Abstract
Background
Identification of increased pulmonary capillary wedge pressure (PCWP) by right heart catheterization (RHC) is the reference standard for the diagnosis of heart failure with preserved ejection fraction (HFpEF). Recently, cardiovascular magnetic resonance (CMR) imaging estimation of PCWP at rest was introduced as a non-invasive alternative. Since many patients are only identified during physiological exercise-stress, we hypothesized that novel exercise-stress CMR-derived PCWP emerges superior compared to its assessment at rest.
Methods
The HFpEF-Stress Trial prospectively recruited 75 patients with exertional dyspnea and diastolic dysfunction who then underwent rest and exercise-stress RHC and CMR. HFpEF was defined according to PCWP (overt HFpEF ≥15 mmHg at rest, masked HFpEF ≥25 mmHg during exercise-stress). CMR-derived PCWP was calculated based on previously published formula using left ventricular mass and either biplane left atrial volume (LAV) or monoplane left atrial area (LAA).
Results
LAV (rest/stress: r = 0.50/r = 0.55, p < 0.001) and LAA PCWP (rest/stress: r = 0.50/r = 0.48, p < 0.001) correlated significantly with RHC-derived PCWP while numerically overestimating PCWP at rest and underestimating PCWP during exercise-stress. LAV and LAA PCWP showed good diagnostic accuracy to detect HFpEF (area under the receiver operating characteristic curve (AUC) LAV rest 0.73, stress 0.81; LAA rest 0.72, stress 0.77) with incremental diagnostic value for the detection of masked HFpEF using exercise-stress (AUC LAV rest 0.54 vs stress 0.67, p = 0.019, LAA rest 0.52 vs stress 0.66, p = 0.012). LAV but not LAA PCWP during exercise-stress was a predictor for 24 months hospitalization independent of a medical history for atrial fibrillation (hazard ratio (HR) 1.26, 95% confidence interval 1.02–1.55, p = 0.032).
Conclusion
Non-invasive PCWP correlates well with the invasive reference at rest and during exercise stress. There is overall good diagnostic accuracy for HFpEF assessment using CMR-derived estimated PCWP despite deviations in absolute agreement. Non-invasive exercise derived PCWP may particularly facilitate detection of masked HFpEF in the future.
Keywords: HFpEF, Cardiovascular magnetic resonance, Estimated pulmonary capillary wedge pressure, Rest and exercise-stress
Graphical Abstract
1. Introduction
To date, heart failure (HF) with preserved or mildly reduced ejection fraction accounts for half of all HF patients [1]. Identification of increased left ventricular filling pressure (LVFP) in diastolic dysfunction by assessment of pulmonary capillary wedge pressure (PCWP) using right heart catheterization (RHC) is the reference standard for diagnosis of heart failure with preserved ejection fraction (HFpEF) [2], [3]. Notwithstanding, due to its invasive nature and previous absence of therapeutic intervention, RHC remains underused. However, especially identification and intervention at an early stage of disease may slow down cardiac remodeling [4], [5], [6], [7], [8]. Furthermore, disease progress and development of latent pulmonary vascular disease in conjunction with post-capillary pulmonary hypertension may not only worsen prognosis but exclude further treatment options [9], [10].
Novel indices [11], [12] aim toward improved non-invasive screening based on clinical as well as echocardiographic morphological and functional parameters to identify increased LVFP. However, these indices suffer from reduced discriminative power especially in the midrange probability.
Cardiovascular magnetic resonance (CMR) imaging emerged as a cornerstone in the diagnosis of HF etiology [1] and remains the reference standard for cardiac morphology and function quantification [13]. Therefore, efforts have been directed for LVFP/PCWP estimation by CMR imaging with promising results [14]. While non-invasive approximation of PCWP at rest emerges feasible, a lesson learned underlines the incremental value of PCWP assessment during exercise-stress for early identification of otherwise masked diastolic dysfunction [3] thus allowing for early treatment intervention. However, the relationship of non-invasive exercise-stress CMR-derived PCWP and the reference standard of RHC-derived PCWP has not yet been established.
Recent advances in CMR imaging technology enabled novel real-time (RT) imaging at higher temporal resolutions [13] which allowed the introduction of free-breathing exercise-stress imaging to CMR. Indeed, exercise-stress CMR has demonstrated high diagnostic accuracy for the diagnosis of HFpEF compared to the reference standard RHC [15]. Consequently, this study sought to assesses the feasibility of rest and exercise-stress PCWP approximation.
2. Methods
The HFpEF Stress trial (NCT03260621) prospectively recruited 75 patients referred for exertional dyspnea (New York Heart Association [NYHA] class ≥ II). If echocardiography showed signs of diastolic dysfunction (E/e′ ≥8) and preserved left ventricular ejection fraction (LVEF), ≥50% patients were addressed for study participation. Exclusion criteria were as follows: contraindications for CMR imaging (cardiac devices, allergy to gadolinium-based contrast agents or renal impairment resulting in inability to administer contrast—glomerular filtration rate (GFR) <30 mL/min/1.73 m2—and claustrophobia) [16], comorbidities resulting in dyspnea, including pulmonary (forced expiratory volume in 1 second or vital capacity <80% of the reference) and cardiac causes (coronary artery disease—stenosis >50% and moderate to severe valvular heart disease). Patients had to be in stable sinus rhythm during CMR imaging and RHC. The study was approved by the local ethics committee at the University of Goettingen. All patients gave written informed consent before participation. The study was conducted according to the principles of the Helsinki Declaration and funded by the German Centre for Cardiovascular Research (DZHK, HFpEF Stress trial DZHK-17). The data underlying the findings are available at the imaging database of the University Hospital Goettingen and access will be granted to researchers that meet the criteria for access upon formal request.
