Abstract
Measles virus (MV) and vesicular stomatitis virus (VSV) are both members of the Mononegavirales but are only distantly related. We generated two genetically stable chimeric viruses. In MGV, the reading frames of the MV envelope glycoproteins H and F were substituted by a single reading frame encoding the VSV G glycoprotein; MG/FV is similar but encodes a G/F hybrid in which the VSV G cytoplasmic tail was replaced by that of MV F. In contrast to MG/FV, MGV virions do not contain the MV matrix (M) protein. This demonstrates that virus assembly is possible in the absence of M; conversely, the cytoplasmic domain of F allows incorporation of M and enhances assembly. The formation of chimeric viruses was substantially delayed and the titers obtained were reduced about 50-fold in comparison to standard MV. In the novel chimeras, transcription and replication are mediated by the MV ribonucleoproteins but the envelope glycoproteins dictate the host range. Mice immunized with the chimeric viruses were protected against lethal doses of wild-type VSV. These findings suggest that it is feasible to construct MV variants bearing a variety of different envelopes for use as vaccines or for gene therapeutic purposes.
Membrane glycoproteins drive the assembly and budding of enveloped RNA viruses (39) and are the key players in determining the host range and tissue tropism. The tropism or host range could be altered by changing the viral envelope protein(s) (pseudotyping). Pseudotyping is critically dependent on the ability of the nucleocapsids of one virus to interact with the envelope proteins specified by the other. Initially, vesicular stomatitis virus (VSV) was found to form pseudotypes with a number of other enveloped viruses including avian and murine retroviruses, herpes simplex virus, and influenza A virus (32, 49). Influenza A virus, on the other hand, did not incorporate envelope protein of VSV (22, 50). Moreover, replication-deficient retroviruses were pseudotyped with the envelope glycoprotein G of VSV or influenza virus hemagglutinin (8, 23, 38, 48).
Pseudotypes have been generated either by mixed infections with two different viruses or by using packaging cells (either transiently or stably transfected) to complement viruses carrying either temperature-sensitive defects or deletions in genes encoding envelope proteins (19, 21, 26). Alternatively, three-plasmid expression systems have been used to generate retroviral vectors. One plasmid drives the expression of viral proteins required in trans (Gag and Pol), the second encodes a heterologous envelope protein for pseudotyping the particles generated, and the third, a transducing plasmid, merely contains the long terminal repeats and a reporter or selection genes preceded by a packaging signal (reference 23 and references therein). In all these cases, pseudotypes were phenotypically complemented, by either temperature-sensitive or completely replication-deficient viruses. In addition, genes were inserted into the VSV genome, rescuing a phenotypically or genotypically mixed VSV (37). However, the VSV/rabies virus G or, at least, the cytoplasmic domain of the G protein was always required for packaging of foreign proteins into the viral envelope (17, 37). Here, we report the construction of true chimeric viruses with completely altered envelope and tropism.
Measles virus (MV), a member of the family Paramyxoviridae, genus Morbillivirus, is a large pleomorphic virus, 150 to 300 nm in diameter. The number of ribonucleoproteins (RNPs) packaged into the virus varies widely (10 to 100 or more per virus) (16). Its genome codes for the nucleocapsid proteins, nucleoprotein (N), phosphoprotein (P), and RNA polymerase (L) (29); the integral membrane proteins, hemagglutinin (H) and fusion protein (F); and the membrane-associated matrix protein (M). The two viral transmembrane glycoproteins, H and F, are both required for virus-host cell membrane fusion, while attachment to host cells is mediated by H alone. M protein, on the other hand, appears to mediate interactions between the RNP and the envelope proteins. Direct evidence has not yet been obtained for the interaction of M with the envelope proteins and its necessity for MV assembly and budding. Only in vitro has it been demonstrated that M binds to the RNP complex and is associated with membranes (13).
In patients with subacute sclerosing panencephalitis (SSPE), a rare but consistently lethal disease affecting the central nervous system and occurring several years after acute MV infection, the M protein was often defective or completely absent (7, 12, 47). Nevertheless, an inexorable spread of replicating RNPs through the entire brain occurs in patients with SSPE, apparently without formation of virions. Sequence analysis of F genes of persistent MVs in all patients with SSPE examined so far showed that the F ectodomain was well conserved whereas the cytoplasmic domain was truncated or extensively mutated (36). However, all SSPE-associated F variants analyzed were efficiently transported to the cell surface and mediated cell-cell fusion activity (6).
Since measles ranks among the major causes of infant death in developing countries, it is urgent to unravel the mechanisms implicated in the formation of virions, the role of the envelope glycoproteins in assembly and budding, and well as the enigmatic consequences of MV infection, such as the commonly detected induction of strong transient immunosuppression, the suspected frequent MV persistence (15), and the rare triggering of SSPE.
Using our recently established MV rescue system (28), we report the construction of chimeric viruses in which the reading frames encoding the MV envelope glycoproteins are replaced by a complete or altered VSV G reading frame. The chimeric viruses (MGV and MG/FV) are autonomous and genetically stable. Mice immunized with these viruses were protected against lethal doses of wild-type VSV. We also show that the cytoplasmic domain of the F glycoprotein is necessary and sufficient for incorporating the MV M protein into the virions and that, at slightly lower yields, virions can also form without M protein. Possible practical applications of such chimeric viruses as vaccines and as vectors for targeted gene delivery are discussed.
MATERIALS AND METHODS
Cells and viruses.
Baby hamster kidney (BHK-21), human embryonic kidney 293, monkey kidney fibroblast (Vero), and mouse fibroblast thymidine kinase-negative (Ltk−) cells were maintained in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal calf serum. For rescue, 293-3-46 cells were used. These cells are helper cells stably expressing the MV N and P proteins as well as T7 RNA polymerase (28).
MV Edmonston B was propagated in 293 or Vero cells. To obtain high-titer stocks, 293 or Vero cells were infected at a multiplicity of infection (MOI) of 0.01 PFU/cell. When more than 80% of cells had formed syncytia, two-thirds of the medium was discarded and the virus was harvested by scraping the cells into the remaining medium and subjected to two rounds of freezing and thawing and low-speed centrifugation (300 × g for 5 min at 4°C) to remove the cell debris. Aliquots were stored at −80°C.
VSV Indiana was propagated in Vero cells at an MOI of 0.01 PFU/cell. The virus was harvested from the supernatants after 80 to 90% of the cells had detached. Supernatants were clarified by centrifugation, and aliquots were stored at −80°C. To prepare stocks of the MGV and MG/FV chimeras, Vero cells were infected at an MOI of 0.01 or 0.1 PFU/ml. When 80% of the cells exhibited cytopathic effects (CPE), supernatants were collected and clarified by centrifugation at 4,500 × g for 20 min. Aliquots were stored at −80°C.
Plasmids.
All cloning procedures were carried out essentially as described by Sambrook et al. (34). PCR amplifications were carried out with the proofreading Pfu DNA polymerase (Stratagene) and primers with a 3′-terminal phosphorothioate bond instead of a phosphodiester bond (40). Our constructs were based on plasmid p(+)MV (28). All MV cDNAs were adjusted to the rule of six (4). DNA sequences of the synthetic oligonucleotides are given in lowercase for non-MV nucleotides and in uppercase for the MV nucleotides; relevant restriction endonuclease recognition sites are underlined in Fig. 1.
