Abstract
Multiple sclerosis (MS) is a chronic, progressive, inflammatory disorder of the central nervous system. Relapsing–remitting MS (RRMS), the most common form of the disease, is characterized by transient neurological dysfunction with concurrent accumulation of disability. Over the past three decades, disease-modifying therapies (DMTs) capable of reducing the frequency of relapses and slowing disability worsening have been studied and approved for use in patients with RRMS. The first DMTs were interferon-betas (IFN-βs), which were approved in the 1990s. Among them was IFN-β-1a for subcutaneous (sc) injection (Rebif®), which was approved for the treatment of MS in Europe and Canada in 1998 and in the USA in 2002. Twenty years of clinical data and experience have supported the efficacy and safety of IFN-β-1a sc in the treatment of RRMS, including pivotal trials, real-world data, and extension studies lasting up to 15 years past initial treatment. Today, IFN-β-1a sc remains an important therapeutic option in clinical use, especially around pregnancy planning and lactation, and may also be considered for aging patients, in which MS activity declines and long-term immunosuppression associated with some alternative therapies is a concern. In addition, IFN-β-1a sc is used as a comparator in many clinical studies and provides a framework for research into the mechanisms by which MS begins and progresses.
Keywords: Disease-modifying therapies, Interferons, Interferon-beta-1a, Interferon-β-1a for subcutaneous injection, Multiple sclerosis, Relapsing–remitting multiple sclerosis
Key Summary Points
| Interferon-β (IFN-β) 1a for subcutaneous injection (sc) (Rebif®) was one of the first disease-modifying therapies (DMTs) approved for multiple sclerosis (MS). |
| Three decades of research and clinical experience have provided a wealth of data on the long-term safety, efficacy, and real-world effectiveness of IFN-β. |
| Through the evolving clinical landscape of MS treatment, IFN-β-1a sc has remained a mainstay of MS treatment and research. |
| IFN-β-1a sc continues to play a role in research into MS pathophysiology and treatment, as an active comparator and as a stable background therapy in clinical trials of other treatments. |
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disorder of the central nervous system (CNS) that generally manifests as one of several phenotypes [1–3]. Approximately 85% of people with MS present with relapses, periods during which new neurologic symptoms appear or existing symptoms become worse, interspersed with remissions, times during which recovery may occur and the disease does not appear to progress [1, 4]. This pattern is defined as relapsing–remitting MS (RRMS) [1, 4]. RRMS was originally defined as one of three distinct clinical courses that included RRMS, primary progressive MS, and secondary progressive MS (SPMS) [4]. Current evidence, however, supports considering MS as a spectrum from the outset of the disease, defined by relative contributions of overlapping pathological and reparative or compensatory processes [4].
MS was first described by Jean-Martin Charcot in 1868, but more than a century passed before the first proven effective disease-modifying therapy (DMT), recombinant interferon-β (IFN-β), became available [5]. Before IFN-β, there were few treatment options for MS, and those that were available had both limited efficacy and significant toxicity [6, 7]. Chemotherapeutic agents like azathioprine or cyclophosphamide that suppress the immune system and are associated with significant toxicity were reserved for more severe cases and, therefore, were not given early in the course of MS. Research was focused on finding a treatment that would modify the course of the disease without compromising overall immune response.
Endogenous IFNs were first identified in the 1950s [8–10]. Initially, IFN-β preparations were investigated for the treatment of MS owing to their known antiviral properties. This was based on the theory that MS might be caused by a chronic viral infection, supported by detection of anti-measles antibodies in cerebrospinal fluid, links to Epstein-Barr virus infection, and evidence of decreased activation of IFN-β pathways in people with MS compared to those without MS [2, 11–14]. Several studies have also reported decreased endogenous type I IFN signaling and secretion in patients with RRMS [15].
Types of IFN
The IFNs identified in humans have been divided into three classes, or types: type I, II, and III [16]. Type I IFNs include IFN-α and IFN-β, while the type II classification includes only IFN-γ (gamma) [16]. IFN-α and IFN-β are induced by viruses and double-stranded RNA and share the same receptor, though their biological activity is slightly different [10]. IFN-α is used for the treatment of chronic viral hepatitis while IFN-β is used as treatment for relapsing forms of MS. Type II IFN has a different receptor, has minimal antiviral activity, and is used for the treatment of chronic granulomatous disease [10, 16]. More recently, type III IFNs, also called IFN-λ (lambda), are being evaluated for the treatment of several viral infections, including chronic viral hepatitis [10, 16].
