Abstract
Background
Long-term care residents were among the most vulnerable during the COVID-19 pandemic. We estimated vaccine effectiveness of mRNA COVID-19 vaccines in Medicare nursing home residents aged ≥65 years during pre-Delta and high Delta periods.
Methods
We conducted a retrospective cohort study from 13 December 2020 to 20 November 2021 using Medicare claims data. Exposures included 2 and 3 doses of Pfizer-BioNTech and Moderna COVID-19 vaccines. We used inverse probability weighting and Cox proportional hazards models to estimate absolute and relative vaccine effectiveness.
Results
Two-dose vaccine effectiveness against COVID-19–related death was 69.8% (95% CI, 65.9%‒73.3%) during the pre-Delta period and 55.7% (49.5%‒61.1%) during the high Delta period, without adjusting for time since vaccination. We observed substantial waning of effectiveness from 65.1% (54.2%‒73.5%) within 6 months from second-dose vaccination to 45.2% (30.6%‒56.7%) ≥6 months after second-dose vaccination in the high Delta period. Three doses provided 88.7% (73.5%‒95.2%) vaccine effectiveness against death, and the incremental benefit of 3 vs 2 doses was 74.6% (40.4%‒89.2%) during high Delta. Among beneficiaries with a prior COVID-19 infection, 3-dose vaccine effectiveness for preventing death was 78.6% (50.0%‒90.8%), and the additional protection of 3 vs 2 doses was 70.0% (30.1%‒87.1%) during high Delta. Vaccine effectiveness estimates against less severe outcomes (eg, infection) were lower.
Conclusions
This nationwide real-world study demonstrated that mRNA COVID-19 vaccines provided substantial protection against COVID-19–related death. Two-dose protection waned after 6 months. Third doses during the high Delta period provided significant additional protection for individuals with or without a prior COVID-19 infection.
Keywords: COVID-19, Delta, nursing home, real-world evidence, vaccine effectiveness
In a nationwide real-world study among nursing home residents, mRNA COVID-19 vaccines provided substantial protection against COVID-19–related death, even among prior COVID-19 survivors. Two-dose protection waned after 6 months. A third dose significantly boosted protection.
BACKGROUND
As of 19 January 2023, >1 million coronavirus disease 2019 (COVID-19) deaths have been reported in the United States [1]. People aged ≥65 years accounted for 75% of all US COVID-19 deaths [2].
On 11 and 18 December 2020, the US Food and Drug Administration (FDA) issued emergency use authorizations (EUAs) for the primary series of BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) mRNA COVID-19 vaccines for the prevention of COVID-19 illness. On 12 August 2021, the FDA amended the EUA for the Pfizer-BioNTech and Moderna vaccines to allow for the use of an additional dose in certain individuals who were immunocompromised. The EUAs were extended in the fall of 2021 to allow for booster doses for people aged ≥65 years (22 September and 20 October 2021 for the Pfizer-BioNTech and Moderna vaccines, respectively). On 29 March 2022, the FDA amended the EUA to allow for a second booster dose of the Pfizer-BioNTech and Moderna vaccines for people aged ≥50 years or certain individuals who were immunocompromised. A bivalent booster (original, Omicron BA.4/BA.5) was authorized on 31 August 2022 for the Pfizer-BioNTech and Moderna vaccines. On 11 September 2023, the FDA approved a monovalent vaccine (2023–2024 formula) for Pfizer-BioNTech and Moderna regardless of prior vaccination status. Nursing home (NH) residents were an extremely vulnerable population during the COVID-19 pandemic [3–8]. We used Medicare claims data to (1) assess 2- and 3-dose vaccine effectiveness (VE) against COVID-19–related outcomes, (2) determine how VE changed for periods with different levels of COVID-19 circulation, (3) compare VE during pre-Delta and high Delta variant circulation periods, (4) calculate brand-specific VE of the Pfizer-BioNTech and Moderna vaccines, and (5) investigate waning effectiveness due to time since second-dose vaccination during the high Delta period among NH long-term care residents.
METHODS
We used a retrospective cohort design to study absolute and relative VE of mRNA COVID-19 vaccines among the elderly (aged ≥65 years) Medicare Fee-for-Service population residing in NHs from 13 December 2020 to 20 November 2021. The analysis used a marginal structural model allowing residents to move from an unvaccinated cohort to 1-, 2-, and 3-dose cohorts. We used a Cox proportional hazards model to estimate VE.
Data Sources
We obtained demographic and enrollment information from the Centers for Medicare and Medicaid Services (CMS), and we derived information on medical conditions and services from Medicare Part A and B claims in Shared Systems Data. We derived NH resident and facility information from the Minimum Data Set and Nursing Home Compare. We used the Centers for Disease Control and Prevention’s COVID Data Tracker to determine COVID-19 variant circulation, and we used the American Community Survey to derive population density and area deprivation index [9, 10].
Study Population and Inclusion Criteria
The study population consisted of Medicare beneficiaries aged ≥65 years who resided in an NH for >100 days and met the specific inclusion and exclusion criteria (supplementary material) [11, 12].
To minimize the impact of potential exposure misclassification due to underreporting of COVID-19 vaccinations in claims, we excluded residents from NHs with <10% of residents vaccinated with at least 1 dose on or before 1 March 2021.
Index, Follow-up, and Exposure
Index dates were assigned on an NH level and represented the first vaccination date within an NH. We followed residents until the occurrence of an outcome, a censoring event, or the end of the study period (20 November 2021), whichever occurred first. Censoring events were vaccination with a non-mRNA COVID-19 vaccine, receipt of multiple vaccine brands, a third dose before the EUA, a fourth dose, dose spacing that did not meet recommendations, loss of Medicare Part A/B coverage or enrollment into Part C, discharge from an NH to the community, or death. For residents with an ongoing COVID-19 episode on the index date, follow-up started after the end of the episode.
We divided follow-up time into time-varying exposure cohorts, so residents could contribute time to the unvaccinated and 1-, 2-, and 3-dose cohorts. Residents moved to each subsequent cohort 14 days after vaccination to allow time for an immune response. We identified vaccinations using Healthcare Common Procedure Coding System codes, Current Procedural Terminology codes, and National Drug Codes in any care setting (Supplementary Table 1). Due to the very limited 1-dose follow-up time, findings for the 1-dose vaccinated cohorts were believed to be less reliable and informative and are not reported in this article.
