Abstract
Introduction
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as an adjunct to CPR for nontraumatic cardiac arrest (NTCA). This translational study investigated the impact of varying low-flow duration (15- vs 30-mins) on REBOA's hemodynamic performance and ability to achieve return of spontaneous circulation (ROSC) in a porcine model.
Methods
Thirty-two pigs were anesthetized and placed into ventricular fibrillation. All animals received a 4-min no-flow period before CPR was initiated. Animals were randomized into four groups: 15- vs 30-minutes of CPR; REBOA vs. no-REBOA. After completion of 15- or 30-minute low-flow, ACLS was initiated and REBOA was inflated in experimental animals.
Results
In the 15-mins groups, there were no differences in the rates of ROSC between REBOA (4/8, 50%) and control (4/8, 50%; p = 0.99). However, in the 30-min groups, the REBOA animals had a significantly higher rate of ROSC (6/8, 75%) compared to control (1/8, 12.5%; p = 0.04). In the 7-mins after REBOA deployment in the 30-min animals there was a statistically significant difference in coronary perfusion pressure (REBOA 42.1 mmHg, control 3.6 mmHg, p = 0.038). Importantly, 5/6 animals that obtained ROSC in the 30-min group with REBOA re-arrested at least once, with 3/6 maintaining ROSC until study completion.
Conclusion
In our porcine model of NTCA, REBOA preferentially improved hemodynamics and ROSC after a 30-mins period of low-flow CPR. REBOA may be a viable strategy to improve ROSC after prolonged downtime, however, more hemodynamic support will be required to maintain ROSC.
Keywords: Cardiopulmonary resuscitation, Aortic occlusion, Endovascular, Intra-aortic balloon, Emergency medical services, Swine
Introduction
Non-traumatic cardiac arrest (NTCA) is associated with significant morbidity, mortality, and financial cost.1, 2 In both the United States and Europe, only 8–10% of patients survive to hospital discharge and a smaller fraction survive with intact neurologic function.3 Standard-of-care treatments of NTCA include cardiopulmonary resuscitation (CPR) and advanced cardiac life support algorithms (ACLS). However, due to anatomic limitations, CPR often fails to sufficiently raise aortic diastolic pressure or maintain coronary artery perfusion pressures (CPP), factors important for a return of spontaneous circulation (ROSC).4 Resuscitative endovascular balloon occlusion of the aorta (REBOA), an intervention designed to treat non-compressible hemorrhage, is a possible augment to traditional CPR.5 REBOA is a balloon-tipped catheter that is inserted into the femoral artery and advanced retrograde into the aorta. By expanding the REBOA balloon in the thoracic aorta (Zone 1, between the left subclavian artery and the coeliac axis) the limited blood flow generated by CPR is restricted to the proximal aorta, improving perfusion of the heart and brain.6 Translational research and early human case series have shown that Zone 1 REBOA placement during CPR increases CPP, cerebral perfusion, end-tidal CO2 (ETCO2), and ROSC.7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Due to these favorable hemodynamic effects, Phase 1 and Phase 2 human clinical trials are being planned across the United States and Europe.17, 18, 19, 20, 21 However, the ideal patient enrollment criteria for these trials are difficult to define, as the translational research using REBOA for NTCA have used inconsistent durations of both cardiac no-flow and low-flow states. The no-flow period lasts from the cessation of blood flow to the heart and brain until the initiation of CPR. The low-flow state, by contrast, is defined as the onset of CPR until ROSC or cessation of resuscitation. Previous translational studies have used no-flow periods ranging from 0 to 8 minutes and low-flow periods ranging from 0 to 24 minutes before aortic occlusion and attempts to achieve ROSC.8, 9, 10, 11, 12, 13, 15, 16, 22, 23 Furthermore, these studies are confounded by the difficulty of performing cardiac arrest research where the timing and ability to achieve ROSC cannot be independently controlled. While this variability is expected in translational animal research, these varying time periods represent vastly different patient populations and clinical data shows that changes in low- and no-flow periods directly impact patient centered outcomes. Using conventional resuscitation techniques without REBOA, 75% of patients that survive with intact neurologic function achieved ROSC prior to 15-minutes of low-flow time. After 15-minutes of low-flow, the probability of functional recovery fell to ∼2%.24 However, a Norwegian case series of REBOA for NTCA showed that neurologically intact survival was possible despite low-flow periods greater than 45 minutes.25.
In order to inform upcoming Phase 2 and 3 clinical trials, we sought to explore the relationship between REBOA and varying NTCA low-flow periods. If REBOA can be deployed in patients with up to 30-minutes of low-flow time, it can likely be applied to out-of-hospital cardiac arrest. However, if limited to only 15-minutes of low-flow time, its application outside of major, academic hospitals may be extremely limited. We sought to fill this knowledge gap by studying the impact of varying low-flow periods (15-mins vs. 30-mins) on REBOA in a porcine model of NTCA. We originally hypothesized that shorter periods of low-flow (15-mins) would preferentially benefit from REBOA compared to longer low-flow periods (30-mins), relative to control animals (no REBOA).
