Abstract
Background
Newborn anemia is among the most common hematological problems and it can cause asymptomatic or severe to acute life-threatening events. It leads to impairment in brain maturation and development, tissue hypoxia, and stunted growth and then arrested growth if left untreated. The prevalence of anemia among newborns ranges from 23.4–66% in sub-Saharan Africa. But, there is limited information in Ethiopia regarding the prevalence of newborn anemia and its risk factors. Therefore, this study aimed to determine the prevalence of newborn anemia and its associated factors at Jimma Medical Center (JMC), South-west Ethiopia.
Methods
A hospital-based cross-sectional study design was implemented from January 14 to February 28, 2021, involving 288 full-term newborns by employing consecutive convenient sampling technique for study participant selection. Socio-demographic data and other associated factors were collected through interviews and a review of medical records by a structured questionnaire. Three mL umbilical cord blood samples from each newborn were collected and analyzed for a complete blood count by an automated hematological analyzer. Data were entered into Epi Data version 3.1 and exported to Statistical Package for Social Science version 20 for analysis. Binary logistic regression were used to identify the predictors of newborn anemia.
Results
The overall prevalence of anemia among newborns was 26.4%; of them, 65.8%, 25%, and 9.2% were mild, moderate, and severe anemia types, respectively. Maternal vegetable consumption habit (AOR = 0.26, 95% CI: 0.11, 0.62) and maternal anemia (AOR = 0.34, 95% CI: 0.17, 0.69) were significantly associated with anemia in newborns.
Conclusion
In general, newborn anemia in this study was a moderate public health problem. Based on this study, early screening of anemia among newborns may reduce further complications. Prevention of maternal anemia during pregnancy by improving their nutritional status especially vegetable consumption had a positive impact on reducing anemia among newborns.
Keywords: newborn, anemia, Jimma, Ethiopia
Introduction
Anemia is defined by the World Health Organization as a reduction in the amount of red blood cells in circulation, which causes the hemoglobin (Hgb) concentration to drop below the necessary level. This impairs the body’s ability to transport oxygen and is insufficient to meet its physiological requirements. Specific physiologic needs vary with a person’s age, gender, altitude, smoking behavior, and different stages of pregnancy.1
Anemia in newborns is defined as a condition in which hemoglobin levels in the body are lower than normal. However, there is no established Hgb limit for cord blood to define anemia in newborns; this study used an Hgb value of less than 14 g/dL, adopted from a study conducted in Ethiopia,2 then anemia in newborns or neonate is divided into three categories based on hemoglobin value as follows: 10 to 13.9 g/dl for mild anemia, 7 to 9. 9 g/dl for moderate anemia and < 7 g/dl is severe anemia. Additionally, maternal anemia is defined as Hgb <11g/dL.3,4
In developing countries, the prevalence of newborn anemia and severe anemia were between 50% and 80%, and 10% to 20%, respectively.5 Because it causes infant mortality and morbidity in the first few months of a baby’s life, newborn anemia is a severe public health concern.5–7 Its prevalence in sub-Saharan Africa is high, ranging from 23.4% to 66%.8,9
The study carried out in Ethiopia indicated that the prevalence of neonatal anemia of 9%, 25%, and 29.1% were reported in Addis Ababa, Gondar, and Nekemte, respectively. Furthermore, those studies found that anemia in the mother, mothers’ age, preterm birth, her habit of consuming vegetables, cesarean delivery, occupation, parasitic infection, vaginal bleeding during pregnancy and her failure to take iron/folate supplements during her pregnancy were all associated with newborn anemia.10–12
In a newborn, anemia is a common and complex problem owing to the unique blood picture because of variations in hematological profiles.13 It may result in an acute, severe, or asymptomatic life-threatening event.14 Anemia negatively impacts a newborn’s short- and long-term mental, physical, and social development. It also affects brain development, delays brain maturation, impairs later-life school performance, stunts growth, causes tissue and organ hypoxia, and worsens cognitive, motor, and social-emotional development. These effects lower earning potential and have a negative impact on the nation’s economy.6,15 Additionally, it is worse in developing nations with low iron intake, ineffective interventional programs, and inadequate infrastructure for early detection and treatment of anemia in health care facilities.16,17
Due to the increased metabolic needs of pregnancy following expanding placenta, a growing fetus, and maternal tissues, pregnant women are especially more susceptible to iron deficiency. It thus raises the possibility of maternal anemia. Since the fetus depends on the mother’s iron levels; women at greater risk of iron deficiency stand a higher chance of having anemic newborns. Because of these, anemia in newborns presents severe public health problems and results in newborn morbidity and mortality in underdeveloped nations.18–21
Although anemia’s national and regional prevalence in various population age groups is known in Ethiopia,4 there is a dearth of information regarding the severity of anemia and its contributing factors in neonates. To prevent, treat, and reduce anemia, it is crucial to research the particular risk factors and prevalence of anemia in a given setting and population group.3 Thus, the purpose of this study was to assess the prevalence of newborn anemia and its associated risk factors at Jimma Medical Center (JMC), south-west Ethiopia.
Materials and Methods
Study Design, Period and Area
A hospital-based cross-sectional study was conducted from January 14 to February 28, 2021 at JMC's delivery ward. The medical center is found at Jimma town, located from 350 km in the south-west of Addis Ababa, the capital city of Ethiopia. The location of the area is 1,780 meters above sea level, with approximate latitude coordinates of 7° 40’N and longitude of 36° 50’E. JMC provides services for approximately 4,500 deliveries in a year and 15 deliveries are expected daily from the catchment population of about 20 million people.
