Abstract
Atherosclerotic cardiovascular disease (ASCVD) continues to be a major health concern globally. Apolipoprotein (Apo) B/A1 ratio is a reliable predictor of ASCVD and an important factor in assessing the risk of myocardial infarction. Tissue prolapse (TP) is defined as the tissue extrusion into the lumen through the stent struts after implantation, which is a significant factor for poor short-term outcomes such as acute and subacute thrombosis, severe myocardial necrosis, and vulnerable plaque. Therefore, the aim of this study was to investigate the relationship between Apo B/A1, plaque vulnerability, and tissue prolapse on optical coherence tomography (OCT). This study enrolled 199 patients with atherosclerotic cardiovascular disease (ASCVD) who underwent percutaneous coronary intervention (PCI). Both pre- and post-procedural optical coherence tomography (OCT) examinations were conducted to assess TP volume and plaque morphology. Logistic regression analyses were performed to identify potential risk factors for tissue prolapse volume. Receiver operator characteristic (ROC) curve analysis was carried out to evaluate the value of the Apo B/A1 ratio for tissue prolapse volume. The high Apo B/A1 ratio group showed a larger TP volume (P = 0.001) and a higher percentage of plaque rupture and erosion in comparison to the low Apo B/A1 ratio group (P = 0.022 and P = 0.008). The high Apo B/A1 ratio group and the high TP volume group also had a higher proportion of thin-cap fibroatheroma (TCFA) (P = 0.046, P = 0.021). Multivariate logistic regression analysis revealed that both Apo B/A1 ratio (odds ratio [OR]: 1.041, 95% confidence interval [CI] 1.007–1.076; P = 0.019) and TCFA (OR: 3.199, 95%CI 1.133–9.031; 0.028) were significantly related to high TP volume. Furthermore, the area under the curve (AUC) for predictive value of TP volume was 0.635 for Apo B/A1 (95% CI 0.554–0.717, P = 0.002) compared to 0.615 for low density lipoprotein cholesterol (LDL-C) (95% CI 0.533–0.697, P = 0.008). The Apo B/A1 ratio is an independent predictor of TP volume on OCT and is related to plaque vulnerability.
Keywords: Apolipoprotein B/A1 ratio, Tissue prolapse, Optical coherence tomography
Background
While statin therapy is effective in achieving treatment objectives, the residual cardiovascular risk remains significantly high at approximately 70% [1, 2]. As a result, researchers have sought to identify more advanced biomarkers to help explain this residual risk. Apolipoproteins B and A1 are the main surface proteins on LDL and HDL particles, respectively. Thus, the apo B/A1 ratio may reflect the cholesterol balance between atherogenic and anti-atherogenic lipoprotein particles. Previous research has shown apo B/A1 ratio can predict CVD and is strongly associated with the risk of myocardial infarction [3, 4]. Additionally, the apo B/A1 ratio is associated with vulnerable plaque, including plaque rupture, erosion, and thrombus [5].
TP refers to refers to tissue extrusion into the lumen through stent struts after implantation [6]. Several intravascular ultrasound (IVUS) studies have demonstrated an association between tissue prolapse and poor short-term prognosis, including acute and subacute thrombosis and more myocardial necrosis [7–9]. OCT has a higher resolution than IVUS and can provide clearer and more reliable information on TP [10, 11]. However, the relationship between apo B/A1 ratio and TP has not been fully investigated. In this study, researchers assessed the TP volume detected by OCT and investigated the association between the apo B/A1 ratio and TP volume.
Methods
Patient population
This study is a single-center, cross-sectional analysis. Researchers retrospectively enrolled patients diagnosed with ASCVD and undergoing PCI with pre- and post-procedural OCT examination from December 2019 to November 2022 at Central China Fuwai Cardiovascular Hospital, and these patients were required to have implanted at least one stent in the target vessel. Exclusion criteria included end-stage renal disease, serious liver dysfunction, hematological disease, malignant tumor disease, allergy to statins, and poor OCT imaging quality. In addition, some AMI patients who potentially had a greater amount of atherosclerotic plaques and intracoronary thrombus were excluded in this study. Patients with severe coronary stenosis which resulted in failure of OCT catheter to pass or poor OCT imaging quality before balloon dilatation were also excluded. The study protocol was approved by the Human Research Committee of Fuwai Central China Cardiovascular Hospital.
Clinical and laboratory data collection
Detailed demographic information, medical history, biochemical examination, drug usage, and angiographic data were obtained from the medical records. Venous blood samples were taken for serum lipid analysis when patients were hospitalized. Relevant lipoprotein markers such as LDL-C, high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglycerides (TG) were measured by electro-chemiluminescence immunoassay. Other laboratory parameters were measured using standard methods upon admission.
Angiographic procedure
All patients were treated with aspirin (300 mg loading dose, followed by 100 mg/day) and clopidogrel (300 mg loading dose, followed by 75 mg/day) or ticagrelor (180 mg loading dose, followed by 180 mg/day). Coronary angiography was performed via the transradial or transfemoral approach with a 6F or 7F sheath. Before PCI, intravascular infusion of 100–120 IU/kg unfractionated heparin was given. The culprit vessel was determined by a combination of coronary angiography, left ventricular wall motion abnormalities, electrocardiogram, and scintigraphic evidence of myocardial ischemia. Experienced interventionists selected the PCI strategy, and all patients underwent stent implantation with less than 25% residual stenosis on quantitative coronary angiography analysis.
OCT image acquisition and analysis
The OCT system used in this study was frequency-domain OCT (C7 ILMIEN system; St. Jude Medical, St. Paul, MN, USA). To avoid the effect of coronary spasms, 0.2 mg nitroglycerin was injected into coronary arteries. After the accomplishment of coronary angiography, OCT examination was performed via an imaging catheter in a virtually blood-free environment. The catheter was advanced distal to the lesion and then pulled back by the machine automatically at a steady rate. OCT images were analyzed at every frame using an offline review workstation.