Methods
Overview
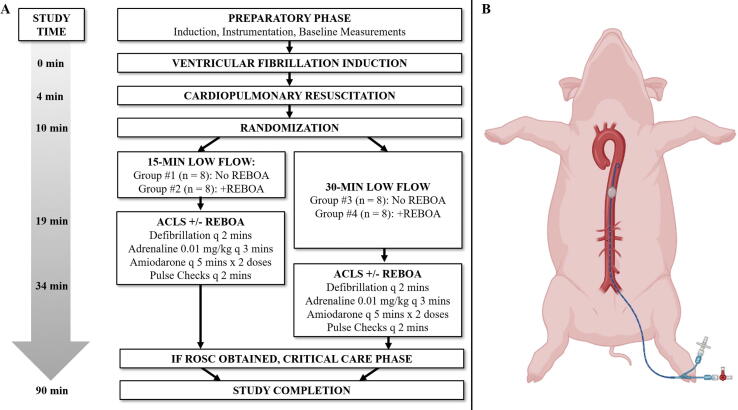
The 59th Medical Wing Institutional Animal Care and Use Committee (IACUC) approved this research (Protocol #FWH20210151AR). All research participants involved in the study obtained appropriate training through the American Association for Laboratory Animal Science (AALAS), and animal care was performed in strict compliance with the Guide for the Care and Use of Laboratory Animals in a facility accredited by AAALAC, International. Healthy, adult, castrated male and non-pregnant female Yorkshire-cross (Sus scrofa) were allowed to acclimate for at least 7-days in temperature- and light controlled pens with access to environmental enrichment. Animals weighed between 70 and 90 kg and were fasted the night before the experiment. The conduct of the protocol is illustrated in Fig. 1.
Fig. 1.
A) Experimental Design. For 15-Min Low Flow Groups #1 and #2, ACLS and REBOA initiates at T = +19-mins, and continuing until ROSC or 30-minutes of CPR. For 30-Min Low Flow Groups 3 and 4 ACLS and REOBA initiates at T = +34 minutes and continuing until ROSC or 30-minutes of continuous CPR; B) Illustration of REBOA’s position in Zone 1 of the porcine model. See Methods Section for full description of animal’s instrumentation.
Animal preparation
Animals were premedicated with 6.6 mg/kg intramuscular tiletamine/zolazepam. Following endotracheal intubation, general anesthesia was maintained with 1.5–2.5% isoflurane in 30–100% oxygen. Pigs received 15 mL/kg of 0.9% sodium chloride intravenously. Animals were mechanically ventilated with tidal volumes of 8–10 mL/kg, a positive end-expiratory pressure of 5 cmH2O, and a respiratory rate of 10–15 breaths per minute, titrated to maintain ETCO2 at 40 ± 5 mmHg.
All vascular access was obtained via Seldinger technique under ultrasound guidance. Both external jugular veins were cannulated. A pressure tipped catheter was placed into the superior vena cava to measure central venous pressure (CVP). A transvenous pacing (TVP) wire was placed through the right external jugular via a 9-Fr resuscitation catheter (Arrowg+ard Blue MAC; Teleflex, Wayne, PA) to induce ventricular fibrillation (VF). The right carotid was cannulated with a 5-Fr arterial line (Micropuncture® Kit, Cook, Bloomington, IN) and the left femoral artery was cannulated with a 7-Fr sheath to monitor proximal and distal blood pressure, respectively. The left brachial was accessed with a 5-Fr cannula to allow for laboratory draws proximal to the REBOA balloon. Fluoroscopy confirmed the position of the uninflated aortic balloon in Zone 1, the TVP within the right ventricle, and to mark the location of the apex of the heart. The animals were connected to an electrocardiogram monitor and defibrillator (R Series, ZOLL, Chelmsford, Massachusetts) with pre-positioned defibrillator pads (Stat-padz, ZOLL, Chelmsford, Massachusetts).
A pneumatic mechanical CPR device (Life-Stat; Grand Rapids, MI) was placed around the chest with the compression device on the marked cardiac apex. Animals were placed in a custom, wedge-shaped surgical table to stabilize animals during chest compressions. Baseline laboratory and arterial blood gas samples were obtained after instrumentation.
Intervention
After the initial setup, the animals were given a dose of diazepam 0.05 mg/kg intravenously (max 3.0 mg) to ensure adequate sedation prior to induction of VF. A 9-V battery was applied to the electrodes of the TVP lead until VF was induced (T = 0 mins). Mechanical ventilation and isoflurane were discontinued and the TVP lead was removed. After 4 mins of no-flow VF (T = 4 mins) mechanical CPR was initiated and the ventilator was set at 10 breaths/min with 100% oxygen. At T = 10 minutes, the animals were randomised using the sealed envelope method26 into one of four groups:
-
•
Group #1: 15-mins low-flow CPR without REBOA (control)
-
•
Group #2: 15-mins low-flow CPR with REBOA
-
•
Group #3: 30-mins low-flow CPR without REBOA (control)
-
•
Group #4: 30-mins low-flow CPR with REBOA
After completion of the low-flow states (T = 19 mins for Groups #1/#2; T = 34 mins for Groups #3/#4), the pre-positioned aortic occlusion balloon was inflated and ACLS algorithms were initiated. Aortic occlusion was confirmed by the loss of distal femoral arterial pressure during CPR. Animals were assessed for ROSC at two-minute intervals and defibrillation was performed (200 joules, biphasic) if VF was observed. Intravenous 0.01 mg/kg adrenaline (epinephrine) was administered in three-minute intervals while the animal remained in cardiac arrest. After five minutes of ACLS, two doses of amiodarone were administered every 5 minutes (5 mg/kg IV for the first dose and 2.5 mg/kg for the second dose), if required. The ACLS phase continued for thirty minutes or until the subject achieved ROSC, defined as visible arterial blood pressure fluctuations consistent with cardiac perfusion lasting at least 30 seconds in duration.