Interferon-Based Therapy Before Use as an MS Treatment
IFNs comprise a large and varied family of signaling proteins or cytokines involved in modulating the immune response, including responses to viral infection and cancer, among other crucial cellular functions [8, 10, 11]. In the 1950s, two teams independently discovered IFN in different experimental contexts and described the newly discovered biological factor in terms of its viral inhibitory (or interfering) function [8–10, 17]. The first group, located in Japan, was studying vaccination with ultraviolet-irradiated vaccinia (inactivated virus) [8–10, 17]. When challenged with live vaccinia at the site of initial inoculation, vaccinia replication was inhibited, and the researchers attributed this to an “inhibitory factor” [8, 10, 17]. A few years later, a UK-based team studying influenza virus found that adding heat-inactivated influenza virus to chorioallantoic membrane fragments from chick embryos could stimulate the production of a factor which interfered with subsequent viral replication—this factor was formally named “interferon” [8–10].
The therapeutic potential of IFN against viral infections soon became apparent [11], but the earliest ambitions of clinical trials to study the antiviral function of IFNs in the 1960s were limited by insufficient quantities of isolated IFN product. With the evolution of gene cloning in the 1980s, sufficient quantities of pure IFN could be produced for clinical investigation [10, 11]. IFN was initially investigated as an antiviral treatment against both DNA and RNA viruses, and it was also explored as an anticancer treatment, notably for leukemia [10, 11]. In 1986, the first approval of an IFN for pharmaceutical purposes by the US Food and Drug Administration (FDA) was for the treatment of cancer, specifically IFN-α2 for hairy cell leukemia [11]. Since then, IFN-α has been approved for the treatment of several infectious diseases including hepatitis B and C, as well as genital warts caused by human papillomavirus (condyloma acuminatum) [3, 11, 18].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
IFN in MS
The hypothesis that viral infection was involved in the etiology of MS led to the examination of IFNs as a potential treatment, although the role of specific viral agents in the pathology of MS still remains unconfirmed [2, 11–13]. Studies of IFNs in MS during the 1970s suggested lower IFN-like activity following viral induction in people with MS compared to controls [11], prompting the evaluation of IFN for the treatment of MS. Type II IFN (IFN-γ) in particular was examined because of evidence of reduced production in people with MS. However, one of the first studies found a more than threefold increase in relapses from recombinant IFN-γ [19]. The authors concluded that this type of IFN was not suited for MS treatment.
In contrast, intrathecal injections of natural IFN-β demonstrated promising early results in reducing MS relapses [11, 12, 20]. First published in 1981, the results of a small study of 10 patients with MS treated with IFN-β (administered intrathecally by serial lumbar puncture) and 10 patients in the control group found clinical benefit was more common in patients who were treated versus those who were untreated [20–22]. In the 1990s, recombinant versions of IFN-β replaced crude extracts of purified natural fibroblast IFN-β such as Frone®, which was administered via subcutaneous (sc) injection [23, 24]. Recombinant IFN-β, administered parenterally, was the first treatment to demonstrate measurable clinical benefit in improving the natural history of MS in patients with relapsing MS [11, 13]; it prevented and shortened relapses, limited the formation of new brain lesions seen on magnetic resonance imaging (MRI), and slowed disability progression [13, 25]. In 1993, IFN-β-1b (Betaseron®, delivered by sc injection) [26] became the first recombinant IFN-β and the first DMT to be approved by the FDA for MS treatment [11, 13].
Several formulations of recombinant IFN-β are approved for the treatment of MS, distinguished by structural differences related to methods of production [2, 13]. IFN-β-1b is obtained from DNA cloned into a bacterial vector and, as a result, is not glycosylated. To reduce misfolding, it also has one fewer amino acid and an amino acid substitution compared to native human IFN-β. In contrast, IFN-β-1a is produced in a mammalian cell, is glycosylated without amino acid substitutions, and is essentially identical to the endogenous form of human IFN-β [2, 13]. The next two interferon DMTs approved by the FDA for MS treatment after IFN-β-1b sc were IFN-β-1a, delivered by intramuscular (im) injection (Avonex®) [27], and IFN-β-1a, delivered by sc injection (Rebif®) [28, 29]. Two formulations of pegylated IFN-β-1a (Plegridy®) have been approved; the sc formulation was approved in 2014, and the im formulation was approved in 2021.
Mechanism of Action of Interferons in MS
The hallmark of MS is autoimmune inflammation that targets the CNS. While the exact cause of MS remains unknown, adaptive immunity appears to play a prominent role in the pathogenesis of RRMS, whereas innate immune responses contribute to SPMS and are likely to contribute to CNS damage throughout different phases of MS [11, 13, 25]. MS is thought to be triggered and perpetuated by overactivity and/or regulatory/homeostatic imbalance of the immune system [13]. Dysregulation of effector as well as regulatory components of the adaptive immune system can result in inflammation, which ultimately causes demyelination and subsequent axonal damage and loss [13]. Specifically, the autoimmune response observed in MS is mediated via T helper (Th)1 and Th17 CD4+ T cells and autoreactive B cells through both direct cytotoxicity and by modulating different facets of the immune response [30, 31].
