in the line of ablation (area between the white dotted lines).
The mean reduction in birefringence during phase 1 in points demonstrating fibrosis in the chronic phase was 63.8%±11.3% versus 9.1%±0.1% in the points that resulted in gaps (Figure 5A and 5B). Using a threshold of reduction of birefringence of ≥20%, changes in acute birefringence predicted a chronic lesion with a sensitivity of 96% and a specificity of 83% (Table). A ROC curve (receiver operating characteristic curve) showed an excellent model performance with an area under the curve of 0.95. The highest sensitivity and specificity values were for a birefringence change between 14.5% and 20.5% (Figure S1).
Figure 5.
Loss of tissue birefringence for lesions displaying fibrosis or recovery. A, Box plot comparing the percentage of loss of tissue birefringence for lesions with chronic electrical gaps vs lesions with chronic fibrosis. B, The loss in tissue birefringence is depicted for each lesion with red lines, indicating that no chronic fibrosis vs black lines indicated chronic fibrosis. One can see grossly that regardless of the initial absolute value, which can vary depending on every animal and the tissue targeted for ablation, small drops in birefringence of <20% do not result in durable lesions, whereas a reduction of ≥20% results in chronic fibrosis. BiR indicates birefringence; and PFA, pulsed field ablation.
Table.
Predictive Value of Loss in Tissue Birefringence for Long-Term Scar Formation
The specificity is the ability of the optical coherence reflectometry system to detect in the acute phase when an ablation will be reversible. Electrogram voltage abatement, on the other hand, had a specificity of only 11%.
Tissue Examination and Histology
During necropsy, SVC lesions were easily identifiable by gross observation, but no lesions or injuries were seen in the surrounding structures (Figure 6).
Figure 6.
Histology four weeks after ablation sparing vessels and surrounding structures. Masson trichrome staining was used in A through C. Hematoxylin and eosin staining was used in D and E. A, Preserved structure of a nerve within the ablated tissue. B, Preserved structure of arterioles and venules within the ablated tissue. C, Lymph node in close proximity to ablated tissue with homogeneous fibrosis. D and E, Preserved structure of the esophagus in close proximity to the ablated superior vena cava. Longitudinal section and transverse section.
Of the 141 lesions analyzed, all were available for histological examination. Chronic PFA lesions exhibited replacement fibrosis. Nerves, lymph nodes, and vessels within the ablated tissue were preserved, as shown in Figure 6. There was no evidence of coagulative necrosis in any of the lesions. The boundaries between healthy and fibrotic tissues were well demarcated. The transition zone between healthy and ablated tissues showed branches of collagen entering the healthy cardiac tissue (Figure 7). All chronic lesions showed transmurality given the limited thickness of the SVC.
Figure 7.
Gross pathology and histology examination of pulsed field ablation lesions. A, Gross pathology of the superior vena cava (SVC) 8 wk after ablation. B, Dashed line showing well-demarcated continuous fibrosis on the line of ablation. C, Masson trichrome stain of the area of ablation showing homogeneous replacement fibrosis in blue with preserved vessels (blue arrow) and nerves (orange arrow). D, Nonablated/healthy myocardial tissue below the line of ablation. E, Transition zone with islands of preserved cardiomyocytes surrounded by fibrosis.
DISCUSSION
This preclinical study looked at the ability of loss of tissue birefringence detected by PS-OCR to predict chronic lesion development after PFA. We found that application of PFA resulted in a loss of tissue birefringence ≥20% for lesions demonstrating replacement fibrosis in contrast to a <20% loss for ablations that did not result in chronic lesion formation. Furthermore, a ≥20% loss in birefringence demonstrated a sensitivity of 96% and a specificity of 83% for lesions creating chronic fibrosis with an overall high positive predictive value of 97%. All the chronic lesions showed transmurality in the SVC. In contrast, acute electrogram voltage abatement occurred after all PFA lesions and to a similar magnitude whether the ablation formed a chronic scar or not.