If ROSC was obtained in a subject randomised to receive REBOA (Groups #2 and #4), the REBOA balloon was gradually deflated over the subsequent five minutes (removing 1/10th of the volume every thirty seconds). After initial ROSC, if pulses were subsequently lost, the ACLS algorithm was re-started and, in Groups #2 and #4, the REBOA balloon was re-inflated. If subsequent ROSC was achieved, the balloon was deflated by the same protocol. If after 30 minutes ROSC had not been achieved, the subjects were euthanized. This pattern was allowed to repeat until a period of 30 minutes passed without ROSC or until completion of the study period (T = 90).
In animals that achieved and sustained ROSC, blood pressure was controlled with adrenaline titration following American Health Association ACLS guidelines for a mean arterial pressure (MAP) goal >65 mmHg. Following 30 seconds of hypotension (MAP <65 mmHg), a continuous infusion of adrenaline was started at a dose of 0.1 mcg/kg/min and increased by 0.1 mcg/kg/min every two minutes to a maximum of 1.0 mcg/kg/min. Once the MAP goal was reached, the infusion was adjusted by 0.1/kg/min mcg to maintain the MAP in the target range (65–75 mmHg). Hypoglycemia and electrolyte disturbances were corrected via predetermined protocols.27.
Outcomes
The primary outcome of this study was ROSC. Pre-determined secondary outcomes include sustained ROSC >5 mins, survival to the end of the protocol, diastolic blood pressure (DBP), CPP (calculated as DBP - minimum CVP)28, 29, arterial serum lactic acid, and total adrenaline requirements.
Analysis
Data are presented in the text as mean +/- standard deviation for continuous variables and fraction (%) for categorical variables. Data in graphs is shown as mean +/- standard error of the mean. Groups were compared using analysis of variance (ANOVA), Kruskal–Wallis, Fisher’s exact, log-rank analysis, or two-way repeated ANOVA, as appropriate. Normality was tested using the Shapiro-Wilk test. Tukey post hoc test was used for multiple comparisons. Differences between groups were considered significant when p < 0.05. All statistical analysis was performed using Sigmaplot 12 (Grafiti, Palo Alto, CA).
Results
Baseline Characteristics
Thirty-two animals were included in the study with no exclusions. Prior to initiation of the experiment, there were no significant baseline differences among groups with regard to weight, sex, hemodynamics, or laboratory results (Table 1).
Table 1.
Demographics, laboratory, and hemodynamic information. Baseline hemodynamics were obtained ten-minutes prior to induction of ventricular fibrillation. Randomization hemodynamics were obtained ten-minutes following ventricular fibrillation, after a 4-minute no-flow period and 6-minutes of CPR. p value represents one-way ANOVA between all four groups except for pCO2 and diastolic blood pressure, as indicated by #, where ANOVA on ranks was used due to non-normal distributions.
| Group 1 | Group 2 | Group 3 | Group 4 | |||
|---|---|---|---|---|---|---|
| n = 8 | n = 8 | n = 8 | n = 8 | |||
