Abstract
While progress has been made in the effort to eradicate malaria, the disease remains a significant threat to global health. Acquired resistance to frontline treatments is emerging in Africa, urging a need for the development of novel antimalarial agents. Repurposing human kinase inhibitors provides a potential expedited route given the availability of a diverse array of kinase-targeting drugs that are approved or in clinical trials. Phenotypic screening of a library of type II human kinase inhibitors identified compound 1 as a lead antimalarial, which was initially developed to target human ephrin type A receptor 2 (EphA2). Here, we report a structure–activity relationship study and lead optimization of compound 1, which led to compound 33, with improved antimalarial activity and selectivity.
Graphical Abstract

INTRODUCTION
Despite significant progress in reducing the global impact of malaria, the mosquito-borne illness caused by parasites of the Plasmodium genus remains a threat in many regions, evidenced by an estimated 247 million cases in 2021.1 One of the most pressing concerns is the emergence of resistance to the frontline treatment, artemisinin-based combination therapy (ACT), in Africa, the region with the most severe burden of malaria.2 New antimalarial drugs, ideally directed against novel targets, are needed. A valuable source of novel antimalarials is to repurpose existing compounds originally developed for other therapeutic applications.3 Protein kinase inhibitors are used extensively for the treatment of cancer and are often designed to target mutationally activated kinases.4 In addition to oncologic applications, protein kinase inhibitors are utilized in the treatment of a range of other conditions, including ocular hypertension and autoimmune disorders like rheumatoid arthritis and ulcerative colitis.4,5 As such, protein kinase inhibitors represent a subset of such compounds that are actively being explored for repurposing as antimalarials.6 Protein kinases facilitate myriad parasite functions at every stage of its complex life cycle, and the ability to inhibit multiple stages of the plasmodial life cycle is a key criterion for a lead antimalarial candidate.7 Additionally, there is significant divergence between the human and Plasmodium kinomes, allowing for selectivity.8 Finally, a tremendous amount of research has been conducted in the pursuit of human kinase inhibitors, which can be leveraged to expedite the development of new antiplasmodial inhibitors.9-11
Most kinase inhibitors are categorized as type I; they act by forming hydrogen bonds to residues in the hinge region of the ATP-binding site, thereby displacing ATP.12 Type II kinase inhibitors, on the other hand, target the inactive conformation, exploiting a hydrophobic pocket near the ATP binding site generated by a conformational rearrangement of the activation loop. Type II inhibitors, although possessing a hinge-binding moiety, also contain a hydrophobic tail component, which usually binds to the allosteric pocket beyond the DFG moiety.13 The additional interaction with the allosteric pocket influences kinase selectivity and can prolong compound residence time in the binding site, which may augment in vivo efficacy.14,15 The type II inhibitors bafetinib, imatinib, and nilotinib are reported to have moderate antimalarial activity, with ring stage EC50s of 1.3, 3.0, and <1 μM, respectively; however, the plasmodial protein target remains unknown.16 In addition, recently, a new type II pharmacophore was identified as an antiplasmodial agent acting beyond its inhibition of designated kinase P. falciparum PK6, although it remains to be seen if the classical type II pharmacophore translated to type II inhibition in Plasmodium.17 Type II kinase inhibitors are an attractive starting point for repurposing for antimalarial activity, given the range of chemical space yet to be explored and the pre-existence of large collections of compounds generated during efforts to target human kinases. In this study, we describe the identification of a type II kinase inhibitor scaffold, exemplified by compound 1, that displays potent dual-stage antiplasmodial activity in vitro and exhibits efficacy in a mouse malaria model. Our structure–activity analysis revealed an essential role of a 3-position substitution on the central ring in preserving antiplasmodial activity and characterizes the influence of other pharmacophore components on both potency and selectivity.
RESULTS AND DISCUSSION
Identification of Compound 1 as a Lead Antimalarial Agent.
To identify type II scaffolds with the potential to be repurposed as antimalarials, we first conducted a broad phenotypic screen of a type II kinase inhibitor library against the multidrug-resistant Dd2 strain of P. falciparum, the most lethal Plasmodium species. Asynchronous P. falciparum Dd2 blood stage cultures were treated with a 1 μM concentration of compound for 72 h, and growth was assessed by a SYBR Green I-based proliferation assay.18 Of the library’s 553 compounds, 58 inhibited parasite growth by at least 90% at 1 μM (Figure S1). When the EC50s of these compounds were obtained, we identified many compounds with a submicromolar EC50. Among them, thiophenylpyridine compound 1 displayed an EC50 of 80 ± 8 nM in asynchronous cultures. Compound 1 was originally created as part of a series designed to target human EphA2, a receptor tyrosine kinase, in the type II manner.19 The library included compounds that follow a common pharmacophore: a heterocyclic head, a central ring flanked by linker regions, and a tail that occupies the hydrophobic pocket present in the inactive conformation of EphA2. This potent inhibition of a human kinase raised the concern that, despite promising antiplasmodial action, the utility of compound 1 would be limited because of poor selectivity.
To investigate this possibility, we compared the antiplasmodial activities of four available analogs of compound 1, which differ only in central ring substitutions in the 2- and 3-positions, for their inhibitory effect on human EphA2, as determined by the SelectScreen Kinase Profiling Service (Invitrogen), an in vitro FRET-based assay. As illustrated in Table 1, substitutions at the 2- and 3-positions in the central ring led to a separation of human EphA2 activity from antiplasmodial activity. Compared to compound 5, which displays potent EphA2 inhibition (IC50 = 11 nM) and poor antiplasmodial activity (Dd2 EC50 = 3.9 μM), compound 1 exhibits an almost 900-fold reduction in human EphA2 activity and over 48-fold increase in antiplasmodial activity. To further assess the human kinase selectivity of compound 1, the binding affinities to a panel of 468 kinases (403 nonmutant kinases) were screened at 1 μM in the KINOMEscan Profiling Service. As we expected, compound 1 demonstrated outstanding selectivity, with an S (10) selectivity score of 0.035 (Figure S2). These encouraging results suggest that central ring modifications have a crucial role in antiplasmodial activity and that there might be an opportunity to improve selectivity by further modifications to other regions of compound 1.
Table 1.

| ||||
|---|---|---|---|---|
| compound | R1 | R2 | human EphA2 IC50 (nM) ± SD |
P. falciparum Dd2 EC50 (nM) ± SD |
| 1 | H | −Cl | >10,000 | 80 ± 8 |
| 2 | H | −OCH3 | >10,000 | 341 ± 47 |
| 3 | H | H | 6730 ± 5530 | 479 ± 98 |
| 4 | −Cl | H | 52 ± 13 | 770 ± 182 |
| 5 | −CH3 | H | 11 ± 0.3 | 3910 ± 1630 |
EphA2 activity determined by SelectScreen kinase profiling.
Antiplasmodial activity determined by SYBR Green I-based proliferation assay in asynchronous parasites.
Structure–Activity Relationship.
Based on previous SAR studies and molecular modeling, thiophenylpyridine of compound 1 binds to the adenine binding region of the ATP pocket, which is a key determinant of potency and selectivity, and therefore, we focused an initial set of analogs to this region (Table 2). We used both the multidrug-resistant P. falciparum Dd2 and the drug-sensitive P. falciparum 3D7 strains to test compounds against ring-stage malaria parasites, and an MTS-based cell proliferation assay in the human hepatocarcinoma line HepG2 and the adenocarcinoma line MCF7 for general cytotoxicity and selectivity. We first removed the thiophene, leading to a pyridine compound 6, which resulted in a 3-fold weaker potency than compound 1, accompanied by marginal improvement in selectivity (indices of 71 and 75). To better understand the effect of the pyridine substitution, analogs with piperazine (compound 7), phenyl group (compound 8), phenylthiophene (compound 9), pyrimidine (compound 10), pyrimidin-2-amine (compound 11), and nicotinonitrile (compound 12) were synthesized. Antiplasmodial activity evaluation demonstrated that replacing the pyridine with a saturated piperazine ring (7) resulted in the loss of potency (EC50 1.2 μM) and selectivity. Removing the nitrogen from pyridine (compound 8) and thiophenylpyridine (compound 9) resulted in a further decrease in the potency by 4- to 5-fold, indicating that the pyridine is essential for antiplasmodial activity, and the nitrogen in the pyridine ring may contribute to hydrogen-bonding with the potential target. Introducing small substitution groups and/or additional nitrogen atoms to the pyridine ring (compound 10, compound 11, compound 12) did not rescue the potency and selectivity from compounds 8 and 9. This implies that the thiophene ring is a major contributor to antimalarial activity, suggesting a π–π interaction between the thiophene and the potential target might exist. Furan (compound 13), phenyl (compound 14), thiazole (compound 15), and benzonitrile (compound 16) were employed to further probe into the potency-enhancing effect of thiophene. As a result, compounds 13, 14, 15, and 16 exhibited comparable antimalarial activity, and interestingly, compounds 13 and 15 displayed improved selectivity compared to compound 1. Compound 16 stood out as the most potent analog in this series, with an EC50 of 36 nM against both the Dd2 and 3D7 strains.
Table 2.
SAR Study of the Solvent-Exposed Region

| ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | A | Antiplasmodial Activity | Cytotoxicity | |||||
| Dd2 EC50 (nM) |
3D7 EC50 (nM) |
RIa | HepG2 EC50 (nM) |
SIb | MCF7 EC50 (nM) |
SIc | ||
| 1 |

|
58 ± 5 | 42 ± 8 | 1.4 | 2590 ± 84 | 45 | 3510 ±727 | 61 |
| 6 |

|
147 ± 23 | 127 ± 13 | 1.2 | 10400 ± 140 | 71 | 11000± 1810 | 75 |
| 7 |
|
1230 ± 123 | 2120 ± 350 | 0.6 | 14000 ± 2030 | 11 | 18800 ±521 | 15 |
| 8 |

|
493 ± 73 | 361 ± 8 | 1.4 | 7240 ± 1360 | 15 | 6070 ± 133 | 12 |
| 9 |

|
253 ± 41 | 196 ± 13 | 1.3 | 5460 ± 302 | 22 | 8360 ± 812 | 33 |
| 10 |

|
520 ± 66 | 395 ± 24 | 1.3 | 17300 ± 2430 | 33 | 15300 ± 699 | 29 |
| 11 |

|
709 ± 118 | 378 ± 62 | 1.9 | 19200 ± 604 | 27 | 13700 ± 349 | 19 |
| 12 |

|
191 ± 12 | 186 ± 9 | 1.0 | 11000 ± 758 | 58 | 7580 ± 165 | 40 |
| 13 |

|
51 ± 5 | 58 ± 8 | 0.9 | 5410 ± 228 | 106 | 6490 ± 637 | 127 |
| 14 |

|
50 ± 3 | 41 ± 4 | 1.2 | 3290 ± 175 | 66 | 4350 ± 811 | 87 |
| 15 |

|
56 ± 7 | 74 ± 1 | 0.8 | 6080 ± 928 | 109 | 8140 ±1600 | 145 |
| 16 |

|
36 ± 3 | 36 ± 2 | 1.0 | 1460 ± 185 | 41 | 2190 ± 292 | 61 |
Resistance Index (RI) = P. falciparum Dd2 EC50/P. falciparum 3D7 EC50.
Selectivity Index (SI) = HepG2 EC50/P. falciparum Dd2 EC50.
Selectivity Index (SI) = MCF7 EC50/P. falciparum Dd2 EC50. Antiplasmodial activity determined by SYBR Green I-based proliferation assay in ring stage parasites. Cytotoxicity evaluated by MTS-based viability assay.
Next, we focused on the amide portions of the molecule (Table 3). We observed previously that the amide close to thiophenylpyridine is essential for potent inhibition of EphA2. Removal or replacement of the carbonyl group with methylene led to a 6-fold loss of potency (17 and 18). Reversing the amide bonds did not improve the potency (19 and 20). The tail on compound 1, trifluoromethyl (−CF3) and piperazine, was positioned to the pocket created by the movement of the activation loop. Truncating the piperazine significantly decreased the potency, with an EC50 of 1110 nM for compound 21. Removal of the CF3 (22) retained the activity but, more importantly, dramatically improved the selectivity. Incorporation of smaller type II tail 4-(pyridin-2-yl) morpho-line decreased potency by 13-fold (compound 23).
Table 3.
SAR Study of Linker and Tail

| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | L1 | L2 | B | Antiplasmodial Activity | Cytotoxicity | |||||
| Dd2 EC50 (nM) |
3D7 EC50 (nM) |
RIa | HepG2 EC50 (nM) |
SIb | MCF7 EC50 (nM) |
SIc | ||||
| 1 |

|

|
|
58 ± 5 | 42 ± 8 | 1.4 | 2590 ± 84 | 45 | 3510 ± 727 | 61 |
| 17 |
|

|
|
296 ± 23 | 253 ± 21 | 1.2 | 3620 ± 670 | 12 | 5530 ± 331 | 19 |
| 18 |
|

|
|
344 ± 52 | 310 ± 42 | 1.1 | 5590 ± 198 | 16 | 8330 ± 1070 | 24 |
| 19 |

|

|
|
104 ± 12 | 73 ± 3 | 1.4 | 9160 ± 1340 | 88 | 7030 ± 488 | 68 |
| 20 |

|

|
|
109 ± 17 | 82 ± 9 | 1.3 | 4240 ± 461 | 39 | 5360 ± 473 | 49 |
| 21 |

|

|

|
1110 ± 154 | 541 ± 106 | 2.1 | >20000 | >18 | > 20000 | >18 |
| 22 |

|

|
|
81 ± 4 | 76 ± 3 | 1.1 | 8770 ± 1090 | 108 | 10300 ± 1090 | 127 |
| 23 |

|

|

|
739 ± 4 | 700 ± 17 | 1.1 | >20000 | 27 | 14700 ± 1400 | 20 |
Resistance Index (RI) = P. falciparum Dd2 EC50/P. falciparum 3D7 EC50.
Selectivity Index (SI) = HepG2 EC50/P. falciparum Dd2 EC50.
Selectivity Index (SI) = MCF7 EC50/P. falciparum Dd2 EC50. Antiplasmodial activity determined by SYBR Green I-based proliferation assay in ring stage parasites. Cytotoxicity evaluated by MTS-based viability assay.
After exploring the hinge region, amide linker, and tail parts, we shifted our focus to the central ring. Encouraged by the preliminary SAR results (Table 1), it was clear that the modification of the central ring provided us with a decent point for further SAR exploration. As shown in Table 4, the EC50 of compound 1 was 2.59 μM in HepG2 and 3.51 μM in MCF7, producing respectable selectivity indices of 45 and 61, respectively. Replacement of the 3-Cl group with 2-F (compound 24) and 2-OMe (compound 25) dramatically reduced the activity and selectivity. Installing the F to the 4-position, affording compound 26, slightly inhibited Plasmodium (EC50 = 867 nM) with poor selectivity. Introducing F to the 4-position combined with the addition of a methyl substitution at the 2-position (compound 27) led to sharply weakened antiplasmodial activity and selectivity, which indicates that 2-position substitution is not tolerated for maintaining antimalarial activity and selectivity. Engagement of a methyl group to the 3-position, affording compound 28, showed similar potency with compound 2 (Table 1), 6-fold less potent than compound 1. To further investigate the effects of the central ring, the phenyl ring was replaced by pyridine, generating compounds 29 and 30. The positions of a nitrogen atom in pyridine exerted a dramatic effect on antiplasmodial potency and selectivity. Moving the nitrogen atom from the ortho position (compound 29) to the meta position (compound 30) led to a more than 7-fold improvement in both potency and selectivity, which proved our speculation that 2-position substitution is not tolerated, and 3-substitution is essential for antimalarial activity. Combined with the above SAR data, these results demonstrated that 3-position substitutions are important for potency, and the 3-Cl substitution displayed the highest potency. The excellent potency of 3-Cl substitution may be attributed to halogen bonding and/or hydrophobic interactions with the potential targets.20-22 To verify our hypothesis, 3-Cl was replaced by 3-F (31), 3-Br (32), and 3-CF3 (33). As we expected, all three of these inhibitors demonstrated similar antiplasmodial activity compared to compound 1, in which compound 33 slightly outperformed on potency (EC50: 31 nM for Dd2, 35 nM for 3D7) and demonstrated boosted selectivity (indices of 92 and 114).
Table 4.
SAR Study of the Central Ring

| ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | C | Antiplasmodial Activity | Cytotoxicity | |||||
| Dd2 EC50 (nM) |
3D7 EC50 (nM) |
RIa | HepG2 EC50 (nM) |
SIb | MCF7 EC50 (nM) |
SIc | ||
| 1 |

|
58 ± 5 | 42 ± 8 | 1.4 | 2590 ± 84 | 45 | 3510 ± 727 | 61 |
| 24 |

|
1320 ± 79 | 763 ± 51 | 1.7 | 3170 ± 332 | 2.4 | 5100 ± 952 | 3.9 |
| 25 |

|
1540 ± 252 | 821 ± 18 | 1.9 | 3240 ± 496 | 2.1 | 5060 ± 978 | 3.3 |
| 26 |

|
981 ± 27 | 491 ± 26 | 2.0 | 6710 ± 907 | 6.8 | 10800 ± 1690 | 11 |
| 27 |

|
1580 ± 108 | 1460 ± 63 | 1.1 | 4050 ± 335 | 2.6 | 6910 ± 640 | 4.4 |
| 28 |

|
372 ± 37 | 235 ± 34 | 1.6 | 3200 ± 238 | 8.6 | 5220 ± 517 | 14 |
| 29 |

|
579 ± 64 | 356 ± 17 | 1.6 | 3030 ± 89 | 5.2 | 4610 ± 708 | 8.0 |
| 30 |

|
76 ± 15 | 70 ± 14 | 1.1 | 2550 ± 77 | 34 | 4710 ± 16 | 62 |
| 31 |

|
135 ± 10 | 113 ± 21 | 1.2 | 6690 ± 744 | 50 | 10200 ± 19 | 76 |
| 32 |

|
61 ± 9 | 39 ± 4 | 1.6 | 4380 ± 387 | 72 | 6590 ± 845 | 108 |
| 33 |

|
31 ± 5 | 35 ± 5 | 0.9 | 2850 ± 66 | 92 | 3540 ± 271 | 114 |
Resistance Index (RI) = P. falciparum Dd2 EC50/P. falciparum 3D7 EC50.
Selectivity Index (SI) = HepG2 EC50/P. falciparum Dd2 EC50.
Selectivity Index (SI) = MCF7 EC50/P. falciparum Dd2 EC50. Antiplasmodial activity determined by SYBR Green I-based proliferation assay in ring stage parasites. Cytotoxicity evaluated by MTS-based viability assay.
In Vitro Metabolic Stability and In Vivo Pharmacokinetics.
Given the potent activity of compound 1 and its analogs, we evaluated all the active compounds with EC50 below 150 nM for their stability in mouse microsomes. As shown in Table 5, compounds 1, 13–16, 22, 32, and 33 exhibit suitable microsomal stability and low clearance, compound 1 being the most stable. By contrast, compounds 6 and 30 are metabolized rapidly/have a fast clearance.
Table 5.
In Vitro Metabolic Stability in Mouse Liver Microsomes
| compound ID | T1/2 (min) | Clint (μL/min/mg) |
|---|---|---|
| 1 | 79.5 | 9 |
| 6 | 15.1 | 46 |
| 13 | 58.1 | 12 |
| 14 | 50.2 | 14 |
| 15 | 52.1 | 13 |
| 16 | 42.5 | 16 |
| 22 | 65.3 | 11 |
| 30 | 19.1 | 36 |
| 31 | 32.4 | 21 |
| 32 | 40.7 | 17 |
| 33 | 41.4 | 17 |
As aforementioned, compound 1 demonstrated the best metabolic stability. To prove the antimalarial efficacy of this scaffold, compound 1 was selected for further in vivo studies. Pharmacokinetic studies of compound 1 in mice are shown in Table 6: it exhibits low bioavailability (4.8%) after oral administration, which may be attributed to its poor solubility and cell permeability (Table S1), leading to slow absorption (Tmax = 4.67 h). Intravenously, compound 1 displayed a short half-life (4.16 h) and a moderate exposure level (volume of distribution 0.84 L/kg), indicating low penetration into tissues. By comparison, the known antiplasmodial compound GNF179 displays a half-life of 8.9 h and a volume of distribution of 11.8 L/kg.23 Scaffold optimization efforts are presently underway to improve metabolic stability and pharmacokinetic properties while retaining high potency and selectivity.
Table 6.
Pharmacokinetic Profile of Compound 1
| subject | T1/2 (h) | Tmax (h) | Cmax (ng/mL) | AUClast (min*ng/mL) | AUCINF_obs (min*ng/mL) | CLobs (mL/min/kg) | Vss (L/kg) | F% |
|---|---|---|---|---|---|---|---|---|
| IV(2mg/kg) | 4.16 | 0.08 | 11,107 | 411,546 | 489,317 | 4.3 | 0.84 | |
| PO (10mg/kg) | 5.71 | 4.67 | 153 | 51,931 | 98,627 | 108.9 | 4.8 |
Compound 1 Displays Both Prophylactic and Therapeutic Efficacy In Vivo.
To determine whether compound 1 possesses the coveted multistage activity present in a lead antimalarial candidate, we evaluated its activity against the liver stage. A liver stage assay utilizing luciferase-expressing P. berghei parasites (P. bergheiLuc) revealed an EC50 of 1.77 ± 0.07 μM (Figure S3). We then evaluated the in vivo efficacy of compound 1 when administered prophylactically and therapeutically. In the prophylactic model, the compound was administered IV as a single dose, 6 h before IV inoculation with P. bergheiLuc sporozoites.23 The therapeutic model utilized the standard 4-day Peter’s test, in which the mice are infected IP with P. bergheiLuc infected red blood cells (iRBCs) and treated IV, once daily, for 4 days.24 Infection was monitored by flow cytometry and by in vivo imaging (Figure S4).25 Both experimental models were conducted utilizing two drug doses, 15 and 50 mg/kg IV, and as a control, we used an antiplasmodial compound with both blood and liver stage activity, GNF179.23 For this proof-of-concept experiment, although not ideal for antimalarial, we used the IV route of administration. In both sets of experiments, by day 12, parasitemia was systemic and widespread in untreated control groups (Figures 1 and S4). When compound 1 was used prophylactically at 15 or 50 mg/kg, mice cleared the infection and showed no recrudescence until sacrifice at 90 days (Figure 1A,C). Similarly, the mice treated therapeutically with 15 mg/kg were cured of infection (Figure 1B,D). The mice treated prophylactically with the lower dose, did show a very weak indication of infection at the injection site, detected by luminesce (Figure S4) on day 2, though parasitemia measurements on day 6 and onward showed no evidence of infection (Figure 1A). We attribute the early luciferase signal to sporozoites being retained at the injection site, potentially in the skin. Taken together, these results indicated that, in the murine model, compound 1 acted both therapeutically, by resolving blood stage infection, and prophylactically, by preventing the establishment of a systemic infection, even at a dose as low as 15 mg/kg.
Figure 1.
Compound 1 prevents blood-stage malaria infection when applied prophylactically (A, C) and cures infection when given therapeutically (B, D) at 15 and 50 mg/kg. For A and C, mice (infected and untreated control group) were pretreated with vehicle alone or given a single 15 or 50 mg/kg dose of a control compound, GNF179,23 or compound 1 via IV injection in the vehicle. After 6 h groups of 5 mice were infected with 105 P. bergheiLuc sporozoites23 suspended in DMEM media via IV injection. For B and D, mice were infected with 107 fresh P. bergheiLuc iRBCs suspended in cryopreservation solution via IP infection and then treated afterward at 1, 2, 3, and 4 days post-infection via IV injection in the standard Peter’s test24 using compounds described above. Parasitemia was determined by flow cytometry25 at the indicated days for A and B. Survival is shown in C and D.
Compound 1 Kills Parasites Rapidly and Targets Ring Stages More Effectively.
Having established the efficacy of compound 1 in vivo, in-depth characterization of the effects of compound 1 on blood-stage P. falciparum was conducted to evaluate its properties as an antimalarial lead candidate. The rate of parasiticidal activity was evaluated using a flow cytometry-based assay, in which asynchronous Dd2 cultures were treated for 12, 24, or 48 h with 10 × EC50 of compound 1, washed, and monitored for 4 days. As shown in Figure 2A, parasites treated with compound 1 for as little as 12 h failed to recover within 4 days. To dissect the parasite clearance kinetics precisely, a parasite reduction ratio assay was performed with both compound 1 and the potent antiplasmodial analog, compound 16.26 Synchronized parasites were exposed to 10 × EC50 of the compound at ring stage, and every 24 h for 120 h, an aliquot of the culture was washed and serially diluted in uninfected red blood cells, and growth was assessed after 21 days. As shown in Figure 2D, both compound 1 and compound 16 are fast-acting, similar to dihydroartemisinin (DHA), with no lag phase detected and a 99.9% parasite clearance time (PCT) of less than 32 h.
Figure 2.
Killing profile of compound 1 in blood-stage P. falciparum. Fast-acting DHA and slow-acting atovaquone are controls. (A–C) Flow-cytometry-based assay evaluated parasite viability after treatment for 12 (A), 24 (B), and 48 (C) hours. Graphs depict the average of 3 biological replicates ± SEM (D) P. falciparum parasite reduction ratio of both compounds 1 and 16 with the accompanying table. The graph presents data from 2 biological replicates ± SEM. The lag phase is the time before the maximal killing rate. PRR (parasite reduction ratio) is the reduction of viable parasites over one life cycle. 99.9% PCT (parasite clearance time) is the time required to clear the initial parasite load by 3-log units.
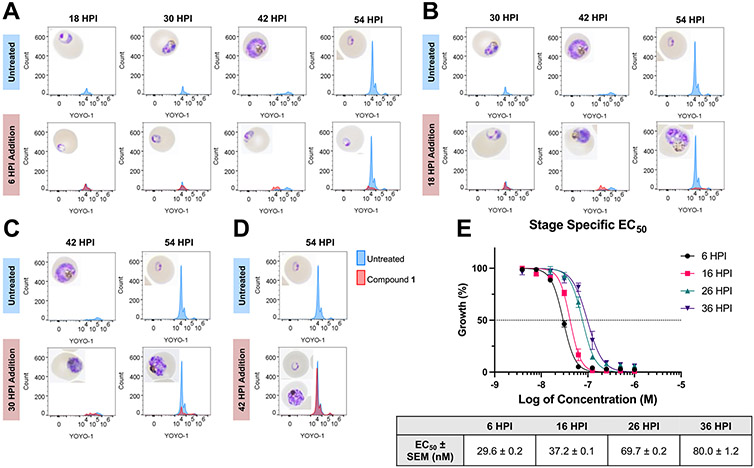
We then sought to identify the stage at which the compound exerted its maximum antimalarial effects through a stage-specific assay. Synchronized Dd2 culture was treated with 5 × EC50 of compound 1 at 6-, 18-, 30-, and 42 h post-invasion (HPI) and monitored until 54 HPI by microscopy and flow cytometry, staining with YOYO-1 for nuclear content. As shown in Figure 3, the histograms in the control reflect a rightward shift in the YOYO-1 peak due to an increase in DNA through schizogony, followed by a leftward peak for reinvasion at 54 h. However, when compound 1 was added at 6 HPI, this early peak failed to shift, and microscopy showed that parasites seemed to remain stunted in the ring stage. When applied at both 18 and 30 HPI, there was a delayed shift, and parasites were clearly malformed. However, by 42 HPI, the effect was much less pronounced. A reinvasion peak was observed, indicating the production of viable merozoites and successful reinvasion, though some parasites still displayed aberrant morphology.
Figure 3.
Stage specific activity of compound 1. (A–D) Flow cytometry to detect DNA replication and Giemsa-stained thin blood smears to monitor morphology, with treatment at 6 HPI (A), 18 HPI (B), 30 HPI (C), and 42 HPI (D). Histograms representative of 3 biological replicates. (E) Stage specific EC50 of compound 1 confirms increased potency in early asexual stage. Graph reflects 3 biological replicates ± SEM.
Finally, to quantitatively evaluate the varying susceptibility of parasites through the life cycle to compound 1, we treated highly synchronized (0–3 HPI) 3D7 parasites at four points in the asexual life cycle (6, 16, 26, and 36 HPI), and after 72 h performed a SYBR Green I-based proliferation assay to obtain EC50 values. We found that compound 1 exhibited a notably lower EC50 in the early ring stage (30 nM) that increased throughout the lifecycle to 80 nM at 36 HPI (Figure 3E).
Compound 1 and Compound 16 are Effective against Artemisinin-Resistant Clinical Isolates.
The evidence that compound 1 is fast-acting, primarily in the ring stage, raised the concern that the compound acts in a manner similar to artemisinin, and may therefore be vulnerable to emerging artemisinin resistance.27 To evaluate the effect of compound 1 and compound 16 on artemisinin-resistant P. falciparum parasites, we assessed the ring stage survival of field isolates that are resistant to artemisinin and piperaquine (PPQ), an analog of chloroquine that is used in an ACT. Three strains from Cambodia were used: one that remains sensitive to artemisinin (IPC 5188), one that is artemisinin-resistant (IPC 5202), and one that is both artemisinin- and piperaquine-resistant (IPC 6261).28-32 Using the ring stage survival assay (RSA),29 highly synchronized early ring stage parasites (0–3 HPI) were treated with 10 × EC50 of compound 1 or compound 16 for 6 h before compound removal, and survival was evaluated at 72 h with Giemsa-stained thin blood smears. As controls, DHA and the vehicle, DMSO, were used. As shown in Figure 4A, compound 1 and compound 16 displayed a similar inhibitory profile to each other in all strains, which would be expected for analogs. However, the survival in all strains, including 3D7, was notably higher than that of the DHA-treated cultures, indicating that 6-h incubation might not be sufficient to exert a maximal effect. We then performed a piperaquine survival assay (PSA).31 This assay is identical to the RSA with the exception that piperaquine (PPQ) served as the control, and the treatment duration is 48 h. In this assay, the parasites treated with either compound 1 or compound 16, regardless of strain, did not survive the 48 h treatment (Figure 4B). Furthermore, the EC50 values of both compounds remained in the low nanomolar range in all strains (Figure 4C). Taken together, these results indicate that compound 1 and compound 16 maintain efficacy against both artemisinin and piperaquine-resistant strains, though a treatment time of between 6 and 48 h is required.
Figure 4.
Compounds 1 and 16 are effective in artemisinin and piperaquine resistant isolates. Parasite lines are resistant (−R) or sensitive (−S) to artemisinin (ART) or piperaquine (PPQ). (A) Ring Stage Survival Assay and (B) Piperaquine Survival Assay graphs reflect average % survival ± SEM of 3 biological replicates. (C, D) EC50 determination, graphs depict average ± SEM of 3 biological replicates.
Compound 1 Maintains Potency in Ex Vivo Ugandan Isolates.
We then evaluated the efficacy of compound 1 in currently circulating parasite populations. Ex vivo P. falciparum isolates were obtained from patients from three locations in northern and eastern Uganda: The Patongo Health Center in Patongo, Agago District; the Tororo District Hospital in Tororo, Tororo District, and the Busiu Health Center in Busiu, Mbale District. Activity, evaluated with the SYBR-Green I proliferation assay, was compared to P. falciparum Dd2 and P. falciparum 3D7 parasites in predominantly ring stage to reflect the stage of the clinical isolates more closely. As shown in Figure 5, Compound 1 maintained very potent activity against all ex vivo isolates, with an average EC50 of 9.21 nM. This suggests that prevailing P. falciparum populations in eastern and northern Uganda lack a genetic background that would reduce compound 1 efficacy, underscoring the compound’s utility as a preclinical candidate.