COVID-19 Episode and Outcome Definitions
COVID-19 episodes must be observed with the COVID-19 diagnosis code U07.1. Because implementation of this code started on 1 April 2020, we identified COVID-19 episodes from that date onward. The COVID-19 episode definition is described in the supplementary material.
The primary outcomes of interest were COVID-19–related death, COVID-19–related hospitalization, and the composite outcome of COVID-19–related death/hospitalization. Because our previous studies showed that a large proportion of NH residents with severe illness were not being admitted to a hospital, we used COVID-19–related death as the main severe illness marker [3]. Secondary outcomes were medically attended COVID-19 infection, COVID-19–related hospitalization complications (intensive/coronary care unit or in-hospital mortality), COVID-19 pneumonia, and COVID-19 acute respiratory failure. Hospital complications were identified by revenue center codes, and all other outcomes were defined through ICD-10-CM diagnosis codes. Outcomes had to be observed with the COVID-19 diagnosis code U07.1 after follow-up began. Definitions and codes for all outcomes are in the supplementary material.
Covariates
We adjusted for factors potentially associated with an increased risk for severe COVID-19 outcomes, using clinical consultation and prior assessments of risk factors for covariate selection [4]. Individual-level characteristics were demographics, socioeconomic status (Medicare-Medicaid dual eligibility), health status (eg, prior COVID-19 status, medical conditions, frailty conditions), influenza vaccination, and mortality risk score [13–15]. We assessed medical conditions in the 6 months prior to the index date, except for prior COVID-19 status for which we used data from 1 April 2020. We controlled for multiple variables related to frailty conditions using methods described in articles by Segal et al [14, 15]. We also controlled for NH characteristics because our previous COVID-19 natural history study showed that they were strong predictors of COVID-19 risk (supplementary material) [4]. To adjust for local COVID-19 circulation and address outcome misclassification during lower circulation periods, we included measures of census tract–level COVID-19 circulation and Delta variant share as time-varying risk factors. We spatially smoothed circulation rates monthly at the census tract level and modeled Delta share with cubic splines using weekly data points at the US Department of Health and Human Services region level (supplementary material) [16]. We also adjusted for socioeconomic status at the geographic level using the area deprivation index. We evaluated covariate balance between vaccinated and unvaccinated cohorts weekly using standardized mean differences. Covariates are listed in the supplementary material.
Statistical Analysis
We used marginal structural Cox regression models to estimate hazard ratios (HRs) for COVID-19 outcomes in the vaccinated cohorts as compared with the unvaccinated cohort [17, 18]. At every 7 days, the marginal structural models assigned weights to observations via inverse probability of treatment weighting, creating a weighted pseudo-population that balanced covariates across the 4 cohorts (Table 1). We used 3 time-varying treatment weights (1-, 2-, and 3-dose weights) and 1 time-varying censoring weight to calculate the overall weight. To construct weights, we derived 4 sets of propensity scores from pooled multinomial logistic regression models with either vaccination status (for the 3 treatment models) or censor status (for the censoring model) as the dependent variable and all covariates as independent variables (covariates listed in the supplementary material). We used a Cox proportional hazards model on the weighted data to estimate HRs between cohorts (2-dose vs unvaccinated, 3-dose vs unvaccinated). We calculated VE as a percentage: 100 × (1 − HR). We also applied doubly robust estimation including all weighting-model covariates in the outcome model; doubly robust results are reported in the Results section [19]. Non-doubly robust VE estimates are in the supplementary material.
Table 1.
Cohort Demographic Distribution of Eligible Beneficiaries for the COVID-19–Related Death Outcome at Study End
| Cohort at Study End, % or Mean (SD) | max SMDa | ||||
|---|---|---|---|---|---|
| Covariate Evaluated at Baseline | Unvaccinated (n = 32 890) | 2-Dose (n = 143 145) | 3-Dose (n = 49 239) | Unweighted | Weighted |
| Sex | |||||
| Female | 68.4 | 69.8 | 71.4 | 0.09 | 0.05 |
| Male | 31.6 | 30.2 | 28.6 | 0.09 | 0.05 |
| Age, y | |||||
| 65–69 | 14.1 | 12.1 | 9.9 | 0.13 | 0.06 |
| 70–74 | 16.8 | 14.9 | 12.8 | 0.11 | 0.05 |
| 75–79 | 16.6 | 15.6 | 14.3 | 0.06 | 0.04 |
| 80–84 | 16.9 | 17.1 | 16.9 | 0.01 | 0.00 |
| 85–89 | 16.3 | 17.8 | 19.3 | 0.08 | 0.03 |
| 90–94 | 12.2 | 14.6 | 17.2 | 0.14 | 0.06 |
| ≥95 | 7.0 | 7.9 | 9.6 | 0.10 | 0.07 |
| Race | |||||
| American Indian/Alaskan Native | 0.5 | 0.5 | 0.4 | 0.03 | 0.01 |
| Asian | 1.4 | 1.4 | 1.5 | 0.06 | 0.03 |
