Abstract
Cardiac amyloidosis (CA) is an infiltrative disease caused by amyloid fibril deposition in the myocardium; the 2 forms that most frequently involve the heart are amyloid light chain (AL) and amyloid transthyretin (ATTR) amyloidosis. Cardiac troponin (cTn) is the biomarker of choice for the detection of myocardial injury and is frequently found to be elevated in patients with CA, particularly with high-sensitivity assays. Multiple mechanisms of myocardial injury in CA have been proposed, including cytotoxic effect of amyloid precursors, interstitial amyloid fibril infiltration, coronary microvascular dysfunction, amyloid- and non-amyloid-related coronary artery disease, diastolic dysfunction, and heart failure. Regardless of the mechanisms, cTn values have relevant prognostic (and potentially diagnostic) implications in both AL and ATTR amyloidosis. In this review, the authors discuss the significant aspects of cTn biology and measurement methods, potential mechanisms of myocardial injury in CA, and the clinical application of cTn in the management of both AL and ATTR amyloidosis.
Key Words: troponin, amyloidosis, myocardial injury, prognosis, diagnosis
Central Illustration
Highlights
-
•
Two forms of cardiac amyloidosis (CA), AL and ATTR affect the heart.
-
•
Therapies are available for both forms of CA.
-
•
High sensitivity cTn a marker of myocardial injury is often elevated with CA.
-
•
Many mechanisms for myocardial injury in CA require recognition and consideration.
-
•
cTn is diagnostic and prognostic aid in CA and in monitoring therapy.
Cardiac amyloidosis (CA) is an infiltrative disease caused by the deposition of amyloid fibrils in the extracellular matrix, progressively leading to organ damage and dysfunction.1 CA now is an increasingly recognized cause of heart failure, particularly with preserved ejection fraction.2 The 2 main types of amyloidosis that can involve the heart, amyloid light chain (AL) and amyloid transthyretin (ATTR) amyloidosis, are pathophysiologically different. In AL amyloidosis, the amyloid precursor proteins are immunologic light chains (LCs) most commonly produced by a plasma cell clone. In ATTR amyloidosis, either variant (hereditary) ATTR (ATTRv) amyloidosis or wild-type ATTR (ATTRwt) amyloidosis,3 the precursor protein is ATTR, which is produced predominantly by the liver and serves as a carrier for thyroxine and retinol. Although the disease management, course, and prognosis of AL and ATTR CA are different, at present, effective treatment options exist for both.4,5 The efficacy of these treatment options is dependent on disease stage at the time of diagnosis, determined mainly by the severity of cardiac involvement,3 which can be assessed using cardiac biomarkers and imaging techniques. Cardiac troponin (cTn) is the biomarker of choice for the detection of myocardial injury,6 and it is frequently found to be elevated in patients with CA, in particular when high-sensitivity assays are used. The extent of abnormalities in cTn is of crucial prognostic significance in these patients.3 In this review, we elucidate the significant aspects of cTn biology, measurement, and interpretation when it is used adjunctively to assist in the management of patients with CA. The multiplicity of possible causes for myocardial injury in these patients are addressed, with particular focus on the relevant pathophysiological mechanisms and common clinical scenarios.
cTn: Basic Concepts
Since the development of cTn assays in the late 1980s and early 1990s, this marker has assumed a progressively more predominant role in cardiovascular care. It is currently the biomarker of choice for the detection of myocardial injury and for the diagnosis of myocardial infarction.6 It is significantly more specific than prior markers and much more sensitive, which allows the identification of many new processes that can damage cardiomyocytes.
The troponin complex consists of 3 different proteins, troponin C, troponin I, and troponin T, which are encoded by separate genes and are essential for proper excitation and contraction in cardiomyocytes (Figure 1).7 Because of their cardiospecific isoform expression, cTnI and cTnT can be used as highly specific markers of myocardial damage.8 Recent data suggest that the re-expression of cTnT (gene TNNT2) in patients with active myopathies and myositis can contribute to cTnT elevations in some patients.9,10 At present, there is no evidence of up-regulation or re-expression of cTnI in diseased skeletal muscle.10 cTn elevations occur not only in acute ischemic disease with overt necrotic cardiac damage but in many other situations (Table 1).6
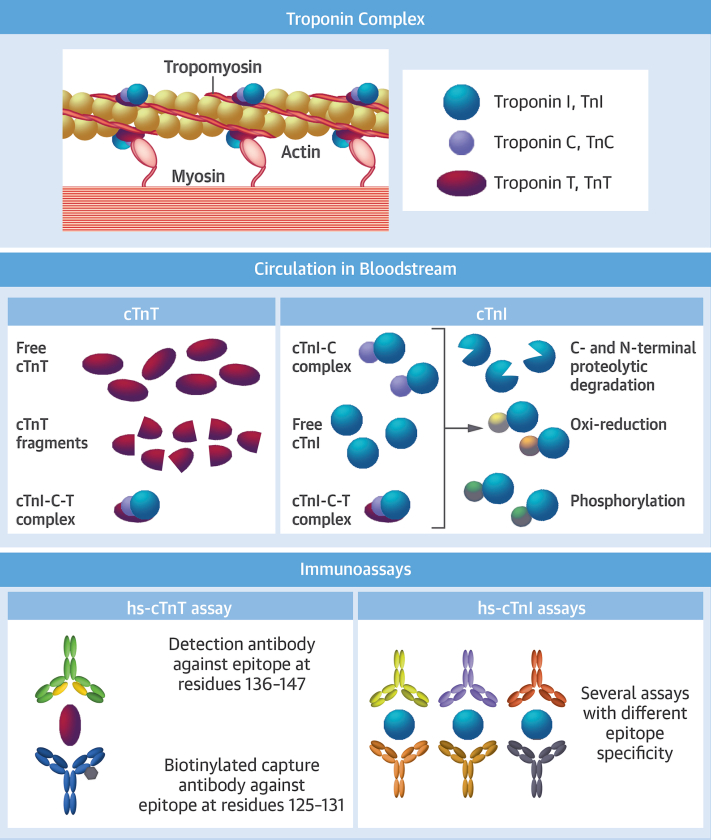
Figure 1.
Cardiac Troponin Structure, Release in Bloodstream, and Methodologies of Measurement
After a myocardial infarction, cardiac troponin I (cTnI) circulates in the blood mainly as a binary complex with cardiac troponin C, but also as free cTnI and a ternary complex, cTnI-C-T. Various molecular modifications and protein fragmentations have been described. cTnT circulates mainly in a free form, but cardiac troponin T (cTnT) fragments as well as the cTnI-C-T complex are also present. The high-sensitivity cTnT (hs-cTnT) assay uses fragment antigen binding portions of 2 cTnT-specific mouse monoclonal antibodies. For the fifth-generation assay, the original antibody has been re-engineered to produce a mouse-human chimeric detection antibody. Several high-sensitivity cTnI (hs-cTnI) assays are approved for clinical use, with a lack of harmonization related to different aspects, starting with the difference in epitope specificity of antibodies produced by different companies. Indeed, immunoassays that use anti-cTnI monoclonal antibodies are dependent on the epitope regions recognized by the antibodies incorporated into each assay. Moreover, cTnI-related characteristics such as biochemical modifications (eg, degradation, phosphorylation, oxide reduction) and different circulating complexes can lead to altered signal generation of sandwich-type cTnI immunoassays that use antibodies directed against these modified regions.15 Reproduced with permission from Brush et al.100
Table 1.
Possible Causes of Myocardial Injury According to the Fourth Universal Definition of Myocardial Infarction
| Myocardial injury related to acute myocardial ischemia | Atherosclerotic plaque disruption with thrombosis |
| Myocardial injury related to acute myocardial ischemia because of oxygen supply/demand imbalance | Reduced myocardial perfusion
|
| Other causes of myocardial injury | Cardiac conditions
|
Ischemic and nonischemic causes of myocardial injury are shown. In each patient with cardiac amyloidosis, myocardial injury can be due to the amyloid infiltration process, but other concomitant factors (such as arrhythmias or hypotension) can also be present. Adapted with permission from Thygesen et al.6
Over time, assays for cTnT and cTnI measurements have improved in terms of analytical performance. The original assays were insensitive, but present-day high-sensitivity cTn (hs-cTn) assays provide increased sensitivity and greater precision at low concentrations, so that they are able to identify patients at higher risk for adverse events in multiple situations.11 Analytical terminology and characteristics of hs-cTn assays are reported in Table 2.12,13 The correlation between values measured with contemporary (non-high-sensitivity) cTn assays and hs-cTn assays is good at higher levels but poor at low concentrations, especially with hs-cTnT.14
Table 2.
