Abstract
BACKGROUND
Hepatocellular carcinoma (HCC) patients complicated with portal vein tumor thrombus (PVTT) exhibit poor prognoses and treatment responses.
AIM
To investigate efficacies and safety of the combination of PD-1 inhibitor, transcatheter arterial chemoembolization (TACE) and Lenvatinib in HCC subjects comorbid with PVTT.
METHODS
From January 2019 to December 2020, HCC patients with PVTT types I-IV were retrospectively enrolled at Beijing Ditan Hospital. They were distributed to either the PTL or TACE/Lenvatinib (TL) group. The median progression-free survival (mPFS) was set as the primary endpoint, while parameters like median overall survival, objective response rate, disease control rate (DCR), and toxicity level served as secondary endpoints.
RESULTS
Forty-one eligible patients were finally recruited for this study and divided into the PTL (n = 18) and TL (n = 23) groups. For a median follow-up of 21.8 months, the DCRs were 88.9% and 60.9% in the PTL and TL groups (P = 0.046), res-pectively. Moreover, mPFS indicated significant improvement (HR = 0.25; P < 0.001) in PTL-treated patients (5.4 months) compared to TL-treated (2.7 months) patients. There were no treatment-related deaths or differences in adverse events in either group.
CONCLUSION
A triplet regimen of PTL was safe and well-tolerated as well as exhibited favorable efficacy over the TL regimen for advanced-stage HCC patients with PVTT types I-IV.
Keywords: Hepatocellular carcinoma, Transcatheter arterial chemoembolization, Lenvatinib, PD-1 inhibitor, Portal vein tumor thrombus
Core Tip: Hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT) has a poor prognosis and treatment responses. Assessment of its survival has important clinical implications. Through our research, we discovered that a triplet regimen of PD-1 inhibitor/transcatheter arterial chemoembolization/Lenvatinib was safe and well-tolerated as well as exhibited favorable efficacy over the transcatheter arterial chemoembolization/Lenvatinib regimen for advanced-stage HCC comorbid with PVTT.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most frequently diagnosed primary malignancies of the liver that leads to high morbidity and mortality rates in adults, especially those with chronic liver diseases and hepatitis infections. In 2020, HCC ranked sixth among all cancers concerning 4.7% of all new cancer cases, and third for 8.3% of all cancer-related deaths[1]. The portal vein tumor thrombus (PVTT) is a commonly occurring complication in at least 16%-30% of HCC patients[2], contributes to poor prognosis, and increases the susceptibility to cancer recurrence, with the median survival of about 2.7-4 months without any interventions[3]. Although both European Association for the Study of the Liver and American Association for the Study of Liver Diseases guidelines suggested that HCC patients complicated with PVTT should be classified as Barcelona Clinic Liver Cancer-C stage and receive systemic treatments[4,5]. However, there is currently no optimal treatment strategy for such patients. Tyrosine kinase and immune checkpoint inhibitors (TKI and ICI, respectively) are generally applied in systemic therapies of HCC, while the combination of transcatheter arterial chemoembolization (TACE) with systemic therapeutics has substantially improved treatment outcomes in advanced-stage patients.
TACE is recommended for treating unresectable HCC[6-8], and its safety and effectiveness have been demonstrated in several clinical trials[9-12]. Despite this, in clinical practices, tumor recurrence and distant metastasis are often encountered in post-TACE HCC patients[13]. Several studies have confirmed better treatment responses when TACE is combined with TKI over TACE alone[14-16]. In the era of rapidly advancing immunotherapy research, studies suggest that ICI and TKI together can promote the recovery of cytotoxic T lymphocytes (CTLs) from exhaustion[17], thereby enhancing antitumor responses[18,19]. Retrospective studies suggest that a triplet regimen of TACE or a combination of hepatic arterial infusion chemotherapy (HAIC), Lenvatinib, and PD-1 inhibitor could be superior to dual therapy in advanced HCC cases[20-22]. However, there is limited data on highly complicated HCC patients, and the success rate of Atezolizumab plus Bevacizumab therapy has not yet been established for this subset of HCC patients[23]. Therefore, we hypothesized that the triplet regimen of TACE plus targeted drugs and ICIs might introduce better prognoses for HCC patients with PVTT. Here, we aimed to compare the safety and efficacy of the triplet regimen of Lenvatinib, PD-1 inhibitor, and TACE vs Lenvatinib plus TACE in advanced-stage HCC with type I- IV PVTT.
MATERIALS AND METHODS
Patient selection
Eligible patients were retrospectively enrolled at Beijing Ditan Hospital affiliated with Capital Medical University, between January 2019 and December 2020. Participants were distributed into two treatment groups: The PD-1 inhibitor/TACE/Lenvatinib (PTL) and TACE/Lenvatinib (TL) groups. HCC patients had different stages of PVTT without obstructing the major vein lumen or inferior vena cava. The Chinese Society of Clinical Oncology guidelines for liver carcinoma were followed to confirm the histological and clinical diagnoses of HCC[24]. The diagnosis of PVTT was confirmed either by radiological (e.g., CT and MRI) or pathological methods. As per Cheng’s classification, the PVTT could be staged in four types. Type I tumor thrombus involves portal vein branches in segments; type II includes left and right branches of the hepatic portal vein; type III involves the main portal vein, and type IV affects the superior mesenteric vein or inferior vena cava[25].
