Abstract
OBJECTIVES:
Alcohol withdrawal syndrome (AWS) may progress to require high-intensity care. Approaches to identify hospitalized patients with AWS who received higher level of care have not been previously examined. This study aimed to examine the utility of Clinical Institute Withdrawal Assessment Alcohol Revised (CIWA-Ar) for alcohol scale scores and medication doses for alcohol withdrawal management in identifying patients who received high-intensity care.
DESIGN:
A multicenter observational cohort study of hospitalized adults with alcohol withdrawal.
SETTING:
University of Chicago Medical Center and University of Wisconsin Hospital.
PATIENTS:
Inpatient encounters between November 2008 and February 2022 with a CIWA-Ar score greater than 0 and benzodiazepine or barbiturate administered within the first 24 hours. The primary composite outcome was patients who progressed to high-intensity care (intermediate care or ICU).
INTERVENTIONS:
None.
MAIN RESULTS:
Among the 8742 patients included in the study, 37.5% (n = 3280) progressed to high-intensity care. The odds ratio for the composite outcome increased above 1.0 when the CIWA-Ar score was 24. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) at this threshold were 0.12 (95% CI, 0.11–0.13), 0.95 (95% CI, 0.94–0.95), 0.58 (95% CI, 0.54–0.61), and 0.64 (95% CI, 0.63–0.65), respectively. The OR increased above 1.0 at a 24-hour lorazepam milligram equivalent dose cutoff of 15 mg. The sensitivity, specificity, PPV, and NPV at this threshold were 0.16 (95% CI, 0.14–0.17), 0.96 (95% CI, 0.95–0.96), 0.68 (95% CI, 0.65–0.72), and 0.65 (95% CI, 0.64–0.66), respectively.
CONCLUSIONS:
Neither CIWA-Ar scores nor medication dose cutoff points were effective measures for identifying patients with alcohol withdrawal who received high-intensity care. Research studies for examining outcomes in patients who deteriorate with AWS will require better methods for cohort identification.
Keywords: alcohol use disorder, benzodiazepines, inpatients, intensive care units, substance withdrawal syndrome
KEY POINTS
Question: What are the withdrawal score and lorazepam milligram equivalent (LME) cutoff points to identify patients who received high-intensity care for alcohol withdrawal syndrome (AWS)?
Findings: In a multicenter observational cohort study, a Clinical Institute Withdrawal Assessment Alcohol Revised (CIWA-Ar) score of 24 and an LME of 15 mg were highly specific but poorly sensitive for identifying patients who received high-intensity care.
Meaning: Neither the CIWA-Ar score nor LME dosing was optimal for the identification of patients with AWS who received high-intensity care. Alternative approaches are needed for cohort identification in studies aimed at preventing deterioration related to withdrawal.
Alcohol withdrawal syndrome (AWS) can progress to more severe and complicated forms of withdrawal such as delirium and seizure that may require mechanical ventilation and prolonged hospitalization (1–3). Nearly half of the patients with AWS in the ICU had extended ICU stays, and of those that required more than seven days of ICU-level care, 16% died during hospitalization (4, 5). Patients with a history of recent heavy alcohol use along with other acute medical conditions, such as pneumonia, cardiac dysfunction, and alcohol-related liver disease, were also at higher risk for higher degree of AWS (4, 6–9), and ultimately, required higher levels of care in the hospital setting.
Accurate identification of patients with AWS who may progress to require a higher intensity of care would provide opportunities to examine the patients at the highest risk for poor health outcomes (3, 10–13). Using a health services approach to study AWS by examining the requirement of high-intensity care, a clinically relevant endpoint that has not been rigorously studied. Identifying patients with more severe forms of AWS remains problematic because there are no unified definitions (2, 14, 15). Furthermore, current definitions were not derived from data, but rather based on expert consensus (3, 15). The American Thoracic Society (ATS) used greater than or equal to 40 mg of diazepam administered within 1 hour to define severe alcohol withdrawal syndrome (SAWS) (16). The American Society of Addiction Medicine (ASAM) and the ATS defined different severities of AWS using the Clinical Institute Withdrawal Assessment Alcohol Revised (CIWA-Ar) which is a widely used scale to guide the management of AWS with medication dosing (17). The ASAM and ATS cutoff for SAWS were CIWA-Ar scores greater than or equal to 19 and greater than or equal to 15, respectively (1, 16). None of these criteria was validated in the acute care setting.
The use of unvalidated severity scores (CIWA-Ar score ≥ 15), medication thresholds (≥ 40 mg of diazepam in 1 hr), and International Classification of Diseases (ICD) codes for delirium tremens or seizures for identifying worsening AWS may miss many patients. Prior work has shown that claims data were poorly sensitive for identifying alcohol-related conditions (18, 19). We aimed to examine existing expert-determined criteria with data-derived criteria in the evaluation of patients with AWS who required high-intensity care, such as intermediate-level care or ICU-level care. We hypothesized that a data-derived cutoff point in CIWA-Ar score or medication dose would have better sensitivity and specificity for receiving high-intensity care than using the expert-determined cutoff points for SAWS.