Study Participants
All mothers with their respective singleton live birth newborns delivered at full-term gestation (37–41 weeks) at JMC during the study period were included in the study. On the other hand, newborns with inaccessible cord blood samples, pregnant mothers who had taken blood and blood products for the last 3 months, who were receiving therapy for anemia, and had taken anti-parasite drugs in the previous two weeks were not included in this study.
Sample Size Determination and Sampling Technique
The minimum sample size was determined using single population proportion formula 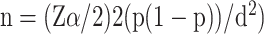 by considering the following assumptions, taking 25% prevalence of newborn anemia from the previous study done at University of Gondars' specialized hospital, Ethiopia.11 Therefore, the final minimum sample size was 288. Mothers with their respective newborns were recruited by consecutive convenient sampling technique.
by considering the following assumptions, taking 25% prevalence of newborn anemia from the previous study done at University of Gondars' specialized hospital, Ethiopia.11 Therefore, the final minimum sample size was 288. Mothers with their respective newborns were recruited by consecutive convenient sampling technique.
Data Collection Methods
A pre-tested structured questionnaire and reviews of medical records using a checklist were used to collect the data. The questionnaire was prepared after reviewing different related literature inside10,11 and outside the country.16,22,23 The questionnaire, first prepared in English language was translated into local language, then it was translated back to English language to ensure its consistency. Maternal socio-demographic characteristics, nutritional characteristics, birth interval, history of intestinal parasite infection (Helminths and protozoan parasite infection diagnosis during pregnancy) and malaria diagnosis during pregnancy, history of miscarriage, history of excess menstrual bleeding, and newborn gender were collected by face-to-face interviews and also by reviewing medical records.
Regarding nutritional characteristics or diet information of the mother, the women were asked about the frequency of consumption of each food (meat or animal product, vegetable and fruit consumption) per day, per week, or per month. In this study, pregnant women were defined as “consumers” of a food item (meat or animal product, vegetable and fruit consumption) when they had consumed those items at least once a week or per week.24,25
In this study vaginal bleeding was referred to as bleeding that was not related to menstruation. Menstrual bleeding was assessed based on asking the mother about the average length of menstruation or assessed by using an average number of sanitary pads changed per day. Menstrual bleeding was considered as excessive when the average length of menstruation was longer than 7 days or an average number of sanitary pads changed per day was greater than 4.
Maternal clinical data, CBC result, and blood group type were collected by reviewing medical records. For mothers who had no CBC results that was performed when the mothers come to delivery and have no blood group records, CBC test and blood grouping was performed by collecting 3ml venous blood. The interview, record review and blood collection were conducted by two trained midwifery professionals.
ABO blood incompatibility was defined in this study when group A or B babies born to group O mothers and also RH blood incompatibility occurs when Rh-positive babies were born to Rh-negative mothers.
The nutritional status of the pregnant mothers was determined by Mid-Upper Arm Circumference (MUAC) between the shoulder (olecranon) and the tip of the elbow (acromion process) of the non-dominant hand using a non-stretchable and non-elastic tape then, the result was interpreted as undernourished and well-nourished if her MUAC was less than 23 cm and greater than or equal to 23 cm respectively.26,27 Gestational age was determined by the last menstrual period but the last menstrual period of the mother was not known, gestational age was estimated by the New Ballard Score for inclusion and exclusion of study participants. The data collectors measured the newborn’s weight using a digital scale and the measurement was performed twice and an average value was taken. A newborn’s weight greater than or equal to 2.5 kg was considered as normal birth weight and less than 2.5 kg was considered as low birth weight.4
About three3 ml of umbilical cord blood sample was collected after the baby was born and the placental side of the umbilical cord was clamped and cut (in <2 minute after birth), and then needle was inserted into pre-identified large vein of the clamped umbilical cord by excluding the placenta. The collected sample was immediately poured into a tri-potassium ethylene diamine tetra-acetic acid (K3-EDTA) test tube and gently mixed to prevent blood clotting. The specimen was labeled and analyzed for CBC within 3–6 hours using Beckman coulter (UniCel® DxH 800, Florida, USA) hematological analyzer machine based on direct current principle for cells and spectrophotometric principle for Hgb. ABO blood group and Rh type of the newborn were determined from blood sample through forward (direct method) using test tube method. Two experienced laboratory technologist performs the CBC and blood grouping by strictly following to standard operating procedures.
Umbilical cord blood hemoglobin value is a dominant hematologic parameter for the diagnosis of newborn anemia.28,29 Hence this parameter was used in this study to define anemia in newborns. In this study, newborn anemia was defined as Hgb value <14g/dL and 10 to 13.9 g/dl as mild anemia, 7 to 9. 9 g/dl as moderate anemia and < 7 g/dl as severe anemia. Also maternal anemia was defined as Hgb <11g/ dL.
Data Quality Assurance
To assure the quality or validity of the data, the questionnaire was pre-tested among 5% of the sample size at Shenen Gibe Hospital, Jimma. The reliability of the tool was determined using Cronbach’s Alpha. Cronbach’s alpha among items in these study questionnaires was 0.892, which exceeded the prescribed threshold of 0.70. Training was given to data collectors, daily cross-checking of collected data with maternal records and daily supervision was made during the data collection period to assure the quality of socio-demographic data, clinical data and laboratory data. Standard operating procedures were also strictly followed during specimen collection and CBC analysis.
A daily background run for the hematological analyzer was carried out to minimize errors. Reagent expiration date was checked before the analysis of the patient sample and appropriate internal quality controls were run before the assay of samples. The test findings were kept confidential. The results of every laboratory test were documented and reported, and the specimens were handled carefully.
