Abstract
Diabetes as a fastest growing diseases worldwide is characterized by elevated blood glucose levels. There’s an enormous financial burden associated with this endocrine disorder, with unequal access to health care between developed and developing countries. PI3Ks (phosphoinositide 3-kinases) have been demonstrated to be crucial for glucose homeostasis, and malfunctioning of these molecules can contribute to an increase in glucose serum levels, the main pathophysiological feature of diabetes. Additionally, recent evidence suggests that miRNAs and lncRNAs are reciprocally interacting with this signaling pathway. It is therefore evident that abnormal regulation of miRNAs/lncRNAs in the lncRNAs/miRNAs/PI3K/AKT axis is related to clinicopathological characteristics and plays a crucial role in the regulation of biological processes. It has therefore been attempted in this review to describe the interaction between PI3K/AKT signaling pathway and various miRNAs/lncRNAs and their importance in DM biology. We also presented the clinical applications of PI3K/AKT-related ncRNAs/herbal medicine in patients with DM.
Keywords: Diabetes mellitus, PI3K/AKT, miRNA, lncRNA
1. Introduction
One of the most common and fastest growing diseases in the world is diabetes, an endocrine disease characterized by abnormally high blood glucose levels [1]. It is estimated that 693 million adults will be affected by diabetes by the year 2045, and both the macrovascular system (cardiovascular disease (CVD)) and microvascular system (diabetic kidney disease (DKD), diabetic retinopathy and neuropathy) will be leading causes of death and morbidity [2]. A significant financial burden is carried by DM, and there is an unequal distribution of health-care expenditures and access to treatment between developed and developing countries. It is estimated that 10% of diabetics suffer from type 1 diabetes (T1D), caused by an autoimmune destruction of pancreatic beta cells, while 90% of diabetics suffer from type 2 diabetes (T2D), which is primarily caused by obesity, inactivity, and smoking. Several clinical trials established the effectiveness of lifestyle interventions as a means of preventing T2D development [3]. The presence of hereditary factors, however, has also been found to be influential in determining the individual’s susceptibility for T2D development and response to lifestyle changes as well. As a consequence of these facts, there is a need for further research regarding the molecular basis of diabetes mellitus as well as deciphering new factors involved in the pathogenesis of this disease.
In order for glucose homeostasis to be achieved, Phosphoinositide 3-kinases (PI3Ks) have been identified as key molecules that regulate glucose levels, and dysregulation of their function can conclude in an increase in glucose serum levels, one of the most significant pathophysiological aspects of diabetes [4]. It is important to note that the significance of PI3Ks for diabetic patients does not merely relate to glucose metabolism. There is considerable evidence that PI3Ks play a crucial role in the damage induced to target organs caused by diabetes, including vessels, the heart, and the brain. In this context, Wang et al. investigated how macrophage autophagy is regulated in diabetic encephalopathy (DE). They found that PI3K/Akt/mTOR signaling may contribute to DE development by suppressing macrophage autophagy [5]. Furthermore, the use of transgenic murine models, where elements of the PI3K/Akt pathway are overexpressed in β-cells, has provided direct evidence that PI3K/Akt signaling is crucial to the development and function of β-cells. Importantly, in vivo findings disclosed that by overexpressing the constitutively active form of Akt1 (CA-Akt) in the β-cells, it is possible to induce a major increase in the size of β-cells and the total mass of the islets, with consequent improvement in glucose tolerance. This treatment protected mice from the effects of streptozotocin (STZ)-induced diabetes (multiple low doses of 40 mg/kg body weight for five consecutive days, which causes autoimmune diabetes). Given that the rate of β-cell proliferation remained unchanged, it was concluded that disease protection was associated with an Akt-mediated preservation of β-cell mass and increased metabolic activity, specifically the anabolic processes that drive cell growth [6]. There are additional mechanisms that can regulate the PI3K/Akt pathway. Simply defined as the transcribed but untranslated component of the genome, the non-coding RNAs mainly comprised of microRNAs (miRNAs) and long noncoding RNAs (lncRNAs). The purpose of this review is to summarize the current studies that investigate how crosstalk between miRNAs/lncRNAs and the PI3K/AKT pathway may contribute to the pathogenesis of diabetes mellitus. We also presented the clinical applications of PI3K/AKT-related ncRNAs/herbal medicine in patients with DM.
2. PI3K/AKT signaling pathway
As a previously unknown phosphoinositide kinase, phosphoinositide 3-kinase (PI3K) was discovered in 1985. Due to its multiple functions, the PI3K/AKT pathway still merits research despite decades of research [7]. The PI3K/AKT pathway is essential for cell physiology as it facilitates growth factor signals during organismal growth as well as crucial cellular processes, including lipid metabolism, glucose homeostasis, cell proliferation, and protein synthesis [8]. In accordance with their structure and substrate selectivity, PI3Ks can be classified into three classes (I-III) [9]. Heterodimers of a regulatory subunit (P85) and a catalytic subunit (P110) are the most commonly studied isoforms of class I that are activated by cell surface receptors. In the amino terminus of P85, there is a Src homology 3 (SH3) domain and two proline-rich regions, whereas in the basal terminus, two SH2 domains and a non-coding region are present, which coordinate with P110. As well, class I isoforms are further classified into class IA (PI3Kα, β and δ) and class IB (PI3Kγ) according to their modes of regulation. Class IA contain catalytic subunits p110α, β, δ and regulatory subunits p85α, β, γ. A subunit of class IB is composed of a catalytic subunit known as p110 and either a regulatory subunit or a catalytic subunit known as p101 or p87. They differ in the manner in which they are activated. PI3Kα, β and δ become activated when extracellular ligands bind to a transmembrane glycoprotein receptor tyrosine kinase (RTK) with enzyme activity, while PI3Kγ is activated by G protein coupled receptors (GPCRs) and Ras family GTP enzymes [10]. There are three class II isoforms, PI3KC2α, 2β, 2γ, which may constitutively bind to membranes and require additional activation signals. There is a single class III PI3K, vacuolar protein sorting 34 (VPS34), which is required for membrane trafficking between the plasma membrane and the early endosomes [11]. PKB is a serine/threonine kinase that functions downstream of PI3K as well as the principal effector kinase for the PI3K/AKT pathway. There are three highly homologous subtypes of AKT encoded by different genes: AKT1/PKBα, AKT2/PKBβ, and AKT3/PKBγ. An N-terminal pleckstrin homology domain (PH), a central fragment, and a C-terminal regulatory domain are contained in each isoform [12]. It is the PH domain which facilitates membrane translocation during AKT activation, and mutations or deletions of this domain can impair AKT activity [13]. In the catalytic domain, the ATP-binding site and Thr308 (AKT1-Thr308, AKT2-Thr309, AKT3-Thr305) serve as phosphorylation sites that activate AKT. There are 40 amino acids in the C-terminal regulatory domain, which houses a hydrophobic region that contains the second phosphorylation site necessary to activate AKT, that is, Ser473 (AKT1-Ser473, AKT2-Ser474, AKT3-Ser472). As soon as PI3K/AKT is phosphorylated by an upstream signal, a variety of biological actions are initiated through the phosphorylation or complex formation with a variety of downstream molecules, including FoxO family members, GSK-3, mTOR, and actin-related proteins [14]. There are several downstream targets of PI3K/AKT signal transduction, including mTOR, which plays an important role in regulating the metabolism of cells. As a result of phosphorylating and inhibiting cyclin-dependent kinase inhibitors p21 and p27, AKT is able to affect cell cycle progression [15]. It is also possible to modulate apoptosis through AKT by inhibiting Bcl-2 antagonist of cell death (Bad), bcl-2-like protein 11 (BIM), caspase-9, and forkhead box protein O1 [16]. Nuclear factor erythrocyte two related factor (Nrf2) plays a major role in regulating oxidative stress, thereby promoting the transcription of detoxification enzyme and antioxidant enzyme genes. Through its adjustment of several downstream molecules, the PI3K/AKT pathway has been implicated in cell proliferation, glucose metabolism, cell survival, cell cycle, and protein synthesis as well as being involved in neuronal morphology and plasticity. It has been suggested that the PI3K/AKT pathway may represent an effective therapeutic target in the treatment of solid tumors, immune-mediated diseases, cardiovascular diseases, diabetes, nervous system diseases, and other diseases due to its imbalanced expression in these diseases. This is an area that deserves further study in order to establish its relevance as a meaningful therapeutic target [17].
2.1. PI3K/AKT pathway in diabetes mellitus and associated complications
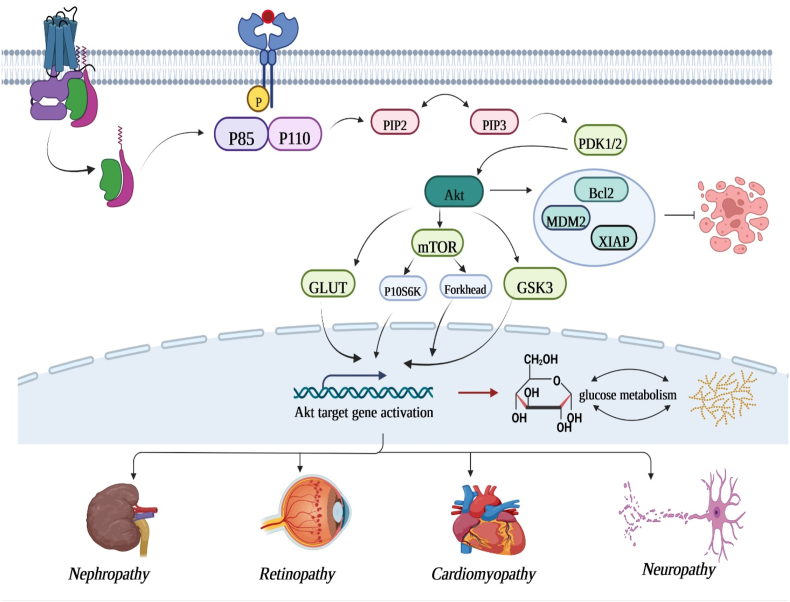
There has been extensive research that indicates that the PI3K/AKT/mTOR pathway plays an important role in glucose homeostasis [18]. As a major cellular signaling pathway, this pathway plays a pivotal role in the cellular response to extracellular stimuli, such as insulin and insulin-like growth factor-1 (IGF-1) [19]. As a result of insulin receptor stimulation, insulin receptor substrate (IRS) is phosphorylated at tyrosine sites, allowing PI3K to be activated. As a result, PIP2 is converted into PIP3 by PI3K. As a result of PIP3, AKT (also referred to as protein kinase B or PKB) is recruited to the membrane. Following activation of AKT in the cell, mTOR is further activated, which consists of two large, distinct multi-protein complexes: the mTOR complex 1 (mTORC1) and the mTOR complex 2 (mTORC2). In response to mTORC1 activation, the ribosomal S6 kinase 1 (S6K1) is phosphorylated, which is crucial for important cellular processes, such as translation, transcription, cell growth, and protein synthesis. Furthermore, mTORC1 inhibits autophagy, one of the basic degradation processes in cells, whereas mTORC2 is regarded as the key downstream AKT Ser473 kinase in response to insulin and growth factors stimuli. As PI3K/AKT/mTOR is crucial for maintaining proper cellular function, disruption of this pathway may contribute to T2DM development [20]. Varma et al. hypothesized that hyperglycemia decreases endothelial cell (EC) proliferation and survival via PI3k and Akt signaling pathways. According to their findings, EC proliferation was significantly impaired by d-glucose (20 and 40 mM) compared to control, but not by mannitol. Additionally, HUVEC exposed to 40 mM d-glucose displayed a significant increase in apoptosis. Additionally, their further investigation revealed that d-glucose at 40 mM significantly reduced PI3K tyrosine-phosphorylation, Akt threonine 308-phosphorylation, and Akt activity in comparison to 5 mM d-glucose as a control. Furthermore, inhibition of PI3k-Akt by pharmacological means reduced the proliferation of ECs in a dose dependent manner, whereas transfection with Akt mutants increased the proliferation of ECs grown with 20 and 40 mM d-glucose. As well, in their study, it appears that d-glucose acts through threonine phosphorylation of Akt to regulate Akt signaling, and that hyperglycemia-related impairments in PI3K-Akt signaling may facilitate diabetic EC proliferative dysfunction [21]. A further study conducted by Yin et al. examined whether polymorphisms in PIK3CA (catalytic subunit of PI3K), AKT1, AKT2, and FRAP1 (mTOR) genes were related to T2DM risk in Chinese individuals. The researchers reported that individuals with the rs2494746 CG/GG or rs2494738 GA/GG genotype in AKT1 were at a greater risk for T2DM than those with homozygous variants. Furthermore, their subsequent study demonstrated that haplotype GC in the AKT1 gene consists of rs2494738 and rs3803304, which indicates a significant association with T2DM. Importantly, the results of the generalized multifactor dimensionality reduction (GMDR) analysis showed that the best interactive model contained three polymorphisms, namely rs2494746 (AKT1), rs4802071 (AKT2), and rs4845856 (FRAP1). Therefore, their study provides evidence that genes involved in the PI3K/AKT/mTOR could play a significant role in the development of T2DM [22]. Furthermore, dysregulation of PI3K/AKT signaling pathway is implicated in the development of vascular complications associated with diabetes such as retinopathy, neuropathy, nephropathy, and cardiomyopathy. This has been discussed completely in the next section.
2.1.1. PI3K/AKT in diabetic cardiomyopathy
One of the leading causes of death associated with diabetes mellitus is cardiovascular disease. In spite of numerous studies that have demonstrated altered metabolic, contractility, and other heart functions associated with diabetes, there is still a debate as to whether diabetes mellitus directly adversely affects the myocardium and is independent of other risk factors, such as hypertension and coronary artery disease, which are particularly prevalent in this patient population [23]. It has also been commonly believed that hyperglycemia itself is a major contributor to cardiovascular disease risk in patients with diabetes mellitus, even those with T2D [24]. Researchers found that an upregulation of the PI3K signaling pathway can result in contractile dysfunction and abnormal glucose/lipid utilization, potentially leading to pathological hypertrophy. In contrast, in diabetic myocardium, PI3K signaling is upregulated, resulting in elevated inflammation and cardiac fibrosis [25]. In spite of this, this result may not be attributed to the activation of the PI3K signaling pathway in cardiac myocytes, but rather to increase PI3Kγ signaling in leukocytes. There are also a number of cardiac ion channels that are regulated by PI3Kα signaling. During diabetes, changes to these ion channels, most importantly an increase in the persistent sodium current, can result in an increase in the QT interval, which may increase the risk of ventricular arrhythmias. It is possible that this mechanism contributes to a higher incidence of sudden cardiac death among diabetic patients [26]. As well, Prakoso et al. conducted a study to determine whether administering a recombinant adeno-associated virus vector containing a constitutively active PI3K construct to a preclinical model of T2D at a clinically relevant time point attenuated diabetic cardiomyopathy. According to their findings, one injection of cardiac targeted rAAV6-caPI3K reduced diabetes-induced cardiac remodeling by reducing cardiomyocyte hypertrophy (reducing cardiomyocyte size and expression of Nppa gene) and cardiac fibrosis (reduced interstitial and perivascular collagen deposition). Furthermore, in their study, rAAV6-caPI3K reversed diabetes-induced LV systolic dysfunction as shown by an improvement in fractional shortening and velocity of circumferential fiber shortening (all P < 0.05 vs pre-AAV measurement). Ultimately, the authors concluded that rAAV6-caPI3K attenuates diabetic cardiomyopathy induced by T2D, providing support for its potential translation into clinical practice [27]. In addition, Wu et al. investigated resveratrol’s effect and mechanism of action in diabetic cardiomyopathy (DCM). In their study, RSV treatment prevented the deterioration of cardiac function and structural cardiomyopathy in a streptozotocin-induced diabetic rat model, as well as reducing myocardial apoptosis. It was also observed that glucose reduced cell viability, inhibited Akt and FoxO3a phosphorylation, and blocked FoxO3a translocation; however, these effects were reversed by RSV at 10 M concentrations. Additionally, their further investigations revealed that the inhibitor of the PI3K pathway, LY294002, abolished the protective effect of RSV in vitro. A further benefit of this compound is the restoration of streptozotocin-impaired phosphorylation of both Akt and FoxO3a (p-Akt and p-FoxO3a) and the suppression of nuclear translocation of FoxO3a in vivo. Taking these data together, the authors conclude that RSV is a potential therapeutic agent against DCM as it inhibits apoptosis through the PI3K/Akt/FoxO3a pathway [28]. It is apparent from these results that PI3K plays a significant role in developing novel therapeutics to treat diabetic cardiomyopathy.
2.1.2. PI3K/AKT in diabetic neuropathy
It is estimated that 25% of diabetics suffer from diabetic neuropathy, and its mechanism is not fully understood. Those with diabetic neuropathy suffer from progressive nerve fiber loss that affects both the autonomic and somatic nervous systems and only a minority experience pain [29]. It has been shown in recent years that neurotrophins interaction with the tyrosine kinase (Trk) receptor activates the PI3K/Akt signal pathway, a pathway that regulates neuron survival, differentiation, axonal growth, regeneration and protects nerve regeneration. Nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are transported retrogradely in sympathetic, sensory and motor neurons through the PI3K/Akt signal pathway, located in the distal axon of neurons [30]. When PI3K is inhibited in distal axons, retrograde transport of NGF is attenuated as well as neuronal apoptosis is induced. A diabetes-induced decrease in PI3K and Akt activity in the vagus nerve does not affect the protein expression of the p85 subunits of PI3K and Akt, or Akt phosphorylation, but increases p70s6 kinase phosphorylation [31]. Liu et al. investigated PI3K/AKT/mTOR signaling pathways in painful diabetic neuropathy (PDN). Upon modeling at 3 weeks, diabetic rats showed significantly reduced mechanical withdrawal thresholds (MWT), an increase in p-PI3K, p-AKT, and p-mTOR protein expression, and a decrease in Beclin1 and LC3-II protein expression. In their study, PI3K inhibitors significantly improved WMW values in DM + LY animals, and the expression of p-PI3K, p-AKT, and p-mTOR proteins in the spinal cord significantly decreased, while Beclin1 and LC3-II were significantly increased. It has been concluded that PDN is associated with activation of the PI3K/AKT/mTOR pathway, which leads to impaired autophagy, suggesting that inhibiting the pathway might improve autophagy activity and mitigate symptoms of the disease [32]. Also, Wang et al. investigated the effects of phillyrin on DN and the mechanisms by which its effects might be exerted. In a mouse model of diabetes, it has been demonstrated that phillyrin significantly decreased fasting blood glucose (FBG), glycosylated haemoglobin A1c (HbA1c), serum and urine microglobulin levels, serum blood urea nitrogen, serum creatinine, as well as improving kidney pathological changes. As well, in their study, phillyrin inhibited Glycogen synthase kinase-3β (GSK-3β) activity through PI3K/Akt signaling, increased Bcl-2/Bax ratios, reduced cytochrome C release from mitochondria to the cytoplasm, inhibited caspase-3 activation, and finally blocked renal cells apoptosis. Thereby, a promising therapeutic strategy for DN may be found in PHILLYRIN, which primarily activates the PI3K/Akt/GSK-3 signaling pathways [33]. Thus, these findings indicate that diabetes-related neuropathy is associated with the impairment of the PI3K/Akt signal pathway.