Analytical Terminology to Describe hs-cTn Assay Performance
| Term | Description |
|---|---|
| Limit of blank | “Noise” signal inherent in the analytical system 95th percentile of analytical signal when no cTn is present, only matrix |
| Limit of detection | Lowest concentration detectable in 95% of measurements Imprecision at the limit of detection is often high High-sensitivity assays should measure cTn above the limit of detection in ≥50% of healthy subjects |
| Limit of quantitation | Lowest cTn concentration that can be reported as a number with specified certainty (typically 20% imprecision) |
| Coefficient of variation | A measure of assay imprecision at any given concentration Coefficient of variation should be ≤10% at the 99th percentile upper reference limit for hs-cTn assays |
| 99th percentile upper reference limit | Universally endorsed as the reference cutoff to aid in the diagnosis of acute myocardial infarction. Key components:
|
Analytical terminology to properly understand and describe the performance of hs-cTn assays is shown, as reported in Table 3.
cTn = cardiac troponin; hs-cTn = high-sensitivity cardiac troponin.
Although only 1 hs-cTnT assay is in use for clinical practice, many hs-cTnI assays have been developed; the same is true for older contemporary assays. Because of the different antibodies used in each assay and the unique mix of cTnI forms in each patient sample (Figure 1), there is no harmonization between the different hs-cTnI assays, and absolute values cannot be directly compared.15 Analytical characteristics of the different hs-cTn assays available on the market are reported in Table 3.
Table 3.
Analytical Characteristics and Reference Values of Available High-Sensitivity Assays
| Company/Platform/Assay | LoB, ng/L | LoD, ng/L | 99th Percentile, ng/L | CV at 99th Percentile |
|---|---|---|---|---|
| Abbott/Alinity i systems/Alinity i STAT High Sensitive Troponin-I; commercial (OUS) | 1.0 | 1.6 | Overall: 26.2 F: 15.6 M: 34.2 |
Overall: 4.0% F: 5.3% M: 3.5% |
| Abbott/ARCHITECT i systems/ARCHITECT STAT High Sensitive Troponin-I; commercial (U.S.) | 0.9 | 1.7 | Overall: 28 F: 17 M: 35 |
Overall: 4.3% F: 5.0% M: 4.1% |
| Abbott/ARCHITECT i systems/ARCHITECT STAT High Sensitive Troponin-I; commercial | 0.7-1.3 | 1.1 | Overall: 26.2 F: 15.6 M: 34.2 |
Overall: 4.0% F: 5.3% M: 3.5% |
| Beckman Coulter/Access 2, DxI/Access hsTnI; commercial (OUS) | 0.0-1.7 | 1.0-2.3 | Overall: 17.5 F: 11.6 M: 19.8 |
Overall: 3.7% F: 4.2% M: 3.6% |
| Beckman Coulter/Access 2/Access hsTnI; commercial (U.S.): LiHep plasma | 0.0-0.8 | 1.0-2.0 | Overall: 17.5 F: 11.6 M: 19.8 |
Overall: 3.7% F: 4.2% M: 3.6% |
| Beckman Coulter/Access 2/Access hsTnI; commercial (U.S.): serum | 0.0-0.8 | 1.0-2.0 | Overall: 18.2 F: 11.8 M: 19.7 |
Overall: 6.0% F: 6.9% M: 5.8% |
| Beckman Coulter/DxI, Access hsTnI; commercial (U.S.): LiHep plasma | 0.0-1.7 | 1.5-2.3 | Overall: 17.9 F: 14.9 M: 19.8 |
Overall: 5.2% F: 5.6% M: 5.0% |
| Beckman Coulter/DxI, Access hsTnI; commercial (U.S.): serum | 0.0-1.7 | 1.5-2.3 | Overall: 18.1 F: 13.6 M: 19.8 |
Overall: 6.2% F: 6.5% M: 6.1% |
| BioMérieux VIDAS High Sensitive Troponin I; commercial | 1.9 | 3.2 | Overall: 19 F: 11 M: 25 |
7.0% |
| ET Healthcare Pylon hsTnI assay; China, FDA approved | 0.8 | 1.2-1.4 | Overall: 27 F: 21 M: 27 |
10% |
| ET Healthcare Pylon hsTnT; research | 0.4 | 0.8 | Overall: 13 F: 13 M: 14 |
4% |
| Fujirebio Lumipulse G G1200 and G600II hsTnI | 1.2 | 2.1 | Overall: 28.6 F: 22.4 M: 32.9 Serum overall: 26.9 F: 21.4 M: 29.4 LiHep plasma overall: 29.6 F: 27.8 M: 32.8 |
≤4.6% |
| LSI Medience PATHFAST cTnI; commercial | NP | 1 | Overall: 15.46 M: 16.91 F: 11.46 |
<6% |
| LSI Medience PATHFAST hs-cTnI/PATHFAST cTnI-II | 1.23 | 2.33 | Overall: 27.9 F: 20.3 M: 29.7 |
6.1% |
| Ortho/VITROS/hsTroponin I; commercial | 0.14-0.51 | 0.39-0.86 | Serum overall: 11 F: 9 M: 12 LiHep plasma overall: 11 F: 9 M: 13 |
<10% |
| Quidel/Alere TriageTrue hs-cTnI | 0.4 (plasma) 0.5-0.8 (whole blood) |
0.7-1.6 (plasma) 1.5-1.9 (whole blood) |
Overall: 20.5 F: 14.4 M: 25.7 |
5.0%-5.9% at 21 ng/L (plasma) 5.9%-6.5% at 22 ng/L (whole blood) |
| Roche/cobas e601, e602, e411/cTnT-hs 18-min; commercial | 2.53 (1.58 for e411) | 3.16 (2.54 for e411) | Overall: 14 F: 9 M:17 |
<10% |
| Roche/cobas e601, e602, e411/cTnT-hs STAT; commercial | 2.36 (2.14 for e411) | 2.85 (3.25 for e411) | Overall: 14 F: 9 M: 17 |
<10% |
| Roche/cobas e801/e402 cTnT-hs 18 min and 9 min; commercial | 18 min: 2.21 9 min: 1.91 |
18 min: 2.97 9 min: 2.72 |
Overall: 14 F: 9 M: 17 |
<10% |
| Roche/cobas e601, e602, e411 cTnT Gen 5 STAT; commercial | 2.5 (3 for e411) | 3 (5 for e411) | Overall: 19 F: 14 M: 22 |
<10% |
| Roche/cobas e801/cTnT Gen 5-9 min; commercial | 2.5 | 3 | Overall: 19 F: 14 M: 22 |
<10% |
| Siemens ATELLICA High-Sensitivity TnI (TnIH) (U.S. and OUS); commerciala | 0.50 | 1.6 | Overall: 45.4 F: 38.6 M: 53.5 |
<4.0% |
| Siemens ATELLICA VTLi hs-cTnIa | 0.55 | 1.2 (plasma) 1.6 (whole blood) |
Overall: 22.9 F: 18.5 M: 27.1 |
6.5% (plasma) 6.1% (whole blood) |
| Siemens ADVIA Centaur XP/XPT/CP High-Sensitivity TnI (TNIH) (U.S. and OUS); commerciala,b | 0.50 | 1.6 | Overall: 46.5 F: 39.6 M: 58.0 |
<4.9% |
| Siemens Dimension VISTA High Sensitivity TnI (TNIH) (OUS); commerciala | 1.0 | 2.0 | Overall: 58.9 F: 53.7 M: 78.5 |
<5.0% |
| Siemens Dimension ExL High Sensitivity TnI (TNIH) (OUS); commerciala | 1.1 | 2.7 | Overall: 60.4 F: 51.4 M: 76.2 |
<5.0% |
| Tosoh CL AIA-PACK cTnI; commercial | NP | 1.61 | Overall: ≤24 (Asian), ≤31 (Caucasian) | NP |
Relevant analytical characteristics of high-sensitivity troponin assays available on the market inside and outside of the United States. Data were extracted from the International Federation of Clinical Chemistry and Laboratory Medicine Task Force on Clinical Applications of Cardiac Bio-Markers (version 052023). All data are listed as provided by the manufacturers.
CV = coefficient of variation; FDA = U.S. Food and Drug Administration; LiHep = lithium heparin; LoB = limit of blank; LoD = limit of detection; NP = not provided; OUS = outside the United States; F= Female; M = Male.
Manufacturers may have submitted assays they claim to be “high sensitivity” that do not meet the International Federation of Clinical Chemistry and Laboratory Medicine requirements of <10% CV at the 99th percentile and ≥50% measurable concentrations greater than or equal to the LoD for both men and women.
The products and features mentioned herein are not commercially available in all countries.
