Abstract
Chagas disease is a serious parasitic infection caused by Trypanosoma cruzi. Unfortunately, the current chemotherapeutic tools are not enough to combat the infection. The aim of this study was to evaluate the trypanocidal activity of benznidazole-loaded microparticles during the acute phase of Chagas infection in an experimental murine model. Microparticles were prepared by spray-drying using copolymers derived from esters of acrylic and methacrylic acids as carriers. Dissolution efficiency of the formulations was up to 3.80-fold greater than that of raw benznidazole. Stability assay showed no significant difference (P > 0.05) in the loading capacity of microparticles for 3 years. Cell cultures showed no visible morphological changes or destabilization of the cell membrane nor haemolysis was observed in defibrinated human blood after microparticles treatment. Mice with acute lethal infection survived 100% after 30 days of treatment with benznidazole microparticles (50 mg kg−1 day−1). Furthermore, no detectable parasite load measured by quantitative polymerase chain reaction and lower levels of T. cruzi-specific antibodies by enzyme-linked immunosorbent assay were found in those mice. A significant decrease in the inflammation of heart tissue after treatment with these microparticles was observed, in comparison with the inflammatory damage observed in both infected mice treated with raw benznidazole and untreated infected mice. Therefore, these polymeric formulations are an attractive approach to treat Chagas disease.
Key words: Benznidazole, Chagas disease, Eudragit®, experimental infection, microparticles, spray-drying
Introduction
Chagas disease is a vector-borne neglected disease widely distributed in Latin America. As reported, about 6–7 million people worldwide, mostly in Latin America, are estimated to be infected with Trypanosoma cruzi, the parasite that causes Chagas disease. In the last decades, Chagas disease has spread to non-endemic countries including USA, Canada, and many European and some African, Eastern Mediterranean and Western Pacific countries, due to migration phenomena of the infected population from endemic countries (WHO, 2020). This parasitic disease presents two phases: acute and chronic, the latter characterized by cardiomyopathy, megaoesophagus, megacolon and alterations of the peripheral nervous system that can cause the death. Despite the high number of people infected with T. cruzi, <1% have been diagnosed and treated, so far (Kratz et al., 2018). Discovered more than 40 years ago, benznidazole (BNZ) and nifurtimox (NFX) are the only approved drugs to treat this parasitic infection (Apt, 2010; Sguassero et al., 2015). In adults, BNZ is commonly prescribed in two daily doses of 5 mg kg−1 day−1 for 60 days while NFX is recommended in three daily doses of 8–10 mg kg−1 day−1 for 60 days (Meymandi et al., 2018). BNZ is the drug of choice in several countries of Latin America, USA and Spain being prescribed to treat the acute infection (Cardoso et al., 2018). However, its efficacy to treat the chronic stage of the disease is still controversial (Pérez-Molina et al., 2009). Despite this, clinical studies showed that BNZ is still used for acute and chronic phases in children (Sosa Estani et al., 1998) and adult patients (Viotti et al., 2009). Recently, the BENEFIT study has shown a trypanocidal effect of BNZ, with decreased parasitic loads in patients with severe chronic disease, but no association with clinical outcomes (Morillo et al., 2015). In addition, it is important to note that BNZ treatment in children showed promising results in terms of efficacy (Sosa Estani et al., 1998; Altcheh et al., 2011). However, the appearance of adverse effects, including hepatic and renal damage, anorexia, dermatitis, nervous and digestive problems, immunosuppression and teratogenicity after BNZ administration, is still a serious concern (Maya et al., 2007; Salomon, 2012; Bonney, 2014). As described, between 20 and 40% of patients must discontinue treatment due to such undesirable effects (Viotti et al., 2009; Vinuesa et al., 2017; Crespillo-Andújar et al., 2018). Taking all these considerations into account, it is imperative to develop chemotherapeutic alternatives to optimize the biological response and minimize the adverse effects, as strongly recommended by other researchers (Kratz et al., 2018; Ribeiro et al., 2020). BNZ is a poorly water-soluble compound (0.4 mg mL−1) and, therefore, high doses are needed to reach therapeutic concentrations in blood following oral administration (Quijia Quezada et al., 2019). Thus, reduction of the particle size constitutes a useful strategy to enhance the aqueous solubility/dissolution and improve the bioavailability after oral administration (Maximiano et al., 2011; Ferraz et al., 2018). Generally, micronization methodologies, including spray-drying devices, lead to the development of drug-loading microparticulate systems with desired functionalities (Piccirilli et al., 2014; Al-Khattawi et al., 2018). Particularly, spray-drying technique transforms liquid products into free-flowing dry powders and such methodology can be scaled up from the laboratory to the industrial production (Poozesh and Bilgili, 2019). Keeping in mind the needs of novel oral formulations for Chagas disease, the aim of this study was to formulate BNZ-loaded microparticles (BNZ-MP) by spray-drying using Eudragit® RL PO (RL) and Eudragit® RS PO (RS) to evaluate their efficacy in an experimental model of acute infection with the virulent T. cruzi Nicaragua (TcN) isolate (Grosso et al., 2010). Dissolution efficiency (DE) and storage stability assays were performed to characterize the formulations. In addition, in vitro studies in cell culture and haemolytic assays of the BNZ-MP were carried out. Then, the levels of T. cruzi-specific antibodies, antiparasitic efficacy and histopathological damages, after oral administration of BNZ-MP, were investigated.
Materials and methods
Materials
BNZ (lot 9978 A, 99% purity; Laboratorios Elea, Buenos Aires, Argentina, was provided by the National Institute of Parasitology ‘Dr. Mario Fatala Chaben’, National Ministry of Health, INP-ANLIS, Buenos Aires, Argentina). Fetal bovine serum (FBS) was purchased from Natocor (Córdoba, Argentina) and horse serum was obtained from Internegocios SA (Córdoba, Argentina). African green monkey kidney epithelial cells (Vero cells) were obtained from Asociación Banco Argentino de Células (ABAC, Pergamino, Argentina). Eudragit® RL PO and RS PO (copolymers of ethylacrylate, methylmethacrylate and methacrylic acid esterified with quaternary ammonium groups) and Sipernat® (speciality silica) were kindly donated by Evonik (Buenos Aires, Argentina). All other reagents and chemicals used for analytical purposes were of chromatography grade.
Formulation of BNZ-MP
BNZ-MP were obtained by spray-drying method (Seremeta et al., 2019). On the one hand, 6.0 g of polymer (RL or RS) and 2.4 g of BNZ were dissolved in absolute ethanol (60 mL) under magnetic stirring (IKA® C-MAG HS 4 digital, Staufen, Germany) overnight (200 rpm). On the other hand, Sipernat® was dispersed in distilled water (540 mL, 7.5% w/v). Then, both phases were mixed, magnetically stirred (1 h) and homogenized (5 min, 17 500 rpm) by using T18 Ultra-Turrax (IKA®-Werke GmbH & Co.KG, Staufen, Germany). This suspension was fed into a Mini Spray Dryer Büchi B-290 (Büchi Labortechnik AG, Flawil, Switzerland) with the co-current flow and open-loop mode. The inner diameter of the spray nozzle tip was 0.7 mm. It should be noted that the sample was maintained under magnetic stirring at 200 rpm throughout the process to ensure homogeneity. The operating parameters were previously adjusted (Seremeta et al., 2019). The resulting air outlet temperature was of 65°C. The BNZ-MP were recovered from the glass collection vessel and stored in caramel colour glass vials sealed with chlorobutyl rubber septa (Labco Limited, Lampeter, UK) and protected from light and moisture, in laboratory cabinets, until further use. The samples were obtained for triplicate using RL and RS. These were namely BNZ-MP-RL and BNZ-MP-RS, respectively. Size and size distribution of the BNZ-MP were estimated by scanning electron microscopy (SEM). An image analysis system (Image Pro-plus® software 6.0) was used to analyse the images of BNZ-MP with 20× magnification and to obtain the average size value.
