Abstract
We compared characteristics of HIV diagnosis and recent HIV infection (ie, likely acquired within the last year) in Cambodia. We included individuals ≥ 15 years old accessing HIV testing. From August 2020 to August 2022, 53 031 people were tested for HIV, 6868 were newly diagnosed, and 192 were recently infected. We found differences in geographical burden and risk behaviors with diagnosis and recency (eg, men who have sex with men, transgender women, and entertainment workers had a nearly 2-fold increased odds of testing positive for recent infection compared to being diagnosed with HIV). Recent infection surveillance may provide unique insights into ongoing HIV acquisition to inform programs.
Keywords: HIV, recent infection, surveillance
Tracking the characteristics of newly diagnosed cases is the foundation of infectious disease surveillance. Many pathogens have a short period between infection and symptom onset, recovery, or death. An individual with an untreated human immunodeficiency virus (HIV) infection may, however, take nearly 10 years to develop AIDS-related symptoms and 12 years before death [1, 2]. Therefore, depending on test-seeking behavior, newly diagnosed HIV cases may represent a mix of people who were infected years ago and those infected more recently. This presents major challenges to national HIV programs, whose goal is to focus prevention programs on the current, rather than past, drivers of their HIV epidemic using national surveillance data.
By measuring the evolution of the antibody avidity after seroconversion during initial HIV infection, laboratory-based tests—termed recency assays—have emerged as an approach to detect recent infection for HIV surveillance [3, 4]. The recent infection testing algorithm (RITA), comprising a rapid test for recent infection (RTRI) and viral load (VL), is an innovative surveillance tool to ascertain whether persons have likely been infected in the previous year (recent) or prior (long term) [5]. We sought to compare risk factors for HIV diagnosis and recent infection among individuals tested for HIV in Cambodia using national surveillance data.
METHODS
Design
This analysis utilized data from Cambodia’s national HIV surveillance system. We analyzed data from individuals aged ≥15 years accessing diagnostic HIV testing services from all 68 public health and nongovernmental organization facilities across all 25 provinces in Cambodia from 1 August 2020 to 11 August 2022. Individuals with newly diagnosed HIV infection, based on the national HIV diagnostic testing algorithm, who provided consent were tested for recent HIV infection using the Asanté HIV-1 rapid recency assay (Sedia Biosciences). Individuals testing RTRI-recent on the assay were tested for VL and those with VL ≥1000 copies/mL were considered RITA-recent.
Cambodia’s National Ethics Committee for Health Research granted ethical approval for this project. This project was reviewed in accordance with Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes.
Statistical Analyses
We managed data in a MySQL database. We compared risk factors for new HIV diagnosis and RITA-recent infections with those for testing negative for HIV, using multivariable generalized estimating equations (GEE) logistic regression models in R version 4.1.2 (R Project for Statistical Computing). Based on characteristics collected during routine HIV infection surveillance, we evaluated 5 characteristics in both the HIV infection analyses and the RITA-recent analyses: sex, age group (20–34 or ≥35 vs 15–19 years), marital status (married or widowed vs single), educational attainment (primary education or higher vs none), and testing services client type (men who have sex with men [MSM], transgender women [TGW], people who use drugs [PWUD], or entertainment workers [EW, ie, those who provide entertainment, including sex, for money] vs others not identified as being a member of these populations [referred to as the general population in routine monitoring and evaluation tools]). The GEE method was used to produce logistic regression estimates for clustered data using R “gee-pack” version 1.3.3. We included maps created in ArcGIS (Esri) of HIV diagnoses and RITA-recent individuals per 100 000 population aged 15 years or older using population estimates from Cambodia’s Ministry of Planning [6].
RESULTS
During the first 2 years of national recent infection surveillance implementation, data were captured on 53 031 individuals tested for HIV infection. Of these, 6868 individuals (13.0%) were newly diagnosed with HIV. Of these, 6180 (90%) consented and were tested for recent infection. Among the 6180 individuals tested for recent infection, 314 (5.2%) individuals tested assay-recent and 237 of these were tested for VL as a part of RITA. Among these, 192 (81.0%) tested RITA-recent, and 45 (19%) of the assay-recent infections were reclassified as long-term. The characteristics of individuals testing HIV positive and RITA-recent are provided in Table 1. Multivariable odds ratios for HIV diagnosis and testing RITA-recent are also in Table 1. New HIV diagnoses per 100 000 population were highest in Phnom Penh, Preah Sihanouk, Koh Kong, Siem Reap, and Banteay Meanchey while the provinces with the highest numbers of RITA-recent individuals per 100 000 population were Phnom Penh, Siam Reap, Banteay Meanchey, Preah Sihanouk, and Oddar Meanchey (Figure 1 and Supplementary Material).