FIG. 1.
Features of plasmids which allow the rescue of the chimeric viruses MGV and MG/FV. The entire MV F and H open reading frame and parts of the untranslated region were substituted by the open reading frame of VSV G. The nucleotides in lowercase are derived from the VSV G gene, and those in uppercase represent MV sequences. The numbering of the nucleotides refers to the MV antigenomic sequence as given by Radecke and Billeter (27). The underlined nucleotides were added during the cloning procedure to comply with the rule of six (4) for p(+)MG/FV and p(+)MGV. The antigenomic sense of the specified RNAs is indicated by (+). Relevant regions of the nucleotide sequences near the ends of the VSV inserts and of the corresponding encoded amino acids are detailed. The sequence tags (MV 1702 and 1805/6) are present in all plasmids. Boxes around nucleotides indicate the nontranscribed gene boundary trinucleotides between genes M and F and between genes H and L, respectively. The MV cDNA sequence is available from the EMBL gene bank under accession no. Z66517.
To obtain p(+)MGV, an intermediate plasmid, p(+)G-CAT, was constructed. The VSV G gene, of VSV Indiana (kindly provided by J. Rose, Yale University) was amplified from the pAR-G plasmid (46) with primers 300 (5′-cctcgatatcggcg ccactatgaagtgccttttgtacttagc-3′) and 301 (5′-ccgctcgagtggactagtagttactttccaagtcggttcatctc-3′) to introduce the EcoRV and XhoI cloning sites. The PCR fragment was digested with EcoRV and XhoI and replaced the EcoRV-SalI fragment in plasmid p(+)NPCAT (28). p(+)NPCAT contains an additional intercistronic region similar to the N-P intergenic boundary and was constructed by inserting (5′-ctaGCCTACCCTCCATCATTGTTATAAAAAACTTAGGAACCAGGTCCACACAGCCGCCAGCCCATCAACgcgtatcgcgata-3′, MV(+) 1717 to 1782) and the internally complementary oligonucleotide into the SpeI site of the P gene followed by the chloramphenicol acetyltransferase coding region. The NarI-SpeI fragment of p(+)G-CAT (42) containing the G gene replaced the NarI-SpeI fragment of p(+)MV containing the F and H genes. The new plasmid was called p(+)MGV (Fig. 1).
To construct p(+)MG/FV, a PCR was performed on pAR-G with primers 291 (5′-cctcgatatcggcgccactatgaagtgccttttgtac-3′) and 297 (5′-ctcctagcctaggaatagtccaatgattaaccctatg-3′). The PCR fragment was digested with PstI and AvrII. Another PCR was performed on peΔ5F1 (43) with primers 298 (5′-cttccactagtgctcaggGGGCGTTGTAATAAAAAGGT-3′) and 299 (5′-ccctcgagactagtctgcaggaTCAGAGCGACCTTACATAGG-3′). The PCR fragment was digested with SpeI. The two PCR fragments were ligated into the PstI-SpeI-cleaved p(+)G-CAT construct, yielding p(+)G/F-CAT. The NarI-SpeI fragment of this plasmid was then used to replace that of p(+)MV, which contains the F and H genes. The new plasmid was designated p(+)MG/FV (Fig. 1).
Rescue of MGV and MG/FV chimeric particles.
The experimental setup, transfection, and rescue of MV were performed exactly as in our previous work (28). Briefly, 293-3-46 cells were transfected with 5 μg of either p(+)MG/FV or p(+)MGV in the presence or absence of 100 ng of plasmid specifying the MV L mRNA. To control the rescue protocol, p(+)MV was used. First, CPE appeared 2 to 4 days later. To allow infection to progress, the confluent monolayers were transferred from 36-mm wells to 100-mm dishes. When they reached confluency, the cells were scraped into 1 to 2 ml of culture medium and subjected to one round of freezing and thawing, and the supernatants were clarified by centrifugation. To produce virus stocks, cleared supernatants were used to infect monolayers of Vero cells. All experiments with live chimeric MVs were performed at biosafety level 2, which complies with the rules of the Swiss Interdisciplinary Committee for Biosafety in Research and Technology.
Western blotting.
Infected cells were washed once with phosphate-buffered saline (PBS) and lysed with 300 μl of reporter lysis buffer (Promega) and 1/60 or 1/6 of the total lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide) and blotted onto Immobilon-P transfer membranes (pore size, 0.45 μm; Millipore). The membranes were washed for 1 h with TBST (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% Tween 20) containing 1% bovine serum albumin and 1% skim milk to block nonspecific binding. As the first antibody, rabbit polyclonal antibodies directed against MV N, P diluted 6,000-fold in TBST buffer, or M diluted 4,000-fold in TBST buffer (24) were used. A rabbit polyclonal anti-Fi antibody raised against the synthetic peptide NH2-CPDLTGTSKSYVRSL-COOH, corresponding to the cytoplasmic domain of MV F protein (14), was used at a 4,000-fold dilution. The rabbit anti-VSV antibody was used at a 1,000-fold dilution. The second antibody was a swine anti-rabbit antibody coupled to horseradish peroxidase, allowing visualization of the proteins by enhanced chemiluminescence (Amersham Life Science).
Growth and infectivity assays of viruses.
Since MV is primarily cell associated, formation of infectious particles was determined at different time points for either cell-free or cell-associated viruses. Equal numbers of Vero cells were cultured in 36-mm dishes 1 day before infection. The cells were then infected with MGV, MG/FV, MV, or VSV at an MOI of 0.1 and incubated at 37°C for 2 h for MGV and MG/FV or 1 h for MV and VSV. Nonadsorbed virus was removed by washing the cells once with PBS. Complete medium was added, and the cells were incubated at 37°C for various times. At each time point (indicated in the figure legends), the supernatant of one 36-mm dish was collected to determine the titer of cell-free virus. To determine the titer of cell-associated virus, cells were scraped into 0.6 ml of OptiMEM and subjected to one round of freezing and thawing. Cell debris were removed by centrifugation. All the supernatants were kept at −80°C until all the samples were collected. The titers of MV were determined by a standard plaque assay on Vero cells. To avoid any discrepancy, the number of freezing and thawing steps for supernatants containing cell-free virus or cell-associated virus was kept the same.
Titers of MGV, MG/FV, and VSV were determined by an immunofluorescence assay. Subconfluent (80%) Vero cells on coverslips were inoculated with the serially diluted virus and incubated for 1 to 2 h at 37°C. The cells were washed once with PBS and overlaid with 1% low-melting-point agarose. At 12 or 20 h later, VSV-infected cultures were fixed with 15% trichloroacetic acid, for 30 min and the overlay was removed. The coverslips were then processed for immunofluorescence with anti-VSV antibody and fluorescein isothiocyanate-conjugated secondary antibody. MGV- or MG/FV-infected cells were treated similarly, except that cells were fixed only at 48, 72, 96, or 144 h postinfection (p.i.) (to assess the parameters for counting the foci formed). The foci of fluorescent G-protein-expressing cells were counted. For comparison, both immunofluorescence and plaque assays were tested for MV and found to be comparable.
Radiolabeling, immunoprecipitation, endoglycosidase treatment, and PAGE.
