Abstract
Purpose
Patients with spinal metastases (SM) from solid neoplasms typically exhibit progression to an advanced cancer stage. Such metastases can either develop concurrently with an existing cancer diagnosis (termed metachronous SM) or emerge as the initial indication of an undiagnosed malignancy (referred to as synchronous SM). The present study investigates the prognostic implications of synchronous compared to metachronous SM following surgical resection.
Methods
From 2015 to 2020, a total of 211 individuals underwent surgical intervention for SM at our neuro-oncology facility. We conducted a survival analysis starting from the date of the neurosurgical procedure, comparing those diagnosed with synchronous SM against those with metachronous SM.
Results
The predominant primary tumor types included lung cancer (23%), prostate cancer (21%), and breast cancer (11.3%). Of the participants, 97 (46%) had synchronous SM, while 114 (54%) had metachronous SM. The median overall survival post-surgery for those with synchronous SM was 13.5 months (95% confidence interval (CI) 6.1–15.8) compared to 13 months (95% CI 7.7–14.2) for those with metachronous SM (p = 0.74).
Conclusions
Our findings suggest that the timing of SM diagnosis (synchronous versus metachronous) does not significantly affect survival outcomes following neurosurgical treatment for SM. These results support the consideration of neurosurgical procedures regardless of the temporal pattern of SM manifestation.
Keywords: Surgery for spinal metastasis, Synchronous versus metachronous tumor occurrence, Survival, Neuro-oncology
Introduction
Systemic tumor disease with singular or multiple spinal metastases (SM) has assumed an increasingly prominent role in the daily clinical practice of spine surgeons and the lives of affected patients (Coleman 2006; Brande et al. 2022). It is estimated that approximately 5–15% of all cancer patients will ultimately develop spinal metastases (Brande et al. 2022; Jenis et al. 1999; Jacobs and Perrin 2001). Among the primary culprits are breast cancer, prostate cancer, and lung cancer, with the primary tumor remaining elusive in 3–10% of cases (Greenlee et al. 2000; Ulmar et al. 2007).
In the therapeutic arsenal for this profoundly affected patient population, surgery stands as a common treatment modality (Furlan et al. 2022). After lungs and liver, skeletal system and bones bear the brunt of systemic metastases (Macedo et al. 2017; Maccauro et al. 2011). Surgical options for managing spinal metastases encompass a spectrum, from biopsy coupled with vertebroplasty or kyphoplasty (Stangenberg et al. 2017; Georgy 2010), to spinal canal decompression in isolation (Patchell et al. 2005), or in conjunction with minimally invasive percutaneous procedures (Miscusi et al. 2015) and open instrumentation with augmented screws (Ringel et al. 2017; Park et al. 2019a), at times necessitating anterior–posterior stabilization (Ulmar et al. 2006; Gezercan et al. 2016). The overarching objective of surgical intervention is the mitigation or prevention of neurological deficits, coupled with a focus on enhancing the patient’s quality of life (Fehlings et al. 2016; Depreitere et al. 2020). Additionally, surgery provides a means to attain a definitive histological diagnosis of the spinal tumor lesion and potentially improves overall survival (OS) (Patchell et al. 2005; Krober et al. 2004).
SM may arise within the context of a previously known and managed systemic cancer disease (metachronous presentation), often preceded by multimodal therapies, such as radiation, systemic chemotherapy, immunotherapy, or specifically targeted therapies (Gerszten et al. 2009; Berger 2008; Choi et al. 2015, 2019). Alternatively, newly diagnosed SM may serve as the inaugural presentation of a previously undiscovered systemically disseminated cancer (synchronous presentation) (Jacobs and Perrin 2001; Bollen et al. 2018; Patnaik et al. 2020).
Despite existing literature, it remains uncertain whether the choice to surgically resect SM in cases of synchronous versus metachronous presentation significantly influences surgical decisions and patient survival. This study seeks to clarify this issue by examining the prognostic implications of synchronous versus metachronous SM diagnoses, measured from the day of neurosurgical SM resection, in patients who underwent surgical intervention for SM.
Methods
Patients and inclusion criteria
This study is based on consecutive patients aged >18 years who had undergone primary spinal canal decompression, with or without instrumentation, for SM between 2015 and 2020 at the neurosurgical department of the University Hospital Bonn. Comprehensive clinical data, including age, gender, primary tumor type, SM location, details of the neurosurgical procedure, the extent of spinal vertebrae involvement, American Society of Anesthesiologists (ASA) score, clinical-neurological assessment, and functional status measured by the American Spinal Injury Association (ASIA) Score (2019), were recorded.
Functional status was further evaluated using the Karnofsky Performance Scale (KPS) upon admission, categorizing patients into KPS ≥ 70% or KPS < 70%, as previously described (Schuss et al. 2021; Hamed et al. 2023; Schweppe et al. 2023; Ilic et al. 2021). The Charlson Comorbidity Index (CCI) was employed to quantify the comorbidity burden of patients before undergoing surgery (Hamed et al. 2022a; Schneider et al. 2020; Lehmann et al. 2023).