Table 1.
Characteristics of Individuals Tested for HIV, Diagnosed With HIV, and Classified as Recent Using the RITA From Cambodia's National HIV Surveillance Systems, August 2020 to August 2022
| Characteristic | Total No. Tested for HIV | No. Diagnosed With HIV | aOR for HIV Diagnosis (95% CI) | P Value | No. RITA-Recent | aOR for RITA-Recent (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sex | |||||||
| Female | 26 002 | 1843 | Ref | ... | 32 | Ref | ... |
| Male | 27 029 | 5025 | 1.73 (1.62–1.86) | < .001 | 160 | 2.04 (1.21–3.42) | .01 |
| Age, y | |||||||
| 15–19 | 3953 | 323 | Ref | ... | 10 | Ref | ... |
| 20–34 | 27 689 | 4002 | 1.48 (1.30–1.68) | < .001 | 143 | 1.35 (.64–2.83) | .42 |
| ≥ 35 | 21 389 | 2543 | 1.55 (1.35–1.78) | < .001 | 39 | 0.82 (.38–1.76) | .61 |
| Marital status | |||||||
| Single | 15 310 | 3341 | Ref | ... | 128 | Ref | ... |
| Married | 35 041 | 2738 | 0.74 (.68-.81) | < .001 | 51 | 0.76 (.45–1.31) | .33 |
| Widowed | 2680 | 789 | 2.63 (2.31–2.98) | < .001 | 13 | 1.56 (.82–2.96) | .17 |
| Education | |||||||
| None | 3105 | 599 | Ref | ... | 11 | Ref | ... |
| Primary | 17 123 | 2072 | 0.59 (.52-.65) | < .001 | 38 | 0.56 (.29–1.09) | .09 |
| Secondary | 15 738 | 1926 | 0.49 (.43-.55) | < .001 | 53 | 0.52 (.27–1.03) | .06 |
| High school | 10 526 | 1396 | 0.36 (.32-.41) | < .001 | 52 | 0.45 (.23-.88) | .02 |
| Above high school | 6539 | 875 | 0.16 (.14-.19) | < .001 | 38 | 0.18 (.09-.38) | < .001 |
| Type of client | |||||||
| General population | 46 963 | 3761 | Ref | ... | 66 | Ref | ... |
| Men who have sex with men | 4078 | 2280 | 15.50 (14.00–17.16) | < .001 | 98 | 27.40 (15.53–48.33) | < .001 |
| Transgender women | 1088 | 510 | 11.00 (9.50–12.75) | < .001 | 21 | 19.05 (9.58–37.88) | < .001 |
| People who use drugs | 125 | 95 | 26.34 (17.22–40.29) | < .001 | 1 | 13.93 (1.94–100.05) | .01 |
| Entertainment worker | 777 | 222 | 3.46 (2.88–4.16) | < .001 | 6 | 6.14 (2.47–15.28) | < .001 |
Multivariable adjusted odds ratios are for individuals diagnosed with HIV and for individuals testing RITA-recent. HIV-negative individuals were the comparator for all odds ratios and all variables listed were included in the multivariable analysis.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; Ref, reference; RITA, recent infection testing algorithm.
Figure 1.