Data Processing and Analysis
The data from both questioner and laboratory were cleaned, edited, checked for completeness manually, and entered into Epi Data 3.1. Then it was transferred into a SPSS version 20 for analysis. Prior to any analysis, the Shapiro–Wilk and Kolmogorov–Smirnov tests were used to confirm that the data were normally distributed. For each independent variables, descriptive analysis were performed and summarized by number, percentage and frequency. The result was also presented with tables and charts. Bivariable logistic regression was performed to identify candidate variables and then variables having p-value less than or equal to 0.25 were candidate for multivariate logistic regression. Multivariate logistic regression was analyzed with backward stepwise likelihood ratio to assess association outcome with independent variable and to control potential confounding variables and then a p-value <0.05 were considered as having a significant relationship with the dependent variables. The Hosmer and Lemeshow test was used to assess the model fitness of the final logistic regression model at a p-value of greater than 0.05.
Results
Socio-Demographic Data of Study Participants
Two hundred eighty-eight (288) newborns, which comprised142 males and 146 females, and their respective mothers were include in the study. The median (IQR) age of the mothers was 25 (23-33) years with the minimum and maximum ages of 18 and 38 years, respectively. About 72.5% of newborns were delivered from maternal age below 30 years. About 72.9% of newborns were delivered through spontaneous vaginal delivery and the majority (88.5%) newborns had normal birth weight. Of the total newborns, 75% and 39.9% were born from mothers living in an urban area and who were housewives by their occupation, respectively. In addition, 80.9% of newborns were born from mothers having >1000ETB family monthly income (Table 1).
Table 1.
Socio-Demographic Characteristics of Study Participants at JMC, South-west Ethiopia from January 14 to February 28, 2021 (n = 288)
| Maternal and Newborn characteristic | Frequency | Percentage (%) | |
|---|---|---|---|
| Newborn gender | Male | 142 | 49.3 |
| Female | 146 | 50.7 | |
| Newborn birth weight | Low birth weight | 33 | 11.5 |
| Normal birth weight | 255 | 88.5 | |
| Maternal age | ≤24 years | 94 | 32.6 |
| 25–29 years | 115 | 39.9 | |
| ≥ 30 years | 79 | 27.4 | |
| Residence | Rural | 72 | 25 |
| Urban | 216 | 75 | |
| Maternal occupation | Housewife | 115 | 39.9 |
| Small scale business | 91 | 31.6 | |
| Employed | 82 | 28.5 | |
| Maternal education level | No formal education | 64 | 22.2 |
| Primary education | 69 | 24 | |
| Secondary education | 88 | 30.6 | |
| Above secondary education | 67 | 23.3 | |
| Family monthly income | <500 ETB | 20 | 6.9 |
| 501–1000 ETB | 35 | 12.2 | |
| >1000 ETB | 233 | 80.9 | |
Clinical Data of Study Participants
All study participant mothers were HIV negative. Of the total study participant, 62.5% were born from multiparous mothers and 64.4% from mothers were greater than 2 years birth interval. In this study, about 9.7% of babies were born to mothers who had no antenatal care follow-up. The majority of mothers (83.3%) had taken iron and folic acid supplementation, mainly (53.3%) at the second trimester of pregnancy. Of the total mothers, 10.1%, 16.7%, and 6.9% had a history of vaginal bleeding, intestinal parasitic infection, and a history of malaria during their pregnancy, respectively (Table 2).
Table 2.
Clinical Characteristics of Study Participants at JMC, South-west Ethiopia from January 14 to February 28, 2021 (n = 288)
| Variables | Frequency | Percent (%) | |
|---|---|---|---|
| Parity | Primiparous | 108 | 37.5 |
| Multiparous | 180 | 62.5 | |
| Birth interval (n = 180) | ≤ 2 | 64 | 35.6 |
| >2 | 116 | 64.4 | |
| ANC follow up | Yes | 260 | 90.3 |
| No | 28 | 9.7 | |
| Iron and folic acid supplementation | Yes | 240 | 83.3 |
| No | 48 | 16.7 | |
| Trimester iron or folic acid supplementation started (n = 240) | First trimester | 84 | 35 |
| Second Trimester | 128 | 53.3 | |
| Third trimester | 28 | 11.7 | |
| History of miscarriage | Yes | 19 | 6.6 |
| No | 269 | 93.4 | |
| History of excess menstrual bleeding | Yes | 33 | 11.5 |
| No | 255 | 88.5 | |
| History of vaginal bleeding during pregnancy | Yes | 29 | 10.1 |
| No | 259 | 89.9 | |
| History of intestinal parasite infection during pregnancy | Yes | 48 | 16.7 |
| No | 240 | 83.3 | |
| History of malaria infection during pregnancy | Yes | 20 | 6.9 |
| No | 268 | 93.1 | |
| Rhesus incompatibility managed | Yes | 8 | 2.8 |
| No | 280 | 97.2 | |
| ABO incompatibility | Yes | 47 | 16.3 |
| No | 241 | 83.7 | |
| Current mode of delivery | Spontaneous vaginal | 210 | 72.9 |
| Cesarean section | 78 | 27.1 | |
Nutrition Data of Study Participants
More than two-thirds (68.4%) of birthing mothers had meal frequency greater than three times per day. However, only 11.5% of mothers were undernourished. More than 84% of the newborns were delivered by mothers who consume meat or animal products, vegetables, and fruit every week. The maternal Hgb value ranges from 6.8–16.8 g/dl with a median (IQR) value of 13 g/dl (12–14.2g/dl). In this study, 58 (20.1%) mothers were anemic and from this 4 (6.9%), 15 (25.9%), and 39 (67.2%) were severe, moderate, and mild anemia type, respectively (Table 3).
Table 3.
