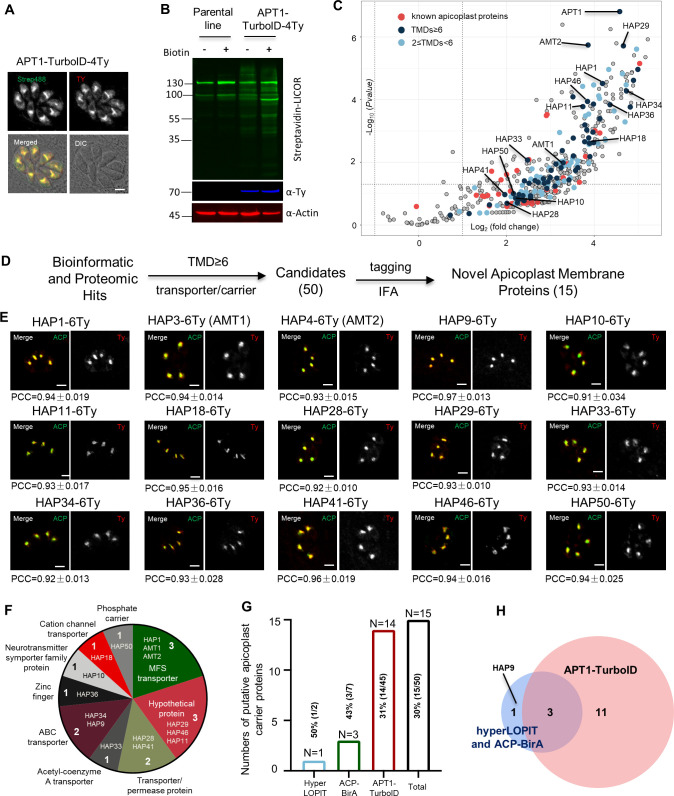
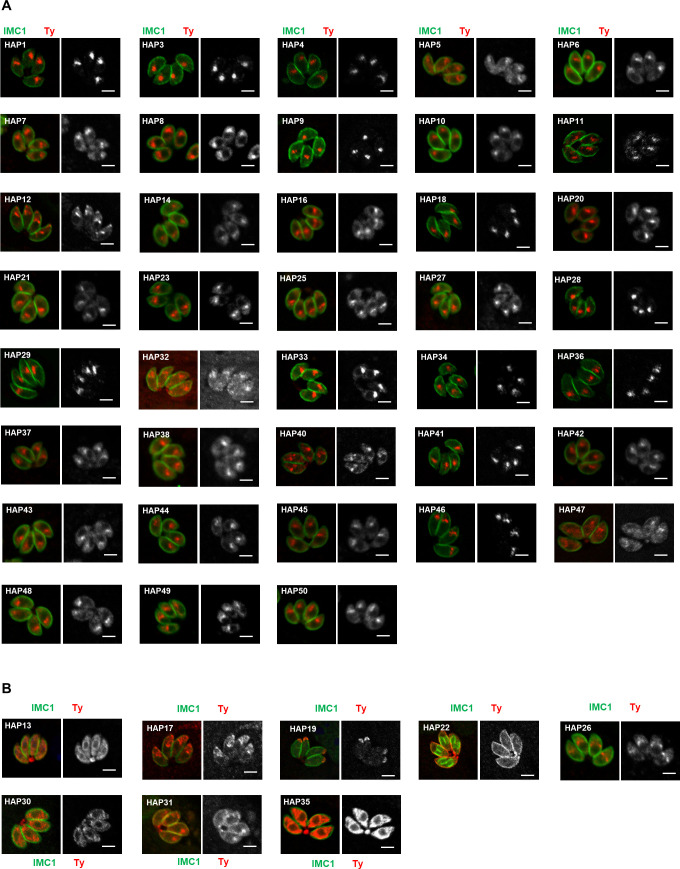
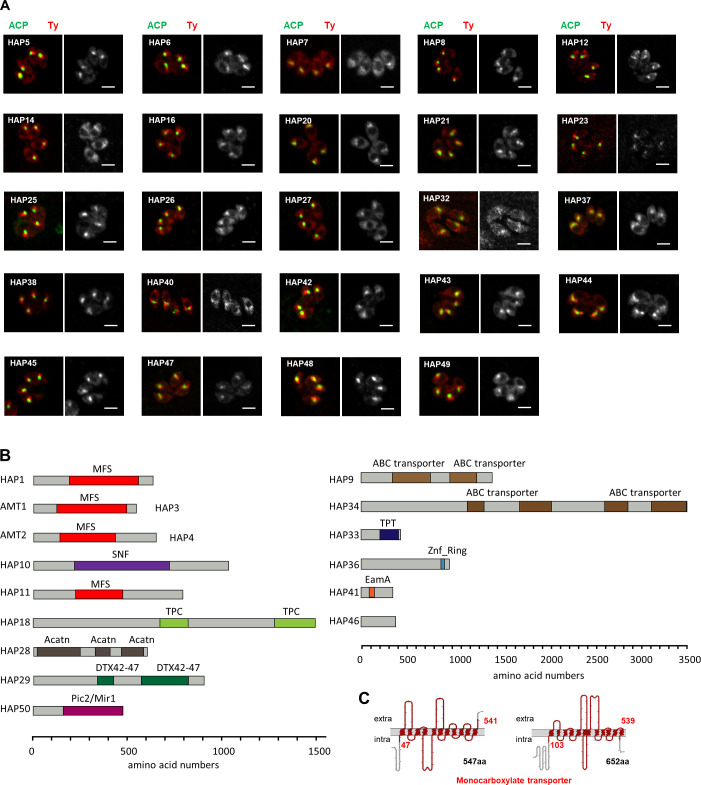
Figure 1. Discovery of apicoplast membrane proteins.
Activity test of biotinylation of proximal proteins by APT1-TurboID-4Ty. Parasites were grown in 500 μM D-biotin for 90 min, followed by detection of biotinylated proteins (green) by streptavidin reagents on indirect fluorescence assay (IFA) (A) and on western blots (B). Actin served as the loading control. Scale = 5 μm. (C) Volcano plot analysis comparing the TurboID fusion to the parental line. Three replicate mass-spectrometry experiments were analyzed by the Student t-test. Known apicoplast proteins (red) and candidates with different numbers of transmembrane domains (TMDs; deep and light blue were indicated, and novel apicoplast proteins were pointed out; see Supplementary file 2a). (D) Workflow of the discovery of novel apicoplast membrane proteins. Candidates from hyperLOPIT (Barylyuk et al., 2020), ACP-BirA (Boucher et al., 2018), and APT1-TurboID (this study) were screened by epitopte tagging and IFA. (E) Confocal co-localization of HAP-6Ty with the apicoplast marker ACP, showing confocal imaging of HAP-6Ty (red) and ACP (green), and Pearson correlation coefficiency (PCC). The PCC values over the merged fluoresent foci for candidate proteins and ACP were analyzed by a co-localization and region of interest (ROI) intensity analysis module in the NIS Elements AR software and shown with mean ± standard error of the mean (SEM; N = 6 for each). Scale = 5 μm. (F–H) Summary and comparison of the screening from three datasets. The putative transporters are grouped into diverse types (F) and identified from three different datasets (G), which shared three novel proteins, as shown by the Venn diagram (H). Three independent experiments were performed with similar outcomes (A, B, E).