Myocardial injury is defined as a cTn value greater than the sex-specific 99th percentile upper reference limit,6 regardless of cause, and it is frequently present in patients with primary cardiovascular disease as well as those whose primary problems can be noncardiac conditions such as critical illness.16 Its prognostic relevance has been established in multiple clinical scenarios.6,16, 17, 18, 19, 20 With the transition from conventional to hs-cTn assays, the frequency of the detection of myocardial injury has increased,21 leading to a more precise stratification of patients at higher risk for adverse events but also escalating the challenge of identifying the reasons of cTn elevations in each patient. In some clinical situations, even values less than the 99th percentile upper reference limit have prognostic information, explaining why cTn helps in risk stratification when analyzed as a continuous variable and not only when relying on thresholds to define a normal range.22,23
Myocardial Injury in CA
Since the 2000s, it has been appreciated that almost invariably, cTn levels are elevated in patients with CA.24 Such elevations are common and manifest important prognostic implications (discussed later). The exact frequency of myocardial injury is difficult to determine definitively and likely relates to differences in the populations studied, differences in the stages of the disease process, and the fact that so many different hs-cTn assays are available with unique cutoffs to define myocardial injury. Nevertheless, multiple pathophysiological mechanisms of myocardial injury in patients with CA have been proposed (Central Illustration). It is important for clinicians to recognize that increases in hs-cTn values may have a multiplicity of causes, ischemic or nonischemic (Table 1); in patients with amyloid infiltration, hs-cTn values reflect the extent of myocardial involvement while also being potentially influenced by all these other causes.
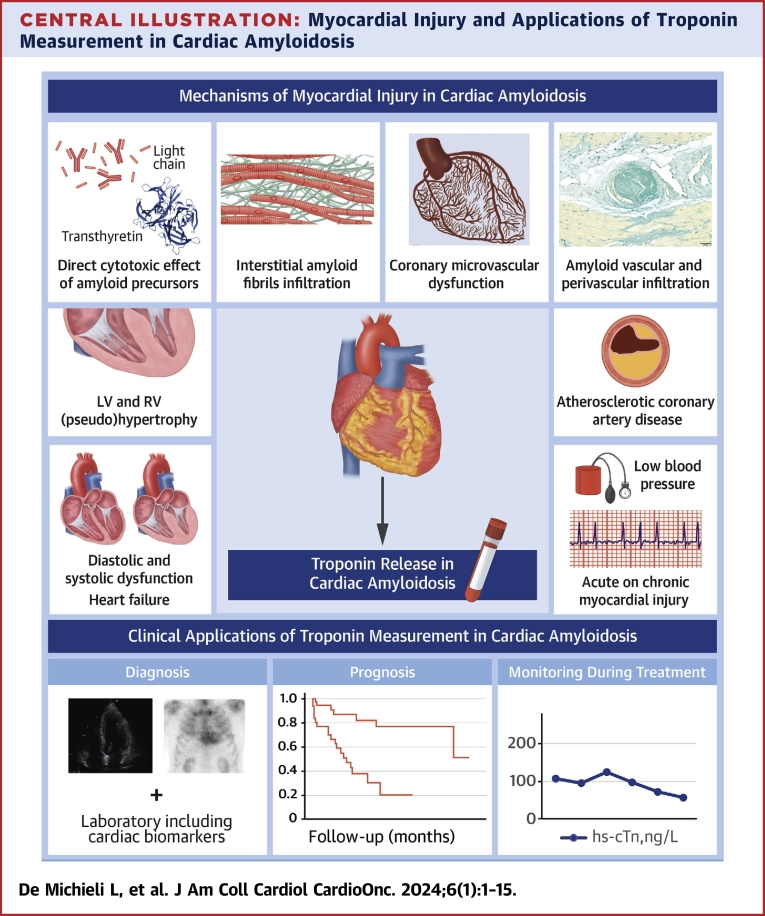
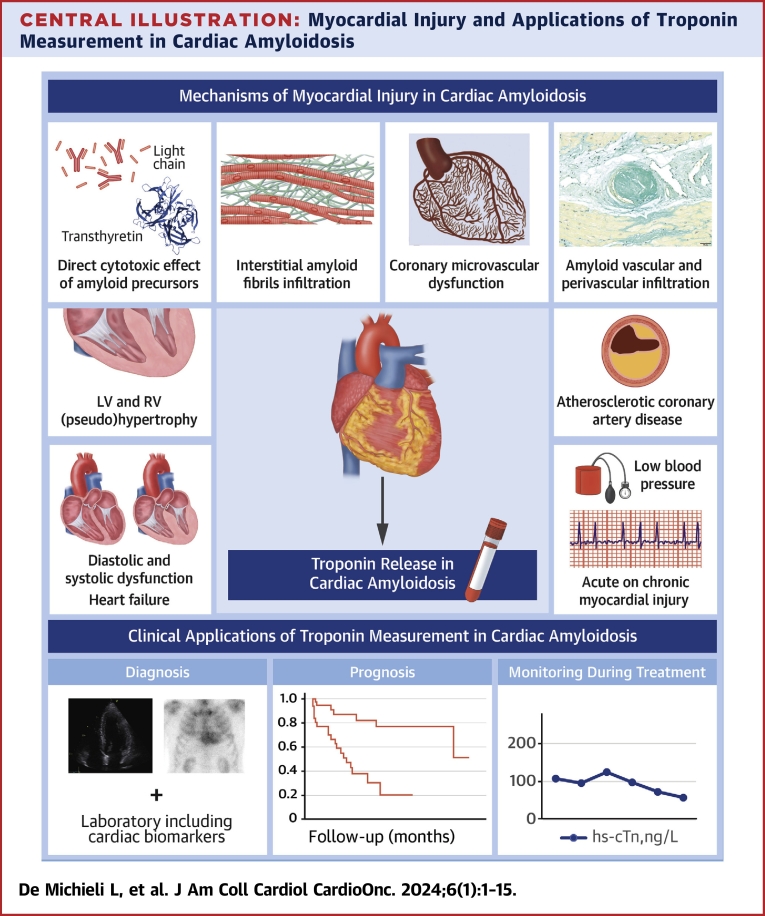
Central Illustration.
Myocardial Injury and Applications of Troponin Measurement in Cardiac Amyloidosis
Several mechanisms are potentially involved in the development of myocardial injury (defined as an increase in cardiac troponin values greater than the 99th percentile sex-specific and assay-specific reference limits) in patients with cardiac amyloidosis. These mechanisms include amyloid-related causes, such as amyloid precursor toxicity, amyloid interstitial infiltration, and amyloid vascular involvement, but also non-amyloid-related causes such as diastolic and systolic dysfunction, heart failure, atherosclerotic coronary artery disease, and other causes of supply-demand imbalance such as tachyarrhythmias and hypotension. Regardless of the cause, measurement of cardiac troponin is important in the management of patients with cardiac amyloidosis for diagnostic, prognostic, and monitoring purposes. hs-cTn = high-sensitivity cardiac troponin; LV = left ventricular; RV = right ventricular.
Direct cytotoxic effect of amyloid precursors
Amyloid precursors can have direct toxic effects on cardiomyocytes. In AL amyloidosis, the proteotoxicity of LCs has been extensively reported.25 Interestingly, infusion of LCs from patients with severe AL amyloidosis with cardiac involvement causes marked impairment of ventricular relaxation on isolated mouse heart models.26 Moreover, human amyloid LCs alter the cellular redox state in isolated cardiomyocytes.27 This results in direct impairment of cardiomyocyte contractility and relaxation, independent of fibril deposition, associated with alterations in intracellular calcium handling.27 Amyloidogenic LCs have been reported to provoke oxidative stress, cellular dysfunction, and apoptosis in isolated adult cardiomyocytes through activation of p38 mitogen-activated protein kinase,28 which also mediates the transcription of brain natriuretic peptide.25 A study performed on human cardiac fibroblasts and Caenorhabditis elegans reported a correlation between the overall conformational properties of native folded proteins (including flexibility, kinetic instability, and dynamic state) and the proteotoxicity of cardiotropic LCs.29 In vitro experiments suggest that whereas LC-derived amyloid fibrils exhibit inhibition of the cell growth and division, soluble LC proteins allow cell growth but cause cellular dysfunction and apoptosis in cardiomyocytes, suggesting that the mechanisms of cytotoxicity differ between soluble proteins and amyloid fibrils.25,30 Clinically, serum free LCs (sFLCs), particularly lambda, significantly correlate reasonably but not exactly with increases in cTnI and N-terminal–pro–brain natriuretic peptide (NT-proBNP) as well as echocardiographic parameters.31,32 Thus, the value of sFLCs provides additional prognostic information in conjunction with cTn and NT-proBNP, and therefore, they have been integrated into multiparametric staging systems.33,34
In ATTR CA, the literature is less extensive. Markers of tissue damage characteristic of inflammation, apoptosis, and the stimulation of reactive oxygen species have been found in tissues of human and transgenic mouse models carriers of mutant transthyretin variants, well before amyloid deposits are detected.35 However, the direct toxicity of AL precursors is thought to be more marked than that of ATTR, possibly contributing to the different clinical profiles of the 2 conditions, such that AL amyloidosis has more rapid progression and worse prognosis if untreated than ATTR amyloidosis.36
Interstitial amyloid fibril infiltration
Amyloid infiltration in the heart results in disruption of tissue architecture and subsequent replacement fibrosis, with cardiomyocyte damage. The extracellular fibrils have a significant impact on the mechanics and physiology of the target tissue.37 In AL CA models, in vitro analyses have shown that amyloid fibrils rapidly surround cultured cardiomyocytes and recruit soluble LCs, triggering cytotoxicity.37 Extracellular amyloid fibrils also appear to disrupt cardiac matrix homeostasis and alter extracellular matrix turnover, which is critical for the maintenance of myocyte-myocyte force coupling and proper myocardial function.38 An overexpression of matrix metalloproteinases39 also has been reported. Moreover, fibroblasts are able to internalize both aggregated transthyretin40 and amyloidogenic LCs.41 These mechanisms contribute to the expansion of extracellular spaces and to the development of interstitial, reactive fibrosis in response to tissue damage, which may contribute further to myocardial disruption and damage.