Plaque morphology was characterized both qualitatively and quantitatively based mainly on previous criteria for OCT plaque characterization [6]. The presence of TP was evaluated immediately after PCI. TP area was assessed at 1-mm intervals throughout the stented segments, and plaque morphology features at the most protruding sites were evaluated, including plaque types, macrophage, cholesterol crystal, microvessels and TCFA. Fibrous plaque was defined as a plaque with homogeneous and highly backscattering regions. Lipid-rich plaque was defined as a plaque with a lipid arc greater than 180°. Calcified plaque was defined as a plaque with a calcification arc greater than 90° at the largest part, and calcium arc was measured by using the offline review workstation at 1-mm intervals. The lipid arc and the overlying fibrous cap thickness at the thinnest part through the whole lesion were measured at 1-mm intervals before PCI. The fibrous cap thickness of a plaque was the average value of three measurements. TCFA was defined as a plaque with a lipid rich arc greater than 180° and the thinnest fibrous cap less than 65 μm. Plaque rupture was defined as the discontinuous fibrous cap with obvious cavity formation, while plaque erosion was defined as the composed of evidence of thrombus, an irregular luminal surface, and no evidence of cap rupture evaluated in multiple adjacent frames. Macrophage was defined as a signal-rich punctuate region with heterogeneous backward shadows. Cholesterol crystal was defined as thin, linear regions of high intensity, usually associated with a fibrous cap or necrotic core. Microvessels were defined as black holes within a plaque with the presence of at least three consecutive frames. Thrombus by OCT appears as a mass attached to luminal surface or floating within the lumen. Red thrombus is highly backscattering and has a high attenuation (resembles blood), and white thrombus is less backscattering, is homogeneous, and has low attenuation. Tissue prolapse was defined as the tissue extrusion into the lumen through the stent struts after implantation. TP area was calculated by subtracting the lumen area from the stent area and TP volume was the sum of TP area measured in the whole stented segment at 1-mm intervals in the stented segment, as shown in Fig. 1. Representative OCT images of the culprit vessel were shown in Fig. 2. All OCT images were analyzed by two independent and experienced investigators who were blinded to the angiographic results and clinical data. A consensus diagnosis was obtained with the help of the third investigator if there was any disagreement between the two observers.
Fig. 1.
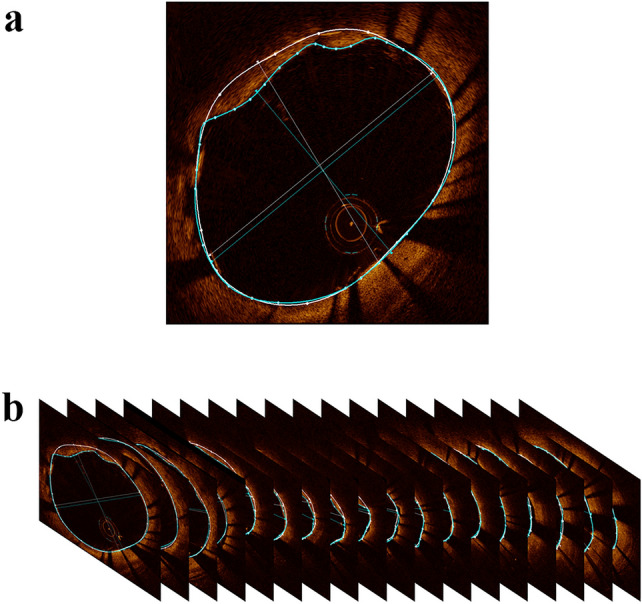
Measurement of TP volume on OCT. A TP area was calculated by subtracting the lumen area (blue line) from the stent area (white line); B TP volume was the sum of TP area measured in the whole stented segment at 1-mm intervals in the stented segment
Fig. 2.
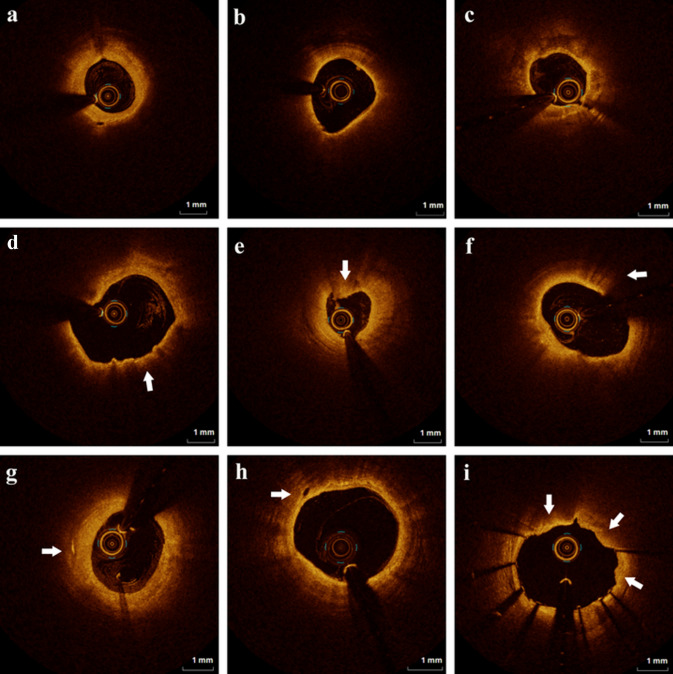
Representative optical coherence tomography images of various culprit vessel morphologies. A The fibrous plaque was defined as a plaque with homogeneous and highly backscattering regions. B Lipid-rich plaque was defined as a plaque with lipid arc > 180°. C Calcified plaque was defined as a plaque with calcification arc > 90° at the largest part. D Thin-cap fibroatheroma (TCFA) was defined as a plaque with lipid rich arc > 180° and the thinnest fibrous cap < 65 μm. E Plaque erosion was defined as the composed of evidence of thrombus, an irregular luminal surface, and no evidence of cap rupture evaluated in multiple adjacent frames. F Macrophage was defined as signal-rich punctuate region with heterogeneous backward shadows. G Cholesterol crystal was defined as thin, linear regions of high intensity, usually associated with a fibrous cap or necrotic core. H Microvessels were defined as black holes within a plaque with the presence on at least three consecutive frames. I Tissue prolapse was defined as the tissue extrusion into the lumen through the stent struts after implantation
Statistical analysis
IBM SPSS Statistics 26.0 software (SPSS Inc, Chicago, IL, USA) was used for all analyses. Categorical data were expressed as absolute frequencies and percentages (%) and compared using the chi-square test or Fisher’s exact test. Continuous data were expressed as mean ± standard deviation (SD) and compared using Student’s t-test, Mann–Whitney test, one-way analysis of variance, or Kruskal–Wallis test between two groups. Correlations between two variables were determined using the Pearson test or Spearman’s rank test as appropriate. The patients were also divided into two groups according to the median value of TP volume to explore the differences of OCT findings [12]. Logistic regression analyses were performed to assess risk factors for tissue prolapse volume. ROC curve analysis were performed to assess the value of the Apo B/A1 ratio for tissue prolapse volume. P value < 0.05 was considered statistically significant.
