Abstract
PURPOSE
Cemiplimab is approved for treating locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC). Solid organ transplant recipients have been excluded from immunotherapy trials, given concern for allograft rejection despite their increased risk of skin cancers. Chronic immunosuppression is necessary to prevent organ rejection but may attenuate antitumor response with PD-1 inhibitors.
METHODS
We report a phase I study of cemiplimab for kidney transplant recipients (KTRs) with advanced CSCC. After cross-taper to a mammalian target of rapamycin (mTOR) inhibitor and pulsed dose corticosteroids (prednisone 40 mg once daily, the day before and on days 1-3 of each cycle, followed by 20 mg once daily on days 4-6, then 10 mg once daily until the day before each subsequent cycle), patients received cemiplimab 350 mg intravenously once every 3 weeks for up to 2 years and were assessed for response every 8 weeks. The primary end point was the rate of kidney rejection, with key secondary end points including rate and duration of response, and survival.
RESULTS
Twelve patients were treated. No kidney rejection or loss was observed. A response to cemiplimab was observed in five of 11 evaluable patients (46%; 90% CI, 22 to 73), including two with durable responses beyond a year. Median follow-up was 6.8 months (range, 0.7-29.8). Treatment-related grade 3 or greater adverse events occurred in five patients (42%), including diarrhea, infection, and metabolic disturbances. One patient died of angioedema and anaphylaxis attributed to mTOR inhibitor cross-taper.
CONCLUSION
mTOR inhibitor and corticosteroids represent a favorable immunosuppressive regimen for KTRs with advanced CSCC receiving immunotherapy. This combination resulted in durable antitumor responses with no kidney rejection events (funded by Regeneron Pharmaceuticals [ClinicalTrials.gov identifier: NCT04339062]).
INTRODUCTION
Although cutaneous squamous cell carcinoma (CSCC) is cured with surgery in most patients, in some, the cancer can become locoregionally recurrent or metastatic.1 Solid organ transplant recipients receiving chronic immunosuppression (IS) have a markedly increased risk of CSCC that is 65-250 times that of the general population, accounting for 40% of all malignancies that develop in organ transplant recipients.2 Many of these patients will develop additional cutaneous lesions, and cancer mortality is high, with few systemic therapeutic options.3,4 Despite the long-term risk of second malignancies after organ transplantation, kidney transplantation is the treatment of choice for most with end-stage kidney disease, and improves quality of life, reduces mortality risk, and is less costly when compared with maintenance dialysis.5
CONTEXT
Key Objective
Can immunotherapy be used to treat kidney transplant recipients (KTRs) with advanced cutaneous squamous cell carcinoma (CSCC)?
Knowledge Generated
In a phase I clinical trial of 12 KTRs with advanced CSCC receiving the PD-1 inhibitor cemiplimab, cross-taper to a mammalian target of rapamycin (mTOR) inhibitor and pulsed dose prednisone with each treatment cycle resulted in no kidney allograft rejection events and favorable antitumor activity.
Relevance (G.K. Schwartz)
-
The use of an mTOR inhibitor may represent a novel means to prevent kidney allograft rejection induced by a PD-1 checkpoint inhibitor in the treatment of CSCC.*
*Relevance section written by JCO Associate Editor Gary K. Schwartz, MD, FASCO.
Although the pathways leading to increased cutaneous malignancy risk in organ transplant recipients are not fully understood, it is accepted that immunosuppressive medications used to control the rejection response leads to reduced immune surveillance and carcinogenic effects.6 Cemiplimab is a monoclonal antibody directed at PD-1, which has demonstrated significant and often durable responses in patients with locally advanced or metastatic CSCC, now representing a standard treatment option.7 However, organ transplant recipients and those treated with systemic immunosuppressive therapy were excluded from studies evaluating PD-1 blockade for CSCC, given the risk of allograft rejection.8 Whether an effective immunosuppressive regimen can be maintained to prevent kidney allograft rejection but permit antitumor benefit with PD-1 inhibitors is not known.
To our knowledge, we report the first prospective phase I study investigating the PD-1 inhibitor cemiplimab in kidney transplant recipients (KTRs) with advanced incurable, or metastatic CSCC. Given the proposed synergistic antitumor role observed with mammalian target of rapamycin (mTOR) inhibitors affecting cell proliferation and immune differentiation in cancer metabolism,9 we standardized our immunosuppressive regimen to include sirolimus or everolimus combined with a pulsed dose and schedule of glucocorticoids.10 The primary objective was to evaluate the rate of kidney allograft rejection, with a key secondary objective of measuring antitumor activity.
METHODS
Study Oversight
The study Protocol (Appendix 1, online only) was approved by the institutional review board at Dana-Farber Cancer Institute (DF/HCC# 19-817). The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent. Deidentified participant data may be shared upon request to the corresponding author.
Study Population
Patients age 18 years or older were eligible for inclusion to the study if they had locoregionally advanced unresectable, incurable or metastatic CSCC with a history of kidney transplantation (at least 6 months prior to enrollment), with one or more sites of measurable disease by RECIST, version 1.1, an Eastern Cooperative Oncology Group performance status of ≤2, and adequate organ function, including an estimated glomerular filtration rate (GFR) ≥30 mL/min/1.73 m2 for patients with creatinine levels above institutional normal range (Chronic Kidney Disease-Epidemiology Collaboration equation) and a urine protein to creatinine ratio <0.5 g/g creatinine (equal to or less than 500 mg of proteinuria per day). Patients had to be off antiproliferative immunosuppressive medications at the time of screening. Any number of lines of previous systemic or radiation therapy were permitted, but previous immunotherapy exposure was exclusionary.
Study Design and Treatment
This phase I, single-arm, single-center, nonrandomized trial enrolled patients at the Dana-Farber Cancer Institute (Boston, MA). The trial design originally included two parallel cohorts, one for allogeneic stem-cell transplant recipients and the other for KTRs. The stem-cell transplant cohort permanently closed for slow accrual with no enrolled patients.
During a screening period of up to 28 days, patients were cross-tapered (7-10 days before starting immunotherapy) from their existing immunosuppressive regimen to an mTOR inhibitor (sirolimus or everolimus) with a goal trough of 4-6 ng/mL and prednisone 10 mg once daily. Once mTOR inhibitor trough levels were achieved, patients received cemiplimab intravenously at a dose of 350 mg every 21 days for up to 35 doses over 2 years or until the occurrence of disease progression (PD), unacceptable toxicity, or withdrawal of consent. Additionally, patients received prednisone 40 mg once daily, the day before and the day of each cemiplimab cycle through day 3, followed by 20 mg once daily on days 4-6, then 10 mg once daily on day 7 continuously until the day before each subsequent cycle in a pulsed fashion. Standard prophylactic antimicrobial and antiviral use was permitted. mTOR inhibitor dose adjustments were made by a transplant nephrologist on the basis of trough levels obtained at least once each cycle, and adherence was documented by drug diary.