A study by Pucci et al42 showed that extracellular volume evaluated on cardiac magnetic resonance correlates with the combination of amyloid deposition and interstitial fibrosis at endomyocardial biopsy of the left ventricle (LV). This combination of amyloid and fibrosis also correlates with hs-cTnT, in both AL CA (r = 0.622) and ATTR CA (r = 0.533), suggesting that infiltration, fibrosis, and extracellular disruption together contribute to myocardial injury. In a cohort of patients with ATTRwt CA, hs-cTnT correlated with native T1 and extracellular volume on cardiac magnetic resonance and modestly, if at all, with amyloid load (r = 0.354) on endomyocardial biopsy of the right ventricle43; this poor correlation with amyloid load on endomyocardial biopsy is probably secondary to the selection bias inherent to biopsy itself, which provides focal information rather than an evaluation of total amyloid load.
Coronary microvascular dysfunction and amyloid- and non-amyloid-related coronary artery disease
Coronary microvascular dysfunction and amyloid- and non-amyloid-related coronary artery disease (CAD) also contribute to myocardial injury in patients with CA. Amyloid deposits can be found in the perivascular regions and in the media of intramyocardial coronary vessels,44 with vascular and perivascular involvement being more frequent in AL CA.45 Coronary microvascular dysfunction can be present, related to 3 possible mechanisms: structural (amyloid deposition in the vessels wall with thickening and stenosis, capillary rarefaction), extravascular (extrinsic compression of the microvasculature), and functional (autonomic and endothelial dysfunction).42,44,46 Dorbala et al44 reported on 21 patients with CA without obstructive epicardial CAD who underwent evaluation of coronary microvascular function with rest and vasodilator stress 13N ammonia positron emission tomography/computed tomography. Compared with 10 patients with LV hypertrophy, patients with CA had lower resting myocardial blood flow, lower stress myocardial blood flow, lower coronary flow reserve, and higher minimal coronary vascular resistance. Coronary microvascular dysfunction was associated with increased LV mass and myocardial relaxation abnormalities. Similarly, on echocardiography, patients with CA had significantly lower coronary flow reserve (together with lower rest and stress global longitudinal strain and lower myocardial work efficiency), and changes in coronary flow reserve and deformation capacity were strongly associated with exercise tolerance.47 Also, in 20 patients with AL CA, stress-induced wall motion abnormalities on echocardiography were frequent (55%) despite the absence of significant epicardial CAD.48 Other cardiovascular and noncardiovascular comorbidities (such as diabetes) can contribute to endothelial abnormalities and coronary microvascular dysfunction, particularly in older and comorbid patients with ATTRwt CA. Therefore, coronary microvascular dysfunction is frequent in CA and likely is an important cause of myocardial injury.49 Amyloid vascular and perivascular infiltration45,50 can also be a cause of chest pain, acute myocardial injury, and myocardial infarction in the absence of atherosclerotic CAD.51, 52, 53 In addition, classic atherosclerotic epicardial CAD also can be present (particularly in older patients with ATTRwt CA2) and may contribute to a lower ischemic threshold. A recent study reported that chest pain is not an uncommon symptom in patients with CA (about 40%), and the etiology seemed to differ, with obstructive CAD more frequent in patients with ATTR CA, whereas amyloid vascular or perivascular involvement was more common in those with AL CA.54 Management of acute coronary syndromes and the differential diagnosis for chest pain in these patients can be challenging because of the underlying chronic myocardial injury. Validated cTn-based algorithms for the rule-in and rule-out of myocardial infarction among patients presenting without ST-segment elevation55 are difficult to apply in those who have baseline hs-cTn increases but are the only guidance available. However, the application of biologically significant relative changes between serial cTn values,6 which help in patients with acute coronary syndromes, have not been validated in those with CA.
Diastolic dysfunction and heart failure
Elevation of LV end-diastolic pressure can lead to apoptosis and cTnI release, as shown in animal models,56 and clinically, cTnT levels correlate with the LV end-diastolic pressure in patients with heart failure.49 Because of the restrictive hemodynamic status of CA, increased LV filling pressure with elevated myocardial wall tension (an obstacle to subendocardial myocardial perfusion) can play an important role in the genesis of myocardial injury. This can result in a vicious circle characterized by elevated LV filling pressures causing subendocardial ischemia, reduced coronary perfusion pressure, and diastolic dysfunction, which in turn can lead to more elevations in LV end-diastolic pressure.57 Moreover, both LV and right ventricular pseudohypertrophy can enhance these mechanisms. Group 2 pulmonary hypertension,58 frequently detected in patients with CA,59 can also contribute to cTn release through myocardial ischemia and cell death due to increased wall tension of the right ventricle with pressure and/or volume overload.60
Acute on chronic myocardial injury
Not only multiple mechanisms can contribute to the genesis and progression of myocardial injury in patients with CA, but these patients are also at risk for acute events related to CA or independent of CA as well. Some of these may be due to ischemia, although clear signs and symptoms of myocardial ischemia are often difficult to appreciate in this complex milieu. Acute exacerbations of heart failure or arrhythmias are frequently observed in CA, both of which are known causes of myocardial injury.6 Patients with CA also are particularly prone to developing atrial fibrillation or flutter and/or bradyarrhythmias.61,62 Nonsustained ventricular arrhythmias are also frequent, particularly in those with more advanced cardiac involvement.63 Hypotension is also frequent in patients with CA, secondary to the primary cardiac disease but also to autonomic dysfunction, and similar to tachyarrhythmias and bradyarrhythmias, prolonged hypotension is counted among the possible causes of myocardial injury related to oxygen supply-demand imbalance.6 All of these are known causes of cTn elevations,6 particularly in individuals with underlying myocardial disease.64
Clinical Use of cTn in CA
The use of cardiac biomarkers like cTn and natriuretic peptides to aid in the evaluation of patients with CA was developed over many years. Today, they have a fundamental role in the prognostic assessment of patients with CA, alone and integrated in multiparametric staging systems. Moreover, they are helpful in monitoring the response to chemotherapy in AL amyloidosis. Emerging data suggest that they can also be of relevance when CA is suspected.65 In the following paragraphs, the role of cTn in the different phases of CA management is discussed (see Table 4 for AL CA and Table 5 for ATTR CA).
Table 4.
Clinical Use of cTn in the Management of Patients With AL Amyloidosis
| Prognosisa |
Response to Therapy | ||
|---|---|---|---|
| Prognostic Variables and Staging Systems | Estimated Survival | ||
Diagnostic score to define cardiac involvement in AL amyloidosis67
|
Mayo Clinic 200473
|
Stage 1: 26.4 mo Stage 2: 10.5 mo Stage 3: 3.5 mo |
Cardiac disease progression89
|
CA very likely in patients with suspected CA65
|
Mayo Clinic 201233
|
Stage 1: 94.1 mo Stage 2: 40.3 mo Stage 3: 14 mo Stage 4: 5.8 mo |
Cardiac disease response89
|
CA unlikely in patients with suspected CA65
|
European 2015 modification of Mayo 200474,75
|
Stage 1: NR Stage 2: 55% 3 y Stage 3a: 52% 3 y Stage 3b: 7 mo |
Graded cardiac response91
|
Boston University staging system76
|
Stage 1: NR Stage 2: 9.4 y Stage 3: 4.3 y Stage 3b: 1 y |
Restaging with Mayo 2004 and 2012 systems94,95 | |
Palladini et al80
|
10.6 mo | ||
ASCT candidates70,82
|
Cutoffs for identification of patients at risk of early mortality after ASCT | ||
A summary of different thresholds for specific biomarkers (particularly cTn evaluated with different assays) for diagnosis, prognosis, and response to therapy in patients with AL amyloidosis is shown. For diagnosis, thresholds of specific markers, potentially useful to predict the likelihood of a final diagnosis of AL cardiac amyloidosis, are reported.