Results
Patient characteristics
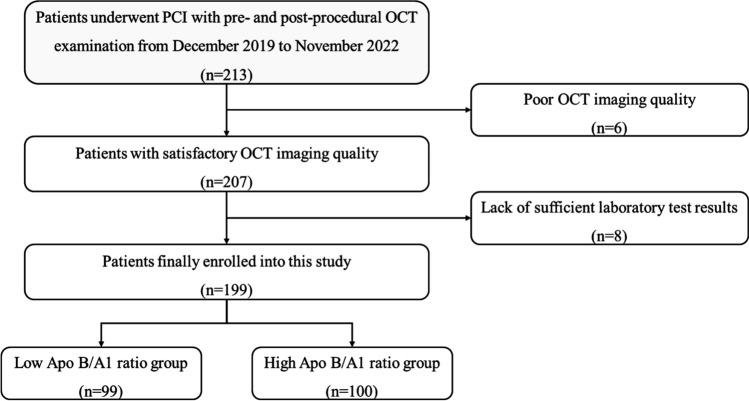
213 patients who underwent PCI with pre- and post-procedural OCT examination from December 2019 to November 2022 in Central China Fuwai Cardiovascular hospital were enrolled in this study. 6 patients with poor OCT imaging quality and 8 patients who had insufficient laboratory test results were excluded. The patients were divided into two groups: the low Apo B/A1 ratio group (n = 99) and the high Apo B/A1 ratio group (n = 100), as shown in Fig. 3.
Fig. 3.
Flow chart of this study. PCI percutaneous coronary intervention, OCT optical coherence tomography, Apo apolipoprotein
Baseline clinical characteristics between the two groups were displayed in Table 1. No significant differences were noted in terms of gender, age, and smoking history. However, a higher number of heart failure patients were observed in the high Apo B/A1 ratio group (P = 0.003). The left ventricular ejection fraction was significantly lower in the high Apo B/A1 ratio group (P = 0.039) and post-PCI CK-MB was significantly higher in the high Apo B/A1 ratio group (P = 0.020). Moreover, renal function biomarkers including creatine and uric acid were higher in the high Apo B/A1 ratio group (P < 0.05). There were no significant differences in statin medication taken at admission between the two groups (P = 0.398).
Table 1.
Baseline characteristic of patients in the low and high Apo B/A1 ratio groups
| Characteristics | All (n = 199) | Low ratio (n = 99) | High ratio (n = 100) | P value |
|---|---|---|---|---|
| Male, n(%) | 142(71.4%) | 67(67.7%) | 75(75%) | 0.253 |
| Age, years, mean ± SD | 60.08 ± 10.52 | 60.32 ± 10.22 | 59.84 ± 10.87 | 0.782 |
| ACS | 86(43.2%) | 38(38.4%) | 48(48.0%) | 0.171 |
| Medical history, n(%) | ||||
| Atrial fibrillation | 1(0.5%) | 1(1, 0%) | 0(0%) | 0.497 |
| Hypertension | 111(55.8%) | 53(53.5%) | 58(58.0%) | 0.526 |
| Diabetes mellitus | 50(25.1%) | 22(22.2%) | 28(28.0%) | 0.347 |
| Heart failure | 15(7.5%) | 2(2.0%) | 13(13.0%) | 0.003 |
| Stoke | 19(9.5%) | 9(9.1%) | 10(10.0%) | 0.827 |
| Prior MI | 24(12.1%) | 14(14.1%) | 10(10.0%) | 0.370 |
| Prior PCI | 31(15.6%) | 18(18.2%) | 13(13.0%) | 0.314 |
| Dyslipidemia | 51(25.6%) | 26(26.3%) | 25(25.0%) | 0.838 |
| Family history | 44(22.1%) | 20(20.2%) | 24(24.0%) | 0.519 |
| Alcohol drinking | 86(43.2%) | 42(42.4%) | 44(44.0%) | 0.822 |
| Smoking | 90(45.2%) | 42(42.4%) | 48(48.0%) | 0.429 |
| Laboratory results, mean ± SD | ||||
| WBC, × 109 | 6.78 ± 2.17 | 6.44 ± 1.76 | 7.12 ± 2.48 | 0.056 |
| Hb, g/L | 136.95 ± 16.51 | 134.49 ± 16.78 | 139.44 ± 15.95 | 0.065 |
| HbA1c, % | 6.14 ± 1.10 | 5.87 ± 0.83 | 6.41 ± 1.27 | 0.005 |
| CK-MB, U/L | 18.81 ± 18.08 | 16.64 ± 13.56 | 20.92 ± 21.55 | 0.251 |
| Post-PCI CK-MB, U/L | 18.50 ± 16.71 | 15.38 ± 12.16 | 21.66 ± 19.90 | 0.020 |
| CRP, mg/L | 2.40 ± 2.41 | 2.37 ± 2.31 | 2.44 ± 2.43 | 0.871 |
| Creatine, μmol/L | 71.19 ± 20.70 | 67.60 ± 16.93 | 74.78 ± 23.45 | 0.035 |
| Uric acid, μmol/L | 312.36 ± 86.31 | 296.87 ± 68.67 | 327.85 ± 98.98 | 0.029 |
| TC, mmol/L | 3.54 ± 0.79 | 3.18 ± 0.65 | 3.89 ± 0.76 | < 0.001 |
| TG, mmol/L | 1.64 ± 1.02 | 1.42 ± 1.00 | 1.87 ± 0.99 | 0.007 |
| HDL-C, mmol/L | 1.00 ± 0.23 | 1.08 ± 0.24 | 0.91 ± 0.17 | < 0.001 |
| LDL-C, mmol/L | 2.01 ± 0.64 | 1.65 ± 0.50 | 2.37 ± 0.57 | < 0.001 |
| Apo A1, g/L | 1.11 ± 0.24 | 1.19 ± 0.26 | 1.02 ± 0.17 | < 0.001 |
| Apo B, g/L | 0.70 ± 0.21 | 0.56 ± 0.13 | 0.84 ± 0.17 | < 0.001 |
| Apo B/A1, % | 0.65 ± 0.23 | 0.48 ± 0.10 | 0.83 ± 0.18 | < 0.001 |