Rest and exercise-stress bicycle ergometry RHC, echocardiography, and CMR imaging were performed in all patients. Data acquisition during exercise-stress was conducted 3 min after reaching an average heart rate between 100 and 110 beats/min at 50–60 rpm [17]. RHC and echocardiography were performed simultaneously, CMR imaging within 24 h in relation to RHC (one case with 2-day interval). Follow-up was conducted after 24 months by medical chart review and telephone interview for acute cardiovascular hospitalization.
2.1. Right heart catheterization
A Swan-Ganz catheter was introduced via the right internal jugular vein using ultrasound guidance and positioned using fluoroscopy [18]. Cardiac pressures were assessed at the level of the right atrium, right ventricle (RV), pulmonary artery (PA), and PCWP position by averaging several respiratory cycles. Oxygen saturations were assessed in the PA. Cardiac output was assessed by the means of thermodilution from at least three valid measurements. The presence of HFpEF was defined according to PCWP (overt HFpEF ≥15 mmHg at rest, masked HFpEF ≥25 mmHg during exercise-stress but <15 mmHg at rest). Patients were classified as non-cardiac dyspnea (NCD) in the absence of cardiac disease based on all available evidence and PCWP below the predefined thresholds.
2.2. Cardiovascular magnetic resonance imaging
CMR imaging was conducted on a clinical 3.0T Magnetom Skyra MRI scanner (Siemens Healthcare, Erlangen, Germany).
Standard cardiac imaging was conducted at rest using steady state free precession (bSSFP) cine sequences for long axis 2-, 3- and 4-chamber view (4-Ch) as well as short axis (SAX) stack acquisitions. Volumetric post-processing included RV and left ventricular (LV) volumes as well as LV mass (LVM) based on SAX analyses.
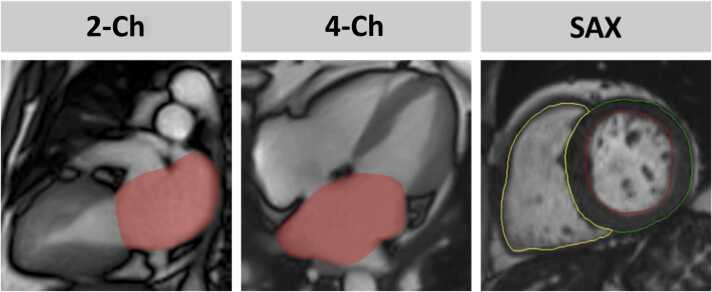
RT imaging was conducted at rest and during exercise-stress employing bSSFP sequences with a strongly undersampled radial encoding scheme and iterative reconstruction [19]. Free-breathing 2-Ch and 4-Ch as well as a SAX stack were acquired at rest and during exercise-stress. Volumetric post-processing included left atrial volume (LAV) based on 2-Ch and 4-Ch biplane Simpson as well as left atrial 4-Ch area (LAA), Fig. 1.
Fig. 1.
CMR-derived PCWP. Cardiovascular magnetic resonance (CMR) imaging derived pulmonary capillary wedge pressure (PCWP) was calculated using left ventricular (LV) mass (LVM) from short axis (SAX) measurements as well as biplane left atrial (LA) volume (LAV) based on long axis 2- and 4-chamber views (Ch) or LA area (LAA) based on monoplane 4 CV analysis. PCWP was calculated according to the formula: 1) 6.1352 + 0.02256 ∗ LVM + 0.07204 ∗ LAV – referred to as LAV PCWP or 2) 4.0584 + 0.02944 ∗ LVM + 0.3148 ∗ LAA – referred to as LAA PCWP.
CMR-derived PCWP was calculated using previously published formula.
2.3. Statistical analysis
Categorical variables are reported as frequencies and were compared using the chi-square test. Continuous variables are reported as medians and associated related interquartile ranges and were compared using the nonparametric Mann-Whitney U test. Dependent continuous parameters were tested using the Wilcoxon signed-rank test. Predictors of invasive PCWP were identified from Spearman rank correlation coefficients and area under the receiver operating characteristic curve (AUC) analyses which are reported with 95% confidence intervals (CI). AUC comparisons were calculated using the method proposed by DeLong et al. [21]. A 2-tailed p-value <0.05 was considered statistically significant. Statistics were calculated using SPSS version 28.0.1.1 (IBM, Armonk, New York, USA) and MedCalc Statistical Software version 22.009 (MedCalc Software Ltd., Ostend, Belgium).
3. Results
3.1. Study population
Baseline characteristics have already been reported elsewhere [15], Tables 1 and 2. Seven patients were excluded from further study participation due to the diagnosis of specific cardiovascular diseases associated to dyspnea (n = 4 significant coronary artery disease, n = 1 amyloidosis, n = 1 hypertrophic cardiomyopathy, n = 1 valvular heart disease). While HFpEF patients were in median 3 years older than NCD patients (p = 0.034), there were no differences in cardiovascular risk factors (p = 0.339). The heavy, hypertensive, atrial fibrillation, pulmonary hypertension, elder, filling pressure (H2FPEF) and Heart Failure Association pre-test, echocardiography and natriuretic peptide, functional testing and final etiology (HFA_PEFF) scores were increased in HFpEF (p ≤ 0.003).
Table 1.