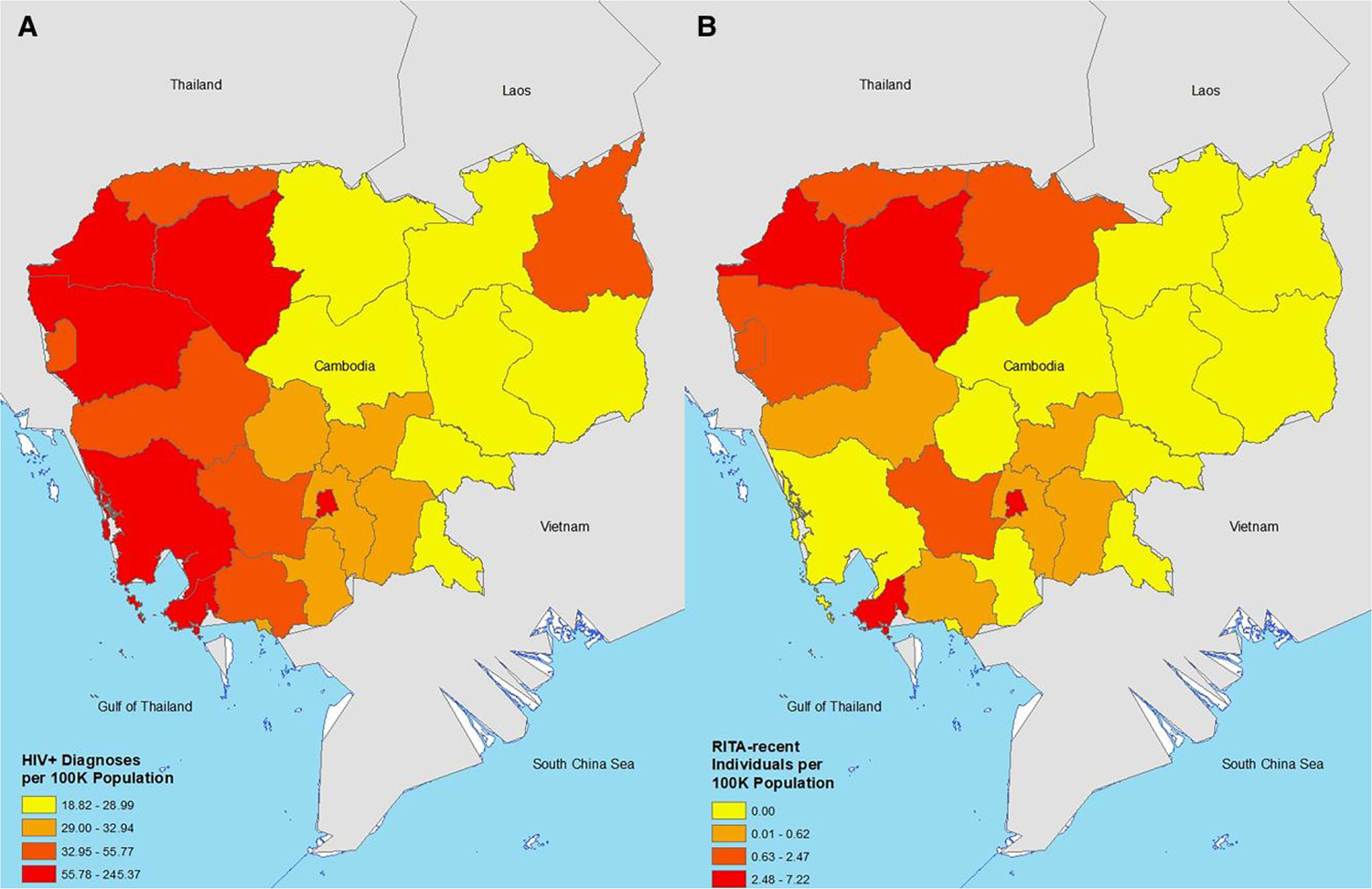
New HIV diagnoses (A) and recent infections (B) standardized per 100 000 population aged 15 years or older, by Cambodian province, August 2020 to August 2022. Abbreviation: RITA, recent infection testing algorithm.
DISCUSSION
In this analysis of national surveillance data, we observed differences in adjusted odds of testing RITA-recent and the adjusted odds for being newly diagnosed with HIV compared to those found to be uninfected. The most pronounced differences related to risk behaviors. Compared with the general population, MSM, TGW, and EW had a nearly 2-fold higher adjusted odds for testing RITA-recent than for being newly diagnosed with HIV. Previous Cambodian biological surveys collected over time may suggest a similar upward trend in acquisition patterns. Specifically, MSM HIV prevalence has increased from 2% in 2011% to 4% in 2019 while the HIV prevalence in TGW increased from 6% in 2015% to 10% in 2019 [7, 8]. Moreover, mathematical modelling found that of the new infections in Cambodia in 2021, 40% are estimated to be among MSM, 14% among the clients and sex partners of key population members, 13% among sex workers, and 7% among transgender people [8].