The inclusion criteria comprised the following: (1) Ages ranged from 18 to 75 years; (2) life expectancy ≥ 3 months; (3) having at least one typical enhanced measurable target lesion of ≥ 1 cm, according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST); (4) Eastern Cooperative Oncology Group-performance status (ECOG-PS) score 0-1; (5) Child-Pugh classification A or B ( ≤ 7 points); (6) non-surgical indications or refusal of surgical treatment; (7) not receiving radiofrequency ablation, TACE, and any other locoregional treatments 4 wk before the admission; (8) no limit on several liver tumors; and (9) might or might not have extrahepatic metastasis. The exclusion criteria included: (1) A history of other cancers; (2) any contraindications for therapies with TACE, Lenvatinib, and/or PD-1 inhibitor; (3) simultaneous ongoing treatment with other drugs; (4) incomplete patient record data; and (5) other conditions considered unsuitable for inclusion in the study by the investigators. Additionally, patients treated with any locoregional therapies e.g., radiofrequency ablation and radiotherapy) for intrahepatic lesions of HCC 4 wk before the study enrollment were eliminated.
Treatments
TACE: TACE was carried out by 2 professional radiologists (Cai L and Guo J). First, the feeding artery of the tumor was determined by angiography, then a super-selective 5-F catheter was inserted. Then, a mixture of Lipiodol (5-20 mL; Lipiodol Ultra-Fluide; André Guerbet Laboratories) and Lobaplatin (20mg/m2; Changan Hainan International Pharmaceutical Co., China) was prepared for embolization using absorbable embospheres (300-500mm; Biosphere Medical Inc). The entire intrahepatic tumor burden was treated by TACE. Conventional TACE (cTACE) was repeated on demand if the patient’s subsequent follow-ups revealed incomplete necrosis or insufficient Lipiodol uptake.
Systemic therapy: Patients with ≥ 60 kg and < 60 kg (or Child-Pugh class B) of body weights, respectively, received 12 mg and 8 mg of Lenvatinib orally once daily until the day of cTACE. In the absence of any post-TACE symptoms (fever, nausea, vomiting, etc.), the medication was resumed after each TACE treatment. Patients in the PTL group received intravenous doses of 200 mg of PD-1 inhibitor (Sintilimab, Camrelizumab, Nivolumab, or Tislelizumab), every 3 wk starting from one to two weeks after TACE. When participants experienced adverse events (AEs) of grade 3 or more, dose modification or temporary interruption of medication was performed, according to the drug labels, until AEs were relieved to grade 1 or none. The drug was discontinued in case of uncontrolled tumor progression or unacceptable AEs.
Follow-up visits
Post-TACE assessments were performed by chest, abdominal, or enhanced CT, MRI, and/or laboratory tests during the follow-up visits every 4-8 wk intervals. Laboratory tests included blood routine, hepatic and kidney function tests, quantitation of urine protein, serum α-fetoprotein, and myocardial enzymes, etc. AEs records were retrieved for analysis from the hospital’s electronic record system, following the Common Terminology Criteria for Adverse Events (v5.0) guidelines. Transient post-TACE AEs, such as elevated live enzymes, abdominal discomforts, and fever, were not recorded. The end-point of the follow-up study was December 1, 2021.
Endpoints
According to mRECIST criteria, median progression-free survival (mPFS) was the primary endpoint, while secondary endpoints included median overall survival (mOS), objective response rate (ORR), disease control rate (DCR), and safety. The mPFS was defined as the duration from the first TACE intervention to the first tumor progression and all-cause death. The mOS was the time from the first TACE to all-cause death. The ORR was the percentage of participants with complete remission (CR) and partial remission (PR). The DCR was referred to as the sum of CR, PR, and stable disease (SD). Tumor progression (20% increase from baseline examination, mRECIST) and transient hepatic dysfunction to Child-Pugh C or emerging extrahepatic metastases were conceived as markers of disease progression[26].
Statistical analysis
Continuous data are presented as median ± interquartile range or mean ± SD. The baseline characteristics of the two groups were evaluated by independent samples t-test or chi-squared (χ2) test. The Kaplan-Meier and log-rank tests were employed to estimate survival curves and corresponding P values. Based on the Cox regression models, multivariate and univariate analyses were performed to identify independent prognostic factors related to mPFS. Any survival-related variables with P < 0.10 from univariate analyses were combined into a multivariate Cox proportional hazards model. A two-tailed P value of < 0.05 was considered statistically significant. The SPSS v 22.0 (IBM, Inc., New York, NY, United States) was exploited for all analyses.