| Black | 18.9 | 12.8 | 9.7 | 0.27 | 0.07 |
| Hispanic | 2.0 | 1.6 | 1.7 | 0.06 | 0.04 |
| White | 75.5 | 82.3 | 85.3 | 0.25 | 0.09 |
| Other/unknown race | 1.7 | 1.5 | 1.5 | 0.02 | 0.02 |
| Medicare-Medicaid dual eligibility | |||||
| Dual | 88.2 | 85.3 | 79.8 | 0.23 | 0.15 |
| Nondual | 11.8 | 14.7 | 20.2 | 0.23 | 0.15 |
| Reason for entering Medicare | |||||
| Aged in without ESRD | 70.2 | 72.3 | 75.7 | 0.12 | 0.07 |
| Disabled without ESRD | 29.5 | 27.4 | 24.0 | 0.12 | 0.07 |
| ESRD only | 0.4 | 0.3 | 0.3 | 0.04 | 0.02 |
| Tract-level population densityb | 10.1 (2.9) | 9.8 (2.7) | 9.9 (2.8) | 0.14 | 0.19 |
| COVID-19 circulation (4-wk rate)b | 9.5 (0.8) | 9.5 (0.8) | 9.6 (0.8) | 0.14 | 0.14 |
| Prior vaccination | |||||
| Influenza | 32.8 | 50.0 | 57.0 | 0.50 | 0.18 |
| Presence of medical condition | |||||
| Diabetes | 42.5 | 42.6 | 40.6 | 0.09 | 0.06 |
| Obesity | 29.6 | 31.6 | 30.9 | 0.04 | 0.03 |
| Chronic liver disease | 2.1 | 2.1 | 1.8 | 0.03 | 0.03 |
| Neurologic/neurodevelopmental | 23.7 | 23.3 | 21.4 | 0.06 | 0.05 |
| Immunocompromised | 4.1 | 3.5 | 3.7 | 0.07 | 0.03 |
| ESRD | 1.4 | 1.2 | 1.4 | 0.07 | 0.02 |
| Essential HTN | |||||
| Without complicated HTN or other CVD | 33.6 | 33.5 | 34.4 | 0.05 | 0.04 |
| With complicated HTN or other CVD | 45.5 | 47.1 | 45.6 | 0.07 | 0.06 |
| Cardiovascular disease | |||||
| Hospitalized stroke/TIA | 0.6 | 0.6 | 0.5 | 0.01 | 0.02 |
| Coronary revascularization | 0.1 | 0.1 | 0.1 | 0.01 | 0.01 |
| Atrial fibrillation | 20.1 | 22.2 | 22.7 | 0.06 | 0.02 |
| Congestive heart failure | 25.2 | 26.4 | 25.8 | 0.03 | 0.02 |
| Hospitalized AMI | 0.4 | 0.4 | 0.4 | 0.02 | 0.03 |
| Other cerebrovascular disease | 20.4 | 20.0 | 18.2 | 0.09 | 0.06 |
| Respiratory disease | |||||
| COPD | 23.2 | 23.3 | 21.1 | 0.07 | 0.05 |
| Asthma without COPD | 1.9 | 2.0 | 2.1 | 0.03 | 0.02 |
| Interstitial lung disease | 1.2 | 1.3 | 1.3 | 0.01 | 0.01 |
| Hypersensitivity pneumonitis | 0.1 | 0.1 | 0.1 | 0.01 | 0.01 |
| Bronchiectasis | 0.2 | 0.3 | 0.3 | 0.01 | 0.01 |
| COVID-19 prior diagnosis | 52.5 | 49.1 | 46.0 | 0.13 | 0.62 |
| Frailty condition | |||||
| Depression | 56.9 | 59.0 | 57.5 | 0.04 | 0.08 |
| Parkinson disease | 7.6 | 8.0 | 8.3 | 0.03 | 0.01 |
| Arthritis | 32.5 | 35.0 | 35.6 | 0.06 | 0.02 |
| Cognitive impairment | 71.3 | 72.5 | 70.8 | 0.04 | 0.03 |
| Paranoia | 22.8 | 19.2 | 17.1 | 0.14 | 0.07 |
| Chronic skin ulcer | 16.0 | 14.1 | 14.0 | 0.06 | 0.06 |
| Skin and soft tissue infection | 11.6 | 10.8 | 11.2 | 0.04 | 0.04 |
| Mycoses | 50.9 | 50.7 | 55.0 | 0.08 | 0.08 |
| Gout | 3.9 | 4.1 | 4.1 | 0.01 | 0.01 |
| Falls | 22.8 | 26.1 | 27.1 | 0.10 | 0.06 |
| Musculoskeletal problems | 52.1 | 55.5 | 57.1 | 0.10 | 0.04 |
| Urinary tract infection | 28.6 | 29.5 | 28.4 | 0.02 | 0.03 |
| Pneumonia | 15.1 | 14.3 | 12.9 | 0.07 | 0.11 |
| Charlson score >0 | 95.3 | 96.3 | 96.2 | 0.05 | 0.04 |
| Hospital admission in past 6 mo | 18.5 | 17.4 | 15.9 | 0.09 | 0.06 |
| ADL score | |||||
| 0–7 | 15.5 | 14.7 | 14.6 | 0.03 | 0.04 |
| 8–14 | 17.9 | 18.6 | 18.4 | 0.02 | 0.02 |
| 15–21 | 47.4 | 49.7 | 50.8 | 0.07 | 0.01 |
| 22–28 | 19.3 | 16.9 | 16.2 | 0.08 | 0.04 |
| MRS3 | |||||
| 0 | 38.4 | 36.7 | 37.3 | 0.03 | 0.01 |
| 1–4 | 43.9 | 46.0 | 46.8 | 0.06 | 0.02 |
| 5–7 | 13.8 | 14.0 | 12.9 | 0.03 | 0.02 |
| 8 | 3.9 | 3.3 | 3.0 | 0.05 | 0.03 |
| Nursing home ownership | |||||
| For profit | 73.6 | 70.0 | 61.4 | 0.26 | 0.18 |
| Nonprofit | 18.3 | 21.0 | 30.5 | 0.29 | 0.14 |
| State-owned veterans’ home | 1.6 | 1.5 | 1.9 | 0.04 | 0.04 |
| Other government-owned facilities | 6.6 | 7.5 | 6.2 | 0.05 | 0.11 |
| Nursing home measures | |||||
| Health inspection 5-star rating | |||||
| 1 star | 24.1 | 20.2 | 15.5 | 0.22 | 0.13 |
| 2 stars | 25.9 | 25.3 | 22.2 | 0.10 | 0.03 |
| 3 stars | 21.6 | 23.4 | 23.5 | 0.05 | 0.02 |
| 4 stars | 20.3 | 22.3 | 27.1 | 0.16 | 0.09 |
| 5 stars | 7.5 | 8.3 | 11.4 | 0.14 | 0.12 |
| Quality measure 5-star rating | |||||
| 1 star | 6.1 | 5.7 | 4.4 | 0.08 | 0.05 |
| 2 stars | 15.5 | 14.5 | 11.8 | 0.11 | 0.09 |
| 3 stars | 22.3 | 21.3 | 17.8 | 0.11 | 0.04 |
| 4 stars | 24.8 | 25.3 | 25.9 | 0.02 | 0.01 |
| 5 stars | 30.7 | 32.7 | 39.7 | 0.19 | 0.10 |
| Nurse aid staffing per resident, h/d | 2.3 (0.6) | 2.4 (0.6) | 2.4 (0.6) | 0.17 | 0.12 |
| Licensed practical nurse staffing, h/d | 0.9 (0.3) | 0.9 (0.3) | 0.9 (0.3) | 0.08 | 0.03 |
| Registered nurse staffing, h/d | 0.6 (0.4) | 0.7 (0.4) | 0.8 (0.4) | 0.29 | 0.14 |
| No. of residents per day | 107.0 (66.2) | 99.6 (60.6) | 109.8 (80.7) | 0.14 | 0.13 |
Abbreviations: ADL, activities of daily living; AMI, acute myocardial infarction; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESRD, end-stage renal disease; h/d, hours per day; HTN, hypertension; MRS3, Mortality Risk Score; SMD, standardized mean difference; TIA, transient ischemic attack.
amax SMD values ≥0.10 indicate that the groups are imbalanced on the specified covariates. SMDs are pairwise comparisons conducted between cohorts. Only the max SMD is displayed here.
bThese two covariates used the continuous log base 2 function.