To measure glycoprotein transport, cell monolayers grown in 36-mm six-well plates were infected with MGV, MG/FV, or VSV at an MOI of 1. At 12 h after VSV infection and 48 h after MGV and MG/FV infection, the cells were washed once with PBS, starved with cysteine- and methionine-free DMEM for 1 h, and pulse-labeled for 20 min in the same medium containing 150 μCi of Tran35S-label (ICN Pharmaceuticals Inc., Irvine, Calif.) per ml. The cells were washed once with warm PBS and chased in prewarmed DMEM, containing additional 50 μM methionine and cysteine, for 0, 60, and 120 min. The chase was terminated by washing the cells with ice-cold DMEM; the cells were kept on ice, lysed with 1 ml of lysis buffer (50 mM Tris [pH 8.0], 10 mM EDTA, 0.1 U of aprotinin per ml, 1% [vol/vol] Nonidet P-40). The lysates were clarified by centrifugation at 10,000 × g at 4°C for 10 min. Half of the lysates were frozen for further use, and the rest were combined with NET-GEL (150 mM NaCl, 5 mM EDTA [pH 8.0], 50 mM Tris-HCl [pH 7.4], 0.05% Nonidet P-40, 0.25% gelatin, 0.02% azide) containing 1:300-diluted anti-VSV antibody and 60 μl of 10% protein A-Sepharose. After incubation on a rocker at 4°C overnight, immunoprecipitates were washed with NET-GEL containing 150 mM NaCl, with NET-GEL containing 0.1% SDS, and finally with 10 mM Tris-HCl (pH 8.0). The proteins were then eluted with 50 mM Tris (pH 7.0)–1% SDS for further analysis. Endoglycosidase H (endo H; Boehringer Mannheim) treatment was performed essentially as described by the manufacturer. Briefly, eluted protein samples were divided into two tubes, and an equal volume (25 μl) of 2× endo H buffer (150 mM citrate buffer [pH 5.5]) was added. To one tube was added 1 to 4 mU of endo H, and the other was kept without enzyme. The samples were incubated at 37°C for 2 to 4 h and then subjected to SDS-PAGE (10 or 12.5% polyacrylamide).
Sucrose gradient centrifugation.
Supernatants from approximately 6 × 106 MGV- or MG/FV-infected cells and 3 × 106 VSV- or MV-infected cells were first clarified by centrifugation at 7,000 × g for 20 min and then layered on a cushion of 1 ml each of 20 and 60% sucrose in TNE (1 mM Tris [pH 7.8], 100 mM NaCl, 10 mM EDTA). After centrifugation at 28,000 rpm for 90 min in an SW-41 rotor, the sucrose interphase (1 ml) was taken, diluted to 20% sucrose, and applied on the top of a 20, 30, 40, 50, and 60% (2 ml each) sucrose step gradient, followed by centrifugation in an SW-41 rotor at 29,000 rpm for 16 h to determine the particle density and contents. Gradients were prepared with Auto Densi-flow (Labconco). A control gradient (containing no virus) was run in parallel to measure the density fluctuation. Fractions of approximately 500 μl were collected, and the density was measured by weighing 100 μl from each fraction twice. Similarly, the densities of the control fractions were determined. Less than 6% differences in density among the same fractions of all the gradients were detected. The profile of a representative gradient is shown in Fig. 5C. The protein content in each fraction was analyzed by SDS-PAGE followed by immunoblotting.
FIG. 5.
Purification and characterization of MGV and MG/FV particles in comparison to MV and VSV. (A) Virus particles were first pelleted on 20% (1.0 ml) and 60% (1.0 ml) sucrose cushions. The interphases were taken and diluted to 20% sucrose, applied to 20 to 60% sucrose gradients, and centrifuged to equilibrium. Fractions were analyzed by SDS-PAGE (12.5% polyacrylamide), immunoblotted, and probed with the antibodies indicated on the right. (B) The blots were reprobed with anti-MV M antibody in addition to anti-VSV. The sucrose density was determined by weighing 100 μl of each fraction. (C) Representative profile of the gradient.
Immunoelectron microscopy.
Cells from monolayers of infected cultures were brought into suspension by a short trypsinization followed by centrifugation in DMEM containing 10% fetal calf serum. The cells were then resuspended in PBS containing 3% freshly made paraformaldehyde and fixed therein for 15 min at room temperature. After fixation, the cells were suspended and incubated for 60 min at room temperature in a 1:50 dilution of primary antibody (anti-VSV), washed in PBS, and further incubated for 60 min at room temperature with a dilution of 1:100 of 6-nm colloidal gold particles coated with protein A (Aurion). After the free protein A-gold complexes were washed out, the cells were fixed for 30 min with 3% glutaraldehyde and then for 30 min with 2% osmium tetroxide. The fixed cells were then dehydrated and embedded for thin sectioning in Epon-Araldite by standard procedures. Thin sections were stained with lead citrate and uranyl acetate and examined with a Philips EM 400 transmission electron microscope.
Animals, immunization, neutralization assay, and challenge.
Inbred C57BL/6 and alpha/beta interferon receptor-deficient mice (A129) mice were obtained from the breeding colony of the Institut für Labortierkunde, Tierspital Zürich, Zürich, Switzerland. The mice were between 8 and 12 weeks of age when used. VSV Indiana was grown on BHK cells in DMEM–5% fetal calf serum. Stocks were diluted so that 200 μl could be injected per mouse. All injections (immunization and challenge) were done intraperitoneally.
The VSV neutralization assay was performed as previously described (9). Briefly, sera from immunized mice were diluted 40-fold in DMEM supplemented with 5% FCS and then heat inactivated. Serial twofold dilutions were mixed with equal volumes of VSV diluted to contain 500 PFU/ml. The mixture was incubated for 90 min at 37°C. Then 100 μl of the serum-virus mixture was transferred to monolayers of Vero cells in 96-well plates and incubated for 1 h at 37°C. An overlay of 100 μl DMEM containing 1% methylcellulose was added. After 24 h at 37°C, the overlay was flicked off and the monolayer was fixed and stained with 0.5% crystal violet. The highest dilution of serum that reduced the numbers of plaques by 50% was taken as the neutralizing titer. Titers are indicated as log2 of 40-fold-prediluted sera. To determine immunoglobulin G titers, undiluted serum was first pretreated with an equal volume of 0.1 M mercaptoethanol in saline.
RESULTS
Construction of plasmids and rescue of MV/VSV chimeric viruses.
Plasmids p(+)MGV and p(+)MG/FV were based on p(+)MV, which allows the production of MV antigenomes and rescue of the virus upon transfection into helper cells (28). In these plasmids, most of the F 5′ nontranslated region, the F reading frame, the F-H intergenic region, and the H reading frame were replaced by either the entire reading frame encoding the VSV G envelope glycoprotein or a hybrid reading frame encoding the ecto- and transmembrane regions of VSV G fused with the cytoplasmic domain of MV F (G/F) (Fig. 1). Rescue of chimeric MVs from these plasmids in the helper cells was successful and could be monitored by detection of foci exhibiting CPE 2 to 4 days after transfection. For a second round of propagation and virus stock preparation, supernatants of the original cell cultures were used to infect BHK-21, 293, and Vero cells. Five days later, particles were harvested from cells and supernatants by freeze-thaw treatment and subjected to titer determination. The infectious agent expressing the VSV-G protein was named MGV (measles-G-virus) and the agent expressing the G/F hybrid was named MG/FV (measles-G/F-virus).