2.1.3. PI3K/AKT in diabetic nephropathy
It is estimated that 30% of T1D and T2D are affected by diabetic nephropathy (DN), which leads to chronic renal failure and end-stage renal disease. This condition is characterized by glomerular hypertrophy, excessive extracellular matrix accumulation, and glomerulosclerosis, and ultimately results in progressive loss of renal function [34]. Lu et al. hypothesized that hyperglycemia-induced ROS via activation of TGF-β1–PI3K–Akt–FoxO3a signaling pathway could negatively regulate manganese superoxide dismutase (MnSOD), resulting in excessive ROS generation and accelerated progression of diabetic nephropathy. Their in vitro analysis in rat mesangial cells disclosed that high glucose (30 mmol l−1), but not equimolar mannitol, is associated with increased the level of phosphorylated Akt, increased the level of FoxO3a, and decreased MnSOD expression. This was followed by an increased level of ROS production, upregulation of TGF-1, and an increase in the phosphorylated Akt/total Akt and FoxO3a phosphorylation/total FoxO3a ratios. Furthermore, these high-glucose-induced changes further boosted the generation of ROS. As well, their in vivo investigation in db/db mice treated with an inhibitor of TGF-β1 (SB431542) or PI3K (LY294002) disclosed that the levels of phosphorylated Akt and phosphorylated FoxO3a in the kidney cortices were decreased, the level of MnSOD expression was increased and the level of the lipid peroxidation end-product, malondialdehyde, was reduced. Based on their findings, overproduction of ROS by a high glucose concentration causes MnSOD expression to decrease via the PI3K–Akt–FoxO3a pathway, further aggravating diabetic nephropathy’s oxidative stress. They concluded that excessive ROS production from high glucose concentrations reduces MnSOD expression via the PI3K–Akt–FoxO3a pathway, further aggravating diabetic nephropathy’s oxidative stress [35]. A similar study was conducted by Lu et al. to determine whether high glucose-induced ROS affected activation of the TGF1-β1/PI3K/Akt/mTOR pathway in both normal rat kidney tubular epithelial cells (NRK-52E) as well as rats with type 1 diabetes. According to their in vitro study, diabetes-induced ROS production increased expression of TGF-β1 and phosphorylation ratios of Akt and mTOR, contributing to EMT. Further, they found that pretreatment of the cells with ROS inhibitors significantly alleviated changes in TGF1, Akt, and mTOR. In addition, the in vivo analysis revealed that diabetic rats were more likely to suffer from renal impairment and renal fibrosis than control rats after streptozocin injection for eight weeks. Importantly, the renal cortex of rats with diabetes showed an increased level of malondialdehyde and activation of the TGF1/PI3K/Akt/mTOR pathway compared to rats without diabetes. They also observed further deterioration of renal fibrosis in DN rats compared with control rats. The authors concluded that ROS, which inhibit activation of the TGF1/PI3K/Akt/mTOR pathways, play an important role in the stimulation of EMT in response to high glucose levels [36]. In this manner, PI3K/AKT play a crucial role in DN progression.
2.1.4. PI3K/AKT in diabetic retinopathy
Diabetic retinopathy (DR) is a common retinal vascular complication of diabetes and one of the most prevalent causes of blindness and visual impairment in working-age adults worldwide. As per the 9th edition of the global diabetes atlas issued by the International Diabetes Federation in 2019, it has been projected that the prevalence of diabetes within the worldwide adult populace aged 20–79 years stands at approximately 9.3%, encompassing a demographic of approximately 463 million individuals [37]. Prior research have ascertained the presence of PI3K/Akt/mTOR proteins within the retinal tissue of rats afflicted with diabetes. Qin et al. explored the levels of laminin, collagen IV, and fibronectin expression, along with the phosphorylated form of protein kinase B (Akt), within retinal pigment epithelial (RPE) cells subjected to elevated glucose conditions. Their investigation revealed that elevated glucose concentrations led to an augmentation in the expression levels of laminin, fibronectin, and collagen IV, concomitant with the activation of Akt within RPE cells. Furthermore, it was observed that pretreatment with LY294002, an inhibitor targeting phosphatidylinositol 3-kinase, effectively inhibited the heightened expression of laminin, fibronectin, and collagen IV induced by elevated glucose concentrations in RPE cells. Thus, elevated glucose levels elicited an upregulation in the expression of laminin, collagen IV, and fibronectin within RPE cells, a phenomenon mediated via the PI3K/Akt signaling cascade. Consequently, the involvement of the PI3K/Akt signaling pathway could potentially underlie the genesis of a fibrotic membrane during the progression of DR [38]. Moreover, Yang et al. examined the potential of crocin to mitigate oxidative stress and the pro-inflammatory reaction within microglial cells prompted by high glucose and free fatty acid (HG-FFA) exposure. In their study, crocin exhibited a notable elevation in the expression levels of PI3K and p-Akt, while the overall Akt expression remained comparatively unaltered. This observation signifies that crocin operates to activate the PI3K/Akt signaling pathway. Furthermore, the utilization of the PI3K inhibitor LY294002 effectively negated the neuroprotective effects conferred by crocin. This outcome serves to illustrate that crocin’s inhibition of microglial cell activation occurs through its modulation of the PI3K/Akt pathway. Thereby, neuroprotective outcomes associated with crocin seem to be mediated via the initiation of the PI3K/Akt signaling cascade [39] (Fig. 1).
Fig. 1.
Schematic representation of PI3K/AKT signaling pathway in DM-related complications.
2.2. Noncoding-RNA
Recent technological advancements have unveiled that mammals generate a multitude of noncoding RNAs, with indications implying that these noncoding RNAs constitute a significant proportion of transcripts within the mammalian genome. It has been proposed that up to 98% of the human genome is responsible for encoding noncoding transcripts [40]. This observation leads to speculation that the notable distinctions in organism complexity between higher and lower organisms could potentially stem from the substantial variations in noncoding transcripts. Many of these noncoding transcripts undergo intricate processing, resulting in the generation of smaller noncoding RNA entities, such as miRNA and lncRNA [41]. By engaging with proteins, RNA, and DNA noncoding RNAs have arisen as pivotal modulators of gene expression in both pathological and physiological conditions.
2.3. MiRNA biosynthesis and function
MicroRNAs constitute a recently identified category of naturally occurring single-stranded RNA molecules (∼22 nt in length) that exert post-transcriptional control over the expression of target genes [42]. Despite their pivotal involvement in biological processes, microRNAs remained undiscovered within the scientific realm until 1993, when the identification of lin-4, a developmental regulator in C. elegans, brought these molecules to light. More than 6000 microRNAs have been detected across an extensive spectrum of both prokaryotic and eukaryotic organisms. While the count of microRNAs might seem modest, their potency lies in their capacity to target numerous genes, and reciprocally, a single target gene can interact with multiple microRNAs in a cooperative manner [43]. This dynamic has led to the prevailing hypothesis that approximately 10%–30% of the entirety of human genes are subject to regulatory influence by microRNAs. MicroRNAs (miRNAs) exhibit a distinct pattern of expression, with some being ubiquitously expressed across various tissues and cells, while others are confined to specific cell types [44]. A substantial portion of human miRNAs is encoded within introns, situated exgenically in non-coding mRNA exons, or embedded within the 3′-UTR sequence of mRNAs. With the exclusion of miRNAs originating from Alu repeat sequences, which are transcribed through Pol-III, microRNAs are transcribed in a Pol II-dependent manner as 5′-capped polyadenylated transcripts, referred to as primary microRNAs (pri-microRNA) [45]. Roughly 40% of human microRNAs are co-transcribed in clusters, encompassing as many as eight discrete microRNA sequences within a solitary transcript, which can extend beyond 1 kilobase in length. Pri-microRNAs are subjected to cleavage by the microprocessor complex, comprising the endoribonuclease Drosha and its co-factor DGCR8 in humans, or Pasha in the context of Drosophila. The outcome is a hairpin structure spanning 60 to 70 nucleotides (pre-microRNA), housing a solitary microRNA sequence. This pre-microRNA is transported to the cytoplasm through Exportin5, relying on a Ran-GTP-mediated mechanism. Within the cytoplasm, pre-microRNAs undergo additional cleavage facilitated by Dicer, in conjunction with co-factors TRBP and PACT (in humans), resulting in the elimination of the loop sequence and formation of a transient asymmetric duplex intermediate. Subsequently, this intermediate structure is incorporated into the miRISC complex, encompassing Argonaut (Ago) proteins [46]. The determination of the active mature miRNA strand is contingent upon the one possessing the least free energy at its 5′ end, whereas the remaining strand is subject to degradation, mediated by an unidentified endoribonuclease. Directed by the mature miRNA sequence, the miRISC complex, once loaded, is guided to its corresponding recognition sequence within the UTR of the target mRNA.
2.4. LncRNA biosynthesis and function
LncRNAs constitute an extensive cohort of transcribed RNA molecules surpassing 200 nucleotides in length, and they possess limited or negligible protein coding capability. These lncRNAs are ubiquitously transcribed across the genome, sharing noteworthy resemblance with conventional mRNAs in terms of their transcriptional initiation by RNA polymerase II [47]. Furthermore, they commonly, though not invariably, undergo alternative splicing and polyadenylation processes. Exhibiting remarkable versatility, lncRNAs possess a diverse array of roles in the regulation of gene expression. In this context, lncRNAs can engage in sequence-specific partial base pairing with RNA or DNA, or they can establish intricate interactions with proteins to form complexes. Recent investigations have sought to classify the diverse array of molecular mechanisms underpinning lncRNA functionality [48]. Given their extensive length, lncRNAs possess the capacity to engender intricate three-dimensional structures through the establishment of multiple intramolecular RNA–RNA interactions. It is possible that the entirety of lncRNAs comprises a multitude of distinct structural or functional domains. Furthermore, in contrast to other RNA categories, a characteristic attribute of lncRNAs is their propensity to encompass an extensive array of binding sites for proteins [47]. Thus, lncRNAs have the potential to serve as a foundation for the oligomerization of proteins or can serve as a framework facilitating the aggregation of numerous proteins into more extensive functional complexes. Empirical evidence has indicated that numerous lncRNAs operate in collaboration with proteins and other nucleic acids, and their secondary or tertiary structures hold significance in facilitating these intricate interactions [49]. Furthermore, complexes formed by lncRNAs and proteins operate to modify chromatin conformation, organize nuclear subdomains, and modulate the regulation of gene expression.
2.5. Dysregulation of non-coding-RNA is a common feature of diabetes
With the advancement of molecular biology techniques, an increasing body of evidence substantiates the pivotal engagement of ncRNAs in the molecular underpinnings of DM. In this context, the study conducted by Zeinali et al. observed elevated levels of miR-122, and diminished levels of miR-126-3p and miR-146a in individuals T2DM and those in a pre-diabetic state, in comparison to healthy individuals. As a result, they deduced that miRNAs hold the potential to play a role in the underlying mechanisms governing the pathogenesis of T2DM [50]. In addition, Majumder et al. investigated the functional implications of HOTAIR within podocytes, both under physiological conditions and when confronted with diabetic challenges. Their findings revealed augmented expression of Glomerular HOTAIR in human DKD, in the renal tissues of mice afflicted with diabetes induced by streptozotocin, and in db/db mice kidneys. Moreover, their study demonstrated that subjecting cultured mouse podocytes to elevated glucose levels led to increased expression of HOTAIR, a process contingent upon p65-dependent signaling. Their discoveries exemplify how the disruption of lncRNA regulation could potentially play a role in the development of DM [51]. Interestingly, recent investigations have documented various signaling molecules and pathways, including NF-κB, PI3K/Akt, mTOR, and STAT pathways, as subjects of regulation by ncRNAs within the domain of DM. In this regard, Zhang investigated the functional attributes of ILF3-AS1 and its potential mechanisms counteracting hypoxia-induced apoptosis in H9c2 cells. They disclosed a reduction in the expression of ILF3-AS1 under hypoxic conditions. Moreover, they observed that ILF3-AS1 upregulation led to mitigation of hypoxia-induced impairment in H9c2 cells, as evidenced by the restoration of cell viability, and migration capabilities, coupled with the repression of apoptosis. Conversely, diminished expression of ILF3-AS1 yielded contrary outcomes. As well, they demonstrated that ILF3-AS1 triggered the activation of the PI3K/Akt signaling cascade. Notably, the administration of the PI3K inhibitor LY294002 effectively nullified the safeguarding effects exerted by ILF3-AS1 in response to hypoxia. They deduced that ILF3-AS1 confers a protective role against hypoxia-induced injury through the PI3K/Akt pathway [52]. Therefore, a complex interaction exists between either miRNA or lncRNA and the PI3K/AKT/mTOR pathway. Therefore, the primary objective of this review is to compile the latest scientific investigations concerning these interplays in the context of DM. This endeavor aims to offer an enhanced understanding, potentially leading to the identification of novel therapeutic targets within this intricate landscape.
2.6. Reciprocal cross-talk between MiRNAs and PI3K/AKT signaling pathway in DM
2.6.1. PI3K/AKT/miR-27a
MicroRNA-27a, derived from the miR-27a gene situated on chromosome 19p13.13, holds significance as a noteworthy constituent within the microRNA family [53]. Chen et al. create an experimental framework encompassing a high-fat diet (HFD)-induced obese mouse model and an insulin resistance (IR) cell culture model utilizing mature 3T3-L1 adipocytes. Their primary aim was to unravel the effects of miR-27a on insulin resistance and glucose metabolism. Their investigation unveiled that miR-27a, exhibiting elevated expression levels in insulin-resistant adipocytes and HFD-induced obese mice, directly interacts with the 3′- UTR of PPAR-γ, consequently exerting suppressive influence on the expression of PPAR-γ. As well, decreased cellular and murine levels of miR-27a led to increased glucose uptake in dose- and time-dependent manner. Furthermore, they revealed that the increased expression of PPAR-γ, subsequent to miR-27a silencing, served to amplify insulin sensitivity. Additionally, the introduction of T0070907, a PPAR-γ inhibitor, suppressed the outcomes brought about by antagomiR-27a transfection. This intervention contributed to the attenuation of glucose levels and the enhancement of insulin sensitivity within adipocytes. In order to validate the involvement of the PI3K/Akt signaling pathway in the regulation of insulin sensitivity mediated by miR-27a-PPAR-γ, they proceeded to administer the PI3K inhibitor wortmannin to AntagomiR-27a-transfected IR cells. They revealed that reduction of glucose levels from antagomiR-27a transfection were subsequently restored following treatment with wortmannin. Furthermore, a lack of alteration in PPAR-γ expression was observed subsequent to the treatment, in contrast to a notable reduction in Akt phosphorylation and GLUT4 expression. Thus, it can be inferred that miR-27a governs insulin sensitivity through PI3K/Akt signaling pathway activity. So, miR-27a assumes a substantial function in IR promotion, partly attributed to its influence on the PPAR-γ-mediated PI3K/Akt signaling cascade. Thereby, miR-27a could be a viable candidate for intervention aimed at enhancing IR and optimizing glucose metabolism in the context of T2DM advancement [54].
2.6.2. PI3K/AKT/miR-351
MiR-351, a recently identified miRNA, exhibits atypical expression patterns within various pathological tissues or cellular contexts. Prior research indicates that suppression of miR-351 leads to increased expression of ITGB3 and triggers activation of the PIK3R1/Akt signaling pathway in endothelial cells [55]. Chen et al. explored the potential engagement of miR-351 within the context of GDM, postulating its interaction with FLOT2 via the PI3K/AKT pathway, thereby potentially yielding consequential functional revelations. Employing initial bioinformatics analysis, they identified that miR-351 is implicated in the modulation of the PI3K/AKT pathway, exerting an influence on the expression of FLOT2 in GDM. As well, their dual luciferase reporter gene assay exhibited the selective binding of miR‐351 to the 3ʹ-UTR of FLOT2. This interaction led to the specific reduction in FLOT2 expression subsequent to transcription. They also substantiated that upregulation of miR‐351 led to a decrease in FLOT2 levels, which, in turn, played a role in mitigating IR and inhibiting gluconeogenesis within the liver of GDM mice. This effect was demonstrated by a decline in phosphoenolpyruvate carboxykinase (PEPCK) and glucose‐6‐phosphatase (G‐6‐Pase) levels, coupled with an increase in GLUT2 expression. Moreover, their subsequent comprehensive functional investigations elucidated that the elevation of miR‐351 levels, achieved through its targeting of FLOT2, led to PI3K/AKT signaling pathway inhibition within GDM mice. In this manner, miR‐351 overexpression led to protection against IR and liver gluconeogenesis. This protective effect occur via the repression of the PI3K/AKT pathway, achieved through the intricate regulation of FLOT2 within the context of GDM mice. Consequently, miR‐351 holding promise for its future application in the clinical management of GDM [56].
2.6.3. PI3K/AKT/miR-26b
The microRNA-26 family, comprising miR-26a, miR-26b, miR-1297, and miR-4465, represents a cluster of highly conserved small RNA molecules characterized by identical sequences within their respective seed regions [57]. Li et al. investigated the impact of miR-26b on GDM in a rat model by focusing on the modulation of the PI3K/AKT signaling pathway. They conducted a random allocation of 60 female rats without pre-existing conditions into three distinct cohorts: group A (normal control), group B (GDM model), and group C (model + miR-26b group). Their qRT-PCR findings unveiled notable downregulation of p-Akt and p-PI3K within group B compared to those in group A. Meanwhile, within group C, these were observed to be diminished in comparison to group B. Their western blot analysis revealed a substantial reduction in the expressions of p-PI3K and p-Akt within group B in contrast to group A. Notably, group C exhibit a notable reduction in p-PI3K and p-Akt than those in group B. they observed the highest expression levels of p-PI3K and p-Akt in group A. Thereby, advancement of GDM is facilitated by miR-26b, which operates through the inhibition of the PI3K/Akt signaling pathway [58].
2.6.4. PI3K/AKT/miR-125a-5p
MicroRNA-125a is evident across all organisms characterized by bilateral symmetry, and it exhibits a conserved nucleotide composition within an 11-base fragment, encompassing the seed region, which remains identical across all examined species [59]. Xu et al. explored the function of miR-125a-5p concerning the modulation of hepatic glycolipid metabolic dysfunction within the context of T2DM, achieved through the targeting of STAT3. They revealed a noteworthy reduction in miR-125a-5p levels within the livers of diabetic mice and rats. Furthermore, they successfully identified STAT3 as the gene targeted by miR-125a-5p. Their findings also demonstrated that miR-125a-5p elevation in C57BL/6 mice resulted in a reduction of STAT3 abundance, accompanied by a decline in the levels of SOCS3 and p-STAT3 expression. So, miR-125a-5p overexpression inhibited and activated SREBP-1c-mediated lipogenesis and PI3K/AKT pathway, respectively. They additionally revealed that the inhibition of miR-125a-5p resulted in a marked elevation in the expression levels of STAT3, p-STAT3, and SOCS3. This led to the activation of the Sterol Regulatory Element-Binding Protein-1c (SREBP-1c) pathway and the suppression of PI3K/AKT pathway. Furthermore, in AML12 cells exposed to palmitic acid, the introduction of a miR-125a-5p mimic significantly enhanced glucose uptake and consumption while simultaneously diminishing the lipid droplets accumulation through the regulation of the STAT3 signaling pathway. Moreover, in their study, elevated expression of miR-125a-5p markedly suppressed STAT3 in diabetic KK-Ay mice, resulting in lowered levels of both blood lipids and glucose. Furthermore, this overexpression led to an augmentation in hepatic glycogen content, alongside a reduction in lipid droplets accumulation within the liver of diabetic mice. Furthermore, miR-125a-5p suppression in KK-Ay mice intensified the impairment of glycolipid metabolism by modulating STAT3 activity. Thus, these findings furnish novel substantiation for the diminished hepatic miR-125a-5p expression in the development of T2DM, underscoring miR-125a-5p′s capability to govern hepatic processes including lipogenesis, gluconeogenesis, and glycogen synthesis by means of STAT3 targeting. Their investigations further substantiated that miR-125a-5p upregulation, primarily through the activation of the PI3K/AKT pathway, holds advantageous implications for maintaining glycolipid metabolism homeostasis in the context of diabetes. As a result, PI3K/AKT/miR-125a-5p axis holds significant promise as a prospective avenue for addressing metabolic disorders [60].