| Parameter | 15 Mins CPR No REBOA | 15 Mins CPR Yes REBOA | 30 Mins CPR No REBOA | 30 Mins of CPR Yes REBOA | p | |
| Demographic | Weight (kg) | 76.6 ± 5.4 | 78.5 ± 7.0 | 77.8 ± 6.3 | 76.6 ± 6.0 | 0.91 |
| Sex (m:f) | 6:2 | 4:4 | 4:4 | 5:3 | 0.455 | |
| Laboratory | pH | 7.47 ± 0.02 | 7.43 ± 0.03 | 7.45 ± 0.06 | 7.47 ± 0.02 | 0.208 |
| pCO2 (mmHg) | 44.13 ± 3.36 | 44.83 ± 1.32 | 44.44 ± 6.63 | 41.93 ± 2.72 | 0.168# | |
| pO2 (mmHg) | 169.2 ± 38.76 | 174.29 ± 43.95 | 157.38 ± 38.21 | 163.88 ± 27.74 | 0.827 | |
| HCO3 (mEq/L) | 44.13 ± 3.36 | 44.83 ± 1.32 | 44.44 ± 6.63 | 41.93 ± 2.72 | 0.344 | |
| Lactate (mmol/L) | 1.18 ± 0.31 | 1.56 ± 0.62 | 1.69 ± 0.51 | 1.4 ± 0.58 | 0.247 | |
| Potassium (mEq/L) | 4.01 ± 0.25 | 4.03 ± 0.30 | 3.98 ± 0.38 | 3.94 ± 0.18 | 0.928 | |
| Glucose (mg/dL) | 72.88 ± 16.42 | 86.13 ± 18.31 | 74.13 ± 22.23 | 72.25 ± 12.07 | 0.362 | |
| Hemoglobin (g/dL) | 8.51 ± 1.12 | 9.55 ± 0.99 | 9.48 ± 0.91 | 8.63 ± 0.76 | 0.07 | |
| Hemodynamics At Baseline (T = −10) | Systolic Blood Pressure (mmHg) | 89.9 ± 13.6 | 86.1 ± 9.7 | 100.9 ± 9.8 | 90.8 ± 12.0 | 0.084 |
| Diastolic Blood Pressure (mmHg) | 55.5 ± 14.8 | 53.8 ± 8.5 | 66.0 ± 11.9 | 53.4 ± 9.1 | 0.108 | |
| Mean Arterial Pressure (mmHg) | 69.3 ± 15.0 | 67.3 ± 10.0 | 80.6 ± 11.4 | 68.5 ± 11.0 | 0.118 | |
| Heart Rate (beats/min) | 42.3 ± 1.8 | 43.1 ± 2.4 | 42.3 ± 2.1 | 41.4 ± 2.1 | 0.442 | |
| End Tidal CO2 (mmHg) | 66.2 ± 13.5 | 74.0 ± 23.6 | 84.0 ± 14.0 | 78.6 ± 17.1 | 0.244 | |
| Hemodynamics At Randomization (T = +10) | Systolic Blood Pressure (mmHg) | 103.2 ± 28.4 | 95.8 ± 28.7 | 102.1 ± 18.4 | 98.8 ± 25.9 | 0.938 |
| Diastolic Blood Pressure (mmHg) | 20.3 ± 16.0 | 21.3 ± 15.6 | 28.0 ± 9.6 | 22.3 ± 10.9 | 0.288# | |
| Mean Arterial Pressure (mmHg) | 48.0 ± 14.3 | 46.1 ± 18.3 | 52.7 ± 11.4 | 47.8 ± 12.1 | 0.815 | |
| End Tidal CO2 (mmHg) | 28.5 ± 10.1 | 32.0 ± 11.4 | 31.3 ± 9.7 | 32.1 ± 11.0 | 0.892 | |
Arrest and Randomization
After induction of VF and initiation of chest compressions, there were physiologic changes in hemodynamics consistent with the initiation of mechanical CPR.4 As a trend, systolic blood pressure (SBP) increased, whereas DBP, MAP, and ETCO2 all decreased. At randomization (T = +10 mins), there were no significant differences between groups in observed hemodynamics with ongoing CPR (Table 1).
Primary Outcome: ROSC
There was no significant difference in the rate of ROSC in the 15-minute groups (Groups #1 and #2), as shown in Table 2, as 50% of both REBOA and non-REBOA groups achieved ROSC (p = 0.999). In both groups #1 and #2, one animal re-arrested after initial ROSC but secondary ROSC was achieved. All animals that achieved ROSC in Group #1 and Group #2 sustained ROSC and survived to the end of the experiment (p = 0.999).
Table 2.
Rates of ROSC and re-arrest, time until ROSC, and total amount of adrenaline used, by group. p value represents Fisher’s exact test or student’s t-test comparing Group #1 vs #2 and #3 vs 4, except for adrenaline as indicated by #, where ANOVA on ranks was used due to non-normal distributions. There is a statistically and clinically significant (p = 0.041) difference in the rate of ROSC between Group 4 (30-minute animal that received REBOA) compared to Group 3, the 30-min control group.^No standard deviation is available for Group 3′s time to ROSC as only one animal obtained ROSC. Data not tested presented on the table as “n.t.”