RESULTS
Patient demographics
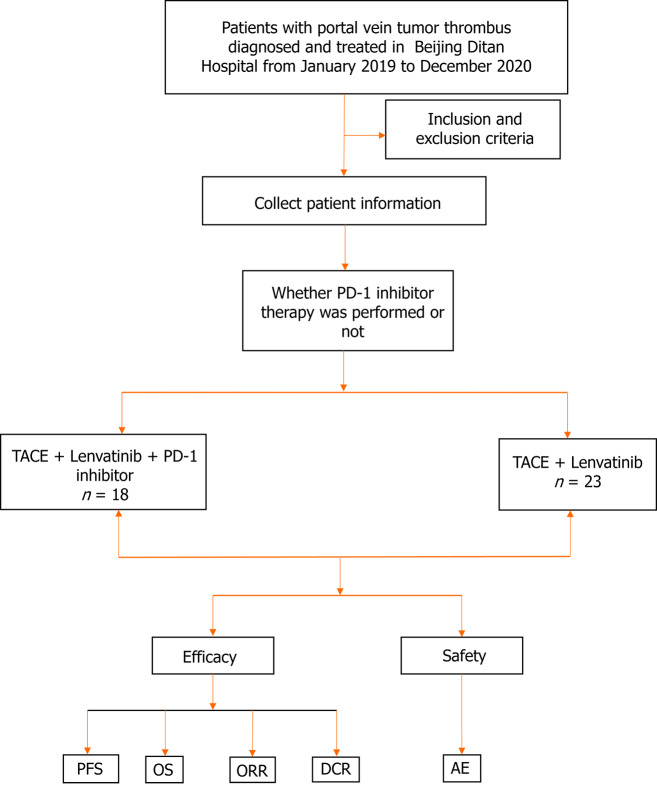
A total of 502 HCC cum PVTT cases were diagnosed in Beijing Ditan Hospital, of which 424 patients were not treated with Lenvatinib, 22 subjects were not treated with TACE, and 15 patients had incomplete follow-up data. Finally, 41 of these patients were enrolled in the study, of which 23 were allotted to the TL group and the rest (n = 18) patients to the PTL group (Figure 1). The baseline data of 41 patients are outlined in Table 1. The median age of the patients was 57.6 ± 8.8 years. Thirty-six percent (n = 15) of patients in both groups had extrahepatic metastases, and most patients presented PVTT types Ⅲ-Ⅳ (63.4%, n = 26). All patients had a Child-Pugh classification of A-B (≤ 7 points) and an ECOG score of 0-1. In the majority of cases (92.7%, n = 38), the etiopathology was hepatitis B virus infection. The baseline characteristics between the two groups were highly comparable in terms of liver function, demographics, and disease characteristics.
Figure 1.
Trial flowchart. Flowchart illustration of patient selection strategy. A total of 41 patients were finally selected, of which 18 patients received treatments with the PD-1 inhibitor/transcatheter arterial chemoembolization (TACE)/Lenvatinib, and 23 patients with the TACE/Lenvatinib regimen. TACE: Transcatheter arterial chemoembolization; AE: Adverse event; PFS: Progression-free survival; OS: Overall survival; ORR: Objective response rate; DCR: Disease control rate.
Table 1.
Baseline clinical characteristics of patients
| Characteristic | PTL (n = 18) | TL (n = 23) | P value |
| Mean age, yr ± SD | 56.9 ± 8.1 | 58.1 ± 9.4 | 0.307 |
| Gender, n () | 0.690 | ||
| Male | 15 (83.3) | 18 (78.3) | |
| Female | 3 (16.7) | 5 (21.7) | |
| Weight, n (%) | 0.794 | ||
| < 60 kg | 7 (38.9) | 8 (34.8) | |
| ≥ 60 kg | 11 (61.1) | 15 (65.2) | |
| Etiology, n (%) | 0.447 | ||
| HBV | 16 (88.9) | 22 (95.7) | |
| Others | 2 (11.1) | 1 (4.3) | |
| ECOG-PS, n (%) | 0.586 | ||
| 0 | 7 (38.9) | 7 (30.4) | |
| 1 | 11 (61.1) | 16 (69.6) | |
| Child-Pugh class, n (%) | 0.209 | ||
| A | 18 (100) | 21 (91.3) | |
| B | 0 (0) | 2 (8.7) | |
| AFP, n (%) | 0.273 | ||
| < 400 ng/mL | 7 (38.9) | 13 (56.5) | |
| ≥ 400 ng/mL | 11 (61.1) | 10 (43.5) | |
| Liver cirrhosis, n (%) | 0.328 | ||
| Absent | 0 (0) | 1 (4.3) | |
| Present | 18 (100) | 22 (95.7) | |
| Extrahepatic metastasis, n (%) | 0.096 | ||
| Absent | 14 (77.8) | 12 (52.2) | |
| Present | 4 (22.2) | 11 (47.8) | |
| Size of largest nodule, n (%) | 0.855 | ||
| < 5 cm | 2 (11.1) | 3 (13.0) | |
| ≥ 5 cm | 16 (88.9) | 20 (87.0) | |
| Tumor thrombus | 0.373 | ||
| Branch of portal vein | 8 (44.4) | 7 (30.4) | |
| Main portal vein and vena cava | 10 (55.6) | 16 (69.6) | |
| Tumor number | 0.415 | ||
| Solitary | 1 (5.6) | 3 (13.0) | |
| Multiple | 17 (94.4) | 20 (87.0) | |
| ALB | 0.740 | ||
| > 3.5 g/dL | 8 (44.4) | 9 (39.1) | |
| ≤ 3.5 g/dL | 10 (55.6) | 14 (60.9) | |
| Treatment history | |||
| Surgery | 0 (0) | 0 (0) | - |
| RFA | 2 (11.1) | 3 (13.0) | 0.856 |
| TACE | 0.123 | ||
| 1-2 | 15 (83.3) | 14 (60.9) | |
| > 2 | 3 (16.7) | 9 (39.1) | |
| ALT (U/L) | 65.8 ± 80.6 | 45.2 ± 26.6 | 0.835 |
| AST (U/L) | 73.6 ± 74.5 | 68.5 ± 46.5 | 0.386 |
| TBIL (mg/dl) | 18.8 ± 7.0 | 17.2 ± 8.9 | 0.780 |
| PT(s) | 12.7 ± 1.2 | 13.1 ± 1.3 | 0.988 |
| PTA(s) | 83.2 ± 12.6 | 83.7 ± 11.3 | 0.640 |
PTL: PD-1 inhibitor/transcatheter arterial chemoembolization/Lenvatinib; TL: Transcatheter arterial chemoembolization/Lenvatinib; HBV: Hepatitis B Virus; ECOG-PS: Eastern Cooperative Oncology Group-performance status; AFP: Alpha fetoprotein; ALB: Albumin; TACE: Transcatheter arterial chemoembolization; ATL: Alanine aminotransferase; AST: Aspartate aminotransferase; TBIL: Total bilirubin; PT: Prothrombin time; PTA: Prothrombin activity.
