Abstract
Background
Primary cardiac soft tissue sarcomas (CSTS) affect young adults, with dismal outcomes.
Objectives
The aim of this study was to investigate the clinical outcomes of patients with CSTS receiving immune checkpoint inhibitors (ICIs).
Methods
A retrospective, multi-institutional cohort study was conducted among patients with CSTS between 2015 and 2022. The patients were treated with ICI-based regimens. The Kaplan-Meier method was used to estimate overall survival (OS) and progression-free survival (PFS). Objective response rates were determined according to Response Evaluation Criteria in Solid Tumors version 1.1. Treatment-related adverse events were graded per the Common Terminology Criteria for Adverse Events version 5.0.
Results
Among 24 patients with CSTS, 17 (70.8%) were White, and 13 (54.2%) were male. Eight patients (33.3%) had angiosarcoma. At the time of ICI treatment, 18 patients (75.0%) had metastatic CSTS, and 4 (16.7%) had locally advanced disease. ICIs were administered as the first-line therapy in 6 patients (25.0%) and as the second-line therapy or beyond in 18 patients (75.0%). For the 18 patients with available response data, objective response rate was 11.1% (n = 2 of 18). The median PFS and median OS in advanced and metastatic CSTS (n = 22) were 5.7 months (95% CI: 2.8-13.3 months) and 14.9 months (95% CI: 5.7-23.7 months), respectively. The median PFS and OS were significantly shorter in patients with cardiac angiosarcomas than in those with nonangiosarcoma CSTS: median PFS was 1.7 vs 11 months, respectively (P < 0.0001), and median OS was 3.0 vs 24.0 months, respectively (P = 0.008). Any grade treatment-related adverse events occurred exclusively in the 15 patients with nonangiosarcoma CSTS (n = 7 [46.7%]), of which 6 (40.0%) were grade ≥3.
Conclusions
Although ICIs demonstrate modest activity in CSTS, durable benefit was observed in a subset of patients with nonangiosarcoma, albeit with higher toxicity.
Key Words: cardiac sarcomas, cardiac tumors, immune checkpoint inhibitors, treatment-related adverse events
Central Illustration
Primary tumors of the heart are rare.1, 2, 3 Approximately 75% of primary cardiac tumors take the form of benign mesenchymal tumors.1 Although uncommon, primary cardiac soft tissue sarcomas (CSTS) rank as the second most common type of primary cardiac neoplasm and account for the majority of malignant primary cardiac tumors.1,3 CSTS may occur at any age but tend to affect individuals in the third to fifth decades of life.1 The clinical presentation of patients with CSTS varies on the basis of tumor size and location, typically involving symptoms such as dyspnea and secondary symptoms such as embolic phenomena, conduction abnormalities, pericardial effusion, and occasional metastases to other sites such as bone, brain, and colon.1 Limited small-scale data exist to guide CSTS management. Although complete tumor excision is the preferred treatment strategy in most cases, depending on the tumor location in the heart, the infiltrative nature of these tumors and the extent of local invasion and metastases can make this approach unfeasible.
Treatment options for recurrent or metastatic CSTS are limited, and responses to anthracycline-based chemotherapy are short lived, with a median progression-free survival (PFS) of 4.4 months in patients treated with first-line palliative chemotherapy.4 Eventually, most patients succumb to metastatic disease,4 and the prognosis of patients with CSTS remains poor, characterized by a median overall survival (OS) of <1 year.1,5
The introduction of immune checkpoint inhibitors (ICIs) has successfully shifted the treatment paradigm in oncology, and these agents have received regulatory approval for a broad spectrum of tumor types.6, 7, 8, 9 In noncardiac sarcomas, clinical trials of ICIs have shown promising results, with response rates ranging between 5% and 37%.10,11 To date, however, there are no reports that examine clinical outcomes and side-effect profiles of patients with primary malignant cardiac tumors treated with ICIs. In this collective effort across multiple institutions, we present an exploration of the outcomes and side-effect profiles of patients with CSTS receiving ICIs.