Nutrition Characteristics of Study Participants at JMC, South-west Ethiopia from January 14 to February 28, 2021 (n = 288)
| Nutritional Characteristics | Frequency | Percent (%) | |
|---|---|---|---|
| Meal frequency within a day | >3 times per day | 197 | 68.4 |
| 3 times per day | 78 | 27.1 | |
| ≤ 2 times per day | 13 | 4.5 | |
| Meat or animal product consumption per week | Yes | 243 | 84.4 |
| No | 45 | 15.6 | |
| Vegetable consumption per week | Yes | 255 | 88.5 |
| No | 33 | 11.5 | |
| Fruit consumption per week | Yes | 260 | 90.3 |
| No | 28 | 9.7 | |
| Nutritional status (MUAC) | Malnourished | 33 | 11.5 |
| Normal | 255 | 88.5 | |
| Maternal anemia | Yes | 58 | 20.1 |
| No | 230 | 79.9 | |
Prevalence of Newborn Anemia
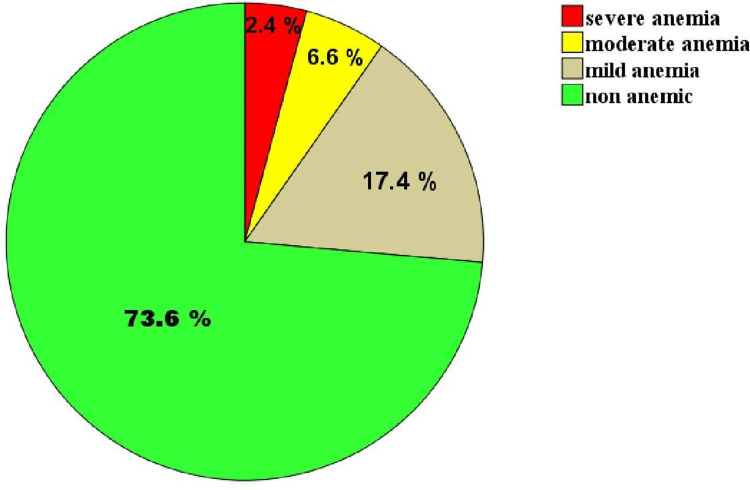
The cord Hgb value ranges from 4.2–21.5 g/dl with a median (IQR) value of 15.8 g/dl (13.8–16. 9 g/dl). The overall prevalence of anemia among full-term newborns at JMC was 26.4% (76/288) and from anemic newborn, 65.8, 25 and 9.2% were mild, moderate, and severe anemia respectively (Figure 1).
Figure 1.
Prevalence of mild, moderate, and severe anemia in the total newborn babies at JMC, South-west Ethiopia from January 14 to February 28, 2021 (n = 288).
Based on red blood cell indices especially MCV value (normal range: 91.6–113.22),2 microcytic, normocytic and macrocytic anemia among newborns were 7.9%, 31.6% and 60.5% respectively. Additionally, about 13 (17.1%) of newborns abies were anemic having ABO incompatibility with their respective mothers but 8 (2.8%) newborns having Rhesus incompatibility with their respective mothers were non anemic due to properly managed during ANC follow up.
Associated Factors of Newborn Anemia
Newborn anemia was high in those babies born from mothers who do not consume vegetables (51.5%), who were anemic (39.7%), who do not take iron or folic acid supplementation (39.6%), who do not consume meat or animal product (37.8%) and who do not follow ANC (35.7%). Based on bivariable logistic regression analysis, maternal consumption habit of vegetable, consumption habit of fruit, iron or folic acid supplementation and maternal anemia were showed significant association with newborn anemia. However, in multivariate binary logistic regression analysis, only mothers’ vegetable consumption habits (AOR = 0.26, 95%CI: 0.11, 0.62) and maternal anemia (AOR = 0.34, 95%CI: 0.17, 0.69) were significantly associated with newborn anemia (Table 4).
Table 4.
Bivariable and Multivariable Binary Logistic Regression Analysis of Factors Associated with Anemia Among Newborn Babies at JMC, South-west Ethiopia from January 14 to February 28, 2021 (n = 288)
| Characteristics of Study Participants | Anemic Newborns | Non-anemic Newborns | COR (95%CI) | AOR (95%CI) | P-value | |
|---|---|---|---|---|---|---|
| Maternal age | ≤24 year | 28(29.8%) | 66(70.2%) | 1 | 1 | 0.242 |
| 25–29 year | 31(27%) | 84(73%) | 0.87(0.48–1.59) | 1.10(0.52–2.30) | ||
| ≥30 year | 17(21.5%) | 62(78.5%) | 0.65(0.32–1.30) | 0.54(0.22–1.30) | ||
| Residence | Rural | 26(36.1%) | 46(63.9%) | 1.88(1.06–3.34) | 0.87(0.28–2.74) | 0.809 |
| Urban | 50(23.1%) | 166(76.9%) | 1 | 1 | ||
| Maternal education level | No formal education | 24(37.5%) | 40(62.5%) | 2.75(1.23–6.14) | 3.43(0.87–13.5) | 0.295 |
| Primary school | 18(26.1%) | 51(73.9%) | 1.62(0.71–3.69) | 2.97(0.83–10.6) | ||
| Secondary school | 22(25%) | 66(75%) | 1.53(0.69–3.36) | 1.86(0.54–6.37) | ||
| Above secondary school | 12(17.9%) | 55(82.1%) | 1 | 1 | ||
| Maternal occupation | Housewife | 34(29.6%) | 81(70.4%) | 1.73(0.88–3.41) | 1.28(0.56–2.94) | 0.171 |
| Small scale business | 26(28.6%) | 65(71.4%) | 1.65(0.81–3.36) | 2.14(0.94–4.88) | ||
| Employed | 16(19.5%) | 66(80.5%) | 1 | 1 | ||
| Family monthly income | <500 ETB | 9(45%) | 11(55%) | 2.65(1.04–6.72) | 1.77(0.50–6.28) | 0.533 |