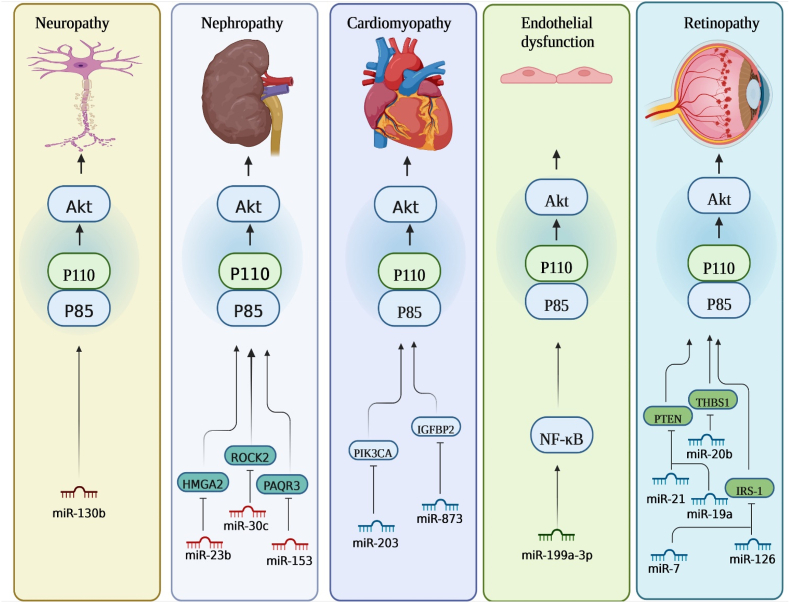
2.6.5. PI3K/AKT/miR-21
MicroRNA-21 (miR-21), extensively investigated and analyzed, plays a crucial role in various distinct pathophysiological mechanisms associated with diabetes mellitus (DM). Lu et al. assess the potential impact of miR-21 suppression on the restoration of PTEN expression and subsequent deactivation of the PI3K/Akt/VEGF pathway, with the overarching goal of impeding DR progression. As an initial step, they unveiled an elevation in the expression of miR-21 within retinal tissues of rats afflicted by DR. They noticed that miR-21 upregulation potentially correlate with PI3K/Akt/VEGF signaling pathway activation and the concurrent PTEN suppression. Subsequently, employing a bioinformatics approach in conjunction with a dual-luciferase reporter gene assay, they confirmed that miR-21 directly target PTEN. Furthermore, through subsequent functional analysis, they validated that the overexpression of miR-21 yielded an upregulation in both mRNA and protein levels of CD34, VEGF, p-Akt/t-Akt, and p-PI3K/t-PI3K. On contrary, they observed a reduction in PTEN mRNA and protein levels. RVECs, upon transfection with a miR-21 inhibitor, demonstrated enhanced cellular viability and angiogenic potential, coupled with the attenuation of apoptotic processes. Consequently, upregulation of miR-21 by activating PI3K/Akt/VEGF and PTEN repression holds the potential to stimulate both the viability and angiogenic capabilities of RVECs in rats afflicted by DR, indicating miR-21 as a targeted approach for the treatment of DR [61]. In addition, Zhang et al. postulated that rapamycin could potentially mitigate the injury inflicted on islets by hypoxia/reoxygenation (H/R) through its influence on miRNA biogenesis regulation. Their initial observations revealed a marked increase in the expression of primary transcripts (pri-miR-21) and precursor molecules (pre-miR-21) of miR-21, as early as 6 h following rapamycin treatment. These findings strongly imply that rapamycin may contribute to the transcriptional induction of miR-21. As well, their transfection assays elucidate the critical role of the mTOR/PI3K/Akt signaling pathways in mediating the rapamycin-induced elevation of miR-21, which is integral for safeguarding against islet injury resulting from H/R. Thereby, inhibitory influence of rapamycin on islet injury due to H/R stress is accomplished through the induction of miR-21 expression mediated by the PI3K/Akt pathway [62]. In addition, Qiu et al. utilized HRMECs as a model to explore the impact of miR-21-5p on angiogenesis induced by elevated glucose levels. They initially established that exposure to elevated glucose levels resulted in an augmented HRMECs proliferation. Furthermore, they observed that miR-21-5p silencing effectively curbed the heightened proliferation induced by high glucose conditions. Additionally, their tube formation assay disclosed that miR-21-5p silencing abrogated the angiogenic response prompted by elevated glucose concentrations. Subsequent to their functional assessments, they observe that the PI3K/AKT and ERK pathways potentially serve as downstream cascades of miR-21-5p in mediating the process of angiogenesis induced by elevated glucose within HRMECs. Ultimately, they illustrated that miR-21-5p potentially contributes to HRMEC angiogenesis through the regulation of the ERK and PI3K/AKT pathways through maspin. Thereby, miR-21-5p silencing resulted in the inhibition of both proliferation and angiogenesis induced by high glucose in HRMECs. These effects were shown to be partially mediated by the modulation of the ERK and PI3K/AKT pathways through its target protein, maspin [63].
2.6.6. PI3K/AKT/miR-130b
MiR-130b is a microRNA that demonstrates notably increased expression levels both within adipose tissue and the systemic circulation during diabetic conditions. Lei et al. explored whether miR-130b plays a role in activating Akt activity to mitigate oxidative stress-induced damage through the modulation of PTEN in the context of diabetic encephalopathy. They initially demonstrated that elevated glucose exposure resulted in the suppression of miR-130b within PC12 and hippocampal cells. As well, miR-130b mimic administration trigger heightened cellular viability in the presence of high glucose stimulation, concurrently inhibited apoptosis. This intervention further led to an augmentation in superoxide dismutase (SOD) activity, coupled with a reduction in malondialdehyde (MDA) levels. Moreover, there was an elevation in Akt protein levels and a concurrent inhibition of the mitochondria-mediated apoptotic pathway. Furthermore, miR-130b inhibitor administration yielded contrasting outcomes, which were subsequently nullified through the utilization of si-PTEN. Furthermore, their in vivo investigation revealed that the agomir-130b administration ameliorated cognitive impairments and neuronal damage. This intervention was concomitantly associated with elevated SOD activity, decreased MDA levels, Akt activation, and inhibition of the mitochondria-mediated apoptotic pathway in DE rats. In this manner, miR-130b involved in PI3K/Akt signaling pathway activation and confer protective effects against oxidative stress-induced injury through the modulation of PTEN in DE rats [64].
2.6.7. PI3K/AKT/miR-195
MiR-195 assumes a significant role as a prominent member within the micro-15/16/195/424/497 family, demonstrating activation across a spectrum of diverse pathological conditions. Xu et al. investigated the function and underlying mechanisms through which miR-195 participates in the process of pancreatic β-cell dedifferentiation triggered by hyperlipidemia in the context of T2DM. In their study, miR-195 overexpression was detected during lipotoxicity-triggered β-cell dedifferentiation, as demonstrated through both in vivo and in vitro experimentation. Moreover, they noticed that miR-195 played a functional role in facilitating β-cell dedifferentiation induced by lipotoxicity. Furthermore, miR-195 restrained the activation of the IRS-1/2/PI3K/Akt pathway, coinciding with β-cell dedifferentiation. Notably, they revealed that miR-195 directly interact with Mfn2. Moreover, it was observed that miR-195 was decreased, exhibiting a correlation with heightened mitochondrial generation of reactive oxygen species during the process of β-cell dedifferentiation. Their investigation further unveiled that silencing miR-195, to a certain extent, counteracted the reduced expression of Mfn2. This restoration, in turn, reinstated the activation of the IRS-1/2/PI3K/Akt pathway, subsequently halting the progression of β-cell dedifferentiation. Consequently, miR-195 was established as a promoter of β-cell dedifferentiation, exerting its effects via negative regulation of Mfn2 and impeding the IRS-1/2/PI3K/Akt pathway. This insight holds the potential to offer a promising avenue for the treatment of T2DM [65]. Additionally, Lai et al. explored the function and mechanism underlying LINC01572 in the advancement of HCC among patients with T2DM. In this regard, they undertook a comprehensive profiling of lncRNAs within HCC tissues and their respective adjacent counterparts derived from patients by both HCC and T2DM. Their findings indicated an aberrant elevation of LINC01572 expression within HCC tissues in comparison to control samples, particularly in cases co-occurring with T2DM. Moreover, elevated LINC01572 levels demonstrated a significant correlation with heightened blood HbA1c concentrations, reduced duration of survival, and advanced tumor staging. Moreover, in their study, LINC01572 elevation substantially facilitated the proliferation, and migration in HCC cells. Conversely, LINC01572 silencing exhibited contrary effects, manifesting inhibitory influences on HCC cell behaviors. Their mechanistic investigation unveiled that the progression of HCC guided by LINC01572 is mediated through its role as a miR-195-5p sponge, leading to an elevation in PFKFB4 levels. This, in turn, fosters an augmentation of glycolytic activity and the activation of the PI3K-AKT signaling pathway. So, LINC01572 functions as a ceRNA for miR-195-5p, thereby alleviating its repression on PFKFB4 and subsequently enhances glycolytic metabolism and initiates the activation of the PI3K/AKT signaling pathway. In this manner, LINC01572/miR-195-5p/PI3K/AKT regulatory network play a crucial role in the promotion of HCC malignancy among individuals with T2DM [66].
2.6.8. PI3K/AKT/miR-7
MiR-7, serving as an exceptional member within the miRNA family, exhibits a substantial level of conserved mature sequences, ranging from 21 to 23 nucleotides in length, across various species. Yang et al. explored the impact of miR-7 on the modulation of cellular proliferation through the HoxB3 gene and the PI3K/AKT/mTOR signaling pathways in DR. Their investigation revealed that miR-7 mimics transfection led to a decrease in apoptosis rates, and upregulation of miR-7. Their subsequent functional assessment unveiled that the introduction of miR-7 mimic resulted in a reduction of Hoxb3, mTOR, p-PI3K, and p-AKT expression, both at the mRNA and protein levels. However, no discernible variation was observed in the expression of PI3K and AKT. In this manner, through the modulation of the PI3K/AKT/mTOR signaling pathways, miR-7 orchestrates the retinal epithelial cells growth, thus emerging as a promising therapeutic target for mitigating and managing DR [67]. In addition, Cao et al. examined the involvement of miR-7 in the context of DR and explored its underlying mechanism. Within their investigation, a noteworthy reduction in miR-7 expression was observed in both ECs and RPs of the experimental group in comparison to the control group. Meanwhile, the mRNA and protein levels of IRS-1 exhibited an elevation. Their experimentation also demonstrated that miR-7 upregulation led to a reduction in cellular activity within both ECs and RPs. Through functional analysis, they ascertained that the miR-7 silencing resulted in an increase in cell viability. Their further examination via western blotting indicated a downregulation in the expressions of VEGF, PI3K, and AKT within ECs and RP cells following the overexpression of miR-7. Significantly, their luciferase reporter assay provided indications that the 3′-UTR region of IRS-1 could interact with miR-7, suggesting that IRS-1 potentially serves as a downstream target gene for miR-7. Additionally, their findings revealed that IRS-1 silencing could counteract the impact of the miR-7 inhibitor on cell proliferation within the diabetic model. Altogether, miR-7 elevation leads to the suppression of VEGF, AKT, and PI3K expression levels through the reduction of its downstream target gene IRS-1, culminating in the inhibition of retinal cell proliferation [68]. Additionally, Ji et al. investigated the role of miR-7a in relation to DR. In their study, a decrease in miR-7a expression was observed, while the expression of IRS-2 was upregulated in both isolated ECs and RPs. Their experimentation also disclosed that miR-7a directly target IRS-2. Their subsequent experimentation unveiled that miR-7a upregulation led to the inhibition of viability and invasive potential in both ECs and RPs. Additionally, this intervention suppressed the protein levels within the PI3K/Akt signaling cascade, as well as IRS-2. Moreover, their findings indicated that the introduction of siRNA directed against IRS-2 led to modifications in the changes facilitated by miR-7a in ECs, implying a potential role for miR-7a in diminishing angiogenesis in DR through the inhibition of IRS-2 levels. In this manner, miR-7a exerts inhibition on the PI3K/Akt signaling pathway by specifically targeting IRS-2, and subsequently led to diminished viability and reduced invasive potential in RPs and ECs [69].
2.6.9. PI3K/AKT/miR-363-3p
Shu et al. explored the mechanisms underlying the ameliorative effects of resveratrol (RSV) on hepatic insulin resistance induced by a high-fat diet (HFD) in murine models, both in vivo and in vitro. Employing high-throughput sequencing analysis, they successfully pinpointed mmu-miR-363-3p as a prominent microRNA integral to the modulatory role of RSV in counteracting insulin resistance. Through comprehensive functional assessment, it was determined that administration of RSV led to down-regulation of G6PC and FOXO1, key components situated downstream within the PI3K-Akt signaling cascade. Furthermore, an in-depth examination conducted in vitro unveiled a noteworthy phenomenon: mmu-miR-363-3p silencing markedly restrained levels of p-PI3K and p-Akt, while concurrently increasing the abundance of G6PC and FOXO1. Furthermore, in their study, administration of mmu-miR-363-3p mimic resulted in a substantial increase in p-PI3K and p-Akt levels, while concurrently exerting an inhibitory influence on the expression of G6PC and FOXO1. This observed outcome closely resemblance the effects achieved through RSV intervention. In this manner, RSV enhances insulin resistance mitigation through the mmu-miR-363-3p overexpression, operating via the PI3K-Akt pathway [70].
2.6.10. PI3K/AKT/miR-19a
The miR-19 family members share identical seed regions and derive from divergent paralogous clusters, namely miR-17-92 (comprising miR-19a and miR-19b-1) and miR-106a-363 (encompassing miR-19-b-2). Zhang et al. investigated the modulatory influence exerted by miR-19a on DR by means of its mediation of the PTEN/Akt signaling pathway. As a primary step, they categorized thirty male Sprague-Dawley rats into three distinct groups: miR-19a inhibitor group, DR group, and Healthy group. It was observed that the miR-19a expression experienced a significant reduction in DR rats following miR-19a inhibitor administration. Concurrently, RGCs exhibited an organized arrangement characterized by attenuated apoptosis and less pronounced necrosis within the group treated with the miR-19a inhibitor. As well, miR-19a inhibitor group displayed a notable reduction in the proportion of cells undergoing apoptosis. Additionally, they revealed that the miR-19a inhibitor group displayed PTEN protein reduction, along with increased activation of Akt pathway in comparison to the DR group. In this manner, miR-19a directly interact with the PTEN protein, thereby orchestrating modulation of the PI3K/Akt pathway, and subsequently influencing the advancement of DR [71].
2.6.11. PI3K/AKT/miR-122
In human, miR-122 derives from a genomic locus situated on chromosome 18. This genomic region, harboring human miR-122, is positioned within a discrete domain comprised of noncoding RNA exons. Initial exploration of miR-122 exhibited its conservation across a spectrum of 12 distinct species, encompassing human, frogs, and zebrafish. Wang et al. explored the function and underlying regulatory pathways governed by miR-122 within the context of diabetes. Their findings demonstrated that the suppressive impact on insulin secretion induced by STZ was alleviated through the implementation of a miR-122 inhibitor. Furthermore, increased activities of antioxidant enzymes, namely GSH-px, CAT, and SOD was observed as a consequence of miR-122 inhibition. As well, miR-122 inhibitor was observed to impede apoptosis and alleviate oxidative stress within STZ-induced INS-1 cells. Conclusively, their investigation revealed that the inclusion of LY resulted in heightened insulin levels, diminished enzymatic activities of SOD, CAT, and GSH-px, and facilitated apoptosis in STZ-induced INS-1 cells. In this manner, disruption of miR-122 can effectively impede apoptosis and oxidative stress in STZ-induced INS-1 cells. This modulation is underpinned by a mechanism intricately associated with the PI3K/AKT pathway [72].
2.6.12. PI3K/AKT/miR-20b
MiR-20b has been extensively investigated as a regulatory entity in both normal and pathological human states. Ma et al. investigated a potential linkage between miR-20b-5p and DR through its interaction with the VEGF/PI3K/Akt signaling pathway, particularly involving the THBS1 gene. Their initial observations revealed increased expressions of eNOS, miR-20b-5p, and CD34 in the retinal tissue of diabetic rats, while THBS1 was inhibited. As well, their further investigation proposed THBS1 as a plausible target for miR-20b-5p. They also revealed that the upregulation of miR-20b-5p led to augmented cell viability in diabetic rats, while miR-20b-5p silencing yielded contrasting outcomes. Moreover, they disclosed that miR-20b-5p elevation induce tube formation in DR rats, while, miR-20b-5p silencing yielded opposite effects. Also, siRNA-THBS1 counteracted the inhibitory impact elicited by the miR-20b-5p inhibitor on the tube formation capacity of cells. Additionally, through a Flow cytometry analysis, it was observed that miR-20b-5p upregulation resulted in a reduction of cell apoptosis among DR rats, while miR-20b-5p silencing displayed contrary outcomes. As well, siRNA-THBS1 was observed to exert a mitigating influence on the suppressive consequences induced by the miR-20b-5p inhibitor in relation to apoptosis in endothelial cells. Therefore, their findings indicated that miR-20b-5p silencing elicits an antiangiogenic influence in the progression of diabetic retinopathy in rats. This is mediated through the suppression of the VEGF/Akt/PI3K signaling pathway, facilitated by THBS1 upregulation [73].
2.6.13. PI3K/AKT/miR-30c
The miR-30 family constitutes a significant and intricate cluster, assuming pivotal functions in both mammals and humans. Comprising five distinct members, this family yields a repertoire of six distinct mature miRNAs (namely miR-30a, −30b, −30c-1, -30c-2, -30d, −30e), each derive from the transcription of six distinct genes situated on human chromosomes 1, 6, and 8, respectively. Cui et al. probe the biological significance of miR-30c-5p in the advancement of human DN through in vitro exploration. Their initial findings highlighted a substantial reduction in miR-30c-5p expression, contrasted by a marked elevation in ROCK2 levels within DN tissues. Their subsequent experimentation unveiled that miR-30c-5p mimic notably enhances cellular proliferation, concurrently impeding apoptosis and EMT within HK-2 cells subjected to HG stimulation. Conversely, the implementation of the miR-30c-5p inhibitor yields a contrary outcome. As well, they successfully detected that ROCK2 functions as a direct downstream target gene of miR-30c-5p. Significantly, their investigation demonstrated that the influence exerted by the miR-30c-5p mimic on cellular proliferation, apoptosis, and EMT was ameliorated upon ROCK2 overexpression within the cellular model of DN induced by HG. Furthermore, they substantiated that both miR-30c-5p and ROCK2 play contributory roles, at least in part, through modulation of the PI3K/AKT pathway during DN pathogenesis. Thereby, manipulation of cellular processes such as cell proliferation, apoptosis, and EMT by miR-30c-5p, achieved through its targeting of ROCK2 within the context of the PI3K/AKT pathway, presents an innovative and prospective therapeutic target for the clinical management of DN [74].