Lesion formation with PFA depends on several parameters, including the intensity of the electric field, duration of exposure, and waveform.7 Good tissue-catheter contact has also been shown to have a direct correlation with lesion size and depth.13–15 However, trabeculated tissue surfaces, unusual anatomies, or cardiorespiratory motion can make stable contact with the endocardial tissue challenging, making energy delivery and lesion formation suboptimal. Furthermore, the shape of larger, multipolar PFA catheters may not be able to contact all aspects of the pulmonary vein antrum during a single application.
Another previously reported limitation of PFA is that it results in almost instantaneous attenuation of electrograms even after subtherapeutic applications.6,16,17 Therefore, loss of electrograms can no longer serve as a reliable ablation end point. Rapid voltage attenuation with PFA likely results because of a combination of edema and cell stunning. Cell excitability is reduced in electroporated cells due to the ion and osmotic imbalance leading to acute hyperpolarization of the cell membranes.18 Edema also results from outward leakage of intracellular components.19 While some of these stunned cells might activate apoptotic cell death, if the magnitude of the PFA field is insufficient due to all the limitations described above, some cells will recover conductivity and repair with time, resulting in reversible electroporation.19–21 To overcome these limitations of PFA, most systems are prescribing empirical recipes of ablation, such as 8 to 12 applications per pulmonary vein with partial rotation of the catheter in between applications.6,16,22 However, the operator can never be certain that this empirical approach will work for varying tissue thickness, scarring, and anatomic shape. Despite optimized pulse voltage and waveforms showing transmurality in animal models, success rates of PFA remain similar to thermal modalities6,23 with enduring pulmonary vein reconnections.24 To date, there are really no other real-time assessments that an operator can use to ensure the durability of any given PFA lesion. The PS-OCR system allows for the assessment of tissue-catheter contact before PFA delivery. It also allows real-time monitoring of a surrogate—in this case, tissue birefringence—to predict chronic lesion formation. The optical coherence reflectometry platform has been adapted to a dedicated point-by-point catheter in this study but could be flexible to any other point-by-point catheter or even a multipoint catheter given the flexibility of the optical hardware.
STUDY LIMITATIONS
The main limitation of this study is the use of SVC isolation instead of pulmonary veins. This catheter prototype had to be positioned parallel to the tissue to deliver PFA because of the bipolar design. This would have been challenging in the small left atrium with the complex venous anatomy in swine. For this reason, we performed SVC isolation because of its similar histological structure to the pulmonary veins. However, the antral tissue can be substantially thinner than the pulmonary vein antrum in the adult human population. Our report of 100% transmurality must also be considered in the context of the thin tissue around the SVC. Areas thinner than the maximum depth of view (1.5 mm) of this system might not always display an accurate calculation of the absolute birefringence value because structures beyond the SVC can be included. Sensitivity and specificity values reported in this study might differ when the system is used in thicker tissues such as the pulmonary veins. In this study, the most common location for electrical gaps was the anterior aspect of the SVC. This was probably related to the catheter design. All ablation lines were delivered right above the right atrial appendage but in close proximity. If the proximal electric ring was not in good contact with the tissue or falling into the right atrial appendage, the electric field delivered might not have been enough to cause cell death. A newer monopolar catheter is being designed to avoid the limitations of a bipolar delivery system. The ability of PS-OCR to detect the same birefringence changes will require further evaluation. We also used a 20% cutoff in birefringence loss based on prior data using radiofrequency as explained in the methods. Other cutoffs could also have been assessed, but as our figures show, ≥20% turned out to be the lower confidence level cutoff for permanently fibrotic lesions. Finally, we took great care to correlate gaps at phase 2 with initial lesions delivered during phase 1 by examining the electroanatomical maps from both phases. However, it is accepted that normal variations in mapping system accuracy and changes in anatomy due to swine growth might have affected the accuracy of our localization.
CONCLUSIONS
This study demonstrated the ability of a PS-OCR-based optical system to predict long-term chronicity of PFA lesions based on a ≥20% drop in tissue birefringence although limited by the tissue thickness.
ARTICLE INFORMATION
Sources of Funding
This study was supported by MedLumics S.L. Spanish state program in the Plan for Scientific, Technical, and Innovation Research framework 2021-2023. CPP2021-008480.