MATERIALS AND METHODS
Patient Selection and Case Definition
This was a multicenter retrospective observational cohort study from the University of Chicago Medical Center and the University of Wisconsin Hospital. The patient cohort was selected using a rule-based approach modified from the ATS definition for patients at risk for severe AWS. The following rules were determined by consensus from several of the ATS authors (T.L.S. and M.A.) for the study cohort with AWS: 1) CIWA order, 2) CIWA greater than 0, and 3) any dose of benzodiazepine or phenobarbital administered within the first 24 hours of arrival. Ordering a CIWA alone, as described in the ATS criteria, led to many encounters with a maximum CIWA-Ar of 0, which likely reflected patients who did not have withdrawal symptoms. The inclusion criteria were applied to adult hospitalizations between November 2008 and February 2022. Uncontrolled AWS that progressed in severity and that further complicated other physical ailments likely required more nursing care, frequent monitoring, continuous infusions, and advanced support with invasive mechanical ventilation; therefore, the receipt of high-intensity care represented by intermediate-level care or ICU care for any reason was selected as the primary outcome. Patients may meet the primary outcome for other contributing reasons outside the progression of AWS alone.
CIWA-Ar Scores and Medication Treatment of AWS
The maximum CIWA-Ar score and the total medication dose for alcohol withdrawal were included as independent variables in the analyses. The following medications qualified for the treatment of AWS: 1) lorazepam, 2) midazolam, 3) diazepam, 4) oxazepam, 5) chlordiazepoxide, and 6) phenobarbital. Other benzodiazepines that were not selected comprised less than 0.1% of observations. The doses of all the benzodiazepines and phenobarbital administered over the study period were converted to Lorazepam Milligram Equivalents (LMEs) (Appendix 1, http://links.lww.com/CCX/B322) (20, 21). Doses exceeding the 99th percentile were deemed biologically implausible as shown in Appendix 2 (http://links.lww.com/CCX/B322), so the data were trimmed at this threshold. A univariable logistic regression was performed to examine the total dose of LME for every hour up to 48 hours and its association with the primary outcome to determine the optimal time for analysis (Appendix 3, http://links.lww.com/CCX/B322). The first 24 hours of presentation, defined as the first 24 hours after the first recorded vital sign at the health system, was selected as the time period for analysis to ensure adequate sample size and showed minimal difference in performance by area under the receiver operating curve when compared with other time points over the first 48 hours.
Analysis Plan
Descriptive statistics between those with and without receipt of high-intensity care were examined. Continuous variables were analyzed using the Kruskal-Wallis test and evaluated as median and interquartile ranges (IQRs). Categorical variables were analyzed using the chi-square test. To describe patient comorbidities, elixhauser disease classification categories included all diagnosis codes that were present at admission (22). The Pearson correlation coefficient between CIWA-Ar score and LME was obtained to determine their correlation.
Logistic regression with restricted cubic spline (RCS) was used to model the nonlinear relationship between the maximum CIWA-Ar score and LME with the primary outcome. RCS was selected because it offers flexibility in capturing complex nonlinear patterns, including multiple inflection points and varying slopes (23). This approach accommodated potential nonlinear associations and identified important thresholds at each knot. The Bayesian Information Criterion (BIC) is a statistical method for model selection that was used to assess the goodness-of-fit and the optimal number of knots in the RCS model (24). The optimal knot was chosen based on the lowest BIC value, considering a range between 3 and 10 knots.
To determine the cutoff points for CIWA-Ar scores and LME, the scores and doses at the RCS knots were examined as well as the point where the odds ratio (OR) exceeded 1.0 in univariable logistic regression for the primary outcome. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), along with their 95% CIs were calculated for the cutoff points.
Additional subgroup analyses were performed for each center to explore possible practice differences between sites. The test characteristics obtained from the RCS cutoff points were compared with test characteristics based on the SAWS criteria set by ASAM and ATS, which were CIWA-Ar scores of 19 and 15, respectively (1, 16). The analyses were performed using R, Version 3.6.3 (RStudio Team, Boston, MA). The institutional review board of the University of Wisconsin Madison approved this study under protocol number 2019–1258, titled Predicting In-hospital Clinical Deterioration, which was approved on November 15, 2019. All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975.