| LVEF, % | 53.35 ± 19.45 | 56.61 ± 16.96 | 50.09 ± 21.27 | 0.039 |
| Culprit vessels, n(%) | ||||
| LAD | 156(78.4%) | 79(79.8%) | 77(77.0%) | |
| LCX | 16(8.0%) | 11(11.1%) | 5(5.0%) | |
| RCA | 37(18.6%) | 18(18.2%) | 19(19.0%) | |
| Lesion site, n(%) | ||||
| Proximal | 128(64.3%) | 68(68.7%) | 60(60.0%) | |
| Middle | 62(31.2%) | 31(31.3%) | 31(31.0%) | |
| Distal | 19(9.5%) | 9(9.1%) | 10(10.0%) | |
| Stent, n(%) | ||||
| 1 | 134(67.3%) | 63(63.6%) | 71(71.0%) | |
| 2 | 51(25.6%) | 29(29.3%) | 22(22.0%) | |
| 3 | 11(5.5%) | 7(7.1%) | 4(4.0%) | |
| 4 | 3(1.5%) | 1(1.0%) | 2(2.0%) | |
| Statins, n(%) | (%) | 68(68.7%) | 63(63.0%) | 0.398 |
ACS acute coronary syndrome, SD standard deviation, MI myocardial infarction, PCI percutaneous coronary intervention, WBC white blood cell, Hb hemoglobin, HbA1c glycosylated hemoglobin, CK-MB creatine kinase-MB, CRP C-reaction protein, TC total cholesterol, TG total triglycerides, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, Apo apolipoprotein, LVEF left ventricular ejection fraction, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery
OCT findings of culprit vessels
OCT findings between the low and high Apo B/A1 ratio groups were shown in Table 2. The percentage of plaque rupture and plaque erosion was higher in the high Apo B/A1 ratio group (P = 0.022 and P = 0.008). Moreover, the proportion of TCFA was also higher in the high Apo B/A1 ratio group (P = 0.046). No significant differences were found among the fibrous plaque type, lipid-rich plaque type, and calcification plaque type. In terms of tissue prolapse, TP volume was larger in the high Apo B/A1 ratio group (P = 0.001).
Table 2.
Optical coherence tomography characteristic of patients in the low and high Apo B/A1 ratio groups
| Characteristics | All (n = 199) | Low ratio (n = 99) | High ratio (n = 100) | P value |
|---|---|---|---|---|
| Plaque morphology, n(%) | ||||
| Plaque rupture | 32(16.1%) | 10(10.1%) | 22(22.0%) | 0.022 |
| Plaque erosion | 32(16.1%) | 9(9.1%) | 23(23.0%) | 0.008 |
| Plaque type, n(%) | ||||
| TCFA | 57(28.6%) | 22(22.2%) | 35(35.0%) | 0.046 |
| Fibrous plaque | 54(27.1%) | 24(24.2%) | 30(30.0%) | 0.361 |
| FCT of fibrous plaque, μm, mean ± SD | 154.50 ± 102.28 | 152.20 ± 101.79 | 156.80 ± 103.98 | 0.846 |
| Lipid-rich plaque | 97(48.7%) | 47(47.5%) | 50(50.0%) | 0.722 |
| FCT of lipid-rich plaque, μm | 94.90 ± 62.56 | 85.60 ± 32.86 | 102.60 ± 79.71 | 0.404 |
| Lipid arc of lipid-rich plaque, ° | 242.22 ± 86.75 | 221.25 ± 92.94 | 259.00 ± 79.86 | 0.199 |
| Calcification | 48(24.1%) | 28(28.3%) | 20(20.0%) | 0.172 |
| Angle, ° | 171.08 ± 84.71 | 188.64 ± 94.88 | 145.33 ± 61.28 | 0.129 |
| Cholesterol crystal | 120(60.3%) | 65(65.7%) | 55(55.0%) | 0.124 |
| Macrophage | 16(8.0%) | 7(7.1%) | 9(9.0%) | 0.617 |
| Micro-vessel | 48(24.1%) | 22(22.2%) | 26(26.0%) | 0.533 |
| Intracoronary thrombus | 55(27.6%) | 24(24.2%) | 31(31.0%) | 0.287 |
| Slow/no flow | 31(15.6%) | 12(12.1%) | 19(19.0%) | 0.181 |
| Quantitative of target vessel | ||||
| MLA, mm2 | 2.04 ± 1.08 | 2.12 ± 1.02 | 1.97 ± 1.15 | 0.408 |
| MLD, mm | 1.55 ± 0.40 | 1.59 ± 0.39 | 1.51 ± 0.41 | 0.241 |
| Proximal reference vessel area, mm2 | 8.49 ± 3.19 | 8.70 ± 3.35 | 8.28 ± 3.02 | 0.418 |
| Proximal reference vessel diameter, mm | 3.30 ± 1.05 | 3.26 ± 0.62 | 3.33 ± 1.34 | 0.671 |
| Distal reference vessel area, mm2 | 6.07 ± 2.38 | 6.44 ± 2.02 | 5.07 ± 2.66 | 0.055 |
| Distal reference vessel diameter, mm | 2.77 ± 0.71 | 2.83 ± 0.44 | 2.72 ± 0.91 | 0.379 |
| Post-stent MLA, mm2 | 5.90 ± 2.41 | 5.74 ± 2.25 | 6.05 ± 2.56 | 0.437 |