Treatment efficacy
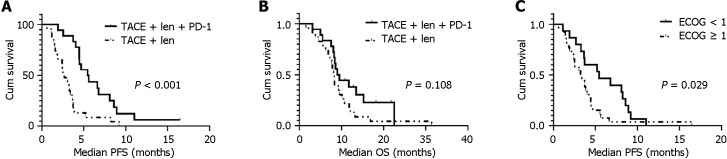
The median follow-up was 21.8 months (95%CI = 14.7-28.8). Patients in the PTL group had significantly longer mPFS (5.4 months; 95%CI = 3.6-7.3) than those in the TL (2.7 months; 95%CI = 1.7-3.6) group (HR 0.25; 95%CI = 0.12-0.52; P < 0.001; Figure 2A and B). However, there was no significant difference in mOS between the PTL (9.0 months; 95%CI = 7.07-10.93) and TL (8.27 months; 95%CI = 7.65-8.89) for groups (P = 0.108); Figure 2A and B). When stratified by ECOG-PS scores, the patients with ECOG score 0 exhibited a longer mPFS than those with ECOG score 1 (Figure 2C). However, no statistical difference in PFS was demonstrated between patients with ≤ 3 tumors vs those with > 3 tumors (5.4 months vs 4.0 months, P = 0.178).
Figure 2.
Kaplan-Meier curves estimate prognosis. A: Kaplan-Meier curves estimate median progression free survival by treatment modality; B: Kaplan-Meier curves estimate median overall survival by treatment modality; C: Kaplan-Meier curves estimate median progression free survival for patients with different Eastern Cooperative Oncology Group scores. TACE: Transcatheter arterial chemoembolization; ECOG: Eastern Cooperative Oncology Group; PFS: Progression-free survival; OS: Overall survival.
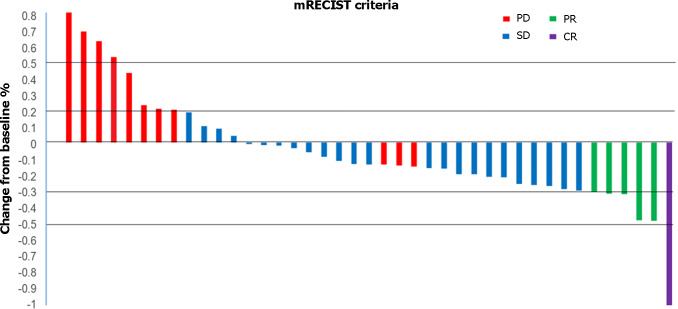
The DCR was superior (P = 0.046) in the PTL group (88.9%; 1 CR, 2 PR, and 13 SD) than that in the TL (60.9%; 0 CR, 3 PR, and 11 SD) groups (Table 2). However, the difference in the ORR was not significant (P = 0.752) between the PTL (16.7%; 1 CR, and 2 PR) and TL (13%; 0 CR and 3 PR) groups. Waterfall analysis revealed a reduction in tumor sizes in 70.7% (29/41) of patients as per the investigator’s assessment (Figure 3).
Table 2.
Therapeutic efficacy of response and conversion therapy, n (%)
|
|
PTL (n = 18)
|
TL (n = 23)
|
P value
|
| CR | 1 (5.6) | 0 (0) | |
| PR | 2 (11.1) | 3 (13.0) | |
| SD | 13 (72.2) | 11 (47.8) | |
| PD | 2 (11.1) | 9 (39.1) | |
| ORR | 3 (16.7) | 3 (13.0) | 0.752 |
| DCR | 16 (88.9) | 14 (60.9) | 0.046 |
PTL: PD-1 inhibitor/transcatheter arterial chemoembolization/Lenvatinib; TL: Transcatheter arterial chemoembolization/Lenvatinib; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease; ORR: Objective response rate; DCR: Disease control rate.
Figure 3.
Waterfall plot of maximum tumor response to triple therapy by investigator using the modified Response Evaluation Criteria in Solid Tumors. Waterfall plots showing the maximum level tumor responses to triple therapy by investigators using the mRECIST approach. mRECIST: Modified Response Evaluation Criteria in Solid Tumors; PD: Progressive disease; SD: Stable disease; PR: Partial remission; CR: Complete remission.
Table 3 presents independent prognostic factors related to mPFS. In univariate analysis, age, gender, cirrhosis, Child-Pugh grade, ECOG performance status, tumor thrombus, bilirubin, prothrombin time, and the intervention method were significantly associated with mPFS (P < 0.05). Finally, we found that the PTL regimen (95%CI = 0.12-0.52; HR = 0.25; P < 0.001), and a lower ECOG score (95%CI = 1.29-6.16; HR = 2.82; P = 0.009) were favorable factors for prolonged PFS.
Table 3.