Overall survival (OS) was calculated from the date of surgical SM resection until death as previously described (Hamed et al. 2022b). Patients for whom no further follow-up information regarding survival was obtainable, typically due to ongoing treatment at external healthcare institutions, were excluded from subsequent statistical survival analysis.
Following histopathological analysis, all patients underwent thorough assessment by our internal Neurooncological Tumor Board, comprised of neurosurgeons, radiation therapists, neurooncologists, and neuroradiologist. Recommendations for post-surgery management were established through interdisciplinary consensus, occasionally coordinated with the treatment plans of referring physicians (Schafer et al. 2021).
Patients were categorized into two distinct cohorts for further analysis: those with SM diagnosed as a manifestation of a previously known cancer (metachronous presentation) and those with a new diagnosis of SM as the initial indication of an undiscovered cancer (synchronous presentation) (Potthoff et al. 2023).
Exclusion criteria encompassed patients classified as non-operable and those lacking complete data or follow-up information. Pertinent clinical parameters, including preoperative functional neurological status, comorbidities, radiological characteristics, primary cancer site, and the timing of diagnosis, were assessed for analysis.
The study adhered to the ethical principles outlined in the 1964 Helsinki Declaration and received approval from the Ethics Committee of the University Hospital Bonn (protocol no. 067/21). Given the retrospective nature of the study, the acquisition of informed consent from participants was not pursued.
Statistical analysis and graphical illustration
Data collection and analysis were conducted utilizing the SPSS computer software package for Windows (Version 27, IBM Corp., Armonk, NY). Categorical variables underwent analysis through contingency tables, employing the Fisher’s exact test when assessing two variables and the chi-square test when evaluating more than two variables. Non-normally distributed data were subjected to the Mann–Whitney U test. Overall survival (OS) rates were assessed using the Kaplan–Meier method, with Graph Pad Prism software for MacOS (Version 9.4.1, Graph pad Software, Inc., San Diego, California, USA) employed for this purpose. Survival rate comparisons were performed utilizing the Gehan–Breslow–Wilcoxon test. To identify predictors of elevated 1-year mortality, a multivariate logistic regression model was constructed using a backward stepwise approach. Statistical significance was determined at p < 0.05. Furthermore, the radar plot was generated using R (Version 3.6.2, Vienna, Austria), as previously outlined in reference (Lehmann et al. 2021).
Results
Patient and tumor characteristics
Between 2015 and 2020, 211 patients had undergone resection of SM at the Neurosurgical Department of the University Hospital Bonn. The median patient age at the day of surgery was 66 years (interquartile range (IQR) 57–74 years) (Table 1). The most common primary tumor site was the lung (23%), followed by the prostate (22%) and the breast (11%). The thoracic spine was the most commonly affected segment of the spine with 56%. Single or dual-level disease was present in 126 patients (60%), whereas multilevel infiltration was present in 85 patients (40%). The majority of patients (62%) underwent decompression and dorsal stabilization, while spinal canal decompression alone was performed in 38% of the patients. Median CCI of the entire patient cohort was 8 (IQR 6–10). 67% of our cohort presented with a preoperative KPS score of ≥ 70. Median OS for the entire study cohort with surgically treated SM was 13 months (IQR 3–23).
Table 1.
Patient characteristics
| n = 211 | |
|---|---|
| Median age (IQR) (in yrs) | 66 (57–74) |
| Female sex | 81 (38.5) |
| Primary tumor site | |
| Lung | 49 (23.0) |
| Breast | 24 (11.4) |
| Prostate | 46 (22.0) |
| Others | 92 (43.6) |
| Location of disease | |
| Cervical | 21 (10.0) |
| Thoracic | 118 (56.0) |
| Lumbar | 36 (17.0) |
| Combined | 36 (17.0) |
| Surgery | |
| Decompression | 81 (38) |
| Stabilization | 130 (62) |
| Levels of disease | |
| 1–2 | 126 (60) |
| ≥3 | 85 (40) |
| Median CCI (IQR) | 8 (6–10) |
| KPS ≥ 70 | 141 (54) |
| Pre-operative neurological deficit (ASIA A-C) | 66 (31) |
| Median OS (IQR) (in months) | 13 (3–23) |
| Time of SM diagnosis | |
| Synchronous | 97 (46) |
| Metachronous | 114 (54) |
Values represent the number of patients unless indicated otherwise (%)
ASA American Society of Anesthesiology physical status classification system, ASIA American Spinal Injury Association, CCI Charlson Comorbidity Index, KPS Karnofsky Performance Scale, IQR interquartile range, n number of patients, OS overall survival, SM spinal metastasis, yrs years
79 of 211 of patients (46%) suffered from synchronous SM, 114 of 211 patients (54%) exhibited metachronous SM. For further more details of patient- and tumor-related characteristics, see Table 1.