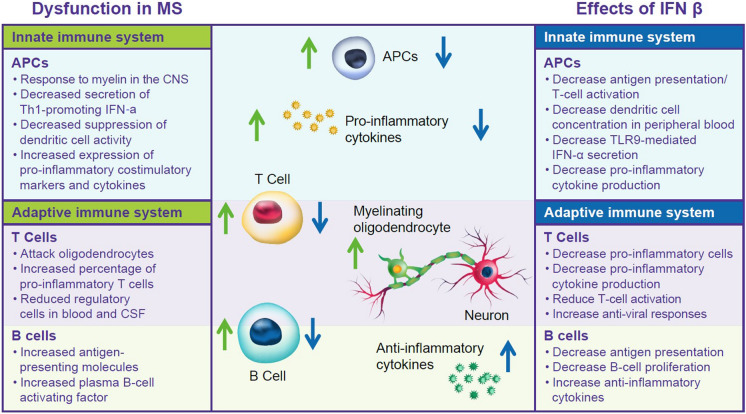
The hypothesized mechanisms of action of IFN-β in the treatment of MS have been reviewed previously [3, 13, 32–34]. Briefly, type I IFNs, which include IFN-β, are crucial players in both innate and adaptive immunity [3, 13, 32–34]. They are expressed in response to viral infections and regulate immunity, cell proliferation, apoptosis, and cell protection [3, 13]. Functional studies reveal reduced IFN responses in people with MS, with downregulation of IFN-β signaling in untreated MS, along with further reduction of IFN-driven genes during MS exacerbations and progression, potentially disrupting adaptive and innate immunity (Fig. 1) [25, 35].
Fig. 1.
Hypothesized pathways of dysfunction in MS and effects of IFN-β. APC antigen presenting cells, CNS central nervous system, CSF cerebrospinal fluid, IFN interferon, MS multiple sclerosis, TLR Toll-like receptors
IFN-β treatment may work to counteract dysfunction in the innate and adaptive immune systems of patients with MS. In the innate immune system, IFN-β treatment decreases T cell activation and antigen presentation, reduces dendritic cell concentrations in peripheral blood, and downregulates TLR9 [13, 32–34]. In the adaptive immune system, IFN-β treatment reduces T cell activation, increases antiviral responses, and decreases pro-inflammatory CD4+ and CD8+ Th17 cells [13, 32–34]. Several studies have reported that IFN-β treatment suppresses Th17 response as well as B cell antigen-presenting capacity and cytokine secretion, findings which were confirmed by ex vivo studies of patients treated with IFN-β [36, 37]. IFN-β treatment impacts both systems by decreasing antigen presentation and reducing pro-inflammatory cytokines [13, 32, 34]. The benefits of IFN-β therapies in MS are thought to stem from their immunomodulatory and antiproliferative virtues, but probably not their antiviral properties (Fig. 1) [2, 13, 33, 38]. However, coming full circle regarding the initial use of IFN-β in MS treatment due to its antiviral properties, the effects of IFN-β on viral infections are once again being considered as a potential mechanism of action [39, 40]. Ongoing research into the pronounced and broad effects of IFN-β on gene expression may lead to further understanding of the mechanism of action of IFN-β therapies and the pathophysiology of MS [35].
IFN-β-1a SC
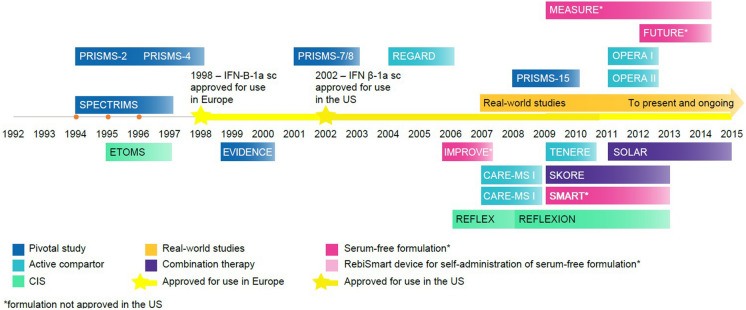
Among the first approved DMTs was IFN-β-1a for sc injection (Rebif®) (Fig. 2) [28, 29]. Currently indicated “for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing–remitting disease, and active secondary progressive disease, in adults” [28]. The recommended dosing according to the prescribing information is either 22 μg or 44 μg injected sc three times per week (tiw) [28]. However, the 44-μg dose is most commonly used in practice, with the 22-μg dose generally reserved for those who do not tolerate the 44-μg dose. Uptitration over a 4-week period is recommended, and patients may be counselled to use analgesics and/or antipyretics to reduce initial flu-like side effects [28, 29].
Fig. 2.