Figure 5.
Compound 1 maintains potency in Ugandan field isolates. Compound 1 was evaluated against P. falciparium ex vivo isolates from patients in the Ugandan towns of Patongo, Tororo, and Busiu. Ring stage Dd2 and 3D7 were also evaluated for comparison with lab-adapted strains. Graph shows individual EC50 values of ex vivo isolates and average of Dd2 and 3D7 EC50 values from biological triplicates.
Compound 1 Maintains Potency in Strains Resistant to Known Antiplasmodials.
Finally, we evaluated the cross-resistance of compound 1 in P. falciparum strains possessing resistance-conferring mutations to antimalarial drug candidates. The purpose of this was 2-fold; cross-resistance would suggest a possible mechanism of action, as well as indicate a looming risk of resistance. The efficacy of compound 1 was assessed against three P. falciparum lines with resistance-causing mutations in the following genes: AcAS, CARL, and PI4K. The line AcAS A597 V has a mutation within the acetyl-coenzyme A synthetase (PF3D7_0627800) which renders parasites resistant to the compound MMV084978, a promising antiplasmodial compound in development.33 CARL I1139K has a mutation in the cyclic amine resistance locus (PF3D7_0321900), providing resistance to imidazolopiperazine antiplasmodials like GNF179.34 PI4K S1320L contains a mutation in the phosphatidylinositol 4-kinase (PF3D7_0509800), conferring resistance to imidazopyrazines such as KDU691.35 As controls, the compounds associated with resistance in the mutants were used, as well as artemisinin. As shown in Figure 6, there was no difference between compound 1 EC50 among the three resistant lines.
Figure 6.
Compound 1 displays no cross-resistance in P. falciparum strains resistant to known antiplasmodials. Results displayed are the average of 2 biological replicates ± SEM.
Compound 1 Is Irresistible to Drug Selection in P. falciparum In Vitro.
To ascertain a mechanism of action, we attempted to generate a resistant line, with the aim of creating clonal populations of resistant parasites. The genomes of the resistant clones would then be sequenced and compared to the parental line to identify resistance-conferring single nucleotide polymorphisms (SNPs) or gene duplication events, possibly revealing causal targets.36 The P. falciparum clonal line, Dd2-B2, was used for resistance line generation.37 Three flasks containing 1 × 108 blood stage Dd2-B2 parasites were treated with 1 × EC50 of compound 1 until death was observed, and one flask of equal parasitemia was left untreated (Figure S5). Flasks 2 and 3 failed to recover after the compound was removed. Only one flask achieved weak resistance, intermittently tolerating 2 × EC50 of compound 1. However, after the second pulse of treatment at this concentration, the parasites failed to rebound, suggesting that the compound kills in a manner that is not easily overcome by genetic mutation. While this hampered our target identification efforts, it also underscored the value of this compound, as one of the strongest exclusion criteria for a prospective antiplasmodial drug is the propensity to develop resistance.
To confirm the “irresistibility” of compound 1, we determined the minimum inoculum of resistance (MIR), a metric used to evaluate the risk of an antiplasmodial compound for inducing resistance.26 The EC50 and EC10 were determined in the Dd2-B2 strain (40.6 and 53.4 nM, respectively) using a flow cytometry-based method on ring-stage parasites. Subsequently, two selections were conducted, one with 1.4 × 107 parasites (1.2 × 106 parasites in 12 wells), and another with 3 × 107 parasites (1 × 107 parasites in 3 wells). After continuous treatment with 3 × EC10 of compound 1, cultures were monitored thrice weekly via thin blood smear. After treatment, parasites were cleared from the culture quickly and failed to recover within 50 days, indicating that compound 1 has an MIR of >7 and a high barrier to resistance.7 While compound 1 shows clear promise as an antimalarial due to its dual therapeutic/prophylactic potential, the cellular mechanism of action remains elusive. The fact that we were unable to generate resistance lines through two different approaches bodes well for the compound as a treatment option; however, the lack of resistant clones obliges us to use other methods for target identification, and these efforts are presently underway.
CONCLUSIONS
A phenotypic screen of a type II library yielded compound 1 with potent antiplasmodial activity in both the asexual blood and liver stages, which was manifested as in vivo efficacy when applied as both a therapeutic and prophylactic. Through extensive structure–activity relationship analysis of its analogs, we deconvoluted the important components of compound 1. The 2-position in the central ring is found to be essential to both selectivity and antimalarial potency, as substitutions at this location are not tolerated; rather, 2-substitution is associated with increased activity against human EphA2. In the 3-position of the central ring, a substitution group, especially a trifluoromethyl group or a halogen is essential for potency. This potency is accentuated by the addition of an additional aromatic moiety to the solvent-exposed region. In addition, the linker and tail portions also influence potency and selectivity: an amide linker adjoining the head region is required for potency, and a reduced type II tail, lacking the trifluoromethyl group improves selectivity. Optimization of the scaffold continues, with the aim of improving pharmacokinetic properties and advancing a lead compound with potential as a preclinical antimalarial candidate.
In addition to gaining valuable structure–activity insight into this scaffold, we also characterized its antiplasmodial activity, thereby evaluating its potential as a lead antimalarial. We found that compound 1 is a fast-acting parasiticidal agent in the asexual blood stages of P. falciparum, and this efficacy extends to strains resistant to current and candidate antimalarials, as well as in ex vivo isolates. Additionally, compound 1 displays promising activity in the liver stage and is efficacious in vivo when applied therapeutically or prophylactically, underscoring its dual-stage utility. Finally, we demonstrated that compound 1 is “irresistible,” failing to generate resistance in two separate attempts. Notably, compound 1 provides the platform to meet Medicines for Malaria Venture’s target candidate profiles because of its therapeutic and prophylactic activities.
While compound 1 was designed as a type II protein kinase inhibitor, it remains to be determined whether the compound inhibits a plasmodial or host kinase, or if it inhibits in a type II fashion. Indeed, it is not unusual for the kinase inhibitors that exert their therapeutic effects via “off-targets,” particularly bromodomain-containing proteins or tubulin.38 Target identification is underway to deconvolute the mechanism of action of compound 1.
CHEMISTRY
Synthetic routes for the preparation of compounds are outlined in Schemes 1-3. The synthesis of derivatives 1, 6, 8–16 commenced with the known commercially available 4-((4-Methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline, which underwent HATU-mediated condensation with benzoic acid derivatives to generate amide S2. Reduction of the resulting nitro compound as aniline analogs followed by a second condensation reaction furnished final products (Scheme 1). Derivatives 7, 17–23 were synthesized based on the synthetic routes described in Scheme 2. With S3 in hand, treatment of S3 with triphosgene provided the active intermediate, which was subject to a condensation reaction with 1-methylpiperazine to afford 7. Analogs 17 and 18 were synthesized from S3 through reductive amination and Pd-mediated amination coupling, respectively. Derivatives 19–23 were prepared in a three-step sequence involving (1) HATU-mediated condensation; (2) Fe-induced reduction of the resulting nitro intermediate; (3) Amide formation of the resulting aniline with 5-(thiophene-2-yl) nicotinoyl chloride. Compounds 2–5, and 24–33 were prepared via similar synthetic routes (Scheme 3). HATU-mediated condensation of S1 with various nitrobenzoic acids gave intermediate S11 and subsequent reduction of resulting nitro compounds and amide formation with nicotinic acid derivatives generating the desired final products.
Scheme 1. Synthesis of Compounds 1, 6, and 8–16.
aReagents and conditions: (a) 3-chloro-5-nitrobenzoic acid, HATU, DIPEA, DMF, 25 °C, 1 h; (b) Fe, NH4Cl, EtOH/H2O, 80 °C, 5 h; (c) acid or acyl chloride, coupling condition.
Scheme 3. Synthesis of Compounds 2–5, 24–33.
aReagents and conditions: (a) 5-nitrobenzoic acid derivatives, HATU, DIPEA, DMF, 25 °C, 12 h; (b) Fe, NH4Cl, EtOH/H2O, 75 °C, 2 h; (c) acid or acyl chloride, coupling condition.
Scheme 2. Synthesis of Compounds 7, 17–23.
aReagents and conditions: (a) Triphosgene, 1-methylpiperazine, DIPEA, CH2Cl2, 0–25 °C (b) 17: 3-bromo-5-(thiophen-2-yl) pyridine, K3PO4, t-BuBrettPhos Pd G3, t-BuBrettPhos, 2Me-THF. 80 °C 18: 5-(thiophen-2-yl)nicotinaldehyde, NaBH3CN, AcOH, 25 °C, 2 h; (c) 3-chloro-5-nitrobenzoic acid, HATU, DIPEA, DMF, 25 °C; (d) Fe, NH4Cl, EtOH/H2O, 75 °C, 3 h; (e) 5-(thiophen-2-yl)nicotinoyl chloride, DIPEA, DMF, 25 °C. (f) NaOH, THF/MeOH/H2O, 25 °C.
EXPERIMENTAL SECTION
Compound Synthesis.
Starting materials, reagents, and solvents were purchased from commercial suppliers and were used without further purification unless otherwise noted. All reactions were monitored using a Waters Acquity UPLC/MS system (Waters PDA eλ Detector, QDa Detector, Sample manager - FL, Binary Solvent Manager) using Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm particle size): solvent gradient = 85% A at 0 min, 1% A at 1.7 min; solvent A = 0.1% formic acid in water; solvent B = 0.1% formic acid in Acetonitrile; flow rate: 0.6 mL/min. Reaction products were purified by flash column chromatography using CombiFlashRf with Teledyne Isco RediSep normal-phase silica flash columns (4, 12, 24, 40 or 80 g) and a Waters HPLC system using a SunFireTM Prep C18 column (19 × 100 mm, 5 μm particle size): solvent gradient = 80% A at 0 min, 10% A at 25 min; solvent A = 0.035% TFA in Water; solvent B = 0.035% TFA in MeOH; flow rate: 25 mL/min. 1H NMR spectra were recorded on 500 MHz Bruker Avance III spectrometers, and 13C NMR spectra were recorded on a 125 MHz Bruker Avance III spectrometer. Chemical shifts are reported in parts per million (ppm, δ) downfield from tetramethylsilane (TMS). Coupling constants (J) are reported in Hz. Spin multiplicities are described as br (broad), s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Purities of assayed compounds were in all cases greater than 95%, as determined by reverse-phase LC–MS analysis. The LC–MS traces and 1H NMR spectra have been provided in Figures S6 and S7, respectively
General Procedure A for Preparation of Compounds 1, 6, 8–16. Step 1:
To a solution of S1 (4.00 g, 13.9 mmol, 1.0 equiv) in DCM (28 mL) were added 3-chloro-5-nitrobenzoic acid (3.15 g, 14.3 mmol, 1.03 equiv) and DIPEA (1.98 g, 15.3 mmol, 2.67 mL, 1.1 equiv) at 0 °C. The mixture was stirred at 25 °C for 0.5 h. LC–MS showed that S1 was consumed completely and one main peak with desired MS was detected. The reaction mixture was poured into water (15 mL) and the reaction was extracted with DCM (50 mL, 30 mL). The organic phase was dried over Na2SO4 and filtered under reduced pressure to give S2 (4.2 g, 8.92 mmol, 64.1% yield) as a white solid. 1H NMR (400 MHz, CD3OD) δ 8.77 (s, 1H), 8.49 (s, 1H), 8.40 (s, 1H), 8.16 (s, 1H), 8.02 (d, J = 8.4 Hz, 1H), 7.80 (d, J = 8.4 Hz, 1H), 3.78 (s, 2H), 2.25–3.24 (m, 10H) 1.35 (t, J = 7.6 Hz, 3H).
Step 2: To a solution of S2 (2.50 g, 5.31 mmol, 1.0 equiv) in EtOH (12 mL) and H2O (4.0 mL) were added NH4Cl (852 mg, 15.9 mmol, 3.0 equiv) and Fe (1.48 g, 26.6 mmol, 5.0 equiv) at 25 °C. The mixture was stirred at 80 °C for 5 h. TLC (DCM: MeOH = 0:1, Product Rf = 0.12) indicated S2 was consumed completely one new spot formed. The reaction was clean according to TLC. The reaction mixture was filtered and concentrated under reduced pressure to remove EtOH. The reaction mixture was poured into water (15 mL) and extracted with ethyl acetate (50 mL, 30 mL). The combined organic phase was washed with saturated brine (10 mL), dried over Na2SO4, filtered, and concentrated in a vacuum to give S3 (2 g, 4.54 mmol, 85.4% yield) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 10.45 (s, 1H), 8.18 (d, J = 2.0 Hz, 1H), 8.06 (d, J = 8.4 Hz, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.04–7.10 (m, 2H), 6.79 (t, J = 2.0 Hz, 1H), 5.72 (s, 2H), 3.65 (s, 2H), 2.75–3.17 (m, 10H), 1.21 (t, J = 7.2 Hz, 3H).
Step 3: Acid chloride way: nicotinic acid derivatives (0.15 mmol) were dissolved in SOCl2 (2 mL) at 25 °C. The mixture was stirred at 80 °C for 2 h. Then, the reaction mixture was concentrated under reduced pressure to remove SOCl2 to give acid chloride as a yellow solid, which was put into the next step directly. A solution of S3 (0.1 mmol, 1.0 equiv) in DMF (2 mL) was added above acid chloride (0.15 mmol, 1.5 equiv) and DIPEA (0.3 mmol, 3.0 equiv) at 0 °C. The mixture was stirred at 25 °C for 30 min. LC–MS showed that S3 was consumed completely and one main peak with the desired MS was detected. The reaction mixture was poured into water (15 mL) and the reaction was extracted with ethyl acetate (50 mL, 30 mL). The reaction mixture was dried over Na2SO4, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by prep-HPLC to give the final compounds.