Patients characteristics.
| Variable | HFpEF | Non-cardiac dyspnea | Significance p |
|---|---|---|---|
| n = 34 | n = 34 | ||
| Age (years) | 69 (67, 77) | 66 (52, 73) | 0.034 |
| Sex male/female | 9/25 | 15/19 | 0.128 |
| NYHA class | 21 x II, 13 x III | 27 x II, 7 x III | 0.110 |
| Atrial fibrillation | 16 | 5 | 0.004 |
| H2FPEF score | 5.0 (3.0, 6.3) | 3.0 (2.0, 5.0) | 0.003 |
| HFA-PEFF score | 5.5 (3.8, 6.0) | 4.0 (2.0, 4.0) | <0.001 |
| Cardiovascular risk factors | |||
| Active smoking | 4 | 5 | 0.720 |
| Hypertension | 27 | 27 | 1.000 |
| Hyperlipoproteinemia | 21 | 21 | 1.000 |
| Diabetes | 5 | 5 | 1.000 |
| Body mass index (kg/m2 BSA) | 28.7 (26.8, 33.2) | 27.6 (25.2, 32.3) | 0.339 |
| Laboratory testing | |||
| NT-proBNP (ng/L) | 255 (102, 606) | 75 (50, 134) | <0.001 |
| Creatinine (mg/dL) | 0.89 (0.74, 1.03) | 0.83 (0.72, 1.04) | 0.995 |
| Echocardiography | |||
| E/e′ rest | 12.5 (9.7, 13.3) | 9.15 (7.5, 10.7) | <0.001 |
| E/e′ stress | 13.8 (10.8, 15.9) | 11.0 (10.0, 14.0) | 0.120 |
| LAVI (mL/m2 BSA) | 43.8 (36.6, 54.2) | 36.2 (29.2, 41.1) | 0.001 |
| TAPSE (mm) | 24 (21.2, 27.2) | 22.5 (20.5, 25.7) | 0.335 |
| PAPsys (mmHg) | 28 (23.5, 33.1) | 22.8 (19.6, 24.7) | 0.001 |
| Right heart catheterization | |||
| PCWP rest (mmHg) | 13 (11, 18) | 8 (6, 10) | <0.001 |
| PCWP stress (mmHg) | 27 (26, 31) | 18 (11, 22) | <0.001 |
| PA rest (mmHg) | 22 (20,28) | 17 (14, 19) | <0.001 |
| PA stress (mmHg) | 44 (39, 52) | 34 (25, 39) | <0.001 |
| PA pO2 rest (%) | 73 (70, 76) | 75 (72, 77) | 0.225 |
| PA pO2 stress (%) | 42 (36, 51) | 48 (43, 52) | 0.118 |
| Cardiac index rest (L/m2 BSA) | 2.9 (2.4, 3.2) | 2.9 (2.6, 3.4) | 0.663 |
| Cardiac index stress (L/m2 BSA) | 5.2 (3.7, 6.1) | 5.8 (4.7, 6.7) | 0.022 |
LAVI left atrial volume index, TAPSE tricuspid annular plane systolic excursion, PAPsys systolic pulmonary artery pressure, STE speckle-tracking echocardiography, LV GLS left ventricular global longitudinal strain, LA Es left atrial reservoir function, PCWP pulmonary capillary wedge pressure, PA pulmonary artery pressure, BSA body surface area, HFpEF heart failure with preserved ejection fraction, NYHA New York Heart Association, BSA body surface area, NT-proBNP N-terminal pro-B-type brain natriuretic peptide, LAVI left atrial volume index. Categorical parameters are reported in absolutes numbers and were compared using the chi-square test. Independent continuous parameters are presented as medians with interquartile ranges and were compared by using the Mann-Whitney U test. Bold p-values indicate statistical significance. This table has been previously published [15].
Table 2.
Cardiovascular magnetic resonance imaging.
| Variable | HFpEF |
Non-cardiac dyspnea |
Significance p |
|---|---|---|---|
| n = 34 | n = 34 | ||
| Left ventricle | |||
| LV mass (g/m2 BSA) | 57.0 (51.0, 66.9) | 55.6 (50.4, 72.0) | 0.932 |
| LV EDV (mL/m2 BSA) | 68.3 (60.7, 77.3) | 68.5 (57.4, 76.8) | 0.741 |
| LV ESV (mL/m2 BSA) | 19.6 (14.8, 25.9) | 20.4 (14.8, 24.3) | 0.917 |
| LV SV (mL/m2 BSA) | 49.6 (42.1, 54.5) | 46.7 (40.1, 53.0) | 0.447 |
| LV EF (%) | 69.0 (66.3, 76.1) | 69.0 (65.0, 75.6) | 0.731 |
| FT LV GLS (%) | −19.9 (−18.8, −22.5) | −21.0 (−19.0, −23.2) | 0.194 |
| FT LV GCS (%) | −35.2 (−30.9, −39.0) | −34.9 (−30.7, −36.9) | 0.516 |
| FT LV GRS (%) | 66.2 (57.7, 74.2) | 63.4 (56.5, 70.1) | 0.275 |
| Left atrium | |||
| FT LA Es (%) | 24.8 (16.7, 30.6) | 35.9 (30.7, 42.3) | <0.001 |
| FT LA Ee (%) | 10.9 (8.56, 16.6) | 16.5 (13.0, 22.1) | <0.001 |
| FT LA Ea (%) | 12.1 (7.82, 16.4) | 18.2 (15.1, 22.4) | <0.001 |
| Right ventricle | |||
| RV EDV (mL/m2 BSA) | 67.7 (54.1, 72.1) | 65.4 (57.9, 76.1) | 0.825 |
| RV ESV (mL/m2 BSA) | 20.1 (16.9, 25.3) | 23.9 (18.8, 28.4) | 0.109 |
| RV SV (mL/m2 BSA) | 44.7 (37.7, 49.6) | 41.8 (37.0, 48.4) | 0.524 |
| RV EF (%) | 67.6 (62.2, 72.1) | 63.8 (60.7, 68.3) | 0.034 |
| FT RV GLS (%) | −22.9 (−20.1, −26.5) | −23.2 (−20.3, −26.6) | 0.912 |
| CMR-derived PCWP | |||
| LAV PCWP rest | 15.8 (14.7, 17.4) | 13.2 (12.4, 15.9) | 0.002 |
| LAV PCWP stress | 17.9 (16.5, 20.6) | 14.5 (13.2, 15.9) | <0.001 |
| LAA PCWP rest | 16.5 (14.3, 17.5) | 14.0 (12.8, 16.0) | 0.002 |
| LAA PCWP stress | 18.0 (16.6, 19.6) | 15.4 (13.6, 17.1) | <0.001 |
LV left ventricular, EDV/ESV end-diastolic/-systolic volume, SV stroke volume, EF ejection fraction, FT Feature-Tracking, GLS/GCS/GRS global longitudinal/circumferential/radial strain, LA left atrium, Es/Ee/Ea atrial reservoir/conduit/booster pump function, HFpEF heart failure with preserved ejection fraction, LAV left atrial volume, PCWP pulmonary capillary wedge pressure, BSA body surface area, CMR cardiovascular magnetic resonance, RV right ventricle. Independent continuous parameters are presented as medians with interquartile ranges and were compared by using the Mann-Whitney U test. Bold p-values indicate statistical significance. This table has in parts been previously published [15].