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
||||
|---|---|---|---|---|---|---|---|
| 15 Mins CPR No REBOA | 15 Mins CPR Yes REBOA | p | 30 Mins CPR No REBOA | 30 Mins of CPR Yes REBOA | p | ||
| Rate of ROSC | 4/8 (50%) | 4/8 (50%) | 0.999 | 1/8 (12.5%) | 6/8 (75%)* | 0.041 | |
| Rearrest | 1/4 (25%) | 1/4 (25%) | 0.999 | 0/1 (0%) | 5/6 (83.3%) | 0.286 | |
| Of Animals with Primary ROSC: | Sustained ROSC >5 Mins | 4/8 (50%) | 4/8 (50%) | 0.999 | 1/1 (100%) | 5/6 (83.3%) | 0.999 |
| Survival to End of Experiment | 4/8 (50%) | 4/8 (50%) | 0.999 | 1/1 (100%) | 3/6 (50%) | 0.999 | |
| Time to ROSC After Initiation of ACLS +/- REBOA (mins) | 12.79 ± 5.37 | 19.25 ± 8.69 | 0.253 | 8.17^ | 12.64 ± 5.43 | n.t. | |
| Total Adrenaline Used (mg/kg) | 0.0778 ± 0.0325 | 0.0955 ± 0.0308 | 0.161# | 0.0868 ± 0.0297 | 0.107 ± 0.0308 | 0.105# | |
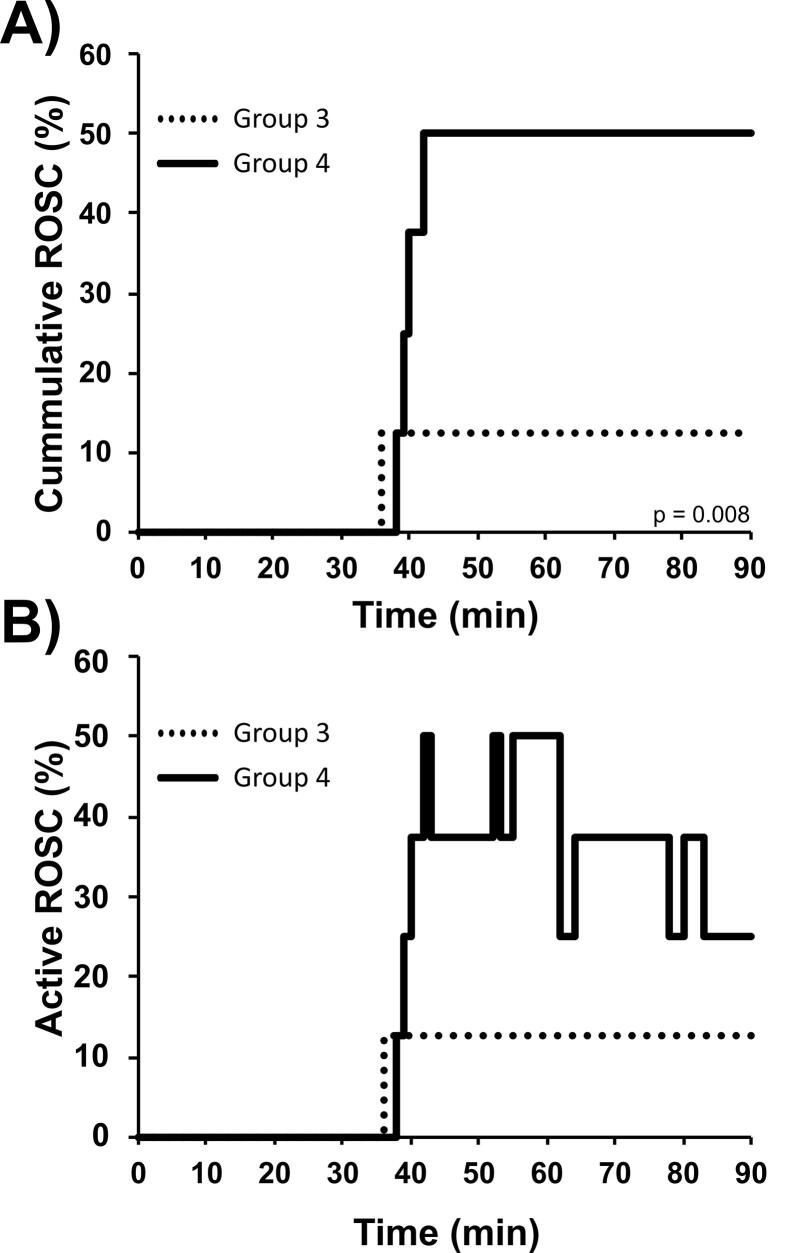
There was a statistically and clinically significant difference in the rate of ROSC in the 30-minute animals that received REBOA (Group #4: 6 / 8, 75%) compared to the 30-minute control (Group #3: 1 / 8, 12.5%; p = 0.041). However, 5 / 6 (83.3%) Group #4 animals re-arrested at least once after preliminary ROSC. A majority sustained ROSC for at least 5-mins and half (3 / 6, 50%) sustained ROSC to the end of the experimental period. The dynamic change of ROSC between groups is visually represented in Fig. 2. Using a log rank analysis comparing Groups 3 and 4, there was a statistically significant difference in the time needed to achieve ROSC (after initiation of ACLS +/- REBOA) in the 30-minute animals that received REBOA (p = 0.008).
Fig. 2.
ROSC rates in Groups 3 (30-Minutes CPR, No REBOA) and Group 4 (30-Minutes CPR, +REBOA). A) Cumulative ROSC (%) independent of rearrest, p = 0.008 results from a log rank analysis between the two groups; B) Active ROSC (%) of each group, showing the dynamic changes of ROSC and re-arrest in Groups 3 and 4. Changes are shown in 30-second increments.
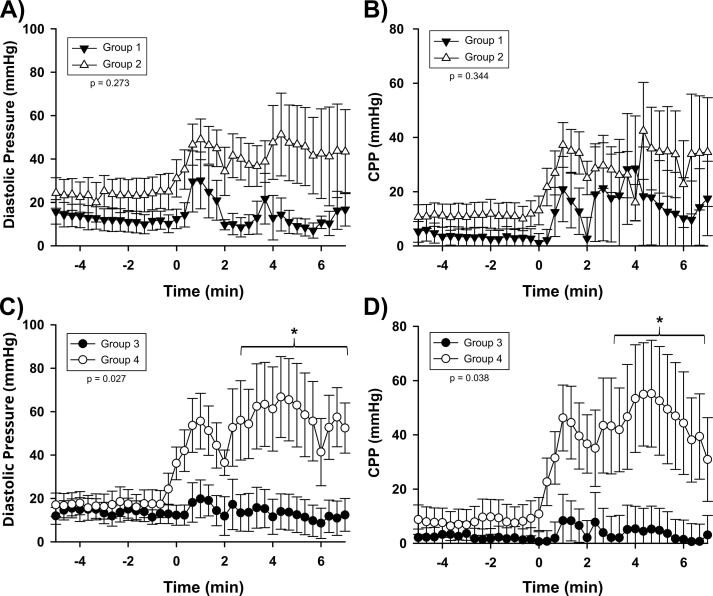
Adrenaline, hemodynamic and laboratory results
In order to compare groups’ hemodynamics without the complication of varying ROSC times, the first seven minutes after intervention was compared among groups, as shown in Fig. 3. No significant differences were found when comparing Groups 1 and 2 with regards to SBP, DBP, or CPP (p = 0.211, 0.273, and 0.3444, respectively). However, in Groups 3 and 4, there was a statistically significant difference observed in DBP (p = 0.027) and CPP (p = 0.038), but not with systolic blood pressure (p = 0.120) in the 30-minute groups. Group 4 had a higher DBP of 54.5 mmHg, compared to 13.7 mmHg in the Group 3 control animals without REBOA. Similarly, the mean CPP was 42.1 mmHg in Group 4 animals with REBOA, compared to 3.6 mmHg in Group 3.
Fig. 3.