RESULTS
Patient and Data Characteristics
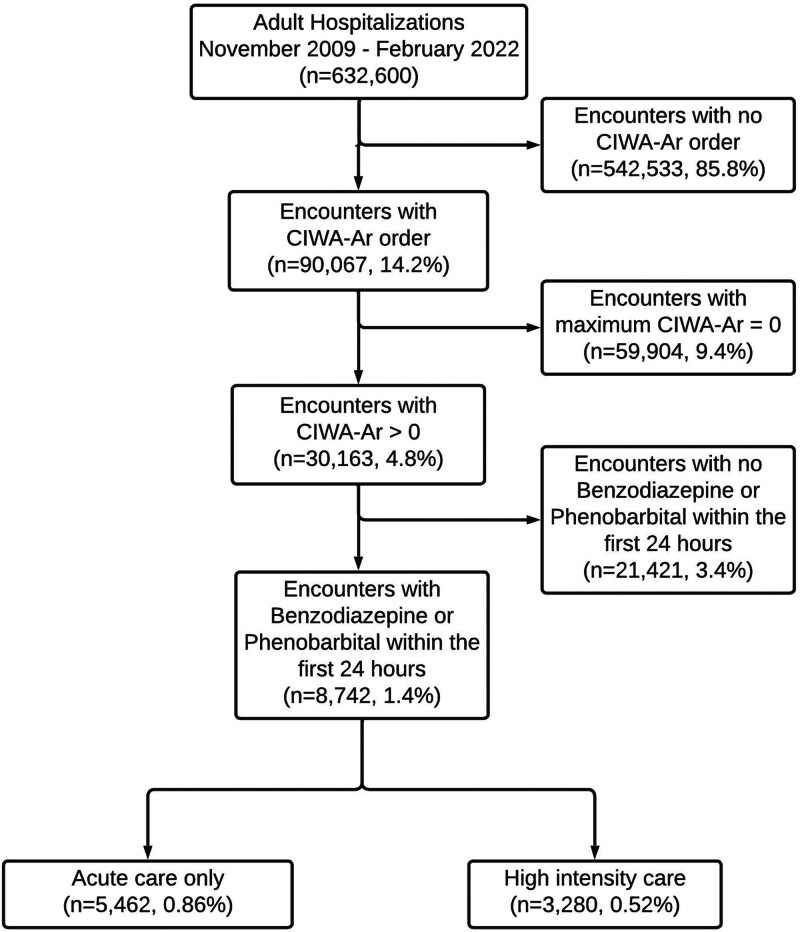
In the multicenter cohort of 632,600 adult hospitalizations, 8,742 (1.4%) encounters met the inclusion criteria for AWS, and 3,280 (0.52%) encounters had the primary outcome of receiving high-intensity care (Fig. 1). The primary outcome consisted of 1452 (0.23%) encounters with intermediate care transfers and 2395 (0.38%) encounters with ICU transfers.
Figure 1.
Patient cohort diagram. Outcomes of patients with Clinical Institute Withdrawal Assessment Alcohol Revised (CIWA-Ar) score greater than 0 and any administration of benzodiazepine or phenobarbital.
Patients receiving high-intensity care were more likely to be older (52 vs. 50 yr, p < 0.001), male sex (73% vs. 63%, p < 0.001), and White race (62% vs. 55%, p < 0.001) (Table 1). The patients at the University of Chicago were predominantly African American (73%) while those at the University of Wisconsin were predominantly White (91%) (Appendix 4, http://links.lww.com/CCX/B322). Patients with the outcome had a higher proportion of comorbidities compared with those without. Notably, patients receiving high-intensity care had a higher proportion of liver disease, coagulopathy, and electrolyte derangements (p < 0.001). ICD codes for alcohol use disorder (AUD) were identified in 77% of encounters in our study cohort (Table 1). At the University of Chicago, only 61% of the study cohort had ICD codes of AUD (Appendix 4, http://links.lww.com/CCX/B322). Of the 3280 admissions that met the outcome criteria, only 113 (3.4%) had ICD codes for complicated alcohol withdrawal, such as delirium tremens and withdrawal seizures. Additionally, the incidence of adjunctive infusion, such as propofol, ketamine, and dexmedetomidine, and mechanical ventilation among patients who met the outcome criteria were 53% (n = 1740) and 35% (n = 1132), respectively.
TABLE 1.
Cohort Patient Characteristics
| Characteristics | Total (n = 8742) | Acute Care (n = 5462) | High-Intensity Care (n = 3280) | p |
|---|---|---|---|---|
| Age, median (IQR) | 51 (40, 59) | 50 (39, 59) | 52 (42, 60) | < 0.001 |
| Male, n (%) | 5854 (67.0) | 3454 (63.2) | 2400 (73.2) | < 0.001 |
| Race, n (%) | < 0.001a | |||
| American Indian or Alaska Native | 73 (0.8) | 47 (0.9) | 26 (0.8) | |
| Asian/Mideast Indian | 54 (0.6) | 39 (0.7) | 15 (0.5) | |
| Black/African American | 3296 (37.7) | 2227 (40.8) | 1069 (32.6) | |
| Pacific Islander/Hawaiian Native | 18 (0.2) | 11 (0.2) | 7 (0.2) | |
| White/Caucasian | 5021 (57.4) | 2977 (54.5) | 2044 (62.3) | |
| Other | 280 (3.2) | 161 (3.0) | 119 (3.7) | |
| Ethnicity, n (%) | < 0.001a | |||
| Declined/unknown | 124 (1.4) | 56 (1.0) | 68 (2.1) | |
| Hispanic/Latino | 343 (3.9) | 207 (3.8) | 136 (4.1) | |
| Non-Hispanic/Latino | 8475 (94.7) | 5199 (95.2) | 3076 (93.8) | |
| Blood alcohol content > 0 | 2896 (50.6) | 1866 (52.0) | 1030 (48.3) | 0.008 |
| Maximum Clinical Institute Withdrawal Assessment Alcohol Revised score in the first 24 hr of admission, median (IQR) | 11 (5, 16) | 10 (5, 15) | 12 (6, 18) | < 0.001 |
| Total LME in the first 24 hr of admission, median (IQR) | 2.5 (1, 6) | 2 (1, 5) | 3 (1, 8) | < 0.001 |
| Total LME in the entire encounter, median (IQR) | 5 (2, 15) | 4 (2, 10) | 8 (3, 32.4) | < 0.001 |
| Elixhauser comorbidities, n (%) | ||||
| Alcohol use disorder | 6747 (77.2) | 4199 (76.9) | 2548 (77.7) | 0.399 |
| Fluid and electrolyte disorders | 5183 (59.3) | 3059 (56.0) | 2124 (64.8) | < 0.001 |
| Hypertension | 4423 (50.6) | 2737 (50.1) | 1686 (51.4) | 0.251 |
| Depression | 3323 (38.0) | 2286 (41.9) | 1037 (31.6) | < 0.001 |
| Arrhythmia | 3297 (37.7) | 1900 (34.8) | 1397 (42.6) | < 0.001 |
| Liver disease | 2874 (32.9) | 1704 (31.2) | 1170 (35.7) | < 0.001 |
| Drug use disorder | 2529 (28.9) | 1699 (31.1) | 830 (25.3) | < 0.001 |
| Chronic pulmonary disease | 2518 (28.8) | 1531 (28.0) | 987 (30.1) | 0.042 |
| Neurologic disorders | 2418 (27.7) | 1376 (25.2) | 1042 (31.8) | < 0.001 |
| Coagulopathy | 1952 (22.3) | 992 (18.1) | 960 (29.3) | < 0.001 |
IQR = interquartile range, LME = lorazepam milligram equivalent.