Patients underwent imaging assessments (computed tomography or positron emission tomography scan) at baseline and every 8 weeks through cycle 10, then every 12 weeks while on treatment. Imaging of externally visible lesions was supplemented with digital medical photography. Target lesions were prespecified at baseline and followed with sequential imaging for response, whereas second primary skin malignancies arising on study were followed as nontarget lesions (and could be treated with local therapy or excision). After treatment discontinuation, patients were followed every 3 months up to 1 year for resolution of toxicity and to document survival.
End Points
The primary end point was safety and toxicity, specifically the rate of kidney allograft rejection or loss. A rejection rate exceeding 60% was considered unacceptable a priori. Stopping rules were developed, on the basis of sequential monitoring and Pocock-type boundaries, such that the probability of stopping the trial would be >90% if the rejection rate exceeded 60%. If the number of patients experiencing kidney rejection after starting cemiplimab was ≥two of three, four of six, or six of 12 patients enrolled to the cohort, the study would close. Adverse events to assess the safety and side-effect profile of cemiplimab were recorded using Common Terminology Criteria for Adverse Events version 5.0. Key secondary end points included objective antitumor response (RECIST 1.1) as best overall response (complete response [CR] or partial response [PR]) determined by investigator assessment; duration of response; and estimates of progression-free survival (PFS) and overall survival (OS).
Exploratory Analyses
Exploratory analyses of potential associations of PD-L1 expression and tumor mutational burden (TMB) with treatment response were performed. Formalin-fixed, paraffin-embedded tumor samples obtained before treatment were submitted for targeted next-generation sequencing with either FoundationOneCDx (Cambridge, MA) or Caris Molecular Intelligence (MI) profile (Newton, MA). PD-L1 tumor proportion or combined positive score was assessed by means of immunohistochemical staining with the use of the 22C3 assay with a score of ≥1% considered positive. Paired pretreatment tumor tissue and peripheral blood multiparametric immune profiling was performed using a BD LSRFortessa instrument (BD Biosciences, San Jose, CA). A custom multiplex cytokine profiling panel (Luminex, EMD Millipore, Burlington, MA) was run on sequential urine samples obtained before and after treatment, as outlined in Appendix 1. The Signatera personalized, tumor-informed assay to detect circulating tumor DNA and Prospera transplant rejection test monitoring donor-derived (dd) cell-free (cf)DNA were each collected in a subset of patients.
Statistical Analysis
A maximum sample size of 12 participants was considered sufficient to assess the trial objective of safety and kidney transplant rejection rate from the time of study treatment and was set empirically. Safety data are summarized descriptively with frequency counts and percentages. Time-to-event end points are summarized using the method of Kaplan-Meier. Point estimates for each end point are presented with 90% CIs derived using log(–log[outcome]) methodology. The change in exploratory immune profiling parameters before and on treatment was compared using a Wilcoxon signed-rank test with a Bonferroni-Dunn correction applied for testing multiple comparisons (two-sided).
The primary analysis was performed when all patients had received treatment with cemiplimab, and when the last patient had first restaging. The efficacy and safety of cemiplimab were assessed in all patients who received at least one dose of the study treatment. All reported data are based on a data-cutoff date of June 14, 2023.
RESULTS
Patients
From November 2020 to March 2023, 12 KTRs enrolled to the trial (Appendix Fig A1). Baseline characteristics are summarized in Table 1. Median age was 62 years (range, 43-86), and 10 patients (83%) were men. The predominant primary anatomic site of tumor was the head and neck (11 patients, 92%), and seven patients (58%) had metastatic disease. All had undergone surgery for CSCC, and 10 patients (83%) had previous radiation therapy. Median time from the last kidney transplant surgery was 7.2 years (range, 2.8-21.1), with four patients having received two kidney allografts, and half (6, 50%) were on a calcineurin inhibitor and antiproliferative agent combined with prednisone for chronic IS before enrollment and cross-taper to our mTOR inhibitor regimen (Appendix 1; Appendix Table A1). Baseline estimated GFR was 49 mL/min (range, 32 to >60).
TABLE 1.
Patient Baseline Characteristics
| Characteristic | Value (n = 12) |
|---|---|
| Age, years, median (range) | 62.5 (43-86) |
| Male sex, No. (%) | 10 (83) |
| Race, No. (%) | |
| White | 11 (92) |
| Not Hispanic or Latino, No. (%) | 11 (92) |
| ECOG PS score, No. (%)a | |
| 0 | 5 (42) |
| 1 | 5 (42) |
| 2 | 2 (17) |
| Time since kidney transplant, years, median (range)b | 7.2 (2.8-21.1) |
| No. of IS agents before enrollment, (%) | |
| 2 | 6 (50) |
| 3 | 6 (50) |
| IS regimen before enrollment, No. (%)c | |
| Calcineurin inhibitors | 7 (58) |
| mTOR inhibitors | 6 (50) |
| Antiproliferative | 6 (50) |
| Prednisone | 10 (83) |
| Baseline renal allograft function, median (range) | |
| Baseline creatinine, mg/dL | 1.51 (0.95-1.86) |
| Estimated GFR, mL/mind | 49 (32-60+) |
| Primary tumor site, No. (%) | |
| Head and necke | 11 (92) |
| Trunk, arms, and legs | 1 (8) |
| Stage group, No. (%)c,f | |
| Tumor stage, T4 | 5 (42) |
| Nodal stage, N2-3 | 8 (67) |
| Metastatic disease (M1) | 7 (58) |
| Site(s) of disease, No. (%)c | |
| Lymph nodes | 8 (67) |
| Lung | 3 (25) |
| Liver | 2 (17) |
| In-transit metastases, skin | 8 (67) |
| Previous therapies, No. (%)c | |
| Surgery (beyond biopsy) | 12 (100) |
| Radiation | 10 (83) |
| Chemotherapy | 3 (25) |
| Cetuximab | 4 (33) |
Abbreviations: AJCC, American Joint Committee on Cancer; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; ECOG PS, Eastern Cooperative Oncology Group performance status; GFR, glomerular filtration rate; IS, immunosuppression; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin.