A) Group 1 vs Group 2 Diastolic Blood Pressure; B) Group 1 vs Group 2 Coronary Perfusion Pressure; C) Group 3 vs Group 4 Diastolic Blood Pressure; D) Group 3 vs Group 4 Coronary Perfusion Pressure. Data is recorded continuously but shown in 10 second increments. Pressure measured in the carotid artery. Error bars are showing standard error of the mean. This shows the improvement REBOA provided to both DBP and CPP in the 30-min animals, compared to the 15-minute animals. *p < 0.05 at specific timepoints.
There were no significant differences in the amount of adrenaline required, lactic acid levels, ETCO2, or arterial blood gas results between groups (Table 2). Across all groups, the animals had severe laboratory derangements consistent with prolonged CPR and resuscitation at T = 60 minutes: pH: 7.05 ± 0.16; lactic acid: 12.54 ± 3.0 mmol/L; HCO3: 12.86 ± 3.72 mEq/L; base excess: −10.60 ± 8.67 mEq/L; K+: 5.74 ± 1.64 mEq/L.
Discussion
Given the relationship between timely interventions in NTCA to ROSC and neurologically intact survival, it is essential to understand the relationship between low-flow CPR period and REBOA prior to large-scale human trials. We developed this pilot study to determine the impacts of REBOA on varying low-flow periods (15- vs 30-mins) We demonstrate that REBOA provides statistically and clinically higher ROSC rates to animals with a 30-min low flow period but not 15-min low flow period.
During in-hospital NTCA, physicians typically arrive 2–5 mins after cardiac arrest.30, 31, 32 By contrast, patient arrival to Emergency Department after out-of-hospital NTCA ranges from 20 to 45 minutes.33, 34 While these scenarios are complicated by varying downtimes, initiation of bystander CPR, etc., they represent a realistic delay in provider arrival before advanced interventions such as REBOA can be placed. REBOA can be deployed in approximately 5–10 minutes by a provider with appropriate training.33 In our study, we selected the 15- and 30-min low flow periods to roughly mimic the low-flow times required for a provider to arrive, successfully place REBOA, and inflate the balloon for in-hospital versus out-of-hospital NTCA.
Previous research has established a direct relationship between higher DBP and/ or CPP and the ability to achieve ROSC.35, 36 The 30-minute low-flow REBOA animals (Group #4) had statistically significant improvements in DBP and CPP, which corresponds with their higher rates of ROSC. By contrast, there were no statistically differences in DBP, CPP, nor ROSC between Groups #1 and #2 after 15-minutes of low-flow. This was contrary to our expected hypothesis that REBOA would provide greater benefits to DBP, CPP, and ROSC after 15-min low-flow, compared to 30-minutes of low-flow. While a review of our data does not elucidate a clear explanation, there are several possible hypotheses for this unexpected phenomenon. First, it is possible that REBOA’s change in DBP provides preferential benefits as aortic tone decreases and ischemic injury accumulates during CPR. We suspected that 15-minutes of low-flow CPR were sufficient to negate these effects, however, there may be complex physiologic mechanisms that occur with increasingly severe metabolic injury. Secondly, there is the possibility that REBOA’s benefits are tempered by corresponding detrimental effects in earlier stages of resuscitation, such as unusual blood flow patterns or left ventricular dilation. In a previous study, early Zone 1 REBOA after 5 mins of low-flow improved DBP but trended towards a delay in achieving ROSC.23 Given the limited power of this study, more research will be required to determine the impacts of REBOA on shorter periods of low-flow CPR.
The 75% rate of ROSC in the 30-minute low flow REBOA group exceeds the human case series data showing that REBOA supports ROSC despite prolonged resuscitation. We believe this data supports the use of REBOA in upcoming Phase 1 and Phase 2 human trials of REBOA in NTCA. Importantly, however, this exciting data is tempered by a high re-arrest rate where only 50% of the animal that achieved initial ROSC survived to the end of the study period. A similar post-ROSC re-arrest phenomenon has been seen in prior REBOA translational series and human trials.6, 23, 25 It is believed that abrupt changes in aortic afterload and blood pressure, especially those associated with balloon deflation, can lead to re-arrest. In this study, we did not perform aggressive post-ROSC interventions in order to limit the number of experimental variables. For example, other than slowly titrating adrenaline and providing treatments for hyperkalemia, we did not provide other interventions such as adaptive REBOA, partial-REBOA, or provide other pharmaceuticals that may have helped prevent re-arrest. As REBOA is a relatively simple intervention for providers to place during CPR, if ROSC can be achieved with REBOA after a prolonged resuscitation, it may help bridge to more aggressive post-ROSC interventions such as adaptive balloon management or extracorporeal membrane oxygenation (ECMO).
These conclusions must take into context the limitations of this study. First, and most importantly, in order to explore the impacts of REBOA on prolonged periods of low-flow, we deployed REBOA at the same time as the first adrenaline dose and before the first defibrillation. This is a side effect of experimental design and we are not suggesting that REBOA should be deployed prior to initiating ACLS. However, had animals achieved ROSC prior to REBOA deployment, they would have been excluded from this study and this would have become cost prohibitive. Secondly, while we delayed randomization of animals until after low-flow CPR had begun (t = 10 minutes), due to the need for REBOA balloon expansion and deflation we were unable to blind the investigators during experimentation, which is a possible source of bias. Next, we used a porcine model of cardiac arrest, which due to differences in chest structure and physiology may impact the effectiveness of mechanical CPR and REBOA relative to humans. Given the complication of comparing groups’ hemodynamics in the setting of varying ROSC and re-arrest times (animals initial ROSC ranged from 3 to 26 mins after initiation of ACLS), we focused our pre-ROSC hemodynamic data from the first 7-minutes after initiation of ACLS +/- REBOA was compared among groups (when >90% of the animals had yet to achieve ROSC, but REOBA was expanded). An analysis of this period limits comparison between hemodynamics of animals under CPR and those having achieved ROSC, but does represent a source of statistical bias., Next, the animals were shocked into ventricular fibrillation with a transvenous pacing wire and did not have pre-existing cardiac injury typical of cardiac arrest. We suspect our overall ROSC rate would be lower with a severe pre-existing cardiac injury. Lastly, the DBP and CPP repeated measures analysis was found to have a non-normal distribution due in part to pauses for defibrillation, potentially leading to type 1 error.