Characterization of proteins expressed from the chimeric viruses.
To analyze the protein components encoded by the new chimeric particles, BHK-21 or 293 cells were infected with either MGV, MG/FV, VSV, or MV at an MOI of 0.1. After 20 h of VSV infection, 24 h of MV infection, or 48 h of MGV and MG/FV infection, the cells were lysed and aliquots were analyzed by Western blotting with a polyclonal antibody directed against all VSV proteins. Figure 2A shows the expression patterns of G-type protein in MGV- and MG/FV-infected cells. Proteins from MV-infected 293 cells, as well as from both controls, uninfected BHK and 293 cells, did not react with the antibody. The differential pattern of the bands in MGV- and MG/FV-infected BHK cells might be due to different maturation states of the proteins. VSV infection showed only the mature G protein in this exposure; however, upon longer exposure, the immature forms of G protein were also visible. In Vero cells, no differences were observed between the protein bands of the three viruses (data not shown).
FIG. 2.
Expression and transport of MGV, MG/FV, VSV, and MV proteins in BHK, 293, Vero, and Ltk− cells. (A to C) Western blot analysis demonstrating the expression of G and G/F proteins as well as MV N, P, and M by the four viruses. Cells were infected with MGV and MG/FV for 48 h. For comparison, cells were infected with VSV and MV for 20 h. They were then lysed, and one-sixth of each lysates was subjected to SDS-PAGE (10% polyacrylamide) and blotted. The membranes were treated with the specified antibodies, polyclonal anti-VSV (A), monoclonal anti-Fi antibody (B), and polyclonal anti-MV N, P, and M antibodies (C). The protein positions are indicated. The various posttranslational forms of the proteins are indicated as c, I, and h. (D) expression of G and G/F in various cells. (E) Cellular transport of envelope glycoproteins encoded by MGV, MG/FV, and VSV. At 12 h after VSV infection and 48 h after chimera infection, cells were pulse-labeled, chased as indicated, and either treated or not treated with endo H. The percentage of glycoprotein sensitivity to endo H was quantitated with a phosphorimager.
An anti-Fi antibody which recognizes the cytoplasmic domain of MV F was used to study the expression of F or G/F hybrid proteins (Fig. 2B). A double band was observed exclusively in MG/FV-infected BHK cells, confirming that MG/FV particles possess the hybrid G/F gene, as expected. In MV-infected 293 cells, as expected, the antibody recognized the F protein precursor F0 and the cleavage product F1, which contains the F cytoplasmic domain. To determine whether the MV nucleocapsid proteins, N, P, and M, were also expressed by MGV and MG/FV, immunoblots of the same lysates were probed with a mixture of the corresponding antibodies. Fig. 2C shows that the three MV proteins, N, P, and M, were expressed in all except VSV infections.
MV grows very poorly in rodent cells, which lack the major MV receptor, CD46. To determine whether exchange of the MV glycoproteins with that of VSV altered the cell specificity, we infected simian Vero cells and mouse Ltk− cells as well as hamster BHK-21 cells and human 293 cells. Lysates were probed with anti-VSV polyclonal antibody. All the cells were efficiently infected with both chimeric viruses, and expression of G and G/F in all cells was comparable to G expression in VSV-infected BHK cells (Fig. 2D). Taken together, these results indicate that the MV chimeras are replication competent, possessing a tropism similar to that of VSV, for which a broad host range is characteristic.
Cellular transport and maturation of glycoproteins G and G/F.
Viral proteins in infected cells bear signals allowing mutual interactions which take place in different cellular compartments. In some cases, transport of the envelope glycoprotein may be affected by the internal virus components (33, 44). It was therefore necessary to compare the transport and maturation rates of the unmodified glycoprotein G and the modified G/F in the presence of either VSV and MV internal proteins N, P, and M. Vero cells were infected with MGV or MG/FV for 48 h or with VSV for 12 h. Infected cells were pulse-labeled for 20 min and chased for 1 or 2 h. Lysates were clarified by centrifugation, and the proteins were immunoprecipitated with anti-VSV polyclonal antibody. Eluted proteins, either untreated or treated with endo H, were analyzed by gel electrophoresis. The endo H sensitivity of glycoproteins was quantitated with a phosphorimager. Figure 2E shows that after 60 min of chase, less than 10% of G in VSV infection remained endo H sensitive whereas the G protein of MGV and the G/F protein of MG/FV remained about 15 and 20% sensitive to endo H, respectively. After 2 h of chase, all the proteins acquired almost complete resistance to endo H. In essence, expression of VSV G in MV did not alter the transport rate of this protein. Furthermore, replacement of the cytoplasmic domain of VSV G with that of MV F reduced the transport efficiency only slightly.
Cytopathogenicity of MGV and MG/FV.
The CPE induced in cells depends on the infecting virus. VSV and MV alter cell morphology differently. The MV F protein, in conjunction with the H protein, is responsible for cell-cell fusion, resulting in syncytium formation, whereas VSV causes cells to round up and detach.
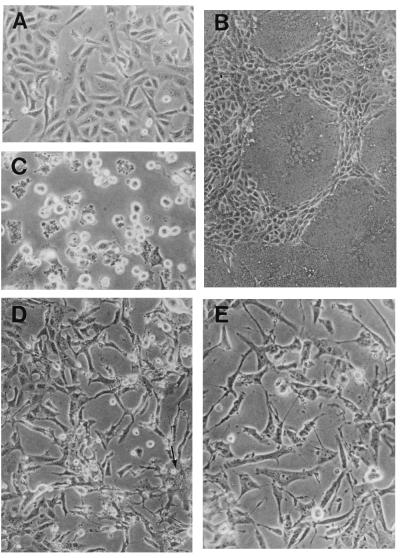
To determine the type of CPE caused by the chimeric viruses, subconfluent Vero cells were infected with either the chimeric agents or the standard viruses, MV and VSV, at an MOI of 0.1. Cells infected with MV underwent the first cell-cell fusion events at 24 h p.i.; syncytia rapidly grew (Fig. 3B) to involve the entire monolayer after 48 to 60 h p.i. At 14 h after VSV infection, cells rounded up and vesiculated before degenerating at 24 to 30 h p.i. (Fig. 3C). In contrast, cells infected with MGV or MG/FV appeared healthy and dividing. Only at about 72 h p.i. did vesiculated and elongated cells slowly appear. In MGV-infected cells, small fusion patches involving only 5 to 10 cell nuclei were visible at 96 h p.i. and did not expand at 150 h p.i. (Fig. 3D). These small syncytia are probably due to the acidification of the medium, which rendered the MGV G protein fusogenic (10). Cell fusions were not observed in MG/FV infections. Around 150 h p.i., cytopathogenic areas were easily visible in the monolayers of cells infected with either virus (Fig. 3D and E). CPE in MGV-infected cells always appeared 12 to 24 h later than did that in MG/FV-infected cells, which is probably due to the slower assembly kinetics of the virus. We also monitored the appearance of CPE with a time-lapse video camera and found that the difference was reproducible among all virus preparations tested. These results show that the newly rescued viruses induced different CPE from those induced by VSV and MV and that the chimeric virions are less virulent in cell culture.