Univariate and multivariate cox proportional hazard model for Median progression-free survival
| Characteristic | N |
Univariate
|
Multivariate
|
||||
|
mPFS
|
95%CI
|
P value |
HR
|
95%CI
|
P value
|
||
| Treatment | 0.000 | 0.25 | 0.12-0.52 | < 0.001 | |||
| PTL group | 18 | 5.4 | 3.61-7.25 | ||||
| TL group | 23 | 2.7 | 1.73-3.61 | ||||
| Gender | 0.162 | - | - | 0.947 | |||
| Male | 33 | 4.00 | 2.70-5.31 | ||||
| Female | 8 | 3.37 | 2.44-4.30 | ||||
| Age | 0.902 | - | - | 0.740 | |||
| ≥ 55 yr | 26 | 3.70 | 3.13-4.28 | ||||
| < 55 yr | 15 | 3.63 | 1.96-5.30 | ||||
| Liver cirrhosis | 0.015 | - | - | - | |||
| Yes | 40 | 3.7 | 3.39-4.01 | ||||
| No | 1 | 1.17 | - | ||||
| Weight | 0.537 | - | - | - | |||
| < 60 kg | 15 | 3.73 | 3.48-3.98 | ||||
| ≥ 60 kg | 26 | 3.27 | 1.87-4.67 | ||||
| Etiology | 0.729 | - | - | - | |||
| HBV | 38 | 3.7 | 3.16-4.24 | ||||
| Others | 3 | 4.47 | 0.36-8.58 | ||||
| PVTT | 0.389 | - | - | 0.704 | |||
| Ⅰ-Ⅱ types | 15 | 3.7 | 0.76-6.64 | ||||
| Ⅲ-Ⅳ types | 26 | 3.7 | 2.95-4.45 | ||||
| TACE | 0.214 | - | - | - | |||
| 1-2 times | 29 | 3.83 | 3.18-4.48 | ||||
| ≥ 2 times | 12 | 3.37 | 2.57-4.17 | ||||
| ECOG-PS | 0.036 | 2.82 | 1.29-6.16 | 0.009 | |||
| < 1 | 14 | 5.43 | 2.55-8.31 | ||||
| ≥ 1 | 27 | 3.27 | 2.69-3.85 | ||||
| Child-Pugh grade | 0.027 | 0.10 | 0.01-1.11 | 0.061 | |||
| A | 39 | 3.73 | 3.28-4.18 | ||||
| B | 2 | 1.83 | - | ||||
| AFP | 0.177 | - | - | - | |||
| < 40 0 ng/mL | 20 | 3.63 | 2.94-4.32 | ||||
| ≥ 400 ng/mL | 21 | 4.43 | 2.79-6.08 | ||||
| Extrahepatic spread | 0.233 | - | - | - | |||
| Yes | 40 | 3.7 | 2.82-4.58 | ||||
| No | 1 | 3.7 | 2.54-4.86 | ||||
| Tumor diameter | 0.959 | - | - | - | |||
| < 5 cm | 5 | 4.43 | 1.94-6.92 | ||||
| ≥ 5 cm | 36 | 3.70 | 3.17-4.23 | ||||
| Anti-viral therapy | 0.714 | - | - | - | |||
| Yes | 38 | 3.7 | 3.01-4.40 | ||||
| No | 3 | 3.7 | 0.82-6.58 | ||||
| ALB | 0.840 | - | - | - | |||
| > 3.5 g/dL | 17 | 3.37 | 2.21-4.53 | ||||
| ≤ 3.5 g/dL | 24 | 3.73 | 3.17-4.30 | ||||
| Family history of HBV | 0.099 | - | - | - | |||
| Yes | 28 | 4.43 | 3.60-5.26 | ||||
| No | 13 | 2.67 | 1.03-4.28 | ||||
| PT | 0.561 | - | - | 0.915 | |||
| < 13 s | 25 | 4.00 | 2.69-5.31 | ||||
| ≥ 13 s | 16 | 2.53 | 0.04-5.02 | ||||
| TBIL | 0.964 | - | - | 0.392 | |||
| < 1.5 mg/dL | 31 | 3.73 | 2.86-4.60 | ||||
| ≥ 1.5 mg/dL | 10 | 2.67 | 1.90-3.45 | ||||
| ALT | 0.907 | - | - | - | |||
| < 40 µg/mL | 22 | 3.73 | 2.11-5.35 | ||||
| ≥ 40 µg/mL | 19 | 3.70 | 3.24-4.16 | ||||
| AST | 0.849 | - | - | - | |||
| < 40 µg/mL | 12 | 3.23 | 1.43-5.03 | ||||
| ≥ 40 µg/mL | 29 | 3.70 | 3.12-4.28 | ||||
PTL: PD-1 inhibitor/transcatheter arterial chemoembolization/Lenvatinib; TL: Transcatheter arterial chemoembolization/Lenvatinib; PVTT: Portal vein tumor thrombus; TACE: Transcatheter arterial chemoembolization; ECOG-PS: Eastern Cooperative Oncology Group-performance status; AFP: Alpha fetoprotein; ALB: Albumin; HBV: Hepatitis B virus; PT: Prothrombin time; TBIL: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate transaminase.
Safety
Since post-TACE symptoms of transient AEs disappeared in a short time in most patients, these did not affect the subsequent treatment. We did not summarize these transient AEs related to TACE. Thirty-two patients (78.0) in the two groups experienced treatment-related AEs, including 12 patients (37.5) with high-grade AEs (≥ grade 3; Table 4). The most frequent treatment-related AEs were asthenia, hand-foot skin reaction, proteinuria, hypertension, decreased white blood cell and platelet counts, hypoproteinemia, ascites, nausea, and decreased weight, and most of these AEs were mild to moderate in severity. The incidence of AEs of ≥ grade 3 was similar between the two groups. Immune-related AEs included myocarditis (1 patient, grade 2) and hypothyroidism (2 patients, grade 2). The incidence of dose reduction/discontinuation during treatment was 30.4% (7/23) in the TL group and 27.8% (5/18) in the PTL group.