| Post-stent MLD, mm | 2.73 ± 0.81 | 2.76 ± 1.01 | 2.71 ± 0.56 | 0.742 |
| Stent diameter, mm | 3.23 ± 0.47 | 3.25 ± 0.48 | 3.21 ± 0.47 | 0.670 |
| Stent length, mm | 25.05 ± 9.02 | 24.08 ± 8.60 | 26.01 ± 9.39 | 0.188 |
| Maximal dilatation pressure, atm | 20.44 ± 3.63 | 20.38 ± 3.83 | 20.50 ± 3.44 | 0.849 |
| TP volume | 1.81 ± 1.55 | 1.47 ± 1.28 | 2.16 ± 1.71 | 0.001 |
SD standard deviation, TCFA thin-cap fibroatheroma, FCT fibrous cap thickness, MLA minimal lumen area, MLD minimal lumen diameter, TP tissue prolapse
Relationship between TP volume and plaque morphologies
Researchers divided the patients into two groups based on TP volume: the low TP volume group (< 1.43 mm3, n = 99) and the high TP volume group (≥ 1.43 mm3, n = 100). The OCT findings of the two groups were displayed in Table 3. The lipid arc and stent diameter were significantly larger in the high TP volume group than in the low TP volume group (P = 0.032 and P = 0.026), and the fibrous cap thickness (FCT) was smaller in the high TP volume group (P = 0.043). Likewise, the percentage of TCFA was also higher in the high TP volume group (P = 0.021), indicating the underlying relationship between TP volume and plaque vulnerability. The high TP volume group was associated with a higher percentage of lipid-rich plaque, intracoronary thrombus and slow/no flow phenomenon (All P value < 0.05). Significant differences were also observed in plaque rupture between the two groups.
Table 3.
OCT findings of patients in low TP group and high TP group
| Characteristics | All (n = 199) | Low TP (< 1.43 mm3, n = 99) | High TP (≥ 1.43 mm3, n = 100) | P value |
|---|---|---|---|---|
| Lipid arc, °, mean ± SD | 190.00 ± 88.40 | 174.31 ± 94.41 | 205.92 ± 79.40 | 0.032 |
| FCT, μm | 133.60 ± 82.94 | 146.80 ± 97.86 | 119.10 ± 60.36 | 0.043 |
| TCFA | 57(28.6%) | 21(21.2%) | 36(36.0%) | 0.021 |
| Lipid-rich plaque, n(%) | 97(48.7%) | 44(44.4%) | 53(53.0%) | 0.227 |
| Fibrous plaque | 54(27.1%) | 20(20.2%) | 34(34.0%) | 0.029 |
| Calcification | 48(24.1%) | 25(25.3%) | 23(23.0%) | 0.710 |
| Plaque rupture | 32(16.1%) | 10(10.1%) | 22(22.0%) | 0.022 |
| MLA, mm2 | 2.04 ± 1.08 | 2.13 ± 1.19 | 1.95 ± 0.96 | 0.337 |
| MLD, mm | 1.55 ± 0.40 | 1.58 ± 0.43 | 1.52 ± 0.37 | 0.375 |
| Stent diameter, mm | 3.23 ± 0.47 | 3.14 ± 0.50 | 3.32 ± 0.43 | 0.026 |
| Stent length, mm | 25.05 ± 9.02 | 24.59 ± 9.21 | 25.51 ± 8.87 | 0.530 |
| Maximal dilatation pressure, atm | 20.44 ± 3.63 | 20.17 ± 3.41 | 20.71 ± 3.84 | 0.365 |
| Intracoronary thrombus | 55(27.6%) | 21(21.2%) | 34(34.0%) | 0.044 |
| Slow/no flow | 31(15.6%) | 10(10.1%) | 21(21.0%) | 0.034 |
FCT fibrous cap thickness, TCFA thin-cap fibroatheroma, MLA minimal lumen area; MLD, minimal lumen diameter
The predictive value of Apo B/A1 for TP
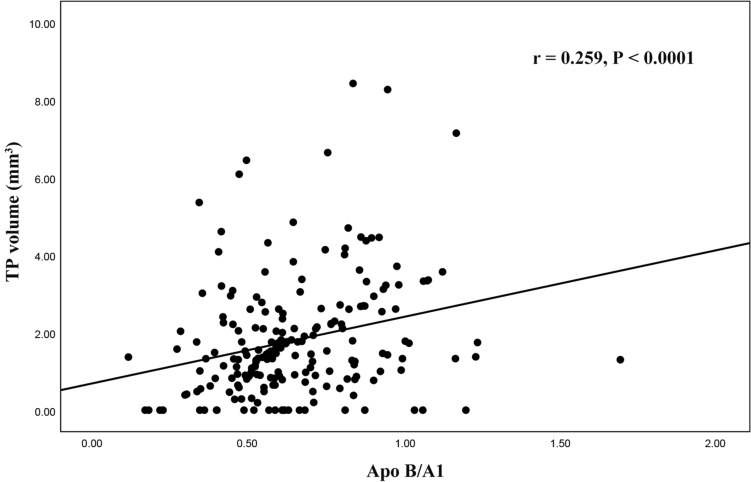
Multivariate logistic regression analysis demonstrated that Apo B/A1 ratio (odd ratio [OR]: 1.041, 95% confidence interval [CI] 1.007–1.076; P = 0.019), TCFA (OR: 3.199, 95%CI 1.133–9.031; P = 0.028) and intracoronary thrombus (OR: 2.866, 95%CI 1.277–6.434; P = 0.011) were significantly related to high TP volume which was shown in Table 4. Additionally, the correlation study showed that the Apo B/A1 ratio was positively related to TP volume (r = 0.259, P < 0.001) (Fig. 4). ROC curve analysis was conducted to evaluate the predictive value of the Apo B/A1 ratio for TP volume. The area under the curve (AUC) was 0.635 (95% CI 0.554–0.717, P = 0.002) for Apo B/A1 and 0.615 (95% CI 0.533–0.697, P = 0.008) for LDL-C, as shown in Fig. 5.