ECOG PS scale ranges from 0 to 5, with higher scores indicating greater disability.
Four patients each received two kidney allografts.
Percentages do not total 100% because components are not mutually exclusive.
Estimated GFR determined by the CKD-EPI equation (60 mL/min implies at that value or greater).
Two patients had HPV-associated disease confirmed by p16 immunohistochemistry and HPV confirmatory testing (in situ hybridization). One of the two was a primary lip cutaneous squamous cell carcinoma and the other an oropharyngeal tumor.
AJCC staging eighth edition, 2017.
Safety
Adverse events of any grade that occurred during the study period, regardless of whether they were attributed to cemiplimab, mTOR inhibitor, or prednisone, were observed in all 12 patients (100%). The most common adverse events of any grade were skin or subcutaneous disorders (10; 83%) and fatigue (9; 75%). Grade 3, 4, and 5 adverse events, regardless of attribution, were observed in 10 (83%), 0, and 3 (25%, occurring during the adverse event reporting period) patients, respectively. Two deaths were attributed to PD, two were attributed to unrelated comorbid medical conditions, and the other is detailed below. Adverse events of any grade that were considered by the investigator to be at least possibly related to treatment occurred in 10 patients (83%; Table 2), with the most common being fatigue (7; 58%). Suspected immune-related adverse events occurred in one (8%) patient who experienced grade 3 diarrhea that resolved without steroid dose adjustment. Five of 12 patients (42%) experienced infections (cellulitis and respiratory illness) requiring hospitalization during the study period.
TABLE 2.
Summary of Treatment-Related Adverse Events
| Adverse Eventa | Patients, No. (%) | |
|---|---|---|
| Any Grade | Grade 3b | |
| Any event | 10 (83) | 5 (42) |
| Serious event | 5 (42) | 5 (42) |
| Event that led to discontinuation of treatment | 1 (8) | 1 (8) |
| Event that led to death | 0 | 0 |
| Event of any grade that occurred in ≥10% of patients or grade 3-5 events that occurred in 1 or more patients |
||
| Endocrine | ||
| Hyperthyroidism | 2 (17) | — |
| GI disorders | ||
| Diarrhea | 2 (17) | 1 (8)c |
| General disorders | ||
| Limb edema | 3 (25) | — |
| Fatigue | 7 (64) | — |
| Infections and infestations | ||
| Lung infection | 1 (8) | 1 (8)d |
| Skin infection | 1 (8) | 1 (8) |
| Upper respiratory infection | 2 (17) | 1 (8)d |
| Metabolism and nutrition disorders | ||
| Acidosis | 1 (8) | 1 (8) |
| Hyperkalemia | 1 (8) | 1 (8) |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 2 (17) | — |
| Skin and subcutaneous tissue disorders | ||
| Maculopapular rash | 2 (17) | — |
| Vascular disorders | ||
| Hypertension | 2 (17) | — |
| Total events | 25 | 6 |
Abbreviation: mTOR, mammalian target of rapamycin.
Safety was assessed in all patients who received at least one dose of cemiplimab, mTOR inhibitor (sirolimus or everolimus), and prednisone. Treatment-related adverse events were coded according to the preferred terms of the Medical Dictionary for Regulatory Activities, version 24.1. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
No grade 4 or 5 treatment-related events were reported.
Grade 3 immune-related adverse event resulting in diarrhea and dehydration.
Grade 3 adverse events attributed to upper respiratory infection and superimposed COVID-19 pneumonia.
No kidney allograft rejection events were observed during the study. No grade ≥3 creatinine elevation was observed throughout the study (Appendix Fig A2). No patients required hemodialysis. Two patients developed hypoperfusion events (one related to angioedema or anaphylaxis, and another due to diarrhea) and one developed allograft pyelonephritis (responsive to antibiotics) leading to elevated creatinine values <three times the upper limit of their baseline values. One adverse event during the study period was fatal and led to study discontinuation. A 58-year-old patient developed acute facial swelling and shortness of breath attributed to angioedema from everolimus and an angiotensin-converting enzyme inhibitor (ACEi) resulting in respiratory failure that did not improve with supportive measures. Nine days after starting cross-taper to everolimus, the patient received one dose of cemiplimab (angioedema began 1 day after PD-1 inhibitor dosing). This death was considered by the investigator to be unlikely related to immunotherapy on the basis of the described incidence of angioedema when mTOR inhibition is combined with an ACEi.11
Clinical Efficacy and Survival
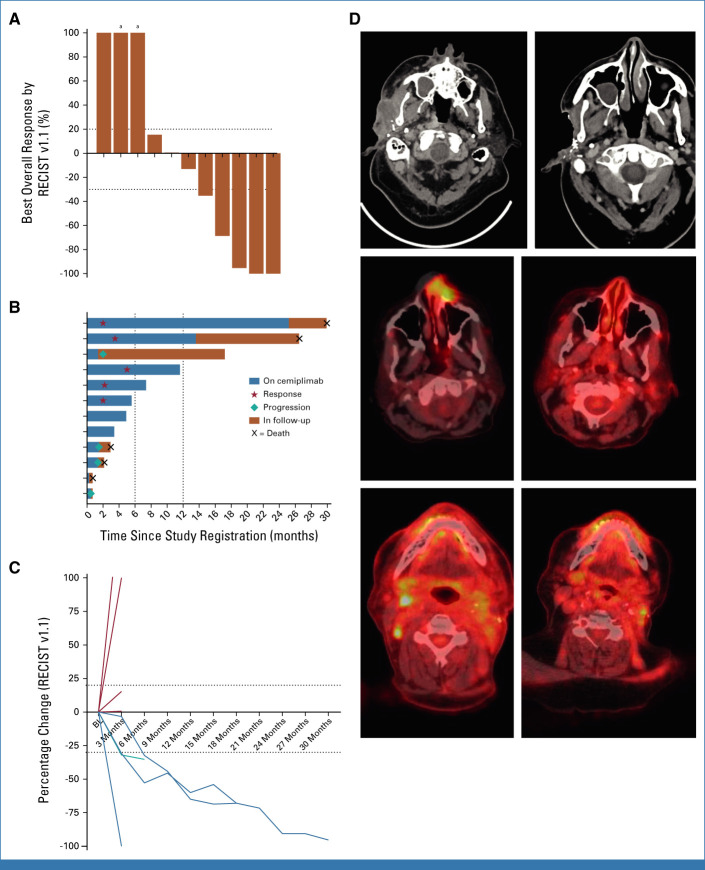
After treatment with cemiplimab and dynamic mTOR inhibitor–based IS with pulsed prednisone, five of 11 evaluable patients (46%; 90% CI, 20 to 73) demonstrated a response, including three CRs and two PRs (Fig 1). Two patients had stable disease for a clinical benefit rate of 64% (90% CI, 35 to 86). Median duration of response was 11.4 months (range, 4.9-29.7) and ongoing among three responders. One patient with a CR ended cemiplimab treatment after 24 months and required excision of a parietal scalp basal cell carcinoma while on study drug. Another patient with a PR stopped cemiplimab after 12 months (patient preference), but was also treated for malignant melanoma of the upper back and required 11 Mohs procedures to clear second primary CSCCs on the torso and extremities while on study drug. Three other responders remain on study treatment at data cutoff.