| 501–1000 ETB | 12(34.3%) | 23(65.7%) | 1.69(0.79–3.61) | 1.63(0.55–4.81) | ||
| >1000 ETB | 55(23.6%) | 178(76.4%) | 1 | 1 | ||
| ANC follow up | No | 10(35.7%) | 18(64.3%) | 1.63(0.72–3.72) | 0.35(0.05–2.69) | 0.312 |
| Yes | 66(25.4%) | 194(74.6%) | 1 | 1 | ||
| Iron and folic acid supplementation | No | 19(39.6%) | 29(60.4%) | 2.10(1.10–4.03) | 1.40(0.05–37.1) | 0.842 |
| Yes | 57(23.8%) | 183(76.2%) | 1 | 1 | ||
| Trimester iron or folic acid supplementation started | First trimester | 14(16.7%) | 70(83.3%) | 1 | 1 | 0.503 |
| Second Trimester | 34(26.6%) | 94(73.4%) | 1.81(0.90–3.62) | 1.45(0.66–3.21) | ||
| Third trimester | 9(32.1%) | 19(67.9%) | 2.37(0.89–6.30) | 1.89(0.59–6.10) | ||
| History of vaginal bleeding during pregnancy | Yes | 11(37.9%) | 18(62.1%) | 1.82(0.82–4.06) | 1.02(0.31–3.32) | 0.977 |
| No | 65(25.1%) | 194(74.9%) | 1 | 1 | ||
| History of intestinal parasite infection during pregnancy | Yes | 17(35.4%) | 31(64.6%) | 1.68(0.87–3.26) | 1.52(0.67–3.50) | 0.320 |
| No | 59(24.6%) | 181(75.4%) | 1 | 1 | ||
| History of Malaria infection during pregnancy | Yes | 8(40%) | 12(60%) | 1.96(0.77–5.00) | 1.90(0.54–6.69) | 0.316 |
| No | 68(25.4%) | 200(74.6%) | 1 | 1 | ||
| Meat or animal product consumption per week | No | 17(37.8%) | 28(62.2%) | 1.89(0.97–3.70) | 1.13(0.40–3.21) | 0.817 |
| Yes | 59(24.3%) | 184(75.7%) | 1 | 1 | ||
| Vegetable consumption per week | Yes | 59(23.1%) | 196(76.9%) | 0.28(0.14–0.60) | 0.26(0.11–0.62) | 0.003 |
| No | 17(51.5%) | 16(48.5%) | 1 | 1 | ||
| Fruit consumption per week | No | 13(46.4%) | 15(53.6%) | 2.71(1.22–6.00) | 0.51(0.10–2.56) | 0.413 |
| Yes | 63(24.2%) | 197(75.8%) | 1 | 1 | ||
| Nutritional status (MUAC) | Malnourished | 12(36.4%) | 21(63.6%) | 1.71(0.80–3.67) | 1.13(0.37–3.49) | 0.829 |
| Normal | 64(25.1%) | 191(74.9%) | 1 | 1 | ||
| Newborn birth weight | Low birth weight | 10(30.3%) | 23(69.7%) | 1.25(0.56–2.75) | 1.82(0.72–4.57) | 0.203 |
| Normal | 66(25.9%) | 189(74.1%) | 1 | 1 | ||
| Maternal anemia | Yes | 23(39.7%) | 35(60.3%) | 1 | 1 | 0.003 |
| No | 53(23%) | 177(77%) | 0.46(0.25–0.84) | 0.34(0.17–0.69) | ||
Note: Bold numeric indicate significant association (p-value <0.05).
Abbreviations: COR, crude odds ratio; AOR, adjusted odds ratio.
Discussion
The overall prevalence of newborn anemia in this study was 26.4% (76/288) and according to WHO public health limits, this result showed that newborn anemia was a moderate public health problem at JMC.3 Several study results reported from different countries such as the Netherlands (21%),30 Brazil (32.6%),22 New York, US (21%),31 Nigeria (34.5%)32 and in Gondar, Ethiopia (25%)11 was consistent with this study result.
However, compared to a study conducted in Addis Ababa, Ethiopia, which found a 9% prevalence, the anemia prevalence among newborns in the current study was higher.10 Sample size variations and study population differences could be the cause of this discrepancy. In the present study, 288 study participants were included, whereas only 89 study participants were included to a study from Addis Ababa. Regarding study population difference, the study conducted from Addis Ababa excludes newborns from mothers who had a history of vaginal bleeding during pregnancy but in this study, 29 newborns from mothers who had a history of vaginal bleeding during pregnancy were included and this may increase the prevalence of anemia in the current study. Besides which, anemia prevalence in our study was higher than a report from Nepal (5.7%),28 the US(14%),33 and Iran (5.8% and 11.7%).34,35 The observed variation in the prevalence of anemia among the studies could potentially be attributed to factors such as low socioeconomic status, inadequate prenatal care coverage and quality, and clinical characteristics of the current study participants relative to those in the aforementioned countries.