Table 4.
Variables related to high TP volume (≥ 1.43mm3)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | OR | 95%CI | P value | |
| ACS | 1.406 | 0.737–2.684 | 0.302 | |||
| Prior MI | 0.566 | 0.210–1.527 | 0.261 | |||
| Dyslipidemia | 0.710 | 0.346–1.454 | 0.349 | |||
| LDL-C | 1.755 | 1.029–2.995 | 0.039 | 0.596 | 0.245–1.446 | 0.252 |
| HDL-C | 0.174 | 0.036–0.828 | 0.028 | 1.436 | 0.159–13.006 | 0.748 |
| Apo B/A1 | 1.043 | 1.023–1.064 | < 0.001 | 1.041 | 1.007–1.076 | 0.019 |
| WBC count | 1.177 | 0.996–1.391 | 0.055 | |||
| CRP | 1.004 | 0.862–1.170 | 0.956 | |||
| LVEF | 0.997 | 0.960–1.036 | 0.892 | |||
| Stent length | 1.026 | 0.990–1.064 | 0.165 | |||
| Stent diameter | 1.533 | 0.776–3.030 | 0.218 | |||
| Maximal dilatation pressure | 1.012 | 0.927–1.106 | 0.789 | |||
| Pre-intervention MLA | 0.884 | 0.655–1.193 | 0.421 | |||
| Plaque rupture | 1.818 | 0.918–3.601 | 0.086 | |||
| Plaque erosion | 2.739 | 1.361–5.510 | 0.005 | 2.013 | 0.917–4.419 | 0.081 |
| TCFA | 6.818 | 2.758–16.854 | < 0.001 | 3.647 | 1.315–10.114 | 0.013 |
| Intracoronary thrombus | 2.267 | 1.150–4.468 | 0.018 | 2.866 | 1.277–6.434 | 0.011 |
ACS acute coronary syndrome, TP tissue prolapse, MI myocardial infarction, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, WBC white blood cell, Apo apolipoprotein, CRP C-reaction protein, LVEF left ventricular ejection fraction, MLA minimal lumen area, TCFA thin-cap fibroatheroma
Fig. 4.
Correlation between Apo B/A1 ratio and TP volume. Apo apolipoprotein, TP tissue prolapse
Fig. 5.
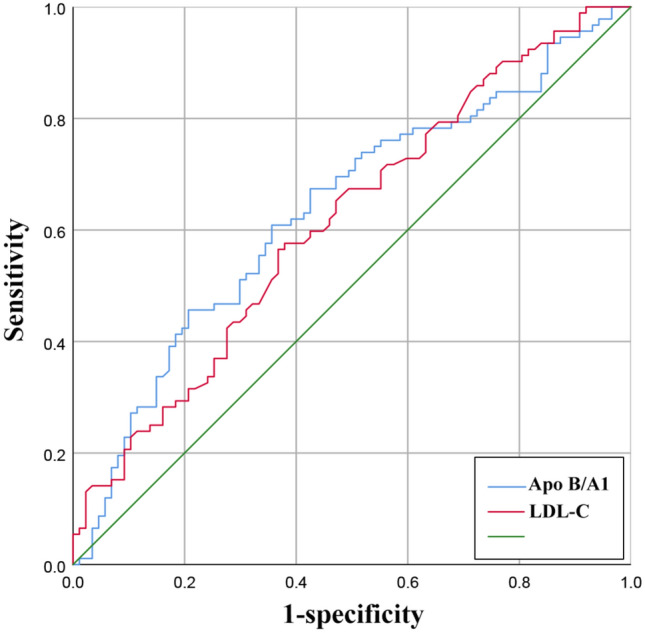
Receiver operating characteristic (ROC) curve for assessing tissue prolapse volume. Apo apolipoprotein, LDL-C low-density lipoprotein cholesterol
Discussion
This present study aimed to examine the underlying relationship among the Apo B/A1 ratio, plaque vulnerability, and TP volume in patients with atherosclerotic coronary disease. The results revealed that individuals with higher Apo B/A1 ratios had a greater incidence of plaque erosion and plaque rupture, indicating that the Apo B/A1 ratio is associated with plaque vulnerability. Additionally, quantitative analysis demonstrated a positive relationship between TP volume after PCI and Apo B/A1 ratio. Furthermore, multivariate analysis revealed that both Apo B/A1 ratio and TCFA were independently associated with TP volume. The Apo B/A1 ratio exhibited predictive value for TP volume, as evidenced by the receiver operator characteristic curve analysis. Therefore, there was underlying relationship between larger TP volume, high Apo B/A1 ratio and plaque instability.
A previous study has demonstrated that TP is an independent factor for the no-flow phenomenon after PCI in patients with acute myocardial infarction (AMI) and plaque rupture [13]. The creatine kinase-myocardial band (CK-MB) levels were greater in patients with TP lesions after stent implanting than in those without TP lesions [7, 9, 12]. Another OCT study examined the relationship between TP and neointimal proliferative restenosis and found that TP volume could predict the degree of neointimal hyperplasia [14].