HATU-mediated amide formation way: To a solution of S3 (0.10 mmol, 1.0 equiv) and nicotinic acid analogs (0.15 mmol, 1.5 equiv) in DMF (2 mL), 1-[bis(dimethylamino)-methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) (76 mg, 0.20 mmol, 2.0 equiv) and N,N-diisopropylethylamine (DIPEA) (64 mg, 0.50 mmol, 5.0 equiv) were added at 0 °C. This mixture then was stirred at 25 °C for 12 h. LC–MS showed that S3 was consumed completely and one main peak with the desired MS was detected. The reaction mixture was poured into water (15 mL) and was extracted with ethyl acetate (3 × 50 mL). The reaction mixture was then dried over Na2SO4, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by preparative HPLC to give the final compounds.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 1).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (400 MHz, DMSO-d6): δ (ppm): 10.87 (s, 1H), 10.64 (s, 1H), 9.12 (d, J = 2.4, 1H), 9.03 (d, J = 2.0 Hz, 1H), 8.53 (t, J = 2.0 Hz, 1H), 8.27 (t, J = 1.6 Hz, 1H), 8.22 (t, J = 2.0 Hz, 1H), 8.18 (d, J = 2.0 Hz, 1H), 8.04–8.07 (m, 1H) 7.86 (t, J = 1.6 Hz, 1H), 7.79 (dd, J = 3.6, 0.8 Hz, 1H), 7.72–7.75 (m, 2H), 7.24–7.27 (m, 1H), 3.57 (s, 2H), 2.29–2.40 (m, 10H), 0.98 (t, J = 7.2 Hz, 3H).
Synthesis of Compound 1, hydrochloride salt. A solution of Compound 1 (1.60 g, 2.55 mmol, 1.0 equiv) in H2O (5 mL) and HCl (0.5 M, 20.4 mL, 4.0 equiv) was stirred at 25 °C for 30 min. The solution then was lyophilized to give compound 1 (1.05 g, 1.42 mmol, 55.8% yield, HCl). 1H NMR (500 MHz, DMSO-d6) δ 11.22 (s, 1H), 10.93 (s, 1H), 9.19 (d, J = 2.2 Hz, 1H), 9.14 (d, J = 2.0 Hz, 1H), 8.78 (t, J = 2.1 Hz, 1H), 8.40 (t, J = 1.7 Hz, 1H), 8.38–8.34 (m, 1H), 8.30 (t, J = 1.9 Hz, 1H), 8.28–8.18 (m, 2H), 7.94 (t, J = 1.7 Hz, 1H), 7.91 (dd, J = 3.6, 1.2 Hz, 1H), 7.79 (dd, J = 5.1, 1.1 Hz, 1H), 7.29 (dd, J = 5.1, 3.6 Hz, 1H), 4.37–4.34 (m, 2H), 3.68 (d, J = 12.0 Hz, 2H), 3.46 (s, 6H), 3.20 (d, J = 8.9 Hz, 2H), 1.29 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, DMSO–D6) δ 164.98, 164.10, 147.76, 146.66, 140.89, 140.41, 138.55, 136.96, 134.20, 133.63, 133.56, 131.08, 130.58, 129.39, 128.62, 127.08, 125.35, 124.03, 123.44, 123.17, 123.14, 119.25, 118.01, 117.96, 55.70, 50.98, 48.79, 48.24, 9.18. MS (ESI): calcd. for C31H30ClF3N5O2S+ [M + H]+ 628.18, found 628.01.
N-(3-(4-(4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)-phenyl)carbamoyl)-5-methoxyphenyl)-5-(thiophen-2-yl) nicotinamide (Compound 2).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (500 MHz, DMSO-d6) δ 10.80 (s, 1H), 10.65 (s, 1H), 9.17 (s, 1H), 9.10 (s, 1H), 8.59 (t, J = 2.1 Hz, 1H), 8.27 (d, J = 2.2 Hz, 1H), 8.17 (dd, J = 8.5, 2.2 Hz, 1H), 8.03 (d, J = 1.7 Hz, 1H), 7.85 (dd, J = 3.6, 1.2 Hz, 1H), 7.79 (ddd, J = 9.3, 4.6, 1.6 Hz, 3H), 7.42–7.37 (m, 1H), 7.34–7.29 (m, 1H), 3.94 (s, 3H), 3.76 (s, 2H), 3.51–3.43 (m, 2H), 3.25–3.13 (m, 2H), 3.12–3.03 (m, 2H), 3.00 (d, J = 13.2 Hz, 2H), 2.49 (t, J = 12.3 Hz, 2H), 1.28 (t, J = 7.3 Hz, 3H). (ESI): calcd. for C32H33F3N5O3S+ [M + H]+ 624.23, found 624.21.
N-(3-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 3).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (500 MHz, DMSO-d6) δ 10.82 (s, 1H), 10.68 (s, 1H), 9.17 (d, J = 2.2 Hz, 1H), 9.10 (d, J = 2.1 Hz, 1H), 8.59 (t, J = 2.2 Hz, 1H), 8.43 (t, J = 2.0 Hz, 1H), 8.29 (d, J = 2.2 Hz, 1H), 8.17 (dd, J = 8.4, 2.2 Hz, 1H), 8.08 (dd, J = 7.8, 2.2 Hz, 1H), 7.85 (dd, J = 3.6, 1.2 Hz, 1H), 7.82 (dt, J = 7.9, 1.2 Hz, 1H), 7.81–7.78 (m, 2H), 7.64 (t, J = 7.9 Hz, 1H), 7.32 (dd, J = 5.1, 3.6 Hz, 1H), 3.76 (s, 2H), 3.60–3.45 (m, 2H), 3.21 (q, J = 7.3 Hz, 2H), 3.07–2.98 (m, 4H), 2.46 (t, J = 12.5 Hz, 2H), 1.27 (t, J = 7.3 Hz, 3H). (ESI): calcd. for C31H31F3N5O2S+ [M + H]+ 594.21, found 594.16.
N-(2-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 4).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (500 MHz, DMSO-d6) δ 10.65 (s, 1H), 10.64 (s, 1H), 9.13 (d, J = 2.3 Hz, 1H), 9.05 (d, J = 2.0 Hz, 1H), 8.55 (t, J = 2.2 Hz, 1H), 8.20 (dd, J = 11.8, 2.2 Hz, 2H), 8.10 (dd, J = 8.6, 2.2 Hz, 1H), 7.95 (dd, J = 8.4, 2.2 Hz, 1H), 7.83–7.76 (m, 2H), 7.75–7.69 (m, 2H), 7.24 (dd, J = 5.1, 3.6 Hz, 1H), 3.69 (s, 2H), 3.45 (d, J = 12.0 Hz, 2H), 3.13 (q, J = 7.3 Hz, 2H), 3.05–2.96 (m, 2H), 2.93 (d, J = 13.9 Hz, 2H), 2.41 (t, J = 12.2 Hz, 2H), 1.20 (t, J = 7.3 Hz, 4H). (ESI): calcd. for C31H30ClF3N5O2S+ [M + H]+ 628.18, found 628.11.
N-(5-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-2-methylphenyl)-5-(thi-ophen-2-yl)nicotinamide (Compound 5).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (500 MHz, DMSO-d6) δ 10.53 (s, 1H), 10.39 (s, 1H), 9.14 (d, J = 2.2 Hz, 1H), 9.07 (d, J = 2.0 Hz, 1H), 8.56 (t, J = 2.2 Hz, 1H), 8.23 (d, J = 2.2 Hz, 1H), 8.14 (dd, J = 8.5, 2.2 Hz, 1H), 8.05 (d, J = 1.9 Hz, 1H), 7.87 (dd, J = 8.0, 1.9 Hz, 1H), 7.80 (dd, J = 3.7, 1.2 Hz, 1H), 7.75 (dd, J =5.1, 1.2 Hz, 1H), 7.73 (d, J =8.6 Hz, 1H), 7.51 (d, J = 8.1 Hz, 1H), 7.26 (dd, J = 5.1, 3.6 Hz, 1H), 3.70 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.16 (q, J = 7.3 Hz, 2H), 3.04–2.93 (m, 4H), 2.41 (d, J = 12.3 Hz, 2H), 2.37 (s, 3H), 1.22 (t, J = 7.3 Hz, 3H). (ESI): calcd. for C32H33F3N5O2S+ [M + H]+ 608.23, found 608.21.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)nicotinamide (Compound 6).
The title compound was prepared according to the general procedure HATU-mediated amide formation pathway. White powder (28 mg, 51%). 1H NMR (500 MHz, DMSO-d6) δ 10.83 (s, 1H), 10.67 (s, 1H), 9.16 (d, J = 2.3 Hz, 1H), 8.82 (dd, J = 4.8, 1.7 Hz, 1H), 8.36 (dt, J = 8.0, 2.0 Hz, 1H), 8.31 (t, J = 1.8 Hz, 1H), 8.22–8.16 (m, 2H), 8.11 (dd, J = 8.4, 2.2 Hz, 1H), 7.84 (t, J = 1.8 Hz, 1H), 7.75 (d, J = 8.5 Hz, 1H), 7.63 (dd, J = 8.0, 4.9 Hz, 1H), 3.71 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.16 (q, J = 7.4 Hz, 2H), 3.08–2.90 (m, 4H), 2.41 (d, J = 12.2 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). (ESI): calcd. for C27H28ClF3N5O2+ [M + H]+ 546.19, found 546.23.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-4-methylpiperazine-1-carboxamide (Compound 7).
To a solution of triphosgene (20 mg, 0.067 mmol, 1.0 equiv) in DCM (2 mL), DIPEA (8.7 mg, 0.067 mmol, 1.0 equiv) was added at 0 °C, and the mixture then was stirred at 25 °C for 5 min. S3 (89 mg, 0.20 mmol, 3.0 equiv) was added to the reaction mixture and stirred at 25 °C for 30 min. 1-methylpiperazine (20 mg, 0.20 mmol, 3.0 equiv) was added to the reaction mixture and stirred for 2 h at 25 °C. LC-MS showed that one main peak was detected with the desired MS. The reaction mixture was poured into water (15 mL) and the reaction was extracted with chloroform/isopropanol (v/v = 4:1, 3 × 50 mL). The reaction mixture was dried over Na2SO4, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by preparative HPLC to give compound 7 (12 mg, 31% yield) as a white powder. 1H NMR (500 MHz, DMSO-d6) δ 10.60 (s, 1H), 9.22 (s, 1H), 8.19 (d, J = 2.2 Hz, 1H), 8.10 (dd, J = 8.5, 2.2 Hz, 1H), 7.99 (t, J = 1.8 Hz, 1H), 7.91 (t, J = 2.0 Hz, 1H), 7.73 (d, J = 8.6 Hz, 1H), 7.69 (t, J = 1.7 Hz, 1H), 4.27 (d, J = 14.3 Hz, 2H), 3.70 (s, 2H), 3.48 (d, J = 11.1 Hz, 4H), 3.21–3.12 (m, 4H), 3.08–2.91 (m, 6H), 2.85 (s, 3H), 2.41 (t, J = 12.3 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C27H35ClF3N6O2 [M + H]+ 567.25, found 567.33.
3-Benzamido-5-chloro-N-(4-((4-ethylpiperazin-1-yl)-methyl)-3-(trifluoromethyl)phenyl)benzamide (Compound 8).
The title compound was prepared according to the general procedure HATU-mediated amide formation pathway. White powder (18 mg, 39%). 1H NMR (500 MHz, DMSO-d6) δ 10.66 (s, 1H), 10.63 (s, 1H), 8.32 (t, J = 1.8 Hz, 1H), 8.18 (dt, J = 3.9, 2.0 Hz, 2H), 8.10 (dd, J = 8.5, 2.2 Hz, 1H), 8.04–7.96 (m, 2H), 7.79 (t, J = 1.7 Hz, 1H), 7.73 (d, J = 8.5 Hz, 1H), 7.66–7.59 (m, 1H), 7.58–7.52 (m, 2H), 3.68 (s, 2H), 3.45 (d, J = 12.0 Hz, 2H), 3.13 (q, J = 7.4 Hz, 2H), 3.03–2.87 (m, 4H), 2.41 (t, J = 12.1 Hz, 2H), 1.21 (t, J = 7.3 Hz, 3H). (ESI): calcd. for C28H29ClF3N4O2+ [M + H]+ 545.19, found 545.53.
3-Chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)-5-(3-(thiophen-2-yl)benzamido)-benzamide (Compound 9).
The title compound was prepared according to the general procedure HATU-mediated amide formation pathway. White powder (15 mg, 23%). 1H NMR (500 MHz, DMSO-d6) δ 10.74 (s, 1H), 10.68 (s, 1H), 8.35 (t, J = 1.8 Hz, 1H), 8.24 (t, J = 1.8 Hz, 1H), 8.22 (q, J = 2.0 Hz, 2H), 8.13 (dd, J = 8.5, 2.2 Hz, 1H), 7.96–7.90 (m, 2H), 7.84 (t, J = 1.7 Hz, 1H), 7.76 (d, J = 8.5 Hz, 1H), 7.69–7.60 (m, 3H), 7.22 (dd, J = 5.1, 3.6 Hz, 1H), 3.71 (s, 2H), 3.49 (s, 2H), 3.16 (q, J = 7.6 Hz, 2H), 3.06–2.92 (m, 4H), 2.46–2.38 (m, 2H), 1.23 (t, J = 7.3 Hz, 3H). (ESI): calcd. for C32H31ClF3N4O2S+ [M + H]+ 627.18, found 627.30.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)pyrimidine-5-carboxamide (Compound 10).
The title compound was prepared according to the general procedure HATU-mediated amide formation pathway. White powder (12 mg, 32%). 1H NMR (500 MHz, DMSO-d6) δ 10.96 (s, 1H), 10.67 (s, 1H), 9.40 (s, 1H), 9.30 (s, 2H), 8.26 (t, J = 1.8 Hz, 1H), 8.19 (d, J = 2.3 Hz, 1H), 8.15 (t, J = 1.9 Hz, 1H), 8.10 (dd, J = 8.5, 2.2 Hz, 1H), 7.86 (t, J = 1.7 Hz, 1H), 7.73 (d, J = 8.6 Hz, 1H), 3.69 (s, 2H), 3.46 (d, J = 12.0 Hz, 2H), 3.14 (q, J = 7.3 Hz, 2H), 3.02–2.91 (m, 4H), 2.43–2.35 (m, 2H), 1.21 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C26H27ClF3N6O2+ [M + H]+ 547.18, found 547.23.
2-Amino-N-(3-chloro-5-((4-((4-ethylpiperazin-1-yl)-methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-pyrimidine-5-carboxamide (Compound 11).
tert-Butyl (tert-butoxycarbonyl)(5-((3-chloro-5-((4-((4-ethylpiperazin-1-yl)-methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-carbamoyl)pyrimidin-2-yl)carbamate (di-Boc-11) was synthesized according to the general procedure HATU-mediated amide formation pathway. To a stirred solution of the above di-Boc-11 in CH2Cl2 (2 mL) was added TFA (2 mL) at 0 °C. The reaction mixture was slowly warmed to ambient temperature and stirred for 1 h. Solvents and volatiles were removed in vacuo. The residue was purified by preparative HPLC to give the final compound 11 as a white solid (28 mg, 27% for 2 steps). 1H NMR (500 MHz, DMSO-d6) δ 10.65 (s, 1H), 10.39 (s, 1H), 8.84 (s, 2H), 8.25 (t, J = 1.8 Hz, 1H), 8.20 (d, J = 2.2 Hz, 1H), 8.16–8.08 (m, 2H), 7.79 (t, J = 1.8 Hz, 1H), 7.74 (d, J = 8.5 Hz, 1H), 7.44 (s, 2H), 3.70 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.16 (q, J = 7.5 Hz, 2H), 2.98 (dd, J = 25.0, 11.4 Hz, 4H), 2.41 (dd, J = 12.2 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C26H28ClF3N7O2+ [M + H]+ 562.19, found 562.23.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-cyanonicotinamide (Compound 12).
The title compound was prepared according to the general procedure acid chloride pathway. White solid (20 mg, 31%). 1H NMR (500 MHz, DMSO-d6) δ 10.96 (s, 1H), 10.69 (s, 1H), 9.36 (d, J = 2.1 Hz, 1H), 9.26 (d, J = 2.0 Hz, 1H), 8.85 (t, J = 2.1 Hz, 1H), 8.28 (t, J = 1.8 Hz, 1H), 8.20 (d, J = 2.2 Hz, 1H), 8.18 (t, J = 1.9 Hz, 1H), 8.11 (dd, J = 8.4, 2.2 Hz, 1H), 7.88 (t, J = 1.7 Hz, 1H), 7.75 (d, J = 8.5 Hz, 1H), 3.71 (s, 2H), 3.48 (d, J = 11.9 Hz, 2H), 3.16 (q, J = 7.3 Hz, 2H), 3.00 (s, 2H), 2.95 (d, J = 13.5 Hz, 2H), 2.41 (t, J = 12.1 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C28H27ClF3N6O2+ [M + H]+ 571.18, found 571.24.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-(furan-2-yl)-nicotinamide (Compound 13).
The title compound was prepared according to the general procedure HATU-mediated amide formation pathway. Pale brown solid (35 mg, 57%). 1H NMR (500 MHz, DMSO-d6) δ 11.01 (s, 1H), 10.79 (s, 1H), 9.25 (d, J = 2.1 Hz, 1H), 9.12 (d, J = 2.1 Hz, 1H), 8.68 (t, J = 2.1 Hz, 1H), 8.41 (t, J = 1.7 Hz, 1H), 8.30 (q, J = 1.8 Hz, 2H), 8.21 (dd, J = 8.5, 2.2 Hz, 1H), 8.01 (dd, J = 1.8, 0.7 Hz, 1H), 7.94 (t, J = 1.7 Hz, 1H), 7.84 (d, J = 8.6 Hz, 1H), 7.36 (dd, J = 3.5, 0.8 Hz, 1H), 6.81 (dd, J = 3.5, 1.8 Hz, 1H), 3.81 (s, 2H), 3.57 (d, J = 11.9 Hz, 2H), 3.24 (q, J = 7.3 Hz, 2H), 3.16–2.99 (m, 4H), 2.54 (d, J = 12.5 Hz, 2H), 1.31 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C31H30ClF3N5O3+ [M + H]+ 612.20, found 612.24.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-phenylnicotinamide (Compound 14).
The title compound was prepared according to the general procedure acid chloride pathway. White solid (19 mg, 27%). 1H NMR (500 MHz, DMSO-d6) δ 10.87 (s, 1H), 10.68 (s, 1H), 9.13 (dd, J = 7.1, 2.2 Hz, 2H), 8.62 (t, J = 2.2 Hz, 1H), 8.33 (t, J = 1.7 Hz, 1H), 8.24–8.18 (m, 2H), 8.12 (dd, J = 8.5, 2.2 Hz, 1H), 7.90–7.84 (m, 3H), 7.75 (d, J = 8.5 Hz, 1H), 7.62–7.55 (m, 2H), 7.54–7.47 (m, 1H), 3.71 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.20–3.06 (m, 2H), 2.98 (dd, J = 27.0, 12.0 Hz, 4H), 2.43–2.33 (m, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C33H32CIF3N5O2+ [M + H]+ 622.22, found 622.25.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-(thiazol-5-yl)-nicotinamide (Compound 15).
The title compound was prepared according to the general procedure acid chloride pathway. White solid (21 mg, 37%). 1H NMR (500 MHz, DMSO-d6) δ 10.90 (s, 1H), 10.69 (s, 1H), 9.26 (s, 1H), 9.18 (d, J = 2.2 Hz, 1H), 9.09 (d, J = 2.0 Hz, 1H), 8.62–8.55 (m, 2H), 8.31 (d, J = 1.8 Hz, 1H), 8.20 (t, J = 1.7 Hz, 2H), 8.15–8.09 (m, 1H), 7.87 (t, J = 1.8 Hz, 1H), 7.75 (d, J = 8.5 Hz, 1H), 3.71 (s, 2H), 3.48 (d, J = 12.0 Hz, 2H), 3.16 (q, J = 7.3 Hz, 2H), 3.01 (s, 2H), 2.95 (d, J = 13.4 Hz, 2H), 2.40 (t, J = 12.0 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, DMSO-d6) δ 164.67, 164.51, 158.68, 158.41, 149.12, 147.89, 140.88, 140.77, 139.12, 134.23, 133.30, 132.34, 132.16, 131.35, 130.97, 129.95, 129.33, 128.19, 126.47, 125.76, 116.05, 115.91, 111.17, 57.01, 51.14, 51.07, 49.95, 9.47. MS (ESI): calcd. for C30H29ClF3N6O2S+ [M + H]+ 629.17, found 629.30.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-(4-cyanophenyl)nicotinamide (Compound 16).