FIG. 3.
Cytopathogenicity of MV, VSV, MGV, and MG/FV. Cells infected with the indicated virus were observed under a light microscope. The progress of the infection was monitored until clear CPE were visible; pictures were taken at different times after infection, as indicated. (A) Noninfected Vero cells; (B) MV infection at 36 h p.i.; (C) VSV infection at 30 h p.i.; (D) MGV infection at 156 h p.i.; (E) MG/FV infection at 144 h p.i. The arrow in panel D shows a fusion patch. Monolayers were photographed with a 40× objective for panels A, C, D, and E and a 20× objective for panel B.
Recovery of infectious MGV and MG/FV particles.
VSV grows to high titers, and the virions are readily released into the medium. In contrast, MV is poorly shed into the medium of infected cells (45). To ascertain whether the chimeric viruses follow the efficient release pattern of VSV or the cell-associated pattern of MV, titers of released or cell-associated MGV, MG/FV, VSV and MV were determined.
Vero cells were infected with the four viruses at an MOI of 0.1. For MV, standard plaque assays were performed and infectivity was calculated as PFU per milliliter. For the other three viruses, titers were determined by direct immunofluorescence. As shown in Fig. 4, both chimeras reached their highest titers at 144 h p.i. (1.0 × 105 to 1.5 × 105 PFU/ml for MGV; 1.6 × 105 to 2.0 × 105 PFU/ml for MG/FV). However, in stock preparations, the titers reached as high as 3.0 × 105 PFU/ml for MGV and 6.0 × 105 PFU/ml for MG/FV. MV and VSV reached their highest titers after 60 and 25 h p.i., respectively. Thus, compared to MV, MGV and MG/FV virion formation was two to three times slower. We did not attempt to determine the factors involved in this delay; however, it is possible that assembly of the internal viral proteins with the viral envelope proteins in the chimeras is not as efficient as in MV or VSV. A comparison of cell-free to cell-associated viruses over the entire growth period showed that an order of magnitude more MV was cell associated than released to medium (Fig. 4D). In contrast, infectious VSV particles in the medium were an order of magnitude more abundant than those bound to cells (Fig. 4C). Interestingly, both MGV and MG/FV were efficiently released to the medium, similar to VSV. The cell-free supernatant of both chimera infections contained titers that were 1 to 2 orders of magnitude higher than those of cell-associated particles (Fig. 4A and B). Compared to MV, the overall titers of chimeric viruses were about 50 times lower and the titers released into the medium were about 10 times lower. These results show that the actual release of these viruses from the cells is dictated mainly by the envelope glycoproteins.
FIG. 4.
Kinetics of infectious-particle formation. Vero cells plated equally in 36-mm well plates were infected with either MGV, MG/FV, VSV, or MV Edmonston B at a MOI of 0.1. At various time points, titers of cell-free and cell-associated virus were determined by immunofluorescence for MGV, MG/FV, and VSV and by plaque assay for MV. The titers of cell-free and cell-associated virus are compared in the same experiment. Data are means of two separate experiments with a deviation of less than 5 to 7%.
Density and composition of MGV and MG/FV infectious particles.
To determine the density of MGV and MG/FV particles in comparison to MV and VSV, supernatants of infected cells were clarified by centrifugation; particles were sedimented first on a cushion of 60% sucrose and then to equilibrium on a 20 to 60% sucrose gradient. Gradient fractions were analyzed by Western blotting and probed with anti-VSV for VSV, MGV, and MG/FV; for MV, a mixture of anti-MV-P, anti-N, and anti-M polyclonal antibodies was used. The anti-VSV antibody revealed the VSV G protein in both MGV and MG/FV particles (Fig. 5A). The MGV particles were detectable in fractions 7 to 10, and the MG/FV particles were detectable in fractions 7 to 11. This is consistent with the infectivity recovered from these fractions. Some G and G/F proteins were detectable in the top fractions of the gradient (fractions 1 to 3 in MGV; fractions 2 and 3 in MG/FV). These could be secreted proteins bound to lipids. When the same blots were probed, in addition to anti-VSV, with the MV anti-M antibody (Fig. 5B), MV M was not incorporated into MGV particles whereas MG/FV contained the M protein. The failure of M protein to be efficiently incorporated into the MGV particle was reproducible in three separate experiments. The MG/FV particles, on the other hand, always contained M protein. These results suggest that infectious particles are formed in the absence of M protein and that exclusion of M from MGV is probably due to the lack of its interaction with the G protein in the envelope. In contrast, MG/FV, which contains a hybrid envelope protein composed of VSV G ecto- and transmembrane domains fused to the cytoplasmic domain of the MV F protein, incorporated M protein, indicating that the F cytoplasmic domain and M interact during assembly.
Determination of the buoyant densities of all the particles showed only small differences between MGV (1.135 to 1.155 g/ml) and MG/FV (1.14 to 1.164 g/ml). The small but reproducibly higher density of MG/FV might be due to the incorporation of M protein into MG/FV but not MGV particles. Compared to MV (1.15 to 1.19 g/ml), both MGV and MG/FV particles are slightly lighter; they show nearly the same density as VSV (1.14 to 1.175 g/ml). Both chimeric viruses are considerably more dense than are infectious particles derived from plasma membrane vesicles containing the VSV-G glycoprotein, which range in density between 1.09 and 1.13 g/ml (11, 31). Note that the densities are averages of three separate experiments; the profile of one experiment is shown in Fig. 5C.
Immunoelectron microscopy of MGV and MG/FV assembly.
To visualize envelope protein localization and distribution as well as potential assembly sites of the chimeric MVs and to corroborate the biochemical data, infected cells were examined by immunoelectron microscopy.