p values assessed the significance of the differences across all groups.
Patients with the outcome received higher doses of LME (p < 0.001) and had higher CIWA-Ar scores (p < 0.001) than patients without the outcome. The median cumulative doses of LME in the first 24 hours for the cases and noncases were 3 mg (IQR 1–8) and 2 mg (IQR 1–5), respectively. The median cumulative doses of LME in the entire encounter for cases and noncases were 8 mg (IQR 3–32.4) and 4 mg (IQR 2–10), respectively.
The median of the maximum CIWA-Ar score in the first 24 hours for the cases and noncases were 12 (IQR 6–18) and 10 (IQR 5–15), respectively. The Pearson correlation coefficient between CIWA-Ar score and LME was 0.48 (p < 0.001). The median time from presentation to the first dose of withdrawal medication for each site was 3 hours (IQR 2–10) and 7 hours (IQR 3–14), respectively. The 99th percentile LME given within the first 24 hours for each site was 40 mg and 80 mg, respectively (Appendix 2, http://links.lww.com/CCX/B322).
Cutoff point Identification
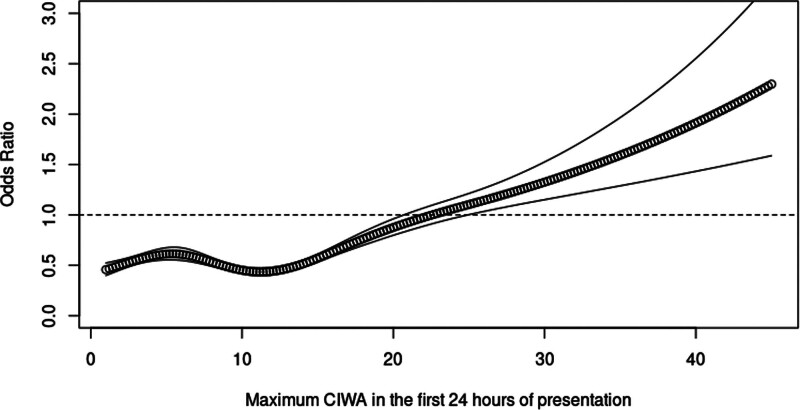
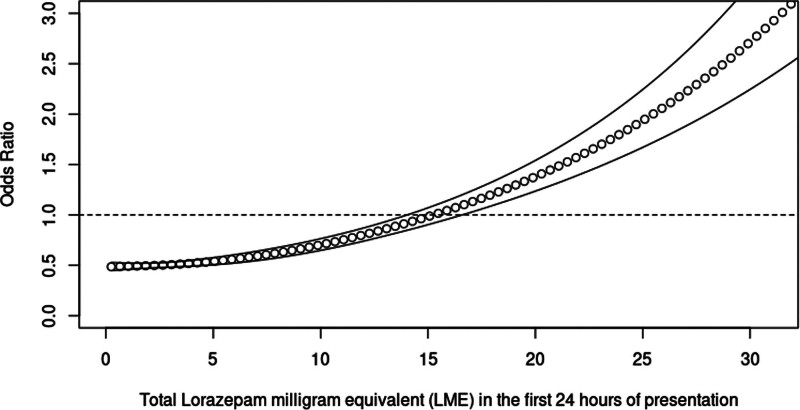
In the RCS regression, five knots and three knots were determined to have the best model fit for CIWA-Ar and LME models, respectively. The logistic regression model with RCS demonstrated a dose-dependent response with increasing scores of CIWA-Ar and LME for the primary outcome with ORs going above 1 at CIWA-Ar score of 24 and LME of 15 mg, respectively (Figs. 2 and 3). The corresponding cutoff points for each knot and their test characteristics are detailed in Table 2.
Figure 2.
Restricted cubic spline regression of the odds ratio of maximum Clinical Institute Withdrawal Assessment Alcohol Revised (CIWA-Ar) scores in the first 24 hours of admission and severe alcohol withdrawal syndrome.
Figure 3.
Restricted cubic spline regression of the odds ratio of the total lorazepam milligram equivalent (LME) in the first 24 hours of admission and severe alcohol withdrawal syndrome.
TABLE 2.