Methods
Patient population
This study was based on an analysis of a retrospective, multicenter database. Data from 8 participating institutions in the United States (Supplemental Table 1) were obtained and are currently housed at the Dana-Farber Cancer Institute. According to the Declaration of Helsinki, this retrospective study was covered by the Institutional Review Board review at the Dana-Farber Cancer Institute (protocol 21-329) and local Institutional Review Boards at participating sites.
Patients included: 1) had cardiac masses identified by transthoracic echocardiography or cardiac magnetic resonance imaging; 2) had histologic diagnoses of CSTS; and 3) had received any line of ICI therapy (≥1 dose), defined as anti–programmed cell death protein-1 (PD-1) or anti–programmed cell death-ligand 1 (PD-L1) alone or in combination with anti–cytotoxic T lymphocyte–associated antigen-4 (CTLA-4) or other anticancer therapies (ie, chemotherapy or targeted agents) between 2015 and 2022. Patients were excluded if the primary site of origin was noncardiac and the pathology was not a sarcoma. CSTS were classified into 1 of 2 histologic cohorts: angiosarcoma and nonangiosarcoma.
Clinical outcomes and toxicity profiles
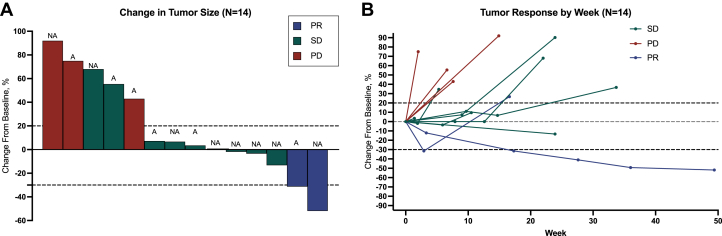
The primary endpoint of this study was OS, defined as the date of ICI initiation to death or censoring at the date of the last follow-up. The secondary endpoints were PFS, objective response rates (ORRs), and treatment-related adverse events (trAEs). PFS measured the time from ICI initiation to radiologic or clinical disease progression, death, or censoring at the date of the last follow-up. ORRs were assessed by board-certified radiologists at respective centers. Radiologists were blinded to clinical data, and RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 was used for ORR determination whenever possible. For 4 patients, serial images were not available for RECIST version 1.1 measurements, and instead, response data from radiology reports were used (Figure 1). For the remaining 4 patients with response data, serial images were unavailable for radiology review, as they were obtained at outside institutions. trAEs were graded per the Common Terminology Criteria for Adverse Events version 5.0.
Figure 1.
Response to Immune Checkpoint Inhibitors Among Patients With Cardiac Soft Tissue Sarcoma
(A) Waterfall plot of best percentage change from baseline in the size of target lesions (sum of diameters). (B) Spider plots of percentage of baseline tumor size (sum of target lesion diameters) at each Response Evaluation Criteria in Solid Tumors version 1.1 assessment of cardiac soft tissue sarcoma. Black-colored dashed lines over −30% and 20% represent partial response (PR) and disease progression thresholds, respectively. A = angiosarcoma; NA = nonangiosarcoma; PD = progressive disease; SD = stable disease.
Statistical analysis
Continuous data are reported as median (Q1-Q3) and categorical data as count (percentage). Kaplan-Meier methods were used to estimate the median OS and PFS using 95% CIs with group comparisons performed using the log-rank test. ORR was defined as the proportion of patients with partial response (PR) or complete response and the percentage reported with exact 95% CI using the exact Clopper-Pearson method. Group comparisons for ORR were performed using the Fisher exact test. Statistical analysis was performed using SAS version 9.4 (SAS Institute), and P values <0.05 were considered to indicate statistical significance.