We used a model selection process with 4 outcome models to determine VE. The base model included vaccine exposure status, Delta variant as a share of circulating COVID-19 variants, and COVID-19 circulation (model 1). Models 2 and 3 included Delta share and COVID-19 circulation, respectively, interacted with exposure status. Model 4 included Delta share and COVID-19 circulation interactions. Decisions regarding interaction terms and the number of knots for Delta share splines were informed with the Akaike information criterion [20].
We assessed brand-specific VE using a similar framework to the primary analysis, but the exposure variable indicated number of doses and vaccine brand. Due to low uptake of third doses of Moderna in our study, we estimated only 2-dose VE for the brand-specific analysis.
We conducted a post hoc waning effectiveness analysis during high Delta circulation (≥95% Delta) with 2 “time since second-dose” categories (<6 or ≥6 months).
Sensitivity Analyses and Quantitative Bias Analysis
We performed several sensitivity analyses and a quantitative bias analysis (QBA) to test the robustness of results and evaluate bias due to exposure misclassification (underreporting of vaccination status). The sensitivity analyses implemented alternate exclusion criteria, incident COVID-19 definitions, and weight truncation methods. The QBA defined low and high exposure misclassification estimates and adjusted VE accordingly (supplementary material).
We conducted all VE analyses using R version 4.1.2 (R Foundation for Statistical Computing) and SAS version 9.4 (SAS Institute Inc).
RESULTS
Among 1 006 664 Medicare NH residents, there were 348 310 study-eligible residents living in 12 111 NHs (Supplementary Figure 1). Of them, 77% were vaccinated with 2 doses of an mRNA COVID-19 vaccine by the end of March 2021 (Supplementary Figure 2). By 20 November 2021, 61% were in the 2-dose cohort, 21% in the 3-dose cohort, and 14% in the unvaccinated cohort. While we observed imbalances among cohorts preweighting, most covariates were well balanced postweighting (Table 1, Supplementary Figure 3). We addressed residual imbalance by reporting doubly robust estimates. Outcome rates are reported in Table 2 and Supplementary Table 2.
Table 2.
Primary and Subgroup Absolute and Relative VE: Primary Outcomes at COVID-19 Circulation Rate of 5000 Infections per 100 000 Persons
| Vaccine Effectiveness, % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Ratea | Pre-Delta | High Delta | ||||||
| Outcome: Cohort | No. of Outcomes | Person Timeb | Rate | 95% CI | VE | 95% CI | VE | 95% CI |
| COVID-19–related death | ||||||||
| Overall | ||||||||
| Unvaccinated | 3483 | 24.8 | 140.2 | 135.6–144.9 | … | … | … | … |
| 2-dose | 4179 | 82.4 | 50.7 | 49.2–52.3 | 69.8 | 65.9–73.3 | 55.7 | 49.5–61.1 |
| 3-dose | 72 | 1.5 | 47.4 | 36.5–58.4 | … | … | 88.7 | 73.5–95.2 |
| 3- vs 2-dose | … | … | … | … | … | … | 74.6 | 40.4–89.2 |
| Had prior COVID-19 | ||||||||
| Unvaccinated | 728 | 10.6 | 68.6 | 63.6–73.5 | … | … | … | … |
| 2-dose | 1776 | 37.3 | 47.7 | 45.4–49.9 | 48.4 | 40.9–55.0 | 28.6 | 18.6–37.4 |
| 3-dose | 33 | 0.7 | 47.9 | 31.6–64.3 | … | … | 78.6 | 50.0–90.8 |
| 3- vs 2-dose | … | … | … | … | … | … | 70.0 | 30.1–87.1 |
| Did not have prior COVID-19 | ||||||||
| Unvaccinated | 2755 | 14.2 | 193.8 | 186.5–201.0 | … | … | … | … |
| 2-dose | 2403 | 45.1 | 53.3 | 51.2–55.4 | 76.2 | 72.8–79.1 | 67.0 | 62.0–71.4 |
| 3-dose | 39 | 0.8 | 47.0 | 32.3–61.8 | … | … | 92.2 | 80.3–96.9 |
| 3- vs 2-dose | … | … | … | … | … | … | 76.3 | 40.3–90.6 |
| Age: 65–74 y | ||||||||
| Unvaccinated | 587 | 7.2 | 81.4 | 74.8–87.9 | … | … | … | … |
| 2-dose | 727 | 20.4 | 35.6 | 33.0–38.2 | 64.5 | 58.8–69.5 | 47.9 | 39.1–55.4 |
| 3-dose | — | — | — | — | … | … | 91.6 | 74.2–97.3 |
| Age: 75–84 y | ||||||||
| Unvaccinated | 1083 | 8.2 | 132.6 | 124.7–140.5 | … | … | … | … |
| 2-dose | 1183 | 26.2 | 45.2 | 42.6–47.8 | 71.1 | 66.8–74.8 | 57.5 | 50.9–63.2 |
| 3-dose | — | — | — | — | … | … | 88.2 | 69.0–95.5 |
| Age: ≥85 y | ||||||||
| Unvaccinated | 1813 | 9.5 | 191.8 | 183.0–200.6 | … | … | … | … |
| 2-dose | 2269 | 35.7 | 63.5 | 60.9–66.1 | 70.9 | 66.8–74.5 | 57.2 | 50.9–62.7 |
| 3-dose | — | — | — | — | … | … | 88.5 | 73.3–95.0 |