Survival rates do not significantly differ between synchronous and metachronous spinal metastases
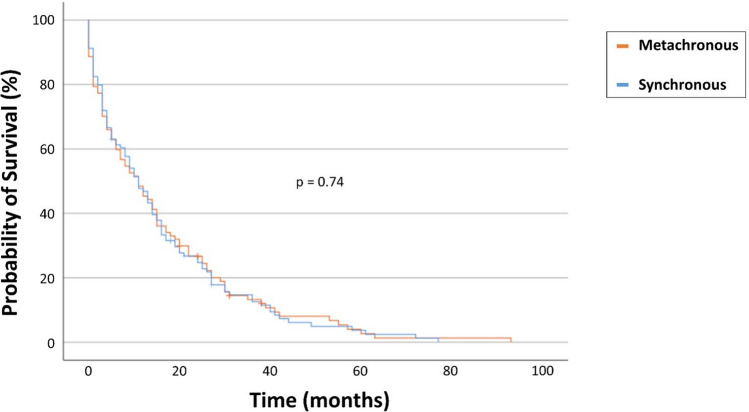
In the synchronous SM group, 50 out of 93 patients (54%) succumbed within 1 year following surgical resection, compared to 60 out of 107 patients (56%) in the metachronous SM group (p = 0.78) (Table 2). The mOS for patients with synchronous SM diagnosis was 13.5 months (95% CI 6.1–15.8), while patients with metachronous SM diagnosis exhibited a mOS of 13.0 months (95% CI 7.7–14.2) when calculated from the day of SM surgical treatment (p = 0.74) (Fig. 1).
Table 2.
Patients with surgically treated SM stratified for synchronous vs. metachronous SM occurrence
| Synchronous diagnosis of SM n = 97 |
Metachronous diagnosis of SM n = 114 |
p value | |
|---|---|---|---|
| Median age (yrs) | 67 (58–73) | 65 (57–75) | 0.8 |
| Female sex | 46 (47) | 35 (31) | 0.02 |
| Pre-operative KPS < 70 | 27 (28) | 43 (38) | 0.1 |
| Primary tumor site | |||
| Lung | 34 (35) | 15 (4) | <0.001 |
| Breast | 20 (21) | 4 (4) | <0.001 |
| Prostate | 14 (14) | 32 (28) | 0.019 |
| Other | 29 (30) | 63 (55) | <0.001 |
| Location of disease | |||
| Cervical | 12 (12) | 9 (8) | 0.4 |
| Thoracic | 50 (52) | 68 (60) | 0.28 |
| Lumbar | 15 (15) | 21 (18) | 0.35 |
| Combined | 20 (21) | 16 (14) | 0.27 |
| Levels of disease | 0.2 | ||
| 1–2 | 53 (55) | 73 (64) | |
| ≥3 | 44 (45) | 41 (36) | |
| Median CCI (IQR) | 8 (7–10) | 8 (6–10) | 0.7 |
| Preoperative neurological deficit (ASIA A-C) | 33 (34) | 33 (29) | 0.5 |
| Surgery | 0.05 | ||
| Decompression | 31 (32) | 50 (44) | |
| Stabilization | 66 (68) | 64 (56) | |
| 1-year mortality | 50/93* (54) | 60/107** (56) | 0.78 |
| Median OS (95% CI) | 13.5 (6.1–15.8) | 13.0 (7.7–14.2) | 0.74 |
Values represent the number of patients unless indicated otherwise (%)
The values in bold are statistically significant
CCI Charlson Comorbidity Index, IQR interquartile range, OS overall survival, SM spinal metastasis, yrs years
* 4 of 97 patients censored with lost to follow-up <12 months
** Median (IQR). 7 of 114 patients censored with lost to follow-up <12 months
Fig. 1.
Kaplan–Meier survival analysis dependent on synchronous vs. metachronous SM occurrence. SM, spinal metastasis; vs., versus
Lung and breast carcinomas were significantly more common in the synchronous group, whereas prostate carcinoma was the most common tumor entity in the metachronous group (Table 2). The female gender was also significantly more frequently affected in the synchronous situation, with breast carcinoma being included. All the other parameters included in Table 2 did not significantly differ between the groups of synchronous and metachronous SM (Fig. 2; Table 2).
Fig. 2.
Radar plot depicting patient- and disease-related characteristics dependent on synchronous vs. metachronous SM occurrence in patients with surgically treated SM. CCI, Charlson comorbidity index; KPS, Karnofsky performance score; mOS, median overall survival; SM, spinal metastasis; vs., versus
Multivariable analysis for predictors of 1-year mortality
We performed a multivariable regression analysis including the variables sex, preoperative KPS, preoperative CCI, tumor entity, and time of diagnosis (synchronous versus (vs.) metachronous) in order to identify independent predictors of 1-year mortality following surgery for SM.
