Abstract
Introduction
The introduction of clacitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) has revolutionized the treatment of migraines. In clinical practice gepants might be considered as a valid option to treat acute attacks in patients with migraine who are treated with mAbs. However, the safety and tolerability of such a combination is not well addressed in the real-world setting. We designed this study to evaluate the safety and tolerability of combining CGRP mAbs with gepants in the management of migraines.
Methods
This was a retrospective, real-world, exploratory study. The participants included within the study were adult (≥ 18 years) patients diagnosed with migraine. Screening for patients who were treated with at least one GCRP mAbs was done. Data was collected from one site, the American Center for Psychiatry and Neurology, Abu Dhabi UAE. A total of 516 patients taking CGRP mAbs were identified. Extracted data from patients’ electronic medical records included patient demographics, migraine characteristics, prescribed treatments, and adverse events (AEs). The tolerability and safety of the combination therapy was evaluated on the basis of documented AEs.
Results
Among the identified 516 patients, 234 were administered gepants in addition to the CRGP mAb (215, rimegepant; 19, ubrogepant). Eleven of the 234 patients switched from rimegepant to urogepant as a result of lack of efficacy; one patient switched from urogepant to zolmitriptan because of the lack of insurance coverage of the former medication. Among all the patients included in this study, three AEs were documented. These AEs were generally mild and transient and hence did not lead to discontinuation of treatment. Moreover, 42 of the 234 (17.9%) patients were switched from one class of CGRP mAbs to another at least once while continuing treatment with the assigned gepants.
Conclusion
The findings of this study demonstrate that combining CGRP mAbs with gepants is a safe and well-tolerated treatment approach for migraine. Future studies are warranted to further validate these findings and explore long-term outcomes.
Keywords: CGRP mAbs, Erenumab, Eptinezumab, Gepants, Migraine, Safety, Tolerability, UAE
Key Summary Points
| Why carry out this study? |
| Migraine is experienced by an estimated 14% of the global population and is responsible for reduced quality of life in those afflicted. |
| There is little reported evidence about the tolerability and safety of combining CGRP mAbs and gepants, particularly in an underreported population. |
| What did this study ask? |
| The current retrospective study sought to investigate the safety and tolerability of combined treatment. |
| What were the study outcomes/conclusions? |
| Three adverse events were reported on this combination; however, they did not lead to the discontinuation of treatment. |
| What was learned from the study? |
| The combination of CGRP mAbs and gepants was well tolerated and safe in the 234 patients included in this study. |
Introduction
Migraine is a debilitating disorder that is usually lifelong and is characterized by recurring attacks of head pain that is often throbbing and frequently unilateral [1]. Migraine is also associated with other symptoms of neurologic dysfunction such as sensitivity to light, sound, or movement [2]. Globally, in 2019, the fifth highest cause of disability-adjusted life-years (DALYs) lost for those of ages 25–49 was headache disorders [3]. Furthermore, from 357 publications, the estimated global prevalence of headache disorders in 2019 was 52.0%, with 14% being migraines [4]. With such a high prevalence of migraines and their known negative impacts on patients, the US Food and Drug Administration (FDA) recently approved a new class of acute and preventive treatments for migraines.
Migraine management can be divided into two categories: acute treatment, which should be offered to all patients with migraine, and preventative treatment, which is reserved for patients with more frequent and severe attacks. Two recently approved classes of treatment for migraines target the calcitonin gene-related peptide (CGRP). CGRPs have been implicated in migraine pathophysiology, during which there is a substantial elevation in the peptide level, in both migraine with and without aura [5]. Since their discovery, there has been interest in how to block the activities of CGRP receptors or ligands using CGRP antagonists and, in turn, improve migraine severity and burden for patients.
Gepants were the first class of CGRP antagonists developed to treat migraines. Gepants are relatively small CGRP receptor antagonists that have been studied for their efficacy in migraine therapy. They have a mass of 0.2–1 kDa and are typically administered orally except for olcegepant [6]; their short half-lives and easy use make them more prone for frequent dosing that is more probable in acute treatments [7]. Fundamentally, gepants bind to the CGRP receptors and inhibit their activation, which reverses CGRP-induced vasodilation, neurogenic inflammation, and sensitization [8]. In contrast with triptans, gepants do not pose the same risk of vasoconstriction, which can affect patients with cardiovascular diseases.
Three drugs have recently been approved by the FDA for acute and preventative treatment: rimegepant, ubrogepant, and atogepant (Table 1).
Table 1.