The title compound was prepared according to the general procedure HATU-mediated amide formation pathway. White solid (27 mg, 46%). 1H NMR (500 MHz, DMSO-d6) δ 10.88 (s, 1H), 10.68 (s, 1H), 9.21 (d, J = 2.3 Hz, 1H), 9.19 (s, 1H), 8.70 (t, J = 2.1 Hz, 1H), 8.31 (t, J = 1.7 Hz, 1H), 8.23–8.18 (m, 2H), 8.15–8.03 (m, 5H), 7.87 (t, J = 1.7 Hz, 1H), 7.75 (d, J = 8.5 Hz, 1H), 3.70 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.19–3.13 (m, 2H), 2.97 (dd, J = 28.4, 12.4 Hz, 4H), 2.40 (d, J = 12.1 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C34H31ClF3N6O2+ [M + H]+ 647.21, found 647.40.
3-Chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)-5-((5-(thiophen-2-yl)pyridin-3-yl)-amino)benzamide (Compound 17).
To a solution of 3-bromo-5-(thiophen-2-yl) pyridine (49 mg, 0.2 mmol) and S3 (60 mg, 0.14 mmol) in 2-MeTHF (3 mL), tribasic potassium phosphate (87 mg, 0.41 mmol), t-BuBrettPhos Pd G3 (23 mg, 0.03 mmol), and t-BuBrettPhos (13 mg, 0.03 mmol) were added at room temperature. The vial was evacuated and purged with nitrogen (3×). The reaction was warmed to 80 °C with stirring and maintained at this temperature overnight. Upon cooling to RT, the mixture was diluted with EtOAc and sat. aq. NH4Cl. The biphasic mixture was transferred to a separatory funnel where the phases were mixed and then separated. The aqueous phase was extracted with additional EtOAc. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and filtered, and the collected filtrate was concentrated to dryness in vacuo. The crude residue was subjected to purification by flash chromatography over silica gel (0–8% MeOH/DCM) to afford the crude compound. Then the crude compound was purified by preparative HPLC to give compound 17 (19 mg, 23.0% yield) as a white powder. 1H NMR (500 MHz, DMSO-d6) δ 10.59 (s, 1H), 9.03 (s, 1H), 8.54 (d, J = 2.0 Hz, 1H), 8.42 (d, J = 2.5 Hz, 1H), 8.19 (d, J = 2.2 Hz, 1H), 8.09 (dd, J = 8.5, 2.2 Hz, 1H), 7.80 (t, J = 2.2 Hz, 1H), 7.73 (d, J = 8.5 Hz, 1H), 7.70–7.66 (m, 2H), 7.65 (t, J = 1.8 Hz, 1H), 7.56 (t, J = 1.6 Hz, 1H), 7.35 (t, J = 2.0 Hz, 1H), 7.21 (dd, J = 5.1, 3.6 Hz, 1H), 3.70 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.15 (q, J = 7.3 Hz, 2H), 3.00 (s, 4H), 2.43–2.35 (m, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C30H30ClF3N6OS+ [M + H]+ 600.18, found 600.20.
3-Chloro-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)-5-(((5-(thiophen-2-yl)pyridin-3-yl)-methyl)amino)benzamide (Compound 18).
To a solution of S3 (22 mg, 0.05 mmol, 1.0 equiv) and 5-(thiophen-2-yl) nicotinaldehyde (14 mg, 0.075 mmol, 1.5 equiv) in AcOH (1 mL), NaBH3CN (9.3 mg, 0.15 mmol, 3.0 equiv) was added at 0 °C. The mixture then was stirred at 25 °C for 2 h. LC–MS showed that S3 was consumed completely and one main peak with desired MS was detected. The reaction mixture was poured into saturated aqueous sodium bicarbonate (20 mL) and the reaction was extracted with chloroform/isopropanol (v/v = 4:1, 3 × 50 mL). The reaction mixture was then dried over Na2SO4, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by preparative HPLC to give compound 18 (15 mg, 49% yield) as a white powder. 1H NMR (500 MHz, DMSO-d6) δ 10.46 (s, 1H), 8.87 (d, J = 2.2 Hz, 1H), 8.55 (d, J = 1.9 Hz, 1H), 8.18 (d, J = 2.2 Hz, 1H), 8.12 (t, J = 2.2 Hz, 1H), 8.06 (dd, J = 8.5, 2.2 Hz, 1H), 7.73–7.63 (m, 3H), 7.24–7.17 (m, 2H), 7.15 (t, J = 1.9 Hz, 1H), 6.89 (t, J = 2.0 Hz, 1H), 4.48 (s, 2H), 3.69 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.15 (q, J = 7.3 Hz, 2H), 3.05–2.88 (m, 4H), 2.44–2.35 (m, 2H), 1.21 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C31H32ClF3N5OS+ [M + H]+ 614.20, found 614.24.
N-(3-Chloro-5-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)benzamido)phenyl)-5-(thiophen-2-yl)-nicotinamide (Compound 19).
Step 1: To a solution of 3-chloro-5-nitroaniline (150 mg, 0.87 mmol) and S4 (302 mg, 0.96 mmol) in DCM (8 mL) was added HATU (661 mg, 1.74 mmol) and DIPEA (561 mg, 4.35 mmol, 0.77 mL) at 0 °C. The mixture was stirred at 25 °C for 4 h. LC–MS showed 3-chloro-5-nitroaniline was consumed completely and one main peak with desired MS was detected. The reaction mixture was poured into water (15 mL) and the reaction was extracted with chloroform/isopropanol (v/v = 4:1, 3 × 60 mL). The organic phase was dried over Na2SO4 and filtered under reduced pressure. The crude residue was subjected to purification by flash chromatography over silica gel (0–5% MeOH/DCM) to afford the nitro compound.
Step 2: To a solution of the above compound (200 mg, 0.43 mmol) in EtOH (4 mL) and H2O (2.0 mL) were added NH4Cl (67.5 mg, 1.27 mmol) and Fe (119 mg, 2.12 mmol) at 25 °C. The mixture was stirred at 80 °C for 2 h. The reaction mixture was filtered and concentrated under reduced pressure to remove EtOH. The reaction mixture was poured into water (15 mL) and extracted with chloroform/isopropanol (v/v = 4:1, 3 × 60 mL). The combined organic phase was washed with saturated brine (30 mL), dried over Na2SO4, filtered, and concentrated in vacuum to give S5 as a white solid.
Step 3: To a solution of S5 (50 mg, 0.11 mmol) and 5-(thiophen-2-yl)nicotinic acid (35 mg, 0.17 mmol) in DMF (2 mL), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) (82 mg, 0.34 mmol) and N,N-diisopropylethylamine (DIPEA) (73 mg, 0.57 mmol) were added at 0 °C. This mixture then was stirred at 25 °C for 12 h. LC–MS showed that S5 was consumed completely and one main peak with the desired MS was detected. The reaction mixture was poured into water (15 mL) and was extracted with ethyl acetate (3 × 50 mL). The reaction mixture was then dried over Na2SO4, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by preparative HPLC to give the final compound 19 (28 mg, 39%) as white powder. 1H NMR (500 MHz, DMSO-d6) δ 10.68 (s, 1H), 10.62 (s, 1H), 9.05 (d, J = 2.3 Hz, 1H), 8.94 (d, J = 2.1 Hz, 1H), 8.43 (t, J = 2.2 Hz, 1H), 8.28 (t, J = 1.9 Hz, 1H), 8.25 (d, J = 1.9 Hz, 1H), 8.20 (dd, J = 8.1, 1.9 Hz, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.72 (dd, J = 3.7, 1.2 Hz, 1H), 7.67 (dd, J = 5.0, 1.1 Hz, 1H), 7.64 (t, J = 1.9 Hz, 1H), 7.60 (t, J = 2.0 Hz, 1H), 7.19 (dd, J = 5.1, 3.6 Hz, 1H), 3.74 (s, 2H), 3.41 (d, J = 12.0 Hz, 2H), 3.14–3.05 (m, 2H), 2.96 (d, J = 11.0 Hz, 2H), 2.88 (d, J = 12.8 Hz, 2H), 2.41–2.32 (m, 2H), 1.15 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 164.68, 164.52, 158.69, 158.42, 149.13, 147.90, 140.89, 140.78, 139.12, 134.23, 133.31, 132.35, 132.17, 131.36, 130.98, 129.96, 129.33, 128.19, 126.47, 125.81, 123.47, 116.06, 115.92, 111.18, 57.01, 51.14, 51.07, 49.95, 9.47. MS (ESI): calcd. for C31H30ClF3N5O2S+ [M + H]+ 628.18, found 628.20.
5-Chloro-N1-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)-N3-(5-(thiophen-2-yl)pyridin-3-yl)-isophthalamide (Compound 20).
Step 1: To a solution of 3-chloro-5-(methoxycarbonyl)benzoic acid (201 mg, 0.94 mmol) and S6 (245 mg, 0.85 mmol) in DCM (8 mL) were added HATU (648 mg, 1.71 mmol) and DIPEA (550 mg, 4.26 mmol, 0.75 mL) at 0 °C. The mixture was stirred at 25 °C for 4 h. LC-MS showed S6 was consumed completely and one main peak with desired MS was detected. The reaction mixture was poured into water (15 mL) and the reaction was extracted with chloroform/isopropanol (v/v = 4:1, 3 × 60 mL). The organic phase was dried over Na2SO4 and filtered under reduced pressure. The crude residue was subjected to purification by flash chromatography over silica gel (0–5% MeOH/DCM) to afford the methyl ester compound.
Step 2: To a solution of the above compound (200 mg, 0.41 mmol) in THF (3 mL) and MeOH (1 mL) was added NaOH (21.5 mg, 0.54 mmol, dissolved in 1 mL H2O) in one portion at 0 °C. The mixture was stirred at room temperature for 2 h. LC–MS showed ester was consumed completely and one main peak with desired MS was detected. The reaction mixture was concentrated in vacuo. The residue was dissolved in H2O (3 mL) and then acidified to pH 5.0 with 1.0 N aq. HCl at 0 °C. The precipitate was collected and dried under vacuum to provide S7 as pale brown solid.
Step 3: To a solution of S7 (52 mg, 0.11 mmol) and 5-(thiophen-2-yl)pyridin-3-amine (15 mg, 0.085 mmol) in DMF (2 mL), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo-[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) (65 mg, 0.17 mmol) and N,N-diisopropylethylamine (DIPEA) (58 mg, 0.43 mmol) were added at 0 °C. This mixture then was stirred at 25 °C for 12 h. LC-MS showed that 5-(thiophen-2-yl)pyridin-3-amine was consumed completely and one main peak with the desired MS was detected. The reaction mixture was poured into water (15 mL) and was extracted with ethyl acetate (3 × 50 mL). The reaction mixture was then dried over Na2SO4, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by preparative HPLC to give the final compound 20 (19 mg, 36%) as white powder. 1H NMR (500 MHz, DMSO-d6) δ 10.60 (d, J = 3.7 Hz, 1H), 9.02 (s, 1H), 8.54 (d, J = 2.2 Hz, 1H), 8.41 (d, J = 2.7 Hz, 1H), 8.19 (d, J = 2.2 Hz, 1H), 8.09 (dd, J = 8.5, 2.2 Hz, 1H), 7.79 (d, J = 2.6 Hz, 1H), 7.73 (d, J = 8.6 Hz, 1H), 7.68 (ddt, J = 6.9, 3.6, 1.8 Hz, 2H), 7.65 (q, J = 1.8 Hz, 1H), 7.56 (q, J = 1.9 Hz, 1H), 7.37–7.32 (m, 1H), 7.21 (ddd, J = 5.0, 3.7, 0.9 Hz, 1H), 3.70 (s, 2H), 3.47 (d, J = 12.0 Hz, 2H), 3.19–3.10 (m, 2H), 3.05–2.89 (m, 4H), 2.41 (d, J = 12.5 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C31H30ClF3N5O2S+ [M + H]+ 628.18, found 628.30.
N-(3-Chloro-5-((3-(trifluoromethyl)phenyl)carbamoyl)-phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 21).
The title compound was prepared according to the general procedure acid chloride pathway. White powder (23 mg, 31%). 1H NMR (500 MHz, DMSO-d6) δ 10.89 (s, 1H), 10.69 (s, 1H), 9.13 (d, J = 2.2 Hz, 1H), 9.04 (d, J = 2.0 Hz, 1H), 8.54 (t, J = 2.2 Hz, 1H), 8.28 (t, J = 1.8 Hz, 1H), 8.23 (dt, J = 5.8, 1.9 Hz, 2H), 8.08 (dd, J = 8.1, 2.0 Hz, 1H), 7.87 (t, J = 1.8 Hz, 1H), 7.80 (dd, J = 3.7, 1.2 Hz, 1H), 7.75 (dd, J = 5.1, 1.1 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.27 (dd, J = 5.0, 3.6 Hz, 1H). MS (ESI): calcd. for C24H16ClF3N3O2S+ [M + H]+ 502.06, found 502.12.
N-(3-Chloro-5-((4-((4-ethylpiperazin-1-yl)methyl)phenyl)-carbamoyl)phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 22).
The title compound was prepared according to the general procedure HATU-mediated amide formation pathway. White powder (15 mg, 34%). 1H NMR (500 MHz, DMSO-d6) δ 10.94 (s, 1H), 10.57 (s, 1H), 9.13 (d, J = 2.2 Hz, 1H), 9.05 (d, J = 2.0 Hz, 1H), 8.57 (t, J = 2.2 Hz, 1H), 8.31 (t, J = 1.7 Hz, 1H), 8.23 (t, J = 2.0 Hz, 1H), 7.89–7.83 (m, 3H), 7.83–7.80 (m, 1H), 7.76 (dd, J = 5.2, 1.1 Hz, 1H), 7.64–7.50 (m, 2H), 7.27 (dd, J = 5.1, 3.6 Hz, 1H), 4.39–3.82 (m, 6H), 3.79–3.12 (m, 6H), 1.24 (t, J = 6.7 Hz, 3H). MS (ESI): calcd. for C30H31ClN5O2S+ [M + H]+ 560.19, found 560.18.
N-(3-Chloro-5-((2-morpholinopyridin-4-yl)carbamoyl)-phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 23).
The title compound was prepared according to the general procedure acid chloride pathway. White powder (21 mg, 27%). 1H NMR (500 MHz, DMSO-d6) δ 11.01 (s, 1H), 10.91 (s, 1H), 9.14 (d, J = 2.2 Hz, 1H), 9.04 (d, J = 2.0 Hz, 1H), 8.53 (t, J = 2.2 Hz, 1H), 8.33 (t, J = 1.8 Hz, 1H), 8.21 (t, J = 1.9 Hz, 1H), 8.08 (d, J = 6.6 Hz, 1H), 7.85 (t, J = 1.7 Hz, 1H), 7.80 (dd, J = 3.6, 1.2 Hz, 1H), 7.76 (dd, J = 5.1, 1.1 Hz, 1H), 7.66 (s, 1H), 7.34 (d, J = 6.7 Hz, 1H), 7.27 (dd, J = 5.1, 3.6 Hz, 1H), 3.81–3.76 (m, 4H), 3.53 (t, J = 4.9 Hz, 4H). MS (ESI): calcd. for C26H23ClN5O3S+ [M + H]+ 520.12, found 520.27.
N-(5-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-2-fluorophenyl)-5-(thio-phen-2-yl)nicotinamide (Compound 24).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (500 MHz, DMSO-d6) δ 10.75 (s, 1H), 10.68 (s, 1H), 9.19 (d, J = 2.2 Hz, 1H), 9.10 (d, J = 2.1 Hz, 1H), 8.60 (t, J = 2.1 Hz, 1H), 8.36 (dd, J = 7.3, 2.3 Hz, 1H), 8.26 (d, J = 2.3 Hz, 1H), 8.15 (dd, J = 8.5, 2.3 Hz, 1H), 8.02 (ddd, J = 8.7, 4.6, 2.3 Hz, 1H), 7.87–7.76 (m, 3H), 7.60 (dd, J = 10.1, 8.6 Hz, 1H), 7.31 (dd, J = 4.9, 3.5 Hz, 1H), 3.75 (s, 2H), 3.60–3.43 (m, 2H), 3.20 (q, J = 7.3 Hz, 2H), 3.03 (dd, J = 26.6, 9.2 Hz, 4H), 2.46 (t, J = 12.3 Hz, 2H), 1.27 (t, J = 7.4 Hz, 3H). MS (ESI): calcd. for C31H30F4N5O2S+ [M + H] + 612.21, found 612.18.
N-(5-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-2-methoxyphenyl)-5-(thiophen-2-yl)nicotinamide (Compound 25).
The title compound was prepared according to the general procedure acid chloride pathway. 1 NMR (500 MHz, DMSO-d6) δ 10.51 (s, 1H), 10.20 (s, 1H), 9.17 (d, J = 2.3 Hz, 1H), 9.08 (d, J = 2.1 Hz, 1H), 8.59 (t, J = 2.2 Hz, 1H), 8.39 (d, J = 2.3 Hz, 1H), 8.27 (d, J = 2.3 Hz, 1H), 8.17 (d, J = 8.6 Hz, 1H), 8.01 (dd, J = 8.6, 2.3 Hz, 1H), 7.84 (d, J = 3.6 Hz, 1H), 7.78 (dd, J = 10.0, 6.7 Hz, 2H), 7.35 (d, J = 8.8 Hz, 1H), 7.31 (dd, J = 5.0, 3.6 Hz, 1H), 4.00 (s, 3H), 3.75 (s, 2H), 3.52–3.44 (m, 2H), 3.20 (q, J = 7.3 Hz, 2H), 3.12–2.94 (m, 4H), 2.44 (dd, J = 16.7, 7.1 Hz, 2H), 1.27 (t, J = 7.3 Hz, 3H). MS (ESi): calcd. for C32H33F3N5O3S+ [M + H]+ 624.23, found 624.11.
N-(3-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-4-fluorophenyl)-5-(thiophen-2-yl)nicotinamide (Compound 26).
The title compound was prepared according to the general procedure acid chloride pathway. White powder (45 mg, 39%). 1 NMR (500 MHz, DMSO-d6) δ 10.81 (s, 1H), 10.78 (s, 1H), 9.12 (d, J = 2.3 Hz, 1H), 9.03 (d, J = 2.1 Hz, 1H), 8.53 (t, J = 2.2 Hz, 1H), 8.19 (d, J = 2.3 Hz, 1H), 8.14 (dd, J = 6.3, 2.7 Hz, 1H), 8.05–7.94 (m, 2H), 7.79 (dd, J = 3.6, 1.2 Hz, 1H), 7.77–7.71 (m, 2H), 7.44 (t, J = 9.3 Hz, 1H), 7.26 (dd, J = 5.1, 3.6 Hz, 1H), 3.73 (s, 2H), 3.48 (d, J = 11.8 Hz, 2H), 3.16 (q, J = 7.3 Hz, 2H), 3.05–2.94 (m, 4H), 2.50–2.33 (m, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C31H30F4N5O2S+ [M + H]+ 612.21, found 612.24.