Conclusions
In our porcine model of NTCA, REBOA preferentially improved hemodynamics and ROSC after a 30-mins period of low-flow CPR, compared to 15-mins of low-flow CPR. REBOA may be a viable strategy to improve ROSC after prolonged downtime, however, more hemodynamic support will be required to maintain ROSC as there was a high re-arrest rate in the 30-min animals that received REBOA.
Author statements
All authors have made substantial contributions to all of the following: the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version which is herein submitted. This manuscript, including related data, figures, and tables, are not under consideration elsewhere.
Dr. Siemieniak presented this data as an oral presentation at the 2023 American Heart Association Resuscitation Science Symposium.
Disclaimer
The opinions expressed in this presentation are solely those of the author(s) and do not represent an endorsement by or the views of the United States Air Force, the Department of Defense, or the United States Government. The view of any manufacturer of products used in this study are not necessarily the official view of, or endorsed by, the U.S. Government, the Department of Defense, or the Department of the Air Force. No Federal endorsement of any manufacturer is intended.
Animal use statement
The experiments reported herein were conducted according to the principles set forth in the National Research Council’s Guide for the Care and Use of Laboratory Animals (8th ed.), and the Animal Welfare Act of 1966, as amended.
CRediT authorship contribution statement
Steven Siemieniak: Writing – review & editing, Investigation. Tanner Greiving: Writing – review & editing, Investigation. Nola Shepard: Writing – review & editing, Resources, Methodology, Investigation, Formal analysis, Data curation. Jason Rall: Writing – review & editing, Writing – original draft, Visualization, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation. Craig Nowadly: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Craig Nowadly previously worked as an independent contractor for Certus Critical Care, Inc. which designs endovascular medical products. Steven Siemieniak, Tanner Greiving, Nola Shepard, and Jason Rall have no conflicts of interest to report.
References
- 1.Yan S., Gan Y., Jiang N., et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24:61. doi: 10.1186/s13054-020-2773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lurie K., Levy M., Swor R., Moore J. The economic impact of out-of-hospital cardiac arrest care. J Emerg Med Serv. 2017:10–16. [Google Scholar]
- 3.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L., Zhang J. Mechanical cardiopulmonary resuscitation for patients with cardiac arrest. World J Emerg Med. 2011;2:165–168. doi: 10.5847/wjem.j.1920-8642.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowadly C.D., Johnson M.A., Hoareau G.L., Manning J.E., Daley J.I. The use of resuscitative endovascular balloon occlusion of the aorta (REBOA) for non-traumatic cardiac arrest: a review. J Am Coll Emerg Physicians Open. 2020;1:737–743. doi: 10.1002/emp2.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowadly C.D., Johnson M.A., Youngquist S.T., Williams T.K., Neff L.P., Hoareau G.L. Automated aortic endovascular balloon volume titration prevents re-arrest immediately after return of spontaneous circulation in a swine model of nontraumatic cardiac arrest. Resusc Plus. 2022;10 doi: 10.1016/j.resplu.2022.100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang W., Weil M.H., Noc M., Sun S., Gazmuri R.J., Bisera J. Augmented efficacy of external CPR by intermittent occlusion of the ascending aorta. Circulation. 1993;88:1916–1921. doi: 10.1161/01.cir.88.4.1916. [DOI] [PubMed] [Google Scholar]
- 8.Gedeborg R., Rubertsson S., Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation. 1999;40:171–180. doi: 10.1016/S0300-9572(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Sesma J., Sara M.J., Espila J.L., Arteche A., Saez M.J., Labandeira J. Effect of Intra-aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med. 2002;20:453–462. doi: 10.1053/ajem.2002.32627. [DOI] [PubMed] [Google Scholar]
- 10.Tiba M.H., McCracken B.M., Cummings B.C., et al. Use of resuscitative balloon occlusion of the aorta in a swine model of prolonged cardiac arrest. Resuscitation. 2019;140:106–112. doi: 10.1016/j.resuscitation.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubertsson S., Bircher N.G., Alexander H. Effects of intra-aortic balloon occlusion on hemodynamics during, and survival after cardiopulmonary resuscitation in dogs. Crit Care Med. 1997;25:1003–1009. doi: 10.1097/00003246-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A., Taki K., Takeshima N. Experimental study on cardiac resuscitation–effectiveness of thoracic aorta occlusion. Masui. 1980;29:677–682. [PubMed] [Google Scholar]
- 13.Spence P.A., Lust R.M., Chitwood W.R., Iida H., Sun Y.S., Austin E.H. Transfemoral balloon aortic occlusion during open cardiopulmonary resuscitation improves myocardial and cerebral blood flow. J Surg Res. 1990;49:217–221. doi: 10.1016/0022-4804(90)90122-I. [DOI] [PubMed] [Google Scholar]
- 14.Brede J. Resuscitative Balloon Occlusion of the Aorta in Non-traumatic Out of Hospital Cardiac Arrest (REBOA). 2018.