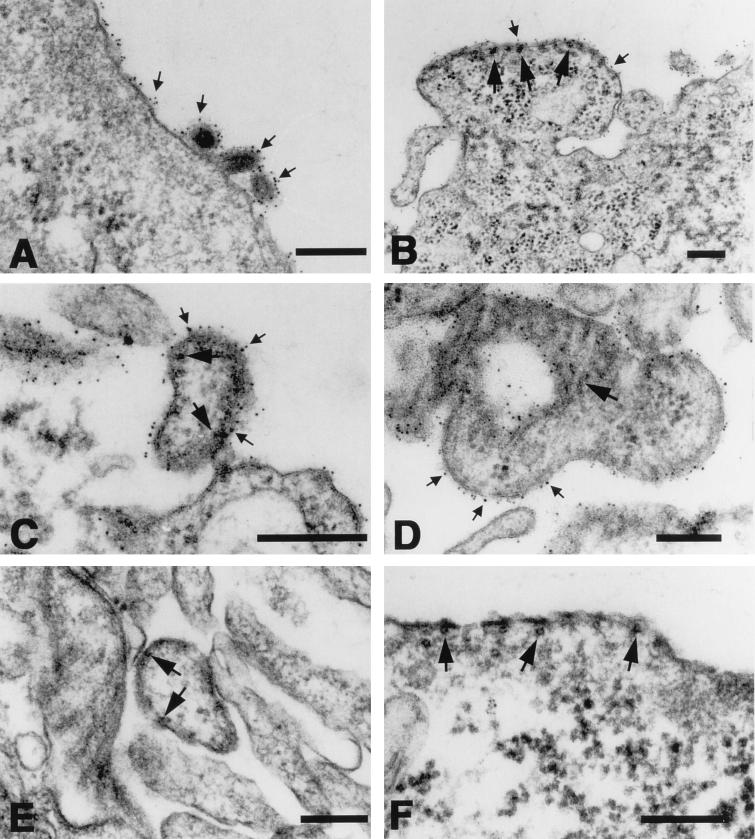
Cells infected with VSV, MGV, or MG/FV were immunolabeled 12 and 96 h p.i., respectively, with an indirect reaction involving anti-VSV antibodies followed by protein A coated with 6-nm colloidal gold. Cell pellets were Epon embedded, thinly sectioned, stained, and visualized by electron microscopy. As shown in Fig. 6A, VSV-infected cells displayed numerous bullet-shaped budding virus particles on their surface, whereas similar particles were not detected in cells infected with the chimeras (Fig. 6B to D). Nevertheless, coating of viral envelopes as well as segments of the plasma membrane of the host cell with colloidal gold particles demonstrated the presence of G or the G/F hybrid proteins (Fig. 6). The antibody was reactive with the envelope of the large, pleomorphic potentially budding chimeric virions, MGV (Fig. 6D) and MG/FV (Fig. 6C), which showed typical electron-dense RNP structures accumulated below the G-containing envelope. In addition, the RNPs detectable in the buds of MG/FV (Fig. 6B) formed similar arrays below the membrane to those detected in MV infections (Fig. 6F). Furthermore, chimeric virions that were released from, or attached to, the cell membrane (Fig. 6C and D) resembled those in MV-infected cells (Fig. 6E). These results confirmed the biochemical analysis, indicating that the rescued particles are viruses or virus-like particles and that the VSV G protein constitutes the envelope protein of these viruses.
FIG. 6.
Immunoelectron microscopy of Vero cells Infected with VSV, MGV, MG/FV, and MV. The infected cells were reacted, prior to their embedding for thin sectioning, with antibodies directed against the G protein of VSV followed by protein A coated with 6-nm colloidal gold. The VSV G protein (small arrows) is detectable on VSV virions (A) as well as on the surfaces of cells infected with MGV (B and C) and MG/FV (D). Dense alignment of gold granules is most prominent in association with segments of the plasma membrane which are associated with dense MV nucleoprotein (large arrows) (B to D), similar to that in MV-infected cells (E and F). Bar, 200 nm.
MGV and MG/FV protect mice against live VSV.
To determine whether the chimeric MV, MGV, and MG/FV could be used as vaccines to protect against lethal infection, we immunized alpha/beta interferon receptor-deficient (A129) mice or C57BL/6 standard mice twice. For safety reasons, UV-inactivated chimeric viruses were used. At 6 days after the booster injection, these mice developed high VSV-neutralizing titers (Table 1). We challenged the A129 or C57BL/6 mice with 103 and 108 PFU, respectively of live VSV. Whereas C57BL/6 mice are quite resistant to VSV infection (50% lethal dose [LD50] > 108 PFU), A129 mice are highly sensitive and die after being given a dose of lower than 20 PFU (20). Therefore, we challenged A129 mice with 103 PFU of live VSV, which corresponds to at least 50× LD50. In this experiment, all the primed mice survived whereas all the unprimed animals died within 6 days. In another experiment, C57BL/6 mice were challenged with 108 PFU of VSV, a dose slightly below the LD50. In this experiment, all the primed mice survived compared to 66% of the unprimed control animals (Table 1).
TABLE 1.
Protection against live VSV after immunization with chimeric MV
| Expt. | Mouse strain | Priminga | Challengeb | Neutralizing titersc | Survivald | Day of death |
|---|---|---|---|---|---|---|
| 1 | A129 | MG/FV | 103 PFU of VSV | 7, 6, 6, 5 | 4/4 | |
| MGV | 103 PFU of VSV | 8, 7, 7, 7 | 4/4 | |||
| none | 103 PFU of VSV | 0, 0, 0, 0 | 0/4 | 5, 5, 6, 6 | ||
| 2 | C57BL/6 | MG/FV | 108 PFU of VSV | 8, 7, 7, 7 | 6/6 | |
| MGV | 108 PFU of VSV | 7, 7, 6, 6 | 6/6 | |||
| none | 108 PFU of VSV | 0, 0, 0, 0 | 4/6 | 12, 14 |
Mice were primed with 5 × 105 PFU of UV-inactivated chimeric viruses and boosted with the same dose 10 days later.
The challenge with the indicated dose of live VSV was done 6 days after the boost.
Neutralizing titers of sera from four mice were measured on the day of challenge. They are indicated as log2 of 40-fold-prediluted sera.
Number of surviving mice/total number of infected mice.
DISCUSSION
Chimeric viruses, combining the replication machinery of one virus with the envelope of another, can be used for a variety of purposes ranging from basic investigations (e.g., studies on virus structure-function relationships and on interactions with the host, in particular with cells of the immune system [35]) to several practical applications including the development of novel live vaccines and of gene delivery systems.
The question is whether the principle of chimera construction established by the present study is widely applicable and could involve a range of different viral envelope proteins. From the results presented here, it is likely that a variety of artificial reading frames for proteins consisting of viral envelope protein ecto- and transmembrane domains fused to an MV-specific cytoplasmic tail(s) could be used to obtain MV-based chimeras. Chimeras containing a single glycoprotein, as used here, or containing two glycoproteins, as in all members of the Paramyxoviridae, might be obtained. VSV or rabies virus G protein is obviously a special case, since it is endowed with the intrinsic function to autonomously form budding vesicles, pinching off from membranes of G-expressing cells (18, 31). Due to this property, the G protein has been used successfully to form different virus pseudotypes, including nonreplicating human immunodeficiency virus type 1 and Moloney murine leukemia virus (23, 25). Therefore, it was not too surprising that Semliki Forest virus replicons expressing VSV G induced the formation of some infectious particles containing replicon RNA (30, 31). However, expression of additional foreign genes was rapidly lost when vector stocks were propagated two or three times (30).
Obviously, for the construction of chimeras, certain constraints on the ability of the envelope of one virus to enclose the nucleocapsid of another virus should be considered. Such constraints are evidenced by the failure to detect pseudotype formation between VSV and Sindbis virus (3), the failure of unaltered human immunodeficiency virus gp120 to pseudotype rabies virus (17), and the exclusion of VSV G from the influenza virus envelope (22, 32, 50). Generally, it can be surmised that the presence of strong and specific interactions between nucleocapsids and the proteins of the envelope minimize the likelihood of pseudotyping. An extreme case involves members of the Togaviridae, in which the symmetry of the icosahedral nucleocapsid is precisely duplicated in the envelope; thus, these viruses can incorporate only very closely related envelope proteins (41). At the other extreme are the pleomorphic or loosely structured viruses, particularly those containing helical RNPs, which seem to more easily tolerate envelope proteins from distantly related viruses.
Our data show that the single VSV envelope glycoprotein was able to replace the two envelope proteins of MV, resulting in the formation of novel viruses with properties distinct from both MV and VSV. Although the formation of chimeric viruses is delayed about twofold, according to the growth curve, and their titer is 10 to 50 times lower than that of the original Edmonston B vaccine strain, these viruses are genetically stable over more than 10 passages, yielding average titers of 1.2 × 105 to 6.0 × 105 PFU/ml. Amplification of these chimeric viruses depends completely on the MV transcription and replication machinery, yet the chimeras are capable of infecting a wider range of cells than is MV. This is in agreement with the idea that the host range of enveloped viruses is determined mainly by their envelope proteins.