FIG 1.
(A) Waterfall plot depicting best overall response (RECIST v1.1) to cemiplimab treatment. Each column represents an individual patient. (B) Swimmer plot showing time on cemiplimab treatment and follow-up period; each row represents an individual patient. (C) Spider plot showing the percentage change (RECIST v1.1) in tumor measurements over time; each line represents an individual patient. (D) Pretreatment (left) and post-treatment (right) CT images showing an infiltrative right preauricular mass from CSCC that resolved over time. Fused PET and CT scans with false color added showing a left dorsal nose CSCC before (left) and after (right) cemiplimab treatment. Fused PET and CT scans showing right level II neck adenopathy (left) before and after (right) treatment. aDenotes the development of a new lesion at first response assessment. BL, baseline; CSCC, cutaneous squamous cell carcinoma; CT, computed tomography; PET, positron emission tomography.
Four patients (40%) experienced disease PD while on treatment with a median time to progression of 1.4 months (range, <1 to 2.1) from the start of cemiplimab. Three of the four were receiving sirolimus with pulsed prednisone for immune suppression. One patient developed rapid clinical PD after one dose of cemiplimab leading to treatment discontinuation, while three others each received three or four doses of cemiplimab before discontinuation. One patient with early progression subsequently received three cycles of carboplatin and paclitaxel, followed by palliative cetuximab (CTX) resulting in a complete CR per the local treating physician and remains on maintenance CTX >6 months. The patient who developed angioedema and subsequent clinical decline was unevaluable.
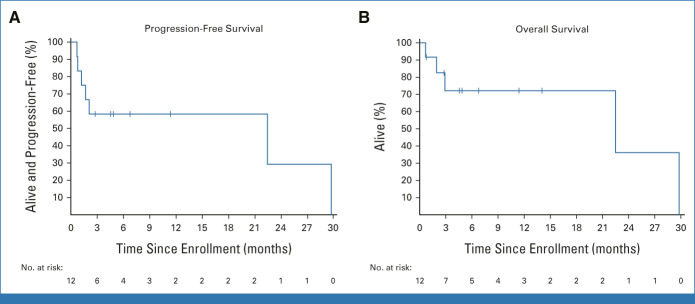
At a median follow-up of 6.8 months (range, 0.7-29.8+), median PFS was 22.5 months (90% CI, 1.2 to 29.8; Fig 2). Median OS was 22.5 months (90% CI, 2.9 to 29.8), with a 3-month OS estimate of 72% (90% CI, 43 to 88). Five patients (42%) had died at the time of data cutoff, while seven patients were censored at last follow-up.
FIG 2.
Kaplan-Meier curves plotting the probability of (A) PFS and (B) OS among kidney transplant recipients with advanced cutaneous squamous cell carcinoma receiving cemiplimab. OS, overall survival; PFS, progression-free survival.
Biomarker Analyses
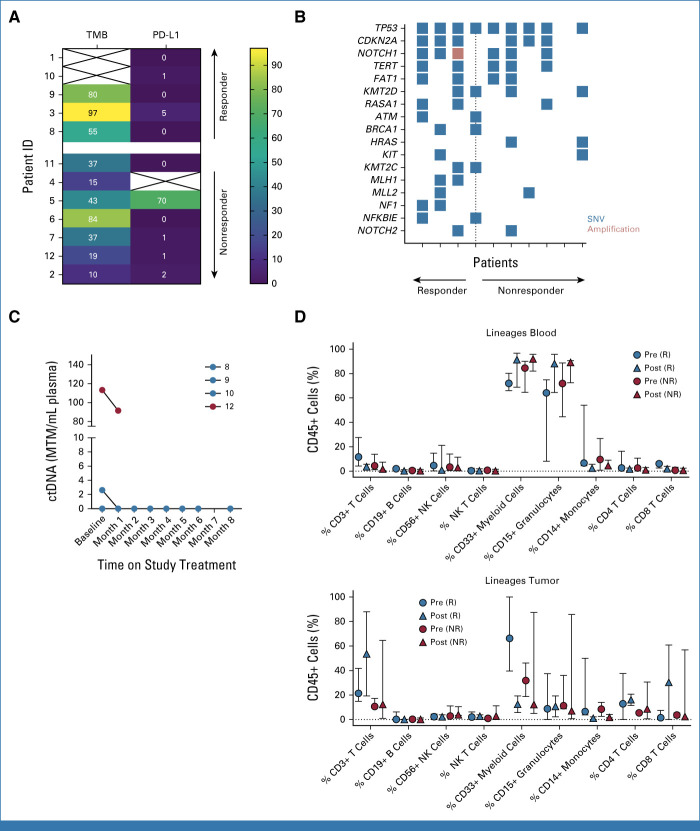
PD-L1 tumor proportion scores on pretreatment biopsy samples (11 evaluable) ranged from 0% to 70%, with a median score of 1 (Fig 3). A PD-L1 score of 5 was noted among a patient with a durable PR, but four other patients in response had a score of 0-1. TMB values ranged from 10 to 97, with a median of 40 mutations per megabase (muts/Mb); median TMB values were higher among responders (80 v 37; P = .05; Mann-Whitney U test). The most common tumor gene alteration (10 evaluable) was in TP53 (90%) from pretreatment samples, while overall tumor mutational profiling showed similar findings regardless of immunotherapy response. One of 10 sequenced tumors demonstrated microsatellite instability, from a responder. Plasma tumor–derived circulating tumor DNA monitoring was detectable at baseline in two of four (50%) evaluable participants (range, 2.62-113.25 mean tumor molecules per mL [MTM/mL]) and decreased or cleared in response to therapy. In-depth multiparametric immune profiling (11 evaluable) revealed no statistically significant differences in major leukocyte populations between pre- and on-treatment samples in blood or tumor. We observed a trend toward increased total CD3+ T lymphocytes, increased CD8+ cytotoxic T lymphocytes, and decreased total myeloid cells in tumor specimens on treatment among responders, which was not observed in nonresponders.