2.6.14. PI3K/AKT/miR-199a-3p
MiR-199 constitutes a markedly conserved microRNA family encompassing two distinct members: miR-199a and miR-199b. Presently, this family is characterized by two variations of precursor miRNAs, namely pre-miR-199a-1 (MI000242) and pre-miR-199a-2 (MI0000281), originating from chromosomal locations 19 and 1, respectively. Zhou et al. investigated the correlation between miR-199a-3p and FGF7, subsequently conducting an in-depth inquiry into the mechanistic aspects of miR-199a-3p and FGF7 within the context of DR. They firstly unveiled a marked reduction in the expression of miR-199a-3p within the ECs and RPs of rats with DR, in comparison to normal rats. As well, their in vitro analysis revealed that miR-199-3p control over critical cellular processes including migration, and proliferation within RPs and ECs. Furthermore, employing bioinformatics analysis coupled with dual-luciferase reporter assays, they established that miR-199a-3p can selectively target FGF7. Furthermore, in their study, elevated FGF7 expression effectively counteracted the suppressive effects induced by miR-199a-3p, particularly proliferation and viability and of both ECs and RPs. Furthermore, they noticed that FGF7 elevation successfully mitigated the impact of miR-199a-3p on impeding the migration of RPs and ECs. They subsequently disclosed that miR-199a-3p upregulation effectively impedes EGFR/PI3K/AKT pathway activation. Notably, this inhibitory impact was partially counteracted by FGF7 upregulation. These findings collectively suggest that the regulatory influence exerted by miR-199a-3p extends to the modulation of the EGFR/PI3K/AKT pathway via its regulation of FGF7. In this manner, in DR rat model, miR-199a-3p through the specific targeting of FGF7 and subsequent inhibition of EGFR/PI3K/AKT pathway exhibited suppressive effects on the processes of cell proliferation, and migration in both ECs and RPs. These effects were achieved through the specific targeting of FGF7 and subsequent inhibition of the activation of the EGFR/PI3K/AKT pathway [75]. In addition, Wang et al. explored the contributions of miR-199a-3p in the advancement of angiogenesis within an in vitro DR model. Their findings unveiled that under HG conditions, miR-199a-3p relative expression level of exhibited a reduction, whereas VEGF expression increased within both APRE-19 and hRMECs cells. As well, miR-199a-3p inhibitor led to enhanced cellular growth, migration, and angiogenesis in hRMECs. Furthermore, their investigation demonstrated that miR-199a-3p elevation significantly mitigated the heightened levels of cell proliferation, migration, and angiogenesis induced by HG conditions. Furthermore, they demonstrated a direct targeting relationship between miR-199a-3p and VEGF. Furthermore, their investigation unveiled a discernible inhibitory effect on the PI3K/AKT signaling pathway and subsequent angiogenesis in response to elevated miR-199a-3p expression under HG conditions. In this manner, miR-199a-3p elevation exhibited a mitigating effect on angiogenesis induced by HG in hRMECs, achieved through the modulation of the PI3K/AKT pathway via VEGF silencing [76]. Wang et al. investigated the functional role and underlying mechanism of miR-199a-3p in the context of vascular endothelial cell injury induced by T2DM. Their investigation revealed a downregulation of miR-199a-3p in peripheral blood samples from T2DM patients when compared to healthy individuals. Moreover, administration of miR-199a-3p mimics via transfection induced a notable enhancement in both the proliferation and migration of HUVECs. In addition, miR-199a-3p upregulation effectively suppressed apoptosis in HUVECs. They subsequently revealed that miR-199a-3p prompted autophagy in HUVECs, and via PI3K/AKT/NF-κB signaling pathway modulates biological activities. Their discoveries imply a potential correlation between the expression of miR-199a-3p and vascular endothelial cell injury, wherein miR-199a-3p appears to enhance the processes of proliferation, migration, and autophagy in HUVECs. This effect is suggested to be mediated through the modulation of the PI3K/AKT/NF-κB signaling pathway. Consequently, miR-199a-3p could potentially serve as a safeguard for vascular endothelial integrity [77].
2.6.15. PI3K/AKT/miR-720
Lu et al. examined whether miR-720 functions to regulate insulin secretion in MIN6 cells by targeting Rab35. In their study, circulating miR-720 were notably elevated within the T2D group in comparison to the control group. Furthermore, miR-720 exhibited a positive correlation with FBG levels, while displaying a negative correlation with fasting insulin (FINS) levels. Moreover, miR-720 upregulation was observed to suppress insulin secretion, while miR-720 reduction exert opposite effect. Furthermore, they observed the regulatory role of miR-720 in insulin secretion within MIN6 cells through its direct interaction with Rab35. Additionally, in comparison to the control group, Rab35 upregulation led to a notable reduction in the levels of mTOR, Akt, and PI3K. Conversely, the suppression of the Rab35 gene resulted in an induction of mTOR, Akt, and PI3K expression. In this manner, miR-720 decrease Rab35 expression, subsequently activating the PI3K/Akt/mTOR signaling pathway, thereby exerting an inhibitory influence on insulin secretion [78].
2.6.16. PI3K/AKT/miR-139-5p
The miR-139 is positioned within the second intronic region of the gene encoding phosphodiesterase 2A, situated on the chromosomal locus 11q13.4. Concurrently, miR-139-5p emerges as a frequently encountered mature microRNA variant originating from the precursor form of miR-139. Qiu et al. investigated the involvement of PICK1 in mediating β-cell dysfunction induced by elevated glucose levels, along with elucidating the mechanistic interplay governing miR-139-5p′s modulation of PICK1 expression within pancreatic β-cells. Their study revealed that upregulating PICK1 expression in diabetic db/db mice yielded notable improvement in glucose tolerance and enhanced insulin secretion. As well, the application of elevated glucose concentrations to Min6 cells resulted in the suppression of PICK1 expression, while PICK1 upregulation exhibited a protective effect against dysfunction in pancreatic cells induced by elevated glucose levels. Furthermore, in their study, PI3K/Akt signaling pathway activation by PICK1 within Min6 cells led to heightened expression of GLUT2, and this was nullified upon administration of a specific inhibitor targeting the PI3K pathway. They subsequently demonstrated that the PICK1 expression is subject to negative regulation by miR-139-5p, achieved through direct interaction with the 3′-UTR of the PICK1 transcript. Their finding also implies that PICK1 potentially engages in preserving the functional integrity of pancreatic β-cells via PI3K/Akt signaling, facilitating insulin secretion, and retarding the progression of diabetes. Additionally, the negative regulation of PICK1 by miR-139-5p, as indicated by their findings, contributes further to elucidating the intricate regulatory framework governing pancreatic β-cell function [79].
2.6.17. PI3K/AKT/miR-24
MiR-24 holds significance as a pivotal constituent within the microRNA family. It is found within two distinct genetic clusters. Among these is the gene cluster miR-23b/miR-27b/miR-24-1, which resides within the transcriptional sequence domain of chromosome 9. Cai et al. observed that subsequent to balloon-induced injury of the carotid artery, the expression level of miR-24 exhibited elevation in normal rats compared to diabetic rats. Conversely, the expression pattern of PIK3R1 demonstrated an inverse outcome. They subsequently disclosed that the elevation of miR-24 yielded a protective influence against the development of neointimal tissue in diabetic rats subsequent to balloon-induced arterial injury. This protective effect was achieved by disrupting the hyperglycemia (HG)-induced processes of migration, proliferation, and excessive collagen synthesis in VSMCs. Their further experimentation demonstrated that miR-24 impeded the activation of PI3K (p85α) and its subsequent effector molecule, Akt, under HG conditions. Furthermore, the introduction of the PI3K p85 agonist, specifically the 740Y-P peptide, led to a significant activation of Akt and PI3K (p85α). This observation serves to indicate that miR-24 is capable of attenuating the proliferation and migration of VSMCs by regulating the signaling pathway associated with PI3K. Thereby, miR-24 overexpression demonstrated a substantial mitigation of vascular remodeling in both VSMCs and balloon-injured diabetic rat models exposed to elevated glucose levels through the repression of proliferation, migration, and collagen accumulation. Importantly, these effects were attributed to the regulatory action of miR-24 on the PIK3R1 gene, thereby influencing the PI3K/Akt/mTOR signaling axes [80].
2.6.18. PI3K/AKT/miR-183
Comprising a trio of constituents - miR-183, miR-96, and miR-182 - the miR-183 cluster’s regulatory implications span a diverse array of conditions, including cancer, and diabetes. Zhang et al. explored the mechanistic involvement of miR-183 in DR, particularly concerning its interplay with the PI3K/Akt/VEGF signaling pathway. Their initial investigations revealed a substantial elevation of miR-183 expression within DR rat models exhibiting an augmented PI3K/Akt/VEGF signaling pathway. As well, upregulation of miR-183 trigger activation of PI3K/Akt/VEGF signaling pathway, mediated by its suppressive impact on BTG1. In addition, their observations indicated that the miR-183 upregulation fosters the proliferation of vascular endothelial cells, while concurrently restraining cellular apoptosis. As well, in their study, knock down of miR-183 yields antiangiogenic effects in the progression of DR in rats. This effect is achieved through the direct elevation of BTG1, leading to the suppression of the PI3K/Akt/VEGF signaling pathway [81].
2.6.19. PI3K/AKT/miR-203
MicroRNA-203, situated at the chromosomal locus 14q32.33, ranks prominently among the frequently cited miRNAs. Yang et al. investigated the regulatory function of miR-203 in the context of myocardial fibrosis in mice afflicted with DCM, with a particular emphasis on its engagement with the PI3K/Akt signaling pathway. By employing online analytical tools and a dual luciferase reporter gene assay, they detected that miR-203 exhibited targeted binding to the 3′-UTR of PIK3CA, leading to a reduction in PIK3CA gene expression. Their findings further revealed that miR-203 overexpression led to a reduction in myocardial apoptosis, myocardial fibrosis, myocardial hypertrophy, and the expression levels of MDA, CoI III, Akt, PIK3CA, PI3K, CoI I, ANP, and ROS within myocardial tissues. This collective effect served to ameliorate the cardiac dysfunction and pathological alterations induced by diabetes mellitus. Thereby, miR-203 upregulation conferring cardioprotection in DCM, achieved through its modulation of PIK3CA via the deactivation of the PI3K/Akt signaling pathway [82].
2.6.20. PI3K/AKT/miR-153-3p
Recently, miR-153, a noteworthy participant in the various biological processes, has emerged as a focal point in various investigations. The two genetic counterparts of miR-153, denoted as miR-153-1 (miR-153-5p) and miR-153-2 (miR-153-3p), each characterized by a conserved sequence, represent evolutionary conserved genes present in diverse taxonomic levels. These genes are located on chromosome 2q35, and on chromosome 7q36.3, respectively [83]. Yang et al. investigated both the impact and the associated molecular mechanism underlying the influence of miR-153-3p on human glomerular mesangial cells subjected to high glucose stimulation. They observed reduced expression of miR-153-3p alongside a concurrent elevation in PAQR3 expression in diabetic nephropathy patients. Furthermore, miR-153-3p overexpression demonstrated the capacity to mitigate mesangial cell proliferation and ECM accumulation. They subsequently revealed a direct regulatory relationship between miR-153-3p and PAQR3. These findings substantiate the role of miR-153-3p in modulating the PI3K/AKT pathway via its interaction with PAQR3. In this manner, miR-153-3p modulates the PI3K/AKT pathway via its interaction with PAQR3, consequently contributing to the regulation of cell proliferation and ECM accumulation within human glomerular MCs exposed to high glucose stimuli [84].
2.6.21. PI3K/AKT/miR-23b
miR-23b, a constituent of the miR-23b∼27b∼24-1 gene cluster situated at locus 9q22.32, has been documented as a multifaceted regulator in diverse pathological and physiological functions. Liu et al. investigated the function of miR-23b in the context of EMT within diabetic nephropathy, elucidating one of the principal molecular targets governed by this specific microRNA. In their study, it was observed that there existed a noteworthy reduction in the expression of miR-23b within human kidney proximal tubular epithelial cells (HK2) subjected to HG induction, as well as in the renal tissues of db/db mice. As well, miR-23b upregulation mitigated the EMT induced by HG conditions, while miR-23b silencing facilitated the EMT process mediated by normal glucose (NG) levels in HK2. Their mechanistic investigation revealed that miR-23b exerts an inhibitory influence on EMT within the context of diabetic nephropathy. This effect is achieved through the specific targeting of high mobility group A2 (HMGA2), consequently leading to the suppression of activation in the PI3K-AKT signaling pathway. Their subsequent functional assays provided additional insights, revealing that high mobility group A2 (HMGA2) silencing or PI3K-AKT signaling pathway suppression via LY294002, replicated the influences of miR-23b upregulation concerning EMT elicited by HG conditions. Conversely, the upregulation of HMGA2 or PI3K-AKT signaling pathway activation using BpV inhibited the impact of miR-23b on EMT induced by HG. Furthermore, they validated that miR-23b upregulation yielded a mitigating effect on EMT, leading to a reduction in the expression levels of genes associated with EMT. This upregulation also demonstrated beneficial outcomes on glycogen deposition, renal histology, fibrotic reactions, and enhanced renal functionality within db/db mice. Taken together, in diabetic nephropathy, miR-23b functions as an EMT inhibitor, operating by impeding the activation of the PI3K-AKT signaling pathway through the specific targeting of HMGA2. This mechanistic insight underscores the prospective viability of miR-23b/HMGA2/PI3K-AKT as a plausible therapeutic target in addressing renal impairment induced by diabetes [85].
2.6.22. PI3K/AKT/miR-126
The microRNA miR-126-3p, often identified as the 3′ segment of the transcript, is localized within the seventh intronic region of EGFL7, a gene situated on the ninth chromosome of the human genome. Fang et al. explored the potential regulatory role of miR-126 in modulating the PI3K-Akt-VEGF signaling pathway within both ECs and RPs. Their investigation demonstrated a notable reduction in miR-126 expression, coupled with an increase in IRS-1 expression, within ECs and RPs derived from a DR mouse model in comparison to those sourced from healthy control subjects. Furthermore, utilizing a luciferase reporter assay, they validated the interaction between miR-126 and IRS-1. Moreover, their experimentation involved the transfection of a miR-126 mimic or miR-126 inhibitor, revealing that upregulated miR-126 expression inhibited the invasiveness and viability of both RPs and ECs, concurrently reducing the protein expression levels of IRS-1 and components within the PI3K/Akt pathway. Conversely, miR-126 silencing led to contrasting outcomes in these parameters. Importantly, administration of small interfering RNA aimed at IRS-1 through transfection induced modifications in the effects elicited by miR-126 within ECs. This observation suggests that miR-126 by inhibiting IRS-1 inhibit angiogenesis in the context of DR. Taken together, their investigation implied that miR-126 exerted an influence on IRS-1 expression, culminating in the reduction of protein expression levels within the PI3K/Akt pathway, and finally led to cell invasion inhibition [86]. In addition, Xin et al. investigate the impact of resveratrol (RSV) on cellular damage and impaired functionality in pancreatic β-cells induced by uric acid (UA). In their study, RSV led to an elevation in the expression level of miR-126, and miR-126 silencing counteracted the safeguarding influence of RSV on Min6 cells impaired by UA. As well, RSV trigger KLF2 overexpression, and the stimulatory impact of RSV on miR-126 expression was reversed upon the silencing of KLF2. Besides, in UA-injured Min6 cells, RSV led to activation of the PI3K/AKT signaling pathway through miR-126 overexpression. They also suggested that RSV could safeguard Min6 cells from injury and impaired functionality caused by UA, achieved through miR-126 modulation and PI3K/AKT signaling pathway activation [87]. Additionally, Yang et al. examined the reciprocal modulation between miR-126 and the VEGF/PI3K/AKT signaling pathway within retinal vascular endothelial cells. Initially, their investigation revealed that HG trigger miR-126 a reduction, concomitant with an upregulation in both PIK3R2 and VEGFA within RF/6A cells. Their subsequent experimentation demonstrated that the upregulation of miR-126 impeded the migratory and sprouting capacities of RF/6A cells prompted by high glucose levels. Conversely, miR-126 silencing yielded contrasting results. Moreover, in their study, miR-126 upregulation demonstrated a suppressive effect on the increased levels of SPRED1, VCAM-1, SDF-1α, VEGFA, and PIK3R2, along with AKT1 activation induced by HG. Conversely, the introduction of a miR-126 inhibitor yielded contrary outcomes. Moreover, utilizing luciferase assays, they identified a direct and inhibitory regulatory relationship between miR-126 and PIK3R2, as well as VEGFA. In this manner, upregulation of miR-126 expression hampers the migratory and sprouting capabilities of RF/6A cells prompted by HG, potentially accomplished through the inhibition of VEGFA and PIK3R2 within the VEGF/PI3K/AKT signaling cascade [88]. Moreover, Lou et al. explored the function of miR-126 in the context of diabetic nephropathy and its underlying mechanism. Within the diabetic nephropathy model, their investigation confirmed a notable reduction in miR-126 expression in comparison to the control group. As well, they revealed that miR-126 silencing increased cellular apoptosis and promoted inflammation (evidenced by elevated levels of TNF-α, IL-18, IL-6, and IL-1β) within an in vitro model of diabetic nephropathy. Conversely, the upregulation of miR-126 resulted in a noteworthy mitigation of cellular apoptosis and a dampening of inflammation. Their subsequent investigational revealed that the reduced expression of miR-126 led to inhibition of p-AKT, PI3K, and VEGF within an in vitro model of diabetic nephropathy. In contrast, miR-126 upregulation increased the protein expression of p-AKT, PI3K, and VEGF in the context of diabetic nephropathy in vitro. In this manner, miR-126 exerts its protective influence on diabetic nephropathy through modulation of the PI3K/AKT signaling pathway [89].
2.6.23. PI3K/AKT/miR-499-5p
MiRNA-499, a recently identified constituent of the microRNA family, is encoded by the myosin gene family and is situated within an intronic region of the Myh7b gene. Wang et al. explored the intricate molecular mechanism underlying the regulatory role of miR-499-5p in insulin resistance. Their findings initially indicated a substantial reduction in miR-499-5p expression within the livers of db/db mice. As well, miR-499-5p silencing hindered the glycogen synthesis and insulin signaling pathway, while miR-499-5p upregulation facilitated the glycogen synthesis and insulin signaling pathway within NCTC1469 cells. Subsequently, they following administration of adenovirus vectors carrying miR-499-5p mimics via tail vein injection noticed that 499-5p directly target PTEN. Importantly, they observed notable improvement in glucose tolerance test (GTT) and insulin tolerance test (ITT) outcomes among mice subjected to a HFD. In this manner, miR-499-5p reduction hindered the PI3K/AKT/GSK signaling pathway and the process of glycogen synthesis, achieved through the specific targeting of PTEN [90].
2.6.24. PI3K/AKT/miR-873
MiR-873 is a microRNA situated on chromosome 9p21.1. Within the miR-873 family, the principal constituents encompass miR-873-5p and miR-873-3p. Han et al. ascertain the potential impact of miR-873 on insulin resistance and myocardial injury within an established rat model of GDM. Their findings revealed that miR-873 silencing led to IGFBP2 overexpression and facilitated PI3K/AKT/mTOR pathway activation. As well, through miR-873 silencing in GDM rats, there was observed improvement in cardiac function and attenuation of myocardial apoptosis, accompanied by increased carbon monoxide synthase and superoxide dismutase activity, along with increased levels of nitric oxide content. Furthermore, in their study, miR-873 inhibition within GDM rats elicited a regulatory effect on insulin resistance and led to a marked reduction in myocardial apoptosis. Overall, their findings revealed that miR-873 silencing by activating PI3K/AKT/mTOR pathway exhibited a regulatory influence on insulin resistance and contributed to the mitigation of myocardial injury in GDM rats [91](Fig. 2).
Fig. 2.
Schematic representation of reciprocal relationship between PI3K/AKT signaling pathway and miRNAs in DM-related complications.
3. Reciprocal cross-talk between LncRNA and PI3K/AKT signaling pathway in DM
3.1. MEG3/PI3K/AKT
Maternally Expressed Gene 3 (MEG3) is positioned on the human chromosome 14q32.3, specifically situated within the DLK1-MEG3 genetic locus. He et al. aimed to elucidate the intrinsic mechanism involving the lncRNA MEG3 in conjunction with DNA methyltransferase 1 (DNMT1) concerning the process of endothelial-mesenchymal transition (endMT) in DR. Their preliminary observations underscored the diminished expression of MEG3 within retinal tissues extracted from rat models of DR and in microvascular endothelial cells from the rat retina cultured under HG conditions. They subsequently unveiled that the enhanced expression of MEG3 effectively impeded endMT, while the suppression of MEG3 expedited the endMT process in DR. This observation implies that the downregulation of MEG3 contributed to the proliferative and migratory behavior of retinal endothelial cells. Their ChIP coupled with RT-qPCR analysis confirmed that DNMT1 operated to decrease MEG3 expression by means of DNA methylation within the rat model of DR. Moreover, they noticed that MEG3 effectively mitigated endMT within cells exposed to HG conditions, achieved through the suppression of the PI3K/Akt/mTOR signaling pathway. In this manner, MEG3 suppression through DNA methylation in DR leads to the induction of endMT through the PI3K/Akt/mTOR pathway. These results underscore the potential therapeutic significance of targeting this pathway and employing strategies based on lncRNA methylation for the purpose of mitigating the progression of DR [92]. Importantly, Ye et al. investigated the involvement of MEG3 in fetal endothelial dysfunction provoked by GDM, along with its corresponding underlying mechanism. Through an array of experimental methodologies, encompassing the tube formation assay and qRT-PCR analysis, they determined that the upregulation of MEG3 resulted in heightened apoptosis, diminished viability, reduced tube formation, and restrained migration of HUVECs. As well, in their study, microRNA-370-3p mimic’s transfection led to a notable decrease in the expression of AFF1, while the augmentation of MEG3 expression resulted in a considerable increase in AFF1 protein levels. Therefore, MEG3 modulates AFF1 expression through an endogenous competitive mechanism. Furthermore, their Western blot analysis indicated a noteworthy inhibition of genes associated with the PI3K/AKT pathway upon the overexpression of MEG3. In this manner, MEG3 detrimentally affects fetal endothelial function by directly targeting microRNA-370-3p and AFF1, employing the PI3K/AKT pathway as a mechanism [93].