Test Characteristics of Clinical Institute Withdrawal Assessment Alcohol Revised and Lorazepam Milligram Equivalent Restricted Cubic Spline Knots and Odds Ratio Equal to 1
| score/dose | Knot/Criteria | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|
| Clinical Institute Withdrawal Assessment-Ar Scores | |||||
| 6 | Second knot | 0.76 (0.74, 0.77) | 0.27 (0.26, 0.28) | 0.38 (0.37, 0.40) | 0.65 (0.63, 0.67) |
| 11 | Third knot | 0.56 (0.55, 0.58) | 0.50 (0.49, 0.52) | 0.40 (0.39, 0.42) | 0.66 (0.64, 0.67) |
| 15 | American Thoracic Society | 0.39 (0.37, 0.41) | 0.73 (0.71, 0.74) | 0.46 (0.44, 0.48) | 0.66 (0.65, 0.68) |
| 16 | Fourth knot | 0.35 (0.33, 0.37) | 0.77 (0.75, 0.78) | 0.47 (0.45, 0.49) | 0.66 (0.65, 0.67) |
| 19 | American Society of Addiction Medicine | 0.24 (0.23, 0.26) | 0.86 (0.85, 0.87) | 0.51 (0.49, 0.54) | 0.65 (0.64, 0.67) |
| 24 | OR = 1 | 0.12 (0.11, 0.13) | 0.95 (0.94, 0.95) | 0.58 (0.54, 0.61) | 0.64 (0.63, 0.65) |
| 26 | Fifth knot | 0.09 (0.08, 0.10) | 0.96 (0.96, 0.97) | 0.60 (0.55, 0.64) | 0.64 (0.63, 0.65) |
| Lorazepam milligram equivalent | |||||
| 2.5 mg | Second knot | 0.58 (0.56, 0.60) | 0.53 (0.51, 0.54) | 0.42 (0.41, 0.44) | 0.68 (0.66, 0.69) |
| 13 mg | Third knot | 0.18 (0.17, 0.19) | 0.94 (0.94, 0.95) | 0.65 (0.62, 0.68) | 0.65 (0.65, 0.67) |
| 15 mg | OR = 1 | 0.16 (0.14, 0.17) | 0.96 (0.95, 0.96) | 0.68 (0.65, 0.72) | 0.65 (0.64, 0.66) |
OR = odds ratio.
For CIWA-Ar RCS, at an OR of 1 and a cutoff point of 24, the sensitivity, specificity, PPV, and NPV were 0.12 (95% CI, 0.11–0.13), 0.95 (95% CI, 0.94–0.95), 0.58 (95% CI, 0.54–0.61), and 0.64 (95% CI, 0.63–0.65), respectively. For LME RCS, at an OR of 1 at 15 mg LME, the sensitivity, specificity, PPV, and NPV were 0.16 (95% CI, 0.14–0.17), 0.96 (95% CI, 0.95–0.96), 0.68 (95% CI, 0.65–0.72), and 0.65 (95% CI, 0.65–0.66), respectively. An analysis combining CIWA-Ar score and LME models did not provide additional performance gain in test characteristics over CIWA-Ar alone.
In the subgroup analysis, the RCS regression plot of CIWA-Ar and LME at each center was similar to the multicenter results (Appendices 5 and 6, http://links.lww.com/CCX/B322). The OR for the composite outcome increased above 1.0 for each center when the CIWA-Ar score reached 28 and 24, respectively. The OR for the composite outcome increased above 1.0 at each center when the 24-hour LME dose reached 15 mg and 16 mg, respectively. The test characteristics are listed in Table 3.
TABLE 3.
Test Characteristics of Clinical Institute Withdrawal Assessment Alcohol Revised and Lorazepam milligram equivalent Restricted Cubic Spline Knots, Odds Ratio Equal to 1, American Thoracic Society Clinical Institute Withdrawal Assessment-Ar Cutoff Points, and American Society of Addiction Medicine Clinical Institute Withdrawal Assessment-Ar Cutoff Points at Each Center
| score/dose | Knot/Criteria | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|
| Clinical Institute Withdrawal Assessment-Ar Scores | |||||
| University of Chicago | |||||
| 8 | Third knot | 0.55 (0.52, 0.57) | 0.47 (0.46, 0.49) | 0.33 (0.31, 0.35) | 0.69 (0.67, 0.71) |
| 12 | Fourth knot | 0.42 (0.40, 0.45) | 0.72 (0.70, 0.74) | 0.38 (0.35, 0.40) | 0.76 (0.74, 0.78) |
| 15 | ATS | 0.26 (0.24, 0.28) | 0.84 (0.83, 0.86) | 0.43 (0.40, 0.47) | 0.71 (0.69, 0.72) |
| 19 | ASAM | 0.16 (0.14, 0.18) | 0.91 (0.90, 0.92) | 0.46 (0.42, 0.51) | 0.70 (0.68, 0.71) |
| 23 | Fifth knot | 0.10 (0.08,0.11) | 0.96 (0.96, 0.97) | 0.55 (0.49, 0.62) | 0.70 (0.68, 0.71) |
| 28 | OR = 1 | 0.05 (0.04, 0.06) | 0.98 (0.98, 0.99) | 0.59 (0.49, 0.68) | 0.69 (0.67. 0.70) |
| University of Wisconsin | |||||
| 13 | Second knot | 0.57 (0.55, 0.59) | 0.47 (0.45, 0.49) | 0.44 (0.43, 0.46) | 0.59 (0.57, 0.62) |
| 15 | ATS | 0.47 (0.45, 0.50) | 0.60 (0.58, 0.62) | 0.47 (0.45, 0.49) | 0.61 (0.59, 0.62) |
| 19 | ASAM | 0.30 (0.28, 0.32) | 0.81 (0.79, 0.82) | 0.53 (0.50, 0.56) | 0.61 (0.59, 0.62) |
| 24 | Third knot, OR = 1 | 0.14 (0.12, 0.15) | 0.93 (0.92, 0.94) | 0.59 (0.54, 0.63) | 0.59 (0.58, 0.61) |
| Lorazepam milligram equivalent | |||||
| University of Chicago | |||||
| 9 mg | Third knot | 0.17 (0.15, 0.20) | 0.93 (0.92. 0.94) | 0.59 (0.54, 0.63) | 0.67 (0.66, 0.69) |
| 15 mg | OR = 1 | 0.11 (0.09, 0.12) | 0.98 (0.97, 0.98) | 0.68 (0.61, 0.74) | 0.70 (0.69, 0.74) |