Key events in the development of subcutaneous IFN-β-1a for MS treatment. CIS clinically isolated syndrome, IFN interferon, sc subcutaneous, US United States
Efficacy in RRMS
Key Trials
Two pivotal studies formed the basis for the approval of IFN-β-1a sc in the USA: the Prevention of Relapses and Disability by Interferon-β-1a Subcutaneously in Multiple Sclerosis (PRISMS) trial and the EVidence of Interferon Dose–response: European North American Comparative Efficacy (EVIDENCE) trial. Both were randomized controlled studies in RRMS (study details are provided in Table 1) [41–52]. As treatment options for MS progressed, pivotal trials gave way to head-to-head studies that included IFN-β-1a sc as well as assessments of a new, serum-free formulation of IFN-β-1a sc.
Table 1.
Efficacy of IFN-β-1a sc: clinical studies
| Study and timing | Design | Study population | Titration and dosing | Outcomes |
|---|---|---|---|---|
| RRMS | ||||
|
PRISMS [41] 1994–1997 |
Double-blind, placebo-controlled, phase 3 |
560 patients with clinically definite or laboratory-supported definite MS of ≥ 1 year duration Kurtzke EDSS scores of 0–5.0 ≥ 2 relapses in the preceding 2 years |
Titration: Dose was gradually increased over 4–8 weeks Dosing: IFN-β-1a sc 22 μg tiw IFN-β-1a sc 44 μg tiw Placebo tiw |
Primary outcome: Relapse count over the course of the study Relapse rate was significantly lower at 2 years with both doses of IFN-β-1a than with placebo Mean number per patient 1.82 and 1.73 for the 22-µg and 44-µg treatment groups, respectively vs 2.56 for the placebo group, (p < 0.005 for both) Risk reductions of 27% [95% CI 14–39] and 33% [21–44] for the 22-µg and 44-µg treatment groups, respectively, vs the placebo group Median time to first relapse was delayed by 3 months in the 22-μg group and 5 months in the 44-μg group vs the placebo group Time to EDSS progression was 21.3, 18.5, and 11.9 months in the IFN 22-μg, 44-μg, and placebo groups, respectively (p < 0.05 vs placebo for both treatment groups) In patients with high baseline EDSS (> 3.5): • Time to confirmed progression was significantly longer only in the 44-μg group compared to the placebo group (7.5, 21.3, and 7.3 months in the, IFN 22-μg, 44-μg, and placebo groups, respectively, p < 0.05 vs placebo for the 44-μg group) Median MRI lesion volume decreased by 1.2% in the 22-µg group and 3.8% in the 44-µg group and increased by 10.9% in the placebo group (p < 0.0001 compared with the placebo group for both IFN doses) T2 new or enlarging lesion number (determined by proton density T2-weighted scans) was reduced by 67% for the 22-µg group and 78% in the 44-µg group compared to the placebo group (p < 0.0001), with a dose effect in favor of the 44-µg tiw group (p = 0.0003) |
|
OWIMS [42] 1995–1996 |
Double-blind, placebo-controlled, phase 3 |
293 patients with clinically definite or laboratory-supported definite RRMS of ≥ 1 year duration Kurtzke EDSS scores of 0–5.0 ≥ 1 relapse in the prior 24 months but not in the 8 weeks before entry ≥ 3 lesions consistent with MS |
Dosing: IFN-β-1a sc 22 μg qw IFN-β-1a sc 44 μg qw Placebo qw |
Primary outcome: Number of CUA lesions at 24 weeks Median CUA lesions of 0.71/scan with placebo, 0.5/scan with 22 μg qw (not significant), and 0.33/scan with 44 μg qw (p = 0.002) T2 new lesion count/scan (mean/median) at 48 weeks was 3.2/1.5 for placebo, 2.4/1.0 for 22 μg (p = 0.03), and 1.5/1.0 for 44 μg (p = 0.0005) BOD at 48 weeks showed a median increase of 5.9% for the placebo group compared with a decrease of 2% in the 22-μg qw group (p = 0.0018) and 1.4% in the 44-μg qw group (p = 0.0058) |
|
1999–2002 |
Randomized, controlled, assessor-blinded |
677 patients with relapsing MS EDSS scores of 0–5.5 ≥ 2 exacerbations in the preceding 2 years |
Titration: In patients randomized to high-dose tiw treatment, the dose was increased over 4 weeks, with patients receiving 8.8 μg tiw for the first 2 weeks and 22 μg tiw for the next 2 weeks Dosing: IFN-β-1a sc 44 μg tiw IFN-β-1a im 30 μg qw |