3.2. PVT1/PI3K/AKT
PVT1, a lncRNA transcribed from the human PVT1 gene, is situated within the widely recognized cancer-associated segment 8q24, also referred to as the 8q24 ′gene desert'. Chen et al. investigated the potential regulatory role of PVT1 in relation to the development and advancement of diabetic peripheral neuropathy (DPN), specifically focusing on its capacity to activate the PI3K/AKT pathway. Their findings demonstrated that PVT1 exerts a downregulatory influence on genes associated with neurodegeneration while concurrently upregulating genes linked to neurogenesis, as evidenced by changes in thermal withdrawal latency (TWL). Furthermore, in their study, notable upregulation of PVT1 led to a marked reduction in both mechanical withdrawal threshold (MWT) and TWL. As well, their western blot analyses yielded results indicating a significant suppression of the PI3K/AKT pathway within the dorsal root ganglia (DRG) of diabetic rats. Furthermore, PVT1 overexpression led to an inhibition of the PI3K/AKT pathway. Thereby, reduced expression of PVT1 within diabetic rats correlated with reduced mechanical withdrawal threshold, thermal withdrawal latency, and sensory nerve conduction velocity. Furthermore, they postulated that PVT1 confers protective effects against diabetic peripheral neuropathy through the activation of the PI3K/AKT pathway [94]. In addition, Wang et al. investigated both the expression pattern and functional implications of the lncRNA PVT1 within human trophoblast cells. Initially, they conducted qRT-PCR analyses to assess the expression levels of PVT1 in a spectrum of cancer cell lines, as well as in HTR8/SVneo cells, HUVEC cells, maternal placental tissue from patients with GDM, patients with preeclampsia (PE), and samples from normal pregnancies. In their study, the comparative expression level of PVT1 in HTR-8/Svneo cells exhibited compared to other cancer cell lines and HUVEC cells. Conversely, the expression was decreased in placentas of patients with GDM and PE as compared to normal placental tissues. Their findings further indicated that PVT1 silencing resulted in a substantial reduction in the proliferative, migratory, and invasive capacities of trophoblast cells. Conversely, apoptosis was significantly increased. Conversely, PVT1 upregulation yielded contrary outcomes. Subsequently, they detected a total of 105 differentially expressed genes (DEGs) subsequent to PVT1 silencing. Their enrichment analysis using GO revealed that these DEGs were intricately associated with the functional alterations of trophoblast cells. Notably, seven of these DEGs exhibited enrichment within the PI3K/AKT pathway. More importantly, they validated that PVT1 silencing distinctly reduced AKT phosphorylation and resulted in reduced expression of the identified DEGs (ITGB8, ITGAV, and GDPD3). Conversely, the elevation of PVT1 expression facilitated the phosphorylation of AKT and led to heightened expression of DEGs (ITGB8, ITGAV, and GDPD3). Therefore, aberrant regulation of PVT1 disrupts the proper functioning of trophoblast cells by perturbing the PI3K/AKT pathway [95].
3.3. SNHG16/PI3K/AKT
Positioned on the chromosomal locus 17q25.1, Small Nucleolar RNA Host Gene 16 (SNHG16) is alternatively designated as ncRAN (Non-Coding RNA Expressed in Aggressive Neuroblastoma). Cai et al. explored the impact of the SNHG16 on modulating the functionalities of human retinal microvascular endothelial cells (hRMECs) exposed to HG. Their findings revealed a notable increase in the expression of SNHG16 in hRMECs subjected to HG treatment. Moreover, SNHG16 was observed to promote the processes of hRMEC angiogenesis, migration, and proliferation. They further proposed that SNHG16 primarily induces dysfunction in hRMECs by activating the PI3K/AKT signaling pathway. Importantly, their study revealed that SNHG16 exerts its influence by sequestering miR-7-5p, functioning as a ceRNA in association with IRS1. In conclusion, our findings elucidated the prospective involvement of SNHG16 in promoting the impairment of hRMECs when subjected to high glucose (HG) conditions, thereby presenting an innovative avenue for the development of therapeutic strategies for DR [96].
3.4. LncRNA RPL13p5/PI3K/AKT
Li et al. explored whether the lncRNA RPL13P5 could potentially serve as a candidate lncRNA associated with GDM. In their study, lncRNA RPL13p5 establishes a co-expression relationship with the TSC2 gene through the modulation of the PI3K-Akt signaling cascade. As well, sequencing analysis of lncRNA RPL13P5 indicated a substantial level of expression, and the subsequent validation through qPCR exhibited conformity with the outcomes of the sequencing data. Furthermore, they identified a correlation between the expression levels of lncRNA RPL13P5 and various clinicopathological characteristics of GDM. Their conclusion posited that LncRNA RPL13P5 engages in a co-expression network with the TSC2 gene, involving the PI3K-Akt signaling pathway, thereby contributing to the insulin resistance within the context of GDM [97].
3.5. UCA1/PI3K/AKT
Initial isolation and characterization of lncRNA UCA1 were accomplished through its cloning and identification in the bladder cancer cell line BLZ-211. Positioned on the positive strand of human chromosome 19p13.12, this RNA entity comprises three exons and two introns. Notably, it contains several stop codons and lacks any conserved lengthy open reading frames (ORFs). Shi et al. explored the impact of the lncRNA UCA1 on glucose metabolism in a rat model of DN, while also delving into the underlying mechanisms governing its regulatory effects. In their study, the administration of an inhibitor targeting the lncRNA UCA1 yielded a notable amelioration in pathological lesions. Furthermore, this intervention led to a noteworthy reduction in serum concentrations of urinary protein (UP), serum creatinine (Scr), and blood urea nitrogen (BUN) within rats afflicted with DN. Through additional analysis using RT-PCR, they revealed a significant upregulation in the mRNA levels of both PI3K and Akt within renal tissue of the model group in contrast to the control group. However, a notable reduction in these parameters was observed within the group treated with the inhibitor targeting lncRNA UCA1, in comparison to model group. In addition, their Western blot analysis revealed a pronounced elevation in the protein abundances of Akt and PI3K within renal tissues of the model group compared to those observed in the control group. In contrast, within the lncRNA UCA1 inhibitor group, there was a noteworthy reduction in the protein expressions of PI3K and Akt in comparison to the model group. In this manner, lncRNA UCA1 by suppressing PI3K-Akt signaling pathway mitigate renal pathological impairments, enhance renal function, and mitigate the inflammatory response within DN rats [98].
3.6. HOTAIR/PI3K/AKT
The genomic location of human HOTAIR is localized within the genomic region of the HOXC locus on chromosome 12q13.13, positioned between the HOXC11 and HOXC12 loci. Qi et al. explored the contribution of the lncRNA HOTAIR in the etiology of DC. In their study, HOTAIR expression levels were notably diminished in both serum and myocardial tissues among individuals afflicted with DC, in contrast to patients diagnosed solely with diabetes and healthy controls. Nonetheless, no statistically significant variations in HOTAIR expression were observed between diabetic patients without cardiomyopathy and the healthy control group. Importantly, they observed that exposure to d-glucose resulted in a notable reduction in the expression of HOTAIR. Furthermore, the decrement in HOTAIR expression appeared to be contingent upon the dosage of d-glucose, with higher doses leading to a more pronounced decrease, suggestive of a dose-dependent relationship. As well, they disclosed that elevated concentrations of d-glucose elicited a decrement in the phosphorylation states of Akt. Moreover, an assessment of the impact of heightened HOTAIR expression on Akt phosphorylation within human cardiomyocytes revealed a propensity of HOTAIR upregulation to enhance the phosphorylation of Akt. In addition, they investigated the impact of HOTAIR upregulation on the viability of AC16 cells subjected to HG conditions and unveiled that HOTAIR by activating PI3K signaling pathway enhance AC16 cells viability. Thereby, their findings propose a potential beneficial role of lncRNA HOTAIR in ameliorating diabetic cardiomyopathy, potentially attributed to its capacity to enhance cardiomyocyte viability by activating PI3K/Akt pathway [99].
3.7. TUG1/PI3K/AKT
Taurine up-regulated 1 (TUG1) is a lncRNA sequence spanning 7598 nucleotides, situated within the genomic region of chromosome 22q12.2. It was initially recognized through a genomic screening of mouse retinal cells treated with taurine. Zang et al. explored the plausible role of lncRNA TUG1 in the advancement of DN and its associated underlying mechanism. Their investigation revealed a decrease in TUG1 expression within diabetic rats and in mesangial cells stimulated by HG levels. As well, in contrast to the control group, diabetic rats exhibited elevated levels of kidney weight, blood urea nitrogen, 24-h urine protein excretion, and serum creatinine levels. These parameters exhibited a substantial reduction following in vivo upregulation of TUG1. They subsequently revealed that the LV-TUG1 transfection resulted in a significant inhibition of the proliferation rate in mesangial cells that had been induced with HG levels, in comparison to the control group. Furthermore, their findings disclosed a significant reduction in protein expressions of p-AKT and p-PI3K within mesangial cells subjected to HG, upon the overexpression of TUG1. This observation indicates a consequential inhibition of the PI3K/AKT pathway. In this manner, TUG1 exerts its influence by impeding the PI3K/AKT pathway, thereby attenuating the proliferation of mesangial cells and mitigating the accumulation of ECM [100].
3.8. LINC00323/PI3K/AKT
Li et al. investigated the impact of LINC00323 on M1 macrophage polarization in the context of DN. In their study, the levels of inflammatory mediators (IL-6 and TNF-α) exhibited an elevation in individuals diagnosed with DN. Moreover, their investigation, employing PCR and western blot analysis, revealed a notable upregulation in the expression of the M1 marker protein CD86. Furthermore, it was revealed that the expression of LINC00323 is elevated in blood samples obtained from patients. Subsequently, a comprehensive investigation into the molecular underpinnings of LINC00323's role in M1 macrophage polarization was conducted, operating at the cellular molecular biology level. The outcomes of this exploration established a significant association between LINC00323 and the PI3K/AKT signaling pathway. Furthermore, through experimentation in animal models, they observed that suppression of LINC00323 expression resulted in a mitigation of DN-associated damage. Therefore, LINC00323 assumes a role as an intermediary in the polarization mechanism of M1 macrophages, primarily through its engagement with the PI3K/AKT signaling pathway. Furthermore, their conclusions underscore the substantial involvement of LINC00323 in the initiation and progression of DN [101].
3.9. HEIH/PI3K/AKT
Zhao et al. investigated into the regulatory interplay involving HEIH and miR-939, while also probing for a potential target relationship between miR-939 and VEGF within ARPE-19 cells. They initially revealed a marked elevation in HEIH expression within the Non-Proliferative Diabetic Retinopathy (NDR) and Proliferative Diabetic Retinopathy (DR) groups in comparison to HC group. As well, the expression of HEIH was notably elevated in the PDR group as compared to the NDR group. Additionally, their investigation revealed that HEIH upregulation markedly suppressed cell viability, instigated apoptosis, facilitated the release of cytochrome C from mitochondria to the cytoplasm, and heightened caspase-3 activity in ARPE-19 cells. Conversely, HEIH suppression yielded contrasting outcomes. Furthermore, their transfection assay provided evidence that the impacts of HEIH inhibition on cell viability, caspase-3 activity, cytochrome C translocation from mitochondria to the cytoplasm, and apoptosis were notably counteracted through the inhibition of miR-939. Moreover, they unveiled VEGF as a plausible target of miR-939. Thus, the modulation of HG-induced injury in ARPE-19 cells by HEIH occurs via the regulatory influence of the miR-939/VEGF axis. In addition, they revealed that exposure to HG led to heightened expression levels of p/t-PI3K and p/t-AKT. This outcome signified the PI3K/AKT signaling pathway activation due to HG stimulation, a trend that was counteracted upon HEIH inhibition. Overall, their investigation unveiled a potential implication of HEIH in the progression of DR, achieved through its role as a miR-939 sponge targeting VEGF expression, as well as its influence on the modulation of PI3K/AKT pathway activation. Collectively, the HEIH/miR-939/VEGF axis may offer a fresh and innovative avenue for the exploration of therapeutic strategies in the context of DR [102] (Fig. 3) (Table 1).
Fig. 3.
Schematic representation of reciprocal relationship between PI3K/AKT signaling pathway and lncRNAs in DM-related complications.
Table 1.
Reciprocal relationship between PI3K/AKT signaling pathway and ncRNAs in DM and its related complications.
| Non-coding RNAs | Name | Expression levels | Interaction with target molecules | Associated complication | Study samples | Effect on PI3K/Akt pathways | Refs. |
|---|---|---|---|---|---|---|---|
| MiRNAs | miR-27a | Upregulated | Directly target PPAR-γ | – | mouse model/3T3-L1 | Inhibit PI3K/Akt pathways | [54] |
| miR-351 | Downregulated | Directly target FLOT2 | GDM | GDM mice | Inhibit PI3K/Akt pathways | [56] | |
| miR-26b | Upregulated | Indirectly target PI3K/Akt signaling pathway | GDM | GDM mice | Inhibit PI3K/Akt pathways | [103] | |
| miR-125a-5p | Downregulated | Directly target STAT3 | – | C57BL/6 mice | Activate PI3K/Akt pathways | [60] | |
| miR-21 | Upregulated | Directly target PTEN | DR | DR models/RVECs | Activate PI3K/Akt pathways | [61] | |
| Downregulated | Indirectly regulated by PI3K/Akt | – | C57BL/6/Islet cell | PI3K/Akt pathways induce miR-21 | [62] | ||
| miR-130b | Downregulated | Directly target PTEN | DE | PC12/hippocampal cells/Male Wistar rats | Activate PI3K/Akt pathways | [64] | |
| miR-195 | Upregulated | Directly target Mfn2 | – | C57BL/6J mice/palmitate-stimulated Min6 cells | Inhibit PI3K/Akt pathways | [65] | |
| Upregulated | Directly target PFKFB4 | – | HCC cell lines/HCC tumor | Inhibit PI3K/Akt pathways | [104] | ||
| miR-7 | Downregulated | Directly target HOXB3 | DR | ARPE-19 cell line | Inhibit PI3K/Akt pathways | [67] | |
| Downregulated | Directly target IRS-1 | DR | diabetic rat model/ECs and RPs cells | Inhibit PI3K/Akt pathways | [68] | ||
| Downregulated | Directly target IRS-2 | DR | mice model of DR/ECs and RPs cells | Inhibit PI3K/Akt pathways | [105] | ||
| miR-363-3p | Downregulated | Indirectly target FOXO1 | insulin resistance | C57BL/6J mice/HepG2 cells | Activate PI3K/Akt pathways | [106] | |
| miR-19a | Upregulated | Directly target PTEN | DR | DR rat model | Inhibit PI3K/Akt pathways | [71] | |
| miR-122 | Upregulated | Indirectly target PI3K/Akt | – | INS-1 | Inhibit PI3K/Akt pathways | [107] | |
| miR-20b-5p | Upregulated | Directly target THBS1 | DR | DR model | Inhibit PI3K/Akt pathways | [108] | |
| miR-30c | Downregulated | Directly target ROCK2 | DN | Human DN tissue/HK-2 cells | Inhibit PI3K/Akt pathways | [74] | |
| miR-199a-3p | Downregulated | Directly target FGF7 | DR | DR rat model//ECs and RPs cells | Inhibit PI3K/Akt pathways | [75] | |
| Downregulated | Directly target VEGF | DR | hRMECs and ARPE-19 cell | Inhibit PI3K/Akt pathways | [76] | ||
| Downregulated | Indirectly target PI3K/Akt | – | Peripheral blood samples/HUVECs | Activate PI3K/Akt pathways | [77] | ||
| miR-720 | Upregulated | Directly target Rab35 | – | MIN6 cells/ | Activate PI3K/Akt pathways | [78] | |
| miR-139-5p | Upregulated | Directly target PICK1 | – | Min6 cells/db/db mice | Inhibit PI3K/Akt pathways | [79] | |
| miR-24 | Downregulated | Indirectly target PI3K/Akt | CAD | diabetic rats/VSMCs | Inhibit PI3K/Akt pathways | [80] | |
| miR-183 | Upregulated | Directly target BTG1 | DR | DR rat model | Activate PI3K/Akt pathways | [81] | |
| miR-203 | Downregulated | Directly target PIK3CA | DCM | DCM mouse model | Inhibit PI3K/Akt pathways | [82] | |
| miR-153 | Downregulated | Directly target PAQR3 | DN | MCs/renal tissue from DN | Inhibit PI3K/Akt pathways | [84] | |
| miR-23b | Downregulated | Directly target HMGA2 | DN | db/db mice/HK-2 cells | Inhibit PI3K/Akt pathways | [85] | |
| miR-126 | Downregulated | Directly target IRS1 | DR | DR rat model/ECs and RPs cells | Inhibit PI3K/Akt pathways | [86] | |
| Downregulated | Indirectly target PI3K/Akt | – | Min6 cells | Activate PI3K/Akt pathways | [87] | ||
| Downregulated | Directly target VEGFA, and PK3R2 | DR | RF/6A cells | Inhibit PI3K/Akt pathways | [88] | ||
| miR-499-5p | Downregulated | Directly target PTEN | insulin resistance | db/db mice/NCTC1469 cells | Activate PI3K/Akt pathways | [90] | |
| miR-873 | Upregulated | Directly target IGFBP2 | myocardial injury/insulin resistance | GDM rat model | Inhibit PI3K/Akt pathways | [91] | |
| lncRNAs | MEG3 | Downregulated | Indirectly target PI3K/Akt | DR | DR Rat Models/Microvascular endothelial cells from rat retina | Inhibit PI3K/Akt pathways | [92] |
| Upregulated | Directly target miR-370-3p | GDM | HUVEC cell | Inhibit PI3K/Akt pathways | [109] | ||
| PVT1 | Downregulated | Indirectly target PI3K/Akt | DPN | DPN Rat Models | Activate PI3K/Akt pathways | [110] | |
| Upregulated | Indirectly target PI3K/Akt | GDM | HTR-8/SVneo cell/trophoblast cells | Activate PI3K/Akt pathways | [95] | ||
| SNHG16 | Upregulated | Directly target miR-146a, and miR-7-5p | DR | hRMECs | Activate PI3K/Akt pathways | [96] | |
| RPL13p5 | Upregulated | Indirectly target PI3K/Akt | GDM | peripheral blood | Activate PI3K/Akt pathways | [111] | |
| UCA1 | Upregulated | Indirectly target PI3K/Akt | DN | DN rat model | Activate PI3K/Akt pathways | [112] | |
| HOTAIR | Downregulated | Indirectly target PI3K/Akt | DCM | Myocardial biopsy/AC16 cell | Activate PI3K/Akt pathways | [99] | |
| TUG1 | Downregulated | Indirectly target PI3K/Akt | DN | DM rats/Mouse-derived mesangial cells | Inhibit PI3K/Akt pathways | [113] | |
| LINC00323 | Upregulated | Indirectly target PI3K/Akt | DN | blood samples of DN patients | Activate PI3K/Akt pathways | [101] | |
| HEIH | Upregulated | Directly target miR-939 | DR | ARPE-19/DR patients | Activate PI3K/Akt pathways | [102] |
4. Diabetes mellitus therapy: from herbal medicine to RNA-based therapy
4.1. Herbal medicine therapy
The primary objective of managing T2DM is the prevention or postponement of associated complications. A pivotal factor for individuals afflicted with diabetes lies in the maintenance of blood glucose concentrations within the established normative range. Currently, the management of T2DM primarily relies on lifestyle modifications encompassing nutritional therapy and physical activity, alongside pharmacological approaches involving oral agents like α-glucoside inhibitors, sulfonylureas, and biguanides, as well as injectable substances such as insulin and agonists of glucagon-like peptide-1 amide (GLP-1). Surgical interventions also constitute a component of the therapeutic treatment. Unfortunately, despite the expansion of our knowledge base, there has been a relatively modest advancement in the domain of therapies and treatments for DM. To address this, conventional herbal medicine and biologically active natural compounds are being employed either as alternative therapeutic options or in a supplementary capacity to enhance the efficacy of established treatments. As well, bioactive constituents present in certain herbal medicines have demonstrated an ability to selectively interact with diverse ncRNAs, particularly miRNAs, lncRNAs, and circRNAs. These ncRNAs have recently garnered attention as novel therapeutic focal points across a spectrum of diseases. Through the modulation of regulatory pathways encompassing anti-atherosclerotic, anti-inflammatory, pro-apoptotic, anti-infectious, and structural remodeling suppression mechanisms, these constituents elicit safeguarding effects within the domains of cancer and DM. Beside, a recent empirical investigation has elucidated that herbs and natural compounds could exert either direct or indirect effect upon the PI3K/AKT signaling cascade. Therefore, in the current work, our focus was directed towards an in-depth analysis of both comprehensive reviews and primary research articles that explore the potential therapeutic bioactive natural compounds and traditional herbal remedies for the management of DM. This exploration centers on their capacity to regulate the PI3K/AKT pathway through interactions with ncRNA mechanisms.