N-(5-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-4-fluoro-2-methylphenyl)-5-(thiophen-2-yl)nicotinamide (Compound 27).
The title compound was prepared according to the general procedure acid chloride pathway. 1 NMR (500 MHz, DMSO-d6) δ 10.76 (s, 1H), 10.43 (s, 1H), 9.17 (d, J = 2.3 Hz, 1H), 9.09 (d, J = 2.1 Hz, 1H), 8.58 (t, J = 2.2 Hz, 1H), 8.21 (d, J = 2.2 Hz, 1H), 8.05 (dd, J = 8.5, 2.2 Hz, 1H), 7.83 (dd, J = 3.6, 1.2 Hz, 1H), 7.80–7.73 (m, 3H), 7.42 (d, J = 10.9 Hz, 1H), 7.33–7.30 (m, 1H), 3.75 (s, 2H), 3.59 (s, 2H), 3.20 (p, J = 6.8 Hz, 2H), 3.08–2.93 (m, 4H), 2.47 (d, J = 17.6 Hz, 2H), 2.40 (s, 3H), 1.27 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C32H32F4N5O2S+ [M + H]+ 626.22, found 626.16.
3-Amino-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)-5-methylbenzamide (Compound 28).
The title compound was prepared according to the general procedure acid chloride pathway. White powder (55 mg, 29%). 1H NMR (500 MHz, DMSO-d6) δ 10.68 (s, 1H), 10.58 (s, 1H), 9.12 (d, J = 2.3 Hz, 1H), 9.04 (d, J = 2.0 Hz, 1H), 8.53 (t, J = 2.2 Hz, 1H), 8.22 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 1.9 Hz, 1H), 8.13 (dd, J = 8.5, 2.2 Hz, 1H), 7.86 (d, J = 2.0 Hz, 1H), 7.80 (dd, J = 3.7, 1.2 Hz, 1H), 7.76–7.71 (m, 2H), 7.60 (s, 1H), 7.26 (dd, J = 5.1, 3.6 Hz, 1H), 3.70 (s, 2H), 3.48 (d, J = 12.0 Hz, 2H), 3.16 (q, J = 7.3 Hz, 2H), 3.04–2.91 (m, 4H), 2.45 (s, 3H), 2.40 (t, J = 11.9 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C32H33F3N5O2S+ [M + H]+ 608.23, found 608.29.
N-(4-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)pyridin-2-yl)-5-(thiophen-2-yl)nicotinamide (Compound 29).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (500 MHz, DMSO-d6) δ 11.54 (s, 1H), 10.94 (s, 1H), 9.16 (d, J = 2.3 Hz, 1H), 9.13–9.06 (m, 1H), 8.75 (t, J = 1.1 Hz, 1H), 8.70 (dd, J = 3.8, 1.5 Hz, 2H), 8.28 (d, J = 2.2 Hz, 1H), 8.16 (dd, J = 8.5, 2.2 Hz, 1H), 7.86 (dd, J = 3.7, 1.2 Hz, 1H), 7.82 (d, J = 8.6 Hz, 1H), 7.79 (dd, J = 5.1, 1.2 Hz, 1H), 7.76 (dd, J = 5.1, 1.6 Hz, 1H), 7.34–7.28 (m, 1H), 3.77 (s, 2H), 3.62–3.42 (m, 2H), 3.21 (q, J = 7.3 Hz, 2H), 3.10–2.98 (m, 4H), 2.51–2.42 (m, 2H), 1.27 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C30H30F3N6O2S+ [M + H]+ 595.21, found 595.11.
N-(4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)-phenyl)-5-(5-(thiophen-2-yl)nicotinamido)nicotinamide (Compound 30).
The title compound was prepared according to the general procedure acid chloride pathway. 1H NMR (500 MHz, DMSO-d6) δ 11.04 (s, 1H), 10.89 (s, 1H), 9.22 (d, J = 2.3 Hz, 2H), 9.13 (d, J = 2.1 Hz, 1H), 9.02 (d, J = 2.0 Hz, 1H), 8.82 (t, J = 2.2 Hz, 1H), 8.62 (t, J = 2.1 Hz, 1H), 8.29 (d, J = 2.2 Hz, 1H), 8.18 (dd, J = 8.5, 2.2 Hz, 1H), 7.87 (dd, J = 3.6, 1.2 Hz, 1H), 7.85–7.79 (m, 2H), 7.33 (dd, J = 5.1, 3.6 Hz, 1H), 3.78 (s, 2H), 3.54 (d, J = 12.0 Hz, 2H), 3.23 (q, J = 7.3 Hz, 2H), 3.13–2.99 (m, 4H), 2.52–2.41 (m, 2H), 1.29 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C30H30F3N6O2S+ [M + H]+ 595.21, found 595.11.
N-(3-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-5-fluorophenyl)-5-(thiophen-2-yl)nicotinamide (Compound 31).
The title compound was prepared according to the general procedure acid chloride pathway. White powder (13 mg, 23%). 1H NMR (500 MHz, DMSO-d6) δ 11.04 (s, 1H), 10.75 (s, 1H), 9.22 (d, J = 2.3 Hz, 1H), 9.13 (d, J = 2.1 Hz, 1H), 8.63 (t, J = 2.2 Hz, 1H), 8.32–8.25 (m, 2H), 8.20 (dd, J = 8.4, 2.2 Hz, 1H), 8.10 (dt, J = 10.9, 2.2 Hz, 1H), 7.89 (dd, J = 3.6, 1.2 Hz, 1H), 7.84 (dt, J = 6.3, 1.5 Hz, 2H), 7.72 (ddd, J = 9.2, 2.4, 1.4 Hz, 1H), 7.35 (dd, J = 5.1, 3.6 Hz, 1H), 3.81 (s, 2H), 3.57 (d, J = 11.9 Hz, 2H), 3.24 (q, J = 7.3 Hz, 2H), 3.14–3.00 (m, 4H), 2.52 (t, J = 12.0 Hz, 2H), 1.31 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C31H30F4N5O2S+ [M + H]+ 612.21, found 612.16.
N-(3-Bromo-5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 32).
The title compound was prepared according to the general procedure acid chloride pathway. White powder (30 mg, 27%). 1H NMR (500 MHz, DMSO-d6) δ 10.88 (s, 1H), 10.69 (s, 1H), 9.14 (d, J = 2.3 Hz, 1H), 9.04 (d, J = 2.0 Hz, 1H), 8.54 (t, J = 2.2 Hz, 1H), 8.36 (t, J = 1.7 Hz, 1H), 8.34 (t, J = 1.9 Hz, 1H), 8.20 (d, J = 2.2 Hz, 1H), 8.12 (dd, J = 8.5, 2.2 Hz, 1H), 7.99 (t, J = 1.6 Hz, 1H), 7.80 (dd, J = 3.6, 1.2 Hz, 1H), 7.78–7.73 (m, 2H), 7.27 (dd, J = 5.1, 3.6 Hz, 1H), 3.72 (s, 2H), 3.48 (d, J = 11.9 Hz, 2H), 3.16 (q, J = 7.3 Hz, 2H), 3.07–2.92 (m, 4H), 2.42 (t, J = 12.3 Hz, 2H), 1.22 (t, J = 7.3 Hz, 3H). MS (ESI): calcd. for C31H30BrF3N5O2S+ [M + H]+ 672.13, found 672.21.
N-(3-((4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-5-(trifluoromethyl)-phenyl)-5-(thiophen-2-yl)nicotinamide (Compound 33).
The title compound was prepared according to the general procedure acid chloride pathway. White powder (11 mg, 20%). 1H NMR (500 MHz, CD3OD) δ 9.01 (t, J = 2.2 Hz, 2H), 8.59 (t, J = 2.1 Hz, 1H), 8.57 (t, J = 1.8 Hz, 1H), 8.36 (t, J = 1.9 Hz, 1H), 8.14 (d, J = 2.2 Hz, 1H), 8.04 (q, J = 1.3 Hz, 1H), 7.96 (dd, J = 8.5, 2.3 Hz, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.65 (dd, J = 3.7, 1.2 Hz, 1H), 7.57 (dd, J = 5.1, 1.1 Hz, 1H), 7.19 (dd, J = 5.1, 3.7 Hz, 1H), 3.68 (d, J = 1.7 Hz, 2H), 2.89–2.41 (m, 10H), 1.16 (t, J = 7.2 Hz, 3H). MS (ESI): calcd. for C32H30F3N6O2S+ [M + H]+ 662.20, found 662.26.
METHODS
Culture Conditions.
P. falciparum strains, unless otherwise noted, were cultured in an adapted Trager and Jansen method.39 Parasites were maintained at 4% hematocrit with A+ erythrocytes in RPMI 1640 medium supplemented with 26 mM sodium bicarbonate, 25 mM HEPES, 2% dextrose, 15 mg/L hypoxanthine, 25 mg/L gentamycin, and 0.5% AlbuMAX II (ThermoFisher, Waltham MA). P. falciparum strains Dd2 (MRA-156) and 3D7 (MRA-152) were incubated at 5% CO2 and 37 °C while Cambodian clinical isolates, strains IPC 5188 (MRA-1239), strain IPC 5202 (MRA-1240), and IPC_6261 (MRA-1284) (BEI Resources, Manassas, VA) were incubated in 90% N2, 5% O2, and 5% CO2 at 37 °C in a humidified modular incubator chamber (Billups Rothenberg, San Diego, CA, USA). Human HepG2 and MCF7 lines were maintained in Eagle’s Minimal Essential Medium, supplemented with 1% antibiotic–antimycotic solution (Gemini Bio, West Sacramento, CA) and 10% fetal bovine serum in 5% CO2 at 37 °C.
SYBR Green I Proliferation Assay.
This method was based on the previously described SYBR Green I-based assay.18 Briefly, intraerythrocytic P. falciparum cultures containing primarily ring stage (except for data reported in Table 1 where asynchronous cultures were used) were plated at 1% hematocrit and 1% parasitemia in 96-well flat-bottomed black plates with compound and incubated at 37 °C in 5% CO2. As a control for no parasite growth, 5 μM chloroquine was used. The final DMSO concentration did not exceed 0.2%. After 72 h, plates were subjected to one freeze–thaw cycle at –80 °C and given 100 μL of lysis buffer (20 mM Tris-HCl, 5 mM EDTA, 0.8% Triton X-100, 0.08% saponin, and 1× SYBR Green I (Thermo Fisher, Waltham, MA)). Plates were incubated at RT, protected from light for 1 h, and read with a BioTek Synergy Neo2 multimode microplate reader (Winooski, VT). Fluorescence emission was detected at 485 nm excitation and 530 nm emission. Resulting values were normalized with full growth untreated controls and no growth chloroquine-treated controls. CDD Vault (Burlingame, CA) was used to calculate EC50 values from at least three biological replicates.
Cytotoxicity Assay.
Human HepG2 hepatocytes (ATCC ID: HB-8065) or human MCF7 mammary gland adenocarcinoma (ATCC ID: HB-22) were seeded in clear flat-bottomed 384-well plates at a density of 2250 cells or 1000 cells per well, respectively. After 24 h of incubation in 5% CO2 at 37 °C, the compound was applied in serial dilution, with DMSO concentrations not exceeding 0.25% and incubated for another 48 h. Negative control wells were treated for 10 min with 5% Triton X-100, and all wells received 10 μL CellTiter 96 AQueous One Solution Reagent MTS 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium reagent (Promega, Madison, WI) and incubated at 37 °C for 3 h. The plate was then read at an absorbance of 490 nm with a BioTek Synergy Neo2 multimode microplate reader (Winooski, VT). Untreated cells and Triton X-100-treated cells were used to normalize the results, and EC50 was calculated with CDD Vault (Burlingame, CA).
Rate of Killing Assay.
The assay used to evaluate the rate of killing was adapted from a previously described method.40 Blood stage P. falciparum Dd2 cultures were plated at 4% hematocrit and 1% parasitemia into a 24-well plate, and wells were given 10 × EC50 of compound 1 and incubated in standard culture conditions for 12, 24, or 48 h. At this point, the culture was washed once with fresh media, and parasites were monitored every 24 h thereafter up to 96 h. Per time point, Giemsa-stained thin blood smears were made, and culture samples were collected, washed in 0.5% BSA, and incubated with 60 μM MitoTracker Deep Red FM (Thermo Fisher, Waltham, MA) and 1× SYBR Green I (Thermo Fisher, Waltham, MA) at 37 °C for 20 min. Samples were again washed in 0.5% BSA and resuspended in PBS. Samples were evaluated with a CytoFLEX S (Beckman Coulter, Brea, CA) flow cytometer, recording 100,000 events per sample. Data analysis was performed with FlowJo Version 10 software (Ashland, OR). The proportion of viable parasites was acquired by calculating the percent of SYBR Green I and MitoTracker double-positive cells. Gating was performed with the aid of four control samples: uninfected erythrocytes, untreated parasite culture, untreated culture stained only with MitoTracker, and DHA-treated culture.
Parasite Reduction Ratio (PRR) Assay.
This assay was based on the previously described Parasite Reduction Ratio (PRR) assay.26 P. falciparum 3D7 blood-stage parasites were synchronized to mature schizonts with Percoll gradient or gelatin flotation.41,42 During reinvasion at 0–3 HPI, culture was shaken in a humidified chamber with 90% N2, 5% O2, and 5% CO2 at 37 °C to prevent multiple infected RBCs, and this was followed by sorbitol synchronization.43 Parasitemia was counted with Giemsa-stained thin blood smears, and the culture was diluted to 2% hematocrit and 0.5% parasitemia (1 × 106 parasites/mL). Culture is treated with 10 × EC50 of the compound, and every 24 h the compound is washed out and media replenished until 120 h, which corresponds to two and a half asexual life cycles. At each time point, samples of the washed culture were serially diluted 1:3 in a round-bottom 96-well plate with uninfected RBCs. These plates were incubated for 21 days, with media change twice a week and hematocrit supplementation once a week. On day 21, plates were frozen, and parasite growth was assessed with a SYBR Green I-based assay as described above. The number of viable parasites per time point was calculated using the following formula: , where is the serial dilution factor and is the number of wells with parasite growth. Using GraphPad Prism 9 (San Diego, CA), the log((# viable parasites) + 1) was graphed as a function of time, and the lag phase, parasite reduction rate (PRR), and 99.9% parasite clearance time (PCT) were determined from the curve.
Stage Specific Assay.
The stage-specific assay was performed as previously described.44 Blood stage P. falciparum Dd2 parasites were synchronized to select early rings, 0–3 HPI. The first synchronization was with a magnetic MACS LD column (Cat. # 130–042–901, Miltenyi Biotec, Gaithersburg, MD), followed by 5% sorbitol treatment 3 h later.45,43 At 6 HPI, parasites were plated onto a 96-well plate at 1% hematocrit and 1% parasitemia, and 5 × EC50 of compound 1 was added to the 6 HPI treatment wells. At intervals of 12 h, samples were washed in PBS and fixed in 4% paraformaldehyde in PBS, supplemented with 0.075% glutaraldehyde. Giemsa-stained thin blood smears were also realized. At 54 HPI, samples were permeabilized with 0.25% Triton X-100 and treated with 50 μg/mL RNase A before being stained with 500 nM YOYO-1 (Thermo Fisher, Waltham, MA). Finally, samples were washed in PBS and analyzed with a CytoFLEX S flow cytometer (Beckman Coulter, Brea, CA). Per sample, 100,000 events were collected, and uninfected RBCs served as a gating control. Analysis of results was performed with FlowJo software Version 10. For microscopy images, thin blood smears were fixed in methanol, stained in a 2% Giemsa solution for 12 min, and rinsed with distilled water. Images were acquired with a Nikon ECLIPSE Si microscope and a Nikon Plan Fluor 100×/1.3 oil immersion objective (Nikon Instruments, Melville, NY) and analyzed with the Nikon NIS-Elements Version 5.30.04 software.
Stage Specific SYBR Green I Proliferation Assay.
Determination of stage-specific EC50 was performed using a modified SYBR Green I proliferation assay, as described above. In this version, P. falciparum 3D7 was tightly synchronized to 0–3 HPI maintained at 37 °C in a humidified chamber with 90% N2, 5% O2, and 5% CO2. Every 10 h, 96-well black plates were given compound and parasites at 1% parasitemia, and 1% hematocrit. After 72 h, plates were frozen at −80 °C and read as described above.
Liver Stage Activity Assay.
Liver stage activity was evaluated as previously described.46 Briefly, 3 × 103 of HepG2-A16-CD81 cells were seeded in 1536-well plates (Greiner Bio, Frickenhausen, Germany). Then, cells were infected with 1 × 103 luciferase-expressing P. berghei sporozoites and incubated for a further 48 h. For both control compounds and compound 1, 50 nL was added in a 1:3 serial dilution of 12 concentrations and incubated for 18 h. Per well, 2 μL of BrightGlo luciferin reagent (Promega, Madison, WI) was added, and luciferase activity was measured with a PerkinElmer Envision multimode plate reader. Values were normalized to maximum inhibition (0.25 μM atovaquone) and minimum inhibition (DMSO). EC50 values were calculated with the CDD Vault (Burlingame, CA).