In vitro BNZ dissolution
Dissolution studies were performed using a method previously reported with slight modifications (Leonardi et al., 2009; Lima et al., 2011). The USP paddle method (apparatus 2) in USP Standard Dissolution Apparatus Hanson Research SR6 Plus (Chatsworth, CA, USA) was used. The dissolution medium was 900 mL of HCl 0.1 N maintained at 37°C and the stirring speed was set at 75 ± 2 rpm. The following samples were tested in triplicate: (i) BNZ-MP-RL equivalent to 50 mg of BNZ, (ii) BNZ-MP-RS equivalent to 50 mg of BNZ and (iii) free BNZ (50 mg). Sink conditions were used to ensure complete dissolution of the drug in the volume of dissolution medium. The volume of the medium used was not <3 times that required to form a saturated solution of the drug substance, according to the United States Pharmacopeia 30 Edition and the National Formulary 25 (USP 30-NF 25). The samples were placed into the flasks containing dissolution medium and the time counter was set to zero. At regular time intervals (10, 15, 20, 30, 45, 60, 90 and 120 min), samples of medium (4 mL) were taken using a filter and replaced by the same amount of fresh medium to keep a constant volume. The samples were diluted with 0.1 N HCl and the concentration of dissolved BNZ was determined by UV-visible spectrophotometry (UV-1800 Shimadzu UV-VIS Spectrophotometer, Kyoto, Japan) at 324 nm and 25°C using a calibration curve of the drug in absolute ethanol/distilled water (97/3 v/v) (5–40 μg mL−1, R2 > 0.9997). The dissolution profiles were compared using the DE at 15, 30 and 90 min (DE15, DE30 and DE90, respectively). Determinations were carried out for triplicate and results were expressed as mean ± s.d. Statistical analysis of the DE was performed using a one-way analysis of variance (ANOVA) followed by a least significant difference.
BNZ-MP stability assay
The samples were placed in caramel colour glass vials sealed with chlorobutyl rubber septa (Labco Limited) and stored at room temperature (25 ± 2°C) in laboratory cabinets for 6, 9, 12, 24 and 36 months. The stability was assessed by measuring the drug content of the BNZ-MP-RL and BNZ-MP-RS before (zero time) and after storage. Thus, each sample of BNZ-MP (0.01 g) was dissolved in 5 mL mixture of absolute ethanol/distilled water (97/3 v/v) and left under moderate magnetic stirring for 30 min. Then, 200 μL of the sample were taken and diluted up to 5 mL with the same solvent mixture. Finally, the BNZ concentration in each sample was determined by UV-visible spectrophotometry (see above). Determinations were carried out for triplicate according to equation (1) and results were expressed as mean ± s.d.:
| 1 |
where WBNZ is the weight of the drug in the BNZ-MP and WMP is the total weight of BNZ-MP obtained.
Encapsulation efficiency of BNZ-MP
The encapsulation efficiency (%EE) of the BNZ-MP was determined according to equation (2):
| 2 |
where DCE and DCT are the experimental and theoretical drug content of the BNZ-MP, respectively. Determinations were carried out for triplicate.
Cell toxicity assay
The viability of the kidney epithelial cells from African green monkey (Vero line) was carried out by reduction of yellow tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide to a blue crystalline formazan product (MTT). A 96-well microplate with 1 × 105 Vero cells per well were incubated with increasing concentrations of different BNZ-MP (0, 10, 25 and 50 μg mL−1) suspended in Roswell Park Memorial Institute medium (RPMI) overnight at 37°C. Then, MTT [in phosphate-buffered saline (PBS), 5 mg mL−1] was added and, after 2–4 h, a solution of dimethyl formamide (DMF) and sodium dodecyl sulphate (SDS) (50% of DMF + 10% of SDS) was added to dissolve the MTT. The colour was measured in a microplate reader (Bio-Rad, model 3550, Buenos Aires, Argentina) at 540 nm. Assays were performed in triplicate.
Haemolytic effect
To determine the possible haemolytic effect of the formulated BNZ particles, increasing concentrations of different BNZ-MP (10, 25, 50 and 100 μg mL−1) were added to a 4% suspension of fresh defibrinated human blood prepared in sterile 5% glucose solution and left in contact for 24 h at 37°C. Then, samples were centrifuged and the supernatant absorbance was determined at 540 nm. The absorbance values indicate the haemolysis per cent. Untreated BNZ and Triton X-100 (10%) were used as the haemolytic reference drug and positive control, respectively.
Parasites
Cultured-derived trypomastigotes of the TcN isolate (Grosso et al., 2010) were maintained in culture using RPMI medium supplemented with 10% of FBS obtained by serial passage through Vero cells (ABAC).
Animal model
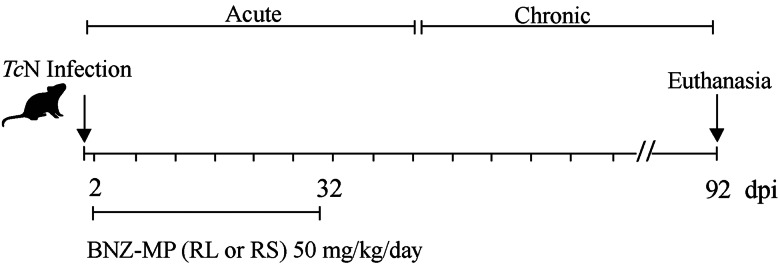
The animals were obtained from the National Institute of Parasitology ‘Dr. Mario Fatala Chaben’ bioterium (Buenos Aires, Argentina), under specific pathogen-free conditions. Mice were in a controlled room with water and food ad libitum, a circadian light cycle and a temperature of 22°C. Animals were randomly selected prior to infection and assignment to the treatment groups. Physical adverse events that were monitored twice a day included inability to move, reduced appetite, extreme pallor, ruffled hair and body weight loss over 16%. Five groups (n = 4–7) of 1-month-old female C3H/HeN mice were inoculated intraperitoneally with 1000 culture-derived trypomastigotes of the TcN isolate. Raw BNZ and BNZ-MP were dispersed in the corresponding volume of olive oil immediately before administration. Formulations were administered to mice through oral gavage, at 2 days post-infection (d.p.i.). The following groups of mice were systematically compared: (1) infected mice and without treatment, (2) infected mice and treated with BNZ for 30 days with daily doses of 50 mg kg−1 day−1 (BNZ 50), (3) infected mice and treated with BNZ-MP-RL for 30 days with daily doses of 50 mg kg−1 day−1 (RL 50), (4) infected mice and treated with BNZ-MP-RS for 30 days with daily doses of 50 mg kg−1 day−1 (RS 50) and (5) mice without infection (Fig. 1). For the untreated group, a larger number of mice (n = 38) was infected to obtain an appropriate survival.
Fig. 1.
Treatment schedule in the experimental murine model of acute TcN infection. TcN-infected mice treated at 2 d.p.i. with BNZ or BNZ-MP at 50 mg kg−1 day−1 (RL 50 or RS 50) for 30 days.
Parasitaemia detected by DNA amplification
First, one volume of blood was collected from euthanized infected and uninfected mice at 3 months post-infection (p.i.) after completing the treatments (n = 4–7 samples per treatment). Then, each sample of blood was mixed with an equal volume of guanidine-HCl 6 M, EDTA 0.1 M, pH 8 and kept for 1 week at room temperature and after at 4°C until further use. Thus, DNA was isolated by means of a quantitative polymerase chain reaction (qPCR) (High Pure PCR Template Preparation Kit, Roche, Mannheim, Germany) from 0.2 mL of guanidine-EDTA buffer B mixture and eluted in 0.2 mL, following the manufacturer's protocol. A bacterial commercial plasmid, pQE (Qiagen, MD, USA) was used as an internal standard of DNA extraction. A T. cruzi satellite DNA flanked by the Sat Fw and Sat Rv oligonucleotides highly conserved in the parasite genome was amplified by ABI 7500 thermocycler (Applied Biosystems, Carlsbad, CA, USA) (Duffy et al., 2009). A commercial kit (SYBR® GreenER® qPCR SuperMix Universal, Invitrogen, Life Technologies, Waltham, MA, USA) was used to run duplicated samples as previously described (Bua et al., 2013). Epimastigotes of the TcN isolate, DTU TcI were used as a standard in artificially spiked mouse blood. In each determination, the parasite curve, negative samples and non-template DNA were included (Grosso et al., 2013). The cut-off value was determined at 0.14 Eq parasites mL−1.