| COVID-19–related hospitalizationc | ||||||||
| Overall | ||||||||
| Unvaccinated | 3360 | 24.6 | 136.8 | 132.1–141.4 | … | … | … | … |
| 2-dose | 2215 | 81.8 | 27.1 | 25.9–28.2 | 65.3 | 60.6–69.4 | 40.4 | 33.8–46.2 |
| 3-dose | 22 | 1.5 | 14.6 | 8.5–20.7 | … | … | 76.8 | 63.2–85.4 |
| 3- vs 2-dose | … | … | … | … | … | … | 61.1 | 38.6–75.4 |
| Had prior COVID-19 | ||||||||
| Unvaccinated | 435 | 10.6 | 41.2 | 37.3–45.0 | … | … | … | … |
| 2-dose | 536 | 37.2 | 14.4 | 13.2–15.6 | 58.1 | 49.7–65.0 | 28.9 | 18.4–38.0 |
| 3-dose | — | — | — | — | … | … | 63.3 | 12.6–84.6 |
| 3- vs 2-dose | … | … | … | … | … | … | 48.3 | −22.7–78.2 |
| Did not have prior COVID-19 | ||||||||
| Unvaccinated | 2925 | 14.0 | 209.0 | 201.4–216.5 | … | … | … | … |
| 2-dose | 1679 | 44.7 | 37.6 | 35.8–39.4 | 66.7 | 62.3–70.6 | 43.6 | 36.9–49.6 |
| 3-dose | — | — | — | — | … | … | 79.8 | 65.4–88.2 |
| 3- vs 2-dose | … | … | … | … | … | … | 64.2 | 38.9–79.0 |
| Age: 65–74 y | ||||||||
| Unvaccinated | 945 | 7.1 | 132.7 | 124.2–141.2 | … | … | … | … |
| 2-dose | 648 | 20.3 | 32.0 | 29.5–34.4 | 61.3 | 54.8–66.8 | 33.3 | 24.0–41.6 |
| 3-dose | — | — | — | — | … | … | 78.5 | 36.8–92.7 |
| Age: 75–84 y | ||||||||
| Unvaccinated | 1205 | 8.1 | 149.4 | 140.9–157.8 | … | … | … | … |
| 2-dose | 770 | 26.0 | 29.6 | 27.5–31.7 | 66.6 | 61.4–71.0 | 42.4 | 34.7–49.3 |
| 3-dose | — | — | — | — | … | … | 64.0 | 32.4–80.9 |
| Age: ≥85 y | ||||||||
| Unvaccinated | 1210 | 9.4 | 129.0 | 121.7–136.3 | … | … | … | … |
| 2-dose | 797 | 35.6 | 22.4 | 20.8–24.0 | 66.9 | 61.9–71.3 | 43.1 | 35.5–49.8 |
| 3-dose | — | — | — | — | … | … | 86.8 | 71.5–93.8 |
| COVID-19–related death or hospitalization | ||||||||
| Overall | ||||||||
| Unvaccinated | 5548 | 24.6 | 225.8 | 219.9–231.8 | … | … | … | … |
| 2-dose | 5436 | 81.8 | 66.4 | 64.7–68.2 | 66.8 | 63.1–70.2 | 52.7 | 47.2–57.7 |
| 3-dose | 82 | 1.5 | 54.4 | 42.7–66.2 | … | … | 83.7 | 66.7–92.0 |
| 3- vs 2-dose | … | … | … | … | … | … | 65.4 | 29.6–83.1 |
| Had prior COVID-19 | ||||||||
| Unvaccinated | 1021 | 10.6 | 96.6 | 90.7–102.5 | … | … | … | … |
| 2-dose | 2144 | 37.2 | 57.7 | 55.2–60.1 | 47.6 | 40.8–53.5 | 28.8 | 20.4–36.4 |
| 3-dose | 34 | 0.7 | 49.6 | 32.9–66.2 | … | … | 70.6 | 40.9–85.4 |
| 3- vs 2-dose | … | … | … | … | … | … | 58.7 | 17.0–79.5 |
| Did not have prior COVID-19 | ||||||||
| Unvaccinated | 4527 | 14.0 | 323.4 | 314.0–332.8 | … | … | … | … |
| 2-dose | 3292 | 44.7 | 73.7 | 71.2–76.2 | 72.5 | 69.2–75.4 | 62.7 | 57.9–66.9 |
| 3-dose | 48 | 0.8 | 58.5 | 42.0–75.1 | … | … | 87.8 | 73.5–94.4 |
| 3- vs 2-dose | … | … | … | … | … | … | 67.4 | 28.9–85.1 |
| Age: 65–74 y | ||||||||
| Unvaccinated | 1248 | 7.1 | 175.2 | 165.5–185.0 | … | … | … | … |
| 2-dose | 1156 | 20.3 | 57.0 | 53.7–60.3 | 64.0 | 59.2–68.2 | 48.7 | 41.7–54.8 |
| 3-dose | — | — | — | — | … | … | 88.7 | 69.2–95.9 |
| Age: 75–84 y | ||||||||
| Unvaccinated | 1837 | 8.1 | 227.7 | 217.3–238.1 | … | … | … | … |
| 2-dose | 1650 | 26.0 | 63.5 | 60.4–66.5 | 68.5 | 64.5–72.0 | 55.0 | 49.2–60.2 |
| 3-dose | — | — | — | — | … | … | 80.8 | 58.0–91.2 |
| Age: ≥85 y | ||||||||
| Unvaccinated | 2463 | 9.4 | 262.6 | 252.2–273.0 | … | … | … | … |
| 2-dose | 2630 | 35.6 | 73.9 | 71.1–76.8 | 67.0 | 62.9–70.6 | 53.0 | 47.0–58.2 |
| 3-dose | — | — | — | — | … | … | 83.8 | 66.8–92.1 |
Circulation rate is over a 28-day period. Dashes (—) indicate outcome counts ≤10, and associated statistics are masked to protect the anonymity of the data.
Abbreviation: VE, vaccine effectiveness.
aPer 100 000 person-weeks.
b100 000 person-weeks.
cBased on model selection with the Akaike information criterion, the best-fitting model for the hospitalization outcome did not include a COVID-19 circulation term. Thus, VE for the hospitalization outcome is the same across all COVID-19 circulation rates.
The best-fitting hazard model for the COVID-19–related death outcome interacted the Delta share and COVID-19 circulation rate with the exposure status. Selected hazard models for all study outcomes are in Supplementary Table 3. We reported estimates in the pre-Delta period (0% Delta circulation) and the high Delta period (99% Delta circulation). To improve the positive predictive value of the outcome definition, we presented results for high COVID-19 circulation rates (Supplementary Figure 4).