The multivariable analysis revealed preoperative KPS < 70 (OR 0.1, 95% CI 0.06–0.2, p < 0.001), preoperative CCI > 10 (OR 0.5, 95% CI 0.2–0.9, p < 0.001), and tumor entity breast (OR 0.2, 95% CI 0.07–0.7, p = 0.01) as significant and independent predictors of 1-year mortality (Table 3). Time of SM diagnosis (synchronous vs. metachronous SM presentation) did not meet statistical significance (OR 0.7, 95% CI 0.4–1.4, p = 0.3).
Table 3.
Multivariable regression analysis for predictors of 1-year mortality
| Factors | Adjusted OR | 95% CI | p value |
|---|---|---|---|
| Female sex | 0.6 | 0.3–1.2 | 0.2 |
| Pre-operative KPS < 70 | 9.8 | 4.6–20.7 | <0.001 |
| CCI > 10 | 0.5 | 0.2–0.9 | 0.03 |
| Tumor entity | |||
| Lung | 1.7 | 0.8–3.6 | 0.2 |
| Breast | 0.2 | 0.07–0.7 | 0.01 |
| Synchronous diagnosis of SM | 0.7 | 0.4–1.4 | 0.3 |
The values in bold are statistically significant
CCI Charlson Comorbidity Index, CI confidence interval, KPS Karnofsky Performance Scale, OR odds ratio, SM spinal metastasis
Discussion
This study analyzes the prognostic impact of metachronous vs. synchronous SM diagnosis in patients who had undergone surgical therapy for SM. We found that the time of SM diagnosis does not impact 1-year mortality and patient survival when measured from the day of SM resection.
In the group of patients with SM from lung and breast cancer, SM significantly more often occurred in the synchronous than in the metachronous situation. Compared with this, SM from prostate and other carcinoma significantly more often occurred in the course of the known underlying cancer disease (metachronous situation). Lung cancer is notably associated with the highest incidence of spinal metastases (SM) and brain metastases (BM). The occurrence of SM in lung cancer patients, as reported in the literature, ranges from 5% to a significant 56%. This variation is influenced by factors, such as the histological type of the cancer, the status of the epidermal growth factor receptor (EGFR) mutation, and the stage of the disease (Berghoff et al. 2016; Nayak et al. 2012; Goncalves et al. 2016; Wang et al. 2017; Zhang et al. 2020; Rizzoli et al. 2013). Similarly, SM is observed in 5–15% of breast and prostate cancer cases, making these two types of cancer among the most common to develop SM (Rizzoli et al. 2013; Hong et al. 2020; Kumar et al. 2020; Park et al. 2019b). The observed difference in the frequency of synchronous versus metachronous spinal metastasis (SM) diagnosis between lung and prostate cancer may be partially attributed to the diagnostic practices for these cancers. Prostate cancer may often be detected during routine medical check-ups for men, leading to earlier diagnosis. In contrast, lung cancer typically remains undetected until it reaches more advanced stages of the disease (Goldsmith 2014; Lux et al. 2019; Vinas et al. 2016).
Our findings regarding the distribution of cancer entities align with those reported in well-established studies (Krober et al. 2004; Hosono et al. 2005; Sciubba and Gokaslan 2006). Consistent with numerous publications, we observed that the thoracic spine was the most frequently affected spinal segment in both synchronous and metachronous SM groups (Bach et al. 1990; Comey et al. 1997). However, our study did not identify a specific dissemination pattern linked to the primary tumor, such as a preference for lung cancer metastases to manifest singularly or multiply in the thoracic spine, as noted in some reports (Schiff et al. 1998; Gilbert et al. 1978). Conversely, other researchers have observed a concentration of bronchial carcinoma in the thoracic spine and a predominance of prostate carcinoma in the lumbar spine (Krober et al. 2004).
In contemporary literature, the incidence of multiple spinal canal metastases in cases of spinal infiltration with SM is reported to be up to 30% (Sande et al. 1990). Our cohort demonstrates a prevalence with 45% in synchronous SM and 36% in metachronous SM involving more than three segments.
To the best of our knowledge, this study is the first to investigate the prognostic impact of synchronous versus metachronous SM. A notable aspect of our approach is the emphasis on postoperative survival in the survival analysis. This focus is crucial as it aligns with the typical juncture at which neurosurgeons encounter patients with spinal metastasis. These findings suggest that the indication for surgery should be considered regardless of whether the SM is synchronous or metachronous. This conclusion is significant for clinical decision-making in neurosurgery, suggesting that the timing of metastasis, in relation to the primary tumor, should not be a deterrent to surgical intervention.
In essence, our findings advocate for a surgical approach in managing spinal metastasis without bias toward the metastasis’ temporal classification. This has direct implications for neurosurgical management, underscoring the importance of considering surgery as a viable treatment option in both synchronous and metachronous scenarios and providing a clear directive for surgical intervention.