4.1.1. Treatment via Rg1
Ginsenoside Rg1 (Rg1), a biologically active constituent derived from Panax ginseng, has been substantiated as conferring protective attributes against oxidative stress, inflammatory processes, cerebral ischemia, diabetes, hepatic functionality impairment, and Alzheimer’s disease. Huang et al. explored the impact of Rg1 on the treatment of DFUs and the associated underlying mechanism. Their initial observations revealed that Rg1 facilitated cellular proliferation, angiogenesis, and migration, while concurrently mitigating cellular apoptosis within high-glucose-induced HUVECs. As well, they revealed that miR-489–3p silencing ameliorated the detrimental effects induced by HG on HUVECs, whereas the upregulation of miR-489–3p undermined the protective influences exerted by Rg1. By means of functional rescue experimentation coupled with western blot analysis, they demonstrated that miR-489–3p upregulation, predominantly achieved through the deactivation of the PI3K/AKT/eNOS signaling cascade and the suppression of NO production, exacerbated the impairment inflicted upon HUVECs by HG. Furthermore, this perturbation even counteracted the cellular protective effects conferred by Rg1. They subsequently revealed that miR-489–3p directly target Sirt1, and upregulation of Sirt1 expression facilitated the process of wound healing within the context of DFUs. Importantly, their animal experimentation disclosed that Rg1 facilitated the progression of wound closure through the modulation of the miR-489–3p/Sirt1 axis. In conclusion, Rg1 ameliorated the condition of DFUs by upregulating Sirt1 expression through the suppression of miR-489–3p, consequently stimulating PI3K/AKT/eNOS signaling pathway activation [114].
4.1.2. Treatment via JTXK granule
JTXK granule constitutes a formulation of a pure Chinese medicinal granules, comprising Rehmannia (DiHuang), Pueraria (Gegen), Fructuscorni (ShanYuRou), Ginseng (RenShen), Radix salviae miltiorrhizae (DanShen), and an additional of five distinct Chinese herbs, all meticulously combined according to specified proportional ratios. Mo et al. explored the impact of JTXK granule on the miRNA expression profile within the pancreatic tissue of KKAy diabetic mice. Their objective was to elucidate both the molecular mechanism and pathways underlying the anti-diabetic efficacy of JTXK granule. Initially, they identified a collective of 45 miRNAs displaying notable disparities between the model group and the cohort treated with JTXK. Furthermore, their miRNA-mRNA investigation illustrated the intimate correlation between the distinctive expression patterns of and mmu-miR-378a-3p, mmu-miR-139-5p, mmu-miR-320-3p, mmu-miR-291a-3p, and mmu-miR-192-5p, with pancreatic histological changes. Moreover, they via pathway analysis disclosed a substantive association between the differentially expressed miRNAs (DEMs) and the PI3K-Akt Signaling Pathway. Additionally, within their investigation, the levels of p-Foxo1 and p-Akt within the INS-1-FOXO1 overexpressing model cells were observed to be lower than those within the control group. Notably, the administration of JTXK granules exhibited the capacity to elevate the expression of p-Foxo1, p-Akt, and Akt. In this manner, JTXK granule could exert an anti-diabetic effect by modulating the expression of both mRNA and miRNAs implicated in the PI3K-Akt pathway within the pancreatic tissue of diabetic mice [115].
4.1.3. Treatment via MFA
Methyl ferulic acid (MFA), a bioactive constituent found within the rhizomes of Securidaca inappendiculata Hassk, demonstrates a diverse array of pharmacological properties. Zhang et al. explored the impact of MFA on insulin sensitivity within ethanol-induced L-02 cells and alcohol-fed mice, and elucidate the role of the miR-378b-mediated PI3K/AKT pathway within this system. In their study, their findings indicated that MFA effectively reduced the heightened expression of miR-378b in alcohol-fed mice and L-02 cells exposed to ethanol. Furthermore, this intervention concurrently restored the expression levels of IR and p110α, both of which are direct targets of miR-378b. Their subsequent investigations conducted both in vivo and in vitro revealed that MFA significantly mitigated the exacerbation of hepatic insulin resistance resulting from the upregulation of miR-378b. Furthermore, miR-378b silencing abolished the favorable impacts of MFA on insulin sensitivity. These findings confirmed the favorable influence of MFA in the context of ALD and elucidate its capacity to modulate the miR-378b-mediated PI3K-AKT pathway, thereby improving hepatic insulin resistance. This proposed mechanism underscores the potential therapeutic efficacy of MFA [116].
4.1.4. Treatment via AST
Astragaloside IV (AST), a prominent constituent derived from Astragalus membranaceus, has demonstrated efficacy in managing DPN. Yin et al. investigated the impact of AST on injury to myelin Schwann cells in the context of DPN and, notably, to explore the mechanistic underpinnings of AST’s therapeutic potential in DPN treatment, marking a novel endeavor in this area of research. Initially, they unveiled that AST led to a reduction in blood glucose levels, ameliorated damage to the peripheral nerve myelin sheath, and enhanced neurological function in rats afflicted with DPN. As well in their study, AST increased autophagic activity and mitigated apoptosis within RSC96 cells. Their mechanistic investigation revealed that AST facilitated autophagy through the regulation of miR-155-mediated PI3K/Akt/mTOR signaling pathways. Furthermore, AST decreased apoptosis in RSC96 cells by enhancing autophagic processes. Thereby, AST by increasing miR-155 expression, and subsequently inhibition of the PI3K/Akt/mTOR signaling pathway ameliorates myelin sheath injury in DPN [117].
4.1.5. Treatment via GA
Plant-derived foods encompass a variety of hydroxybenzoic acid derivatives, encompassing compounds such as vanillic acids, p-hydroxybenzoic, protocatechuic, syringic, and Gallic. Typically, these derivatives manifest in a bound form within foods, often constituting integral elements of complex structures like hydrolyzable tannins and lignins, or being attached to cell walls and proteins. Lee et al. conducted an assessment to examine the potential anti-diabetic effects of hydroxybenzoic acid derivatives and investigated the role played by miR-1271 in this context. Their study revealed that gallic acid (GA), classified as a derivative of hydroxybenzoic acid, effectively ameliorated hepatic IR induced by free fatty acids (FFAs). This was evidenced by enhanced glucose consumption and a reduction in reactive oxygen species levels. As well, in their study, GA suppressed the elevation of miR-1271 induced by FFAs, concurrently enhancing the expression of its target molecules, including p-PI3K, p-FOXO1, p-AKT, and p-IRS. This modulation was accompanied by the regulation of genes involved in glucose metabolism. They subsequently provided further validation of the participation of miR-1271 in the defensive role of GA against IR, substantiated through the utilization of both miR-1271 mimic and miR-1271 inhibitor interventions. In this manner, GA mitigates IR through modulation of the miR-1271/IRS/PI3K/AKT/FOXO1 pathway, potentially rendering it a viable candidate for the treatment of IR [118].
4.1.6. Treatment via Nar
Naringenin, a flavonoid categorized within the subclass of flavanones, is ubiquitously present in various Citrus fruits, bergamot, tomatoes, and other fruit varieties. Additionally, it exists in its glycosidic form, predominantly as naringin. Diverse biological functions have been linked to this phytochemical, encompassing antiadipogenic, anti-inflammatory, antioxidant, antiviral, antitumor, antibacterial, and cardioprotective properties. Li et al. explored the safeguarding potential of naringenin (Nar) against injury resulting from myocardial ischemia reperfusion (MI/R), while also examining the involvement of the miR-126-PI3K/AKT axis in this context. Diabetic rats were subjected to oral administration of either distilled water or naringenin (25 or 50 mg/kg) for a duration of 30 days, followed by induction of MI/R. Their findings demonstrated the ameliorative impact of Nar on MI/R injury in diabetic rats induced by streptozotocin (STZ). This effect was evidenced through quantification of myocardial enzyme levels via spectrophotometric analysis, determination of enhanced cardiac viability using the MTT assay, measurement of suppressed myocardial oxidative stress through spectrophotometric assessment, and the augmentation of cardiac function monitored via a hemodynamic monitoring system. Furthermore, their investigation unveiled that Nar increased myocardial miR-126-PI3K/AKT axis within D-MI/R rats. So, Nar mitigated MI/R-induced injury by enhancing the myocardial miR-126-PI3K/AKT axis in STZ-induced diabetic rats. Their conclusion suggests that Nar could offer a viable strategy for addressing diabetic ischemic heart disease (IHD) [119].
4.1.7. Treatment via Triptolide
Tripterygium wilfordii Hook F (TwHF), an indigenous Chinese herbal plant, has been employed in clinical practice for the management of diabetic kidney disease (DKD) over a span of years, attributed to its immunosuppressive and anti-inflammatory characteristics. Triptolide, a bioactive compound derived from the root of Tripterygium wilfordii Hook F (TwHF), serves as a pharmacologically significant component. Xue et al. explored whether triptolide enhances the EMT in both rats exhibiting DKD and human proximal tubular epithelial (HK-2) cells. Their observations revealed that triptolide led to a decline in albuminuria, improved the renal structure, and diminished renal EMT within rats afflicted with DKD. As well, in their study, PI3K/AKT signaling pathway activation was observed in diabetic rats, a phenomenon that was partially ameliorated through the administration of triptolide. Furthermore, their investigation revealed that triptolide attenuated the elevated expression of miR-188-5p induced by HG levels in HK-2 cells. This modulation was attributed to the direct interaction of miR-188-5p with the 3′-UTR of the PTEN gene, resulting in the inhibition of PTEN expression. Significantly, their findings revealed that the reduction in miR-188-5p expression, mirroring the impact of triptolide, mitigated PI3K/AKT pathway activation as well as HG-induced EMT. Conversely, overexpression of miR-188-5p counteracted the effects of triptolide on the PI3K/AKT pathway and EMT. Thereby, triptolide improves renal EMT by modulating the PI3K/AKT signaling pathway, facilitated by the interplay between miR-188-5p and PTEN. This highlights the potential for miR-188-5p to be a target of triptolide in the treatment of DKD [120].
4.1.8. Treatment via Shengjie Tongyu decoction
Shengjie Tongyu decoction (SJTYD) is a traditional Chinese herbal formulation known for its multifaceted biological characteristics, exhibiting potent anti-inflammatory and antioxidant properties. In this regard, a recent investigation delved into the therapeutic potential of SJTYD concerning Diabetic Cardiomyopathy (DCM). Computational investigation revealed that SJTYD had a notable effect on lncRNA H19, the mTOR pathway, and effectively ameliorated cardiac dysfunction parameters observed in Diabetic Cardiomyopathy (DCM). Further experimental investigation demonstrated that SJTYD exhibited the capacity to inhibit myocardial injury areas, reduce both the quantity of autophagosomes, and modulate the expression levels of proteins associated with autophagy. In this manner, SJTYD shows potential in safeguarding against diabetic myocardial injury by suppressing cardiomyocyte autophagy via activating lncRNA H19, regulating ROS, and modulating the PI3K/Akt/mTOR signaling pathway [121].
4.1.9. Treatment via Nutt
Pharmacological investigations have indicated that the alcoholic extract derived from Coreopsis tinctoria Nutt exhibits properties encompassing hypoglycemic, antihyperlipidemic, and antihypertensive effects, along with antifibrotic and anti-inflammatory attributes within the context of DN. Yu et al. explored the impacts of the alcoholic extract of Coreopsis tinctoria Nutt (AC) on mice with DN. They divided a cohort of 30 db/db mice with DN into three distinct groups, each receiving treatment via oral gavage: AC at a dosage of 300 mg/kg/day, metformin at a dosage of 180 mg/kg/day, or a saline control, spanning a period of 10 weeks. Upon the administration of AC, noteworthy reductions were observed in HbA1c levels, 24 h UAE, 24 h UV, FBG levels, and the K/W among mice with DN. Furthermore, substantial improvements were noted in renal structural abnormalities and fibrotic conditions, signifying a pronounced kidney-protective influence of AC in DN-afflicted mice. Their findings indicated that AC intervention led to a decrease in the expression of miR-200b and miR-192, an elevation in ZEB2 expression, and a reduction in the expression of COL4 α1. So, the potential mechanism through which AC confers protection against DN could involve the modulation of miRNA expression levels. Additionally, their further experimentation via western blotting analysis implies that the anti-renal fibrosis efficacy of AC might be associated with an elevated expression of ZEB2 and the suppression of PTEN/PI3K/AKT signaling pathway activation. In this manner, the potential renal protective effect of AC in DN mice might stem from its capacity to reduce the levels of miR-200b and miR-192. These miRNAs, in turn, could exert regulatory influence over the expression of their respective target genes and subsequently regulate the activity of the PTEN/PI3K/AKT pathway, thereby contributing to the attenuation of renal fibrosis [122] (Fig. 4).
Fig. 4.
Herbal medicine relieve DM-related complications through regulating miRNAs-mediated PI3K/Akt signaling pathway.
4.2. RNA-based therapy
Research investigations have showcased that miRNAs actively participate in nearly all physiological processes, thereby establishing a correlation between dysregulated miRNAs and numerous human pathologies [123]. Hence, there is significant demand for therapeutic approaches involving miRNA mimics and anti-miRNAs, aiming to either reinstate or reduce the expression of miRNAs, representing crucial strategies for precisely modulating miRNA levels in therapeutic. Therefore, therapeutic strategies involving miRNA mimics to reinstate miRNA expression or anti-miRNAs to reduce aberrantly expressed miRNAs are greatly desired for precisely controlling miRNA levels in effective therapeutic interventions [124]. In particular, non-viral, polymer-based carriers have shown advantages such as versatility of structural modifications and protection of unstable miRNA [125]. Research has focused on three primary categories of polymeric nanocarriers for miRNA transport, encompassing polyplexes, polylactic-co-glycolic acid (PLGA) nanoparticles, and dendrimers. The integration of miRNA into delivery systems can be achieved through complexation (involving electrostatic interactions), conjugation (utilizing covalent linkers), or encapsulation methods. These nanotechnological constructs shield synthetic miRNAs from nuclease degradation, enhancing their stability and enabling precise dosage delivery to attain the intended clinical impact within target cells [126]. Despite the promising long-term prospects and notable strides in clinical translation achieved through RNA-targeted interventions, significant challenges persist, particularly in the context of recent technological innovations. These challenges pertain to the critical aspects of clinical safety and efficacy, necessitating comprehensive scrutiny and discourse. Furthermore, genome editing in the human context also elicits ethical considerations that warrant thorough evaluation. So, the fundamental shared factors that serve as critical benchmarks for assessing the clinical effectiveness and safety of these emerging technologies encompass: (i) absence of pertinent adverse effects, (ii) robust specificity of the therapeutic agent toward its molecular target, (iii) proficient delivery to the intended organ, and (iv) requisite stability tailored to the specific clinical intent. Additionally, it is noteworthy that numerous animal studies predominantly involve young and generally healthy subjects, thereby often diverging from the complexities encountered in clinical reality. In addition, it is noteworthy to underscore that RNA-based therapeutic approaches possess the capacity to markedly enhance clinical outcomes for individuals afflicted with DM. The benefits associated with RNA-based therapeutic interventions encompass their capability to achieve a highly discerning reduction in the concentration of nearly any targeted protein. This facilitates a more intricate and accurate regulation of metabolic pathways compared to the often limited scope achievable through conventional small molecule-based pharmaceutical agents. RNA-based therapeutic modalities additionally enable the attenuation of target protein expression even in cases where conventional small molecule inhibitors are unavailable. RNA-based therapeutic approaches also offer the potential to alleviate the burden of medication consumption, given their dosing regimen commonly spans from weekly administrations to twice yearly injections. Beside, in light of the plausible involvement of PI3K in DM, recent scholarly investigations have focused on therapeutic agents aimed at targeting this signaling pathway. An increasing number of ncRNAs associated with the PI3K/AKT pathway has been identified as promising biomarkers for the management of DM. In this regard, Wang et al. explored the potential involvement of exosomes derived from hypoxic adipose stem cells (HypADSCs-exo) in adapting to hypoxia conditions and expediting the healing process of diabetic wounds. Through transwell assay, they detected a substantial enhancement in fibroblast proliferation induced by HypADSCs-exo. Moreover, their investigations revealed that HypADSCs-exo regulated the synthesis of extracellular proteins and chemokines within fibroblasts, thereby exerting potential impacts on angiogenesis. They subsequently unveiled that the proliferation and migratory behavior of fibroblasts, under the influence of HypADSCs-exo, could potentially be contingent upon the PI3K/AKT signaling pathway. Their discovery further unveiled the capacity of HypADSCs-exo to modulate inflammatory factors, chemokines, and the formation of the extracellular matrix in diabetic mice. In conclusion, HypADSCs-exo facilitates fibroblast proliferation and migratory behavior through the activation of the PI3K/AKT pathway, consequently accelerating diabetic wound healing [127]. In addition, Li et al. investigated an effective approach to facilitate the healing of chronic wounds in a rat model of diabetes. In their study, exosomes originating from synovial mesenchymal stem cells (SMSCs) exhibiting overexpression of miR-126-3p (SMSCs-126-Exos), characterized by a particle size of 85 nm, were embedded within hydroxyapatite/chitosan (HAP-CS) composite hydrogels (HAP–CS–SMSCs-126-Exos) to serve as wound dressings. Their transwell assays demonstrated a substantial promotion in the migratory capability of human dermal microvascular endothelial cells (HMEC-1) induced by SMSCs-126-Exos nanoparticles, compare to the HAP-CS, SMSCs-Exos, and control groups. Furthermore, they revealed that HMEC-1 cells incubated with control medium, HAP-CS, and SMSCs-Exos exhibited sparse or incomplete tube network formation. Conversely, the inclusion of SMSCs-126-Exos notably augmented the proficiency of HMEC-1 cells in generating capillary networks. As well, their western blot analysis demonstrated a pronounced activation of AKT and ERK1/2 signaling pathways by SMSCs-126-Exos. As a result, HAP–CS–SMSCs-126-Exos holds considerable promise for the advancement of therapeutic strategies in the context of diabetic chronic wounds healing [128]. In addition, Tao et al. investigated the competence of extracellular vesicles as transporters of LncRNA to neutralize the regeneration-inhibiting effect of hyperglycemia and thus constitute a potential treatment strategy for diabetic wounds. In an effort to optimize the generation of vesicles for effective delivery of LncRNA-H19, their investigation exploited a high-capacity extracellular vesicle-mimetic nanovesicles (EMNVs), exhibiting a production rate that surpasses the spontaneous release of extracellular vesicles from cells by more than a hundredfold. Their findings revealed that H19 was effectively transported into HMEC-1 cells via H19EMNVs, thereby mitigating the deficiency of H19 induced by hyperglycemia. Subsequent experimental investigations confirmed that hyperglycemia led to compromised Akt activation, confirmed that the fundamental mechanism responsible for this phenomenon involves the inhibition of LncRNA-H19. Conversely, H19EMNVs introduction successfully reinstated the functional activity of Akt. As well, their discovery also demonstrated that H19EMNVs exhibited a robust capacity to counteract the regenerative impediments induced by hyperglycemia, leading to a notable acceleration in the restorative progression of chronic wound healing. Thereby, bioengineered EMNVs hold significant potential as a potent tool for proficiently conveying LncRNA, thus positioning them as an exceedingly promising multifunctional platform for drug delivery in the foreseeable future [129] (Fig. 5).