FIG 3.
(A) Tumor mutational burden (mutations per megabase) and PD-L1 TPS plotted for patients with evaluable pretreatment tumor samples arranged by best response to treatment (complete or partial response reflects responders; progressive disease reflects nonresponders). (B) Tumor mutational plot depicting gene alterations among each patient (columns) with evaluable pretreatment tumor material for targeted next-generation sequencing arranged by best response to treatment (only those mutations occurring in at least 20% of samples are depicted). (C) Summary plot of baseline and monthly on-treatment Signatera ctDNA values (MTM/mL) for four participants with available testing results. Patient numerical identifier shown in the legend and color-coded by best overall response (red = responder; blue = unevaluable). (D) In-depth multiparametric immune profiling by flow cytometry on matched or paired blood and tumor specimens before (pre-) and on (post-)treatment separated by response to immunotherapy. ctDNA, circulating tumor DNA; ID, identifier; MTM/ml, mean tumor molecules per mL; NR, nonresponder; R, responder; SNV, singe-nucleotide variant; TPS, tumor proportion score.
Plasma dd-cfDNA monitoring demonstrated reassuring values <1% among three of five (60%) study participants when obtained at baseline and after cemiplimab administration over time (Appendix Fig A2). One patient with a history of recurrent urinary tract infections experienced a rise in dd-cfDNA to 1.08% (baseline 0.42%) with a stable mild, transient creatinine elevation because of allograft pyelonephritis that resolved with antibiotics. Another patient had elevated baseline dd-cfDNA at 1.43% before starting cemiplimab, which later declined on therapy; notably, the patient had received two (double) renal allografts, for which dd-cfDNA testing has not been validated.
DISCUSSION
Immune checkpoint inhibitors targeting PD-1 are widely used to treat many solid tumor malignancies, but the risk of immune-mediated organ rejection has previously excluded kidney and other transplant recipients from trials investigating their efficacy. To our knowledge, we report the results of the first prospective clinical trial of cemiplimab coupled with mTOR inhibitor and pulsed dose corticosteroid IS to treat advanced, metastatic CSCC. We demonstrate no organ rejection events and a manageable safety profile among 12 PD-1 inhibitor–treated patients on standardized IS. Furthermore, we observed durable antitumor activity among this critical population with high unmet need.
In retrospective series, kidney allograft rejection rates approach 50% among nonmelanoma skin cancer patients treated with immunotherapy.12 Rejection events are often acute in onset (most occurring <4 weeks from initial exposure) and severe with mixed cellular and antibody-mediated rejection that involves kidney vasculature. Preexisting, graft antigen-specific memory T cells are thought to be activated and proliferate once immune suppression is deintensified, leading to rapid and severe kidney allograft rejection.13 None of our 12 treated patients experienced evidence of graft rejection during this critical period, which is encouraging.
In patients with CSCC, conversion from a calcineurin to mTOR inhibitor has been effective as secondary prevention.14 An mTOR inhibitor with careful trough level monitoring paired with pulsed prednisone around cemiplimab dosing promoted antitumor efficacy while also permitting allograft tolerance, as proposed by others.15 Plasma dd-cfDNA was available in only a subset of patients and seems useful to monitor the risk of kidney rejection.16 We advocate for weekly laboratory assessments to monitor renal function and urine protein levels, and careful mTOR inhibitor trough monitoring with plasma dd-cfDNA assessments at least every other week for the first 2-3 months of treatment. We recognize that mTOR inhibitors are not well tolerated by all patients, and ACEi should be stopped during cross-taper. In some cases, calcineurin inhibitors might offer better protection against organ rejection but recent data have suggested tacrolimus with prednisone is insufficient to prevent allograft rejection in KTRs with advanced CSCC treated with nivolumab with or without ipilimumab.17 Although our sample size is limited, given the risk imposed to this patient population, we observed comparable response rates compared with the broader advanced CSCC population treated with cemiplimab.7 Future trials might reconsider pulsed prednisone dosing as dose equivalents of 10 mg once daily or greater have been associated with worse outcomes in anti–PD-1–treated patients,18 and might explore the role of mTOR inhibitor conversion in organ transplant recipients with other advanced cancers.
Furthermore, we aimed to incorporate potential biomarkers of response and kidney rejection. Similar to previous reports,19 higher TMB and increased CD8+ cytotoxic T lymphocytes were potential predictors of response but even patients with low PD-L1–expressing tumors experienced clinical benefit from cemiplimab in this study—acknowledging our limited sample size. It was also interesting to note that baseline tumor-derived ctDNA was not readily detectable in all patients but did seem to track with response when detected. The impact of allograft status, ongoing immune suppression, and the marked molecular heterogeneity of cutaneous squamous cancers likely reflects the variability among these preliminary observations. Urine cytokine profiling results will be reported separately.
In conclusion, among KTRs with advanced CSCC, cemiplimab resulted in durable antitumor responses, and no kidney rejection was observed when coupled with mTOR inhibition and pulsed dose corticosteroids to maintain IS. Combining mTOR inhibition and pulsed corticosteroids is a favorable IS regimen when KTRs require anti–PD-1 therapy. Future trials are warranted to confirm these findings and might consider a comparator arm with an alternative IS regimen or a combinatorial immunotherapy approach.
APPENDIX 1. TUMOR AND PERIPHERAL BLOOD IMMUNE PROFILING
Fresh tissue biopsies were enzymatically disaggregated in a 5-cm dish in dissociation buffer consisting of RPMI (Life Technologies, Carlsbad, CA) + 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 100 U/mL collagenase type IV (Life Technologies), and 50 μg/mL DNase I (Roche, Basel, Switzerland). Suspension was incubated at 37°C for 45 minutes and then further mechanically dissociated by pipetting. RBCs were removed from samples using red blood cell lysis buffer (BioLegend). Samples were pelleted and then resuspended in fresh RPMI + 10% FBS and strained through a 40-μm filter. Fresh blood samples were centrifuged for 5 minutes at 2,000 rpm and peripheral blood mononuclear cell layer was isolated. Cells were incubated with the Live/Dead Zombie NIR (BioLegend, San Diego, CA) for 5 minutes in the dark at room temperature. Fc receptors were blocked for 10 minutes on ice before surface antibody staining using Human FcR Blocking Reagent (Miltenyi, Bergisch-Gladbach, Germany). Cells were stained for 15 minutes on ice in the dark and washed 2× with phosphate-buffered saline + 2% FBS. Cells were analyzed on a BD LSRFortessa with FACSDiva software (BD Biosciences). Data was analyzed using FlowJo software version 10.8.1. Antibodies were specific for the following human markers: CD3 (UCHT1), CD4 (RPA-T4), CD14 (M5E2), CD15 (W6D3), CD16 (3G8), CD19 (HIB19), CD33 (WM53), CD38 (HIT2), CD45 (HI30), CD45RA (HI100), CD56 (B159), CD69 (FN50), PD-1 (EH12.1), CTLA-4 (L3D10), PD-L1 (29E.2A3), PD-L2 (24F.10C12), TIM-3 (F38-2E2), and CD31 (WM59) from BioLegend; and CCR7 (150503), HLA-DR (G46-6), and CD8 (RPA-T8) from Thermo Fisher Scientific (Waltham, MA).