Limitations
This study is subject to a number of limitations. First, the data collection was retrospective in nature, and there was no randomization of patients; instead, treatment decisions were made based on the individual preferences of physicians at our institution. Additionally, the study population of patients with SM is notably diverse, encompassing a range of underlying cancer types and varying pre-treatment histories. Despite these limitations, our findings might provide a basis for the establishment of multicenter registries and the development of further prospective studies.
Conclusions
The present study indicates that the timing of SM diagnosis, whether synchronous or metachronous, does not substantially influence patient survival following surgical treatment. These findings imply that decisions regarding neurosurgical intervention should be considered independently of the temporal classification of SM.
Abbreviations
- ASA
American Society of Anesthesiologists
- ASIA
American Spinal Injury Association
- CCI
Charlson Comorbidity Index
- CI
Confidence interval
- KPS
Karnofsky Performance Scale
- SM
Spinal metastases
- OS
Overall survival
Author contributions
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. The study design was approved by the appropriate ethics review board. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MB, and MS. The first draft of the manuscript was written by MB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Informed consent was not sought as a retrospective study design was used.
Consent to publish
All authors agreed to the publication of the manuscript.
Ethics approval
The local ethics committee at the University of Bonn (protocol no. 067/21) approved the present study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- ASIA and ISCoS International Standards Committee (2019) The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)—What’s new? Spinal Cord 57(10):815–817. 10.1038/s41393-019-0350-9 [DOI] [PubMed] [Google Scholar]
- Bach F, Larsen BH, Rohde K, Borgesen SE, Gjerris F, Boge-Rasmussen T, Agerlin N, Rasmusson B, Stjernholm P, Sorensen PS (1990) Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir 107(1–2):37–43. 10.1007/BF01402610 [DOI] [PubMed] [Google Scholar]
- Berger AC (2008) Introduction: role of surgery in the diagnosis and management of metastatic cancer. Semin Oncol 35(2):98–99. 10.1053/j.seminoncol.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Berghoff AS, Schur S, Fureder LM, Gatterbauer B, Dieckmann K, Widhalm G, Hainfellner J, Zielinski CC, Birner P, Bartsch R, Preusser M (2016) Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open 1(2):e000024. 10.1136/esmoopen-2015-000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen L, Dijkstra SPD, Bartels R, de Graeff A, Poelma DLH, Brouwer T, Algra PR, Kuijlen JMA, Minnema MC, Nijboer C, Rolf C, Sluis T, Terheggen M, van der Togt-van Leeuwen ACM, van der Linden YM, Taal W (2018) Clinical management of spinal metastases-The Dutch national guideline. Eur J Cancer 104:81–90. 10.1016/j.ejca.2018.08.028 [DOI] [PubMed] [Google Scholar]
- Choi D, Pavlou M, Omar R, Arts M, Balabaud L, Buchowski JM, Bunger C, Chung CK, Coppes MH, Depreitere B, Fehlings MG, Kawahara N, Lee CS, Leung Y, Martin-Benlloch JA, Massicotte EM, Mazel C, Meyer B, Oner FC, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Ulbricht C, Verlaan JJ, Wang M, Crockard HA (2019) A novel risk calculator to predict outcome after surgery for symptomatic spinal metastases; use of a large prospective patient database to personalise surgical management. Eur J Cancer 107:28–36. 10.1016/j.ejca.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Choi D, Fox Z, Albert T, Arts M, Balabaud L, Bunger C, Buchowski JM, Coppes MH, Depreitere B, Fehlings MG, Harrop J, Kawahara N, Martin-Benlloch JA, Massicotte EM, Mazel C, Oner FC, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Verlaan JJ, Wang M, Crockard HA (2015) Prediction of quality of life and survival after surgery for symptomatic spinal metastases: a multicenter cohort study to determine suitability for surgical treatment. Neurosurgery 77(5):698–708; discussion 708. doi:10.1227/NEU.0000000000000907 [DOI] [PubMed]
- Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12(20 Pt 2):6243s–6249s. 10.1158/1078-0432.CCR-06-0931 [DOI] [PubMed] [Google Scholar]
- Comey CH, McLaughlin MR, Moossy J (1997) Anterior thoracic corpectomy without sternotomy: a strategy for malignant disease of the upper thoracic spine. Acta Neurochir 139(8):712–718. 10.1007/BF01420043 [DOI] [PubMed] [Google Scholar]
