Sudden cardiac death (SCD) causes approximately 350,000 deaths annually in the United States, accounting for 42% of all cardiovascular deaths (1). Sudden cardiac arrest (SCA) can in some cases be aborted with early resuscitation, including cardiopulmonary resuscitation (CPR) and early defibrillation where indicated (2). Unfortunately, about 90% of SCA events do progress to SCD (3). Numerous interventions have been studied to improve the survival of SCA patients and prevent SCD. Early bystander CPR, early defibrillation, therapeutic hypothermia, and emergent coronary intervention are among the beneficial treatments (3).
Concerning the latter treatment, coronary artery disease accounts for a majority of SCA events, especially in individuals older than age 40 years. Acute coronary occlusion is often complicated by ventricular fibrillation leading to SCA. Accordingly, for patients resuscitated from SCA who exhibit ST-segment elevation (STE) on electrocardiogram, guidelines recommend urgent coronary angiography and percutaneous coronary intervention, if amenable (4). Extending this approach, several large observational studies have found an acute culprit lesion in 25% to 35% (especially including an occluded artery) of patients not manifesting STE. In these retrospective studies, an emergent catheterization approach was associated with a mortality benefit (5–8). Against the backdrop of these promising retrospective data, Lemkes et al. (9) randomized SCA patients without STE to emergent or delayed catheterization, and did not find an improvement in survival with emergent coronary angiography. Notably, only 5% of SCA patients without STE had acute thrombotic occlusion; a further 15% displayed a nonocclusive, but unstable lesion (9). Additional studies are underway regarding these SCA patients without STE (10).
Given the poor survival of SCA patients, even in those achieving restoration of spontaneous circulation (ROSC), in 2015, the American College of Cardiology Interventional Council sought to risk-stratify SCA patients expected to benefit from coronary angiography versus those with unfavorable prognosis who may benefit least (11). Predictors of poor outcome were diverse and included 10 characteristics. However, the approach was qualitative, and the number of adverse factors that should deter an urgent invasive catheterization in a given patient was unclear.
In this issue of the Journal, Harhash et al. (12) endeavor to provide more quantitative guidance in excluding SCA victims from an emergent trip to the catheterization laboratory (Figure 1). The authors performed a retrospective analysis of the large INTCAR (International Cardiac Arrest Registry). This registry includes U.S. and Northern European patients who experienced aborted SCA from presumed cardiac causes between 2007 and 2017 and who survived to hospital admission. Harhash et al. (12) analyzed 8 potential unfavorable features (11) for predicting the rate of survival to hospital discharge. In total, 7 of the variables were drawn from the earlier work (i.e., unwitnessed arrest, no bystander CPR, nonshockable rhythm, age >85 years, ROSC achieved more than 30 min into resuscitation, lactate >7 mmol/l, pH <7.2) (11). They added the variable chronic kidney disease (CKD) as a surrogate for end-stage renal disease (not available in INTCAR).
FIGURE 1. Identifying and Improving the Prognosis for Sudden Cardiac Arrest Victims.
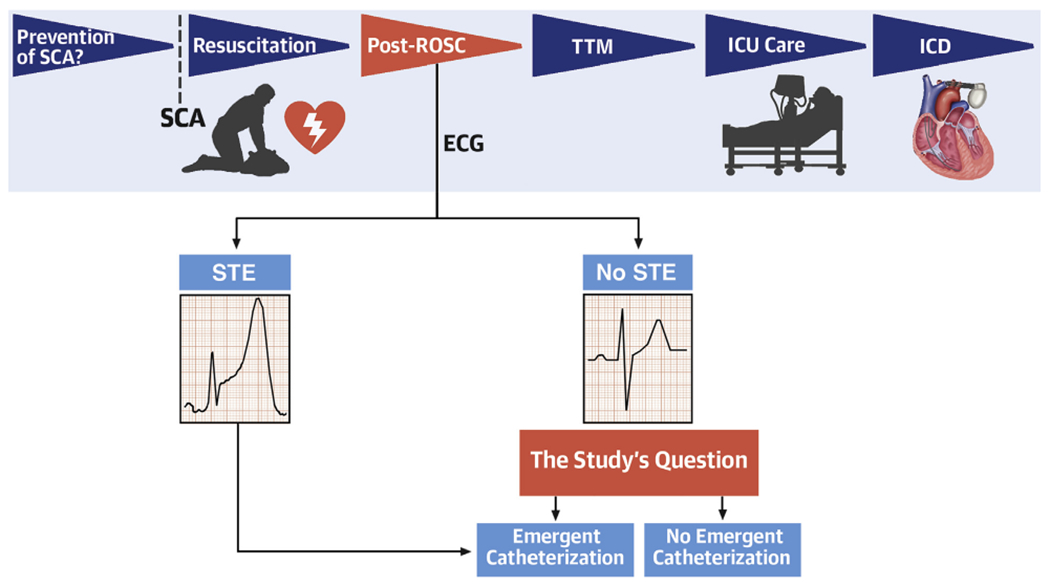
Phases in the management of sudden cardiac arrest (SCA) shown in temporal sequence on the top row, focusing on elements that impact outcome. Harhash et al. (12) focus on patients achieving restoration of spontaneous circulation (ROSC) and, in those lacking ST-segment elevation (STE) on the electrocardiogram (ECG), whether prognosis is improved by emergent catheterization or whether comorbid risks dilute the potential benefits. ICD = implantable cardioverter defibrillator; ICU = intensive care unit; TTM = therapeutic temperature modulation.