Specific anti-T. cruzi humoral response
A sample of blood (500 μL) from uninfected mice, treated infected mice and untreated infected mice was collected from the orbital venous sinus at 3 months p.i. Then, the serum of the collected samples was analysed for IgG antibody levels by the enzyme-linked immunosorbent assay (ELISA). As a source of antigen, a lysate preparation derived from epimastigotes of the strain, Tul2 stock (20 μg mL−1) was used. Briefly, antigen diluted in carbonate buffer pH = 9.6 was used to coat flat-bottomed (96-well, 50 μL well−1) plates at 4°C overnight. Then, 5% skimmed milk in PBS was used to block the plates (100 μL well−1) at room temperature for 1 h. Plates were washed three times with PBS-0.05% Tween 20 (PBS-T) and incubated with sera samples (50 μL well−1, 1:50–1:400 dilution) at 37°C for 30 min. Then, plates were washed with PBS-T and 50 μL well−1 of horseradish peroxidase-labelled goat anti-mouse IgG (Jackson ImmunoResearch Inc, West Grove, PA, USA) were added at room temperature for 30 min. O-phenylenediamine dihydrochloride (50 μL well−1) and 2 N sulfuric acid were used to develop and stop the reaction, respectively. Optical density (OD) was read with an ELISA microplate reader (Dynatech Laboratories, Alexandria, VA, USA) at 490 nm. The mean minus two standard deviations of OD obtained from sera of untreated infected control mice was set up as a cut-off value for significant decreases in antibody levels.
Histopathological studies
Histopathological analysis was carried out with heart tissues removed from treated and untreated infected mice. These tissues were fixed in 10% formaldehyde solution and embedded in paraffin. Then, tissue sections (5 μm) were stained with Haematoxylin and Eosin (H&E) stain and observed by light microscopy. Finally, according to the extension of inflammation, tissues from eight different areas of the heart (left and right atria, upper and lower halves of each ventricular wall and upper and lower septum) were scored. Different numbers were assigned to each section according to the degree of inflammation: (0) absent/none, (1) focal or mild myocarditis with one foci, (2) moderate with two inflammatory foci, (3) extensive with generalized coalescing of inflammatory foci or disseminated inflammation with minimal necrosis and retention of tissue integrity, and (4) severe with diffused inflammation, interstitial oedema and loss of tissue integrity (Gupta and Garg, 2010). An estimate of the degree of cardiac tissue inflammation was obtained from an average of the values found in the eight sections of the heart.
Statistical methods
The normality of the variable distribution was assessed by using the Shapiro–Wilk normality test. Differences among groups were evaluated by ANOVA or Kruskal–Wallis test as appropriately followed by Bonferroni or Dunn's multiple comparison tests. Statistical significance was considered at P < 0.05 (two-tailed). Data analyses and graphs were performed with GraphPad Prism 7.0 software.
Results
Formulation of BNZ-MP
The corresponding BNZ formulations were obtained in nearly 70% yield with a media size of 0.87–1.08 μm. The %EE was of 95.41% (2.31) and 96.21% (6.11) for BNZ-MP-RL and BNZ-MP-RS, respectively. It is important to mention that BNZ-MP were obtained as free-flow powders and no aggregation phenomena were observed. In contrast, as reported by Cortesi et al. (2007), the preparation of acyclovir-containing MP by spray-drying with Eudragit® RS lead to the formation of aggregates, mainly.
In vitro BNZ dissolution
Dissolution studies showed that both BNZ microparticulate polymeric formulations exhibited faster dissolution rates than the raw drug. DE at 15 min (DE15) indicated a 3.80 and 3.12-fold increase in dissolution rate for BNZ-MP-RL and BNZ-MP-RS, respectively. Furthermore, DE at 30 min (DE30) was 2.39 and 2.07-fold greater for BNZ-MP-RL and BNZ-MP-RS, respectively, to the raw drug (Table 1). This indicates that the DE of the drug from the microparticulate systems was greater in the first minutes and decreasing with the passage of time with respect to untreated BNZ.
Table 1.
In vitro BNZ dissolution efficiency and stability of BNZ-MP stored at room temperature up to 36 months
| Sample | Dissolution efficiency (±s.d.) | (%) Drug content (±s.d.) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Time (month) | |||||||||||||
| 10 | 15 | 20 | 30 | 45 | 60 | 90 | 120 | 0 | 6 | 9 | 12 | 24 | 36 | |
| BNZ-MP-RLa | 83.56**** (9.97) | 86.68**** (8.03) | 89.64**** (7.41) | 92.28**** (6.37) | 91.51**** (5.67) | 91.52*** (4.98) | 91.31** (5.28) | 92.73* (4.98) | 22.90 (0.55) | 22.88 (0.57) | 22.76 (0.48) | 22.92 (0.41) | 21.52 (0.96) | 21.86 (1.44) |
| BNZ-MP-RSb | 58.95*** (6.83) | 71.13*** (6.01) | 78.92*** (8.05) | 80.00**** (3.42) | 81.88*** (3.63) | 82.59** (3.05) | 83.59 (2.37) | 83.80 (2.01) | 23.09 (1.47) | 23.05 (0.40) | 22.00 (0.81) | 21.63 (0.29) | 21.53 (1.28) | 21.64 (0.83) |
| BNZc | 15.57 (2.61) | 22.82 (2.62) | 29.62 (3.89) | 38.59 (4.43) | 51.37 (4.11) | 60.36 (4.48) | 74.40 (3.58) | 81.79 (3.31) | – | – | – | – | – | – |
BNZ-MP-RL: microparticles of Eudragit® RL PO loaded with benznidazole.
BNZ-MP-RS: microparticles of Eudragit® RS PO loaded with benznidazole.
BNZ: raw benznidazole.
Difference is statistically significant with respect to BNZ: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
BNZ-MP stability assay
The stability assay of BNZ-MP was carried out by measuring the drug content at zero time (i.e. immediately after their fabrication) and after storage at room temperature for 6, 9, 12, 24 and 36 months. To provide long shelf-life, it is necessary to avoid the leakage of the drug from the MP for a long period. In this case, the results showed no significant changes (P > 0.05) in drug content values under storage (Table 1). After 36 months, the drug content values slightly decreased to 95.46 and 93.72% of initial values for BNZ-MP-RL and BNZ-MP-RS, respectively (Table 1).
Cell toxicity assay
Due to the clinical interest of BNZ for treatment of a neglected disease as Chagas disease, the biocompatibility and cell toxicity of the novel formulations should be evaluated. Thus, a cell viability study was carried out by MTT assay to evaluate the possible toxicity associated with the BNZ-MP. After incubation of Vero cell culture with the BNZ-MP, there were no visible morphological changes or cell membrane destabilization. The OD values showed no significant differences after the addition of such formulations with respect to untreated cells (Supplementary Table 1 1). As seen, BNZ-MP-RL 50, 25 and 10 showed a toxicity percentage of 7.7, 8.4 and 27%, respectively. In a similar fashion, BNZ-MP-RS 50, 25 and 10 exhibited 3.7, 9.6 and 26%, respectively.
Haemolytic effect
Due to the potential interest in clinical research, an in vitro haemolytic assay was performed to evaluate whether BNZ-MP might damage the red blood cells. BNZ was used as the haemolytic reference drug and Triton X-100 (10%) as the positive control. Low percentage of haemolysis (4.6–8%) was observed in defibrinated human blood incubated with BNZ or different concentrations of BNZ-MP (10, 25, 50 and 100 μg mL−1). Thus, such formulations did not promote any alteration or degradation in erythrocyte membranes suggesting that RL and RS may be used as carriers for BNZ microencapsulation. As expected, Triton X-100 produced complete lysis of the erythrocytes (Supplementary Table 1 2).