Fig. 5.
A schematic representation of RNA-base therapy in DM.
5. Conclusion
T2DM and its comorbidities have reached epidemic proportions. The prevalence and incidence of T2D, representing >90% of all cases of diabetes, are increasing rapidly throughout the world. PI3K/AKT pathway is one of the most important intracellular pathways involved in glucose homeostasis, lipid metabolism, protein synthesis and cell proliferation and survival. Recent investigation disclosed that PI3K play a crucial role in the development of diabetes and diabetes-related complications, either on the level of tissue inflammation or in the regulation of energy homeostasis. Therefore, PI3K/AKT pathway is a prospective biological target for treating diabetes. In the current work we demonstrated that ncRNAs such as miR-499, miR-126, miR-23b, miR-153-3p, PVT1, SNHG16, UCA1, and so forth could interplay with component of PI3K/AKT pathway, and thereby contribute to diabetes pathogenesis. Several ongoing experimental studies are indicating the effectiveness of using therapeutic techniques that focus on ncRNAs-PI3K/AKT regulatory network as a potent strategy to combat diabetes. In this regard, natural products like Rg1, JTXK, MFA, Triptolide, and so forth through ncRNAs have shown significant potential in regulating PI3K/AKT pathway which can lead to manage diabetes mellitus and its complications. In addition, numerous ongoing experimental research are demonstrating the efficacy of therapeutic strategies based on ncRNAs as powerful therapeutic agents against T2DM and related complications. Here we demonstrated that ncRNA-based exosome by modulating PI3K/AKT pathway could be used as a therapeutic options for diabetic complications. The recognition that certain miRNAs possess multiple targets could offer a strategic advantage in the formulation of therapeutic approaches, given the potential to concurrently modulate the expression of multiple genes. Yet, the induction of off-target effects should also be taken into account as a plausible adverse outcome of these therapeutic interventions. Such therapies for the treatment of DM or its associated complications is currently in its nascent stages. Subsequent research ought to evaluate the efficacy, bioavailability, and long-term effects of these approaches in animal models, thereby expediting the translation of these findings into practical clinical implementations.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to publish this manuscript.
Funding
Not applicable.
Author’s contributions
SMAZ collected and analyzed the literature, drafted the figures and wrote the paper; SMAZ, conceived and gave the final approval of the submitted version. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
CRediT authorship contribution statement
Seyed Mohsen Aghaei-Zarch: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that there is no competing interests.
Acknowledgements
Not applicable.
References
- 1.Mirzavandi F., Babaie S., Rahimpour S., Razmpoosh E., Talenezhad N., Zarch S.M.A., et al. The effect of high dose of intramuscular vitamin D supplement injections on depression in patients with type 2 diabetes and vitamin D deficiency: a randomized controlled clinical trial. Obes. Med. 2020;17 [Google Scholar]
- 2.Mousavinasab F., Karimi R., Taheri S., Ahmadvand F., Sanaaee S., Najafi S., et al. Microbiome modulation in inflammatory diseases: progress to microbiome genetic engineering. Cancer Cell Int. 2023;23(1):271. doi: 10.1186/s12935-023-03095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dehghan M., Ghorbani F., Najafi S., Ravaei N., Karimian M., Kalhor K., et al. Progress toward molecular therapy for diabetes mellitus: a focus on targeting inflammatory factors. Diabetes Res. Clin. Pract. 2022;189 doi: 10.1016/j.diabres.2022.109945. [DOI] [PubMed] [Google Scholar]
- 4.Rathinaswamy M.K., Burke J.E. Class I phosphoinositide 3-kinase (PI3K) regulatory subunits and their roles in signaling and disease. Adv. Biol. Regul. 2020;75 doi: 10.1016/j.jbior.2019.100657. [DOI] [PubMed] [Google Scholar]
- 5.Wang B., Zhong Y., Li Q., Cui L., Huang G. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy. Aging (Albany NY) 2018;10(10):2772. doi: 10.18632/aging.101586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camaya I., Donnelly S., O’Brien B. Targeting the PI3K/Akt signaling pathway in pancreatic β‐cells to enhance their survival and function: an emerging therapeutic strategy for type 1 diabetes. J. Diabetes. 2022;14(4):247–260. doi: 10.1111/1753-0407.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan H., Singh A., Thapa K., Garg N., Grewal A.K., Singh T.G. Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res. 2021;1761 doi: 10.1016/j.brainres.2021.147399. [DOI] [PubMed] [Google Scholar]
- 8.Wu W., Xia X., Tang L., Luo J., Xiong S., Ma G., et al. Phosphoinositide 3-kinase as a therapeutic target in angiogenic disease. Exp. Eye Res. 2023 doi: 10.1016/j.exer.2023.109646. [DOI] [PubMed] [Google Scholar]
- 9.Heng E.Y.Z., Maffucci T. 2022. An Overview of Class II Phosphoinositide 3-kinases. PI3K and AKT Isoforms in Immunity: Mechanisms and Therapeutic Opportunities; pp. 51–68. [DOI] [PubMed] [Google Scholar]
- 10.Molinaro A. 2020. Investigating the Role of Class-1 Phosphoinositide 3 Kinases (PI3Ks) in Insulin Signaling and Obesity. [Google Scholar]
- 11.Yoshioka K. Class II phosphatidylinositol 3-kinase isoforms in vesicular trafficking. Biochem. Soc. Trans. 2021;49(2):893–901. doi: 10.1042/BST20200835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida M., Harari P., Wheeler D., Toulany M. Targeting AKT/PKB to improve treatment outcomes for solid tumors. Mutat. Res. Fund Mol. Mech. Mutagen. 2020;819 doi: 10.1016/j.mrfmmm.2020.111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calleja V., Laguerre M., Parker P.J., Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7(1) doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risso G., Blaustein M., Pozzi B., Mammi P., Srebrow A. Akt/PKB: one kinase, many modifications. Biochem. J. 2015;468(2):203–214. doi: 10.1042/BJ20150041. [DOI] [PubMed] [Google Scholar]
- 15.Glaviano A., Foo A.S., Lam H.Y., Yap K.C., Jacot W., Jones R.H., et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer. 2023;22(1):138. doi: 10.1186/s12943-023-01827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Chen J., Chen H., Duan Z., Xu Q., Wei M., et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J. Biosci. 2012;37:91–101. doi: 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- 17.Long H.-Z., Cheng Y., Zhou Z.-W., Luo H.-Y., Wen D.-D., Gao L.-C. PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.648636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Liu X., Han L., Gao X., Liu E., Wang T. Regulation of lipid and glucose homeostasis by mango tree leaf extract is mediated by AMPK and PI3K/AKT signaling pathways. Food Chem. 2013;141(3):2896–2905. doi: 10.1016/j.foodchem.2013.05.121. [DOI] [PubMed] [Google Scholar]
- 19.Gabbouj S., Ryhänen S., Marttinen M., Wittrahm R., Takalo M., Kemppainen S., et al. Altered insulin signaling in Alzheimer’s disease brain–special emphasis on PI3K-Akt pathway. Front. Neurosci. 2019;13:629. doi: 10.3389/fnins.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnuson B., Ekim B., Fingar D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012;441(1):1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 21.Varma S., Lal B.K., Zheng R., Breslin J.W., Saito S., Pappas P.J., et al. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005;289(4):H1744–H1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin X., Xu Z., Zhang Z., Li L., Pan Q., Zheng F., et al. Association of PI3K/AKT/mTOR pathway genetic variants with type 2 diabetes mellitus in Chinese. Diabetes Res. Clin. Pract. 2017;128:127–135. doi: 10.1016/j.diabres.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Dillmann W.H. Diabetic cardiomyopathy: what is it and can it Be Fixed? Circ. Res. 2019;124(8):1160–1162. doi: 10.1161/CIRCRESAHA.118.314665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borghetti G., Von Lewinski D., Eaton D.M., Sourij H., Houser S.R., Wallner M. Diabetic cardiomyopathy: current and future therapies. Beyond glycemic control. Front. Physiol. 2018;9:1514. doi: 10.3389/fphys.2018.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q., Xu T., Li D., Pan D., Wu P., Luo Y., et al. JNK/PI3K/Akt signaling pathway is involved in myocardial ischemia/reperfusion injury in diabetic rats: effects of salvianolic acid A intervention. Am. J. Tourism Res. 2016;8(6):2534. [PMC free article] [PubMed] [Google Scholar]
- 26.Lin R.Z. Phosphoinositide 3-kinases and diabetic cardiomyopathy. J. Cardiovasc. Pharmacol. 2017;70(6):420–421. doi: 10.1097/FJC.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 27.Prakoso D., De Blasio M.J., Tate M., Kiriazis H., Donner D.G., Qian H., et al. Gene therapy targeting cardiac phosphoinositide 3-kinase (p110α) attenuates cardiac remodeling in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2020;318(4):H840–H852. doi: 10.1152/ajpheart.00632.2019. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z., Huang A., Yan J., Liu B., Liu Q., Zhang J., et al. Resveratrol ameliorates cardiac dysfunction by inhibiting apoptosis via the PI3K/Akt/FoxO3a pathway in a rat model of diabetic cardiomyopathy. J. Cardiovasc. Pharmacol. 2017;70(3):184–193. doi: 10.1097/FJC.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 29.Feldman E.L., Callaghan B.C., Pop-Busui R., Zochodne D.W., Wright D.E., Bennett D.L., et al. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019;5(1):41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M., Shi X., Luo M., Lan Q., Ullah H., Zhang C., et al. Taurine ameliorates axonal damage in sciatic nerve of diabetic rats and high glucose exposed DRG neuron by PI3K/Akt/mTOR-dependent pathway. Amino Acids. 2021;53:395–406. doi: 10.1007/s00726-021-02957-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen S.-P., Zhou Y.-Q., Liu D.-Q., Zhang W., Manyande A., Guan X.-H., et al. PI3K/Akt pathway: a potential therapeutic target for chronic pain. Curr. Pharmaceut. Des. 2017;23(12):1860–1868. doi: 10.2174/1381612823666170210150147. [DOI] [PubMed] [Google Scholar]
- 32.Liu K., Yang Y., Zhou F., Xiao Y., Shi L. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy and relieves hyperalgesia in diabetic rats. Neuroreport. 2020;31(9):644–649. doi: 10.1097/WNR.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 33.Wang T., Wen X., Zhang Z., Xie M., Zhou J. Phillyrin ameliorates diabetic nephropathy through the PI3K/Akt/GSK-3β signalling pathway in streptozotocin-induced diabetic mice. Hum. Exp. Toxicol. 2021;40(12_suppl):S487–S496. doi: 10.1177/09603271211051598. [DOI] [PubMed] [Google Scholar]
- 34.Tziomalos K., Athyros V.G. Diabetic nephropathy: new risk factors and improvements in diagnosis. The review of diabetic studies. Reg. Dev. Stud. 2015;12(1–2):110. doi: 10.1900/RDS.2015.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Q., Zhai Y., Cheng Q., Liu Y., Gao X., Zhang T., et al. The Akt–FoxO3a–manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp. Physiol. 2013;98(4):934–945. doi: 10.1113/expphysiol.2012.068361. [DOI] [PubMed] [Google Scholar]
- 36.Lu Q., Wang W.W., Zhang M.Z., Ma Z.X., Qiu X.R., Shen M., et al. ROS induces epithelial-mesenchymal transition via the TGF-β1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Exp. Ther. Med. 2019;17(1):835–846. doi: 10.3892/etm.2018.7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W., Lo A.C. Diabetic retinopathy: pathophysiology and treatments. Int. J. Mol. Sci. 2018;19(6):1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin D., Zhang G-m, Xu X., Wang L-y. The PI3K/Akt signaling pathway mediates the high glucose-induced expression of extracellular matrix molecules in human retinal pigment epithelial cells. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/920280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X., Huo F., Liu B., Liu J., Chen T., Li J., et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J. Mol. Neurosci. 2017;61(4):581–589. doi: 10.1007/s12031-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 40.Nia A.H.S., Reyhani-Ardabili M., Sheikhvand M., Bagheri-Mohammadi S., Niknejad H., Rasoulzadeh H., et al. Non-coding RNAs: a new frontier in Benzene-mediated toxicity. Toxicology. 2023 doi: 10.1016/j.tox.2023.153660. [DOI] [PubMed] [Google Scholar]
- 41.Huang C., Azizi P., Vazirzadeh M., Aghaei-Zarch S.M., Aghaei-Zarch F., Ghanavi J., et al. Non-coding RNAs/DNMT3B axis in human cancers: from pathogenesis to clinical significance. J. Transl. Med. 2023;21(1):621. doi: 10.1186/s12967-023-04510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fardi F., Khasraghi L.B., Shahbakhti N., Naseriyan A.S., Najafi S., Sanaaee S., et al. An interplay between non-coding RNAs and gut microbiota in human health. Diabetes Res. Clin. Pract. 2023 doi: 10.1016/j.diabres.2023.110739. [DOI] [PubMed] [Google Scholar]
- 43.Aghaei-Zarch S.M., Alipourfard I., Rasoulzadeh H., Najafi S., Aghaei-Zarch F., Partov S., et al. Non-coding RNAs: an emerging player in particulate matter 2.5-mediated toxicity. Int. J. Biol. Macromol. 2023;235 doi: 10.1016/j.ijbiomac.2023.123790. [DOI] [PubMed] [Google Scholar]
- 44.Aghaei Zarch S.M., Vahidi Mehrjardi M.Y., Babakhanzadeh E., Nazari M., Talebi M., Zeniali F., et al. MiR-181b expression levels as molecular biomarker for type 2 diabetes. J. Mazandaran Univ. Med. Sci. 2019;29(176):195–201. [Google Scholar]
- 45.Lu T.X., Rothenberg M.E. MicroRNA. J. Allergy Clin. Immunol. 2018;141(4):1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohr A.M., Mott J.L., editors. Overview of microRNA biology. Thieme Medical Publishers; 2015. (Seminars in Liver Disease). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herman A.B., Tsitsipatis D., Gorospe M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell. 2022;82(12):2252–2266. doi: 10.1016/j.molcel.2022.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Q., Ma H., Chen H., Zhang Z., Xue Q. LncRNA in tumorigenesis of non-small-cell lung cancer: from bench to bedside. Cell Death Discov. 2022;8(1):359. doi: 10.1038/s41420-022-01157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badmalia M.D., Sette Pereira H., Siddiqui M.Q., Patel T.R. A comprehensive review of methods to study lncRNA–protein interactions in solution. Biochem. Soc. Trans. 2022;50(5):1415–1426. doi: 10.1042/BST20220604. [DOI] [PubMed] [Google Scholar]
- 50.Zeinali F., Aghaei Zarch S.M., Jahan-Mihan A., Kalantar S.M., Vahidi Mehrjardi M.Y., Fallahzadeh H., et al. Circulating microRNA-122, microRNA-126-3p and microRNA-146a are associated with inflammation in patients with pre-diabetes and type 2 diabetes mellitus: a case control study. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0251697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majumder S., Hadden M.J., Thieme K., Batchu S.N., Niveditha D., Chowdhury S., et al. Dysregulated expression but redundant function of the long non-coding RNA HOTAIR in diabetic kidney disease. Diabetologia. 2019;62(11):2129–2142. doi: 10.1007/s00125-019-4967-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J., Yang Z., Fang K., Shi Z., Ren D., Sun J. Long noncoding RNA ILF3-AS1 regulates myocardial infarction via the miR-212-3p/SIRT1 axis and PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24(5):2647–2658. doi: 10.26355/eurrev_202003_20534. [DOI] [PubMed] [Google Scholar]
- 53.Bi W., Li J., Xiong M., Pan B., Zhang Z., Nasifu L., et al. The diagnostic and prognostic role of miR-27a in cancer. Pathol. Res. Pract. 2023 doi: 10.1016/j.prp.2023.154544. [DOI] [PubMed] [Google Scholar]
- 54.Chen T., Zhang Y., Liu Y., Zhu D., Yu J., Li G., et al. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging (Albany NY) 2019;11(18):7510. doi: 10.18632/aging.102263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H., Song D., Liu Q., Li L., Sun X., Guo J., et al. miR-351 promotes atherosclerosis in diabetes by inhibiting the ITGB3/PIK3R1/Akt pathway and induces endothelial cell injury and lipid accumulation. Mol. Med. 2022;28(1):120. doi: 10.1186/s10020-022-00547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen S.H., Liu X.N., Peng Y. MicroRNA‐351 eases insulin resistance and liver gluconeogenesis via the PI3K/AKT pathway by inhibiting FLOT2 in mice of gestational diabetes mellitus. J. Cell Mol. Med. 2019;23(9):5895–5906. doi: 10.1111/jcmm.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C., Li Y., Lu Y., Niu Z., Zhao H., Peng Y., et al. miR-26 family and its target genes in tumorigenesis and development. Crit. Rev. Oncol.-Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103124. [DOI] [PubMed] [Google Scholar]
- 58.Li H., Li X., Jiang J., Shi P., Zhang X., Tian M. Effect of miR-26b on gestational diabetes mellitus in rats via PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24(4):1609–1615. doi: 10.26355/eurrev_202002_20335. [DOI] [PubMed] [Google Scholar]
- 59.Russo A., Potenza N. Antiproliferative activity of microRNA-125a and its molecular targets. MicroRNA. 2019;8(3):173–179. doi: 10.2174/2211536608666181105114739. [DOI] [PubMed] [Google Scholar]
- 60.Xu L., Li Y., Yin L., Qi Y., Sun H., Sun P., et al. miR-125a-5p ameliorates hepatic glycolipid metabolism disorder in type 2 diabetes mellitus through targeting of STAT3. Theranostics. 2018;8(20):5593. doi: 10.7150/thno.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu J.-M., Zhang Z.-Z., Ma X., Fang S.-F., Qin X.-H. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Exp. Eye Res. 2020;190 doi: 10.1016/j.exer.2019.107886. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y., He S., Du X., Jiang Y., Tian B., Xu S. Rapamycin suppresses hypoxia/reoxygenation‐induced islet injury by up‐regulation of miR‐21 via PI 3K/Akt signalling pathway. Cell Prolif. 2017;50(1) doi: 10.1111/cpr.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiu F., Tong H., Wang Y., Tao J., Wang H., Chen L. Inhibition of miR-21-5p suppresses high glucose-induced proliferation and angiogenesis of human retinal microvascular endothelial cells by the regulation of AKT and ERK pathways via maspin. Biosci., Biotechnol., Biochem. 2018;82(8):1366–1376. doi: 10.1080/09168451.2018.1459179. [DOI] [PubMed] [Google Scholar]