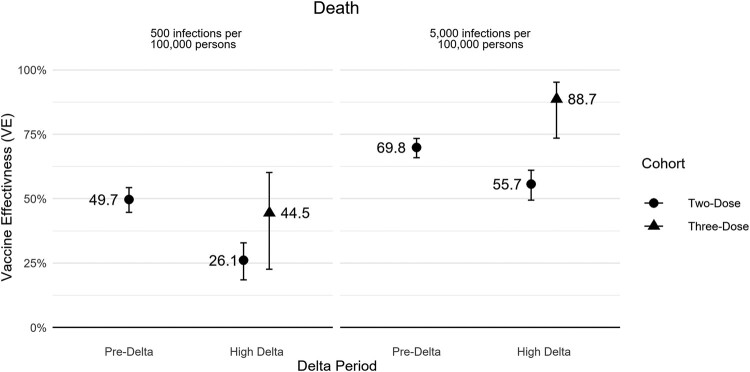
In the pre-Delta and high Delta periods, 2-dose VE against COVID-19–related death was 69.8% (95% CI, 65.9%–73.3%) and 55.7% (95% CI, 49.5%–61.1%), respectively (Table 2, Figure 1). In the high Delta period, 3-dose VE against death was 88.7% (95% CI, 73.5%–95.2%), and the additional protection of 3 vs 2 doses was 74.6% (95% CI, 40.4%‒89.2%; Table 2). Two- and 3-dose VE for death was higher during periods of high-level COVID-19 circulation. VE against less severe outcomes (eg, medically attended infection) was lower across all periods and more reduced in the high Delta period than more severe outcomes (eg, death and hospitalization complications; Supplementary Table 2, Supplementary Figure 5).
Figure 1.
Absolute vaccine effectiveness for COVID-19–related death by Delta share and COVID-19 circulation rate. Circulation rate is over a 28-day period. Error bars indicate 95% CI.
Among those with a prior COVID-19 infection, 2-dose VE for preventing COVID-19–related death was 48.4% (95% CI, 40.9%–55.0%) in the pre-Delta period and 28.6% (95% CI, 18.6%–37.4%) during high Delta. Three-dose VE for preventing death among this group was 78.6% (95% CI, 50.0%‒90.8%), and the additional protection of 3 vs 2 doses was 70.0% (95% CI, 30.1%‒87.1%) during high Delta. Results of additional subgroup analyses are displayed in Table 2.
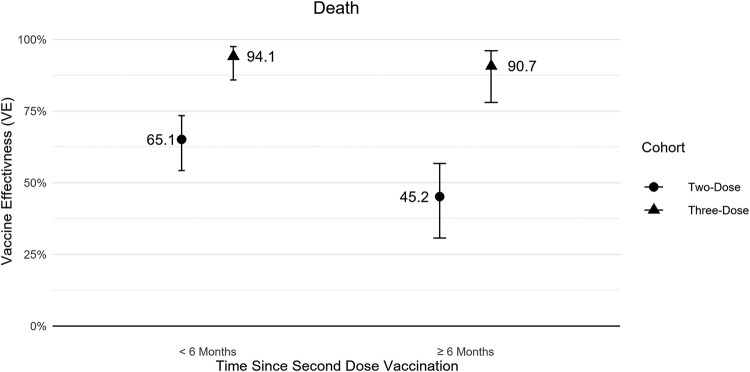
For the waning effectiveness analysis during the high Delta period, the 2-dose VE against COVID-19–related death was 65.1% (95% CI, 54.2%‒73.5%) within 6 months following the second dose. After 6 months, the 2-dose VE fell to 45.2% (95% CI, 30.6%‒56.7%). All 3-dose follow-up time was <3 months in the study, and the 3-dose VE against death was high regardless of time since prior vaccination: the 3-dose VE was 94.1% (95% CI, 85.8%‒97.5%) at <6 months following the second dose and 90.7% (95% CI, 78.0%‒96.1%) at ≥6 months following the second dose (Figure 2, Supplementary Table 4).
Figure 2.
Absolute 2- and 3-dose vaccine effectiveness for COVID-19–related death by time since second dose at COVID-19 circulation rate of 5000 infections per 100 000 persons during high Delta period. Circulation rate is over a 28-day period. Error bars indicate 95% CI.
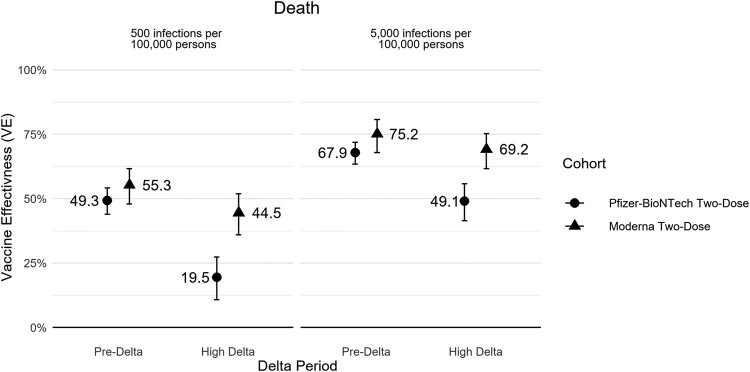
Brand-specific VE analyses showed higher VE point estimates for 2-dose Moderna when compared with 2-dose Pfizer-BioNTech for all outcomes during pre-Delta, although 95% CIs overlapped substantially. During high Delta, VE estimates were significantly higher for 2-dose Moderna than 2-dose Pfizer-BioNTech for all outcomes (Figure 3, Supplementary Table 5). Moderna VE additionally appeared more stable across variant periods vs Pfizer-BioNTech. Moderna 2-dose VE against COVID-19–related death was 75.2% (95% CI, 67.9%–80.8%) during the pre-Delta period and 69.2% (95% CI, 61.6%–75.2%) during the high Delta period. Pfizer-BioNTech 2-dose VE against death was 67.9% (95% CI, 63.5%–71.9%) during the pre-Delta period and decreased to 49.1% (95% CI, 41.4%–55.7%) in the Delta period.
Figure 3.
Brand-specific absolute 2-dose vaccine effectiveness for COVID-19–related death by Delta share and COVID-19 circulation rate. Circulation rate is over a 28-day period. Error bars indicate 95% CI.