Summary of gepants
| Drug | Generation | Administration | Indication | Status | Availability in the UAE |
|---|---|---|---|---|---|
| Olcegepant | 1st | Intravenous | Acute treatment | Development stopped | – |
| Telcagepant | 1st | Oral | Acute treatment | Development stopped | – |
| MK-3207 | 1st | Oral | Acute treatment | Development stopped | – |
| BI44370 | 1st | Oral | Acute treatment | Development stopped | – |
| Rimegepant | 2nd | Oral | Acute and preventative treatment | February 2020 | Yes |
| Ubrogepant | 2nd | Oral | Acute treatment | December 2019 | Yes |
| Atogepant | 2nd | Oral | Preventative treatment | September 2021 | No |
| Zavegepant | 3rd | Nasal | Acute treatment | March 2023 | No |
Monoclonal antibodies (mAbs), unlike small molecules, have an atomic mass of 150 kDa [9]. As large molecules, mAbs do not typically cross the blood–brain barrier unless it has been compromised; therefore, they must be administered by injection, either subcutaneously or intravenously [7]. The dosing intervals of the mAb drug depend on its half-life and is administered between 4 weeks and 3 months [10]. mAbs, unlike small molecules, are not eliminated via hepatic, biliary, or renal routes and thus raise few concerns about hepatotoxicity and drug interaction [11]. Four mAbs are currently approved by the FDA for the prevention of migraine, with clinical trials in both episodic and chronic migraines: erenumab, galcanezumab, fremanezumab, and eptinezumab (Table 2).
Table 2.
Summary of CGRP mAbs
| Drug | Mechanism | Indication | Dosing | FDA approved | Availability in the UAE |
|---|---|---|---|---|---|
| Erenumab | Block CGRP receptor | Prophylactic | Monthly, subcutaneous | 2018 | Yes |
| Eptinezumab | Bind to CGRP ligand | Prophylactic | Quarterly, intravenous | 2020 | Yes |
| Glacanezumab | Bind to CGRP ligand | Prophylactic | Monthly, subcutaneous | 2018 | Yes |
| Fremanezumab | Bind to CGRP ligand | Prophylactic | Monthly or quarterly, subcutaneous | 2018 | No |
There is little reported evidence about the tolerability and safety of combining CGRP mAbs and gepants. Most studies available, although small, discuss the long-term safety of using them concomitantly, and they conclude that the combination is probably safe and well tolerated. In this study we aimed to assess any potential adverse effects and evaluate the overall tolerability and safety of this combination of treatment approach.
Methods
Study Design
This is a descriptive-retrospective observational study based on medical records which was conducted in a single site, the American Center for Psychiatry and Neurology (ACPN), Abu Dhabi, UAE.
A total of 516 patients with either episodic migraine (EM) or chronic migraines (CM) who received CGRP mAbs from 2017 to end of August 2023 were screened for eligibility for inclusion in the study. Of the 516 patients, analysis was done on data from 234 patients who received combined treatment of gepant and a CGRP mAb. Data was gathered from patients’ clinical records which contain all the required demographic information, diagnosis, and medication history. Safety and tolerability of their treatment plan was assessed through the recorded adverse events on each patient’s clinical records.
Ethics
This study was conducted in accordance with the Helsinki Declaration of 1964 and consistent with Good Clinical Practice (GCP). Compliance with all ethical guidelines, health authority regulations, and data privacy laws was ensured. Prior to the start of the study all relevant approvals were obtained from ACPN’s Institutional Review Board (IRB), a waiver of informed consent from the corresponding ethics committee was obtained. To ensure transparency and accuracy all authors were given access to the study data.
Sample
Data included 516 male and female patients from January 2017 to August 2023 who are adults (≥ 18 years), and who had a recorded diagnosis of either EM or CM, as per the International Classification of Headache Disorders (ICHD-3) criteria.
Patients were included in the detailed study analysis if they had been prescribed both a CGRP mAbs and a gepant for their migraine. The patients were administered either rimegepant or ubrogepant by their treating neurologists in ACPN.
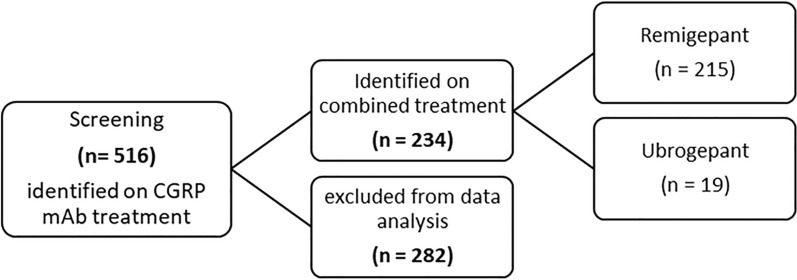
With the two gepants and three CGRP mAbs currently available in UAE, there are various combinations that could take place with the treatment plans of patients: any subject independently taking only one of the two drug classes, or neither drug class, was excluded from the study analysis (Fig. 1).