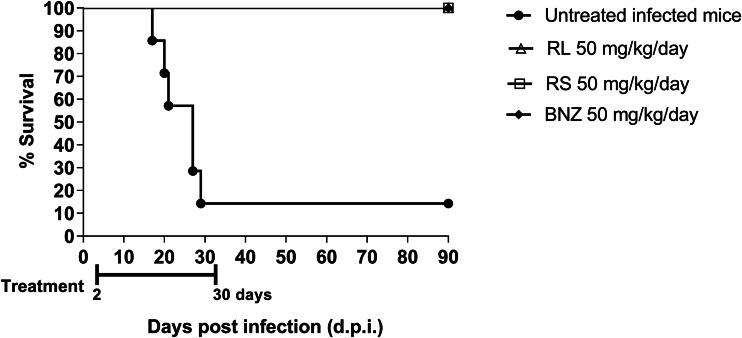
Course of infection
An assay in the acute phase of TcN-infected mice was carried out with different BNZ-MP to evaluate the effectiveness of this novel treatment for Chagas disease. All infected mice (100%) treated with 50 mg kg−1 day−1 of BNZ-MP (RL 50 or RS 50) for 30 days survived as seen in Fig. 2. This acute infection shows a peak of parasitaemia 30–35 d.p.i. in untreated C3H/HeN mice inoculated with 1000 TcN trypomastigotes, with a survival rate of 15% (Grosso et al., 2013). Additionally, untreated infected mice and infected mice treated with BNZ-MP 50 mg kg−1 day−1 showed the same appearance, behaviour and weight (data not shown).
Fig. 2.
Survival rate curve of TcN-infected C3H/HeN mice. Mice were infected with 1000 trypomastigotes of TcN and the survival rates were checked daily up to 90 days. Each line indicates the survival rate of untreated infected mice and mice treated with 30 oral doses of BNZ-MP or BNZ at 50 mg kg−1 day−1 (RL 50, RS 50 or BNZ 50, respectively). To assess differences among survival curves, a log-rank test of Kaplan–Meier was performed.
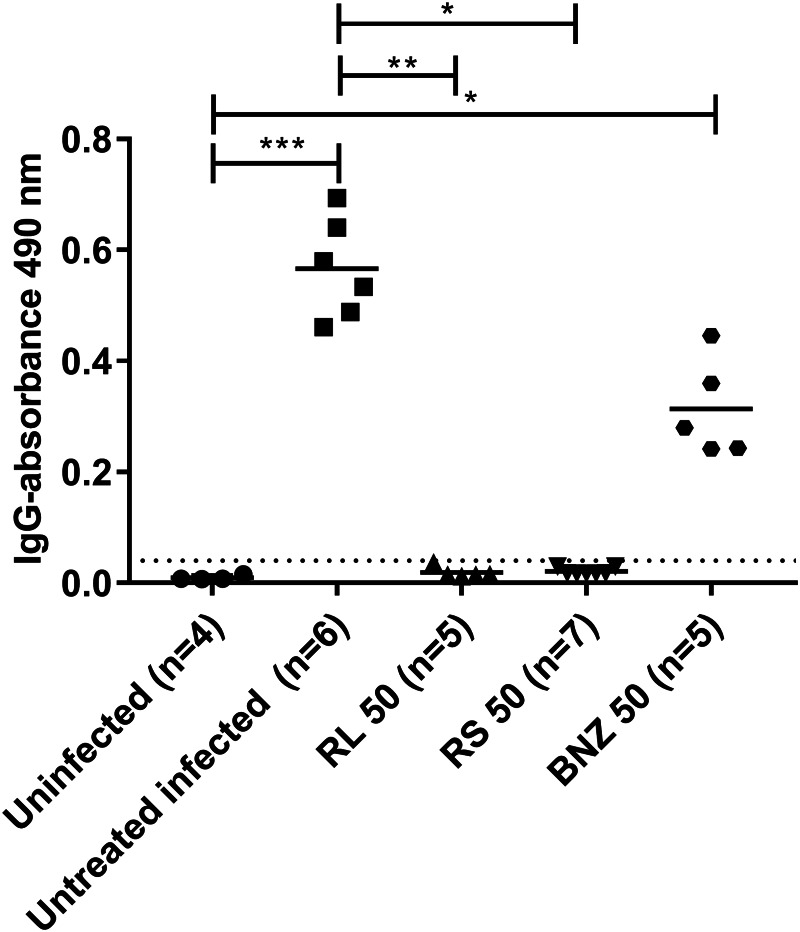
Specific anti-T. cruzi humoral response
Trypanosoma cruzi-specific IgG antibody levels were measured in uninfected mice, untreated infected mice and infected mice treated with BNZ-MP or raw BNZ. As shown in Fig. 3, treatment with BNZ-MP (50 mg kg−1 day−1) led to a decrease in T. cruzi-specific antibodies of all mice compared to untreated infected control mice. No antibodies were detected in all animals treated with BNZ-MP. These treatments were significantly more effective than those with the same doses of raw BNZ.
Fig. 3.
Trypanosoma cruzi-specific antibody levels in serum samples from TcN-infected untreated and treated mice after 3 months of follow-up. Serum samples from TcN-infected untreated mice and treated with BNZ-MP or BNZ at 50 mg kg−1 day−1 (RL 50, RS 50 or BNZ 50, respectively) were analysed for IgG antibody levels specific for T. cruzi antigens by ELISA. The cut-off value for negative antibody levels, as described in the Materials and methods section, is represented by the horizontal dotted line. Each dot represents an individual mouse. *P < 0.05; ***P < 0.001 as determined by the Kruskal–Wallis test.
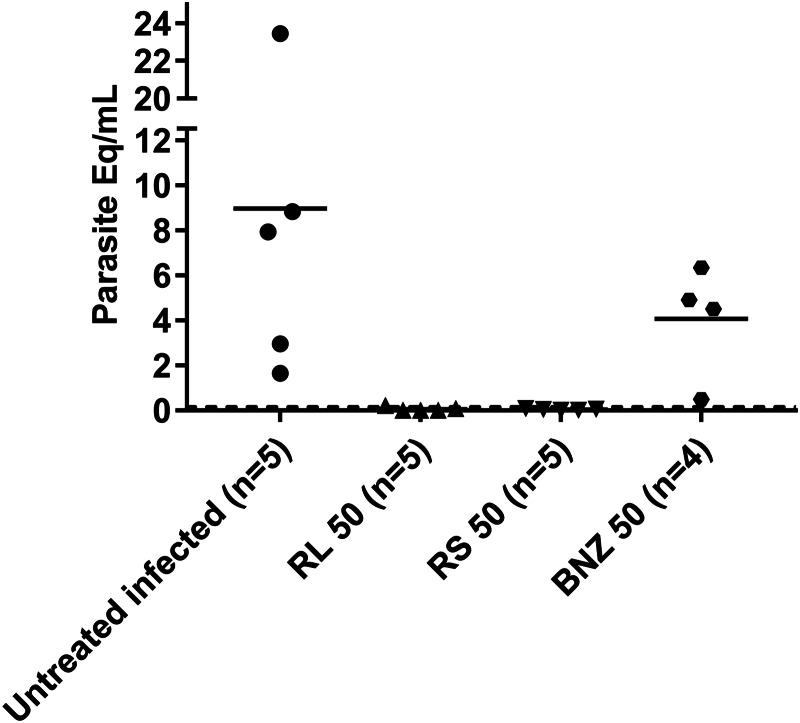
Parasitaemia detected by DNA amplification
As seen in Fig. 4, the results of this assay indicated that no detectable parasitaemia was observed in mice treated with BNZ-MP (RL 50 or RS 50) at 3 months p.i. In contrast, raw BNZ-treated mice reduced their parasite load by 50%.
Fig. 4.
Parasitaemia by qPCR. Quantitative PCR amplification of the T. cruzi satellite DNA from the blood of TcN-infected untreated mice and treated with BNZ-MP or BNZ at 50 mg kg−1 day−1 (RL 50, RS 50 or BNZ 50, respectively). Each symbol represents the parasite equivalents mL−1 (Eq mL−1) at 92 d.p.i. measured by qPCR in each mouse as described in the Materials and methods section. The dotted line represents the limit of detection of the technique.