Patients with HFpEF (5 and 6 points) according to the HFA_PEFF score had significantly higher calculated PCWP compared to patients within the borderline area (2–4 points) (LAV PCWP rest p = 0.002, stress p = 0.001; LAA PCWP rest p < 0.001, stress p < 0.001). There was no difference comparing NCD and borderline patients (p = 0.618–0.981).
3.2. CMR for the prediction of invasive PCWP
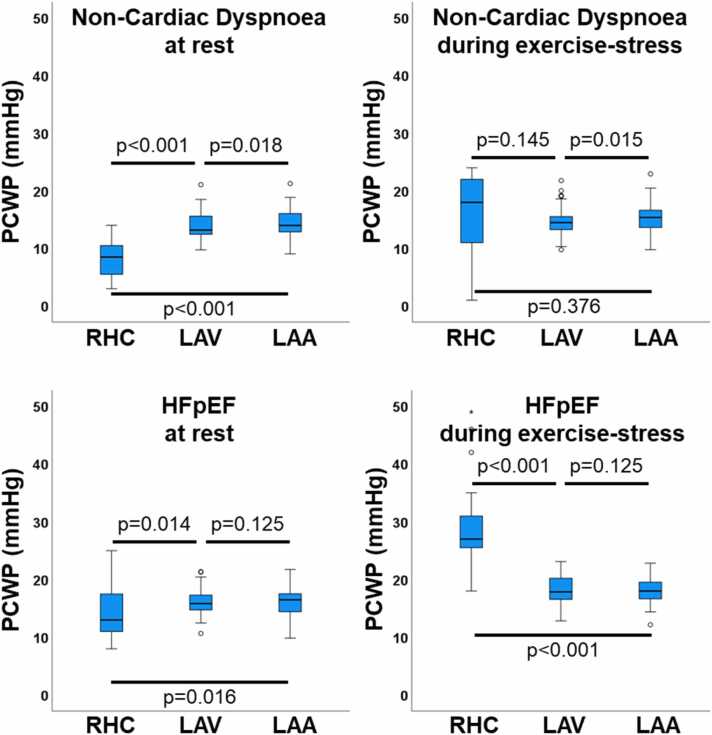
At rest, non-invasive PCWP consistently overestimated (p < 0.001) invasive PCWP in median by LAV 4.1 (1.8, 6.8) and LAA 4.2 (1.9, 7.2). This relationship reversed during exercise-stress with distinctly increased underestimation (p < 0.001) in median by LAV −6.2 (−2.7, −10.1) and LAA −5.5 (−2.0, −9.7). This relationship is further demonstrated in Fig. 2, highlighting the highest deviation in HFpEF during exercise-stress. Changes from rest to exercise-stress for CMR metrics are reported in Table 3.
Fig. 2.
Pulmonary capillary wedge pressure comparisons. The boxplots show the median, 1/3 interquartile, and 1.5×IQR whiskers for pulmonary capillary wedge pressure (PCWP) according to right heart catheterization (RHC) as well as left atrial volume (LAV) and left atrial area (LAA) cardiovascular magnetic resonance (CMR) derived calculated PCWP. Statistics were calculated using the Wilcoxon signed-rank test. HFpEF: heart failure with preserved ejection fraction, IQR: interquartile range.
Table 3.
Rest and exercise-stress metrics.
| Variable | Rest | Exercise-stress | Significance p |
|---|---|---|---|
| Cardiovascular risk factors | |||
| LA volume (mL) | 88.1 (58.3, 104.8) | 109.2 (74.0, 135.1) | <0.001 |
| LA area (cm2) | 24.5 (19.4, 30.0) | 29.4 (23.1, 34.2) | <0.001 |
| PCWP LA volume | 14.9 (12.8, 16.5) | 16.3 (14.1, 18.6) | <0.001 |
| PCWP LA area | 15.7 (13.1, 16.8) | 16.6 (15.2, 18.5) | <0.001 |
| Right heart catheterization | |||
| PCWP (mmHg) | 11 (8, 14) | 23 (18, 27) | <0.001 |
LA left atrium, PCWP pulmonary capillary wedge pressure. Independent continuous parameters are presented as medians with interquartile ranges and were compared by using the Mann-Whitney U test. Bold p-values indicate statistical significance.