VE results for the composite COVID-19–related death/hospitalization outcome and all secondary outcomes followed similar trends as death and hospitalization (Table 2; Supplementary Figure 7; Supplementary Tables 2, 5, and 6). The VE and brand-specific VE analyses exhibited mild sensitivity to the inclusion of covariates in the outcome model (Supplementary Table 7). The QBA-adjusted 2-dose VEs based on the low estimate of exposure misclassification were largely contained within the originally estimated 95% CIs. The high estimate of exposure misclassification for the 2-dose VE consistently increased VE outside of the originally reported CIs. The 3-dose VE was not substantially affected by exposure misclassification according to the QBA (Supplementary Table 8). Analyses were otherwise insensitive to changes in specifications (Supplementary Tables 9–13).
DISCUSSION
This nationwide large cohort study showed that among 348 310 study-eligible long-term care NH residents aged ≥65 years, 2 doses of an mRNA COVID-19 vaccine provided significant protection against severe COVID-19 outcomes, particularly COVID-19–related death. The addition of a third dose substantially increased VE, often higher than the original 2-dose VE estimate. Moreover, we found that vaccination provided an additional benefit of preventing death among individuals who had survived a prior COVID-19 infection, which highlights the benefit of vaccination regardless of prior infection.
We showed lower 2-dose VE during the high Delta period than the pre-Delta period, without accounting for a potential differential effect of time since vaccination during the 2 periods, and the results are consistent with other studies reporting VE across variant periods [5, 21–23]. However, protection against death remained relatively high in the high Delta period, as observed in other studies [24, 25].
The observed lower VE during the high Delta period vs the pre-Delta period is a mixed effect of time since vaccination and differential protection against a new strain. Because most NH residents were fully vaccinated with COVID-19 vaccines during a short time frame early in the study period and the high Delta period occurred later in the study, it is difficult to distinguish between the effects of the Delta variant and the effects of waning immunity in the same model. While there is evidence suggesting that VE decreases over time, very few studies are able to isolate the effect of variant periods vs time since vaccination [5, 21–23, 26]. One study was able to identify negative impacts on immunity from variant strain and time since second-dose vaccination, suggesting that both factors contribute to the decrease in VE across time [25]. A study conducted on a smaller population demonstrated that VE of Pfizer-BioNTech was high in the first month following vaccination in any variant period but decreased rapidly in the following months [27].
We conducted a post hoc waning effectiveness analysis during only the high Delta period to investigate the impact of time since second-dose vaccination. We observed substantial waning of 2-dose protection against COVID-19–related death after 6 months from a second-dose vaccination, which demonstrates that time since vaccination accounts for the majority of the decrease in VE across time. The finding that VE drops off around 6 months is supported by various studies in the literature [25, 28, 29].
Consistent with other studies, we found that the VE of the Moderna vaccine was higher than that of the Pfizer-BioNTech for all COVID-19–related outcomes during the high Delta period [30, 31]. While many factors could contribute to the observed difference between the vaccines, some studies suggest that this may be due to the longer dose interval between Moderna primary series doses when compared with Pfizer-BioNTech (28 vs 21 days) or the higher dosage of the primary series of Moderna (100 µg) as compared with Pfizer-BioNTech (30 µg) [32, 33].
We found higher VE against COVID-19–related outcomes during high COVID-19 circulation periods than low COVID-19 circulation periods. This finding suggests that COVID-19 diagnosis codes in claims are more reliable during periods of high circulation, which may be attributed to higher positive predictive values (lower false-positive rates). We encountered similar concerns in our previous influenza VE studies [34]. For future claims-based real-world VE studies, we recommend using only periods of moderate to high disease circulation.
We made significant efforts to minimize bias in our analysis, particularly bias from vaccine misclassification. Because Medicare beneficiaries might have received vaccines outside the Medicare system (eg, at mass vaccination centers) or because vaccinators were not always submitting claims, there are substantial concerns of underreporting COVID-19 vaccinations in medical claims. As a result, the unvaccinated cohort likely included some vaccinated beneficiaries for whom we could not observe vaccinations through claims, and the 2-dose cohort likely included some beneficiaries who had received 3 doses. To reduce exposure misclassification, we restricted our analysis to the NH population because federal programs structured the rollout of vaccines among NHs, resulting in more reliable vaccine reporting. We additionally excluded NHs with <10% of residents reportedly vaccinated with at least 1 dose of a COVID-19 vaccine by 1 March 2021. A cross-examination of Medicare claims data with public use NH-level vaccination rates from the CMS showed that this exclusion criteria effectively excluded many NHs with significant underreporting of the primary series vaccines. Furthermore, the QBA suggested that exposure misclassification had a limited impact on 2- and 3-dose VE estimates.
Our study has several strengths. First, this nationwide real-world study was conducted on a large cohort of NH residents, producing VE estimates among a very vulnerable population. We used propensity score models to control for individual-, geographic-, and NH-level characteristics, which our previous COVID-19 natural history study identified as strong predictors of COVID-19 risk [4]. We additionally included COVID-19 circulation and Delta variant share exposure interactions in the hazards model, allowing us to directly observe the effect that the terms had on VE. In addition, we investigated waning effectiveness during the high Delta period. Our use of time-varying cohorts allowed residents to contribute follow-up time to multiple-dose cohorts, affording greater person time and statistical power within cohorts. Finally, this study examined VE estimates against death, including among persons who survived a prior COVID-19 infection.
Our study has some limitations. The precision of the 3-dose VE estimate may be limited by low 3-dose person time and low COVID-19 circulation toward the end of the study period. We could not investigate 3-dose waning effectiveness because follow-up time was <3 months for all the beneficiaries. Further research on the added benefit of the third dose and waning effectiveness during the Omicron period is necessary. Our study investigated COVID-19–related outcomes rather than outcomes due to COVID-19. Because our prior investigations found that a significant proportion of NH residents who died had not been hospitalized, we considered that hospitalizations may not accurately account for disease severity among NH residents [3]. To address this, we included a combined outcome of death and hospitalization. While we accounted for several facility-level risk factors, we were unable to directly control for facilities’ infection prevention practices, which may affect a resident’s likelihood to be infected within a facility. We additionally did not differentiate between third-dose vaccinations authorized for certain individuals who were immunocompromised and booster vaccinations authorized for the general population because our investigations have shown that the third-dose administration codes were applied to a much broader population than the immunocompromised population. Finally, our study is limited by the lack of laboratory-confirmed diagnoses in Medicare claims data. As such, some claims-based diagnoses of COVID-19 may not be true cases.