Since it is thought that the interaction of the cytoplasmic domains of MV H and F with the internal viral components is necessary to initiate budding, we wondered whether VSV G protein could replace the entire envelope proteins (H and F) of MV to form chimeric particles in high yield. Therefore, we designed two constructs in which one contained the complete VSV G coding sequence and the second contained a hybrid sequence in which the cytoplasmic domain of G was replaced by that of F. Interestingly, viruses were recovered from both constructs, MGV and MG/FV. Both viruses grew in all the cells tested, with MGV growth being slightly slower than that of MG/FV. MGV did not incorporate the MV M protein, although M was always detectable in large quantities in MGV-infected cells. In contrast, the presence of the cytoplasmic domain of F in the G/F hybrid of MG/FV enabled this virus to incorporate M protein. This confirms that the cytoplasmic domain of F protein contains determinants recognized by the M protein sufficient for its incorporation into the virus envelope (5).
The fairly efficient budding and release of the virions lacking M absolutely depends upon the intrinsic “pull-capacity” of the envelope protein. The VSV G protein used in this study is ideal in this sense. In persistent MVs isolated from many patients with SSPE, M protein is defective and virus particles cannot be recovered (1). It is likely that in SSPE the defective MV spreads by local cell-cell fusion. The MV envelope proteins, F and H, are not endowed with a pull-function of similar strength as that of VSV G. Thus, the absence of M essentially aborts the formation of the virus particles, favoring cell-cell fusion. This was directly demonstrated by constructing an MV without M, which spreads mainly by cell-cell fusion and forms only very few virions (5a). In this context, the inefficient release of MV, which might be due to an intrinsically weak pinching-off from the cell surface, should be recalled. It is interesting that the chimeric virions, despite their pleomorphic MV-like appearance, were released efficiently from cells, in this respect mimicking the VSV particles.
It is not yet established whether MV RNPs directly contribute to the budding process of the virus, but this is suggested from microscopic observations. As shown by electron microscopy, RNPs appeared to be concentrated at the internal leaflet of the membranes of budding virus and in the released particles (Fig. 6B). This is confirmed by confocal microscopy, where partial colocalization of N protein with the VSV G in cells infected with MGV and with MG/FV was evident (data not shown). This favors the idea that incorporation of RNPs into virions is driven by structural features of the RNP and is enhanced by the M protein.
The densities of chimeric viruses are slightly lower than that of the parental MV. This could be due to the packaging of fewer RNPs in both MGV and MG/FV than in MV. The fact that MGV exhibits a slightly lower density than MG/FV could be due merely to the exclusion of M from MGV virions.
The new chimeras induced slightly different CPEs in cell culture, which are profoundly distinct from those induced by MV or by VSV. The difference from both VSV and MV could be anticipated on the basis of previous observations; on the one hand, the VSV M causes typical rounding of cells (2), and on the other hand, it is well known that MV F and H envelope glycoproteins mediate cell-cell fusion at neutral pH, forming syncytia, in contrast to VSV-G, which fuses at low pH after endocytic uptake of the virion.
Finally, neither chimeric virus is as virulent as MV or VSV in cell culture, as demonstrated by the delay in appearance of CPE and progeny formation.
Practical applications of propagation-competent MV-based chimeras can be readily envisaged. The protection experiment against VSV challenge, carried out with UV-inactivated MGV and MG/FV, provides the first experimental hint in this direction. For decades, MV Edmonston B has been used as an inexpensive, safe, and efficient live human vaccine, generally inducing lifelong protection against MV. Since MV has the potential of accommodating in its genome at least three additional transcription units, together comprising more than 5,000 bases (11a), it is feasible to add to the viral genome additional open reading frames to express antigens of other pathogens. Given that the expression of such additional open reading frames is stable over many generations, it is reasonable to surmise that long-lasting immunity against the additionally expressed antigens might ensue from such constructed di- or multivalent MV-based vaccines, similar to the lifelong immunity induced against MV.
The ability to construct new chimeric viruses containing a different envelope adds yet another dimension to the development of new vaccines and virus-based vectors. Such chimeras should escape neutralization by preexisting anti-MV antibodies. MV-based chimeric constructs with envelopes of closely related morbilliviruses such as canine distemper virus or more distantly related viruses such as mumps virus, appear both feasible and promising. It can even be envisaged, mainly for the purpose of destructive gene delivery, that our system could be used for the construction of chimeric viruses containing engineered envelope proteins to target only specific cells.
ACKNOWLEDGMENTS
We thank Adrian Ochsenbein for help with animal experiments and Roland Naef for assistance with confocal microscopy. We are grateful to Frank Radecke for helpful discussions, to Hassan Y. Naim and Mike G. Roth for critical reading of the manuscript, and to Fritz Ochsenbein for expert photographic work.
This investigation was supported by grants from the Swiss National Science Foundation and the NIH to M.A.B.
REFERENCES
- 1.Billeter M A, Cattaneo R, Schmid A, Eschle D, Kaelin K, Rebmann G, Udem S A, Sheppard R D, Baczko K, Liebert U G, Schneider-Schaulies S, Brinckmann U, ter Meulen V. Host and viral features in persistent measles virus infections of the brain. In: Kolakofsky D, Mahy B W J, editors. Genetics and pathogenicity of negative strand viruses. Amsterdam, The Netherlands: Elsevier Science Publishers BV; 1989. pp. 356–366. [Google Scholar]
- 2.Blondel D, Harmison G G, Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J Virol. 1990;64:1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge B W, Pfefferkorn E R. Phenotypic mixing between group A arboviruses. Nature. 1966;210:1397–1399. doi: 10.1038/2101397a0. [DOI] [PubMed] [Google Scholar]
- 4.Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cathomen T, Naim H Y, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Cathomen, T., et al. Unpublished data.
- 6.Cattaneo R, Rose J K. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J Virol. 1993;67:1493–1502. doi: 10.1128/jvi.67.3.1493-1502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter M A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J, Roth M G, Hunter E. A chimeric avian retrovirus containing the influenza virus hemagglutinin gene has an expanded host range. J Virol. 1992;66:7374–7382. doi: 10.1128/jvi.66.12.7374-7382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehr T, Bachmann M F, Bluethmann H, Kikutani H, Hengartner H, Zinkernagel R M. T-independent activation of B cells by vesicular stomatitis virus: no evidence for the need of a second signal. Cell Immunol. 1996;168:184–192. doi: 10.1006/cimm.1996.0065. [DOI] [PubMed] [Google Scholar]
- 10.Fredericksen B L, Whitt M A. Mutations at two conserved acidic amino acids in the glycoprotein of vesicular stomatitis virus affect pH-dependent conformational changes and reduce the pH threshold for membrane fusion. Virology. 1996;217:49–57. doi: 10.1006/viro.1996.0092. [DOI] [PubMed] [Google Scholar]
- 11.Graham J M, Winterbourne D J. Subcellular localization of the sulphation reaction of heparan sulphate synthesis and transport of the proteoglycan to the cell surface in rat liver. Biochem J. 1988;252:437–445. doi: 10.1042/bj2520437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Hangartner, L., et al. Unpublished data.