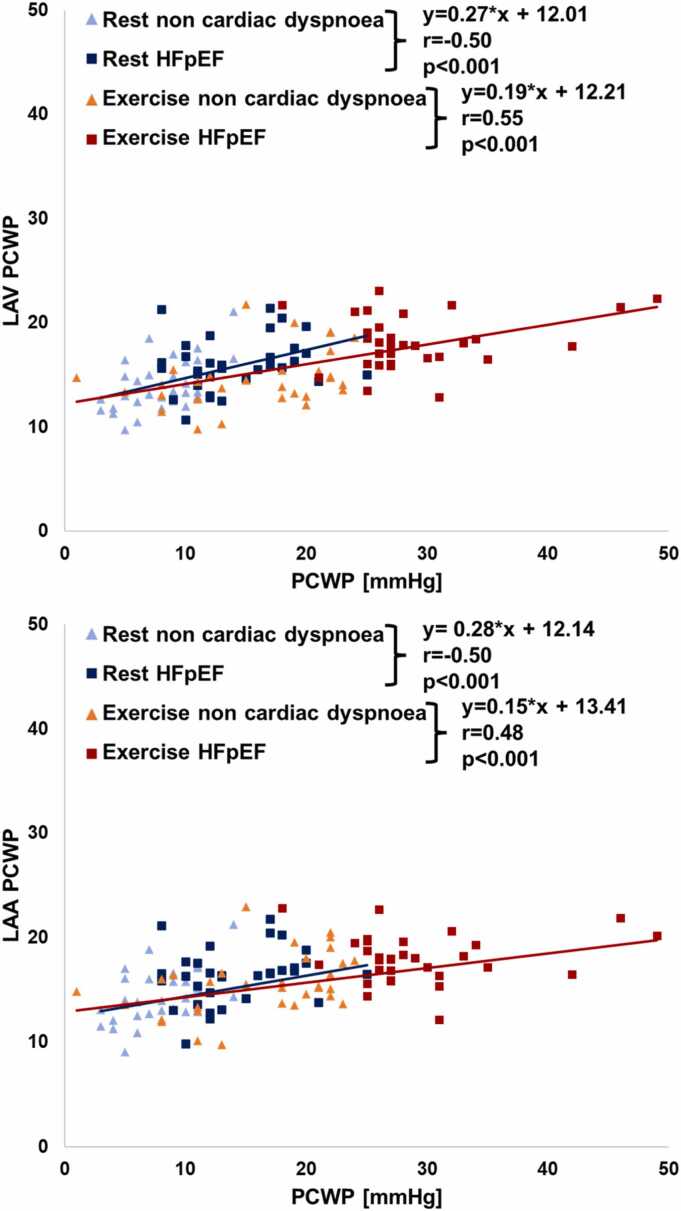
Resting LAV PCWP correlated significantly with RHC-derived PCWP at rest (r = 0.50, p < 0.001) and during exercise-stress (r = 0.44, p < 0.001). Exercise-stress LAV PCWP showed improved correlation to exercise-stress PCWP (r = 0.55, p < 0.001). Similarly, resting LAA PCWP correlated significantly with PCWP at rest (r = 0.50, p < 0.001) and during exercise-stress (r = 0.39, p = 0.001). Again, exercise-stress LAV PCWP showed improved correlation to exercise-stress PCWP (r = 0.48, p < 0.001) Fig. 3.
Fig. 3.
Correlation of CMR-derived estimated PCWP and RHC PCWP. The graphs show the correlation of cardiovascular magnetic resonance (CMR) left atrial volume (LAV) or left atrial area (LAA) derived pulmonary capillary wedge pressure (PCWP) and right heart catheterization (RHC) derived PCWP at rest (blue) and during exercise stress (red). Correlations were assessed using Spearman rank correlation coefficients. HFpEF: heart failure with preserved ejection fraction.
ROC analyses revealed good diagnostic accuracy to detect HFpEF using LAV and LAA PCWP with numerical but non-significant improvement using exercise-stress (LAV AUC rest 0.73 vs stress 0.81, p = 0.123, LAA AUC rest 0.72 vs stress 0.77, p = 0.360), Table 4. Diagnostic accuracy was distinctly lower to detect masked HFpEF compared to overt HFpEF, but diagnostic accuracy significantly increased for the detection of masked HFpEF by using exercise-stress testing (LAV AUC rest 0.54 vs stress 0.67, p = 0.019, LAA AUC rest 0.52 vs stress 0.66, p = 0.012).
Table 4.
Diagnostic accuracy to detect invasively proven HFpEF by CMR-derived PCWP.
| Variable | AUC (95% CI) | Significance | AUC (95% CI) | Significance | AUC (95% CI) | Significance |
|---|---|---|---|---|---|---|
| HFpEF | Rest vs stress | Masked HFpEF | Rest vs stress | Overt HFpEF | Rest vs stress | |
| Left atrial volume | ||||||
| LAV PCWP rest | 0.73 (0.60-0.86) | 0.123 | 0.54 (0.39-0.70) | 0.019 | 0.79 (0.67-0.90) | 0.498 |
| LAV PCWP stress | 0.81 (0.70-0.92) | 0.67 (0.53-0.81) | 0.75 (0.63-0.88) | |||
| Left atrial area | ||||||
| LAA PCWP rest | 0.72 (0.60-0.85) | 0.360 | 0.52 (0.37-0.68) | 0.012 | 0.80 (0.68-0.92) | 0.147 |
| LAA PCWP stress | 0.77 (0.65-0.89) | 0.66 (0.52-0.81) | 0.71 (0.57-0.84) |
HFpEF heart failure with preserved ejection fraction, CMR cardiovascular magnetic resonance, PCWP pulmonary capillary wedge pressure, CI confidence intervals, LAV/LAA left atrial volume/area. The table shows the area under the receiver operating characteristic curve (AUC) for the differentiation of patients with and without HFpEF and subgroups of masked and overt HFpEF. AUC analyses were compared using the nonparametric approach introduced by De Long et al.
Results on LA longitudinal deformation (long axis strain [LAS]) have previously been published [15]. At rest, LA LAS and LAV PCWP performed equally well to diagnose invasively proven HFpEF (AUC LA LAS 0.81 vs LAV PCWP 0.73, p = 0.250). In contrast, LA LAS outperformed LAV PCWP during exercise-stress testing (AUC LA LAS 0.93 vs LAV PCWP 0.81, p = 0.007).