On the other hand, the prevalence of anemia in the current study was lower than in the previously published study from Iran (53%),36 Benin (61.1%),37 Nigeria (65.6%),38 and Ghana (57.3%).23 The possible reason for the difference of this study from Iran could be ascribed to differences in research subjects way of delivery. The majority of our study participants (210/ 72.9%) were born vaginally and because of this 45 (59.2%) newborns were anemic, as well as 78 (27.1%) who were born through cesarean section and of these, 31 (40.8%) newborns were anemic but all study participants in Iran study were born by cesarean section. The placental transfusion force and duration may be weak during a cesarean section. This could be because of the effect of anesthesia, the uterine incision, and the immediate clamping of the umbilical cord, as well as the lack of uterine or vaginal pressure, which forces fluids out of the fetus’s lungs and supports breathing39 and compared to a vaginal delivery, an inadvertent incision of the placenta causes hemorrhage and anemia.11,36
The possible difference between our study and Benin might be attributed to that the Benin study was conducted among most of the newborns delivered to malaria-infected mothers. However, only 6.9% of newborns born from mothers having a history of malaria were included in the current study. Due to placental barrier disruption or damage, malaria parasites enter the fetus through congenital and resulting in fetal immune activation in response to maternal malarial antigens and causing the fetal RBC to be destroyed and then this resulted in lower Hgb (anemia).40
Another possible reason for the difference between the current study and the Benin study could be the inclusion of preterm newborn, whereas the current study only included term newborns. Premature babies are not yet of full gestational age, most of the iron needed by the newborn is stored during the third (last) trimester. As a result, premature newborn have a negative iron balance, leading to the development of anemia.41 Additionally, compared to full-term newborns, premature newborns' kidneys are still immature and cannot produce enough erythropoietin, leading to the development of anemia.42
The deviation of this study from the study conducted in Nigeria and Ghana could be due to all study newborns being born to HIV negativemothers. However, study participants born to HIV positive mothers were included in studies conducted in Ghana and Nigeria. In comparison to our study, HIV may increase the prevalence of anemia in newborns. According to a report from Kenya, having an HIV positive mother increases the risk of newborn anemia both directly through mother-to-child HIV transmission and indirectly through the fact that babies of HIV positive mothers have worse anemia than babies of HIV negative mothers.43
In the current study, based on red blood cell indices especially MCV value (normal range: 91.6–113.22),2 microcytic, normocytic and macrocytic anemia among newborns were 7.9%, 31.6%, and 60.5% respectively. Additionally, 183 (76.2%) babies born to mothers taking iron and folic acid supplementation were non-anemic but, 57 (23.8%) babies born to mothers not taking iron and folic acid supplementation were anemic. The possible reason for those may be due to anemia in a newborn can be grouped into three major classes based on specific causes; which is anemia caused by blood loss, impaired RBC production, and increased destruction of RBC. Blood loss is the commonest cause of neonatal anemia including obstetrical causes (placental abruption, placenta previa, and trauma to placenta or umbilical cord during delivery), feto-maternal hemorrhage, fetoplacental transfusion, twin-twin transfusion, and internal hemorrhage. Increased destruction of RBC can happen as a result of immune hemolysis (blood group incompatibility), acquired hemolysis (like due to infection), and rarely hereditary RBC disorders including RBC enzyme defects, RBC membrane defects, hemoglobinopathies, congenital hemolytic anemia (α-thalassemia, membrane disorders). Impaired RBC production can happen as a result of aplastic or hypoplastic anemia and Bone marrow suppression.13,44
Based on this finding, maternal dietary habit of eating vegetables during pregnancy was significantly associated with newborn anemia, which is supported by studies published in Qatar16 and Gondar.11 The results showed that babies born from mothers who ate vegetables were 74% less likely to develop anemia than babies born from mothers who ate no vegetables. This may be because of that vegetables are important sources of micronutrients such as non-heme iron and folic acid, which are used by the mother and her newborn to synthesize Hgb and new red blood cells. And also vegetarians (who consume vegetables at least once per week) generally have enough iron because they get a lot of vitamin C from their diet, which helps with non-heme iron absorption. A reduced development of anemia in the newborn is the result of the fetus’s active parasitic uptake of iron from the mother’s circulation, which it then transfers into the fetal circulation through the placenta for Hgb synthesis.45
The other variable that showed a significant association with newborn anemia was maternal anemia which is supported by a study reported in Nepal,28 Benin,37 Nigeria,32 and Ghana.23 Babies born to non-anemic mothers were 34% less likely to develop newborn anemia as compared to newborns born from anemic mothers. This could be because low levels of Hgb are a late symptom of iron deficiency, and the low levels of Hgb in these newborns could be a sign of low iron stores at birth. This suggests that a mother’s anemia during childbirth affects the hemoglobin levels of her newborn.
Hence, this observation contradicted the common belief that the fetus continues to extract and receive iron to meet its needs even if the mother is anemic. However, in severe maternal anemia, the placental mechanism of iron transport from the mother to the fetus may be impaired and leading to decreased cord blood hemoglobin levels.10,46 The relationship between maternal anemia and newborn anemia is a complex one, and there are many unanswered questions. To fully understand this relationship, more research using molecular biological tools is required.
Overall, this study yielded important insights about the prevalence and associated factors of anemia in newborns at JMC. The results of this study can be used by health professionals and policymakers to plan improvements at this age. Therefore, effective intervention packages need to reduce anemia among pregnant women through iron or folic acid supplementation, improve maternal dietary habit of vegetable consumption and properly evaluated, control and treated anemia during pregnancy in the study area may reduce anemia in pregnant women and their babies.
The major limitation of this study was serum ferritin concentration test was not performed, which suggests that iron deficiency may be the cause of anemia in newborns. Furthermore, due to the cross-sectional nature of the study design and the absence of an assessment of the occurrence of hemorrhage between the fetus and the mother prior to delivery, it is also unable to infer direct biological causality.
Conclusion and Recommendations
Overall, newborn anemia was a moderate public health problem in this study based on WHO cut of values. Maternal vegetable consumption habits and maternal anemia were the factors significantly associated with anemia. Efforts should be made to reduce its burden on a newborn. The prevention of maternal and newborn anemia was positively impacted by improving maternal nutritional status, particularly vegetable consumption during pregnancy. To prevent negative effects (like anemia) on a newborn, anemia during pregnancy should be appropriately assessed, controlled, and treated accordingly. Furthermore, large-scale longitudinal studies with larger samples are needed to identify the specific etiology and causes of neonatal anemia.