Conversely, compared with the general population, PWUD had about 50% lower adjusted odds for testing RITA-recent than for being newly diagnosed with HIV. The biological surveys collected over time may also reaffirm this observation: the HIV prevalence in people who inject drugs decreased from 24% in 2011 to 15% in 2017 [7, 8]. Moreover, mathematical modelling suggests that people who inject drugs currently represent 3% of new HIV infections [8]. Collectively, the recent infection data, biological surveillance data, and the national-level mathematical modelling appear be providing us with data consistent with those from elsewhere in the Asia-Pacific region suggesting that MSM and transgender people are increasingly infected with HIV while changes in drugs being used, from heroin to amphetamines, is becoming more common and may be affecting HIV risk related to drug use [9–12].
We also observed differences in age-stratified adjusted odds for HIV diagnosis and RITA-recent status. Specifically, 15–19 year olds had lower adjusted odds for HIV diagnosis compared to individuals aged 20–34 years and to individuals aged 35 years or more. Although the sample sizes were small, and statistical significance was not achieved, individuals aged 20–34 years had higher RITA-recent odds compared to 15–19 year olds while the RITA-recent odds for individuals 15–19 years and individuals aged 35 years or older appeared similar. Given the small sample sizes of RITA-recent by age, and possible differences in risk in underlying populations seeking HIV testing across these age bands, further studies are needed to inform age-stratified risks of RITA-recent infection in Cambodia and elsewhere.
Although general population members had the lowest adjusted odds of being newly diagnosed with HIV, or being RITA-recent, their risk was not negligible. This may be related to a limitation in the general population classification. For example, this group may include some MSM, TGW, PWUD, and EW who may not want to provide information about their potential risks for infection. Risk elicitation may also be very poor in some testing sites compared with others. This could attenuate the differences between the general population and key population members. Nonetheless, relatively high RITA-recent rates were observed in several rural provinces without known large numbers of key population members. A prior outbreak among the general population in rural Cambodia has demonstrated the importance of ensuring medical injection safety to reduce the risk of HIV transmission across all populations [13]. Since this time, major strides have been made in improving regulations for practitioners and training health care providers [14]. Future efforts may be helpful to characterize other major risks of HIV transmission in the general population nationally and across populations in rural provinces of Cambodia.
We did observe some differences in the geographical distribution of HIV diagnoses and RITA-recent individuals. For example, Koh Kong ranked third out of 25 provinces for the number of diagnoses per 100 000 population but none of them tested RITA-recent, suggesting that individuals in this province acquired HIV long ago. Conversely, Preah Vihear ranked 19th out of 25 provinces for the number of diagnoses per 100 000 population but ranked 8th out of 25 provinces for the number of RITA-recent individuals per 100 000 population, suggesting that many individuals have acquired HIV recently. This suggests that there are unique geographical insights emerging from recent infection surveillance data that would not have otherwise been gleaned from diagnostic HIV case surveillance data.
The overall yield of 13% of testers being diagnosed was relatively high and may be explained by referrals from screening testing modalities and possible repeat testing amongst previously diagnosed individuals. Specifically, although all 68 facilities offering HIV diagnostic testing in Cambodia were included, there are other self-testing, community-based testing, and screening testing venues across the country. The individuals presenting for diagnostic testing therefore represents a mix of those who are seeking HIV testing for the first time at a diagnostic site and those who have screened positive elsewhere and were referred for diagnostic testing. Repeat testers could also be included with newly diagnosed individuals if previously diagnosed individuals disengage with care and provide different information upon re-engagement.
National estimates from mathematical modelling in Cambodia suggest that approximately 1100 individuals were newly infected with HIV in 2021 [8]. Over the course of 2 calendar years, among 53 031 persons who were tested for HIV, 6868 individuals were diagnosed and 192 were RITA-recent in this analysis. This suggests that the vast majority of individuals being diagnosed are not incident cases but rather acquired HIV years ago and that there remains a potential gap between the estimated number of newly infected individuals with HIV forecasted over the 2-year timeframe (approximately 2200) and the number of individuals whose new HIV infections are being diagnosed (192). This gap may be further increased if potential repeat testers are identified and removed from national surveillance data. Identifying newly infected people with HIV early in the course of infection and immediately initiating treatment is a core intervention to reduce morbidity, HIV-related death, and transmission. Continuing to provide a mix of self-, community-, and facility-based testing models may help bridge the diagnostic gap in Cambodia, particularly among Cambodian key populations, including MSM, TGW, PWUD, and EW, who may experience higher levels of stigma and discrimination than the general population [15].