3.3. Association of CMR PCWP and hospitalization
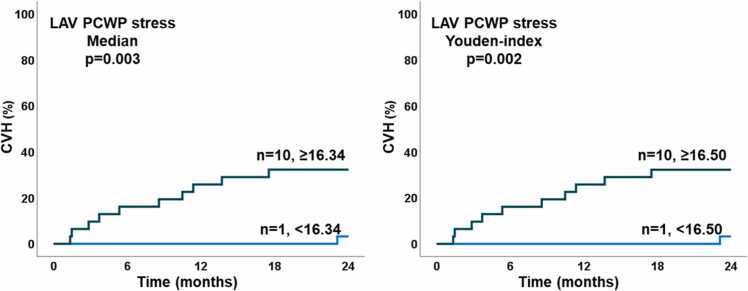
Eight HFpEF and three NCD patients were hospitalized for acute cardiovascular reasons during 24 months follow-up (p = 0.123). Two patients were lost to follow-up. Among CMR PCWP parameters, only LAV PCWP during exercise-stress was associated with hospitalization during 24 months follow-up (HR 1.32, 95% CI 1.07–1.63, p = 0.009) while LAV PCWP at rest (p = 0.073) as well as LAA PCWP at rest and during exercise-stress (p = 0.086 and 0.067) showed statistical trends only. LAV PCWP during exercise-stress remained a predictor for hospitalization independent of a history of atrial fibrillation (HR 1.26, 95% CI 1.02–1.55, p = 0.032). Kaplan Meier curves confirmed the association of LAV PCWP during exercise-stress and hospitalization after dichotomization at the median (p = 0.003) and the Youden index (p = 0.002), Fig. 4. In line, LAV PCWP during exercise stress showed the highest AUC for event prediction with numerical but statistically non-significant increase compared to rest (LAV PCWP rest AUC 0.64 vs stress 0.76, p = 0.137). Prognostic power was lower for LAA PCWP (AUC rest 0.67 vs stress 0.68, p = 0.870). CMR-derived LAV PCWP performed equally for event prediction compared to RHC-derived PCWP (rest CMR 0.64 vs RHC 0.65, p = 0.198 and stress CMR 0.76 vs RHC 0.63, p = 0.180).
Fig. 4.
Cardiovascular hospitalization during follow-up. The graphs show the number of patients with cardiovascular hospitalization (CVH) in patients with exercise-stress cardiovascular magnetic resonance derived pulmonary capillary wedge pressure (PCWP) based on left ventricular mass and atrial volume, left dichotomized at the median, right dichotomized at the Youden index. LAV: left atrial volume.
4. Discussion
Based on the prospectively recruited study population of the HFpEF Stress trial, the present substudy elaborates on non-invasive rest and exercise-stress CMR-derived PCWP calculations based on previously developed models using LV mass as well as LAV or LAA for PCWP calculation [14], [20].
Firstly, the present study confirms feasibility of non-invasive PCWP calculation and demonstrates similar diagnostic accuracy using free-breathing real-time CMR imaging. Secondly, non-invasively estimated PCWP during exercise-stress CMR shows equally good correlation to invasive exercise-stress PCWP compared to resting conditions. Thirdly, exercise-stress shows improved diagnostic accuracy compared to rest, especially for masked HFpEF. Lastly, non-invasive PCWP during exercise-stress is associated to heart failure hospitalization.
Based on a derivation and separate validation study population, Garg et at. [14] previously proposed a non-invasive method for PCWP calculation based on simple LV mass and LAV calculation. This resulted in a correlation of r = 0.55 to invasive PCWP and a diagnostic accuracy for HFpEF with an AUC of 0.81. In line with these results, in the present population, LAV PCWP shows a similar correlation coefficient (r = 0.50, p < 0.001) and diagnostic accuracy to detect HFpEF (AUC 0.73). On the one hand, this confirms the ability of RT free breathing data acquisition for non-invasive PCWP calculation compared to the reference standard of bSSFP cine sequences at rest. On the other hand, correlation is, in line with previously published data, modest and estimated PCWP by CMR may not be interchangeable with RHC-derived PCWP. Notwithstanding, current guideline recommendations have introduced exercise-stress tests in HFpEF in case of borderline screening results at rest [11], the reference-standard still being invasive exercise-stress RHC [3]. Indeed, correlation of resting CMR-derived PCWP was lower compared to invasive exercise-stress as opposed to invasive resting RHC-derived PCWP. This demonstrates that non-invasive testing at rest alone cannot predict hemodynamic responses to exercise stress accurately and therefore does not yield optimal diagnostic accuracy. Indeed, the HFpEF Stress trial demonstrated incremental value for exercise-stress CMR to detect invasively proven HFpEF [15]. Importantly, exercise-stress CMR-derived PCWP showed similarly high correlation to exercise-stress RHC-derived PCWP compared to the resting situation, thus highlighting feasibility of exercise-stress non-invasive PCWP calculation. In the present study population, more than half of all patients were diagnosed as HFpEF patients according to exercise-stress thresholds only, referred to as masked HFpEF. Noteworthy, compared to testing at rest, exercise-stress CMR-derived PCWP showed significant incremental diagnostic value for the identification of these masked HFpEF patients.
However, diagnostic accuracy to detect masked HFpEF was distinctly lower compared to overt HFpEF. One potential underlying reason may be the following: While calculated PCWP at rest mildly overestimates invasive PCWP, this relationship reversed during exercise-stress showing a distinct underestimation of invasive PCWP. Non-invasive CMR-derived PCWP calculation is based on LV mass and atrial size. Assuming that LV mass does not change in response to exercise-stress, LA size remains as the only parameter to reflect dynamic hemodynamic changes. However, atrial remodeling in response to increased LVFP is a chronic process, and acute changes of LVFP in response to exercise-stress may not be adequately reflected in atrial size alone. Although all morphological CMR-derived parameters showed a significant increase from rest to exercise-stress, especially the out-of-proportion increase of PCWP linked to HFpEF was not simulated by CMR-estimated PCWP. Indeed, rather the inability of atrial size to compensate for acutely induced congestion by exercise-stress may result in the typical out-of-proportion increase of PCWP in HFpEF. Consequently, because atrial size does not and may not be able to increase in parallel with the out-of-proportion increase in PCWP or even is a reason for the former in HFpEF it may not emerge as the best diagnostic tool for exercise-stress testing in HFpEF. This underlines that equations based on morphology alone need further amendments to be applicable to dynamic exercise-stress testing in HFpEF.