Fig. 1.
Flowchart of patients included
Fundamentally, the study looked to assess the tolerability and safety of the combined usage of CGRP antagonists by looking for any potential adverse effects that may result from using the two classes of medications together.
Results
Demographic and Baseline Characteristics
Among the 234 patients receiving CGRP mAbs, 45 (19.2%) were male and 189 (80.8%) were female. The majority of patients (54.3%) were diagnosed with episodic migraine. The mean age of migraine onset was 29.31 ± 10.0 years. More than half of the patients (59.4%) had no previous preventative migraine treatment. Most patients (68.7%) had no family history of migraine. When considering psychiatric comorbidities, 59 had major depression disorder, 48 had general anxiety disorder, and 1 had a history of panic attacks. Other comorbidities included epilepsy (16 cases), sleeping disorders (13 cases), thyroid disorders (2 cases), Crohn’s disease (1 case), and fibromyalgia (1 case).
Furthermore, 234 out of 516 patients were prescribed gepants in combination with mAbs as an abortive migraine treatment. Specifically, 215 patients were prescribed rimegepant and 19 patients were prescribed ubrogepant. Forty-two of all the patients on CGRP mAbs switched at some point in their treatment from one class of CGRP mAbs to another because of either lack of efficacy or intolerability and remained on gepants. Thirty-nine of them switched from one class of CGRP mAb to another because of lack of efficacy, two requested to be switched from erenumab to eptinezumab as a personal preference, and two switched because of lack of compliance with dosage timings. Out of the 215 who initially took rimegepant, 11 patients were later switched to ubrogepant because of lack of efficacy, while 1 out of the 19 patients switched from ubrogepant to zolmitriptan as a result of ubrogepant no longer being covered by their insurance. Emirati nationals accounted for most of the sample size (89.3%).
Fort-two patients switched from one CGRP mAb to another. Out of these 42 patients, 5 also switched from one gepant to another. Details of these patients’ switching patterns are shown in Table 3. Out of the 234 patients on combined therapy (CGRP mAbs and gepants), 3 (1.2%) patients reported side effects. Further information on these three patients is provided in Table 4. The average treatment duration for the 234 patients in the study was 2.55 months. The maximum duration of combined treatment was 13 months, while the minimum duration included was 1 month. Notably, one patient remained on combined treatment for an extended period of 22 months, during which a total of 168 pills were consumed (Table 5). This patient reported no side effects throughout the treatment. The average number of pills consumed was divided into three groups: rimegepant, rimegepant to ubrogepant, and ubrogepant ; the average of pills consumed for each group was 32.04, 38.91, and 27 respectively (Table 5). As for the three patients who reported side effects, two were on a combined treatment of erenumab and rimegepant. One reported constipation as a side effect due to erenumab during the first month, which improved during the second month without requiring any medical intervention; the other patient who was on combined treatment for 6 months, consuming 64 pills throughout the treatment duration, reported nausea and vomiting during the first day of the month of Ramadan while fasting, hence leading to more frequent debilitating migraine attacks, but they reported no further nausea symptoms on the following days of fasting. This patient has a known history of migraine with symptoms of nausea and photophobia. The third patient who was initially on a combined treatment of erenumab and rimegepant and later switched from erenumab to eptinezumab reported peri-labial numbness and a slight cough during infusion of eptinezumab; they were discharged safely after reassessment by a physician and symptoms went (Table 4). None of these reported adverse events led to discontinuation of combined treatment.
Table 3.
Patients’ switching patterns
| CGRP mAbs switching profile | Gepants switching profile | |
|---|---|---|
| Patient 1 | Erenumab to eptinezumab | Rimegepant to ubrogepant |
| Patient 2 | Galcanezumab to eptinezumab | Rimegepant to ubrogepant |
| Patient 3 | Erenumab to eptinezumab | Rimegepant to ubrogepant |
| Patient 4 | Erenumab to eptinezumab | Rimegepant to ubrogepant |
| Patient 5 | Eptinezumab to erenumab | Rimegepant to ubrogepant |
Table 4.