Acknowledgments
We would like to acknowledge Hematology and Immunohematology unit of Jimma Medical Center, Jimma University, study participants, data collectors and other individuals who directly or indirectly contributed to this study.
Funding Statement
The authors received no specific funding for this study.
Abbreviations
AOR, Adjusted odds ratio; CBC, Complete blood count; g/ dl, Gram per deciliter; Hgb, Hemoglobin; HIV, Human immunodeficiency virus; JMC, Jimma Medical Center; LBW, Low birth weight; MCH, Mean Corpuscular Hemoglobin; MCHC, Mean Corpuscular Hemoglobin Concentration; MCV, Mean Corpuscular Volume; MUAC, Mid-Upper Arm Circumference; RBC, Red Blood Cell; RDW, Red Cell Distribution Width; WBC, White blood cell; WHO, World Health Organization.
Data Sharing Statement
All important data are within the manuscript but the datasets used during analysis are available from the corresponding author on reasonable request (in SPSS code).
Ethics approval and consent to participate
Ethical clearance was obtained from the institutional review board (IRB) of Jimma University, Institute of Health with letter protocol number IHRPGD928/20 and our research protocol meets ethical standards outlined by the declaration of Helsinki, national and international guidelines. After ethical approval was received, permission to conduct the study was obtained from the Head of the School of Medical Laboratory Science and the chief clinical director of the JMC.
Written informed consent was obtained from each mother for participation in this study after explaining the objective and purpose of the study. All methods include umbilical cord blood sample collection from newborns were performed in accordance with the relevant guidelines and regulations that meets national and international guidelines as approved by an appropriate ethics committee. Any abnormal hematological test results of study subject were communicated to their attending physician immediately proper management and treatment.
Disclosure
The authors reported that they have no competing interests and no need of consent for publication.
References
- 1.World health organization. Hemoglobin Concentrations for the Diagnosis of Anemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Tiruneh T, Kiros T, Getu S. Hematological reference intervals among full-term newborns in Ethiopia: a cross-sectional study. BMC Pediatr. 2020;20(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mclean E, Cogswell M, Egli I, Wojdyla D, Benoist B. Worldwide prevalence of anemia, WHO vitamin and mineral nutrition information system, 1993 – 2005. Public Heal Nutr. 2009;12(4):444–454. [DOI] [PubMed] [Google Scholar]
- 4.Central Statistical Agency (CSA). Ethiopia] and ICF. Ethiopia Demographic and Health Survey, 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2017. [Google Scholar]
- 5.Osungbade KO, Oladunjoye AO. Anemia in developing countries: burden and prospects of prevention and control. Anemia. 2012;2012:120–125. [Google Scholar]
- 6.Ewusie JE, Ahiadeke C, Beyene J, Hamid JS. Prevalence of anemia among under-5 children in the Ghanaian population: estimates from the Ghana demographic and health survey. BMC Public Health. 2014;14(1):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dairo MD, Lawoyin TO. Socio-demographic determinants of anemia in pregnancy at primary care level: a study in urban and rural Oyo state, Nigeria. Afr J Med Med Sci. 2004;33(3):213–217. [PubMed] [Google Scholar]
- 9.Brabin B, Kalanda BF, Verhoeff FH, Chimsuku L, Broadhead R. Risk factors for fetal anemia in a malarious area of Malawi. Annals Tropic Pedi. 2013;24(4):311–321. [DOI] [PubMed] [Google Scholar]
- 10.Terefe B, Birhanu A, Nigussie P, Tsegaye A. Effect of maternal iron deficiency anemia on the iron store of newborns in Ethiopia. Anemia. 2015;2015(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiruneh A, Shiferaw E, Enawgawu B. Prevalence and associated factors of anemia among full-term newborn babies at university of Gondar comprehensive specialized hospital, northwest Ethiopia: a cross-sectional study. Italian J Pedi. 2020;46(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dereje I, Etefa T, Gebremariam T, Getaye A, Tunta A, Gerbi A. Prevalence of anemia and associated factors among term newborns in nekemte specialized hospital, western Ethiopia. J Multidiscip Healthc. 2021;14(1):2607–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lokeshwar M, Singhal T, Shah N. Anemia in the newborn. Indian J Pediatrics. 2003;70(11):893–902. [DOI] [PubMed] [Google Scholar]
- 14.Arora S, Doda V, Maria A, Kotwal U, Goyal S. Maternal anti-M induced hemolytic disease of newborn followed by prolonged anemia in newborn twins. Asian J Transfusion Sci. 2015;9(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Jin L, Liu J-M, Ye R, Ren A. Maternal hemoglobin concentration during gestation and risk of anemia in infancy: secondary analysis of a randomized controlled trial. J Pediatrics. 2016;175:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zainel AJAL, Osman SRO, Al-Kohji SMS, Selim NA. Iron deficiency, its epidemiological features and feeding practices among infants aged 12 months in Qatar: a cross-sectional study. BMJ Open. 2018;8(5):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter RC, Jacobson JL, Burden MJ, et al.. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 2010;126:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Hawsawi ZM, Al-Rehali SA, Mahros AM, Al-Sisi AM, Al-Harbi KD, Yousef AM. High prevalence of iron deficiency anemia in infants attending a well-baby clinic in northwestern Saudi Arabia. Saudi med j. 2015;36(9):1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mhanna RG, Rahal M, Iskandarani M, Hammoudi D. Incidence and risk factors associated with iron deficiency anemia among hospitalized Lebanese infants. Int J Pharm Pract. 2016;24(3):203–208. [DOI] [PubMed] [Google Scholar]