Histopathology
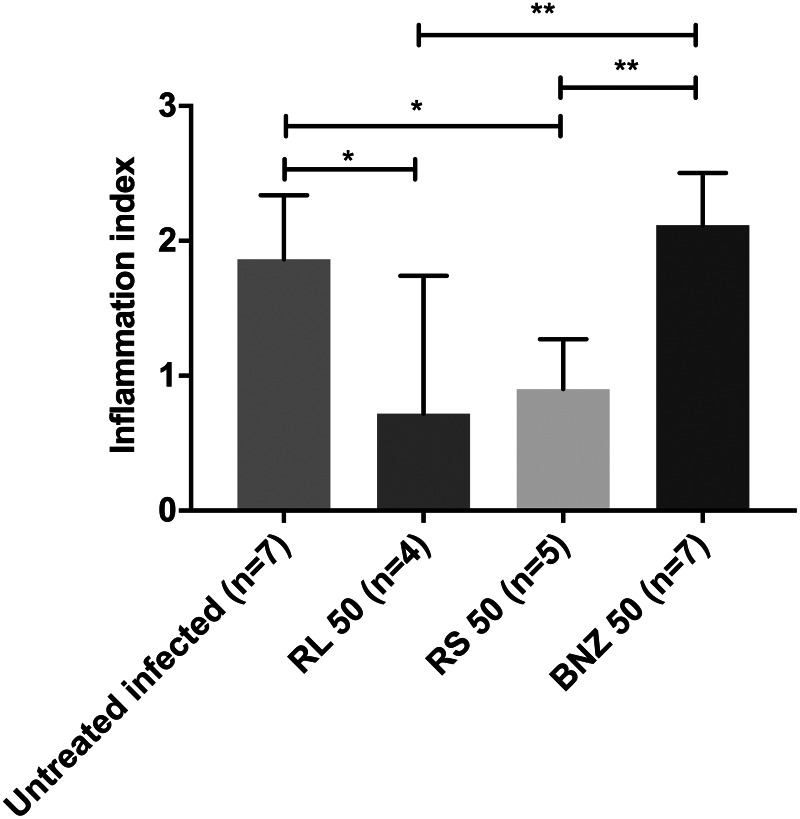
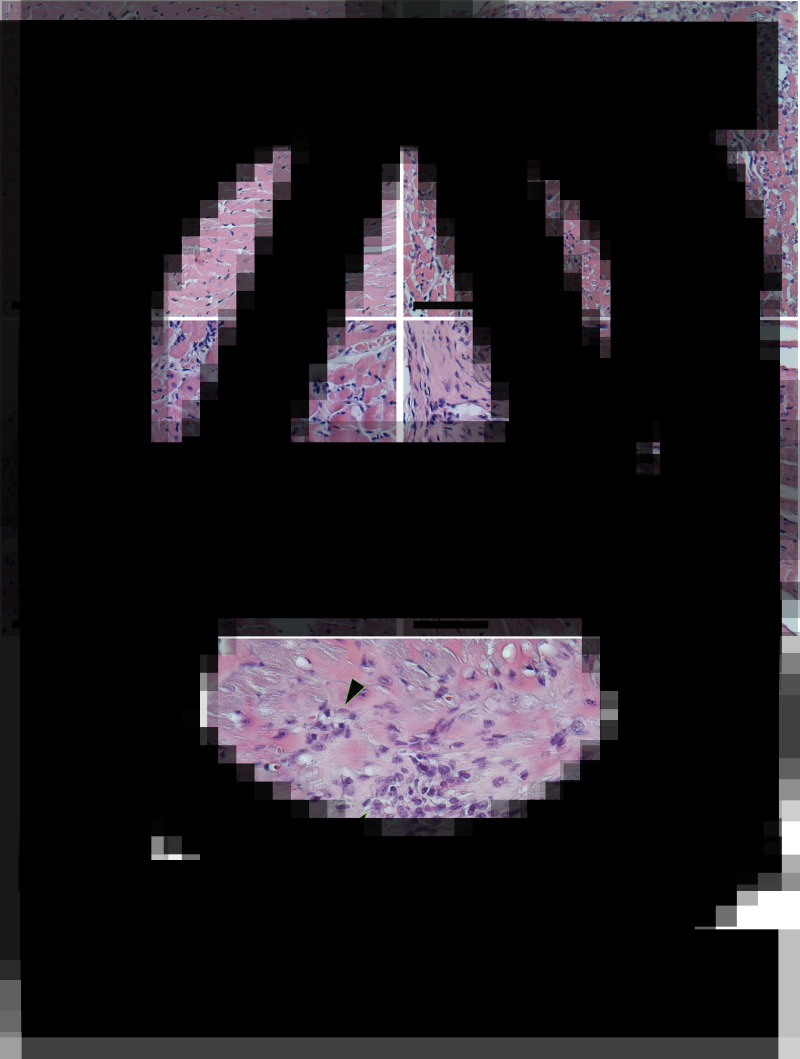
The results of this study showed that infected mice treated with raw BNZ exhibited similar heart tissue inflammatory damage to that of the untreated infected group. In contrast, it is important to note that a significant decrease of inflammatory cells in the heart tissue was observed after treatment with BNZ-MP (RL 50 and RS 50) (Fig. 5). In Fig. 6A, it is possible to see the cardiac tissue of uninfected mice without any sign of inflammation. On the other hand, the cardiac tissues of both untreated infected mice (Fig. 6B) and infected mice treated with the raw drug (Fig. 6E) showed amastigotes nests, extensive and multiple inflammatory foci of mononuclear cell infiltrates and some necrotic areas with structural alterations and fibrotic foci (Grosso et al., 2013). In contrast, the cardiac tissues of mice treated with BNZ-MP-RL (Fig. 6C) and BNZ-MP-RS (Fig. 6D) were not damaged and only minor inflammatory foci with respect to untreated infected animals were detected (Fig. 6B).
Fig. 5.
Evaluation of inflammation in the heart of acute T. cruzi-infected mice treated with BNZ or BNZ-MP. Animals were treated at 2 d.p.i. with BNZ-MP or BNZ at 50 mg kg−1 day−1 (RL 50, RS 50 or BNZ 50, respectively) for 30 days. Data represent the morphometric quantification of inflammatory cells in heart tissue stained with Haematoxylin and Eosin (H&E). *P < 0.05; **P < 0.01, analysed by the Kruskal–Wallis test, bars represent mean + s.d.
Fig. 6.
Haematoxylin and Eosin (H&E) staining of heart tissues from mice infected with T. cruzi and treated with BNZ-MP at 3 months post-infection. (A) Uninfected mice, (B) untreated infected mice, arrows show amastigotes nests, (C) infected mice treated with RL 50, (D) infected mice treated with RS 50 and (E) infected mice treated with BNZ 50. (A–D) Magnification: 20× and (E) 40×. Scale bars: 25 μm. This figure appears in colour in the online version and in black and white in the printed version.