AL = amyloid light chain; ASCT = autologous stem cell transplantation; BNP = brain natriuretic peptide; CA = cardiac amyloidosis; CarCR = complete cardiac response; CarNR = cardiac nonresponse; CarPR = cardiac partial response; CarVGPR = cardiac very good partial response; cTnI = cardiac troponin I; cTnT = cardiac troponin T; dFLC = difference between involved and uninvolved free light chains; GLS = global longitudinal strain; hs-cTnT = high sensitivity cardiac troponin T; LV = left ventricular; NR = not reached; NT-proBNP = N-terminal pro–brain natriuretic peptide; RELAPS = relative apical sparing; mo = months; y = years.
In prognostic staging systems, the stage for each patient is defined on the basis of the number of variables above the specified thresholds tabulated in the table. For some staging system, alternative cutoffs for BNP (instead of NT-proBNP) are available. Criteria for response to therapy in AL amyloidosis are reported for completion, even if they do not include cTn or high-sensitivity cTn. Criteria for hematological response are not reported.
Table 5.
Clinical Use of cTn in the Management of Patients With ATTR CA
| Prognosisa |
Response to Therapy | ||
|---|---|---|---|
| Prognostic Variables and Staging Systems | Estimated Survival | ||
CA very likely in patients with suspected CA65
|
Grogan et al85 for ATTRwt CA
|
Stage 1: 66 mo Stage 2: 40 mo Stage 3: 20 mo |
Disease progression in ATTR CA96: at least 1 marker in each domain
|
CA unlikely in patients with suspected CA65
|
Nakashima et al88 for ATTRwt CA
|
32 mo for high-risk group (score 2 or 3) | |
NAC staging system for ATTRwt and ATTRv CA86
|
Stage 1: 69.2 mo Stage 2: 46.7 mo Stage 3: 24.1 mo |
||
A summary of different thresholds for specific biomarkers (particularly cTn evaluated with different assays) for diagnosis, prognosis, and response to therapy in patients with ATTR CA is shown. For diagnosis, thresholds of specific biomarkers, potentially useful to predict the likelihood of a final diagnosis of ATTR CA, are reported.
ATTR = amyloid transthyretin; ATTRv = variant amyloid transthyretin; ATTRwt = wild-type amyloid transthyretin; eGFR = estimated glomerular filtration rate; NAC = National Amyloidosis Center; other abbreviations as in Table 4.
In prognostic staging systems, the stage for each patient is defined on the basis of the number of variables above the specified thresholds tabulated in the table. The NAC staging system has been reported for completeness, even if it does not include cTn or high-sensitivity cTn.
Diagnosis
Compared with its role in the prognostic assessment of patients with CA, the diagnostic value of cTn has been less extensively investigated, in part because the metrics necessary to distinguish CA from other disease entities that can cause myocardial dysfunction has not been probed adequately. In addition, there are many assays for cTnI, both conventional and high sensitivity, and finding exact thresholds for diagnosis requires large studies for each.
In patients at risk for developing AL amyloidosis, including those with monoclonal gammopathy of uncertain significance, periodical screening for potential cardiac involvement can be performed with NT-proBNP and cardiovascular imaging parameters.66 Some studies have started investigating the role of hs-cTnT in the diagnostic algorithm of CA.67, 68, 69 Recently, Vergaro et al65 reported that hs-cTnT, alone and in combination with NT-proBNP, was useful when CA was suspected to identify patients in whom the diagnosis is unlikely and those in whom it is much more likely. They suggested cutoffs of <180 ng/L for NT-proBNP and <14 ng/L for hs-TnT as optimal rule-out thresholds. hs-TnT ≥ 86 ng/L was a good rule-in threshold, but the pretest probability of CA was high; about 60% of patients had confirmed CA in both derivation and validation cohorts. In the validation cohort, 74 patients had both biomarkers less than the cutoff values, with 4 false negatives. For the rule-in cutoff, about 143 patients were correctly classified, with 17 false positives. Further studies are needed to validate these findings in populations with lower prevalence of CA. Thus, for diagnosis, other features must be present at least adjunctively.
Prognosis
AL amyloidosis
The prognostic role of cTn, particularly cTnT, in CA has been extensively validated.70 Dispenzieri et al71 showed that survival of patients with AL amyloidosis was significantly worse among those with increased cTnT and cTnI values compared with those with undetectable cTn. On multivariable analysis, cTnT was a better predictor than cTnI. With the evidence that NT-proBNP also had a strong prognostic value in AL amyloidosis,72 a staging system was developed integrating cTnT and cTnI with NT-proBNP.73 Patients were stratified in 3 stages (I, II, and III), with median survival times of 26.4, 10.5, and 3.5 months, respectively. The proposed cutoffs were ≥332 ng/L for NT-proBNP, ≥0.035 μg/L for cTnT, and ≥0.1 μg/L for cTnI (using the Stratus CS assay). Subsequently, sFLC levels (particularly the difference between involved and uninvolved sFLCs) were integrated in the model (Mayo 2012 model), with stratification of patients in 4 groups. In this model, cTnT was used at a threshold cutoff of ≥0.025 μg/L.33 A European modification of the first Mayo Clinic stage was also developed, subclassifying stage III patients in stages IIIa and IIIb according to NT-proBNP levels using a threshold of 8,500 ng/L.74,75 The Boston University staging system, instead, was based on brain natriuretic peptide (81 ng/L) and cTnI (0.1 ng/mL).76 Comparing the available staging systems suggests that the European 2015 model had better prediction for 1-year mortality, but the Mayo 2012 model increased the ability to predict long-term survival.77
When the hs-cTnT assay became available for clinical practice, a study from the Mayo Clinic78 demonstrated that hs-cTnT numerical values could not merely be substituted for cTnT measurements in the original Mayo Clinic staging system. A threshold of 50 ng/L was derived with a quartic formula70 and was integrated into the Mayo 2004 model. For the updated Mayo 2012 model, which is currently widely used, a threshold of hs-cTnT ≥40 ng/L was validated.79 Because of the improved analytical performance of the high-sensitivity assays,78 the prognostic significance of this biomarker improved. Palladini et al80 concurred and reported that a prognostic cutoff of 77 ng/L for hs-cTnT best predicted mortality at presentation. After chemotherapy, changes in NT-proBNP and a >75% increase in hs-cTnT were independent prognostic determinant. Thus, they suggested that hs-cTnT at baseline could be a simple and powerful tool for single subjects risk assessment and for patient stratification in clinical trials.80
Dispenzieri et al81 reported also on the clinical use of soluble suppression of tumorigenicity 2 in the prognosis of patients with AL amyloidosis, together with cTnT, NT-proBNP, and differential sFLCs. It is unclear, however, how much these add to hs-cTnT alone.
Autologous stem cell transplantation has been shown to be an effective therapy for patients with AL amyloidosis, but only selected patients can undergo this procedure.5 Cardiac biomarkers at baseline are useful for risk stratification of early death following autologous stem cell transplantation. Specifically, cTnT >0.06 μg/L (or hs-cTnT of 73/75 ng/L) and NT-proBNP >5,000 ng/L manifest optimal discrimination.70,82
Even though in some of these approaches, thresholds for cTnI have been proposed,75,77,78,80 only 1 study has reported on a hs-cTnI assay (the Siemens Advia Centaur CP assay at a threshold of 100 ng/L). No other data exist for the other high-sensitivity assays, which are now the state of the art in clinical practice. It is likely that over time, with large studies, the optimal threshold values for each of the hs-cTnI assays will be defined.
ATTR CA
cTn, particularly cTnT and hs-cTnT, is now a recognized prognostic factor in ATTR CA, especially ATTRwt CA.83,84 Grogan et al85 developed a prognostic staging system for ATTRwt CA on the basis of a cTnT threshold of 0.05 ng/mL and an NT-proBNP threshold of 3,000 pg/mL. The 4-year survival estimates were 57%, 42%, and 18% for stages I, II, and III, respectively, with stage III patients having an increased risk for mortality after adjustment for age and sex compared with stage I patients. A widely used staging system that avoids the issue of so many different cTn assays, was developed by Gillmore et al86 for ATTRwt CA and ATTRv CA. It is based on NT-proBNP and estimated glomerular filtration rate. In 175 patients with ATTR CA (133 with ATTRwt CA and 42 with ATTRv CA), this staging system provided better prognostic accuracy compared with one using NT-proBNP and contemporary cTnI.87 Recently, a staging system combining hs-cTnT (>50 ng/L), brain natriuretic peptide (>250 pg/mL), and estimated glomerular filtration rate (<45 mL/min/1.73 m2) was published, with good prediction of prognosis in 176 Japanese patients with ATTRwt CA.88 As of now, consistent data on the prognostic value of hs-cTnI assays and of cTn when integrated with disease-specific biomarkers such as transthyretin and retinol-binding protein are not available.