Characteristics of patients who experienced side effects on combined therapy (CGRP mAbs and gepants)
| Age/gender | Class of migraine | CGRP mAb | Gepants | Side effect | |
|---|---|---|---|---|---|
| Patient X | 22/female | Episodic | Erenumab then switched to eptinezumab | Rimegepant | Peri-labial numbness and cough |
| Patient Y | 42/female | Chronic | Erenumab | Rimegepant | Nausea and vomiting |
| Patient Z | 55/female | Chronic | Erenumab | Rimegepant | Constipation |
Table 5.
Average number of pills consumed by patients on combined treatment
| Gepant | Number of patients | Mean | Minimum | Maximum |
|---|---|---|---|---|
| Rimegepant | 215 | 32.04 | 8 | 168 |
| Rimegepant to ubrogepant | 11 | 38.91 | 8 | 96 |
| Ubrogepant | 8 | 27 | 8 | 64 |
Discussion
To our best knowledge, this is the largest real-world descriptive study that reports the adverse effects experienced by patients who were prescribed CGRP antagonists and gepants concurrently. In this study 234 patients were on combination therapy and 1.28% of them reported non-serious adverse events. In a previous multicenter study including 13 patients with combination therapy of rimegepant and mAbs, 23% of them experienced adverse effects related to their combination therapy; however, none of these patients discontinued their treatment [12]. In that study there were no distinctions based on which CGRP mAb was co-administered with rimegepant, whereas in our study two patients who reported side effects were concurrently on erenumab with rimegepant and one was on eptinezumab and rimegepant. We observed similar rates of severe adverse effects leading to discontinuation of combined treatment to those seen with standalone CGRP mAbs [13] and CGRP antagonist [14, 15]. Sowers et al. [16] also reported no increase in adverse events when combining gepants with a CGRP mAb. While nausea and constipation have been reported also with this combination therapy, peri-labial tenderness and cough experienced in one patient in our study, to the best of our knowledge, they had not been reported with this combination therapy before [17]. Although in clinical practice specialists are considering this combination therapy for migraine treatment on the basis of a limited number of studies that endorse its safety [12, 17, 18], only case series so far have documented its effectiveness [19]. Delving into the pharmacodynamic perspective, because gepants can potentially penetrate the blood–brain barrier while CGRP monoclonal antibodies function in the peripheral system, it is logical to contemplate that combining the two could result in a synergistic impact, potentially enhancing effectiveness [20]. In a mini-review by Shah et al., 13 patients were on combined treatment with an average treatment duration of 2.20 months, which is quite similar to the average duration of this study (2.55 months); similarly adverse events were reported among 5 patients, but they were not serious and, therefore, combined treatment was continued. The indication to use gepants in our study is treatment of acute attacks, and as such they were used infrequently on an as-needed basis, while mAbs, as indicated for preventive therapy, are used for long-term therapy. This raises the question of the validity of our findings. However, we believe the average number of utilized gepants pills in our study, along with the average months of follow-up, which are larger than any of the reported figures in the literature, would further support our findings of the safety and tolerability of this combination. On the other hand, as a result of CGRP’s involvement in regulating various organ systems, there is a valid concern that the combination of two CGRP antagonists might negatively impact the normal physiological function of CGRP, causing harmful events [18]. Gepants selectively block CGRP receptors while on the other hand CGRP mAbs such as eptinezumab and galcanezumab directly target the CGRP ligand. Both pathways aim to regulate the CGRP pathway. However, the potential synergy in combining gepants and CGRP mAbs in migraine treatment depends on points of intervention within the CGRP pathway. Blockade of ligand and receptor could possibly lead to complex outcomes that are challenging to predict accurately. Therefore, further studies are needed to study the synergistic effect of combined treatment which may possibly lead to improved efficacy or potential adverse events.
Limitations
While this study included a medium number of patients who were administered various CGRP mAbs with CGRP antagonists, there are clear limitations of this study to acknowledge. This study was retrospective, with a potential for recollection bias, and being a single-center study may limit the generalizability of our findings. Additionally, the effectiveness of using this combination treatment was not assessed. We strongly believe that a larger sample, more real-world data, and randomized clinical trials are needed to fully characterize the effectiveness, safety, and tolerability of this combination treatment.
Conclusion
Our study provides insight into a novel migraine treatment approach, combining CGRP mAbs as a preventive strategy with CGRP antagonists as an abortive intervention. This combination therapy seems to be well tolerated in the 234 patients included in this study. Further research is required to validate this finding, as it has the potential to significantly benefit patients with migraine, particularly those who are currently undergoing suboptimal migraine treatment regimens.