- Depreitere B, Ricciardi F, Arts M, Balabaud L, Bunger C, Buchowski JM, Chung CK, Coppes MH, Fehlings MG, Kawahara N, Martin-Benlloch JA, Massicotte EM, Mazel C, Meyer B, Oner FC, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Verlaan JJ, Wang M, Crockard HA, Choi D (2020) How good are the outcomes of instrumented debulking operations for symptomatic spinal metastases and how long do they stand? A subgroup analysis in the global spine tumor study group database. Acta Neurochir 162(4):943–950. 10.1007/s00701-019-04197-5 [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Nater A, Tetreault L, Kopjar B, Arnold P, Dekutoski M, Finkelstein J, Fisher C, France J, Gokaslan Z, Massicotte E, Rhines L, Rose P, Sahgal A, Schuster J, Vaccaro A (2016) Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol 34(3):268–276. 10.1200/JCO.2015.61.9338 [DOI] [PubMed] [Google Scholar]
- Furlan JC, Wilson JR, Massicotte EM, Sahgal A, Fehlings MG (2022) Recent advances and new discoveries in the pipeline of the treatment of primary spinal tumors and spinal metastases: a scoping review of registered clinical studies from 2000 to 2020. Neuro Oncol 24(1):1–13. 10.1093/neuonc/noab214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgy BA (2010) Vertebroplasty technique in metastatic disease. Neuroimaging Clin N Am 20(2):169–177. 10.1016/j.nic.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Gerszten PC, Mendel E, Yamada Y (2009) Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine 34(22 Suppl):S78–S92. 10.1097/BRS.0b013e3181b8b6f5 [DOI] [PubMed] [Google Scholar]
- Gezercan Y, Cavus G, Okten AI, Menekse G, Cikili M, Adamhasan F, Arslan A, Acik V (2016) Single-stage posterolateral transpedicular approach with 360-degree stabilization and vertebrectomy in primary and metastatic tumors of the spine. World Neurosurg 95:214–221. 10.1016/j.wneu.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Gilbert RW, Kim JH, Posner JB (1978) Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol 3(1):40–51. 10.1002/ana.410030107 [DOI] [PubMed] [Google Scholar]
- Goldsmith SM (2014) A unifying approach to the clinical diagnosis of melanoma including “D” for “Dark” in the ABCDE criteria. Dermatol Pract Concept 4(4):75–78. 10.5826/dpc.0404a16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves PH, Peterson SL, Vigneau FD, Shore RD, Quarshie WO, Islam K, Schwartz AG, Wozniak AJ, Gadgeel SM (2016) Risk of brain metastases in patients with nonmetastatic lung cancer: analysis of the Metropolitan Detroit Surveillance, Epidemiology, and End Results (SEER) data. Cancer 122(12):1921–1927. 10.1002/cncr.30000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S (2000) Wingo PA (2000) Cancer statistics. CA Cancer J Clin 50(1):7–33. 10.3322/canjclin.50.1.7 [DOI] [PubMed] [Google Scholar]
- Hamed M, Potthoff AL, Layer JP, Koch D, Borger V, Heimann M, Scafa D, Sarria GR, Holz JA, Schmeel FC, Radbruch A, Guresir E, Schafer N, Schuss P, Garbe S, Giordano FA, Herrlinger U, Vatter H, Schmeel LC, Schneider M (2022a) Benchmarking safety indicators of surgical treatment of brain metastases combined with intraoperative radiotherapy: results of prospective observational study with comparative matched-pair analysis. Cancers 14(6):1515. 10.3390/cancers14061515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed M, Brandecker S, Rana S, Potthoff AL, Eichhorn L, Bode C, Schmeel FC, Radbruch A, Schafer N, Herrlinger U, Koksal M, Giordano FA, Vatter H, Schneider M, Banat M (2022b) Postoperative prolonged mechanical ventilation correlates to poor survival in patients with surgically treated spinal metastasis. Front Oncol 12:940790. 10.3389/fonc.2022.940790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed M, Potthoff AL, Heimann M, Schafer N, Borger V, Radbruch A, Herrlinger U, Vatter H, Schneider M (2023) Survival in patients with surgically treated brain metastases: does infratentorial location matter? Neurosurg Rev 46(1):80. 10.1007/s10143-023-01986-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Youk T, Lee SJ, Kim KM, Vajdic CM (2020) Bone metastasis and skeletal-related events in patients with solid cancer: a Korean nationwide health insurance database study. PLoS ONE 15(7):e0234927. 10.1371/journal.pone.0234927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono N, Ueda T, Tamura D, Aoki Y, Yoshikawa H (2005) Prognostic relevance of clinical symptoms in patients with spinal metastases. Clin Orthop Relat Res 436:196–201. 10.1097/01.blo.0000160003.70673.2a [DOI] [PubMed] [Google Scholar]