Response to treatment
AL amyloidosis
Cardiac response to chemotherapy has been defined as a >30% decrease with a total decline of >300 ng/L in NT-proBNP or a decrease of at least two NYHA functional classes (if baseline class III or IV).89 Caution should be applied, however, in interpreting changes of NT-proBNP values <50% because of its high biological variability.90 A recent paper reported that graded cardiac response (on the basis of the extent of NT-proBNP reduction) allowed a better assessment of cardiac improvement than the traditional binary response system.91 However, cTn was proposed to define cardiac disease progression at 6 months, on the basis of an increase of ≥33%.89 However, the assays used were contemporary cTnT and cTnI assays, and the cTnI assay was not identified. Other criteria for disease progression were an NT-proBNP increase >30% and >300 ng/L or a reduction of ≥10% in LV ejection fraction.89
The current Mayo Clinic staging systems also are useful for restaging during treatment at 3 and 6 months after chemotherapy initiation. A worsening stage at 3 and 6 months is associated with worse survival than maintenance at the same stage.92 In patients with disease relapse after first-line therapy, both the Mayo 2004 and 2012 staging systems are useful for prognostic stratification with second-line therapy.93
ATTR CA
A multiparametric approach is recommended to evaluate disease progression in ATTR CA; among the various criteria, an increase in cTn of >30% (which, however, is less than the reference change value, ie, the amount of change explained by conjoint analytical and biological variation) is considered indicative of disease progression.94 Specific therapy for patients with ATTR CA has recently become available,3 but few studies have investigated the changes in cardiac biomarkers over time to verify the response to treatment. Most trials and clinical studies report the trend of NT-proBNP, not cTn, over time.4,95 Recently, a single-center French study96 showed that tafamidis therapy stabilizes NT-proBNP and hs-cTnT levels over time, especially in patients with higher values at baseline. However, further studies on the role of hs-cTn for the monitoring of response to therapy in ATTR CA are needed.
Future Directions
The field of cardiac and noncardiac biomarkers in the clinical management of patients with CA continues to evolve. Many research gaps remain to be addressed. With regard to hs-cTn, hs-cTnI assays have not yet been validated in patients with CA. The specific metrics derived will vary depending on the assay being used. Moreover, new and improved assays will be available on the market in the near future, potentially further refining risk stratification in this and other settings. In addition, novel markers are being elucidated as potential indicators of disease pathogenesis, course, and progression, for both AL CA (eg, LC glycosylation)97,98 and ATTR CA (including transthyretin, retinol-binding protein 4, non-native transthyretin, and neurofilament LC).99 At present, their interactions with available prognostic biomarkers are yet to be determined. Finally, validated and new staging systems for prognostication will need to be explored in newer cohorts of patients with CA now that earlier diagnosis is more frequent and multiple effective therapies are available for all forms.
Conclusions
cTn has dramatically changed clinical practice in cardiology in the past 30 years. It is now the biomarker of choice for the detection of myocardial injury, and high-sensitivity assays can identify even modest increases in a multiplicity of clinical conditions. Myocardial injury is particularly frequent in patients with CA because of multiple synergistic mechanisms that contribute to cardiomyocytes damage. Evaluation of cTn, alone and integrated in staging systems, is helpful in the management of patients with CA, who are now being increasingly recognized and treated. There are still many potential areas of research in this field, including further addressing the diagnostic significance of cTn in suspected CA and its prognostic and monitoring role now that new effective therapies are available not only for AL CA but also for ATTR CA. Assay-specific thresholds (other than for cTnT and hs-cTnT) are also essential to allow a wider dissemination of troponin-based diagnostic and prognostic scores.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with CA, both AL and ATTR often manifest increases in cTn and natriuretic peptides. The causes for these increases include the cytotoxic effects of amyloid precursors, infiltration of interstitial amyloid fibrils, coronary microvascular dysfunction, amyloid/nonamyloid-related CAD, diastolic dysfunction, and heart failure. In addition, many clinical conditions, such as arrhythmias, hypotension, critical illness and decompensated heart failure, can add acute onto the chronic myocardial injury.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: There are a variety of algorithms that can assist in diagnosing CA, helping to assess its prognosis and to allow one to follow patients’ responses to a variety of treatments.
TRANSLATIONAL OUTLOOK: The metrics for the use of cTn and natriuretic peptides in this setting are distinct from the metrics applicable to patients with possible myocardial infarction. This needs to be kept in mind by clinicians in using the criteria proposed.
Funding Support and Author Disclosures
Dr De Michieli has received honoraria from Pfizer, Alnylam Pharmaceuticals, and AstraZeneca. Dr Cipriani has received honoraria from Pfizer, Alnylam Pharmaceuticals, and AstraZeneca. Dr Dispenzieri has received research support from Alnylam Pharmaceuticals, Pfizer, Takeda, and Bristol Myers Squibb; participates on the data and safety monitoring board for Oncopeptides and Sorrento; and is on the advisory board and independent review committee for Janssen. Dr Jaffe has consulted or presently consults for most of the major diagnostics companies, including Beckman-Coulter, Abbott, Siemens, Ortho Diagnostics, ET Healthcare, Roche, Radiometer, SphingoTec, Amgen, and Novartis; and he has stock options in RCE Technologies.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Muchtar E., Dispenzieri A., Magen H., et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289(3):268–292. doi: 10.1111/joim.13169. [DOI] [PubMed] [Google Scholar]
- 2.AbouEzzeddine O.F., Davies D.R., Scott C.G., et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6(11):1267. doi: 10.1001/jamacardio.2021.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Pavia P., Rapezzi C., Adler Y., et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2021;42(16):1554–1568. doi: 10.1093/eurheartj/ehab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 5.Gertz M.A. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97(6):818–829. doi: 10.1002/ajh.26569. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K., Mair J., Katus H., et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31(18):2197–2204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G., Targher G., Franchini M., Plebani M. Genetic and biochemical heterogeneity of cardiac troponins: clinical and laboratory implications. Clin Chem Lab Med. 2009;47(10):1183–1194. doi: 10.1515/CCLM.2009.322. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann L.H., Heckmann M.B., Bailly G., et al. Cardiomuscular biomarkers in the diagnosis and prognostication of immune checkpoint inhibitor myocarditis. Circulation. 2023;148(6):473–486. doi: 10.1161/CIRCULATIONAHA.123.062405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Fay de Lavallaz J., Prepoudis A., Wendebourg M.J., et al. Skeletal muscle disorders: a noncardiac source of cardiac troponin T. Circulation. 2022;145(24):1764–1779. doi: 10.1161/CIRCULATIONAHA.121.058489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo R., De Michieli L., Jaffe A.S. Sex-specific 99th percentile URLs for cardiac troponin assays—their time has come. Clin Chem. 2021;67(1):197–200. doi: 10.1093/clinchem/hvaa204. [DOI] [PubMed] [Google Scholar]
- 12.Apple F.S., Jaffe A.S., Collinson P., et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem. 2015;48(4-5):201–203. doi: 10.1016/j.clinbiochem.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Januzzi J.L., Mahler S.A., Christenson R.H., et al. Recommendations for institutions transitioning to high-sensitivity troponin testing. J Am Coll Cardiol. 2019;73(9):1059–1077. doi: 10.1016/j.jacc.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Hammerer-Lercher A., Ploner T., Neururer S., et al. High-sensitivity cardiac troponin t compared with standard troponin T testing on emergency department admission: how much does it add in everyday clinical practice? J Am Heart Assoc. 2013;2(3) doi: 10.1161/JAHA.113.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apple F.S. Counterpoint: standardization of cardiac troponin I assays will not occur in my lifetime. Clin Chem. 2012;58(1):169–171. doi: 10.1373/clinchem.2011.166165. [DOI] [PubMed] [Google Scholar]
- 16.Babuin L., Vasile V.C., Rio Perez J.A., et al. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Crit Care Med. 2008;36(3):759–765. doi: 10.1097/CCM.0B013E318164E2E4. [DOI] [PubMed] [Google Scholar]