Fibrous cap thickness is thought to be a resistive factor against tissue prolapse resulting from high mechanical stress during stent expansion that can lead to plaque rupture and tissue prolapse [15]. An OCT study confirmed that the plaques in patients with acute coronary syndrome exhibited more vulnerable features than those with non-acute coronary syndromes, such as wider lipid arc, longer lipid length, thinner fibrous cap, and more TCFA [16]. This study found that the fibrous cap thickness was smaller in the high TP volume group and the percentage of plaque rupture was also larger in the high TP volume group, which was consistent with the above theory and study.
While OCT has a higher resolution than IVUS [17] and can accurately identify plaque components and microstructure, it is an invasive procedure with inevitable limitations such as high cost, and potential complication risks [5]. Therefore, effective biomarkers that can predict TP need to be explored. This study quantitatively analyzed TP volume and plaque morphologies using OCT and performed a logistic analysis to determine independent factors of the severity of tissue prolapse. The results showed that TCFA and Apo B/A1 ratio were associated with the severity of tissue prolapse after excluding some clinical and imaging factors. Recent studies showed that the apo B/A1 ratio may reflect the cholesterol balance and the risk of major adverse cardiovascular events. An observational study found that the Apo B/A1 ratio is an independent predictor for complicated lesions and future myocardial infarction in patients with diabetes and acute coronary syndrome, which was consistent with our findings [18]. This study also revealed that HbA1c in the high Apo B/A1 ratio group was significantly higher than that in the low Apo B/A1 ratio group. There was a significant association between the Apo B/A1 ratio and the severity of coronary artery stenosis detected by multidetector computed tomography or coronary angiography [19–21]. The Apo B/A1 ratio was associated with diacron-reactive oxygen metabolites, which can reflect oxidative stress, endothelial dysfunction, and inflammation and high C-reaction protein level with high Apo B/A1 ratio was related to a high risk of ASCVD [22, 23]. Therefore, the Apo B/A1 ratio plays a crucial role in the genesis and development of ASCVD, and the ratio demonstrated good predictive value for the prognosis of patients with ASCVD [24].
Correlation study showed that the Apo B/A1 ratio was positively related to TP volume, and the results revealed that a higher Apo B/A1 ratio may reflect a bigger TP volume. Furthermore, the ROC curve of Apo B/A1 demonstrated predictive value for bigger TP volume and the AUC of the ROC curve of Apo B/A1 was even higher than that of LDL-C which can help to explain the high residual cardiovascular risk [2]. In patients with ACS, especially ST segment elevation myocardial infarction (STEMI), more patients had a large amount of thrombus [25]. However, even using OCT, it can sometimes be difficult to determine the difference between plaque prolapse and thrombus prolapse. To lessen the influence of thrombus prolapse, some AMI patients who potentially had a greater amount of atherosclerotic plaques and intracoronary thrombus were excluded in this study. This may explain the discrepancy that ACS patients don’t have larger tissue prolapse volume in our study.
There are inevitably several limitations of this study that should be acknowledged. First, this was a single-center, retrospective study with small sample size. Second, patients with poor OCT imaging quality and without post-OCT examination were excluded, which may result in selection bias. Third, data regarding short and long-term outcomes are lacking in this study. Finally, follow-up OCT and laboratory examination were not performed to explore the effects of TP on myocardial damage and in-stent restenosis. Thus, future studies will enroll more samples, follow-up OCT examinations and data regarding outcomes also need to be collected.
Conclusion
This study demonstrated that TP volume was larger in the high Apo B/A1 ratio group, and the presence of TCFA was significantly related to high TP volume. The Apo B/A1 ratio is an independent predictor for TP volume on OCT which was related to plaque vulnerability.
Acknowledgements
Not applicable.
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease;
- Apo
Apolipoprotein
- TP
Tissue prolapse
- OCT
Optical coherence tomography
- PCI
Percutaneous coronary intervention
- ROC
Receiver operator characteristic
- TCFA
Thin-cap fibroatheroma
- OR
Odds ratio; CI: confidence interval
- AUC
Area under the curve
- LDL-C
Low density lipoprotein cholesterol
- IVUS
Intravascular ultrasound
- HDL-C
High-density lipoprotein cholesterol
- TC
Total cholesterol
- TG
Triglycerides
- SD
Standard deviation
- LVEF
Left ventricular ejection fraction
- FCT
Fibrous cap thickness
- AMI
Acute myocardial infarction
- CK-MB
Creatine kinase-myocardial band
- CABG
Coronary artery bypass grifting
- WBC
White blood cell
- Hb
Hemoglobin
- HbA1c
Glycosylated hemoglobin
- LAD
Left anterior descending artery
- LCX
Left circumflex artery
- RCA
Right coronary artery
- MLA
Minimal lumen area
- MLD
Minimal lumen diameter
Author contributions
YD: study design, collection of data, statistical analyses, drafting of the manuscript. BZ, YL, ZD, JZ, WY, and HL: collection of data, statistical analyses. CG: study conception and design, critical revision for important intellectual content, and final approval of the submitted manuscript. Each author agreed to be responsible for the article and agreed on the final manuscript.
Funding
The authors report no conflicts on interests in regard to this manuscript.