This study uses real-world data to demonstrate the substantial protection that mRNA vaccines provide against COVID-19 outcomes. We found that while 2-dose protection was lower during the high Delta circulation period than the pre-Delta period, the decreased protection was mostly due to waning effectiveness, where we found that 2-dose protection waned substantially ≥6 months following vaccination in the high Delta period. Our finding that vaccination provided added protection to NH residents who had survived a prior COVID-19 infection indicates the additional benefit of vaccination among those with immunity derived from a prior infection. Our study additionally provides information to better understand real-world data for NH residents and improve the design of postmarketing VE claims-based studies.
Supplementary Material
Contributor Information
Yun Lu, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Arnstein Lindaas, Acumen LLC, Burlingame, California, USA.
Kathryn Matuska, Acumen LLC, Burlingame, California, USA.
Hector S Izurieta, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Rowan McEvoy, Acumen LLC, Burlingame, California, USA.
Mikhail Menis, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Xiangyu Shi, Acumen LLC, Burlingame, California, USA.
Whitney R Steele, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Michael Wernecke, Acumen LLC, Burlingame, California, USA.
Yoganand Chillarige, Acumen LLC, Burlingame, California, USA.
Hui Lee Wong, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Jeffrey A Kelman, Center for Medicare, Centers for Medicare and Medicaid Services, Washington, DC, USA.
Richard A Forshee, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge Jessica Hervol for assistance with coordination and manuscript writing and review and Carla Gomez Victor for assistance with manuscript writing. Coauthor Jeffrey A. Kelman, MD, passed away on February 8, 2024. He was a good friend, a brilliant collaborator, a compassionate caregiver, and an extraordinary human being. We will miss him greatly.
Author contributions. A. L., K. M., R. M., X. S., M. W., and Y. C. contributed to study design, data collection and analysis, results interpretation, and manuscript writing and review. Y. L., H. S. I., M. M., W. R. S., J. A. K., and R. A. F. contributed to study design, results interpretation, and manuscript writing and review. H. L. W. contributed to study design and manuscript writing and review.
Patient consent statement. This study did not require full US Food and Drug Administration institutional review board review and approval because it was determined to be exempt from the requirements of 45 CFR 46.104(d)(2) under the 2018 Common Rule. The use of Medicare administrative data was approved by the US Centers for Medicare and Medicaid Services privacy board under a data use agreement. The analyses utilize only existing records, and the participants cannot be identified. Patient consent was not required since our study is based on the US Centers for Medicare and Medicaid Services claims data and thus does not include factors necessitating patient consent.
Financial support. This work was funded by the US Food and Drug Administration through an interagency agreement (IAA number: O2309-075-075-013007) between the US Food and Drug Administration and the US Centers for Medicare and Medicaid Services for which Acumen LLC is the contractor.
References
- 1. Johns Hopkins University Center for Systems Science and Engineering . Coronavirus COVID-19 global cases. Available at: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed 19 January 2023.
- 2. Centers for Disease Control and Prevention . COVID mortality overview. Available at: https://www.cdc.gov/nchs/covid19/mortality-overview.htm. Accessed 19 January 2023.
- 3. Izurieta HS, Graham DJ, Jiao Y, et al. Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 million US Medicare beneficiaries. J Infect Dis 2021; 223:945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu Y, Jiao Y, Graham DJ, et al. Risk factors for COVID-19 deaths among elderly nursing home medicare beneficiaries in the prevaccine period. J Infect Dis 2022; 225:567–77. [DOI] [PubMed] [Google Scholar]
- 5. Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prasad N, Derado G, Nanduri SA, et al. Effectiveness of a COVID-19 additional primary or booster vaccine dose in preventing SARS-CoV-2 infection among nursing home residents during widespread circulation of the Omicron variant—United States, 14 February–27 March 2022. MMWR Morb Mortal Wkly Rep 2022; 71:633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McConeghy KW, White EM, Blackman C, et al. Effectiveness of a second COVID-19 vaccine booster dose against infection, hospitalization, or death among nursing home residents—19 states, 29 March–25 July 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldin S, Adler L, Azuri J, Mendel L, Haviv S, Maimon N. BNT162b2 mRNA COVID-19 (Comirnaty) vaccine effectiveness in elderly patients who live in long-term care facilities: a nationwide cohort. Gerontology 2022; 68:1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . COVID data tracker variant proportions. Available at: https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed 26 January 2022.
- 10. University of Wisconsin School of Medicine . Neighborhood atlas: area deprivation index. Available at: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed 28 August 2020.
- 11. Centers for Medicare and Medicaid Services. MDS 3.0 quality measures user’s manual. Vol 14.0. Centers for Medicare and Medicaid Services, 2020.
- 12. Goodwin JS, Li S, Zhou J, Graham JE, Karmarkar A, Ottenbacher K. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res 2017; 17:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas KS, Ogarek JA, Teno JM, Gozalo PL, Mor V. Development and validation of the nursing home minimum data set 3.0 Mortality Risk Score (MRS3). J Gerontol A Biol Sci Med Sci 2019; 74: 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care 2017; 55:716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Segal JB, Huang J, Roth DL, Varadhan R. External validation of the claims-based frailty index in the National Health and Aging Trends Study cohort. Am J Epidemiol 2017; 186:745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services. Regions . Available at: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/hhs_regions_map.pdf. Accessed 23 May 2023.
- 17. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
- 18. Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 19. Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akaike H. A new look at the statistical model identification. IEEE 1974; 19:716–23. [Google Scholar]
- 21. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021; 385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plumb ID, Feldstein LR, Barkley E, et al. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19–associated hospitalization among adults with previous SARS-CoV-2 infection—United States, June 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med 2021; 27:2136–43. [DOI] [PubMed] [Google Scholar]
- 25. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun 2022; 13:3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021; 398:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menegale F, Manica M, Zardini A, et al. Evaluation of waning of SARS-CoV-2 vaccine-induced immunity: a systematic review and meta-analysis. JAMA Netw Open 2023; 6:e2310650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chemaitelly H, Ayoub HH, Tang P, et al. Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population-based cohort study. Lancet Infect Dis 2023; 23:816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in US veterans. N Engl J Med 2022; 386:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. Jama 2021; 326:1533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science (New York, NY) 2021; 374:eabj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Izurieta HS, Thadani N, Shay DK, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis 2015; 15:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.