Primary clinical endpoint: Proportion of patients who remained free from relapses The proportion of patients who remained relapse-free was significantly greater in the high-dose tiw treatment group vs the low-dose qw treatment group (adjusted odds ratio 1.5; 95% CI 1.1–2.0; p = 0.023) After 24 weeks, 74.9% of the IFN-β-1a sc 44-µg tiw group and 63.3% of the IFN-β-1a im 30-µg qw group remained free from relapses (p = 0.0005) At the end of the 64-week comparative phase • A higher proportion of patients in the IFN-β-1a sc 44-µg tiw group remained free from relapses compared with the IFN-β-1a im 30-µg qw group (p = 0.023) • ARR was 17% lower (p = 0.033) in the IFN-β-1a sc 44-µg tiw group vs the IFN-β-1a im 30-µg qw group • Time to first relapse was longer (hazard ratio [HR] 0.70; p = 0.002) in the IFN-β-1a sc 44-µg tiw group compared with the IFN-β-1a im 30-µg qw group at the end of the comparative phase During the second phase The group that changed from qw im to tiw sc dosing had • A 50% reduction in mean relapse rates (p < 0.001) • Significant reductions in MRI activity vs the comparative phase of the study The group that had remained with tiw sc dosing from the comparison phase had • A 26% reduction in mean relapse rates (p < 0.028) |
|
REGARD [44] 2004–2006 |
Multicenter, randomized, parallel, open-label |
764 patients with RRMS EDSS scores of 0–5.5 ≥ 1 attack in the previous 12 months Clinically stable or neurologically improving during the 4 weeks before randomization |
Titration: Patients who received IFN-β-1a had a standard dose titration for the first 4 weeks Dosing: IFN-β-1a sc 44 μg tiw Glatiramer acetate sc 20 mg od |
Primary outcome measure: Time to first relapse over 96 weeks No significant difference in time to first relapse between the treatment groups (HR 0.94; 95% CI 0.74–1.21; p = 0.64) No significant differences between groups were observed in • Numbers of T2 active lesions (defined as new or enlarging T2 lesions), • Proportions of scans with T2 active lesions, change in T2 lesion volume There were significantly fewer Gd+ lesions with IFN-β-1a compared with GA (0.24 vs 0.41 lesions per patient per scan, 95% CI − 0.4 to 0.1; p = 0.0002) |
|
IMPROVE (Investigating MRI Parameters with Rebif® imprOVEd formulation) [45] 2006–2009 |
Randomized, double-blind (16 weeks) and rater-blind (24 weeks) phases |
180 patients with RRMS EDSS scores of 0–5.5 Active disease ≥ 1 clinical event and ≥ 1 Gd+ MRI lesion within the previous 6 months |
Dosing: New formulation of IFN-β-1a sc 44 μg tiw |
Primary endpoint: The number of CUA MRI brain lesions at week 16 At week 16, the mean number of CUA lesions was lower with IFN-β-1a sc 44 μg tiw vs placebo (p < 0.001; 69% fewer lesions) No CUA lesions were observed in 53.3% of patients receiving IFN-β-1a vs 16.7% of those receiving placebo |
| Extension studies | ||||
|
PRISMS-4 [46] 1997–1999 |
Long-term, double-blind extension | 533 patients with RRMS who had participated in the PRISMS study |
Dosing: IFN-β-1a sc 22 μg tiw IFN-β-1a sc 44 μg tiw Crossover: Placebo, followed by IFN-β-1a sc 22 μg tiw Placebo, followed by IFN-β-1a sc 44 μg tiw |
Primary outcome: Relapse count per patient over 4 years Relapse rates for 4 years were • Crossover (placebo/IFN-β-1a sc): 1.02 • IFN-β-1a sc 22 μg tiw: 0.80 (p < 0.001 vs crossover) • IFN-β-1a sc 44 μg tiw: 0.72 (p < 0.001 vs crossover) |
| PRISMS-7/8 [59] | Long-term open-label follow-up of a double-blind initial study | 382 patients with RRMS who had participated in the PRISMS study |
Dosing: IFN-β-1a sc 22 μg tiw IFN-β-1a sc 44 μg tiw |
Compared with patients in the late treatment group, patients originally randomized to IFN-β-1a 44 μg sc tiw demonstrated • Less EDSS progression • Lower relapse rates • Less T2 burden of disease |
| PRISMS-15 [60] | 290 patients with RRMS who had participated in the PRISMS study |
Dosing: IFN-β-1a sc 22 μg tiw IFN-β-1a sc 44 μg tiw |
Higher cumulative dose exposure and longer treatment times (i.e., the highest dose quartile) were associated with better outcomes compared with the lowest dose quartile as measured by: • Annualized relapse rate (0.37 and 0.50, respectively) • Number of relapses (5.8 and 7.8) • 3-month confirmed EDSS progression (38% vs 50%) • Mean change in EDSS score (1.15 and 2.53) • Conversion to SPMS (20.8% and 52.1%) |
|
| Active comparator | ||||
|
CARE-MS I [47] Enrollment: 2007–2009 |
Randomized rater-masked, controlled phase 3 trial |
581 patients with RRMS with disease duration ≤ 5 years ≥ 2 relapses in the previous 2 years and ≥ 1 in the previous year EDSS score ≤ 3.0 Cranial abnormalities on MRI attributable to MS |
Dosing: IFN-β-1a sc 44 μg tiw Alemtuzumab 12 mg iv od (od for 5 days at baseline and od for 3 days at 12 months) |