These data could be triangulated with other national surveillance data to guide programmatic decision-making as there are advantages and disadvantages of each data source. Because HIV diagnosis was required for recent infection testing, populations with higher health literacy and HIV testing utilization could be overrepresented in recent infection surveillance data while those with poorer health literacy and testing utilization could be underrepresented. However, prevalence data collected from biological surveys using community- and network-based screening approaches found similar trends [7, 8]. Moreover, not all with newly diagnosed HIV were tested for recent infection and not all RTRI-recent individuals were tested for VL, making use of RITA-recent absolute numbers less reliable. Moreover, the RITA-recent analysis had lower statistical power than the HIV diagnosis analysis (eg, a single PWUD client tested RITA-recent). Continued data collection for national recent HIV infection surveillance may help improve overall statistical power and our confidence in these findings.
National recent infection surveillance likely provides unique insights compared to using new HIV diagnosis alone. These data, along with other surveillance and data sources, can help identify outbreaks or clusters and signal potential gaps in prevention, testing, and key population programs.
Supplementary Material
Financial support.
This research was supported by the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of Cooperative Agreement number NU2GGH002170–02–00.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the funding agencies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copy-edited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative group on AIDS incubation and HIV survival including the CASCADE EU concerted action. Concerted action on SeroConversion to AIDS and death in Europe. Lancet 2000; 355:1131–7. [PubMed] [Google Scholar]
- 2.Todd J, Glynn JR, Marston M, et al. Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy. AIDS 2007; 21(Suppl 6):S55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The TRACE Initiative. Tracking with recency assays to control the epidemic. https://trace-recency.org/. Accessed 9 September 2022.
- 4.World Health Organization. Using recency assays for HIV surveillance: 2022 technical guidance. https://www.who.int/publications/i/item/9789240064379. Accessed 21 March 2023.
- 5.Facente SN, Grebe E, Maher AD, et al. Use of HIV recency assays for HIV incidence estimation and other surveillance use cases: systematic review. JMIR Public Health Surveill 2022; 8:e34410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cambodia Ministry of Planning. Population estimates. https://mop.gov.kh/en-us/aboutMoP/aboutMoPList?MenuName=AboutMoP. Accessed 15 November 2022.
- 7.Joint United Nations Programme on HIV/AIDS. Key populations atlas. https://kpatlas.unaids.org/dashboard. Accessed 22 November 2022.
- 8.Joint United Nations Programme on HIV/AIDS. Cambodia. https://www.aidsdatahub.org/country-profiles/cambodia. Accessed 22 November 2022. [Google Scholar]
- 9.United Nations Office on Drugs and Crime. World drug report; 2022. https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2022.html. Accessed 8 September 2022. [Google Scholar]
- 10.Joint United Nations Programme on HIV/AIDS. Key populations. https://www.aidsdatahub.org/populations. Accessed 8 September 2022.
- 11.Hser YI, Liang D, Lan YC, Vicknasingam BK, Chakrabarti A. Drug abuse, HIV, and HCV in Asian countries. J Neuroimmune Pharmacol 2016; 11:383–93. [DOI] [PubMed] [Google Scholar]
- 12.van Griensven F, Guadamuz TE, de Lind van Wijngaarden JW, Phanuphak N, Solomon SS, Lo YR. Challenges and emerging opportunities for the HIV prevention, treatment and care cascade in men who have sex with men in Asia Pacific. Sex Transm Infect 2017; 93:356–62. [DOI] [PubMed] [Google Scholar]
- 13.Vun MC, Galang RR, Fujita M, et al. Cluster of HIV infections attributed to unsafe injection practices—Cambodia, December 1, 2014-February 28, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:142–5. [DOI] [PubMed] [Google Scholar]
- 14.Kanagasabai U, Singh A, Shiraishi RW, et al. Improving injection safety practices of Cambodian healthcare workers through training. PLoS One 2020; 15:e0241176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Consolidated guidelines on HIV testing services. https://www.who.int/publications/i/item/978-92-4-155058-1. Accessed 8 September 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