Results
CSTS cohort characteristics
Among the 24 patients diagnosed with CSTS, the median age was 45 years (Q1-Q3: 37-54 years), with 13 men (54.2%) and 5 being Asian or Black patients (20.8%) (Table 1, Supplemental Table 2). The median follow-up time was 17.9 months (95% CI: 13.3 to not reached). The median cardiac mass diameter was 6 cm (Q1-Q3: 4.2-6.7 cm). The most common histologic subgroup was angiosarcoma (n = 8 [33.3%]). The remaining CSTS cases were grouped into the nonangiosarcoma CSTS cohort (n = 16 [66.7%]) (Supplemental Figure 1). Among the patients, 18 (75.0%) had metastatic CSTS, while 4 (16.7%) had locally advanced disease. Thirteen patients (54.2%) had histories of surgery with curative intent. Complications related to the cardiac mass developed in approximately 16 patients (66.7%), including heart failure (12.5%), valvulopathies (12.5%), and cardiac tamponade (8.3%) (Table 1). Baseline cardiac magnetic resonance imaging was performed on 20 patients (83.3%) with CSTS, revealing cardiac thrombi in 7 (29.2%). Cardio-oncology referrals were made for 11 of 24 patients (45.8%). The baseline demographics, clinicopathological characteristics, and treatment plans for each patient are shown in Table 1 and Supplemental Table 2. Among the 23 patients with available data, 4 had their PD-L1 status assessed, with 2 having tumor proportion scores of 0% and 2 with scores of 1% (Supplemental Table 2).
Table 1.
Baseline Clinical Characteristics (N = 24)
| Age at ICI start, y | 45 (37-54) |
| Female | 11 (45.8) |
| Race | |
| Asian | 3 (12.5) |
| Black or African American | 2 (8.3) |
| White | 17 (70.8) |
| Other | 2 (8.3) |
| Ethnicity | |
| Hispanic/Latinx | 4 (16.7) |
| Non-Hispanic/non-Latinx | 20 (83.3) |
| Region of United States | |
| Northeast | 2 (8.3) |
| Midwest | 12 (50.0) |
| South | 9 (37.5) |
| West Coast | 1 (4.2) |
| Smoking | |
| Never | 16 (66.7) |
| Former | 8 (33.3) |
| Type of malignancy | |
| Angiosarcoma | 8 (33.3) |
| Nonangiosarcoma cardiac soft tissue sarcomaa | 16 (66.7) |
| Diameter of cardiac mass, cm | 6.0 (4.2-6.7) |
| Complications from cardiac mass | |
| None | 8 (33.3) |
| Cardiac tamponade | 2 (8.3) |
| Arrhythmia | 4 (16.7) |
| HF | 3 (12.5) |
| Syncope | 1 (4.2) |
| Valvulopathy | 3 (12.5) |
| Dysphagia | 1 (4.2) |
| Pericarditis/pericardial effusion | 2 (8.3) |
| Obstruction-related processb | 3 (12.5) |
| Cardiac MRI | 20 (83.3) |
| Cardiac consult at ICI initiation | 11 (45.8) |
| Location of cardiac mass | |
| Atrial | 18 (75.0) |
| Ventricular | 1 (4.2) |
| Multiple | 5 (20.8) |
| Cardiac thrombus at ICI initiation | 7 (29.2) |
| Number of systemic therapy lines prior to ICI initiation | |
| 0 | 4 (16.7) |
| 1 | 12 (50) |
| ≥2 | 8 (33.3) |
| Class of ICI used | |
| Anti-PD-1 | 17 (70.8) |
| Anti-PD-1 + anti-CTLA-4 | 3 (12.5) |
| Anti-PD-1 + non-ICI-based therapyc | 4 (16.7) |
| ECOG status at ICI start | |
| 0 | 9 (37.5) |
| 1 | 12 (50) |
| ≥2 | 2 (8.3) |
| Unavailable | 1 |
Values are median (Q1-Q3) or n (%).
HF = congestive heart failure; CTLA-4 = cytotoxic T lymphocyte–associated antigen 4; ECOG = Eastern Cooperative Oncology Group; ICI = immune checkpoint inhibitor; MRI = magnetic resonance imaging; PD-1 = programmed cell death protein 1; trAE = treatment-related adverse event.
8 pleomorphic cardiac sarcoma, 4 spindle cell sarcoma, 1 liposarcoma, 1 chondroblastic osteosarcoma, 2 intimal sarcoma.
Includes mitral valve obstruction and superior vena cava syndrome.
1 patient of each: pembrolizumab plus paclitaxel, pembrolizumab plus pazopanib, pembrolizumab + ribociclib, and pembrolizumab + interleukin 2 therapy.