- Ilic I, Faron A, Heimann M, Potthoff AL, Schafer N, Bode C, Borger V, Eichhorn L, Giordano FA, Guresir E, Jacobs AH, Ko YD, Landsberg J, Lehmann F, Radbruch A, Herrlinger U, Vatter H, Schuss P, Schneider M (2021) Combined assessment of preoperative frailty and sarcopenia allows the prediction of overall survival in patients with lung cancer (NSCLC) and surgically treated brain metastasis. Cancers 13(13):3353. 10.3390/cancers13133353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WB, Perrin RG (2001) Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus 11(6):e10. 10.3171/foc.2001.11.6.11 [DOI] [PubMed] [Google Scholar]
- Jenis LG, Dunn EJ, An HS (1999) Metastatic disease of the cervical spine. A review. Clin Orthop Relat Res 359:89–103. 10.1097/00003086-199902000-00010 [DOI] [PubMed] [Google Scholar]
- Krober MW, Guhring T, Unglaub F, Bernd L, Sabo D (2004) Outcome between surgical and non-surgical treatment of metastatic tumors of the spine: a retrospective study of 259 patients. Z Orthop Ihre Grenzgeb 142(4):442–448. 10.1055/s-2004-822796 [DOI] [PubMed] [Google Scholar]
- Kumar N, Tan WLB, Wei W, Vellayappan BA (2020) An overview of the tumors affecting the spine-inside to out. Neurooncol Pract 7(Suppl 1):i10–i17. 10.1093/nop/npaa049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann F, Schenk LM, Ilic I, Putensen C, Hadjiathanasiou A, Borger V, Zimmermann J, Guresir E, Vatter H, Bode C, Schneider M, Schuss P (2021) Prolonged mechanical ventilation in patients with deep-seated intracerebral hemorrhage: risk factors and clinical implications. J Clin Med 10(5):1015. 10.3390/jcm10051015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann F, Potthoff AL, Borger V, Heimann M, Ehrentraut SF, Schaub C, Putensen C, Weller J, Bode C, Vatter H, Herrlinger U, Schuss P, Schafer N, Schneider M (2023) Unplanned intensive care unit readmission after surgical treatment in patients with newly diagnosed glioblastoma—forfeiture of surgically achieved advantages? Neurosurg Rev 46(1):30. 10.1007/s10143-022-01938-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux MP, Emons J, Bani MR, Wunderle M, Sell C, Preuss C, Rauh C, Jud SM, Heindl F, Langemann H, Geyer T, Brandl AL, Hack CC, Adler W, Schulz-Wendtland R, Beckmann MW, Fasching PA, Gass P (2019) Diagnostic accuracy of breast medical tactile examiners (MTEs): a prospective pilot study. Breast Care 14(1):41–47. 10.1159/000495883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA (2011) Physiopathology of spine metastasis. Int J Surg Oncol 2011:107969. 10.1155/2011/107969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, Goncalves F (2017) Bone metastases: an overview. Oncol Rev 11(1):321. 10.4081/oncol.2017.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miscusi M, Polli FM, Forcato S, Ricciardi L, Frati A, Cimatti M, De Martino L, Ramieri A, Raco A (2015) Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine 22(5):518–525. 10.3171/2014.10.SPINE131201 [DOI] [PubMed] [Google Scholar]
- Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1):48–54. 10.1007/s11912-011-0203-y [DOI] [PubMed] [Google Scholar]
- Park SJ, Lee KH, Lee CS, Jung JY, Park JH, Kim GL, Kim KT (2019a) Instrumented surgical treatment for metastatic spinal tumors: is fusion necessary? J Neurosurg Spine 32(3):456–464. 10.3171/2019.8.SPINE19583 [DOI] [PubMed] [Google Scholar]
- Park JS, Park SJ, Lee CS (2019b) Incidence and prognosis of patients with spinal metastasis as the initial manifestation of malignancy: analysis of 338 patients undergoing surgical treatment. The Bone & Joint Journal 101-B(11):1379–1384. 10.1302/0301-620X.101B11.BJJ-2018-1600.R2 [DOI] [PubMed] [Google Scholar]
- Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Mohiuddin M, Young B (2005) Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 366(9486):643–648. 10.1016/S0140-6736(05)66954-1 [DOI] [PubMed] [Google Scholar]
- Patnaik S, Turner J, Inaparthy P, Kieffer WK (2020) Metastatic spinal cord compression. Br J Hosp Med 81(4):1–10. 10.12968/hmed.2019.0399 [DOI] [PubMed] [Google Scholar]