Author Contributions
Ibrahim Al Qaisi, Princess Carmina and Vanessa Santos helped collecting data. Batool Al Daher, Shadi Haddad and Yazan Bader helped analysing data and Medline search. Reem Suliman and Taoufik AlSaadi helped interpreting data, write up and overseeing the project. All authors approved the final manuscript.
Funding
The study received no funding. The journal’s Rapid Service Fee was funded by the authors.
Data Availability
All data generated or analyzed during this study are included in this published article. No data repository is available.
Declarations
Conflict of Interest
Batool Al Daher, Ibrahim Al Qaisi, Princess Carmina, Reem Suliman, Shadi Haddad, Taoufik AlSaadi, and Yazan Bader have no competing interests and nothing to disclose.
Ethical approval
The research was conducted in accordance with the Declaration of Helsinki and consistent with GCP and the applicable regulatory requirements. The American Center for Psychiatry and Neurology (ACPN) Institutional Review Board has waived the need for informed consent for this study as it involved a retrospective analysis.
Footnotes
Prior Presentation: Preliminary data of this study were orally presented as an abstract at the European Headache Conference (EHC) 2023 on 7 December 2023, in Barcelona, Spain.
Contributor Information
Taoufik Alsaadi, Email: talsaadi@live.ca.
Reem Suliman, Email: Rk.suliman16@gmail.com.
References
- 1.Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346(4):257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 2.Amiri P, Kazeminasab S, Nejadghaderi SA, et al. Migraine: a review on its history, global epidemiology, risk factors, and comorbidities. Front Neurol. 2022 doi: 10.3389/fneur.2021.800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23(1):34. doi: 10.1186/s10194-022-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 6.Rissardo JP, Caprara ALF. Gepants for acute and preventive migraine treatment: a narrative review. Brain Sci. 2022;12(12):1612. doi: 10.3390/brainsci12121612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hindiyeh N, Hovren H. Advances in migraine therapeutics: the role of calcitonin gene-related peptide. In: Smith RA, Kaspar BK, Svendsen CN, editors. Neurotherapeutics in the era of translational medicine. London: Academic; 2021. p. 181–201.
- 8.Yuan H, Spare NM, Silberstein SD. Targeting CGRP for the prevention of migraine and cluster headache: a narrative review. Headache. 2019;59:20–32. doi: 10.1111/head.13583. [DOI] [PubMed] [Google Scholar]
- 9.Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52:83–124. doi: 10.1007/s40262-012-0027-4. [DOI] [PubMed] [Google Scholar]
- 10.Diener HC, Förderreuther S, Gaul C, et al. Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor. Neurol Res Pract. 2020;2(1):1–6. doi: 10.1186/s42466-020-00057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giamberardino MA, Affaitati G, Costantini R, Cipollone F, Martelletti P. Calcitonin gene-related peptide receptor as a novel target for the management of people with episodic migraine: current evidence and safety profile of erenumab. J Pain Res. 2017;10:2751–2760. doi: 10.2147/JPR.S128143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berman G, Croop R, Kudrow D, et al. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. 2020;60(8):1734–1742. doi: 10.1111/head.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene KA, Gentile CP, Szperka CL, et al. Calcitonin gene-related peptide monoclonal antibody use for the preventive treatment of refractory headache disorders in adolescents. Pediatr Neurol. 2021;114:62–67. doi: 10.1016/j.pediatrneurol.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair HA. Rimegepant: a review in the acute treatment and preventive treatment of migraine. CNS Drugs. 2023;37(3):255–265. doi: 10.1007/s40263-023-00988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10268):51–60. doi: 10.1016/S0140-6736(20)32544-7. [DOI] [PubMed] [Google Scholar]
- 16.Sowers LP, Waite J, Rea B, Russo AF. 63rd Annual Scientific Meeting American Headache Society ®. Headache. 2021;61(S1):1–178. 10.1111/head.14130.
- 17.Jakate A, Blumenfeld AM, Boinpally R, et al. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: a randomized phase 1b drug–drug interaction study. Headache. 2021;61(4):642–652. doi: 10.1111/head.14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellesi L. Combining two CGRP inhibitors to treat migraine. Expert Opin Drug Saf. 2022;21(9):1135–1136. doi: 10.1080/14740338.2022.2130890. [DOI] [PubMed] [Google Scholar]
- 19.Mullin K, Kudrow D, Croop R, et al. Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology. 2020;94(20):e2121–e2125. doi: 10.1212/WNL.0000000000008944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah T, Bedrin K, Tinsley A. Calcitonin gene relating peptide inhibitors in combination for migraine treatment: a mini-review. Front Pain Res. 2023;4:1130239. doi: 10.3389/fpain.2023.1130239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. No data repository is available.