Discussion
BNZ, like many other benzimidazole derivatives, is poorly water-soluble and exhibits a low dissolution rate which may require the administration of high doses to achieve therapeutic effects (Buckley et al., 2013). Thus, BNZ belongs to Class II of the Biopharmaceutical Classification System (BCS) (Del Moral Sanchez et al., 2018; Ferraz et al., 2018). However, according to the experimental data provided by Maximiano et al. (2011), it is classified as Class IV, which indicate a reduced solubility and permeability with a Log P in distilled water, gastric fluid and enteric fluid of 0.77, 0.78 and 0.76, respectively (Maximiano et al., 2011). In this regard, the reduction of particle size and increase of the surface area of such lipophilic compounds through the micronization process is one of the most effective methods to overcome such drawbacks (Singh and Van den Mooter, 2016; Abuzar et al., 2018). Although there are several technological tools to produce MP (Paulo and Santos, 2017; Barrera et al., 2020) the spray-drying technique is a versatile and efficient method to produce particles <10 μm when spray-dryers with two-fluid nozzles are used (Kemp et al., 2016; Davis and Walker, 2018). It is important to note that MP prepared by spray-drying are usually organic solvent-free, in contrast to other methodologies that use toxic organic solvents and/or toxic reagents (Li et al., 2008; Shim and Sah, 2020). Furthermore, the selection of a suitable carrier is also a key step during the development of final products (Lengyel et al., 2019). In general, most of the synthetic polymers are dissolved in volatile hazardous chlorinated organic solvents immiscible with water. Thus, the solvents are removed by either evaporation at elevated temperatures or extraction in a large amount of water, resulting in the formation of MP. However, the traces of such hazardous solvents are serious concerns during the development of pharmaceutical, cosmetic and food products. Additionally, the rate and temperature of solvent elimination are reported to affect the morphology of the product and/or the stability of the drug (Dixit et al., 2015; Haque et al., 2018). Taking all these considerations into account, methacrylate copolymers (Eudragit®) were selected as polymeric carriers to formulate BNZ-MP, because of their solubility and harmless properties and ability to encapsulate hydrophobic substances (Thakral et al., 2013; Higashi et al., 2015). Because these carriers are easily dissolved in ethanol, a Generally Recognized As Safe (GRAS) solvent, it is not necessary to use hazardous solvents to solubilize the carriers. In particular, Eudragit® derivatives (RL and RS) were proved to be able to encapsulate BNZ in a very effective manner, providing interesting mechanical and stability characteristics to the final products (Seremeta et al., 2019). However, there are many other polymers with good capability as carriers for hydrophobic drugs. Therefore, in the future, other carriers will be used in our research group in order to encapsulate BNZ and compare them with Eudragit® RS and RL. It is important to note that the combination of the methodology and the selected carriers allowed an excellent drug encapsulation efficiency (95–96%). As can be seen in Table 1, there was a significant difference in the dissolution of BNZ from both polymeric MP compared to the raw drug during the first 60 min of the assay. It could be due to the reduced size of the BNZ-MP (<2 μm) after the process of spray-drying and the increased surface area which lead to a fast drug dissolution. These results are consistent with those using chitosan as a polymeric carrier to encapsulate BNZ by the coacervation methodology (Leonardi et al., 2009; Barrera et al., 2020). In this regard, it should be mentioned that the dissolution rate of BNZ was also increased using solid dispersions (SDs). Lima et al. (2011) obtained SDs of BNZ with hydrophilic polymers. Dissolution study showed a significant increase up to 8-fold in dissolution rate of s.d. vs standard BNZ at 30 min. In addition, complexation with supramolecular hosts, such as cyclodextrins (CDs), was also applied for this aim. Leonardi et al. (2013) prepared BNZ-CDs complexes able to improve the dissolution rate of BNZ up to 4.3-fold in comparison with the untreated drug. Another approach to improved BNZ solubility and dissolution was proposed Maximiano et al. (2011) who increased the drug performance by preparing BNZ microcrystals using different hydrophilic stabilizing agents. Taking into account such results, in this work, BNZ loaded into RL and RS-MP lead to a faster drug release as compared to the raw drug. It was found that RL formulation released a higher amount of drug than RS formulation, particularly in the first 10 min of the assay. It could be because RL is more soluble in water and more permeable than RS, which can facilitate the drug release (Apu et al., 2009). Stability of pharmaceutical products is one of the critical aspects of ensuring the safety and efficacy of these products (Wu et al., 2011; Sosnik and Seremeta, 2015). In particular, the problems of instability of liquid formulations upon storage could be avoided by converting them to solid products (Sanchez-Vazquez et al., 2019). As reported, several drugs are more stable in the solid state than the liquid state at ambient temperature (Broeckx et al., 2017; Emami et al., 2018; Ziaee et al., 2019). In this study, stability assay showed no significant difference (P > 0.05) in the drug content of BNZ-MP for 3 years. This finding is in agreement with a report of Cilurzo et al. (2008), who found no variation of nifedipine content after 3 months storage of fast-dissolving MP. These results confirm that spray-drying process is a suitable method to keep the drug content in MP for a long period of time. Due to the potential importance in clinical research, in vitro haemolytic and cytotoxicity assay was performed to evaluate whether BNZ-MP might damage cells. The results showed intact red blood cells and no toxic effects as no morphological change of the mammalian cell membrane, as occurs with other nanoparticles evaluated as carriers for cancer therapy (Liu et al., 2010), indicating that such BNZ-MP would present desirable properties in terms of cell viability. In addition, it was not observed in any BNZ-MP-treated mice physical alterations nor any change in their behaviour during the course of our experiments. We used as standard 50 mg kg−1 day−1 of BNZ with the aim to compare the same amount of drug used in the BNZ-MP formulation, based on the effects observed with BNZ 50 mg kg−1 day−1 on the parasitaemia and parasite in hearts, in the same mice model (Grosso et al., 2013) and other models (Strauss et al., 2013; Rial et al., 2019). Oral administration of drugs to the animal of experimentation is usually performed using an aqueous solution with different excipients (cellulose derivatives or surfactants) (Piccirilli et al., 2014). However, when BNZ-MP were dispersed in water, a small precipitate was observed and, therefore, it was decided to disperse those MP in oil instead of water, as already reported (Rial et al., 2017). In agreement with the results of qPCR assay, mice treated with BNZ-MP did not exhibit parasitic infection in comparison to the mice treated with the raw drug (Fig. 4). This finding could be due to the faster release of BNZ from the Eudragit® formulations (Table 1), which could improve its absorption and further biological activity. A similar finding was reported by Piccirilli et al. who described a faster release and improved pharmacokinetic properties of albendazole from the polymeric microparticles, in comparison with the raw drug (Piccirilli et al., 2014). Thus, the decrease in the levels of specific antibodies after BNZ-MP administration could be directly related to the decrease or absence of the parasite load as has been seen in other BNZ treatments using the same experimental model (Scalise et al., 2016; Rial et al., 2017). One of the most important and serious events of Chagas disease is the damage of the cardiac tissue which can lead to myocarditis and pericarditis (Tanowitz et al., 2015). Thus, the reduction or elimination of any inflammation of the cardiac tissue produced by T. cruzi infection by novel BNZ formulations would be a valuable result. As seen in Fig. 5, the histopathology result of mice heart tissue treated with BNZ-MP showed a clear decrease in inflammation, compared to those treated with raw BNZ and untreated infected mice, probably due to the capacity of these polymeric formulations to improve the delivery of BNZ to those tissues. Thus, such lower levels of inflammation could prevent a possible progression towards fibrosis in the chronic stage of the infection, avoiding cardio pathological complications. Similar findings were observed in previous studies during the development of nanocrystals of BNZ (Rial et al., 2017, 2020), confirming the significance of reformulated BNZ, as novel and efficient treatment for Chagas disease. In summary, fast-release BNZ-MP with a mean particle size of <2 μm and long-term storage stability (3 years) were produced by spray-drying methodology using as carriers Eudragit® RL PO and RS PO. Neither detectable morphological change or cell membrane destabilization nor haemolysis in defibrinated human blood was observed after incubation with such BNZ formulations. Preclinical studies in the murine model showed that BNZ-MP exhibited a remarkable effect on the acute T. cruzi infection. Infected mice survived an acute infection with 30 doses of BNZ-MP (50 mg kg−1 day−1). Also, a decrease of T. cruzi-specific antibody levels and absence of heart tissue damages were observed. Therefore, these fast-release BNZ-MP, prepared by spray-drying, are a very convenient approach to successfully treat Chagas disease in an experimental acute T. cruzi infection.
Acknowledgements
We thank the animal facility staff of INP, Gabriela Barja, Laura Potenza and Leticia Orellana. We are also very grateful to Claudia Nose for her excellent technical assistance.
Author contributions
L.E.F. and C.J.S. conceived and designed the study. M.S.R., K.P.S., M.I.E. and J.B. performed the studies. M.S.R., K.P.S., L.E.F. and C.J.S. analysed and interpreted the results. C.J.S., K.P.S., M.S.R. and L.E.F. wrote the first draft of the manuscript and supplied all test compounds. All authors read and approved the final manuscript.
Ethical standards
All procedures involving animals were approved by the Bioethics Committee of the National Institute of Parasitology ‘Dr. Mario Fatala Chaben’ (Register RENIS N°: 000028, Buenos Aires, Argentina) and followed the rules of the ethical legislation and regulatory entities established in Argentina. The international recommendations for the use of laboratory animals (World Medical Association in the Declaration of Helsinki) were also followed.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002310.
click here to view supplementary material
Financial support
This work was partially supported by the National Institute of Parasitology ‘Dr. Mario Fatala Chaben’, National Ministry of Health, INP-ANLIS, Buenos Aires, Argentina (Grant FOCANLIS 2017, NRU: 1704 to M.S.R.), by CONICET (Argentina), ASACTEI-SANTA FE (Argentina), National University of Rosario (Argentina), Universidad Nacional del Chaco Austral (Argentina) (PI N°63).