- 17.De Michieli L., Ola O., Knott J.D., et al. High-sensitivity cardiac troponin T for the detection of myocardial injury and risk stratification in COVID-19. Clin Chem. 2021;67(8):1080–1089. doi: 10.1093/clinchem/hvab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omland T., Pfeffer M.A., Solomon S.D., et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61(12):1240–1249. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Sarkisian L., Saaby L., Poulsen T.S., et al. Prognostic impact of myocardial injury related to various cardiac and noncardiac conditions. Am J Med. 2016;129(5):506–514.e1. doi: 10.1016/j.amjmed.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Ford I., Shah A.S.V., Zhang R., et al. High-sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol. 2016;68(25):2719–2728. doi: 10.1016/j.jacc.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ola O., Akula A., De Michieli L., et al. Clinical impact of high-sensitivity cardiac troponin T implementation in the community. J Am Coll Cardiol. 2021;77(25):3160–3170. doi: 10.1016/j.jacc.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bularga A., Lee K.K., Stewart S., et al. High-sensitivity troponin and the application of risk stratification thresholds in patients with suspected acute coronary syndrome. Circulation. 2019;140(19):1557–1568. doi: 10.1161/CIRCULATIONAHA.119.042866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamson P.D., Anderson J.A., Brook R.D., et al. Cardiac troponin I and cardiovascular risk in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2018;72(10):1126–1137. doi: 10.1016/j.jacc.2018.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller W.L., Wright R.S., McGregor C.G., et al. Troponin levels in patients with amyloid cardiomyopathy undergoing cardiac transplantation. Am J Cardiol. 2001;88(7):813–815. doi: 10.1016/S0002-9149(01)01877-X. [DOI] [PubMed] [Google Scholar]
- 25.Merlini G., Dispenzieri A., Sanchorawala V., et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. doi: 10.1038/s41572-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 26.Liao R., Jain M., Teller P., et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001;104(14):1594–1597. doi: 10.1161/circ.104.14.1594. [DOI] [PubMed] [Google Scholar]
- 27.Brenner D.A., Jain M., Pimentel D.R., et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94(8):1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 28.Shi J., Guan J., Jiang B., et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38α MAPK pathway. Proc Natl Acad Sci U S A. 2010;107(9):4188–4193. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maritan M., Romeo M., Oberti L., et al. Inherent biophysical properties modulate the toxicity of soluble amyloidogenic light chains. J Mol Biol. 2020;432(4):845–860. doi: 10.1016/j.jmb.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Marin-Argany M., Lin Y., Misra P., et al. Cell damage in light chain amyloidosis. J Biol Chem. 2016;291(38):19813–19825. doi: 10.1074/jbc.M116.736736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czyżewska E., Wiśniewska A., Waszczuk-Gajda A., Ciepiela O. The role of light kappa and lambda chains in heart function assessment in patients with AL amyloidosis. J Cardiovasc Med. 2021;10(6):1274. doi: 10.3390/jcm10061274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S., Dispenzieri A., Katzmann J.A., et al. Serum immunoglobulin free light chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116(24):5126–5129. doi: 10.1182/blood-2010-06-290668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S., Dispenzieri A., Lacy M.Q., et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dispenzieri A., Lacy M.Q., Katzmann J.A., et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107(8):3378–3383. doi: 10.1182/blood-2005-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manral P., Reixach N. Amyloidogenic and non-amyloidogenic transthyretin variants interact differently with human cardiomyocytes: insights into early events of non-fibrillar tissue damage. Biosci Rep. 2015;35(1) doi: 10.1042/BSR20140155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapezzi C., Merlini G., Quarta C.C., et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 37.Lavatelli F. Mechanisms of organ damage and novel treatment targets in AL amyloidosis. Hemato. 2022;3(1):47–62. doi: 10.3390/hemato3010005. [DOI] [Google Scholar]
- 38.Seldin D.C., Berk J.L., Sam F., Sanchorawala V. Amyloidotic cardiomyopathy: multidisciplinary approach to diagnosis and treatment. Heart Fail Clin. 2011;7(3):385–393. doi: 10.1016/j.hfc.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka K., Essick E.E., Doros G., et al. Circulating matrix metalloproteinases and tissue inhibitors of metalloproteinases in cardiac amyloidosis. J Am Heart Assoc. 2013;2(2) doi: 10.1161/JAHA.112.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misumi Y., Ando Y., Gonçalves N.P., Saraiva M.J. Fibroblasts endocytose and degrade transthyretin aggregates in transthyretin-related amyloidosis. Lab Invest. 2013;93(8):911–920. doi: 10.1038/labinvest.2013.83. [DOI] [PubMed] [Google Scholar]
- 41.Trinkaus-Randall V., Walsh M.T., Steeves S., Monis G., Connors L.H., Skinner M. Cellular response of cardiac fibroblasts to amyloidogenic light chains. Am J Pathol. 2005;166(1):197–208. doi: 10.1016/S0002-9440(10)62244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pucci A., Aimo A., Musetti V., et al. Amyloid deposits and fibrosis on left ventricular endomyocardial biopsy correlate with extracellular volume in cardiac amyloidosis. J Am Heart Assoc. 2021;10(20) doi: 10.1161/JAHA.120.020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morioka M., Takashio S., Nakashima N., et al. Correlation between cardiac images, biomarkers, and amyloid load in wild-type transthyretin amyloid cardiomyopathy. J Am Heart Assoc. 2022;11(12) doi: 10.1161/JAHA.121.024717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorbala S., Vangala D., Bruyere J., et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. J Am Coll Cardiol HF. 2014;2(4):358–367. doi: 10.1016/j.jchf.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen B.T., Mereuta O.M., Dasari S., et al. Correlation of histomorphological pattern of cardiac amyloid deposition with amyloid type: a histological and proteomic analysis of 108 cases. Histopathology. 2016;68(5):648–656. doi: 10.1111/his.12793. [DOI] [PubMed] [Google Scholar]
- 46.Al Suwaidi J., Velianou J.L., Gertz M.A., et al. Systemic amyloidosis presenting with angina pectoris. Ann Intern Med. 1999;131(11):838–841. doi: 10.7326/0003-4819-131-11-199912070-00007. [DOI] [PubMed] [Google Scholar]
- 47.Clemmensen T.S., Eiskjær H., Mølgaard H., et al. Abnormal coronary flow velocity reserve and decreased myocardial contractile reserve are main factors in relation to physical exercise capacity in cardiac amyloidosis. J Am Soc Echocardiogr. 2018;31(1):71–78. doi: 10.1016/j.echo.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Ong K.C., Wells Askew J., Dispenzieri A., et al. Abnormal stress echocardiography findings in cardiac amyloidosis. Amyloid. 2016;23(2):124–131. doi: 10.1080/13506129.2016.1176020. [DOI] [PubMed] [Google Scholar]
- 49.Takashio S., Yamamuro M., Izumiya Y., et al. Coronary microvascular dysfunction and diastolic load correlate with cardiac troponin T release measured by a highly sensitive assay in patients with nonischemic heart failure. J Am Coll Cardiol. 2013;62(7):632–640. doi: 10.1016/j.jacc.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 50.Neben-Wittich M.A., Wittich C.M., Mueller P.S., Larson D.R., Gertz M.A., Edwards W.D. Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am J Med. 2005;118(11):1287.e1–1287.e7. doi: 10.1016/j.amjmed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 51.Mueller P.S., Edwards W.D., Gertz M.A. Symptomatic ischemic heart disease resulting from obstructive intramural coronary amyloidosis. Am J Med. 2000;109(3):181–188. doi: 10.1016/S0002-9343(00)00471-X. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen H.T., Nguyen C.T.H. Cardiac amyloidosis mimicking acute coronary syndrome: a case report and literature review. Eur Heart J Case Rep. 2020;4(6):1–7. doi: 10.1093/ehjcr/ytaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai S.B., Seldin D.C., Wu H., O’Hara C., Ruberg F.L., Sanchorawala V. Myocardial infarction with “clean coronaries” caused by amyloid light-chain AL amyloidosis: a case report and literature review. Amyloid. 2011;18(3):160–164. doi: 10.3109/13506129.2011.571319. [DOI] [PubMed] [Google Scholar]
- 54.De Michieli L., De Gaspari M., Sinigiani G., et al. Chest pain in cardiac amyloidosis: occurrence, causes and prognostic significance. Int J Cardiol. 2023;389 doi: 10.1016/j.ijcard.2023.131204. [DOI] [PubMed] [Google Scholar]
- 55.Collet J.P., Thiele H., Barbato E., et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 56.Weil B.R., Suzuki G., Young R.F., Iyer V., Canty J.M. Troponin release and reversible left ventricular dysfunction after transient pressure overload. J Am Coll Cardiol. 2018;71(25):2906–2916. doi: 10.1016/j.jacc.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanhecke T.E., Kim R., Raheem S.Z., McCullough P.A. Myocardial ischemia in patients with diastolic dysfunction and heart failure. Curr Cardiol Rep. 2010;12(3):216–222. doi: 10.1007/s11886-010-0101-1. [DOI] [PubMed] [Google Scholar]