Urine Cytokine Profiling
Urine samples were processed within 4 hours of collection. The samples were centrifuged at 2,000×g for 20 minutes and the supernatant were stored at –80°C until use. The batched urine samples from C1D1, C2D1, and C3D1 time points for each patient were thawed at 4°C and used immediately for multiplex Luminex analysis according to the manufacturer's protocol (25-plex*, HCYTOMAG-60K, Millipore). Briefly, 25 μL of urine sample was added to the bead mix and incubated for 16 hours at 4°C. After wash, the plate was run on a Luminex 200 analyzer and data were collected using Luminex xPONENT software (Luminex, Austin, TX) and the standard curves were fitted using Belysa (v.1.1.0; MilliporeSigma, Darmstadt, Germany). Cytokine measurements that were below quantitative limits were analyzed as half the detection limit values. Measurements were performed by technical duplicates and average ± standard error of the mean values were displayed. *GRO, IFN-g, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17A/CTLA8, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8/CXCL8, IL-9, IP-10/CXCL10, MCP- 1/CCL2, MCP-3/CCL7, MIG/CXCL9, TNF-a, VEGF-A, sCD40L, IL-1ra, and IL-27.
FIG A1.
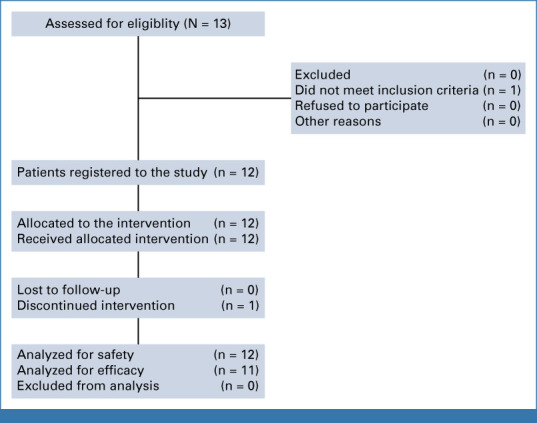
Patient screening and allocation diagram.
FIG A2.
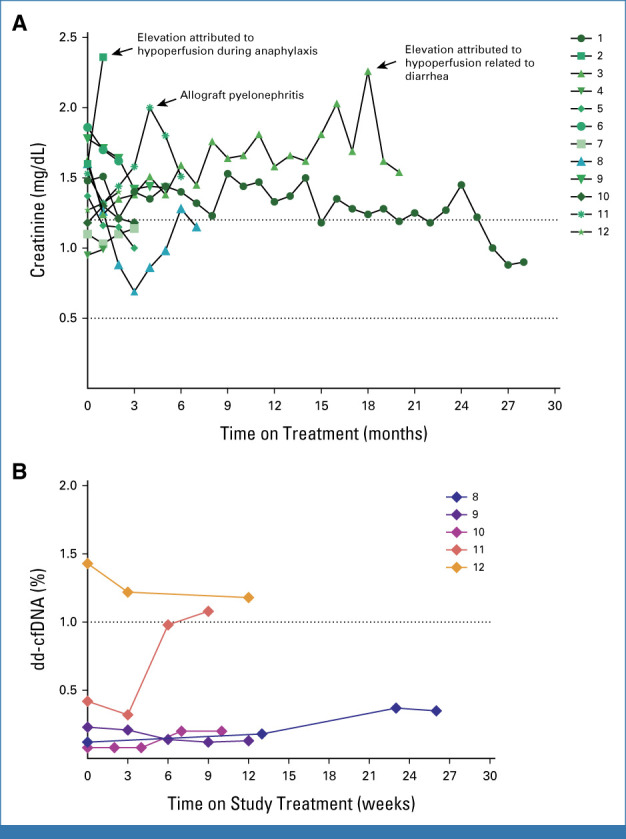
(A) Graphical plot showing serum creatinine (mg/dL) trend over time from baseline and while on treatment at monthly intervals for all study participants (arranged by participant identifier with annotation for significant events). (B) Prospera dd-cfDNA (%) plotted over time from study baseline (in weeks) for participants with available testing (N = 5; arranged by participant identifier). cfDNA, cell-free DNA; dd, donor-derived.
TABLE A1.