Conflict of interest
None.
References
- Abuzar SM, Hyun SM, Kim JH, Park HJ, Kim MS, Park JS and Hwang SJ (2018) Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. International Journal of Pharmaceutics 538, 1–13. [DOI] [PubMed] [Google Scholar]
- Al-Khattawi A, Bayly A, Phillips A and Wilson D (2018) The design and scale-up of spray dried particle delivery systems. Expert Opinion on Drug Delivery 15, 47–63. [DOI] [PubMed] [Google Scholar]
- Altcheh J, Moscatelli J, Moroni S, Garcia-Bournissen F and Freilij H (2011) Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 127, e212–e218. [DOI] [PubMed] [Google Scholar]
- Apt W (2010) Current and developing therapeutic agents in the treatment of Chagas disease. Drug Design, Development and Therapy 4, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apu AS, Pathan AH, Shrestha D, Kibria G and Jalil R (2009) Investigation of in vitro release kinetics of carbamazepine from Eudragit® RS PO and RL P matrix tablets. Tropical Journal of Pharmaceutical Research 8, 145–152. [Google Scholar]
- Barrera MG, Tejada G, Leonardi D, Lamas MC and Salomon CJ (2020) A novel prototype device for microencapsulation of benznidazole: in vitro/in vivo studies. AAPS PharmSciTech 21, 112. [DOI] [PubMed] [Google Scholar]
- Bonney KM (2014) Chagas disease in the 21st century: a public health success or an emerging threat? Parasite 21, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckx G, Vandenheuvel D, Henkens T, Kiekens S, van den Broek MFL, Lebeer S and Kiekens F (2017) Enhancing the viability of Lactobacillus rhamnosus GG after spray drying and during storage. International Journal of Pharmaceutics 534, 35–41. [DOI] [PubMed] [Google Scholar]
- Bua J, Volta BJ, Perrone AE, Scollo K, Velázquez EB, Ruiz AM, De Rissio AM and Cardoni RL (2013) How to improve the early diagnosis of Trypanosoma cruzi infection: relationship between validated conventional diagnosis and quantitative DNA amplification in congenitally infected children. PLoS Neglected Tropical Diseases 7, e2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley ST, Frank KJ, Fricker G and Brandl M (2013) Biopharmaceutical classification of poorly soluble drugs with respect to ‘enabling formulations’. European Journal of Pharmaceutical Sciences 50, 8–16. [DOI] [PubMed] [Google Scholar]
- Cardoso CS, Ribeiro ALP, Oliveira CDL, Oliveira LC, Ferreira AM, Bierrenbach AL, Silva JLP, Colosimo EA, Ferreira JE, Lee T-H, Busch MP, Reingold AL and Sabino EC (2018) Beneficial effects of benznidazole in Chagas disease: NIH SaMi-Trop cohort study. PLoS Neglected Tropical Diseases 12, e0006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilurzo F, Selmina F, Minghettia P, Gennaria CGM, Demartin F and Montanaria L (2008) Characterization and physical stability of fast-dissolving microparticles containing nifedipine. European Journal of Pharmaceutics and Biopharmaceutics 68, 579–588. [DOI] [PubMed] [Google Scholar]
- Cortesi R, Lahm Ajanji SC, Sivieri E, Manservigi M, Fundueanu G, Menegatti E and Esposito E (2007) Eudragit® microparticles as a possible tool for ophthalmic administration of acyclovir. Journal of Microencapsulation 24, 445–456. [DOI] [PubMed] [Google Scholar]
- Crespillo-Andújar C, Chamorro-Tojeiro S, Norman F, Monge-Maillo B, López-Vélez R and Pérez-Molina JA (2018) Toxicity of nifurtimox as second-line treatment after benznidazole intolerance in patients with chronic Chagas disease: when available options fail. Clinical Microbiology and Infection 24:1344.e1–1344.e4. [DOI] [PubMed] [Google Scholar]
- Davis M and Walker G (2018) Recent strategies in spray drying for the enhanced bioavailability of poorly water-soluble drugs. Journal of Controlled Release 269, 110–127. [DOI] [PubMed] [Google Scholar]
- Del Moral Sanchez JM, Gonzalez-Alvarez I, Cerda-Revert A, Gonzalez-Alvarez M, Navarro-Ruiz A, Almidon GL and Bermejo M (2018) Biopharmaceutical optimization in neglected diseases for paediatric patients by applying the provisional paediatric biopharmaceutical classification system. British Journal of Clinical Pharmacology 84, 2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit K, Athawale RB and Singh S (2015) Quality control of residual solvent content in polymeric microparticles. Journal of Microencapsulation, Micro and Nano Carriers 32, 107–122. [DOI] [PubMed] [Google Scholar]
- Duffy T, Bisio M, Altcheh J, Burgos JM, Diez M, Levin MJ, Favaloro RR, Freilij H and Schijman AG (2009) Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Neglected Tropical Diseases 3, e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami F, Vatanara A, Park EJ and Na DH (2018) Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics 10, E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz LRM, Alves AÉG, Nascimento DDSDS, Amariz IAE, Ferreira AS, Costa SPM, Rolim LA, Lima ÁAN and Rolim Neto PJ (2018) Technological innovation strategies for the specific treatment of Chagas disease based on benznidazole. Acta Tropica 185, 127–132. [DOI] [PubMed] [Google Scholar]
- Grosso NL, Bua J, Perrone AE, Gonzalez MN, Bustos PL, Postan M and Fichera LE (2010) Trypanosoma cruzi: biological characterization of an isolate from an endemic area and its susceptibility to conventional drugs. Experimental Parasitology 126, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso NL, Alarcon ML, Bua J, Laucella SA, Riarte A and Fichera LE (2013) Combined treatment with benznidazole and allopurinol in mice infected with a virulent Trypanosoma cruzi isolate from Nicaragua. Parasitology 140, 1225–1233. [DOI] [PubMed] [Google Scholar]
- Gupta S and Garg NJ (2010) Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Neglected Tropical Diseases 4, e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S, Boyd BJ, McIntosh MP, Pouton CW, Kaminskas LM and Whittaker M (2018) Suggested procedures for the reproducible synthesis of poly(d,l-lactide-co-glycolide) nanoparticles using the emulsification solvent diffusion platform. Current Nanoscience 14, 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K, Hayashi H, Yamamoto K and Moribe K (2015) The effect of drug and EUDRAGIT® S 100 miscibility in solid dispersions on the drug and polymer dissolution rate. International Journal of Pharmaceutics 494, 9–16. [DOI] [PubMed] [Google Scholar]
- Kemp IC, Hartwig T, Herdman R, Hamilton P, Bisten A and Bermingham S (2016) Spray drying with a two-fluid nozzle to produce fine particles: atomization, scale-up, and modeling. Drying Technology 34, 1243–1252. [Google Scholar]
- Kratz JM, Garcia Bournissen F, Forsyth CJ and Sosa-Estani S (2018) Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Review of Clinical Pharmacology 11, 943–957. [DOI] [PubMed] [Google Scholar]
- Lengyel M, Kállai-Szabó N, Antal V, Laki AJ and Antal I (2019) Microparticles, microspheres, and microcapsules for advanced drug delivery. Scientia Pharmaceutica 87, 20. [Google Scholar]
- Leonardi D, Salomón CJ, Lamas MC and Olivieri AC (2009) Development of novel formulations for Chagas’ disease: optimization of benznidazole chitosan microparticles based on artificial neural networks. International Journal of Pharmaceutics 367, 140–147. [DOI] [PubMed] [Google Scholar]
- Leonardi D, Bombardiere ME and Salomon CJ (2013) Effects of benznidazole: cyclodextrin complexes on the drug bioavailability upon oral administration to rats. International Journal of Biological Macromolecules 62, 543–548. [DOI] [PubMed] [Google Scholar]
- Li M, Rouaud O and Poncelet D (2008) Microencapsulation by solvent evaporation: state of the art for process engineering approaches. International Journal of Pharmaceutics 363, 26–39. [DOI] [PubMed] [Google Scholar]
- Lima AAN, Soares-Sobrinho JL, Silva JL, Hernandes MZ, Rolim LA and Rolim-Neto PJ (2011) The use of solid dispersion systems in hydrophilic carriers to increase benznidazole solubility. Pharmaceutical Technology 100, 2443–2451. [DOI] [PubMed] [Google Scholar]