| University of Wisconsin | |||||
| 4 mg | Second knot | 0.53 (0.51, 0.55) | 0.54 (0.52, 0.56) | 0.46 (0.44, 0.48) | 0.61 (0.59, 0.63) |
| 16 mg | OR = 1 | 0.18 (0.17, 0.20) | 0.94 (0.93, 0.95) | 0.71 (0.67, 0.75) | 0.61 (0.59, 0.62) |
| 17 mg | Third knot | 0.17 (0.16, 0.19) | 0.95 (0.94, 0.96) | 0.74 (0.69, 0.78) | 0.61 (0.59, 0.62) |
ASAM = American Society of Addiction Medicine, ATS = American Thoracic Society, OR = odds ratio.
For CIWA scores, the ASAM and ATS criteria for SAWS had specificities above 0.60 but with sensitivities below 0.47 in the multicenter and single-center analyses. When compared with the test characteristics of the ASAM and ATS criteria, the data-derived test characteristics were similar when the OR was above 1.0 for receiving high-intensity care.
DISCUSSION
This study highlights the challenges and limitations of the current AWS criteria using alcohol scales and medication doses to identify patients with AWS who progress to high-intensity care. From a health services perspective, predicting the need for high-intensity care is important. However, previous studies using expert-determined medication dosing and CIWA-Ar scores to define SAWS do not perform well in identifying individuals who progress to high-intensity care (1, 16, 25). In a data-driven manner, our results showed that medication doses and CIWA-Ar scores have high specificity but very poor sensitivity when used for predicting higher-intensity care. CIWA-Ar scores and medication doses are not reliable measures for identifying patients who progress to high-intensity care and are likely to lead to many missed cases (e.g., false negatives) in cohort identification for clinical studies.
In the literature, there is no consensus on the formal definition of severe forms of AWS (2, 14, 15). Most studies used 8 mg of LME, in an hour as the cutoff for SAWS because patients may require a second agent such as phenobarbital or propofol for continuous sedation, an indication for high-intensity care, to manage AWS (11, 16). We also found patients who progressed to high-intensity care received a median of 8 mg LME during their hospital stay. Another study used 10 mg of lorazepam as a cutoff before starting a second agent (26). These patients required escalation of care to the ICU for invasive mechanical ventilation. In the study by Gold et al (11), patients who required invasive mechanical ventilation for AWS received an average of 50 mg LME in 24 hours. However, our study showed a lower threshold of 15 mg LME in the first 24 hours of presentation, but we had a broader catchment than only patients receiving mechanical ventilation and included other patients admitted to the ICU or intermediate care units.
ASAM and ATS guidelines recommend using CIWA-Ar scores of 19 and 15, respectively, both of which were derived by an expert panel for symptom-based treatment (1, 16). ASAM uses AWS resistant to benzodiazepine as the definition of SAWS to derive the CIWA-Ar score cutoff. ASAM notes that the CIWA-Ar score cutoff is a suggestion that varies between institutions and should be clinically determined by the clinician. ATS derived their cutoff based on a prospective study that found that AWS patients had CIWA-Ar scores of 10–15 when they developed delirium tremens (27). In our study, there was a poor correlation between CIWA-Ar scores and LME seen in our study. This could be explained by the fact that patients with more severe AWS may require higher doses of benzodiazepines, leading to sedation and an artificially low CIWA-Ar score despite physiologic manifestations of severe withdrawal.
Although ASAM and ATS SAWS criteria were not specifically intended to identify patients who progress to high-intensity care, we used a health services approach in this study by examining the criteria as cutoff points for the primary outcome. We found the criteria set forth by ASAM and ATS were highly specific but poorly sensitive for the identification of patients who progressed to high-intensity care and thus missed many patients. The lack of a CIWA-Ar score and LME cutoff point with useful test characteristics suggests that both criteria are not reliable for identifying patients with AWS who require high-intensity care for the purpose of cohort identification in clinical studies.