- 12.Hirano A. Subacute sclerosing panencephalitis virus dominantly interferes with replication of wild-type measles virus in a mixed infection: implication for viral persistence. J Virol. 1992;66:1891–1898. doi: 10.1128/jvi.66.4.1891-1898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano A, Ayata M, Wang A H, Wong T C. Functional analysis of matrix proteins expressed from cloned genes of measles virus variants that cause subacute sclerosing panencephalitis reveals a common defect in nucleocapsid binding. J Virol. 1993;67:1848–1853. doi: 10.1128/jvi.67.4.1848-1853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu A, Cathomen T, Cattaneo R, Norrby E. Influence of N-linked oligosaccharide chains on the processing, cell surface expression and function of the measles virus fusion protein. J Gen Virol. 1995;76:705–710. doi: 10.1099/0022-1317-76-3-705. [DOI] [PubMed] [Google Scholar]
- 15.Katayama Y, Hotta H, Nishimura A, Tatsuno Y, Homma M. Detection of measles virus nucleoprotein mRNA in autopsied brain tissues. J Gen Virol. 1995;76:3201–3204. doi: 10.1099/0022-1317-76-12-3201. [DOI] [PubMed] [Google Scholar]
- 16.Lund G A, Tyrrell D L J, Bradley R D, Scraba D G. The molecular length of measles virus RNA and the structural organization of measles nucleocapsids. J Gen Virol. 1984;65:1535–1542. doi: 10.1099/0022-1317-65-9-1535. [DOI] [PubMed] [Google Scholar]
- 17.Mebatsion T, Conzelmann K K. Specific infection of CD4(+) target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc Natl Acad Sci USA. 1996;93:11366–11370. doi: 10.1073/pnas.93.21.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mebatsion T, Konig M, Conzelmann K K. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996;84:941–951. doi: 10.1016/s0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- 19.Mebatsion T, Schnell M J, Cox J H, Finke S, Conzelmann K K. Highly stable expression of a foreign gene from rabies virus vectors. Proc Natl Acad Sci USA. 1996;93:7310–7314. doi: 10.1073/pnas.93.14.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 21.Naim H Y, Amarneh B, Ktistakis N T, Roth M G. Effects of altering palmitylation sites on biosynthesis and function of the influenza virus hemagglutinin. J Virol. 1992;66:7585–7588. doi: 10.1128/jvi.66.12.7585-7588.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naim H Y, Roth M G. Basis for selective incorporation of glycoproteins into the influenza virus envelope. J Virol. 1993;67:4831–4841. doi: 10.1128/jvi.67.8.4831-4841.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 24.Oervell C, Norrby E. Immunological relationships between homologous structural polypeptides of measles and canine distemper virus. J Gen Virol. 1980;50:231–245. doi: 10.1099/0022-1317-50-2-231. [DOI] [PubMed] [Google Scholar]
- 25.Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens R J, Rose J K. Cytoplasmic domain requirement for incorporation of a foreign envelope protein into vesicular stomatitis virus. J Virol. 1993;67:360–365. doi: 10.1128/jvi.67.1.360-365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radecke F, Billeter M A. Appendix. Measles virus antigenome and protein consensus sequences. In: ter Meulen V, Billeter M A, editors. Measles virus, CTMI 191. Berlin, Germany: Springer-Verlag KG; 1995. pp. 181–192. [DOI] [PubMed] [Google Scholar]
- 28.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C, Christiansen G, Billeter M A. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rima B, Alexander D J, Billeter M A, Collins P L, Kingsbury D W, Lipkind M A, Nagai Y, Örvell C, Pringle C R, ter Meulen V. Paramyxoviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy. Classification and nomenclature of viruses. Sixth Report of the International Committee on Taxonomy of Viruses. Vienna, Austria: Springer-Verlag KG; 1995. pp. 268–274. [Google Scholar]
- 30.Rolls M M, Haglund K, Rose J K. Expression of additional genes in a vector derived from a minimal RNA virus. Virology. 1996;218:406–411. doi: 10.1006/viro.1996.0211. [DOI] [PubMed] [Google Scholar]
- 31.Rolls M M, Webster P, Balba N H, Rose J K. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 32.Roth M G, Compans R W. Delayed appearance of pseudotypes between vesicular stomatitis virus influenza virus during mixed infection of MDCK cells. J Virol. 1981;40:848–860. doi: 10.1128/jvi.40.3.848-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi T, Leser G P, Lamb R A. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J Cell Biol. 1996;133:733–747. doi: 10.1083/jcb.133.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Schlender J, Schnorr J J, Spielhoffer P, Cathomen T, Cattaneo R, Billeter M A, ter Meulen V, Schneider-Schaulies S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid A, Spielhofer P, Cattaneo R, Baczko K, ter Meulen V, Billeter M A. Subacute sclerosing panencephalitis is typically characterized by alterations in the fusion protein cytoplasmic domain of the persisting measles virus. Virology. 1992;189:910–915. doi: 10.1016/0042-6822(92)90552-z. [DOI] [PubMed] [Google Scholar]
- 37.Schnell M J, Buonocore L, Kretzschmar E, Johnson E, Rose J K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnitzer T J, Weiss R A, Zavada J. Pseudotypes of vesicular stomatitis virus with the envelope properties of mammalian and primate retroviruses. J Virol. 1977;23:449–454. doi: 10.1128/jvi.23.3.449-454.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simons K, Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980;50:1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- 40.Skerra A. Phosphorothioate primers improve the amplification of DNA sequences by DNA polymerases with proofreading activity. Nucleic Acids Res. 1992;20:3551–3554. doi: 10.1093/nar/20.14.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smyth J, Suomalainen M, Garoff H. Efficient multiplication of a Semliki Forest virus chimera containing Sindbis virus spikes. J Virol. 1997;71:818–823. doi: 10.1128/jvi.71.1.818-823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spielhofer P. Ph.D. thesis. Zurich, Switzerland: University of Zurich; 1995. [Google Scholar]
- 43.Spielhofer P. Diploma thesis. Zurich, Switzerland: University of Zurich; 1990. [Google Scholar]
- 44.Tashiro M, McQueen N L, Seto J T, Klenk H D, Rott R. Involvement of the mutated M protein in altered budding polarity of a pantropic mutant, F1-R, of Sendai virus. J Virol. 1996;70:5990–5997. doi: 10.1128/jvi.70.9.5990-5997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Udem S A. Measles virus: conditions for the propagation and purification of infectious virus in high yield. J Virol Methods. 1984;8:123–136. doi: 10.1016/0166-0934(84)90046-6. [DOI] [PubMed] [Google Scholar]
- 46.Whitt M A, Chong L, Rose J K. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J Virol. 1989;63:3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong T C, Ayata M, Ueda S, Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991;65:2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zavada J. Viral pseudotypes and phenotypic mixing. Arch Virol. 1976;50:1–15. doi: 10.1007/BF01317996. [DOI] [PubMed] [Google Scholar]
- 50.Zavada J, Rosenbergova M. Phenotypic mixing of vesicular stomatitis virus with fowl plague virus. Acta Virol. 1972;16:103–114. [PubMed] [Google Scholar]