Evaluation in Ex Vivo Clinical Isolates.
The method of evaluating the effect of compound 1 ex vivo was based on a previous study.47 Clinical isolates of P. falciparum were obtained from patients from the Masafu General Hospital in the Busia district of Uganda, the Tororo District Hospital, and the Patongo Health Center. All patients provided written consent, with the consent for minors provided by a parent/guardian, and assent was provided by patients between 8 and 17 years old. Approval for this study was provided by the Uganda National Council for Science and Technology, the Makerere University Research and Ethics Committee, and the University of California Committee on Human Research. Patients were at least 6 months old and had clinical, but not severe, malaria symptoms and confirmation of infection via Giemsa-stained blood smears. Patients were excluded from the study if they had received treatment for malaria within the past 30 days or were suspected to be infected with other Plasmodium species. Prior to patient treatment, 2–5 mL of patient blood was collected in a heparin collection tube. Parasitemia was determined by counting at least 1000 RBCs on Giemsa-stained blood smears, and only samples with a parasitemia of at least 0.3% were utilized. Blood was washed 3× with RPMI 1640 at 37 °C. Pellets were resuspended in RPMI 1640 supplemented with 25 mM HEPES, 24 mM sodium bicarbonate, 10 μg/mL gentamicin, 0.1 mM hypoxanthine, 0.5% AlbuMAX II (ThermoFisher, Waltham MA) to a hematocrit of 50%. To assess compound efficacy, compound 1 was serially diluted in 96-well plates. As controls, there were wells without compounds and wells without parasites. In addition to the clinical isolates, the lab-adapted strains P. falciparum Dd2 and 3D7 are used as controls. Since the clinical isolates are primarily ring stage, 3D7, and Dd2 cultures are synchronized by collecting flow-through from a magnetic MACS column (Miltenyi Biotec, Gaithersburg, MD), as previously described, to isolate ring stage parasites for use in the assay.45 Per well, parasites were plated at 0.2% parasitemia and 2% hematocrit, using O+ uninfected RBCs. Plates were incubated for 72 h in 90% N2, 5% O2, and 5% CO2 at 37 °C in a humidified modular chamber (Billups Rothenberg, San Diego, CA, USA). Per well, 100 μL of culture was added to a black 96-well plate with 100 μL lysis buffer (20 mM Tris, 5 mM EDTA, 0.08% Triton X-100, 0.008% saponin, and 0.2 μL/mL SYBR Green I (Thermo Fisher, Waltham, MA)). Plates were mixed and incubated at RT for 1 h, protected from light. Fluorescence was read at 485 nM excitation and 530 nm emission with a FLUOstar Omega multimode microplate reader (BMG LabTech, Cary, NC).
Ring Stage Survival Assay (RSA) and Piperaquine Survival Assay (PSA).
The ring-stage survival assay (RSA) and piperaquine survival assay (PSA) are based on a previously described assay.29,31 Briefly, the P. falciparum strains 3D7 (MRA-152) and the Cambodian clinical isolates, strains IPC 5188 (MRA-1239), strain IPC 5202 (MRA-1240), and IPC 6261 (MRA-1284) (BEI Resources, Manassas, VA) were cultured in general conditions, as previously described, incubated in a humidified chamber in 90% N2, 5% O2, and 5% CO2 at 37 °C. Parasites were tightly synchronized as via gelatin flotation to enrich in schizonts, and merozoites were allowed to reinvade for 3 h before sorbitol treatment.42 Cultures were then diluted to 1% parasitemia and 2% hematocrit for compound treatment. For the RSA, 700 nM of dihydroartemisinin (DHA) was used as a control, and for the PSA, 200 nM piperaquine (PPQ) was used. For both compounds 1 and 16, 10 × EC50 was used. For the RSA, parasites were treated for 6 h before the compound was washed out and media replenished, whereas the treatment time was 48 h for the PSA. For both assays, parasitemia was determined via Giemsa-stained thin blood smears 72 h after the start of the assay. The parasite survival rate, represented as % survival, is calculated as follows:
Cross Resistance Evaluation.
Compound efficacy was tested in P. falciparum lines with mutations to known antiplasmodials, and these compounds were used as corresponding controls: AcAS A597 V (compound MMV084978), CARL I1139K (compound GNF179), and PI4K S1320L (compound KDU961). Dd2 was used as a wildtype control, and artemisinin was used as an antiplasmodial control compound. The evaluation was performed using the SYBR Green I assay, as previously described. Briefly, strains were cultivated at 5% hematocrit in media comprised of RPMI with L-glutamine, 38.4 mM HEPES, 3.4 mM NaOH, 0.2% sodium bicarbonate, 0.05 mg/mL gentamicin, 0.2% glucose, 0.2% Albumax (ThermoFisher, Waltham MA), and 4.3% human serum. For screening, compounds were dispensed into a 1536-well black, clear-bottomed plate, and parasites were seeded in screening media (lacking phenol red) to a final hematocrit of 2.5%, final parasitemia of 0.3%, and total volume of 8 μL. Plates were cultured for 72 h in 93% N2/3% CO2/1% CO2 at 37 °C. Then per well, 2 μL of SYBR Green I in lysis buffer (20 mM Tris-HCl, 1.6% Triton-X, 0.16% saponin, 5 mM EDTA, and 10× SYBR Green I), and plates are incubated in the dark for 24 h at RT. In a 2104 EnVision Multilabel Reader (PerkinElmer, Waltham, MA), fluorescence is detected in 485 nm excitation and 530 nm emission. The assay was performed in a biological duplicate.
Resistance Line Generation.
Four flasks were each inoculated with 1 × 108 blood-stage P. falciparum Dd2-B2 parasites at 1% hematocrit. One flask remained untreated, and three were given 1 × EC50 of compound 1, as determined by a SYBR Green I-based assay on primarily ring-stage parasites. Parasitemia was monitored every 48/72 h, and if parasitemia increased, parasite levels were renormalized to 1.2 × 108 parasites per flask.
Minimum Inoculum of Resistance (MIR) Assay.
Blood stage P. falciparum Dd2-B2 cultures were maintained in RPMI-1640 media that has been supplemented with 0.225% sodium bicarbonate, 25 mM HEPES, 50 mg/L hypoxanthine, 2 mM l-glutamine, 0.5% (w/v) AlbuMAX II (Invitrogen, Waltham, MA), 10 μg/mL gentamycin, and at 3% hematocrit in human O+ erythrocytes. Cultures were incubated at 37 °C in 5% CO2, 5% O2, and 90% N2. To determine the EC10 to be used in the minimum inoculum of resistance assay, a flow cytometry-based drug susceptibility assay was performed. Ring-stage P. falciparum Dd2-B2 parasites were diluted to 1% hematocrit and 0.2% parasitemia and exposed for 72 h to compound 1 in a 1:2 serial dilution of 10 concentrations, with final DMSO concentration not exceeding 0.5%. As a control, untreated compounds were used. After 72 h, parasite viability was evaluated by staining parasites with SYBR Green I nucleic acid dye and MitoTracker Deep Red mitochondrial vitality dye and quantified with a flow cytometer (Sartorius, Göttingen, Germany). The minimum inoculum of resistance was determined by exposing two different inoculum sizes to 3 × IC10 (160.32 nM). One selection was composed of 1.2 × 106 parasites in 12 wells (1.4 × 107 total) and 1 × 107 parasite in 3 wells (3 × 107 total). Following exposure, parasites were monitored via thin blood smear 3× per week for 50 days.
Metabolic Stability in Mouse Liver Microsome and Pharmacokinetics Evaluation.
Metabolic Stability in liver microsome data and pharmacokinetic data were obtained as a fee-for-service from UF Scripps Biomedical Research.
Kinetic Solubility.
Kinetic solubility was performed as a fee-for-service from Analiza, Inc., using a miniaturized shake-flask method. Briefly, 50-fold dilutions of the compound were added to the assay buffer (1× PBS, pH 7.4) and incubated with 200 rpm rotary shaking for 24 h at room temperature. The buffer was vacuum-filtered and the filtrate was quantitated by chemiluminescent nitrogen detection (CLND).
Caco-2 Permeability.
Permeability in Caco-2 cells was determined as a fee-for-service by WuXi AppTec Co, Ltd. In brief, Caco-2 cells were seeded onto polyethylene membranes in 96-well plates and treated with 2 μM compound diluted in Hank’s buffered saline solution with 10 mM HEPES, pH 7.4. As controls, 10 μM digoxin and 2 μM each of metorolol and nadolol were used. After incubation for 2 h at 37 °C in 5% CO2, samples were treated with acetonitrile and centrifuged. For the controls, the supernatant was diluted with ultrapure water. Using LC-MS/MS and the peak area ratio of analyte/internal standard, sample, and control compounds were quantified in the starting, donor, and receiver solutions. Afterward, the integrity of the Caco-2 layer was evaluated with a lucifer yellow rejection assay.
In Vivo Evaluation.
Compound 1 was evaluated for efficacy as a prophylactic (single dose through IV injections, 6 h before infection) and therapeutic (4-day Peters test, four doses administered intravenously at 1-, 2-, 3-, and 4- days postinfection). Two doses were tested: 15 and 50 mg/kg. Groups consisted of 5 female Swiss webster mice (Charles River Laboratories International, Inc.), 6 weeks old, and luciferase-expressing rodent malaria parasites, P. berghe-ANKA-GFP-Luc SMcon (P. bergheiLuc) were used.48 P. bergheiLuc parasites come from infected Anopheles stephensi mosquitos raised at the SporoCore, University of Georgia. As controls, an uninfected group, an infected and untreated group (injected only with 200 μL of the vehicle resuspension: 27% DMSO, 1% methylcellulose, 0.5% Tween-80, and 71.5% ultrapure water), and a group treated with the known antiplasmodial compound GNF179 (Chemstep, CS-2796) were used. Compound 1 was dissolved in a vehicle containing 27% DMSO, 1% methylcellulose, 0.5% Tween-80, and 71.5% ultrapure water, and GNF179 was resuspended in 34% DMSO, 25% PEG400, 5% Tween-80, and 36% saline. For the prophylactic model, mice were infected with 105 P. bergheiLuc sporozoites by IV injection (parasites resuspended in 200 μL of DMEM media (DMEM media supplemented with 5% FBS, 146 mg/mL glutamine, 500 units of penicillin, and 500 μg/mL streptomycin)). For the therapeutic model, mice were infected with 107 infected RBCs (200 μL of cryopreserved solution of infected RBC) by IP injection (from a cryopreserved infected blood solution containing ~1000 μL mouse infected RBC (10% parasitemia) resuspended in 300 μL heparin (1 unit/μL) and 4000 μL of freezing solution (15% glycerol, 2.5% FBS and 80% RPMI media)). The parasitemia was monitored from 2 days post-infection (DPI) to 16 DPI by flow cytometry (BD FACSCanto II Flow Cytometry System and FlowJo Software), 6.4 μL of whole blood obtained from the tail of each mouse was resuspended in 320 μL PBS 1× with heparin (0.5 units/ml), the sample was then stained with PBS-SYBR Green I 0.125× (S7567, Invitrogen) for 30 min at 37 °C, and the readouts were performed by flow cytometry. The images were obtained through an In Vivo Imaging Instrument (IVIS); mice anesthetized with isoflurane in the vaporizer system (Caliper Life Sciences, XGI-8, gas anesthesia system) were imaged during 120 s using IVIS equipment (IVIS Lumina LT Series III, PerkinElmer, Waltham, MA, and Living Image 4.3.1 Software). In vivo assays were performed following an approved protocol (S13013) by the Institutional Animal Care and Use Committee (IACUC) from UCSD.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the Thermo Fisher Scientific Center for Multiplexed Proteomics at Harvard Medical School (http://tcmp.hms.edu), as well as the lab of David Fidock of Columbia University Irving Medical Center. This work was supported by a grant from NIH AI172066 (to DC, NSG, and EAW). The following reagent was obtained through BEI Resources, NIAID, NIH: Plasmodium falciparum, Strain Dd2, MRA-156, contributed by Thomas E. Wellems. The following reagent was obtained through BEI Resources, NIAID, NIH: Plasmodium falciparum, Strain 3D7, MRA-102, contributed by Daniel J. Carucci. The following reagents were obtained through BEI Resources, NIAID, NIH: Plasmodium falciparum, Strain IPC_6261, MRA-1284; Plasmodium falciparum, Strain IPC 5202, MRA-1240; and Plasmodium falciparum, Strain IPC 5188, MRA-1239; all of which were contributed by Didier Ménard. Infected mosquitos from the SporoCore, University of Georgia, and in vivo assays were supported by the grants from NIH 1R01Al152533 (EAW), R01Al169892, and from Bill and Melinda Gates Foundation grant number INV-039628 (EAW).
ABBREVIATIONS
- ACT
artemisinin combination therapy
- DHA
dihydroartemisinin
- DPI
days post infection
- EC50
half maximal effective concentration
- HPI
hours post invasion
- IC50
half maximal inhibitory concentration
- MIR
minimum inoculum of resistance
- Pf
Plasmodium falciparum
- PPQ
piperaquine
- PRR
parasite reduction ratio
- PSA
piperaquine survival assay
- RBC
red blood cell
- RI
resistance index
- RSA
ring stage survival assay
- RT
room temperature
- SD
standard deviation
- SEM
standard error of the mean
- SI
selectivity index
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c02046.
LC-MS data for compounds 1–33. 1H NMR spectra for compounds 1–33 (PDF)
Molecular formula strings(CSV)
The authors declare the following competing financial interest(s): Nathanael Gray is a founder, science advisory board member (SAB) and equity holder in Syros, C4, Allorion, Lighthorse, Voronoi, Inception, Matchpoint, CobroVentures, GSK, Larkspur (board member), Shenandoah (board member) and Soltego (board member). The Gray lab receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Jansen, Kinogen, Arbella, Deerfield, Springworks, Interline and Sanofi.
●Affiliations for K.A.S., L.M., N.M., and F.R. are those in which the research was conducted.