- Potthoff AL, Heimann M, Lehmann F, Ilic I, Paech D, Borger V, Radbruch A, Schafer N, Schuss P, Vatter H, Herrlinger U, Schneider M (2023) Survival after resection of brain metastasis: impact of synchronous versus metachronous metastatic disease. J Neurooncol 161(3):539–545. 10.1007/s11060-023-04242-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel F, Ryang YM, Kirschke JS, Muller BS, Wilkens JJ, Brodard J, Combs SE, Meyer B (2017) Radiolucent carbon fiber-reinforced pedicle screws for treatment of spinal tumors: advantages for radiation planning and follow-up imaging. World Neurosurg 105:294–301. 10.1016/j.wneu.2017.04.091 [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Body JJ, Brandi ML, Cannata-Andia J, Chappard D, El Maghraoui A, Gluer CC, Kendler D, Napoli N, Papaioannou A, Pierroz DD, Rahme M, Van Poznak CH, de Villiers TJ, El Hajj FG, International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone D (2013) Cancer-associated bone disease. Osteoporos Int 24(12):2929–2953. 10.1007/s00198-013-2530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer N, Bumes E, Eberle F, Fox V, Gessler F, Giordano FA, Konczalla J, Onken J, Ottenhausen M, Scherer M, Schneider M, Vatter H, Herrlinger U, Schuss P (2021) Implementation, relevance, and virtual adaptation of neuro-oncological tumor boards during the COVID-19 pandemic: a nationwide provider survey. J Neurooncol 153(3):479–485. 10.1007/s11060-021-03784-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff D, O’Neill BP, Wang CH, O’Fallon JR (1998) Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer 83(8):1593–1601 [PubMed] [Google Scholar]
- Schneider M, Heimann M, Schaub C, Eichhorn L, Potthoff AL, Giordano FA, Guresir E, Ko YD, Landsberg J, Lehmann F, Radbruch A, Schwab KS, Weinhold L, Weller J, Wispel C, Herrlinger U, Vatter H, Schafer N, Schuss P (2020) Comorbidity burden and presence of multiple intracranial lesions are associated with adverse events after surgical treatment of patients with brain metastases. Cancers 12(11):3209. 10.3390/cancers12113209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuss P, Schafer N, Bode C, Borger V, Eichhorn L, Giordano FA, Guresir E, Heimann M, Ko YD, Landsberg J, Lehmann F, Potthoff AL, Radbruch A, Schaub C, Schwab KS, Weller J, Vatter H, Herrlinger U, Schneider M (2021) the impact of prolonged mechanical ventilation on overall survival in patients with surgically treated brain metastases. Front Oncol 11:658949. 10.3389/fonc.2021.658949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe JA, Potthoff AL, Heimann M, Ehrentraut SF, Borger V, Lehmann F, Schaub C, Bode C, Putensen C, Herrlinger U, Vatter H, Schafer N, Schuss P, Schneider M (2023) Incurring detriments of unplanned readmission to the intensive care unit following surgery for brain metastasis. Neurosurg Rev 46(1):155. 10.1007/s10143-023-02066-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciubba DM, Gokaslan ZL (2006) Diagnosis and management of metastatic spine disease. Surg Oncol 15(3):141–151. 10.1016/j.suronc.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Stangenberg M, Viezens L, Eicker SO, Mohme M, Mende KC, Dreimann M (2017) Cervical vertebroplasty for osteolytic metastases as a minimally invasive therapeutic option in oncological surgery: outcome in 14 cases. Neurosurg Focus 43(2):E3. 10.3171/2017.5.FOCUS17175 [DOI] [PubMed] [Google Scholar]
- Ulmar B, Catalkaya S, Naumann U, Gerstner S, Cakir B, Schmidt R, Reichel H, Huch K (2006) Surgical treatment and evaluation of prognostic factors in spinal metastases of renal cell carcinoma. Z Orthop Ihre Grenzgeb 144(1):58–67. 10.1055/s-2006-921465 [DOI] [PubMed] [Google Scholar]
- Ulmar B, Huch K, Kocak T, Catalkaya S, Naumann U, Gerstner S, Reichel H (2007) The prognostic influence of primary tumour and region of the affected spinal segment in 217 surgical patients with spinal metastases of different entities. Z Orthop Ihre Grenzgeb 145(1):31–38. 10.1055/s-2007-960506 [DOI] [PubMed] [Google Scholar]
- Van den Brande R, Cornips EM, Peeters M, Ost P, Billiet C, Van de Kelft E (2022) Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: a systematic review. J Bone Oncol 35:100446. 10.1016/j.jbo.2022.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sande JJ, Kroger R, Boogerd W (1990) Multiple spinal epidural metastases; an unexpectedly frequent finding. J Neurol Neurosurg Psychiatry 53(11):1001–1003. 10.1136/jnnp.53.11.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C (2016) Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J 10(3):267–271. 10.1111/crj.12217 [DOI] [PubMed] [Google Scholar]
- Wang BX, Ou W, Mao XY, Liu Z, Wu HQ, Wang SY (2017) Impacts of EGFR mutation and EGFR-TKIs on incidence of brain metastases in advanced non-squamous NSCLC. Clin Neurol Neurosurg 160:96–100. 10.1016/j.clineuro.2017.06.022 [DOI] [PubMed] [Google Scholar]
- Zhang HR, Qiao RQ, Yang XG, Hu YC (2020) A multicenter, descriptive epidemiologic survey of the clinical features of spinal metastatic disease in China. Neurol Res 42(9):749–759. 10.1080/01616412.2020.1773630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.