- Liu F, Park JY, Zhang Y, Conwell C, Liu Y, Bathula SR and Huang L (2010) Targeted cancer therapy with novel high drug-loading nanocrystals. Journal of Pharmaceutical Sciences 99, 3542–3551. [DOI] [PubMed] [Google Scholar]
- Maximiano FP, de Paula LM, Figueiredo VP, de Andrade IM, Talvani A, Sá-Barreto LC, Bahia MT and Cunha-Filho MS (2011) Benznidazole microcrystal preparation by solvent change precipitation and in vivo evaluation in the treatment of Chagas disease. European Journal of Pharmaceutics and Biopharmaceutics 78, 377–384. [DOI] [PubMed] [Google Scholar]
- Maya JD, Cassels BK, Iturriaga-Vásquez P, Ferreira J, Faúndez M, Galanti N, Ferreira A and Morello A (2007) Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comparative Biochemistry and Physiology – Part A: Molecular & Integrative Physiology Special Issues 146, 601–620. [DOI] [PubMed] [Google Scholar]
- Meymandi S, Hernandez S, Park S, Sanchez DR and Forsyth C (2018) Treatment of Chagas disease in the United States. Current Treatment Options in Infectious Diseases 10, 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ and Yusuf S and BENEFIT Investigators (2015) Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. The New England Journal of Medicine 373, 1295–1306. [DOI] [PubMed] [Google Scholar]
- Paulo F and Santos L (2017) Design of experiments for microencapsulation applications: a review. Materials Science and Engineering C: Materials for Biological Applications 77, 1327–1340. [DOI] [PubMed] [Google Scholar]
- Pérez-Molina JA, Pérez-Ayala A, Moreno S, Fernández-González MC, Zamora J and López-Velez R (2009) Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta-analysis. Journal of Antimicrobial Chemotherapy 64, 1139–1147. [DOI] [PubMed] [Google Scholar]
- Piccirilli GN, García A, Leonardi D, Mamprin ME, Bolmaro RE, Salomón CJ and Lamas MC (2014) Chitosan microparticles: influence of the gelation process on the release profile and oral bioavailability of albendazole, a class II compound. Drug Development and Industrial Pharmacy 40, 1476–1482. [DOI] [PubMed] [Google Scholar]
- Poozesh S and Bilgili E (2019) Scale-up of pharmaceutical spray drying using scale-up rules: a review. International Journal of Pharmaceutics 562, 271–292. [DOI] [PubMed] [Google Scholar]
- Quijia Quezada C, Azevedo CS, Charneau S, Santana JM, Chorilli M, Carneiro MB and Bastos IMD (2019) Advances in nanocarriers as drug delivery systems in Chagas disease. International Journal of Nanomedicine 14, 6407–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial MS, Scalise ML, Arrúa EC, Esteva MI, Salomon CJ and Fichera LE (2017) Elucidating the impact of low doses of nano-formulated benznidazole in acute experimental Chagas disease. PLoS Neglected Tropical Diseases 11, e0006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial MS, Scalise ML, López Alarcón M, Esteva MI, Búa J, Benatar AF, Prado NG, Riarte AR and Fichera LE (2019) Experimental combination therapy using low doses of benznidazole and allopurinol in mouse models of Trypanosoma cruzi chronic infection. Parasitology 146, 305–313. [DOI] [PubMed] [Google Scholar]
- Rial MS, Arrua EC, Natale MA, Bua J, Esteva MI, Prado NG, Laucella SA, Salomon CJ and Fichera LE (2020) Efficacy of continuous versus intermittent administration of nanoformulated benznidazole during the chronic phase of Trypanosoma cruzi Nicaragua infection in mice. Journal of Antimicrobial Chemotherapy 75, 1906–1916. [DOI] [PubMed] [Google Scholar]
- Ribeiro V, Dias N, Paiva T, Hagström-Bex L, Nitz N, Pratesi R and Hecht M (2020) Current trends in the pharmacological management of Chagas disease. International Journal for Parasitology: Drugs and Drug Resistance 12, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon CJ (2012) First century of Chagas’ disease: an overview on novel approaches to nifurtimox and benznidazole delivery systems. Journal of Pharmaceutical Sciences 101, 888–894. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vazquez B, Lee JB, Strimaite M, Buanz A, Bailey R, Gershkovich P, Pasparakisa P and Williams GR (2019) Solid lipid nanoparticles self-assembled from spray dried microparticles. International Journal of Pharmaceutics 572, 118784. [DOI] [PubMed] [Google Scholar]
- Scalise ML, Arrúa EC, Rial MS, Esteva MI, Salomon CJ and Fichera LE (2016) Promising efficacy of benznidazole nanoparticles in acute Trypanosoma cruzi murine model: in-vitro and in-vivo studies. The American Journal of Tropical Medicine and Hygiene 95, 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seremeta KP, Arrúa EC, Okulik NB and Salomon CJ (2019) Development and characterization of benznidazole nano- and microparticles: a new tool for pediatric treatment of Chagas disease? Colloids and Surfaces B: Biointerfaces 177, 169–177. [DOI] [PubMed] [Google Scholar]
- Sguassero Y, Cuesta CB, Roberts KN, Hicks E, Comandé D, Ciapponi A and Sosa-Estani S (2015) Course of chronic Trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS ONE 10, e0139363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H and Sah H (2020) Qualification of non-halogenated organic solvents applied to microsphere manufacturing process. Pharmaceutics 12, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A and Van den Mooter G (2016) Spray drying formulation of amorphous solid dispersions. Advanced Drug Delivery Reviews 100, 27–50. [DOI] [PubMed] [Google Scholar]
- Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM and Yampotis C (1998) Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas’ disease. The American Journal of Tropical Medicine and Hygiene 59, 526–529. [DOI] [PubMed] [Google Scholar]
- Sosnik A and Seremeta KP (2015) Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Advances in Colloid and Interface Science 223, 40–54. [DOI] [PubMed] [Google Scholar]
- Strauss M, Lo Presti S, Bazán PC, Baez A, Fauro R, Esteves B, Sanchez Negrete O, Cremonezzi D, Paglini-Oliva PA and Rivarola HW (2013) Clomipramine and benznidazole association for the treatment of acute experimental Trypanosoma cruzi infection. Parasitology International 62, 293–299. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Machado FS, Spray DC, Friedman JM, Weiss OS, Lora JN, Nagajyothi J, Moraes DN, Garg NJ, Nunes MC and Ribeiro AL (2015) Developments in the management of Chagas cardiomyopathy. Expert Review of Cardiovascular Therapy 13, 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral S, Thakral NK and Majumdar DK (2013) Eudragit®: a technology evaluation. Expert Opinion on Drug Delivery 10, 131–149. [DOI] [PubMed] [Google Scholar]
- Vinuesa T, Herráez R, Oliver L, Elizondo E, Acarregui A, Esquisabel A, Pedraz JL, Ventosa N, Veciana J and Viñas M (2017) Benznidazole nanoformulates: a chance to improve therapeutics for Chagas disease. The American Journal of Tropical Medicine and Hygiene 97, 1469–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G and Armenti A (2009) Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Review of Anti-infective Therapy 7, 157–163. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020) Chagas disease (also known as American trypanosomiasis). Available at https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis).
- Wu L, Zhang J and Watanabe W (2011) Physical and chemical stability of drug nanoparticles. Advanced Drug Delivery Reviews 63, 456–469. [DOI] [PubMed] [Google Scholar]
- Ziaee A, Albadarin AB, Padrela L, Femmer T, O'Reilly E and Walker G (2019) Spray drying of pharmaceuticals and biopharmaceuticals: critical parameters and experimental process optimization approaches. European Journal of Pharmaceutical Sciences 127, 300–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002310.
click here to view supplementary material