ICD codes for delirium tremens or seizures are another data element used for complex AWS which may require high-intensity care for sedation and mechanical ventilation. However, previous studies have shown that using ICD codes has poor sensitivity (range, 2–35%) to identify AUD (18, 19). Although AWS and AUD are different diagnoses, we expect that most of the patients experiencing AWS would carry a diagnosis of AUD. At the University of Chicago, only about half of the cohort, which comprised of patients actively being treated with AWS, had ICD codes for AUD possibly because the withdrawal was not prioritized and other physical ailments were the primary focus of admission. In our cohort, only about 3% of the patients who met the outcome criteria had ICD codes for complex withdrawal consistent with previous studies that noted poor sensitivity.
Using the transfer to ICU as a proxy for clinical deterioration has been employed in other clinical studies for hospitalized adults (28–31). Our study outcome included intermediate-level care and ICU-level care for any medical reason, including patients who may be decompensating from acute medical conditions other than AWS. We used high-intensity care in AWS patients as the primary outcome because it likely represents the complex manifestations of withdrawal alone or in combination with other physical ailments. Solely including patients who are admitted to the ICU for alcohol withdrawal as their primary diagnosis would have a small sample size and biased results that are not generalizable for most complex ICU patients. Two studies that investigated patients in the ICU whose sole indication for ICU admission was management of AWS only recruited around 50 subjects due to strict exclusion criteria (12, 13). Despite broadly using receipt of high-intensity care as the outcome, our results showed a dose-dependent response, both within the first 24 hours of presentation and throughout the whole encounter, that suggests a higher severity of alcohol withdrawal correlated with high-intensity care. This is consistent with previous literature where patients with AWS are at an increased risk of complications and prolonged ICU stays (4). In the study cohort, patients who received high-intensity care had a higher frequency of comorbidities, consistent with previous literature suggesting that acute medical illness may precipitate and increase the severity of withdrawal (5–10). The cohort who received high-intensity care had a higher frequency of liver disease, coagulopathy, and electrolyte derangements, which provides good face validity.
The multicenter design of the study afforded a more diverse representation of race and ethnicity, with one center predominantly non-Hispanic Black adults. There were also practice variations across centers. One of the centers had more than double the time for median time to the first dose of benzodiazepine. The 99th percentile LME was also double. Furthermore, each center likely has a different threshold for escalation of care. Despite these significant differences, the CIWA-Ar score and LME at which patients were at increased risk for high-intensity care were similar which supports the generalizability of this study’s findings.
There are several limitations in this study. First, the inclusion criteria of a CIWA-Ar score greater than 0 and receipt of benzodiazepine or phenobarbital allows for the possibility of misclassification bias. Although the intention is to capture patients being actively treated for alcohol withdrawal, it is possible that some patients may have received benzodiazepines or phenobarbital for other conditions, such as anxiety or nonalcohol-related seizures. Next, in this study, we examined high-intensity care as a manifestation of SAWS. Although we compared our spline-derived cutoffs to the ATS and ASAM criteria derived for SAWS, it is important to note that the ATS and ASAM criteria were not intended to identify patients who require high-intensity care. It is also important to note that our cohort may be diluted with patients who require escalation of care for medical conditions not associated with AWS. Our results showed that the first 24-hour LME and total LME were significantly higher in patients who met the primary outcome compared with those who did not. This suggests that the cohort comprised patients with higher severity of AWS. Finally, CIWA-Ar scores may be confounded by symptoms or physiologic changes seen in acute medical conditions in hospitalized patients that may mimic alcohol withdrawal and may lead to overestimation of CIWA-Ar scores and higher doses of benzodiazepine.
CONCLUSIONS
Our study showed that neither expert-determined nor data-driven approaches with CIWA-Ar scores and medication doses are reliable measures for identifying patients with AWS who progress to high-intensity care. Further research is needed for better methods in the prediction of patients who progress to high-intensity care.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Steel received funding from the National Institute on Alcohol Abuse and Alcoholism K23AA030588. Dr. Mayampurath received funding from the National Heart, Lung, and Blood Institute (NHLBI) K01HL148390. Dr. Matthew Churpek received funding from NHLBI R01HL157262. Dr. Afshar received funding from the National Institute on Drug Abuse R01DA051464. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Alvanzo A, Kleinschmidt K, Kmiec JA, et al. : The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med 2020; 14:1–72 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen TA, Lam SW: Phenobarbital and symptom-triggered lorazepam versus lorazepam alone for severe alcohol withdrawal in the intensive care unit. Alcohol 2020; 82:23–27 [DOI] [PubMed] [Google Scholar]
- 3.Nisavic M, Nejad SH, Isenberg BM, et al. : Use of phenobarbital in alcohol withdrawal management—a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics 2019; 60:458–467 [DOI] [PubMed] [Google Scholar]