- 15.Gamberini L., Coniglio C., Lupi C., et al. Resuscitative endovascular occlusion of the aorta (REBOA) for refractory out of hospital cardiac arrest. An Utstein-based case series. Resuscitation. 2021;165:161–169. doi: 10.1016/j.resuscitation.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Olsen M.H., Olesen N.D., Karlsson M., et al. Randomized blinded trial of automated REBOA during CPR in a porcine model of cardiac arrest. Resuscitation. 2021;160:39–48. doi: 10.1016/j.resuscitation.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Daley J.I. The Use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) as an adjunct to advanced cardiac life support in non-traumatic cardiac arrest: an early feasibility trial. clinicaltrials.gov. 2020 [Google Scholar]
- 18.Brede J.R., Skulberg A.K., Rehn M., et al. REBOARREST, resuscitative endovascular balloon occlusion of the aorta in non-traumatic out-of-hospital cardiac arrest: a study protocol for a randomised, parallel group, clinical multicentre trial. Trials. 2021;22:511. doi: 10.1186/s13063-021-05477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levis A., Greif R., Hautz W., Hunziker L., Fehr T., Hänggi M. Feasibility of resuscitative endovascular balloon occlusion (REBOA) in patients with refractory cardiac arrest. Resuscitation. 2018;130:e4–e5. doi: 10.1016/j.resuscitation.2018.07.315. [DOI] [Google Scholar]
- 20.Kristiansen A. REBOA for Out-of-hospital Cardiac Arrest. clinicaltrials.gov. 2021 [Google Scholar]
- 21.Kim H.E., Chu S.-E., Jo Y.H., et al. Effect of resuscitative endovascular balloon occlusion of the aorta in nontraumatic out-of-hospital cardiac arrest: a multinational, multicenter, randomized, controlled trial. Trials. 2024;25:118. doi: 10.1186/s13063-024-07928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dogan E.M., Beskow L., Calais F., Hörer T.M., Axelsson B., Nilsson K.F. Resuscitative endovascular balloon occlusion of the aorta in experimental cardiopulmonary resuscitation: aortic occlusion level matters. Shock. 2019;52:67. doi: 10.1097/SHK.0000000000001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowadly C.D., Hoareau G.L., Grayson J.K., Johnson M.A. Zone 3 REBOA does not provide hemodynamic benefits during nontraumatic cardiac arrest. Am J Emerg Med. 2020;38:1915–1920. doi: 10.1016/j.ajem.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds J.C., Frisch A., Rittenberger J.C., Callaway C.W. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation. 2013;128:2488–2494. doi: 10.1161/CIRCULATIONAHA.113.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brede J.R., Lafrenz T., Klepstad P., et al. Feasibility of pre-hospital resuscitative endovascular balloon occlusion of the aorta in non-traumatic out-of-hospital cardiac arrest. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doig G.S., Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20:187–191. doi: 10.1016/j.jcrc.2005.04.005. discussion 191-193. [DOI] [PubMed] [Google Scholar]
- 27.Tibbits E.M., Hoareau G.L., Simon M.A., et al. Location is everything: the hemodynamic effects of REBOA in Zone 1 versus Zone 3 of the aorta. J Trauma Acute Care Surg. 2018;85:101–107. doi: 10.1097/TA.0000000000001858. [DOI] [PubMed] [Google Scholar]
- 28.Balzer C., Eagle S.S., Yannopoulos D., Aufderheide T.P., Riess M.L. High central venous pressure amplitude predicts successful defibrillation in a porcine model of cardiac arrest. Resuscitation. 2023;185 doi: 10.1016/j.resuscitation.2023.109716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otlewski M.P., Geddes L.A., Pargett M., Babbs C.F. Methods for calculating coronary perfusion pressure during CPR. Cardiovasc Eng. 2009;9:98–103. doi: 10.1007/s10558-009-9079-y. [DOI] [PubMed] [Google Scholar]
- 30.Ezzati E., Mohammadi S., Karimpour H., et al. Assessing the effect of arrival time of physician and cardiopulmonary resuscitation (CPR) team on the outcome of CPR. Interv Med Appl Sci. 2020;11:139–145. doi: 10.1556/1646.10.2018.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stærk M., Lauridsen K.G., Støtt C.T., Riis D.N., Løfgren B., Krogh K. Inhospital cardiac arrest - the crucial first 5 min: a simulation study. Adv Simul (Lond) 2022;7:29. doi: 10.1186/s41077-022-00225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris N.A., Couperus C., Dezman Z., et al. Feasibility of accelerated code team activation with code button triggered smartphone notification. Resuscitation. 2023;187 doi: 10.1016/j.resuscitation.2023.109752. [DOI] [PubMed] [Google Scholar]
- 33.Chien C., Tsai S., Tsai L., et al. Impact of transport time and cardiac arrest centers on the neurological outcome after out-of-hospital cardiac arrest: a retrospective cohort study. J Am Heart Assoc. 2020;9:e015544. doi: 10.1161/JAHA.119.015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vukmir R.B. Survival from prehospital cardiac arrest is critically dependent upon response time. Resuscitation. 2006;69:229–234. doi: 10.1016/j.resuscitation.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Paradis N.A., Martin G.B., Rivers E.P., et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 36.Niemann J.T., Criley J.M., Rosborough J.P., Niskanen R.A., Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14:521–528. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]