PCWP, especially a disproportionate increase in response to exercise-stress, is associated to symptom severity in HFpEF [22] and associated with outcome. In line, non-invasively CMR-derived PCWP has also demonstrated prognostic relevance [14]. In the present study, only exercise-stress LAV PCWP demonstrated this relationship. This may have different underlying factors: Firstly, as outlined above, more than half of HFpEF patients were classified as masked HFpEF and identified during exercise-stress only. Reduced cardiovascular reserve as reflected in an out of proportion increase in PCWP may thus emerge as a sensitive parameter to predict adverse events. Secondly, as discussed previously, atrial size may not reflect congestion and atrial dysfunction as accurately as a functional parameter. However, the biplane approach (LAV) may be more sensitive to minor changes compared to monoplane LAA assessment alone. Notwithstanding, low patient numbers and few events with regards to early-stage HFpEF underline the hypothesis-generating nature of these results and will need further confirmation in larger multi-center approaches. However, considering emerging therapeutic strategies such as sodium glucose co-transporter 2 (SGLT2)-Inhibitors, which for dapagliflozin also demonstrated reduction of rest and exercise-stress PCWP during 24 weeks follow-up [23], follow-up surveys for assessment of PCWP become of clinical interest, especially when non-invasive tools were available.
The HFpEF-Stress trial identified changes in atrial longitudinal deformation, a functional parameter for atrial dysfunction, as highly predictive of LVFP and out-of-proportion increases in response to exercise-stress [15]. Indeed, atrial longitudinal deformation as a diagnostic and prognostic marker has come to the fore in an array of cardiovascular disease [15], [24], [25], [26], [27]. Hence, atrial function rather than size might more dynamically reflect changes in PCWP caused by exercise-stress induced congestion. Indeed, at resting conditions, AUC analyses revealed morphology (LAV) derived PCWP performed equally well compared to LA LAS. In contrast, during exercise-stress, LA function outperformed morphology derived PCWP. This highlights the need for functional parameters in the diagnosis of HFpEF which is also reflected in an overall poorer diagnostic accuracy for masked HFpEF using LAV PCWP. This may point toward a new approach for non-invasive PCWP calculation in the future, including a more dynamic parameter, such as LAS which can be measured as easily as LAV or LAA [28].
5. Limitations
The HFpEF Stress Trial was a monocentric study performed in an experienced CMR core-laboratory to evaluate the feasibility of a newly developed imaging technique. While the in great detail characterized study population allowed the validation of CMR parameters in the context of clinical reference standards, a monocentric approach with low patient numbers is hypothesis generating only. Furthermore, recruited patients were highly selected to avoid hemodynamic disruptive factors in the interpretation of diastolic dysfunction.
6. Conclusion
CMR-derived estimated PCWP is not interchangeable with RHC-derived PCWP due to overestimation at rest and distinct underestimation during exercise-stress. However, due to good correlation, estimated PCWP accurately identifies HFpEF patients with incremental value of exercise-stress to detect masked HFpEF. In the future, incorporation of functional parameters to the equation may further increase reflection of invasive PCWP.
Clinical Trial Registration
Clinicaltrials.gov, NCT03260621.
Funding
The study was funded by a grant from the German Centre for Cardiovascular Research (DZHK).
Author contributions
Andreas Schuster: Writing – review and editing, Writing – original draft, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Gerd Hasenfuß: Writing – review and editing, Resources, Project administration, Conceptualization. Johannes Kowallick: Writing – review and editing, Supervision, Software. Ruben Evertz: Formal analysis. Torben Lange: Writing – review and editing, Formal analysis, Data curation. Alexander Schulz: Writing – review and editing, Formal analysis, Data curation. Sören Jan Backhaus: Writing – review and editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Ethics approval and consent
The study was approved by the Ethics Committee of the University Hospital Goettingen and complied with the Declaration of Helsinki. All individuals gave written informed consent before participating in the study.
Consent for publication
Not applicable.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Andreas Schuster reports financial support was provided by German Center for Cardiovascular Disease. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Sören J. Backhaus, Email: s.backhaus@kerckhoff-klinik.de.
Alexander Schulz, Email: alexander.schulz@med.uni-goettingen.de.
Torben Lange, Email: torben.lange@med.uni-goettingen.de.
Ruben Evertz, Email: ruben.evertz@med.uni-goettingen.de.
Johannes T. Kowallick, Email: johannes.kowallick@med.uni-goettingen.de.
Gerd Hasenfuß, Email: hasenfus@med.uni-goettingen.de.
Andreas Schuster, Email: andreas_schuster@gmx.net.
Availability of data and materials
Regarding data availability, we confirm that all relevant data are within the paper and all data underlying the findings are fully available without restriction and can be accessed at the University Medical Centre Goettingen by researchers who meet the criteria for access to confidential data.