Disclosures
Drs Herranz and Bailleul are employees of MedLumics S.L. Drs Terricabras, Martins, Peinado, Derejko, Mont, Ernst, and Verma are MedLumics S.L. consultants.
Supplemental Material
Supplemental Methods
Supplemental S1 and S2
Figure S1
Video S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- PFA
- pulsed field ablation
- PS-OCR
- polarization-sensitive optical coherence reflectometry
- SVC
- superior vena cava
For Sources of Funding and Disclosures, see page 133.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.123.012255.
Contributor Information
Maria Terricabras, Email: mariaterricabras@gmail.com.
Raphael P. Martins, Email: raphael.pmartins@gmail.com.
Rafael Peinado, Email: rafael.peinado@uam.es.
Paweł Derejko, Email: pderejko@yahoo.com.
Lluís Mont, Email: LMONT@clinic.cat.
Sabine Ernst, Email: s.ernst@rbht.nhs.uk.
David Herranz, Email: dherranz@medlumics.com.
Christophe Bailleul, Email: cbailleul@medlumics.com.
REFERENCES
- 1.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, Roux JF, Yung D, Skanes A, Khaykin Y, et al. ; EARLY-AF Investigators. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980 [DOI] [PubMed] [Google Scholar]
- 3.Inoue K, Hikoso S, Masuda M, Furukawa Y, Hirata A, Egami Y, Watanabe T, Minamiguchi H, Miyoshi M, Tanaka N, et al. ; OCVC Arrhythmia Investigators. Pulmonary vein isolation alone vs. more extensive ablation with defragmentation and linear ablation of persistent atrial fibrillation: the EARNEST-PVI trial. Europace. 2021;23:565–574. doi: 10.1093/europace/euaa293 [DOI] [PubMed] [Google Scholar]
- 4.Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey DJ, Pathak RK, Kumar S, Munawar DA, Young GD, Kalman JM, et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace. 2018;20:f366–f376. doi: 10.1093/europace/eux297 [DOI] [PubMed] [Google Scholar]
- 5.Verma MS, Terricabras M, Verma A. The cutting edge of atrial fibrillation ablation. Arrhythm Electrophysiol Rev. 2021;10:101–107. doi: 10.15420/aer.2020.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma A, Boersma L, Haines DE, Natale A, Marchlinski FE, Sanders P, Calkins H, Packer DL, Hummel J, Onal B, et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF pilot trial. Circ Arrhythm Electrophysiol. 2022;15:e010168. doi: 10.1161/CIRCEP.121.010168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma A, Asivatham SJ, Deneke T, Castellvi Q, Neal RE. Primer on pulsed electrical field ablation. Circ Arrhythm Electrophysiol. 2021;14:e010086. doi: 10.1161/CIRCEP.121.010086 [DOI] [PubMed] [Google Scholar]
- 8.Fu X, Wang Z, Wang H, Wang YT, Jenkins MW, Rollins AM. Fiber-optic catheter-based polarization-sensitive OCT for radio-frequency ablation monitoring. Opt Lett. 2014;39:5066–5069. doi: 10.1364/OL.39.005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Roy P, Shao Q, Jiang C, Choi J, Chung C, Mehra D, Bischof JC. The role of protein loss and denaturation in determining outcomes of heating, cryotherapy, and irreversible electroporation on cardiomyocytes. J Biomech Eng. 2018;140:061007. doi: 10.1115/1.4039375. [DOI] [PubMed] [Google Scholar]
- 10.Fleming CP, Wang H, Quan KJ, Rollins AM. Real-time monitoring of cardiac radio-frequency ablation lesion formation using an optical coherence tomography forward-imaging catheter. J Biomed Opt. 2010;15:030516. doi: 10.1117/1.3459134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herranz D, Lloret J, Jiménez-Valero S, Rubio-Guivernau JL, Margallo-Balbás E. Novel catheter enabling simultaneous radiofrequency ablation and optical coherence reflectometry. Biomed Opt Express. 2015;6:3268–3275. doi: 10.1364/BOE.6.003268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González‐Suárez A, Herranz D, Berjano E, Rubio‐Guivernau JL, Margallo‐Balbás E. Relation between denaturation time measured by optical coherence reflectometry and thermal lesion depth during radiofrequency cardiac ablation: Feasibility numerical study. Lasers Surg Med. 2018;50:222–229. doi: 10.1002/lsm.22771 [DOI] [PubMed] [Google Scholar]