In the present study, these 8 unfavorable features in patients of INTCAR2.0 were analyzed, individually and in 256 different combinations, to find the strength of association to hospital discharge survival. The study’s 2 threshold markers are: 1) hospital discharge rate of 40%, the average rate in the INTCAR2.0 registry; and 2) markedly reduced survival of 10%. Ten percent survival was put forward as a threshold below which an invasive approach might be deemed futile. The INTCAR2.0 registry yielded 2,508 patients who met these criteria, of whom approximately three-fourths had experienced out-of-hospital SCA, with the remainder an in-hospital SCA. The post-ROSC electrocardiogram showed STE in 455 patients (19%). Notably, a relatively high 41% of patients exhibited VF as a presenting rhythm. About 75% had a witnessed SCA, and ~75% received bystander CPR. Catheterization was performed in 43% of the patients, with one-half of those undergoing percutaneous coronary intervention. Overall survival to hospital discharge was 39% in this subpopulation of the INTCAR registry.
On univariate analysis, all 8 selected variables were associated with reduced survival. After adjustment for the other 7 though, CKD fell out as a predictor. Three variables emerged as the strongest markers of adverse outcome: time-to-ROSC >30 min, age >85 years, and nonventricular tachycardia/ventricular fibrillation presenting rhythm. Achieving the benchmark for dismal prognosis (<10% survival) required any 7 variables or almost any combination of 6 factors. Patients with all 3 of the strongest negative predictors had a bleak 7% survival to hospital discharge.
This study validates 7 risk factors predicting adverse outcome from SCA in an independent database, quantitatively ranks the relative prognostic value of these risk factors, and hence enables identification of patients potentially ill-suited for emergent catheterization. Some limitations deserve attention. First, one-quarter of all patients were excluded through lack of data. Second, angiography was not performed in all patients, but rather was applied by uncertain criteria in 86% of SCA early survivors with STE versus only 33% of those without STE. Third, the database lacked granularity regarding CKD severity, perhaps explaining why this marker did not independently predict survival. Fourth, the threshold for emergent catheterization undoubtedly should be different in patients with STE, as opposed to those without STE, given the neutral results of 1 randomized trial in the latter population (9). Indeed, emergent catheterization may differentially impact prognosis in patients with varying combinations of adverse risk factors.
Thus, these data provide clinical guidance to decide on when to—or not to—perform emergent catheterization. In patients without STE, in particular, further analysis of these risk factors is warranted in data from randomized trials, and eventually in pooled data from multiple trials. Even though a factor or combination of factors predicts poor prognosis, invasive intervention may nevertheless markedly improve outcome. Similar analyses should also be conducted in datasets of SCA patients exhibiting STE.
In conclusion, this analysis from the INTCAR SCA registry (12) is insightful and clinically relevant. It enables us to quantitatively weigh prognostic factors when considering emergent catheterization in victims of SCA, and is a strong platform from which to generate novel hypotheses for future studies. Further clinical guidance is urgently needed in how to manage coronary revascularization in SCA victims, because this is 1 step whereby we can readily make a difference in this common condition with otherwise high mortality!
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, et al. heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–528. [DOI] [PubMed] [Google Scholar]
- 2.Merchant RM, Topjian AA, Panchal AR, et al. Part 1: Executive summary: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020;142:S337–57. [DOI] [PubMed] [Google Scholar]
- 3.Narayan SM, Wang PJ, Daubert JP. New concepts in sudden cardiac arrest to address an intractable epidemic: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:70–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary. J Am Coll Cardiol 2013;61:485–510. [DOI] [PubMed] [Google Scholar]
- 5.Yannopoulos D, Bartos JA, Aufderheide TP, et al. The Evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation 2019;139:e530–52. [DOI] [PubMed] [Google Scholar]
- 6.Dumas F, Bougouin W, Geri G, et al. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-segment elevation pattern: insights from the PROCAT II Registry. J Am Coll Cardiol Intv 2016;9:1011–8. [DOI] [PubMed] [Google Scholar]
- 7.Kern KB, Lotun K, Patel N, et al. Outcomes of comatose cardiac arrest survivors with and without ST-segment elevation myocardial infarction: importance of coronary angiography. J Am Coll Cardiol Intv 2015;8:1031–40. [DOI] [PubMed] [Google Scholar]
- 8.Vyas A, Chan PS, Cram P, Nallamothu BK, McNally B, Girotra S. Early coronary angiography and survival after out-of-hospital cardiac arrest. Circ Cardiovasc Interv 2015;8:e002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemkes JS, Janssens GN, van der Hoeven NW, et al. Coronary angiography after cardiac arrest without ST-segment elevation. N Engl J Med 2019;380:1397–407. [DOI] [PubMed] [Google Scholar]
- 10.Hauw-Berlemont C, Lamhaut L, Diehl JL, et al. EMERGEncy versus delayed coronary angiogram in survivors of out-of-hospital cardiac arrest with no obvious non-cardiac cause of arrest: Design of the EMERGE trial. Am Heart J 2020;222:131–8. [DOI] [PubMed] [Google Scholar]
- 11.Rab T, Kern KB, Tamis-Holland JE, et al. Cardiac arrest: a treatment algorithm for emergent invasive cardiac procedures in the resuscitated comatose patient. J Am Coll Cardiol 2015;66:62–73. [DOI] [PubMed] [Google Scholar]
- 12.Harhash AA, May TL, Hsu C-H, et al. Risk stratification among survivors of cardiac arrest considered for coronary angiography. J Am Coll Cardiol 2021;77:360–71. [DOI] [PubMed] [Google Scholar]