References
- 1.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Schulz A., Schuster A. Visualizing diastolic failure: non-invasive imaging-biomarkers in patients with heart failure with preserved ejection fraction. eBioMedicine. 2022;86 doi: 10.1016/j.ebiom.2022.104369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obokata M., Kane G.C., Reddy Y.N.V., Olson T.P., Melenovsky V., Borlaug B.A. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer M., Butler J., Zannad F., Filippatos G., Ferreira J.P., Pocock S.J., et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and a preserved ejection fraction: the EMPEROR-preserved trial. Circulation. 2021;144:1284–1294. doi: 10.1161/CIRCULATIONAHA.121.056824. Published online ahead of print 29 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravassa S., Trippel T., Bach D., Bachran D., González A., López B., et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. Eur J Heart Fail. 2018;20:1290–1299. doi: 10.1002/ejhf.1194. [DOI] [PubMed] [Google Scholar]
- 6.Cleland J.G.F., Ferreira J.P., Mariottoni B., Pellicori P., Cuthbert J., Verdonschot J.A.J., et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart 'OMics' in AGEing (HOMAGE) randomized clinical trial. Eur Heart J. 2021;42:684–696. doi: 10.1093/eurheartj/ehaa758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelmann F., Wachter R., Schmidt A.G., Kraigher-Krainer E., Colantonio C., Kamke W., et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 8.Anker S.D., Butler J., Filippatos G., Ferreira J.P., Bocchi E., Böhm M., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 9.Borlaug B.A., Blair J., Bergmann M.W., Bugger H., Burkhoff D., Bruch L., et al. Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation. 2022;145:1592–1604. doi: 10.1161/CIRCULATIONAHA.122.059486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster A., Schulz A., Lange T., Evertz R., Hartmann F., Kowallick J.T., et al. Concomitant latent pulmonary vascular disease leads to impaired global cardiac performance in heart failure with preserved ejection fraction. Eur J Heart Fail. 2023;25:322–331. doi: 10.1002/ejhf.2781. [DOI] [PubMed] [Google Scholar]
- 11.Pieske B., Tschöpe C., Boer R.A., de, Fraser A.G., Anker S.D., et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 12.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennell D.J. Cardiovascular magnetic resonance. Circulation. 2010;121:692–705. doi: 10.1161/CIRCULATIONAHA.108.811547. [DOI] [PubMed] [Google Scholar]
- 14.Garg P., Gosling R., Swoboda P., Jones R., Rothman A., Wild J.M., et al. Cardiac magnetic resonance identifies raised left ventricular filling pressure: prognostic implications. Eur Heart J. 2022 doi: 10.1093/eurheartj/ehac207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backhaus S.J., Lange T., George E.F., Hellenkamp K., Gertz R.J., Billing M., et al. Exercise-stress real-time cardiac magnetic resonance imaging for non-invasive characterisation of heart failure with preserved ejection fraction: the HFpEF stress trial. Circulation. 2021;143:1484–1498. doi: 10.1161/CIRCULATIONAHA.120.051542. [DOI] [PubMed] [Google Scholar]
- 16.Kramer C.M., Barkhausen J., Bucciarelli-Ducci C., Flamm S.D., Kim R.J., Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22:17. doi: 10.1186/s12968-020-00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdei T., Smiseth O.A., Marino P., Fraser A.G. A systematic review of diastolic stress tests in heart failure with preserved ejection fraction, with proposals from the EU-FP7 MEDIA study group. Eur J Heart Fail. 2014;16:1345–1361. doi: 10.1002/ejhf.184. [DOI] [PubMed] [Google Scholar]
- 18.Rosenkranz S., Preston I.R. Right heart catheterisation: best practice and pitfalls in pulmonary hypertension. Eur Respir Rev. 2015;24:642–652. doi: 10.1183/16000617.0062-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uecker M., Zhang S., Voit D., Karaus A., Merboldt K.-D., Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010;23:986–994. doi: 10.1002/nbm.1585. [DOI] [PubMed] [Google Scholar]
- 20.Grafton-Clarke C., Matthews G., Gosling R., Swoboda P., Rothman A., Wild J.M., et al. The left atrial area derived cardiovascular magnetic resonance left ventricular filling pressure equation shows superiority over integrated echocardiography. Medicina (Kaunas) 2023;59:1952. doi: 10.3390/medicina59111952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837. [PubMed] [Google Scholar]
- 22.Hasenfuß G., Hayward C., Burkhoff D., Silvestry F.E., McKenzie S., Gustafsson F., et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387:1298–1304. doi: 10.1016/S0140-6736(16)00704-2. [DOI] [PubMed] [Google Scholar]
- 23.Borlaug B.A., Reddy Y.N.V., Braun A., Sorimachi H., Omar M., Popovic D., et al. Cardiac and metabolic effects of dapagliflozin in heart failure with preserved ejection fraction: the CAMEO-DAPA trial. Circulation. 2023;148:834–844. doi: 10.1161/CIRCULATIONAHA.123.065134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Backhaus S.J., Schulz A., Lange T., Schmidt-Schweda L.S., Evertz R., Kowallick J., et al. Real-time cardiovascular magnetic resonance imaging for non-invasive characterisation of heart failure with preserved ejection fraction: final outcomes of the HFpEF stress trial. Clin Res Cardiol. 2024;113:496–508. doi: 10.1007/s00392-023-02363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backhaus S.J., Rösel S.F., Schulz A., Lange T., Hellenkamp K., Gertz R.J., et al. RT-CMR imaging for noninvasive characterization of HFpEF: medium-term outcomes HFpEF stress trial. JACC Cardiovasc Imaging. 2022;15:943–945. doi: 10.1016/j.jcmg.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Schuster A., Backhaus S.J., Stiermaier T., Navarra J.-L., Uhlig J., Rommel K.-P., et al. Left atrial function with MRI enables prediction of cardiovascular events after myocardial infarction: insights from the AIDA STEMI and TATORT NSTEMI trials. Radiology. 2019;293:292–302. doi: 10.1148/radiol.2019190559. [DOI] [PubMed] [Google Scholar]
- 27.Guichard J.-B., Nattel S. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol. 2017;70:756–765. doi: 10.1016/j.jacc.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Backhaus S.J., Rösel S.F., Stiermaier T., Schmidt-Rimpler J., Evertz R., Schulz A., et al. Left-atrial long-axis shortening allows effective quantification of atrial function and optimized risk prediction following acute myocardial infarction. Eur Heart J Open. 2022;2 doi: 10.1093/ehjopen/oeac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Regarding data availability, we confirm that all relevant data are within the paper and all data underlying the findings are fully available without restriction and can be accessed at the University Medical Centre Goettingen by researchers who meet the criteria for access to confidential data.