- 64.Lei Y., Yang M., Li H., Xu R., Liu J. miR-130b regulates PTEN to activate the PI3K/Akt signaling pathway and attenuate oxidative stress-induced injury in diabetic encephalopathy. Int. J. Mol. Med. 2021;48(1):1–14. doi: 10.3892/ijmm.2021.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y., Tang Z., Dai H., Hou J., Li F., Tang Z., et al. MiR‐195 promotes pancreatic β‐cell dedifferentiation by targeting Mfn2 and impairing Pi3k/Akt signaling in type 2 diabetes. Obesity. 2022;30(2):447–459. doi: 10.1002/oby.23360. [DOI] [PubMed] [Google Scholar]
- 66.Lai S., Quan Z., Hao Y., Liu J., Wang Z., Dai L., et al. Long non-coding RNA LINC01572 promotes hepatocellular carcinoma progression via sponging miR-195-5p to enhance PFKFB4-mediated glycolysis and PI3K/AKT activation. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.783088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Z., Hu H., Zou Y., Luo W., Xie L., You Z. miR-7 reduces high glucose induced-damage via HoxB3 and PI3K/AKT/mTOR signaling pathways in retinal pigment epithelial cells. Curr. Mol. Med. 2020;20(5):372–378. doi: 10.2174/1566524019666191023151137. [DOI] [PubMed] [Google Scholar]
- 68.Cao Y., Liu D., Zhang H. MiR-7 regulates the PI3K/AKT/VEGF pathway of retinal capillary endothelial cell and retinal pericytes in diabetic rat model through IRS-1 and inhibits cell proliferation. Eur. Rev. Med. Pharmacol. Sci. 2018;22(14):4427–4430. doi: 10.26355/eurrev_201807_15493. [DOI] [PubMed] [Google Scholar]
- 69.Ji Z., Luo J., Su T., Chen C., Su Y. miR-7a targets insulin receptor substrate-2 gene and suppresses viability and invasion of cells in diabetic retinopathy mice via PI3K-Akt-VEGF pathway. Diabetes, Metab. Syndrome Obes. Targets Ther. 2021;14:719. doi: 10.2147/DMSO.S288482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shu L., Zhao H., Huang W., Hou G., Song G., Ma H. Resveratrol upregulates mmu-miR-363-3p via the PI3K-Akt pathway to improve insulin resistance induced by a high-fat diet in mice. Diabetes, Metab. Syndrome Obes. Targets Ther. 2020;13:391. doi: 10.2147/DMSO.S240956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X., Liu Z. MiR-19a inhibitor improves diabetic retinopathy in rats through PTEN/Akt/P-Akt signaling pathway. J. Biol. Regul. Homeost. Agents. 2020;34(2):509–515. doi: 10.23812/20-77-A-60. [DOI] [PubMed] [Google Scholar]
- 72.Wang J., Dong Z., Lou L., Yang L., Qiu J. MiR-122 participates in oxidative stress and apoptosis in STZ-induced pancreatic β cells by regulating PI3K/AKT signaling pathway. Int. J. Endocrinol. 2021:2021. doi: 10.1155/2021/5525112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma Y., Dong C., Chen X., Zhu R., Wang J. Silencing of miR-20b-5p exerts inhibitory effect on diabetic retinopathy via inactivation of THBS1 gene induced VEGF/Akt/PI3K pathway. Diabetes, Metab. Syndrome Obes. Targets Ther. 2021;14:1183. doi: 10.2147/DMSO.S299143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui L., Yu M., Cui X. MiR-30c-5p/ROCK2 axis regulates cell proliferation, apoptosis and EMT via the PI3K/AKT signaling pathway in HG-induced HK-2 cells. Open Life Sci. 2020;15(1):959–970. doi: 10.1515/biol-2020-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou L., Zhang S., Zhang L., Li F., Sun H., Feng J. MiR-199a-3p inhibits the proliferation, migration, and invasion of endothelial cells and retinal pericytes of diabetic retinopathy rats through regulating FGF7 via EGFR/PI3K/AKT pathway. J. Recept. Signal Transduction. 2021;41(1):19–31. doi: 10.1080/10799893.2020.1783556. [DOI] [PubMed] [Google Scholar]
- 76.Wang L., Liu W.-X., Huang X.-G. MicroRNA-199a-3p inhibits angiogenesis by targeting the VEGF/PI3K/AKT signalling pathway in an in vitro model of diabetic retinopathy. Exp. Mol. Pathol. 2020;116 doi: 10.1016/j.yexmp.2020.104488. [DOI] [PubMed] [Google Scholar]
- 77.Wang H., Wang Z., Tang Q. Reduced expression of microRNA-199a-3p is associated with vascular endothelial cell injury induced by type 2 diabetes mellitus. Exp. Ther. Med. 2018;16(4):3639–3645. doi: 10.3892/etm.2018.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu C., Wang D., Feng Y., Feng L., Li Z. miR-720 regulates insulin secretion by targeting Rab35. BioMed Res. Int. 2021:2021. doi: 10.1155/2021/6662612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu H., Ma L., Feng F. PICK1 attenuates high glucose-induced pancreatic β-cell death through the PI3K/Akt pathway and is negatively regulated by miR-139-5p. Biochem. Biophys. Res. Commun. 2020;522(1):14–20. doi: 10.1016/j.bbrc.2019.11.051. [DOI] [PubMed] [Google Scholar]
- 80.Cai W., Zhang J., Yang J., Fan Z., Liu X., Gao W., et al. MicroRNA-24 attenuates vascular remodeling in diabetic rats through PI3K/Akt signaling pathway. Nutr. Metabol. Cardiovasc. Dis. 2019;29(6):621–632. doi: 10.1016/j.numecd.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z.-Z., Qin X.-H., Zhang J. MicroRNA-183 inhibition exerts suppressive effects on diabetic retinopathy by inactivating BTG1-mediated PI3K/Akt/VEGF signaling pathway. Am. J. Physiol. Endocrinol. Metabol. 2019;316(6):E1050–E1060. doi: 10.1152/ajpendo.00444.2018. [DOI] [PubMed] [Google Scholar]
- 82.Yang X., Li X., Lin Q., Xu Q. Up-regulation of microRNA-203 inhibits myocardial fibrosis and oxidative stress in mice with diabetic cardiomyopathy through the inhibition of PI3K/Akt signaling pathway via PIK3CA. Gene. 2019;715 doi: 10.1016/j.gene.2019.143995. [DOI] [PubMed] [Google Scholar]
- 83.Yousefnia S. A comprehensive review on miR-153: mechanistic and controversial roles of miR-153 in tumorigenicity of cancer cells. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.985897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang H., Fang X., Shen Y., Yao W., Chen D., Shen L. MiR-153-3p reduces extracellular matrix accumulation in high glucose-stimulated human glomerular mesangial cells via targeting PAQR3 in diabetic nephropathy. Endocrinol., Diabetes Nutricion. 2022;69(1):34–42. doi: 10.1016/j.endien.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Liu H., Wang X., Liu S., Li H., Yuan X., Feng B., et al. Effects and mechanism of miR-23b on glucose-mediated epithelial-to-mesenchymal transition in diabetic nephropathy. Int. J. Biochem. Cell Biol. 2016;70:149–160. doi: 10.1016/j.biocel.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Fang S., Ma X., Guo S., Lu J. MicroRNA-126 inhibits cell viability and invasion in a diabetic retinopathy model via targeting IRS-1. Oncol. Lett. 2017;14(4):4311–4318. doi: 10.3892/ol.2017.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xin Y., Zhang H., Jia Z., Ding X., Sun Y., Wang Q., et al. Resveratrol improves uric acid-induced pancreatic β-cells injury and dysfunction through regulation of miR-126. Biomed. Pharmacother. 2018;102:1120–1126. doi: 10.1016/j.biopha.2018.03.172. [DOI] [PubMed] [Google Scholar]
- 88.Yang W.-Z., Yang J., Xue L.-P., Xiao L.-B., Li Y. MiR-126 overexpression inhibits high glucose-induced migration and tube formation of rhesus macaque choroid-retinal endothelial cells by obstructing VEGFA and PIK3R2. J. Diabetes Complicat. 2017;31(4):653–663. doi: 10.1016/j.jdiacomp.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Lou Z., Li Q., Wang C., Li Y. The effects of microRNA-126 reduced inflammation and apoptosis of diabetic nephropathy through PI3K/AKT signalling pathway by VEGF. Arch. Physiol. Biochem. 2020:1–10. doi: 10.1080/13813455.2020.1767146. [DOI] [PubMed] [Google Scholar]
- 90.Wang L., Zhang N., Pan H-p, Wang Z., Cao Z-y. MiR-499-5p contributes to hepatic insulin resistance by suppressing PTEN. Cell. Physiol. Biochem. 2015;36(6):2357–2365. doi: 10.1159/000430198. [DOI] [PubMed] [Google Scholar]
- 91.Han N., Fang H.-Y., Jiang J.-X., Xu Q. Downregulation of microRNA-873 attenuates insulin resistance and myocardial injury in rats with gestational diabetes mellitus by upregulating IGFBP2. Am. J. Physiol. Endocrinol. Metabol. 2020;318(5):E723–E735. doi: 10.1152/ajpendo.00555.2018. [DOI] [PubMed] [Google Scholar]
- 92.He Y., Dan Y., Gao X., Huang L., Lv H., Chen J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am. J. Physiol. Endocrinol. Metabol. 2021;320(3):E598–E608. doi: 10.1152/ajpendo.00089.2020. [DOI] [PubMed] [Google Scholar]
- 93.Ye H., Yang S., Zhang Y. MEG3 damages fetal endothelial function induced by gestational diabetes mellitus via AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(24):8553–8560. doi: 10.26355/eurrev_201812_16617. [DOI] [PubMed] [Google Scholar]
- 94.Chen L., Gong H., Xu L. PVT1 protects diabetic peripheral neuropathy via PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(20):6905–6911. doi: 10.26355/eurrev_201810_16160. [DOI] [PubMed] [Google Scholar]
- 95.Wang Q., Lu X., Li C., Zhang W., Lv Y., Wang L., et al. Down-regulated long non-coding RNA PVT1 contributes to gestational diabetes mellitus and preeclampsia via regulation of human trophoblast cells. Biomed. Pharmacother. 2019;120 doi: 10.1016/j.biopha.2019.109501. [DOI] [PubMed] [Google Scholar]
- 96.Cai F., Jiang H., Li Y., Li Q., Yang C. Upregulation of long non-coding RNA SNHG16 promotes diabetes-related RMEC dysfunction via activating NF-κB and PI3K/AKT pathways. Mol. Ther. Nucleic Acids. 2021;24:512–527. doi: 10.1016/j.omtn.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Cheng X., Li D. LncRNA RPL13p5 gene expression promotes insulin resistance in patients with gestational diabetes. Ann. Palliat. Med. 2021;10(10):11024–11034. doi: 10.21037/apm-21-2940. [DOI] [PubMed] [Google Scholar]
- 98.Shi C., Huang Y., Li W., Chen R. Influence of LncRNA UCA1 on glucose metabolism in rats with diabetic nephropathy through PI3K-Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(22):10058–10064. doi: 10.26355/eurrev_201911_19573. [DOI] [PubMed] [Google Scholar]
- 99.Qi K., Zhong J. LncRNA HOTAIR improves diabetic cardiomyopathy by increasing viability of cardiomyocytes through activation of the PI3K/Akt pathway. Exp. Ther. Med. 2018;16(6):4817–4823. doi: 10.3892/etm.2018.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zang X., Li L., Du X., Yang B., Mei C. LncRNA TUG1 inhibits the proliferation and fibrosis of mesangial cells in diabetic nephropathy via inhibiting the PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(17):7519–7525. doi: 10.26355/eurrev_201909_18867. [DOI] [PubMed] [Google Scholar]
- 101.Li K., Li Q. LINC00323 mediates the role of M1 macrophage polarization in diabetic nephropathy through PI3K/AKT signaling pathway. Hum. Immunol. 2021;82(12):960–967. doi: 10.1016/j.humimm.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Zhao C., Fei X., Xu B., Lu Y., Zhang Q. Long non-coding RNA HEIH contributes to diabetic retinopathy by regulating miR-939/VEGF axis. Int. J. Clin. Exp. Pathol. 2019;12(6):2022. [PMC free article] [PubMed] [Google Scholar]
- 103.Li H.-X., Li X.-H., Jiang J., Shi P.-X., Zhang X.-G., Tian M. Effect of miR-26b on gestational diabetes mellitus in rats via PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24(4) doi: 10.26355/eurrev_202002_20335. [DOI] [PubMed] [Google Scholar]
- 104.Lai S., Quan Z., Hao Y., Liu J., Wang Z., Dai L., et al. Long non-coding RNA LINC01572 promotes hepatocellular carcinoma progression via sponging miR-195-5p to enhance PFKFB4-mediated glycolysis and PI3K/AKT activation. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.783088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji Z., Luo J., Su T., Chen C., Su Y. miR-7a targets insulin receptor substrate-2 gene and suppresses viability and invasion of cells in diabetic retinopathy mice via PI3K-Akt-VEGF pathway. Diabetes Metab. Syndr. Obes. 2021:719–728. doi: 10.2147/DMSO.S288482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shu L., Zhao H., Huang W., Hou G., Song G., Ma H. Resveratrol upregulates mmu-miR-363-3p via the PI3K-Akt pathway to improve insulin resistance induced by a high-fat diet in mice. Diabetes Metab. Syndr. Obes. 2020:391–403. doi: 10.2147/DMSO.S240956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J., Dong Z., Lou L., Yang L., Qiu J. MiR-122 participates in oxidative stress and apoptosis in STZ-induced pancreatic β cells by regulating PI3K/AKT signaling pathway. Int. J. Endocrinol. 2021;2021:1–11. doi: 10.1155/2021/5525112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma Y., Dong C., Chen X., Zhu R., Wang J. Silencing of miR-20b-5p exerts inhibitory effect on diabetic retinopathy via inactivation of THBS1 gene induced VEGF/Akt/PI3K pathway. Diabetes Metab. Syndr. Obes. 2021:1183–1193. doi: 10.2147/DMSO.S299143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye H.-H., Yang S.-H., Zhang Y. MEG3 damages fetal endothelial function induced by gestational diabetes mellitus via AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(24) doi: 10.26355/eurrev_201812_16617. [DOI] [PubMed] [Google Scholar]
- 110.Chen L., Gong H., Xu L. PVT1 protects diabetic peripheral neuropathy via PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(20) doi: 10.26355/eurrev_201810_16160. [DOI] [PubMed] [Google Scholar]
- 111.Li Y., Cheng X., Li D. LncRNA RPL13p5 gene expression promotes insulin resistance in patients with gestational diabetes. Ann. Palliat. Med. 2021;10(10):11024–11034. doi: 10.21037/apm-21-2940. [DOI] [PubMed] [Google Scholar]
- 112.Shi C.-H., Huang Y., Li W.-Q., Chen R.-G. Influence of LncRNA UCA1 on glucose metabolism in rats with diabetic nephropathy through PI3K-Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(22) doi: 10.26355/eurrev_201911_19573. [DOI] [PubMed] [Google Scholar]
- 113.Zang X.-J., Li L., Du X., Yang B., Mei C.-L. LncRNA TUG1 inhibits the proliferation and fibrosis of mesangial cells in diabetic nephropathy via inhibiting the PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(17) doi: 10.26355/eurrev_201909_18867. [DOI] [PubMed] [Google Scholar]
- 114.Huang L., Cai H.-A., Zhang M.-S., Liao R.-Y., Huang X., Hu F.-D. Ginsenoside Rg1 promoted the wound healing in diabetic foot ulcers via miR-489–3p/Sirt1 axis. J. Pharmacol. Sci. 2021;147(3):271–283. doi: 10.1016/j.jphs.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 115.Mo F.-F., An T., Zhang Z.-J., Liu Y.-F., Liu H.-X., Pan Y.-Y., et al. Jiang Tang Xiao Ke granule play an anti-diabetic role in diabetic mice pancreatic tissue by regulating the mRNAs and MicroRNAs associated with PI3K-Akt signaling pathway. Front. Pharmacol. 2017;8:795. doi: 10.3389/fphar.2017.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y., Lu J., Zhong Y-j, Yang C-f, Chen L., Wu D., et al. Methyl ferulic acid ameliorates alcohol-induced hepatic insulin resistance via miR-378b-mediated activation of PI3K-AKT pathway. Biomed. Pharmacother. 2022;145 doi: 10.1016/j.biopha.2021.112462. [DOI] [PubMed] [Google Scholar]
- 117.Yin Y., Qu H., Yang Q., Fang Z., Gao R. Astragaloside IV alleviates Schwann cell injury in diabetic peripheral neuropathy by regulating microRNA-155-mediated autophagy. Phytomedicine. 2021;92 doi: 10.1016/j.phymed.2021.153749. [DOI] [PubMed] [Google Scholar]
- 118.Lee H., Lee J. Anti‐diabetic effect of hydroxybenzoic acid derivatives in free fatty acid‐induced HepG2 cells via miR‐1271/IRS1/PI3K/AKT/FOXO1 pathway. J. Food Biochem. 2021;45(12) doi: 10.1111/jfbc.13993. [DOI] [PubMed] [Google Scholar]
- 119.Li S.-H., Wang M.-S., Ke W.-L., Wang M.-R. Naringenin alleviates myocardial ischemia reperfusion injury by enhancing the myocardial miR-126-PI3K/AKT axis in streptozotocin-induced diabetic rats. Exp. Ther. Med. 2021;22(2):1–9. doi: 10.3892/etm.2021.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xue M., Cheng Y., Han F., Chang Y., Yang Y., Li X., et al. Triptolide attenuates renal tubular epithelial-mesenchymal transition via the MiR-188-5p-mediated PI3K/AKT pathway in diabetic kidney disease. Int. J. Biol. Sci. 2018;14(11):1545. doi: 10.7150/ijbs.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang S., Duan J., Liao J., Wang Y., Xiao X., Li L., et al. Shengjie Tongyu decoction regulates cardiomyocyte autophagy through modulating ROS-PI3K/Akt/mTOR Axis by LncRNA H19 in diabetic cardiomyopathy. Alternative Ther. Health Med. 2023;29(6):280–287. [PubMed] [Google Scholar]
- 122.Yu S., Zhao H., Yang W., Amat R., Peng J., Li Y., et al. The alcohol extract of Coreopsis tinctoria Nutt ameliorates diabetes and diabetic nephropathy in db/db mice through miR-192/miR-200b and PTEN/AKT and ZEB2/ECM pathways. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/5280514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ji C., Guo X. The clinical potential of circulating microRNAs in obesity. Nat. Rev. Endocrinol. 2019;15(12):731–743. doi: 10.1038/s41574-019-0260-0. [DOI] [PubMed] [Google Scholar]
- 124.Zhang S., Cheng Z., Wang Y., Han T. The risks of miRNA therapeutics: in a drug target perspective. Drug Des. Dev. Ther. 2021:721–733. doi: 10.2147/DDDT.S288859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kapadia C.H., Luo B., Dang M.N., Irvin‐Choy N.D., Valcourt D.M., Day E.S. Polymer nanocarriers for MicroRNA delivery. J. Appl. Polym. Sci. 2020;137(25) doi: 10.1002/app.48651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng L., Wagner E. Polymeric carriers for nucleic acid delivery: current designs and future directions. Biomacromolecules. 2019;20(10):3613–3626. doi: 10.1021/acs.biomac.9b00999. [DOI] [PubMed] [Google Scholar]
- 127.Wang J., Wu H., Peng Y., Zhao Y., Qin Y., Zhang Y., et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnol. 2021;19(1):1–13. doi: 10.1186/s12951-021-00942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li M., Ke Q.-F., Tao S.-C., Guo S.-C., Rui B.-Y., Guo Y.-P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B. 2016;4(42):6830–6841. doi: 10.1039/c6tb01560c. [DOI] [PubMed] [Google Scholar]
- 129.Tao S.-C., Rui B.-Y., Wang Q.-Y., Zhou D., Zhang Y., Guo S.-C. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25(1):241–255. doi: 10.1080/10717544.2018.1425774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.