Co-primary endpoints: Relapse rate and time to 6-month sustained accumulation of disability 75 (40%) patients in the IFN-β-1a sc 44-μg tiw group relapsed (122 events) compared with 82 (22%) patients in the alemtuzumab group (119 events; rate ratio 0.45; 95% CI 0.32–0.63; p < 0.0001) 20 (11%) of the IFN-β-1a sc 44-μg tiw group had sustained accumulation of disability vs 30 (8%) in the alemtuzumab group (HR 0.70; 95% CI 0.40–1.23; p = 0.22) |
|
CARE-MS II [47] Enrollment: 2007–2009 |
Rater-masked, randomised controlled phase 3 |
581 patients with RRMS with disease duration ≤ 10 years ≥ 2 relapses in the previous 2 years and ≥ 1 in the previous year ≥ 1 relapse on IFN-β or glatiramer after ≥ 6 months of treatment EDSS score ≤ 5.0 Cranial and spinal MRI lesions fulfilling protocol-defined criteria |
Dosing: IFN-β-1a sc 44 μg tiw Alemtuzumab 12 mg iv od (od for 5 days at baseline and od for 3 days at 12 months) Alemtuzumab 24 mg iv od—the 24 mg od group was discontinued to aid recruitment, but data were included for safety assessments |
Co-primary endpoints: Relapse rate and time to 6-month sustained accumulation of disability Relapse rate was lower with alemtuzumab 12 mg vs IFN-β-1a 104 (51%) patients in the IFN-β-1a group relapsed (201 events) compared with 147 (35%) patients in the alemtuzumab group (236 events; rate ratio 0.51; 95% CI 0.39–0.65; p < 0.0001) Risk of sustained accumulation of disability was lower with alemtuzumab 12 mg vs IFN-β-1a 40 (20%) patients in the IFN-β-1a group had sustained accumulation of disability compared with 54 (13%) in the alemtuzumab group (HR 0.58; 95% CI 0.38–0.87; p = 0.008) |
|
TENERE (TErifluNomidE and REbif®) [48] 2009–2011 |
Rater-blinded phase 3 |
324 patients with RRMS Relapsing clinical course with or without progression EDSS score ≤ 5.5 Relapse-free for 30 days prior to randomization |
Dosing: IFN-β-1a sc 44 μg tiw Orally administered teriflunomide 7 mg Orally administered teriflunomide 14 mg |
Primary composite endpoint: Time to failure, defined as first occurrence of confirmed relapse or permanent treatment discontinuation for any cause No difference in time to failure was observed between either dose of teriflunomide and IFN-β-1a |
|
OPERA I/II Randomization: 2011–2013 |
Two identical phase 3, double-blind, Double-dummy, active-controlled, randomized trials |
1656 patients with relapsing MS n = 821 in OPERA I n = 835 in OPERA II EDSS score ≤ 5.5 ≥ 2 relapses in the previous 2 years or ≥ 1 in the previous year MRI showing abnormalities consistent with MS No neurologic worsening for ≥ 30 days before both screening and baseline |
Dosing: Ocrelizumab at a dose of 600 mg iv every 24 weeks IFN-β-1a sc at a dose of 44 μg tiw for 96 weeks |
Primary endpoint: Annualized relapse rate at 96 weeks ARR was lower with ocrelizumab than with IFN-β-1a in OPERA I (0.16 vs 0.29; 46% lower rate with ocrelizumab; p < 0.001) and OPERA II (0.16 vs 0.29; 47% lower rate; p < 0.001 for both trials) In pooled analyses • The percentage of patients with confirmed disability progression at 12 weeks was lower for ocrelizumab: 9.1% for ocrelizumab vs 13.6% for IFN-β-1a, p < 0.001 • The percentage of patients with confirmed disability progression at 24 weeks was lower for ocrelizumab: 6.9% for ocrelizumab vs 10.5% for IFN-β-1a, p = 0.003 |
| Other MS settings | ||||
|
ETOMS [66] 1995 and 1997 |
Double-blind, placebo-controlled, randomized study |
278 patients who had a first episode of neurological dysfunction suggesting MS At least 1 abnormality evident during neurological examination A positive brain MRI scan (≥ 4 white matter lesions on T2-weighted scans, or ≥ 3 white matter lesions, if ≥ 1 was infratentorial or Gd +) |
Dosing: Low dose of 22 µg IFN-β-1a sc qw Placebo |
Primary outcome measure: conversion to CDMS, defined by the occurrence of a second exacerbation The time at which 30% of patients had converted to CDMS was longer in patients treated with IFN-β-1a (569 days) vs placebo (252 days, p = 0.034) Fewer participants in the IFN group developed CDMS compared with the placebo group (52/154 [34%] vs 69/154 [45%]; p = 0.047) |
|
REFLEX [49] 2006–2010 |
Randomized, controlled, double-blind, phase 3 trial |
517 participants with a first clinical demyelinating event suggestive of MS ≥ 2 clinically silent T2 lesion |