- 20.Elshazzly M, Anekar AA, Shumway KR, et al. Physiology, Newborn. Stat pearls publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499951/.Accessed March 04, 2024 [PubMed]
- 21.Gedefaw L, Ayele A, Asres Y, Mossie A. Anemia and associated risk factors among Pregnant women attending antenatal care clinic in wolayita sodo town, southern Ethiopia. Ethiop J Health Sci. 2015;25(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asá S, Erica W, Tatiane AD. Anemia in pregnancy: impact on weight and in the development of anemia in newborn in Brazil. Nutrición Hospitalaria. 2015;32(5):2071–2079. [DOI] [PubMed] [Google Scholar]
- 23.Laar AK, Grant FE, Addo Y, et al. Predictors of fetal anemia and cord blood malaria parasitemia among newborns of HIV positive mothers. BMC Res Notes. 2013;6(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demilew YM, Alene GD, Belachew T. Dietary practices and associated factors among pregnant women in west gojjam zone, northwest Ethiopia. BMC Pregnant Childb. 2020;20:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Workicho A, Belachew T, Feyissa GT, et al. Household dietary diversity and animal source food consumption in Ethiopia: evidence from the 2011 welfare monitoring survey. BMC Public Health. 2016;16:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang AM, Determining a Global Mid-Upper Arm Circumference Cutoff to Assess Malnutrition in Pregnant Women. Washington, DC: FHI 360/Food and Nutrition Technical Assistance III Project (FANTA); 2016. [Google Scholar]
- 27.Diddana TZ. Factors associated with dietary practice and nutritional status of pregnant women in Dessie town, northeastern Ethiopia: a community based cross-sectional study. BMC Pregnancy Childbirth. 2019;19(1):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timilsina S, Karki S, Gautam A, Bhusal P, Paudel G, Sharma D. Correlation between maternal and umbilical cord blood in pregnant women of pokhara valley: a cross-sectional study. BMC Pregnancy Childbirth. 2018;18(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ad P, Ph R, Ra P, et al. Relationship between the iron status of pregnant women and their newborns. Rev Saude Publica. 2007;41:321–327. [DOI] [PubMed] [Google Scholar]
- 30.Kalteren WS, Horst HJH, de Vetten L, Kooi EM, Bos AF. Perinatal anemia is associated with neonatal and neurodevelopmental outcomes in infants with moderate to severe perinatal asphyxia in the Netherlands. Neonatology. 2018;114(4):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Guillet R, Cooper EM, et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;79(1):1–42. [DOI] [PubMed] [Google Scholar]
- 32.Adediran A, Gbadegesin A, Adeyemo TA, et al. Cord blood hemoglobin and ferritin concentrations in newborns of anemic and non-anemic mothers in Lagos, Nigeria. Nigerian Med J. 2013;54(1):22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ru Y, Pressman EK, Guillet R, Katzman PJ, Bacak SJ, O’Brien KO. Predictors of anemia and iron status at birth in neonates born to women carrying multiple fetuses. Pediatric Res. 2018;84(2):199. [DOI] [PubMed] [Google Scholar]
- 34.Esmailnasab N, Afkhamzadeh A, Delpisheh A. Prevalence of maternal anemia and its association with hemoglobin levels of newborn babies. Arch Dis Child. 2012;97(2):218–220. [Google Scholar]
- 35.Mamoury G, Hamedy A, Akhlaghi F. Cord hemoglobin in newborns in correlation with maternal hemoglobin in northeastern Iran. Iranian J Med Sci. 2015;28(4):166–168. [Google Scholar]
- 36.Baharvand P, Fathi M, Eliyasy H, Abdolkarimi B, Kiani AA. The effect of delivery type on neonatal blood indices in an Iranian population. Biomed Res Ther. 2018;5(10):2768–2775. [Google Scholar]
- 37.Koura GK, Ouedraogo S, Le PA, et al. Anemia during pregnancy: impact on birth outcome and infant hemoglobin level during the first 18 months of life. Tropical Med Int Health. 2012;17(3):283–291. [DOI] [PubMed] [Google Scholar]
- 38.Uneke C, Iyare F, Sunday-Adeoye I, Asiegu O, Nwosu K, Ajayi J. Effects of maternal plasmodium falciparum malaria. Anemia HIV Infec Fetal Hemo Lev Nigeria J Gyn Obst. 2008;12(1):1–7. [Google Scholar]
- 39.Zhou Y-B, Zhu L-P, Liu J-M, Liu J-M. Impact of cesarean section on placental transfusion and iron-related hematological indices in term neonates: a systematic review and meta-analysis. Placenta. 2014;35(1):1–8. doi: 10.1016/j.placenta.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 40.Bs H, Ngeleza S, Jj A, et al. Histopathologies, immunolocalization, and a glycan binding screen provide insights into Plasmodium falciparum interactions with the human placenta. Biol Reprod. 2013;88(6):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domellöf M, Georgieff MK. Postdischarge iron requirements of the preterm infant. J Pediatric. 2015;167(4):31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asada N. Tubular immaturity causes erythropoietin-deficiency anemia of prematurity in preterm neonates. Sci Rep. 2018;8(1):4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Eijk AM, Ayisi JG, Ter Kuile FO, et al. Malaria and human immunodeficiency virus infection as risk factors for anemia in infants in Kisumu, western Kenya. Am J Trop Med Hyg. 2002;67:44–53. [DOI] [PubMed] [Google Scholar]
- 44.Kumar K, Nagar N, Sarnadgouda P. Anemia in new born. Pediatr Dimensions. 2016;1(4):87–90. [Google Scholar]
- 45.Marangoni F, Cetin I, Verduci E, et al. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients. 2016;8(10):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal R, Srivastava P. Cord blood hemoglobin levels in relation to maternal anemia. Int J Pediatr Res. 2018;5(7):351–354. [Google Scholar]