Kidney Transplant History Details
| ID | Transplant Type | Transplant IS Regimen Details | Primary Kidney Disease | HLA Mismatches | CMV Status | EBV Status | Last Transplant Date to Immunotherapy Start, Yearsa | Squamous Cell Diagnosis | Treatment Details |
|---|---|---|---|---|---|---|---|---|---|
| 01 | DDKT (first 1979) | MMF, prednisone, mTORi | Familial cystinosis | A0B0DR0 (11 antigen matches) | D–/R+ | D+/R+ | 2.8 | Scalp, head and neck | S + RT, CTX |
| 02 | DDKT (first 1991) | Tacrolimus, prednisone | Hypertension | A1B2DR2 | D–/R+ | D+/R+ | 13.5 | Scalp/face, head and neck | S + RT, CTX |
| 03 | LRKT | Tacrolimus, prednisone | HUS/TTP | A0B0DR0 (two haplotype match) | D?/R+ | D?/R+ | 3.5 | Scalp, face, head and neck, extremities | S, CTX |
| 04 | DDKT | Tacrolimus, prednisone | Chronic glomerulitis | A1B2DR0 | D–/R– | D+/R+ | 7.2 | Scalp, face, head and neck | S + RT |
| 05 | LRKT | MMF, prednisone, belatacept | Hypertension | A1B1DR1 (1 haplotype match) | D+/R+ | D+/R+ | 7.2 | Scalp, face, head and neck | S + RT |
| 06 | LUKT | Tacrolimus, prednisone, mTORi | Chronic glomerulitis | A1B2DR1 | D–/R+ | D–/R+ | 4.6 | Scalp, face, head and neck | S + CRT |
| 07 | LUKT (first 1991) | mTORi, prednisone | IgA nephropathy | A1B1DR2 | NA | NA | 21.1 | Scalp, face, head and neck | S + RT, C |
| 08 | DDKT | Cyclosporin, mTORi, MMF | Polycystic kidney disease | A1B1DR0 | D+/R– | D?/R+ | 20.6 | Scalp, face, head and neck | S |
| 09 | LRKT | Tacrolimus, prednisone, azathioprine | Primary FSGS | A1B1DR1 (one haplotype match) | D+/R– | D+/R+ | 9.6 | Scalp, face, head and neck | S + RT |
| 10 | LUKT | Tacrolimus, MMF | Secondary FSGS | A1B2DR2 | D–/R– | D+/R+ | 3.9 | HPV + oropharynx | RT + CTX, S |
| 11 | LRKT | MMF, prednisone, mTORi | Unknown | A1B2DR1 | D+/R– | D+/R+ | 8.5 | Scalp, face, head and neck | S + RT |
| 12 | LUKT (first 1997) | mTORi, prednisone | IgA nephropathy, Alport syndrome | A2B2DR2 | D–/R– | D+/R+ | 4.6 | HPV + oropharynx, lip/face | S + CRT |
Abbreviations: C, chemotherapy; CMV, cytomegalovirus; CRT, concurrent chemoradiation; CTX, cetuximab; D, donor; DDKT, deceased donor kidney transplant; EBV, Epstein-Barr virus; FSGS, focal segmental glomerulosclerosis; HLA, human leukocyte antigen; HUS/TTP, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome; ID, identifier; IgA, immunoglobulin A; IS, immunosuppression; LRKT, living related kidney transplant; LUKT, living unrelated kidney transplant; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitor; NA, not available; R, recipient; RT, radiotherapy; S, surgery.
Timing from most recent transplant date in the case of patients who underwent two kidney transplants.
Glenn J. Hanna
Employment: Dana-Farber Cancer Institute
Honoraria: Bristol Myers Squibb, Kura Oncology
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Kura Oncology, Sanofi, Prelude Therapeutics, Bicara Therapeutics, Naveris, Exicure, Remix Therapeutics, General Catalyst, Boxer Capital, Rain Therapeutics, KSQ Therapeutics, SIRPant Immunotherapeutics
Research Funding: Bristol Myers Squibb (Inst), Regeneron (Inst), Kartos Therapeutics (Inst), Exicure (Inst), GlaxoSmithKline (Inst), Elevar Therapeutics (Inst), Conquer Cancer, The ASCO Foundation, V Foundation, Gateway for Cancer Research, Genzyme (Inst), Kite/Gilead (Inst), NantKwest (Inst), Actuate Therapeutics (Inst), Bicara Therapeutics (Inst), Secura Bio (Inst)
Expert Testimony: Aaronson Rappaport Feinstein & Deutsch, Ahmuty, Demers, & McManus, Wilson Elser Moskowitz Edelman & Dicker, LLP
Vatche Tchekmedyian
Stock and Other Ownership Interests: Infinity Pharmaceuticals, Hookipa Biotech, Mersana, Aprea Therapeutics, Myovant Sciences, Otonomy, Bluebird Bio, Relmada Therapeutics
Other Relationship: Netflix
Chrysalyne D. Schmults
Employment: Brigham and Women's Hospital
Stock and Other Ownership Interests: Chronicle Medical Software
Consulting or Advisory Role: Castle Biosciences
Research Funding: Castle Biosciences, Regeneron, Merck
Other Relationship: NCCN, SCOUT Health, ISDS Vice President, ASDS Board of Directors
Leonardo V. Riella
Consulting or Advisory Role: Visterra, Apellis Pharmaceuticals, Veloxis, Pirche
Research Funding: Sanofi, AstraZeneca, CareDX, Natera
Patrick Lizotte
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Bicara Therapeutics
Cloud P. Paweletz
Stock and Other Ownership Interests: Xsphera Biosciences
Honoraria: Bio-Rad, Thermo Fisher Scientific
Consulting or Advisory Role: Xsphera Biosciences
Research Funding: Janssen Oncology, Bicara Therapeutics, TargImmune Therapeutics, Takeda, CUE Biopharma, Daiichi Sankyo, Resolution Bioscience, Mirati Therapeutics, Bristol Myers Squibb, Transcenta, Thermo Fisher Scientific, Bicycle Therapeutics, GlaxoSmithKline, Pfizer, Kinnate Biopharma, Ridgeline Discovery, AstraZeneca, Puma Biotechnology
Anil K. Chandraker
Honoraria: Natera
Consulting or Advisory Role: Sanofi
Speakers' Bureau: Allovir
Research Funding: Amgen
Naoka Murakami
Honoraria: Calliditas Therapeutics, Visterra, Akebia Therapeutics, Natera (Inst), CareDX (Inst)
Consulting or Advisory Role: Calliditas Therapeutics, Kyowa Kirin International
Ann W. Silk
Consulting or Advisory Role: Instil Bio, Signatera
Research Funding: Biohaven Pharmaceuticals (Inst), Replimune (Inst), Morphogenesis (Inst), Shattuck Labs (Inst), Checkmate Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 981
PRIOR PRESENTATION
Presented in part at the 2023 ASCO Annual Meeting, Chicago, IL, June 2-6, 2023.
SUPPORT
Supported in part by Regeneron Pharmaceuticals.