Dosing: Serum-free formulation of IFN-β-1a sc 44 μg tiw (n = 171) Serum-free formulation of IFN-β-1a sc 44 μg qw (n = 175) Placebo (n = 171) |
Primary endpoint: Time to a diagnosis of MS as defined by the 2005 McDonald criteria Two-year cumulative probability of a diagnosis of McDonald MS was significantly lower in patients treated with IFN-β-1a sc tiw (62.5%; p < 0.0001; HR 0.49; 95% CI 0.38–0.64; or qw (75.5%, p = 0.008, HR 0.69; 0.54–0.87) vs placebo (85.8%) Risk of conversion to a diagnosis of MS was significantly lower in the IFN-β-1a sc tiw group compared with the qw group (p = 0.0087; HR 0.71; [0.54–0.91]) compared with the placebo group Two-year rates of conversion to clinically definite MS were lower for both IFN-β-1a sc tiw (20.6%; p = 0.0004; HR 0.48 [0.31–0.73]) and qw (21.6%; p = 0.0023; HR 0.53 [0.35–0.79]) than for placebo (37.5%) |
| REFLEXION [50] | Extension study | Participants who completed the 24-month double-blind REFLEX study |
Dosing: Participants who received IFN-β-1a sc in the REFLEX study and did not reach a diagnosis of clinically definite MS continued original treatment Delayed treatment group: participants who received placebo in the REFLEX study who did not reach a diagnosis of clinically definite MS were switched to IFN-β-1a sc 44 μg tiw Participants with clinically definite MS received IFN-β-1a sc 44 μg tiw |
Primary endpoint: Time to a diagnosis of MS as defined by the 2005 McDonald criteria Two-year cumulative probability of a diagnosis of McDonald MS was significantly lower for the IFN-β-1a sc 44-μg tiw group compared with the DT group (44.6% for DT, 40.7% for IFN-β-1a sc 44 μg qw [nominal p = 0.084 vs DT], 39.2% for IFN-β-1a sc 44 μg tiw [nominal p = 0.032 vs DT]) The cumulative probability of meeting McDonald MS 2005 criteria was lower in the IFN-β-1a sc 44-μg tiw group compared with the DT group Mean cumulative numbers of new T2, Gd+, and T1 hypointense lesions as well as T2 and T1 hypointense lesion volume changes were lower in the IFN-β-1a sc 44-μg tiw group compared with the DT group (nominal p < 0.05 for all) |
| SPECTRIMS [51] | Randomized, placebo-controlled trial |
Patients with clinically definite SPMS, with or without exacerbations, following an initial relapsing–remitting course Baseline EDSS of 3.0–6.5 Pyramidal functional score ≥ 2 |
Dosing: IFN-β-1a sc 22 μg tiw IFN-β-1a sc 44 μg tiw Placebo |
Primary endpoint: Time to confirmed progression in disability Time to confirmed progression in disability was not different between the IFN-β-1a sc group and placebo group Relapse rates were reduced for both IFN-β-1a sc dose groups (p < 0.001 for both) Exploratory subanalyses suggested a greater benefit of treatment with IFN-β-1a sc in patients with ≥ 1 exacerbation in the 2 years before the study |
ARR annualized relapse rate, BOD burden of disease, CDMS clinically definite MS, CI confidence interval, CUA combined unique active, DT delayed treatment, EDSS Expanded Disability Status Scale, Gd+ gadolinium-enhancing, HR hazard ratio, IFN interferon, im intramuscular, iv intravenous, MRI magnetic resonance imaging, MS multiple sclerosis, od once per day, qw once per week, RRMS relapsing–remitting MS, sc subcutaneous, SPMS secondary progressive MS, tiw three times per week
The PRISMS phase 3 trial began in 1994, when effective treatment options for MS were limited [2, 41, 53]. The primary efficacy measure was relapse rate over the 2 years of the study, which was significantly reduced in both the IFN-β-1a sc 22-µg tiw (lower dose) and 44-µg tiw (higher dose) groups compared to the placebo group (p < 0.005 for both) [41]. Relapse rates were reduced by 27% (p < 0.05) in the 22-µg group and 32% (p < 0.005) in the 44-µg group compared with the placebo group. Both median time to first relapse and time to Expanded Disability Status Scale (EDSS) progression were delayed in both treatment groups compared with the placebo group. In patients with high baseline EDSS (> 3.5), time to confirmed progression was significantly longer only in the 44-μg group compared to the placebo group. The median MRI lesion volume decreased in the treatment groups and increased in the placebo group, while T2 new or enlarging lesion number (determined by proton density T2-weighted scans) was reduced for both treatment groups compared to the placebo group (p < 0.0001), with a dose effect observed in favor of the 44-µg tiw group (p = 0.0003) [41].