- 4.Vigouroux A, Garret C, Lascarrou J-B, et al. : Alcohol withdrawal syndrome in ICU patients: Clinical features, management, and outcome predictors. PLoS One 2021; 16:e0261443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awissi D-K, Lebrun G, Fagnan M, et al. ; Regroupement de Soins Critiques, Réseau de Soins Respiratoires, Québec: Alcohol, nicotine, and iatrogenic withdrawals in the ICU. Crit Care Med 2013; 41:S57–S68 [DOI] [PubMed] [Google Scholar]
- 6.Moss M, Burnham EL: Alcohol abuse in the critically ill patient. Lancet 2006; 368:2231–2242 [DOI] [PubMed] [Google Scholar]
- 7.O’Brien JM, Jr, Lu B, Ali NA, et al. : Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med 2007; 35:345–350 [DOI] [PubMed] [Google Scholar]
- 8.Schuckit MA, Tipp JE, Reich T, et al. : The histories of withdrawal convulsions and delirium tremens in 1648 alcohol dependent subjects. Addiction 1995; 90:1335–1347 [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Jang MK, Lee JY, et al. : Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol 2005; 20:1833–1837 [DOI] [PubMed] [Google Scholar]
- 10.Mirijello A, D’Angelo C, Ferrulli A, et al. : Identification and management of alcohol withdrawal syndrome. Drugs 2015; 75:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold JA, Rimal B, Nolan A, et al. : A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med 2007; 35:724–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spies CD, Otter HE, Hüske B, et al. : Alcohol withdrawal severity is decreased by symptom-orientated adjusted bolus therapy in the ICU. Intensive Care Med 2003; 29:2230–2238 [DOI] [PubMed] [Google Scholar]
- 13.Yanta J, Swartzentruber G, Pizon A: Alcohol withdrawal syndrome: improving outcomes in the emergency department with aggressive management strategies. Emerg Med Pract 2021; 23:1–41 [PubMed] [Google Scholar]
- 14.Williams D, Lewis J, McBride A: A comparison of rating scales for the alcohol-withdrawal syndrome. Alcohol Alcohol 2001; 36:104–108 [DOI] [PubMed] [Google Scholar]
- 15.Maldonado JR, Sher Y, Ashouri JF, et al. : The “Prediction of Alcohol Withdrawal Severity Scale” (PAWSS): Systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol 2014; 48:375–390 [DOI] [PubMed] [Google Scholar]
- 16.Steel TL, Afshar M, Edwards S, et al. : Research needs for inpatient management of severe alcohol withdrawal syndrome: An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2021; 204:e61–e87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pribék IK, Kovács I, Kádár BK, et al. : Evaluation of the course and treatment of alcohol withdrawal syndrome with the Clinical Institute Withdrawal Assessment for alcohol—revised: A systematic review-based meta-analysis. Drug Alcohol Depend 2021; 220:108536. [DOI] [PubMed] [Google Scholar]
- 18.Boscarino JA, Moorman AC, Rupp LB, et al. ; Chronic Hepatitis Cohort Study (CheCS) Investigators: Comparison of ICD-9 codes for depression and alcohol misuse to survey instruments suggests these codes should be used with caution. Dig Dis Sci 2017; 62:2704–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel AM, Lukasiewicz AM, Webb ML, et al. : ICD-9 diagnosis codes have poor sensitivity for identification of preexisting comorbidities in traumatic fracture patients: A study of the National Trauma Data Bank. J Trauma Acute Care Surg 2015; 79:622–630 [DOI] [PubMed] [Google Scholar]
- 20.Smith DE: Benzodiazepine dependence potential: Current studies and trends. J Subst Abuse Treat 1984; 1:163–167 [DOI] [PubMed] [Google Scholar]
- 21.Guina J, Merrill B: Benzodiazepines II: Waking up on sedatives: Providing optimal care when inheriting benzodiazepine prescriptions in transfer patients. J Clin Med Res 2018; 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharabiani MTA, Aylin P, Bottle A: Systematic review of comorbidity indices for administrative data. Med Care 2012; 50:1109–1118 [DOI] [PubMed] [Google Scholar]
- 23.Croxford R: Restricted cubic spline regression: A brief introduction. Available at: https://support.sas.com/resources/papers/proceedings16/5621-2016.pdf. Accessed June 15, 2023
- 24.Chakrabarti A, Ghosh J: AIC, BIC and recent advances in model selection. In: Handbook of the Philosophy of Science. Volume 7: Philosophy of Statistics. Bandyopadhyay PS, Gabbay DM, Forster MR, et al. (Eds): North Holland, 2011, pp 602–624 [Google Scholar]
- 25.Steel TL, Bradley KA: Improving care for inpatient alcohol withdrawal syndrome: Addressing the lack of rigorous research on a common condition. Ann Am Thorac Soc 2021; 18:1622–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hack JB, Hoffmann RS, Nelson LS: Resistant alcohol withdrawal: Does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol 2006; 2:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burapakajornpong N, Maneeton B, Srisurapanont M: Pattern and risk factors of alcohol withdrawal delirium. J Med Assoc Thai 2011; 94:991–997 [PubMed] [Google Scholar]
- 28.Campbell V, Conway R, Carey K, et al. : Predicting clinical deterioration with Q-ADDS compared to NEWS, between the flags, and eCART track and trigger tools. Resuscitation 2020; 153:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartkowiak B, Snyder AM, Benjamin A, et al. : Validating the Electronic Cardiac Arrest Risk Triage (eCART) score for risk stratification of surgical inpatients in the postoperative setting: Retrospective cohort study. Ann Surg 2019; 269:1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnolds DE, Carey KA, Braginsky L, et al. : Comparison of early warning scores for predicting clinical deterioration and infection in obstetric patients. BMC Pregnancy Childbirth 2022; 22:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kipnis P, Turk BJ, Wulf DA, et al. : Development and validation of an electronic medical record-based alert score for detection of inpatient deterioration outside the ICU. J Biomed Inform 2016; 64:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]