CLINICAL TRIAL INFORMATION
N.M. and A.W.S. contributed equally to this work as senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Glenn J. Hanna, Anita Giobbie-Hurder, Leonardo V. Riella, Naoka Murakami, Ann W. Silk
Financial support: Glenn J. Hanna
Administrative support: Glenn J. Hanna, Zixi Liao, Cloud P. Paweletz
Provision of study materials or patients: Glenn J. Hanna, Vatche Tchekmedyian, Emily S. Ruiz, Chrysalyne D. Schmults, Ann W. Silk
Collection and assembly of data: Glenn J. Hanna, Harita Dharanesswaran, John J. Harran, Zixi Liao, Vatche Tchekmedyian, Emily S. Ruiz, Patrick Lizotte, Naoka Murakami, Ann W. Silk
Data analysis and interpretation: Glenn J. Hanna, Harita Dharanesswaran, Anita Giobbie-Hurder, Lori Pai, Vatche Tchekmedyian, Abigail H. Waldman, Chrysalyne D. Schmults, Leonardo V. Riella, Patrick Lizotte, Cloud P. Paweletz, Anil K. Chandraker, Naoka Murakami, Ann W. Silk
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cemiplimab for Kidney Transplant Recipients With Advanced Cutaneous Squamous Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Glenn J. Hanna
Employment: Dana-Farber Cancer Institute
Honoraria: Bristol Myers Squibb, Kura Oncology
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Kura Oncology, Sanofi, Prelude Therapeutics, Bicara Therapeutics, Naveris, Exicure, Remix Therapeutics, General Catalyst, Boxer Capital, Rain Therapeutics, KSQ Therapeutics, SIRPant Immunotherapeutics
Research Funding: Bristol Myers Squibb (Inst), Regeneron (Inst), Kartos Therapeutics (Inst), Exicure (Inst), GlaxoSmithKline (Inst), Elevar Therapeutics (Inst), Conquer Cancer, The ASCO Foundation, V Foundation, Gateway for Cancer Research, Genzyme (Inst), Kite/Gilead (Inst), NantKwest (Inst), Actuate Therapeutics (Inst), Bicara Therapeutics (Inst), Secura Bio (Inst)
Expert Testimony: Aaronson Rappaport Feinstein & Deutsch, Ahmuty, Demers, & McManus, Wilson Elser Moskowitz Edelman & Dicker, LLP
Vatche Tchekmedyian
Stock and Other Ownership Interests: Infinity Pharmaceuticals, Hookipa Biotech, Mersana, Aprea Therapeutics, Myovant Sciences, Otonomy, Bluebird Bio, Relmada Therapeutics
Other Relationship: Netflix
Chrysalyne D. Schmults
Employment: Brigham and Women's Hospital
Stock and Other Ownership Interests: Chronicle Medical Software
Consulting or Advisory Role: Castle Biosciences
Research Funding: Castle Biosciences, Regeneron, Merck
Other Relationship: NCCN, SCOUT Health, ISDS Vice President, ASDS Board of Directors
Leonardo V. Riella
Consulting or Advisory Role: Visterra, Apellis Pharmaceuticals, Veloxis, Pirche
Research Funding: Sanofi, AstraZeneca, CareDX, Natera
Patrick Lizotte
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Bicara Therapeutics
Cloud P. Paweletz
Stock and Other Ownership Interests: Xsphera Biosciences
Honoraria: Bio-Rad, Thermo Fisher Scientific
Consulting or Advisory Role: Xsphera Biosciences
Research Funding: Janssen Oncology, Bicara Therapeutics, TargImmune Therapeutics, Takeda, CUE Biopharma, Daiichi Sankyo, Resolution Bioscience, Mirati Therapeutics, Bristol Myers Squibb, Transcenta, Thermo Fisher Scientific, Bicycle Therapeutics, GlaxoSmithKline, Pfizer, Kinnate Biopharma, Ridgeline Discovery, AstraZeneca, Puma Biotechnology
Anil K. Chandraker
Honoraria: Natera
Consulting or Advisory Role: Sanofi
Speakers' Bureau: Allovir
Research Funding: Amgen
Naoka Murakami
Honoraria: Calliditas Therapeutics, Visterra, Akebia Therapeutics, Natera (Inst), CareDX (Inst)
Consulting or Advisory Role: Calliditas Therapeutics, Kyowa Kirin International
Ann W. Silk
Consulting or Advisory Role: Instil Bio, Signatera
Research Funding: Biohaven Pharmaceuticals (Inst), Replimune (Inst), Morphogenesis (Inst), Shattuck Labs (Inst), Checkmate Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kauvar AN, Arpey CJ, Hruza G, et al. : Consensus for nonmelanoma skin cancer treatment, part II: Squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg 41:1214-1240, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Euvrard S, Kanitakis J, Claudy A: Skin cancers after organ transplantation. N Engl J Med 348:1681-1691, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Wisgerhof HC, Edelbroek JR, de Fijter JW, et al. : Subsequent squamous- and basal-cell carcinomas in kidney-transplant recipients after the first skin cancer: Cumulative incidence and risk factors. Transplantation 89:1231-1238, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Chockalingam R, Downing C, Tyring SK: Cutaneous squamous cell carcinomas in organ transplant recipients. J Clin Med 4:1229-1239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suthanthiran M, Strom TB: Renal transplantation. N Engl J Med 331:365-376, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Athar M, Walsh SB, Kopelovich L, et al. : Pathogenesis of nonmelanoma skin cancers in organ transplant recipients. Arch Biochem Biophys 508:159-163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migden MR, Rischin D, Schmults CD, et al. : PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 379:341-351, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Grob JJ, Gonzalez R, Basset-Seguin N, et al. : Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase II trial (KEYNOTE-629). J Clin Oncol 38:2916-2925, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou Z, Tao T, Li H, et al. : mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci 10:31, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett R, Barta VS, Jhaveri KD: Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med 376:191-192, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Duerr M, Glander P, Diekmann F, et al. : Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 5:703-708, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami N, Mulvaney P, Danesh M, et al. : A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int 100:196-205, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlap GS, DiToro D, Henderson J, et al. : Clonal dynamics of alloreactive T cells in kidney allograft rejection after anti-PD-1 therapy. Nat Commun 14:1549, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Euvrard S, Morelon E, Rostaing L, et al. : Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 367:329-339, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Esfahani K, Al-Aubodah TA, Thebault P, et al. : Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun 10:4712, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halloran PF, Reeve J, Madill-Thomsen KS, et al. : The trifecta study: Comparing plasma levels of donor-derived cell-free DNA with the molecular phenotype of kidney transplant biopsies. J Am Soc Nephrol 33:387-400, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schank KM, Stein JE, Chandra S, et al. : Nivolumab (NIVO) + tacrolimus (TACRO) + prednisone (PRED) +/- ipilimumab (IPI) for kidney transplant recipients (KTR) with advanced cutaneous cancers. J Clin Oncol 40, 2022. (suppl 16; abstr 9507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbour KC, Mezquita L, Long N, et al. : Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36:2872-2878, 2018 [DOI] [PubMed] [Google Scholar]
- 19.García-Sancha N, Corchado-Cobos R, Bellido-Hernández L, et al. : Overcoming resistance to immunotherapy in advanced cutaneous squamous cell carcinoma. Cancers (Basel) 13:5134, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]