Clinical outcomes
All patients received anti-PD-1-based therapy, with ICIs administered as the first-line therapy in 4 patients and as the second-line therapy in 20 patients. The median number of treatment doses was 5 (Q1-Q3: 2-7). Supplemental Table 2 provides details on the treatment drug, including dosing, schedule, and the number of treatment cycles. Among the 24 patients, 22 (91.7%) received ICIs in the metastatic or locally advanced setting and were included in the subsequent analyses of clinical outcomes and toxicity (Supplemental Figure 1). For all 7 patients with advanced angiosarcoma, the reason for discontinuation was tumor progression, necessitating subsequent therapy or leading to death. In contrast, only 7 of 15 patients (46.7%) with advanced nonangiosarcoma discontinued ICI because of tumor progression. Among the 18 CSTS with response data, the ORR was 11.1% (2 of 18; 95% CI: 3.1%-33%), with response observed in 1 intimal sarcoma (nonangiosarcoma) and 1 angiosarcoma. Serial imaging–based RECIST version 1.1 measurements were available for 14 patients (Figure 1). For the remaining 4 patients with response data, serial images were unavailable for radiologic review, as they were obtained at outside institutions. When stratifying by administered treatment regimens, 1 of 3 patients with CSTS on nivolumab and ipilimumab achieved a durable PR. Among the 4 patients with CSTS on anti-PD-1 therapy combined with non-ICI regimens, 1 patient on pembrolizumab and paclitaxel achieved a PR. None of the 11 patients with CSTS treated with anti-PD-1 monotherapy responded, with 6 patients achieving stable disease as the best response.
The median time on ICI treatment was longer for nonangiosarcoma CSTS compared with cardiac angiosarcomas (5.5 months [Q1-Q3: 3.3-13.5 months] vs 1.1 months [Q1-Q3: 0.6-3.8 months], respectively). The median PFS and OS in the overall cohort were 5.7 months (95% CI: 2.8-13.3 months) and 14.9 months (95% CI: 5.7-23.7 months), respectively (Figures 2A and 2B, Supplemental Table 3). In an exploratory analysis, median PFS was significantly shorter for cardiac angiosarcoma vs nonangiosarcoma CSTS (1.7 vs 11 months; P < 0.0001) (Figure 2C). Similarly, median OS was significantly shorter among patients with cardiac angiosarcomas vs nonangiosarcoma CSTS (3.0 vs 24 months, respectively; P = 0.008) (Figure 2D, Central Illustration). To better understand the role of treatment effect vs underlying disease biology, we calculated the PFS of both ICI treatment and the immediate prior systemic line of therapy (prior to ICI) whenever possible (Figure 3). Otherwise, we included the PFS of the subsequent systemic line of therapy after ICI as a comparator if there was no prior line. For the nonangiosarcoma histology, treatment with ICIs, on average, led to similar PFS compared with the prior systemic or subsequent lines of therapy. In contrast, all patients with angiosarcoma histology derived more benefit from the prior systemic line compared with ICI therapy, as shown by the PFS treatment intervals (Figure 3).
Figure 2.
Survival Outcomes of Patients With CSTS Treated With Immune Checkpoint Inhibitors
(A) Progression-free survival (PFS) of patients with advanced or metastatic cardiac soft tissue sarcoma (CSTS). (B) Overall survival (OS) of patients with advanced and metastatic CSTS. (C) Progression-free survival of patients with advanced and metastatic CSTS by histology. (D) Overall survival of patients with advanced and metastatic CSTS by histology.
Central Illustration.
Survival Outcomes and Safety Profiles With Cardiac Soft Tissue Sarcoma Treated With Immunotherapy
The illustration presents the 2 broad histologic categories (angiosarcoma and nonangiosarcoma). The Kaplan-Meier method was used to estimate the median overall survival (OS) using the log-rank test. Treatment-related adverse events (trAEs) were categorized as grade <3, grade ≥3, or none.
Figure 3.
Swimmer Plot: 15 Patients With Nonangiosarcoma and 7 Patients With Angiosarcoma
Comparison of progression-free survival between 1) an intrapatient control group, defined as the immediate prior non–immune checkpoint inhibitor (ICI) systemic therapy (if available) or post-ICI therapy if prior non-ICI systemic therapy was not available; and 2) the treatment group of interest (ie, ICI therapy). The goal of this swimmer plot is to characterize whether the treatment effect is due to the underlying behavior of the tumor compared with true treatment-specific efficacy. CDK4/6inh = cyclin-dependent kinase 4/6 inhibitor; CTLA-4 = cytotoxic T lymphocyte–associated antigen 4; IL = interleukin; NE = not evaluable; PD-1 = programmed cell death protein 1; PR = partial response; TKI = tyrosine kinase inhibitor; tx = therapy; other abbreviations as in Figure 1.