Contributor Information
Lushun Wang, Department of Chemical and Systems Biology, ChEM-H, Stanford Cancer Institute, School of Medicine, Stanford University, Stanford, California 94305, United States.
Monica J. Bohmer, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, University of Central Florida, Orlando, Florida 32826, United States.
Jinhua Wang, Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02215, United States; Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States.
Flore Nardella, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, University of Central Florida, Orlando, Florida 32826, United States.
Jaeson Calla, Department of Pediatrics, School of Medicine, University California, San Diego, La Jolla, California 92093, United States.
Mariana Laureano De Souza, Department of Pediatrics, School of Medicine, University California, San Diego, La Jolla, California 92093, United States.
Kyra A. Schindler, Department of Microbiology and Immunology, Columbia University Irving Medical Center, New York, New York 10032, United States
Lukas Montejo, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, University of Central Florida, Orlando, Florida 32826, United States.
Nimisha Mittal, Department of Pediatrics, School of Medicine, University California, San Diego, La Jolla, California 92093, United States.
Frances Rocamora, Department of Pediatrics, School of Medicine, University California, San Diego, La Jolla, California 92093, United States.
Mayland Treat, School of Public Health, University of California, Berkeley, California 94704, United States.
Jordan T. Charlton, Department of Natural Sciences and Mathematics, Dominican University of California, San Rafael, California 94901, United States
Patrick K. Tumwebaze, Infectious Disease Research Collaboration, Kampala, Uganda
Philip J. Rosenthal, Department of Medicine, University of California, San Francisco, California 94110, United States
Roland A. Cooper, Department of Natural Sciences and Mathematics, Dominican University of California, San Rafael, California 94901, United States
Ratna Chakrabarti, Division of Cancer Research, Burnett School of Biomedical Sciences, University of Central Florida, Orlando, Florida 32826, United States.
Elizabeth A. Winzeler, Department of Pediatrics, School of Medicine, University California, San Diego, La Jolla, California 92093, United States
Debopam Chakrabarti, Division of Molecular Microbiology, Burnett School of Biomedical Sciences, University of Central Florida, Orlando, Florida 32826, United States.
Nathanael S. Gray, Department of Chemical and Systems Biology, ChEM-H, Stanford Cancer Institute, School of Medicine, Stanford University, Stanford, California 94305, United States
REFERENCES
- (1).World Malaria Report 2022; World Health Organization, 2022. [Google Scholar]
- (2).Uwimana A; Legrand E; Stokes BH; Ndikumana JM; Warsame M; Umulisa N; Ngamije D; Munyaneza T; Mazarati JB; Munguti K; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med 2020, 26 (10), 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Diallo BN; Swart T; Hoppe HC; Tastan Bishop O; Lobb K Potential repurposing of four FDA approved compounds with antiplasmodial activity identified through proteome scale computational drug discovery and in vitro assay. Sci. Rep 2021, 11 (1), 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ayala-Aguilera CC; Valero T; Lorente-Macias A; Baillache DJ; Croke S; Unciti-Broceta A Small Molecule Kinase Inhibitor Drugs (1995–2021): Medical Indication, Pharmacology, and Synthesis. J. Med. Chem 2022, 65 (2), 1047–1131. [DOI] [PubMed] [Google Scholar]
- (5).Ferguson FM; Gray NS Kinase inhibitors: the road ahead. Nat. Rev. Drug Discov 2018, 17 (5), 353–377. [DOI] [PubMed] [Google Scholar]
- (6).Derbyshire ER; Zuzarte-Luis V; Magalhaes AD; Kato N; Sanschagrin PC; Wang J; Zhou W; Miduturu CV; Mazitschek R; Sliz P; et al. Chemical interrogation of the malaria kinome. Chembiochem 2014, 15 (13), 1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Burrows JN; Duparc S; Gutteridge WE; Hooft van Huijsduijnen R; Kaszubska W; Macintyre F; Mazzuri S; Mohrle JJ; Wells TNC New developments in anti-malarial target candidate and product profiles. Malar J. 2017, 16 (1), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Adderley J; Williamson T; Doerig C Parasite and Host Erythrocyte Kinomics of Plasmodium Infection. Trends Parasitol 2021, 37 (6), 508–524. [DOI] [PubMed] [Google Scholar]
- (9).Arendse LB; Wyllie S; Chibale K; Gilbert IH Plasmodium Kinases as Potential Drug Targets for Malaria: Challenges and Opportunities. ACS Infect Dis 2021, 7 (3), 518–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Cabrera DG; Horatscheck A; Wilson CR; Basarab G; Eyermann CJ; Chibale K Plasmodial Kinase Inhibitors: License to Cure? J. Med. Chem 2018, 61 (18), 8061–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ong HW; Adderley J; Tobin AB; Drewry DH; Doerig C Parasite and host kinases as targets for antimalarials. Expert Opin. Ther. Targets 2023, 27 (2), 151–169, DOI: 10.1080/14728222.2023.2185511. [DOI] [PubMed] [Google Scholar]
- (12).Wang B; Wu H; Hu C; Wang H; Liu J; Wang W; Liu Q An overview of kinase downregulators and recent advances in discovery approaches. Signal Transduct Target Ther 2021, 6 (1), 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Liu Y; Gray NS Rational design of inhibitors that bind to inactive kinase conformations. Nat. Chem. Biol 2006, 2 (7), 358–364. [DOI] [PubMed] [Google Scholar]
- (14).Schneider EV; Bottcher J; Huber R; Maskos K; Neumann L Structure-kinetic relationship study of CDK8/CycC specific compounds. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (20), 8081–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Copeland RA; Pompliano DL; Meek TD Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discov 2006, 5 (9), 730–739. [DOI] [PubMed] [Google Scholar]
- (16).Kesely KR; Pantaleo A; Turrini FM; Olupot-Olupot P; Low PS Inhibition of an Erythrocyte Tyrosine Kinase with Imatinib Prevents Plasmodium falciparum Egress and Terminates Parasitemia. PLoS One 2016, 11 (10), No. e0164895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ong HW; Truong A; Kwarcinski F; de Silva C; Avalani K; Havener TM; Chirgwin M; Galal KA; Willis C; Kramer A; et al. Discovery of potent Plasmodium falciparum protein kinase 6 (PfPK6) inhibitors with a type II inhibitor pharmacophore. Eur. J. Med. Chem 2023, 249, No. 115043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Dery V; Duah NO; Ayanful-Torgby R; Matrevi SA; Anto F; Quashie NB An improved SYBR Green-1-based fluorescence method for the routine monitoring of Plasmodium falciparum resistance to anti-malarial drugs. Malar J. 2015, 14, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Choi Y; Syeda F; Walker JR; Finerty PJ Jr.; Cuerrier D; Wojciechowski A; Liu Q; Dhe-Paganon S; Gray NS Discovery and structural analysis of Eph receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett 2009, 19 (15), 4467–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chiodi D; Ishihara Y ″Magic Chloro″: Profound Effects of the Chlorine Atom in Drug Discovery. J. Med. Chem 2023, 66 (8), 5305–5331. [DOI] [PubMed] [Google Scholar]
- (21).Lin FY; MacKerell AD Jr. Do Halogen-Hydrogen Bond Donor Interactions Dominate the Favorable Contribution of Halogens to Ligand-Protein Binding? J. Phys. Chem. B 2017, 121 (28), 6813–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zhu Z; Xu Z; Zhu W Interaction Nature and Computational Methods for Halogen Bonding: A Perspective. J. Chem. Inf Model 2020, 60 (6), 2683–2696. [DOI] [PubMed] [Google Scholar]
- (23).Meister S; Plouffe DM; Kuhen KL; Bonamy GM; Wu T; Barnes SW; Bopp SE; Borboa R; Bright AT; Che J; et al. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 2011, 334 (6061), 1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Peters W. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol 1975, 69 (2), 155–171. [PubMed] [Google Scholar]
- (25).Somsak V; Srichairatanakool S; Yuthavong Y; Kamchonwongpaisan S; Uthaipibull C Flow cytometric enumeration of Plasmodium berghei-infected red blood cells stained with SYBR Green I. Acta Trop 2012, 122 (1), 113–118. [DOI] [PubMed] [Google Scholar]
- (26).Sanz LM; Crespo B; De-Cozar C; Ding XC; Llergo JL; Burrows JN; Garcia-Bustos JF; Gamo FJP falciparum in vitro killing rates allow to discriminate between different antimalarial mode-of-action. PLoS One 2012, 7 (2), No. e30949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Klonis N; Xie SC; McCaw JM; Crespo-Ortiz MP; Zaloumis SG; Simpson JA; Tilley L Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (13), 5157–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ariey F; Witkowski B; Amaratunga C; Beghain J; Langlois AC; Khim N; Kim S; Duru V; Bouchier C; Ma L; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505 (7481), 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Witkowski B; Amaratunga C; Khim N; Sreng S; Chim P; Kim S; Lim P; Mao S; Sopha C; Sam B; et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 2013, 13 (12), 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Witkowski B; Khim N; Chim P; Kim S; Ke S; Kloeung N; Chy S; Duong S; Leang R; Ringwald P; et al. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob. Agents Chemother 2013, 57 (2), 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Duru V; Khim N; Leang R; Kim S; Domergue A; Kloeung N; Ke S; Chy S; Eam R; Khean C; et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 2015, 13, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Leang R; Taylor WR; Bouth DM; Song L; Tarning J; Char MC; Kim S; Witkowski B; Duru V; Domergue A; et al. Evidence of Plasmodium falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment. Antimicrob. Agents Chemother 2015, 59 (8), 4719–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Summers RL; Pasaje CFA; Pisco JP; Striepen J; Luth MR; Kumpornsin K; Carpenter EF; Munro JT; Lin; Plater A; et al. Chemogenomics identifies acetyl-coenzyme A synthetase as a target for malaria treatment and prevention. Cell Chem. Biol 2022, 29 (2), 191–201.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).LaMonte G; Lim MY; Wree M; Reimer C; Nachon M; Corey V; Gedeck P; Plouffe D; Du A; Figueroa N; et al. Mutations in the Plasmodium falciparum Cyclic Amine Resistance Locus (PfCARL) Confer Multidrug Resistance. mBio 2016, 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).McNamara CW; Lee MC; Lim CS; Lim SH; Roland J; Simon O; Yeung BK; Chatterjee AK; McCormack SL; Manary MJ; et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature 2013, 504 (7479), 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Cowell AN; Istvan ES; Lukens AK; Gomez-Lorenzo MG; Vanaerschot M; Sakata-Kato T; Flannery EL; Magistrado P; Owen E; Abraham M; et al. Mapping the malaria parasite druggable genome by using in vitro evolution and chemogenomics. Science 2018, 359 (6372), 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Duffey M; Blasco B; Burrows JN; Wells TNC; Fidock DA; Leroy D Assessing risks of Plasmodium falciparum resistance to select next-generation antimalarials. Trends Parasitol 2021, 37 (8), 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Munoz L. Non-kinase targets of protein kinase inhibitors. Nat. Rev. Drug Discov 2017, 16 (6), 424–440. [DOI] [PubMed] [Google Scholar]
- (39).Trager W; Jensen JB Human malaria parasites in continuous culture. Science 1976, 193 (4254), 673–675. [DOI] [PubMed] [Google Scholar]
- (40).Clements RL; Streva V; Dumoulin P; Huang W; Owens E; Raj DK; Burleigh B; Llinas M; Winzeler EA; Zhang Q; et al. A Novel Antiparasitic Compound Kills Ring-Stage Plasmodium falciparum and Retains Activity Against Artemisinin-Resistant Parasites. J. Infect Dis 2020, 221 (6), 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Dluzewski AR; Ling IT; Rangachari K; Bates PA; Wilson RJ A simple method for isolating viable mature parasites of Plasmodium falciparum from cultures. Trans R Soc. Trop Med. Hyg 1984, 78 (5), 622–624. [DOI] [PubMed] [Google Scholar]
- (42).Jensen JB Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am. J. Trop Med. Hyg 1978, 27 (6), 1274–1276. [DOI] [PubMed] [Google Scholar]
- (43).Lambros C; Vanderberg JP Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol 1979, 65 (3), 418–420. [PubMed] [Google Scholar]
- (44).Wright AE; Collins JE; Roberts B; Roberts JC; Winder PL; Reed JK; Diaz MC; Pomponi SA; Chakrabarti D Antiplasmodial Compounds from Deep-Water Marine Invertebrates. Mar. Drugs 2021, 19 (4), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Mata-Cantero L; Lafuente MJ; Sanz L; Rodriguez MS Magnetic isolation of Plasmodium falciparum schizonts iRBCs to generate a high parasitaemia and synchronized in vitro culture. Malar J. 2014, 13, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Antonova-Koch Y; Meister S; Abraham M; Luth MR; Ottilie S; Lukens AK; Sakata-Kato T; Vanaerschot M; Owen E; Jado JC; et al. Open-source discovery of chemical leads for next-generation chemoprotective antimalarials. Science 2018, 362, 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Tumwebaze PK; Katairo T; Okitwi M; Byaruhanga O; Orena S; Asua V; Duvalsaint M; Legac J; Chelebieva S; Ceja FG; et al. Drug susceptibility of Plasmodium falciparum in eastern Uganda: a longitudinal phenotypic and genotypic study. Lancet Microbe 2021, 2 (9), e441–e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Janse CJ; Franke-Fayard B; Mair GR; Ramesar J; Thiel C; Engelmann S; Matuschewski K; van Gemert GJ; Sauerwein RW; Waters AP High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol. Biochem. Parasitol 2006, 145 (1), 60–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.