Table 4.
Adverse events, n (%)
| Adverse events |
Any grades
|
High grades (≥ 3)
|
||||
|
PTL (n = 18)
|
TL (n = 23)
|
P value
|
PTL (n = 18)
|
TL (n = 23)
|
P value
|
|
| Asthenia | 7 (38.9) | 5 (21.7) | 0.186 | 0 (0.0) | 0 (0.0) | - |
| ALT elevation | 14 (77.8) | 18 (78.3) | 0.665 | 2 (11.1) | 3 (13.0) | 0.851 |
| AST elevation | 13(72.2) | 18 (78.3) | 0.971 | 2 (11.1) | 3 (13.0) | 0.851 |
| Hand-foot skin reaction | 1 (5.6) | 3 (13.0) | 0.435 | 0 (0.0) | 0 (0.0) | - |
| Hypertension | 2 (11.1) | 3 (13.0) | 0.856 | 1 (5.6) | 1 (4.3) | 0.863 |
| Hypothyroidism | 2 (11.1) | 0 (0.0) | 0.163 | 0 (0.0) | 0 (0.0) | - |
| Proteinuria | 2 (11.1) | 3 (13.0) | 0.856 | 0 (0.0) | 1 (4.3) | 0.383 |
| Dysphonia | 0 (0.0) | 1 (4.3) | 0.383 | 0 (0.0) | 0 (0.0) | - |
| Decreased WBC | 5 (27.8) | 4 (17.4) | 0.438 | 1 (5.6) | 0 (0.0) | 0.264 |
| Decreased PLT | 6 (33.3) | 4 (17.4) | 0.249 | 0 (0.0) | 1 (4.3) | 0.383 |
| Hypoproteinemia | 4 (22.2) | 3 (13.0) | 0.451 | 0 (0.0) | 0 (0.0) | - |
| Infection | 1 (5.6) | 1 (4.3) | 0.863 | 0 (0.0) | 1 (4.3) | 0.383 |
| Diarrhea | 3 (16.7) | 2 (8.7) | 0.452 | 0 (0.0) | 0 (0.0) | - |
| Hepatic encephalopathy | 0 (0.0) | 1 (4.3) | 0.383 | 0 (0.0) | 1 (4.3) | 0.383 |
| Myocarditis | 1 (5.6) | 0 (0.0) | 0.264 | 1 (5.6) | 0 (0.0) | 0.264 |
| Anorexia | 3 (16.7) | 4 (17.4) | 0.953 | 0 (0.0) | 0 (0.0) | - |
| Ascites | 4 (22.2) | 3 (13.0) | 0.451 | 0 (0.0) | 0 (0.0) | - |
| Acute kidney injury | 1 (5.6) | 1 (4.3) | 0.863 | 1 (5.6) | 1 (4.3) | 0.863 |
| Nausea | 3 (16.7) | 5 (21.7) | 0.693 | 0 (0.0) | 0 (0.0) | - |
| Decreased weight | 2 (11.1) | 4 (17.4) | 0.584 | 0 (0.0) | 0 (0.0) | - |
| Elevated bilirubin | 1 (5.6) | 1 (4.3) | 0.863 | 1 (5.6) | 0 (0.0) | 0.264 |
| Alimentary tract hemorrhage | 0 (0.0) | 1 (4.3) | 0.383 | 0 (0.0) | 1 (4.3) | 0.383 |
PTL: PD-1 inhibitor/transcatheter arterial chemoembolization/Lenvatinib; TL: Transcatheter arterial chemoembolization/Lenvatinib; ALT: Alanine aminotransferase; AST: Aspartate transaminase; WBC: White blood cell; PLT: Platelet.
DISCUSSION
HCC patients complicated with PVTT have a short life expectancy. Due to a high susceptibility toward intravascular and extrahepatic metastases, increased risk of other serious complications, including bleeding from esophageal varices, and impaired liver function caused by primary hepatic occlusion and worsening hypertension[27]. The updated recommendations of the National Comprehensive Cancer Network for advanced-stage HCC refer to systemic therapy[28]. In the era of combination drug therapies, TKIs combined with ICIs, such as Lenvatinib combined with Pembrolizumab or Nivolumab, Cabozantinib combined with Nivolumab or Atezolizumab, have been proven to offer higher efficacy and longer overall survival in HCC patients[29]. The addition of TACE further improves the response of this combination (TKI plus ICI) therapy for advanced-stage HCC subjects[21,22,30]. However, there are no head-to-head studies on the efficacy of TACE or HAIC added with Lenvatinib/PD-1 inhibitor vs TACE plus Lenvatinib in a subset of advanced HCC patients complicated with PVTT. Our study identified an advantage of triplet therapy in terms of mPFS (5.4 months vs 2.7 months, P < 0.001) as well as DCR (88.9% vs 60.9%, P = 0.046) in HCCs with PVTT types I-IV. And no new additional toxicities were found. These results were complementary to the previous findings[21,22,30], suggesting the triplet regimen could be utilized in advanced HCC cases, including those with PVTT types I-IV.
The probable mechanism underlying the superiority of the triplet regimen over the dual regimen in treating PVTT in terms of prognosis could be as follows: TACE-induced release of inflammatory factors might have activated the adaptive immunity, or TACE might induce spontaneous T cell responses to regulate the immune environment in the tumor microenvironment. Simultaneously, its combination with ICIs may be more effective in promoting antitumor immune reconstitution[31]. Previous findings have highlighted that tumor immune escape mainly occurs when CTLs are depleted. The immune-promoting activities of anti-VEGF drugs can suppress the expression of PD-1 as well as TIM-3 (mucin domain-containing protein 3) on CTLs, thereby rescuing the CTL population from the depleted state and forming a newly balanced immune environment during treatments[17]. A single-arm investigation has indicated that the PTL regimen could achieve an ORR of 80.6% with manageable toxicity[29].