Toxicity profiles
trAEs occurred only in patients with nonangiosarcoma CSTS. trAEs of any grade occurred in 7 of 22 patients (31.8%) (Supplemental Table 4). Grade 3 and 4 trAEs were reported in 6 patients (27.3%), and 1 grade 5 pneumonitis was reported, leading to death. None had cardiac trAEs. Five patients (23%) required steroids to manage trAEs. ICIs were discontinued because of trAEs in 5 of 22 patients (22.7%). Six patients were hospitalized because of trAEs. The distributions of trAEs by histology, use of steroids, and discontinuation because of toxicity are shown in Supplemental Tables 2 and 4.
Discussion
Given the extent of potential person-years lost to related deaths, CSTS can have a considerable societal impact.12 Because of the rarity of CSTS, no randomized clinical trials have been performed to identify an optimal systemic treatment regimen, and chemotherapy protocols are extrapolated from extracardiac soft tissue sarcoma counterpart data. Additionally, no case reports or other low-evidence data are available regarding patients with CSTS treated with ICIs. For the first time, our data suggest that a subset of patients with primary CSTS can achieve clinical benefit from ICI therapy. Both patients with CSTS whose tumors responded were treated with anti-PD-1-based combinations. Our exploratory analysis revealed that clinical benefit among patients with CSTS treated with ICIs is histology specific, whereas cardiac angiosarcomas showed dismal outcomes compared with patients with nonangiosarcoma CSTS. The swimmer plot analysis of ICI and non-ICI PFS outcomes suggests that anti-PD-1 and anti-PD-L1 ICIs are not as effective for patients with angiosarcoma but likely have a role as a combination approach with anti-CTLA-4 or chemotherapy in the treatment of patients with nonangiosarcoma CSTS histologies. Moreover, most patients with nonangiosarcoma histologies achieved durable benefit (12-month stable disease or complete response or PR as the best response) when treated with ICIs (1 of 2 intimal, 3 of 7 pleomorphic, and 2 of 4 spindle cell sarcoma).
Prior studies have demonstrated remarkable responses to ICIs in patients with cutaneous angiosarcomas.13 Although the UV mutation signature may explain responses to cutaneous angiosarcoma,13 this phenomenon likely does not apply to angiosarcomas of visceral organs, such as the heart. Moreover, the aggressive clinical course of visceral angiosarcoma compared with cutaneous angiosarcoma probably makes it less suitable for ICI treatment. In the SARC028 phase 2 study of the anti-PD-1 antibody pembrolizumab, ORRs of 18% (soft tissue) and 5% (bone) were observed among 84 patients with advanced or metastatic soft tissue and bone sarcoma.10 Although monotherapy with the anti-CTLA-4 inhibitor ipilimumab in patients with synovial sarcoma was disappointing, with no responses observed in 6 patients, leading to study closure,14 ipilimumab in combination with nivolumab resulted in an ORR of 16%, compared with 5% with nivolumab monotherapy.13,15 The ICI combination led to a median PFS of 4.1 months and OS of 14.3 months.
trAE rates were higher in patients with nonangiosarcoma CSTS compared with those with angiosarcoma. This may be related to a significantly longer time on ICI treatment in the nonangiosarcoma group. Importantly, none of the patients experienced major adverse cardiac events such as myocarditis. In our study, two-thirds of patients had complications related to their cardiac masses, with nearly 30% having cardiac thrombi. In addition, referrals to cardio-oncology were <50%, thus highlighting the critical need for multidisciplinary care in patients with CSTS.