- 58.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 59.Martens P., Bhattacharya S., Longinow J., et al. Hemodynamic profiling and prognosis in cardiac amyloidosis. Circ Heart Fail. 2023;16(3) doi: 10.1161/CIRCHEARTFAILURE.122.010078. [DOI] [PubMed] [Google Scholar]
- 60.Eggers K.M., Nygren M., Venge P., Jernberg T., Wikström B.G. High-sensitive troponin T and I are related to invasive hemodynamic data and mortality in patients with left-ventricular dysfunction and precapillary pulmonary hypertension. Clin Chim Acta. 2011;412(17-18):1582–1588. doi: 10.1016/j.cca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Martini N, Sinigiani G, De Michieli L, et al. Electrocardiographic features and rhythm disorders in cardiac amyloidosis. Trends Cardiovasc Med.https://doi.org/10.1016/j.tcm.2023.02.006. [DOI] [PubMed]
- 62.Porcari A., Rossi M., Cappelli F., et al. Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. Eur J Heart Fail. 2022;24(7):1227–1236. doi: 10.1002/ejhf.2533. [DOI] [PubMed] [Google Scholar]
- 63.Cappelli F., Cipriani A., Russo D., et al. Prevalence and prognostic role of nonsustained ventricular tachycardia in cardiac amyloidosis. Amyloid. 2022;29(3):211–212. doi: 10.1080/13506129.2022.2060073. [DOI] [PubMed] [Google Scholar]
- 64.De Michieli L., Lobo R., Babuin L., et al. Structural cardiac abnormalities in patients with atrial fibrillation/flutter and myocardial injury. Am J Med. 2022;135(12):1488–1496.e5. doi: 10.1016/j.amjmed.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Vergaro G., Castiglione V., Aimo A., et al. N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T hold diagnostic value in cardiac amyloidosis. Eur J Heart Fail. 2023;25(3):335–346. doi: 10.1002/ejhf.2769. [DOI] [PubMed] [Google Scholar]
- 66.Palladini G., Milani P., Merlini G. Management of AL amyloidosis in 2020. Blood. 2020;136(23):2620–2627. doi: 10.1182/blood.2020006913. [DOI] [PubMed] [Google Scholar]
- 67.Nicol M., Baudet M., Brun S., et al. Diagnostic score of cardiac involvement in AL amyloidosis. Eur Heart J Cardiovasc Imaging. 2020;21(5):542–548. doi: 10.1093/ehjci/jez180. [DOI] [PubMed] [Google Scholar]
- 68.Hu K., Liu D., Salinger T., et al. Value of cardiac biomarker measurement in the differential diagnosis of infiltrative cardiomyopathy patients with preserved left ventricular systolic function. J Thorac Dis. 2018;10(8):4966–4975. doi: 10.21037/jtd.2018.07.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takashio S., Yamamuro M., Izumiya Y., et al. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis: diagnostic utility of hs-cTnT in cardiac amyloidosis. ESC Heart Fail. 2018;5(1):27–35. doi: 10.1002/ehf2.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muchtar E., Kumar S.K., Gertz M.A., et al. Staging systems use for risk stratification of systemic amyloidosis in the era of high-sensitivity troponin T assay. Blood. 2019;133(7):763–766. doi: 10.1182/blood-2018-10-875252. [DOI] [PubMed] [Google Scholar]
- 71.Dispenzieri A., Kyle R.A., Gertz M.A., et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003;361(9371):1787–1789. doi: 10.1016/S0140-6736(03)13396-X. [DOI] [PubMed] [Google Scholar]
- 72.Palladini G., Campana C., Klersy C., et al. Serum N-terminal pro–brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107(19):2440–2445. doi: 10.1161/01.CIR.0000068314.02595.B2. [DOI] [PubMed] [Google Scholar]
- 73.Dispenzieri A., Gertz M.A., Kyle R.A., et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 74.Wechalekar A.D., Schonland S.O., Kastritis E., et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420–3427. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 75.Palladini G., Sachchithanantham S., Milani P., et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612–615. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 76.Lilleness B., Ruberg F.L., Mussinelli R., Doros G., Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood. 2019;133(3):215–223. doi: 10.1182/blood-2018-06-858951. [DOI] [PubMed] [Google Scholar]
- 77.Muchtar E., Therneau T.M., Larson D.R., et al. Comparative analysis of staging systems in AL amyloidosis. Leukemia. 2019;33(3):811–814. doi: 10.1038/s41375-018-0370-z. [DOI] [PubMed] [Google Scholar]
- 78.Dispenzieri A., Gertz M.A., Kumar S.K., et al. High sensitivity cardiac troponin T in patients with immunoglobulin light chain amyloidosis. Heart. 2014;100(5):383–388. doi: 10.1136/heartjnl-2013-304957. [DOI] [PubMed] [Google Scholar]
- 79.Kumar S.K., Gertz M.A., Dispenzieri A. Validation of Mayo Clinic staging system for light chain amyloidosis with high-sensitivity troponin. J Clin Oncol. 2019;37(2):171–173. doi: 10.1200/JCO.18.01398. [DOI] [PubMed] [Google Scholar]
- 80.Palladini G., Barassi A., Klersy C., et al. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood. 2010;116(18):3426–3430. doi: 10.1182/blood-2010-05-286567. [DOI] [PubMed] [Google Scholar]
- 81.Dispenzieri A., Gertz M.A., Saenger A., et al. Soluble suppression of tumorigenicity 2 (sST2), but not galactin-3, adds to prognostication in patients with systemic AL amyloidosis independent of NT-proBNP and troponin T: sST2 in AL Amyloidosis. Am J Hematol. 2015;90(6):524–528. doi: 10.1002/ajh.24001. [DOI] [PubMed] [Google Scholar]
- 82.Gertz M.A., Lacy M.Q., Dispenzieri A., et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013;48(4):557–561. doi: 10.1038/bmt.2012.170. [DOI] [PubMed] [Google Scholar]
- 83.Kreusser M.M., Volz M.J., Knop B., et al. A novel risk score to predict survival in advanced heart failure due to cardiac amyloidosis. Clin Res Cardiol. 2020;109(6):700–713. doi: 10.1007/s00392-019-01559-y. [DOI] [PubMed] [Google Scholar]
- 84.Kristen A.V., Scherer K., Buss S., et al. Noninvasive risk stratification of patients with transthyretin amyloidosis. J Am Coll Cardiol Img. 2014;7(5):502–510. doi: 10.1016/j.jcmg.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Grogan M., Scott C.G., Kyle R.A., et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–1020. doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 86.Gillmore J.D., Damy T., Fontana M., et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 87.Cappelli F., Martone R., Gabriele M., et al. Biomarkers and prediction of prognosis in transthyretin-related cardiac amyloidosis: direct comparison of two staging systems. Can J Cardiol. 2020;36(3):424–431. doi: 10.1016/j.cjca.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 88.Nakashima N., Takashio S., Morioka M., et al. A simple staging system using biomarkers for wild-type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2022;9(3):1731–1739. doi: 10.1002/ehf2.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palladini G., Dispenzieri A., Gertz M.A., et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–4549. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 90.Apple F.S., Wu A.H.B., Jaffe A.S., et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine practice guidelines: analytical issues for biomarkers of heart failure. Circulation. 2007;116(5):e95–e98. doi: 10.1161/CIRCULATIONAHA.107.185266. [DOI] [PubMed] [Google Scholar]
- 91.Muchtar E., Dispenzieri A., Wisniowski B., et al. Graded cardiac response criteria for patients with systemic light chain amyloidosis. J Clin Oncol. 2023;41(7):1393–1403. doi: 10.1200/JCO.22.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abdallah N., Dispenzieri A., Muchtar E., et al. Prognostic restaging after treatment initiation in patients with AL amyloidosis. Blood Adv. 2021;5(4):1029–1036. doi: 10.1182/bloodadvances.2020003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hwa Y.L., Gertz M.A., Kumar S.K., et al. Prognostic restaging at the time of second-line therapy in patients with AL amyloidosis. Leukemia. 2019;33(5):1268–1272. doi: 10.1038/s41375-019-0400-5. [DOI] [PubMed] [Google Scholar]
- 94.Garcia-Pavia P., Bengel F., Brito D., et al. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2021;23(6):895–905. doi: 10.1002/ejhf.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adams D., Gonzalez-Duarte A., O’Riordan W.D., et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 96.Oghina S., Josse C., Bézard M., et al. Prognostic value of N-terminal pro-brain natriuretic peptide and high-sensitivity troponin T levels in the natural history of transthyretin amyloid cardiomyopathy and their evolution after tafamidis treatment. J Cardiovasc Med. 2021;10(21):4868. doi: 10.3390/jcm10214868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dispenzieri A., Larson D.R., Rajkumar S.V., et al. N-glycosylation of monoclonal light chains on routine MASS-FIX testing is a risk factor for MGUS progression. Leukemia. 2020;34(10):2749–2753. doi: 10.1038/s41375-020-0940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nevone A., Girelli M., Mangiacavalli S., et al. An N-glycosylation hotspot in immunoglobulin κ light chains is associated with AL amyloidosis. Leukemia. 2022;36(8):2076–2085. doi: 10.1038/s41375-022-01599-w. [DOI] [PubMed] [Google Scholar]
- 99.Hood C.J., Hendren N.S., Pedretti R., Roth L.R., Saelices L., Grodin J.L. Update on disease-specific biomarkers in transthyretin cardiac amyloidosis. Curr Heart Fail Rep. 2022;19(5):356–363. doi: 10.1007/s11897-022-00570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brush J., Kaul S., Krumholz H. Troponin testing for clinicians. J Am Coll Cardiol. 2016;68(21):2365–2375. doi: 10.1016/j.jacc.2016.08.066. [DOI] [PubMed] [Google Scholar]