- 13.Mattison L, Verma A, Tarakji KG, Reichlin T, Hindricks G, Sack KL, Önal B, Schmidt MM, Miklavčič D, Sigg DC. Effect of contact force on pulsed field ablation lesions in porcine cardiac tissue. J Cardiovasc Electrophysiol. 2023;34:693–699. doi: 10.1111/jce.15813 [DOI] [PubMed] [Google Scholar]
- 14.Howard B, Verma A, Tzou WS, Mattison L, Kos B, Miklavčič D, Onal B, Stewart MT, Sigg DC. Effects of electrode-tissue proximity on cardiac lesion formation using pulsed field ablation. Circ Arrhythm Electrophysiol. 2022;15:e011110. doi: 10.1161/CIRCEP.122.011110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meckes D, Emami M, Fong I, Lau DH, Sanders P. Pulsed-field ablation: computational modeling of electric fields for lesion depth analysis. Heart Rhythm O2. 2022;3:433–440. doi: 10.1016/j.hroo.2022.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, Sediva L, Chovanec M, Dukkipati SR, Jais P. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–326. doi: 10.1016/j.jacc.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 17.Turagam MK, Neuzil P, Schmidt B, Reichlin T, Neven K, Metzner A, Hansen J, Blaauw Y, Maury P, Arentz T, et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: one-year outcomes from the MANIFEST-PF registry. Circulation. 2023;148:35–46. doi: 10.1161/CIRCULATIONAHA.123.064959 [DOI] [PubMed] [Google Scholar]
- 18.Nikolski VP, Efimov IR. Electroporation of the heart. Europace. 2005;7:S146–S154. doi: 10.1016/j.eupc.2005.04.011 [DOI] [PubMed] [Google Scholar]
- 19.Batista Napotnik T, Polajžer T, Miklavčič D. Cell death due to electroporation - a review. Bioelectrochemistry. 2021;141:107871. doi: 10.1016/j.bioelechem.2021.107871 [DOI] [PubMed] [Google Scholar]
- 20.Xie F, Varghese F, Pakhomov AG, Semenov I, Xiao S, Philpott J, Zemlin C. Ablation of myocardial tissue with nanosecond pulsed electric fields. PLoS One. 2015;10:e0144833. doi: 10.1371/journal.pone.0144833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beebe SJ, Sain NM, Ren W. Induction of cell death mechanisms and apoptosis by nanosecond pulsed electric fields (nsPEFs). Cells. 2013;2:136–162. doi: 10.3390/cells2010136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duytschaever M, De Potter T, Grimaldi M, Anic A, Vijgen J, Neuzil P, Van Herendael H, Verma A, Skanes A, Scherr D, et al. Paroxysmal atrial fibrillation ablation using a novel variable-loop biphasic pulsed field ablation catheter integrated with a 3-dimensional mapping system: 1-year outcomes of the multicenter inspIRE study. Circ Arrhythm Electrophysiol. 2023;16:e011780. doi: 10.1161/CIRCEP.122.011780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FE, Calkins H, Sanders P, Packer DL, Kuck KH, et al. ; PULSED AF Investigators. Influence of monitoring and atrial arrhythmia burden on quality of life and health care utilization in patients undergoing pulsed field ablation: a secondary analysis of the PULSED AF trial. Heart Rhythm. 2023;20:1238–1245. doi: 10.1016/j.hrthm.2023.05.018 [DOI] [PubMed] [Google Scholar]
- 24.Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner A, Hansen J, Blaauw Y, Maury P, Arentz T, et al. ; MANIFEST-PF Cooperative. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace. 2022;24:1256–1266. doi: 10.1093/europace/euac050 [DOI] [PMC free article] [PubMed] [Google Scholar]