Although our study demonstrated the superiority of the PTL regimen over the TL, the mPFS was shorter in our study than in the previous study[21]. The following two reasons were considered: (1) This study used advanced-stage HCC patients (63.4% with types III-IV PVTT) with poor prognoses as a sample, while the previous study using a triple regimen did not include all patients with PVTT; and (2) the small sample size used in this study might have caused some variations in the results. We noticed that the mOS of patients treated with PTL was not statistically different compared to that of TL-treated patients. We speculated that the second-line treatment after progression might have exerted a certain impact on the overall survival of patients. For example, the combination with PD-1 inhibitors of patients in the dual group after progression further prolonged the survival time of patients. At the same time, for more accurate and reliable results, randomized controlled trials (RCTs) are needed in the future.
Furthermore, we analyzed the prognostic factors of PFS of the whole cohort and concluded that the treatment mode and the ECOG-PS score were independent factors that could modulate the prognosis of HCC patients. When the ECOG-PS score was lower, the mPFS of patients was better. Moreover, the triplet therapy of the PTL regimen could provide better mPFS to patients than the dual therapy with the TL regimen.
Consistently, the PTL regimen has been shown to offer a similar safety profile in this study[21]. The incidences of treatment-related AEs did not differ considerably between the two treatment modalities. Notably, the triplet therapy regimen was not associated with any recurrences of liver injuries, as well as no grade 3 ir-AEs in our study. A recent study has demonstrated that transient transaminase elevation (e.g., 52% in ALT, or 46% in AST) after TACE could be associated with objective responses[32], which can guide clinical practice. It means that patients with severe liver injury may have limited efficacy from TACE, maybe the severe liver injuries induce liver function deterioration which can hinder the administration of systemic drugs. However, due to limited data sources, no such association was investigated in this study.
Some critical limitations of this study are as follows. First, it was a single-center, small-sample, retrospective, and investigator-biased study. Second, the variability in TACE frequency might have affected the results of this study. Third, the cohort size was too small to obtain reliable statistical power for this study. And lastly, the use of different PD-1 blockers could have influenced the consistency of treatment procedures.
CONCLUSION
In conclusion, we found that the triple therapy with PTL could not only significantly improve mPFS and DCR in advanced HCC patients with PVTT but also prove to be better safety and tolerability. Therefore, based on the previous as well as current findings, it may be concluded that the PTL regimen has powerful potential in obtaining satisfactory treatment outcomes in advanced-stage HCC patients complicated with PVTT types I-IV. Prospective multi-center RCTs are warranted to further confirm the clinical efficacy of the triplet regimen over conventional procedures for HCC with PVTT.
ARTICLE HIGHLIGHTS
Research background
The incidence and mortality of hepatocellular carcinoma (HCC) are among the highest in the world. There are a large number of advanced liver cancer patients with portal vein cancer embolus, and the prognosis is worse. Therefore, it is necessary to explore the treatment plan that can prolong the survival of liver cancer patients with portal vein cancer embolus.
Research motivation
To compare the efficacies and safety levels of the PD-1 inhibitor/TACE/Lenvatinib (PTL) regimen and TACE/Lenvatinib (TL) regimen for HCC subjects comorbid with portal vein tumor thrombus (PVTT), providing a choice for exploring the combination drug regimen that can prolong the survival of HCC with PVTT.
Research objectives
Our research aims to compare the efficacies and safety levels of the PTL regimen and TL regimen for HCC subjects comorbid with PVTT. We found a triplet regimen of PTL was safe and well-tolerated as well as exhibited favorable efficacy over the TL regimen for advanced-stage HCC patients with PVTT types I-IV. The triple therapy regimen may better improve the prognosis of advanced liver cancer patients with portal vein cancer suppository and extend their survival time, which is of great significance.
Research methods
We selected HCC patients with PVTT type Ⅰ-Ⅳ, TACE was carried out by 2 professional radiologists.
Research results
The triple therapy regimen may better improve the prognosis of advanced liver cancer patients with portal vein cancer suppository and extend their survival time. Large-scale prospective studies are needed to further validate the efficacy and safety of the triple therapy regimen in the future.
Research conclusions
A triplet regimen of PTL was safe and well-tolerated as well as exhibited favorable efficacy over the TL regimen for advanced-stage HCC patients with PVTT types I-IV. The combination therapy of TACE, TKI and PD-1 inhibitor was used for advanced liver cancer patients with portal vein cancer embolus.
Research perspectives
Large-scale prospective studies are needed to further validate the efficacy and safety of the triple therapy regimen in the future.
ACKNOWLEDGEMENTS
We thank all patients for their endeavors and unparalleled contributions to this study.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Beijing Ditan Hospital,Capital Medical University Institutional Review Board (Approval No. JDLC 2021-003 -02).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All authors certify that there is no conflict of interest related to the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 27, 2023
First decision: December 4, 2023
Article in press: January 25, 2024
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beenet L, United States S-Editor: Lin C L-Editor: A P-Editor: Chen YX
Contributor Information
Hong-Xiao Wu, Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Xiao-Yan Ding, Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Ya-Wen Xu, Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Ming-Hua Yu, Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Xiao-Mi Li, Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Na Deng, Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Jing-Long Chen, Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China. cjl6412@ccmu.edu.cn.