Data availability
The data used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Zhengzhou University Central China Fuwai Hospital. All procedures were in accordance with institutional guidelines.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zethelius B, Berglund L, Sundström J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 2.Wong ND, Zhao Y, Quek RGW, et al. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: the Multi-Ethnic Study of Atherosclerosis. J Clin Lipidol. 2017;11:1223–1233. doi: 10.1016/j.jacl.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappelle PJWH, Gansevoort RT, Hillege JL, et al. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J Intern Med. 2011;269:232–242. doi: 10.1111/j.1365-2796.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MV, Millwood IY, Kartsonaki C, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–632. doi: 10.1016/j.jacc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng F, Li D, Lei L, et al. Association between apolipoprotein B/A1 ratio and coronary plaque vulnerability in patients with atherosclerotic cardiovascular disease: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2021;20:188. doi: 10.1186/s12933-021-01381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 7.Hong YJ, Jeong MH, Choi YH, et al. Impact of tissue prolapse after stent implantation on short- and long-term clinical outcomes in patients with acute myocardial infarction: an intravascular ultrasound analysis. Int J Cardiol. 2013;166:646–651. doi: 10.1016/j.ijcard.2011.11.092. [DOI] [PubMed] [Google Scholar]
- 8.Choi S-Y, Witzenbichler B, Maehara A, et al. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: a harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS-AMI) substudy. Circ Cardiovasc Interv. 2011;4:239–247. doi: 10.1161/CIRCINTERVENTIONS.110.959791. [DOI] [PubMed] [Google Scholar]
- 9.Hong YJ, Jeong MH, Ahn Y, et al. Plaque prolapse after stent implantation in patients with acute myocardial infarction: an intravascular ultrasound analysis. JACC Cardiovasc Imaging. 2008;1:489–497. doi: 10.1016/j.jcmg.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Jang IK, Tearney G, Bouma B. Visualization of tissue prolapse between coronary stent struts by optical coherence tomography: comparison with intravascular ultrasound. Circulation. 2001;104:2754. doi: 10.1161/hc4701.098069. [DOI] [PubMed] [Google Scholar]
- 11.Bouma BE, Tearney GJ, Yabushita H, et al. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart Br Card Soc. 2003;89:317–320. doi: 10.1136/heart.89.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama T, Kimura S, Akiyama D, et al. Quantitative assessment of tissue prolapse on optical coherence tomography and its relation to underlying plaque morphologies and clinical outcome in patients with elective stent implantation. Int J Cardiol. 2014;176:182–190. doi: 10.1016/j.ijcard.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Hong YJ, Jeong MH, Choi YH, et al. Predictors of no-reflow after percutaneous coronary intervention for culprit lesion with plaque rupture in infarct-related artery in patients with acute myocardial infarction. J Cardiol. 2009;54:36–44. doi: 10.1016/j.jjcc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama T, Kimura S, Ohtani H, et al. Relationship between quantities of tissue prolapse after percutaneous coronary intervention and neointimal hyperplasia at follow-up on serial optical coherence tomography examination. Int J Cardiol. 2017;241:470–477. doi: 10.1016/j.ijcard.2017.01.155. [DOI] [PubMed] [Google Scholar]
- 15.Hajiali Z, Dabagh M, Debusschere N, et al. Tissue prolapse and stresses in stented coronary arteries: a computer model for multi-layer atherosclerotic plaque. Comput Biol Med. 2015 doi: 10.1016/j.compbiomed.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Kato K, Yonetsu T, Kim S-J, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. doi: 10.1161/CIRCIMAGING.112.973701. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Yuan S, Qi J, et al. Advantages and prospects of optical coherence tomography in interventional therapy of coronary heart disease (Review) Exp Ther Med. 2022;23:255. doi: 10.3892/etm.2022.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Jia S, Yuan D-S, et al. Apolipoprotein B/A-I ratio predicts lesion severity and clinical outcomes in diabetic patients with acute coronary syndrome. Circ J Off J Jpn Circ Soc. 2020;84:1132–1139. doi: 10.1253/circj.CJ-19-1097. [DOI] [PubMed] [Google Scholar]
- 19.Hua R, Li Y, Li W, et al. Apolipoprotein B/A1 ratio is associated with severity of coronary artery stenosis in CAD patients but not in non-CAD patients undergoing percutaneous coronary intervention. Dis Markers. 2021 doi: 10.1155/2021/8959019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung CH, Hwang JY, Shin MS, et al. Association of apolipoprotein b/apolipoprotein A1 ratio and coronary artery stenosis and plaques detected by multi-detector computed tomography in healthy population. J Korean Med Sci. 2013;28:709–716. doi: 10.3346/jkms.2013.28.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S-H, Oh D, Jung KS, et al. The association between the apolipoprotein B/A-I ratio and coronary calcification may differ depending on kidney function in a healthy population. PLoS ONE. 2017;12:e0185522. doi: 10.1371/journal.pone.0185522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian YF, Zhou YP, Zhong CK, et al. C-reactive protein level, apolipoprotein B-to-apolipoprotein A-1 ratio, and risks of ischemic stroke and coronary heart disease among inner Mongolians in China. Biomed Environ Sci BES. 2016;29:467–474. doi: 10.3967/bes2016.062. [DOI] [PubMed] [Google Scholar]
- 23.Emoto T, Sawada T, Morimoto N, et al. The apolipoprotein B/A1 ratio is associated with reactive oxygen metabolites and endothelial dysfunction in statin-treated patients with coronary artery disease. J Atheroscler Thromb. 2013;20:623–629. doi: 10.5551/jat.16824. [DOI] [PubMed] [Google Scholar]
- 24.Tian M, Li R, Shan Z, et al. Comparison of apolipoprotein B/A1 ratio, Framingham risk score and TC/HDL-c for predicting clinical outcomes in patients undergoing percutaneous coronary intervention. Lipids Health Dis. 2019;18:202. doi: 10.1186/s12944-019-1144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prati F, Romagnoli E, Gatto L, et al. Clinical impact of suboptimal stenting and residual intrastent plaque/thrombus protrusion in patients with acute coronary syndrome: the CLI-OPCI ACS substudy (Centro per la Lotta Contro L’Infarto-Optimization of Percutaneous Coronary Intervention in Acute Coronary Syndrome) Circ Cardiovasc Interv. 2016;9:e003726. doi: 10.1161/CIRCINTERVENTIONS.115.003726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analysed during the current study are available from the corresponding author on reasonable request.