Study limitations
Our study was limited by its retrospective nature and potential selection bias from large academic centers. Our cohort of patients receiving ICI constituted only a small fraction of those receiving other systemic therapies for cardiac sarcomas, likely representing a relatively healthier population. Moreover, the majority of patients in our study received ICIs as the second-line therapy or beyond for metastatic CSTS, for which clinical outcomes may be dismal. Therefore, the results of our study should be interpreted cautiously in this context. It is worth mentioning that we lacked biomarker data, and only a limited number of patients underwent next-generation sequencing because, in the current landscape, no known biomarkers have been associated with therapeutic implications in soft tissue sarcoma in general. For example, more than 80% of the patients who received immunotherapy did not have tumors testing for PD-1 or PD-L1 status. Only 3 patients had available genomic data; therefore, we excluded the genomic data from the present study.
trAEs were common among patients with nonangiosarcoma histology, but this may be confounded by the longer duration of treatment. A competing risk analysis (considering ICI discontinuation because of progression or other reasons) would account for this but could not be performed because of limited data on the time of occurrence of trAEs. Nevertheless, given the lack of treatment options and limitations to clinical trials specifically for this rare tumor patient population, we hope our work further augments the evaluation of anti-PD-1-based combination approaches that could benefit patients with CSTS.
Conclusions
In this first ever report of ICIs in patients with CSTS, a subset of patients with nonangiosarcoma histology treated with anti-PD-1-based combinations derived clinical benefit, compounded by higher trAEs. The high rate of cardiac mass–related complications highlights the need for multidisciplinary interventions to support patients as they receive cancer-directed therapy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: ICIs are associated with durable clinical benefit in a subset of patients with CSTS of nonangiosarcoma histology.
TRANSLATIONAL OUTLOOK: Future research is warranted to determine genomic and clinical biomarkers that can predict response to ICIs among patients with CSTS.
Funding Support and Author Disclosures
Dr Abdel-Wahab is supported by a K01 Mentored Research Scientist Development Award from the National Institute of Allergy and Infectious Diseases (grant K01AI163412) and has received the University of Texas MD Anderson Cancer Center Institutional Research Grant, Division of Internal Medicine Development Award, Survivorship Seed Money Award, Prioritizing Research Innovation and Mentoring Excellence Award, and Melanoma SPORE Career Enhancement Program Award. Dr Nassar has received honoraria from OncLive, TEMPUS, and the Korean Society for Medical Oncology; and has received consulting fees from Guidepoint Global. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Contributor Information
Toni K. Choueiri, Email: toni_choueiri@dfci.harvard.edu.
Abdul Rafeh Naqash, Email: abdulrafeh-naqash@ouhsc.edu.
Appendix
References
- 1.Butany J., Nair V., Naseemuddin A., Nair G.M., Catton C., Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg A.D., Blankstein R., Padera R.F. Tumors metastatic to the heart. Circulation. 2013;128:1790–1794. doi: 10.1161/CIRCULATIONAHA.112.000790. [DOI] [PubMed] [Google Scholar]
- 3.Burke A.P., Cowan D., Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–395. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Chen T.W., Loong H.H., Srikanthan A., et al. Primary cardiac sarcomas: a multi-national retrospective review. Cancer Med. 2019;8:104–110. doi: 10.1002/cam4.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlandi A., Ferlosio A., Roselli M., Chiariello L., Spagnoli L.G. Cardiac sarcomas: an update. J Thorac Oncol. 2010;5:1483–1489. doi: 10.1097/JTO.0b013e3181e59a91. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi F.S., O’Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 10.Tawbi H.A., Burgess M., Bolejack V., et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen A.P., Sharon E., O’Sullivan-Coyne G., et al. Atezolizumab for advanced alveolar soft part sarcoma. N Engl J Med. 2023;389:911–921. doi: 10.1056/NEJMoa2303383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quiroga D., Liebner D.A., Philippon J.S., et al. Activity of PD1 inhibitor therapy in advanced sarcoma: a single-center retrospective analysis. BMC Cancer. 2020;20:527. doi: 10.1186/s12885-020-07021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florou V., Rosenberg A.E., Wieder E., et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213. doi: 10.1186/s40425-019-0689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maki R.G., Jungbluth A.A., Gnjatic S., et al. A pilot study of anti-CTLA4 antibody ipilimumab in patients with synovial sarcoma. Sarcoma. 2013;2013 doi: 10.1155/2013/168145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Angelo S.P., Mahoney M.R., Van Tine B.A., et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.