Data sharing statement
The dataset used for this study is available from the corresponding author upon reasonable request.
References
- 1.International Agency for Research on Cancer. Global Cancer Observatory. [cited 3 October 2021]. Available from: https://gco.iarc.fr/
- 2.Khan AR, Wei X, Xu X. Portal Vein Tumor Thrombosis and Hepatocellular Carcinoma - The Changing Tides. J Hepatocell Carcinoma. 2021;8:1089–1115. doi: 10.2147/JHC.S318070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, Qi X, Cheng SQ, Teng GJ. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721–730. doi: 10.1016/S2468-1253(19)30178-5. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 6.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2019;20:1042–1113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, Lau WY, Wu M. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition) Liver Cancer. 2020;9:28–40. doi: 10.1159/000503685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 9.Choi JW, Kim HC, Lee JH, Yu SJ, Kim YJ, Yoon JH, Jae HJ, Hur S, Lee M, Chung JW. Transarterial chemoembolization of hepatocellular carcinoma with segmental portal vein tumour thrombus. Eur Radiol. 2017;27:1448–1458. doi: 10.1007/s00330-016-4511-3. [DOI] [PubMed] [Google Scholar]
- 10.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 11.Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, White S, Rilling W, Gamblin TC. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford) 2017;19:659–666. doi: 10.1016/j.hpb.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Xiang X, Lau WY, Wu ZY, Zhao C, Ma YL, Xiang BD, Zhu JY, Zhong JH, Li LQ. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: a multicenter study. Eur J Surg Oncol. 2019;45:1460–1467. doi: 10.1016/j.ejso.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Lin W, Wang H, Zhong M, Yu S, Zhao S, Liang S, Du J, Cheng B, Gu W, Ling C. Effect and Molecular Mechanisms of Jiedu Recipe on Hypoxia-Induced Angiogenesis after Transcatheter Arterial Chemoembolization in Hepatocellular Carcinoma. Evid Based Complement Alternat Med. 2021;2021:6529376. doi: 10.1155/2021/6529376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T, Zhang Y, Wu H, Liao Z. TACE Plus Lenvatinib Versus TACE Plus Sorafenib for Unresectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Prospective Cohort Study. Front Oncol. 2021;11:821599. doi: 10.3389/fonc.2021.821599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, Liu L, Zhang X, Zhai J, Qu Z. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663–675. doi: 10.1007/s12072-021-10184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, Liu X, Zheng L, Chen J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer. 2021;127:3782–3793. doi: 10.1002/cncr.33677. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Fang P, Wang C, Gu M, Pan B, Guo W, Yang X, Wang B. The immunomodulatory activity of lenvatinib prompts the survival of patients with advanced hepatocellular carcinoma. Cancer Med. 2021;10:7977–7987. doi: 10.1002/cam4.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Jiang M, Zhu J, Qu J, Qin K, Zhao D, Wang L, Dong L, Zhang X. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacother. 2020;132:110797. doi: 10.1016/j.biopha.2020.110797. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, Li Q, Luo M, Liang R, Ye J. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:447. doi: 10.1186/s13046-019-1412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, Guo RP. Hepatic Arterial Infusion Chemotherapy Combined With PD-1 Inhibitors Plus Lenvatinib Versus PD-1 Inhibitors Plus Lenvatinib for Advanced Hepatocellular Carcinoma. Front Oncol. 2021;11:618206. doi: 10.3389/fonc.2021.618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, Zhuang W, Chen X, Chen H, Xu B, Lai J, Guo W. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2022;148:2115–2125. doi: 10.1007/s00432-021-03767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, Feng D, Chen Y, Zheng J. The Efficacy of TACE Combined With Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. Front Oncol. 2021;11:783480. doi: 10.3389/fonc.2021.783480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin S, Ren Z, Feng YH, Yau T, Wang B, Zhao H, Bai Y, Gu S, Li L, Hernandez S, Xu DZ, Mulla S, Wang Y, Shao H, Cheng AL. Atezolizumab plus Bevacizumab versus Sorafenib in the Chinese Subpopulation with Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer. 2021;10:296–308. doi: 10.1159/000513486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, Wenming C, Peijun W, Yuxiang Z. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499–502. [PubMed] [Google Scholar]
- 26.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zane KE, Makary MS. Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers (Basel) 2021;13 doi: 10.3390/cancers13215430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. Hepatobiliary Carcinoma (Version 2.2023). Sep 14, 2023. [cited 3 August 2022]. Available online. Available at: https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf .
- 29.Stefanini B, Ielasi L, Chen R, Abbati C, Tonnini M, Tovoli F, Granito A. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2023;23:279–291. doi: 10.1080/14737140.2023.2181162. [DOI] [PubMed] [Google Scholar]
- 30.Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, Zhou JY, Li YN, Qiu FN, Li B, Yan ML. Lenvatinib Combined with Anti-PD-1 Antibodies Plus Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi: 10.2147/JHC.S332420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, Boyton RJ, Altmann DM, Goldin RD, Akarca AU, Marafioti T, Mauri FA, Casagrande E, Grillo F, Giannini E, Bhoori S, Mazzaferro V. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granito A, Facciorusso A, Sacco R, Bartalena L, Mosconi C, Cea UV, Cappelli A, Antonino M, Modestino F, Brandi N, Tovoli F, Piscaglia F, Golfieri R, Renzulli M. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J Pers Med. 2021;11 doi: 10.3390/jpm11101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used for this study is available from the corresponding author upon reasonable request.