Detailed analysis of the MRI results from the PRISMS study included a cohort of 205 patients who had MRIs performed monthly for the first 11 months of the study [54]. The median number of combined unique active (CUA) lesions and the percentage of CUA scans were both reduced. CUA lesions included both T1 gadolinium-enhancing (Gd+) lesions or new/enlarging T2 lesions, as well as lesions that were identified in both scans, which were counted only once. The median number of CUA lesions per patient per scan was 0.88 for the placebo group, 0.17 for the 22-µg tiw group, and 0.11 for the 44-µg tiw group, representing reductions of 80.7% and 87.5% for the 22-µg and 44-µg groups, respectively, compared with placebo group. The median percentages of CUA scans were 44% in the placebo group, 12.5% in the 22-µg group, and 11% in the 44-µg group, representing reductions of 71.5% and 75% for the 22-µg and 44-µg groups, respectively. Reductions in the progressive accumulation of CUA lesions were observed as early as 2 months after the start of treatment (p = 0.0065 for 22 µg; p = 0.0008 for 44 µg).
Many findings from the PRISMS trial suggested a dose- and/or frequency-dependent response to IFN-β [2, 41]. Furthermore, the Once Weekly Interferon for MS (OWIMS) study found limited clinical benefits of IFN-β-1a treatment given once weekly (qw), particularly at the lowest dose (22 µg qw) (Table 1) [42]. Across the MRI endpoints examined, the 22-µg qw dose only showed significant improvement on two measures (p < 0.01), while the 44-µg qw dose showed significant improvements over placebo on all measures (p < 0.01). No significant effects of once-weekly treatment were shown at either dose for clinical outcome measures. These results indicated greater clinical benefits of treatment with IFN-β-1a when administered tiw than qw [2, 42]. Given these and other findings from pharmacodynamic studies, the EVIDENCE trial was designed to compare the efficacy of the sc preparation of IFN-β-1a dosed tiw to the im preparation of IFN-β-1a dosed qw [2, 41].
EVIDENCE was an active-controlled, assessor-blinded study that aimed to determine the impact of dosage, dosing frequency, and administration method on the efficacy of two forms of IFN-β-1a [43]. People with relapsing MS were randomized to a “high-dose” regimen of IFN-β-1a sc 44 µg tiw or a “low-dose” regimen of IFN-β-1a via im injection 30 µg qw for 1–2 years [43]. The primary clinical endpoint was the proportion of patients who remained free from relapses, and a higher proportion of patients in the IFN-β-1a sc 44-µg tiw group were found to remain relapse-free compared with the IFN-β-1a im 30-µg qw group at both 24 and 64 weeks [55]. Annualized relapse rate (ARR) was lower and time to first relapse was longer in the IFN-β-1a sc 44-µg tiw group compared with the IFN-β-1a im 30-µg qw group at the end of the comparative phase. MRI measures of disease activity were similarly reduced with the high-dose high-frequency regimen. The study also included a second phase, during which the group that changed from qw im to tiw sc dosing had a 50% reduction in mean relapse rates (p < 0.001) and significant reductions in MRI activity versus the comparative phase of the study. During the same time period, the group that had remained with tiw sc dosing from the comparison phase had a 26% reduction in mean relapse rates (p < 0.028) [43].
The REbif vs Glatiramer Acetate in Relapsing MS Disease (REGARD) study, a head-to-head comparison of two available options for MS treatment, compared IFN-β-1a to glatiramer acetate (GA) over 96 weeks in patients with relapsing MS [44]. Eligibility criteria included a diagnosis of RRMS, and patients were required to have at least one relapse within 12 months previous to the study [44]. Participants were randomized to open-label IFN-β-1a sc 44 µg tiw (n = 386) or GA sc 20 mg once daily (n = 378) [44]. Time to first relapse, the primary efficacy measure, was not significantly different between the treatment groups. Likewise, no significant differences were observed in the numbers of T2 active lesions (defined as new or enlarging T2 lesions), the proportion of scans with T2 active lesions, or change in T2 lesion volume between groups, although there were significantly fewer Gd+ lesions with IFN-β-1a compared with GA. It should be noted, however, that approximately one-third (34%) of patients had only one attack in the 24 months before enrollment and that the overall relapse rate observed in this study was lower than expected. With some patients likely having less disease activity at baseline due to changed diagnostic criteria as well as the low relapse rates observed during the study, the study may have been underpowered to detect differences between the two treatment groups.
A new formulation of IFN-β-1a sc was developed without serum-derived components (i.e., human serum albumin and fetal bovine serum) in order to improve tolerability and reduce immunogenicity [56, 57]. A single-arm study of the new formulation found that, compared with historical data from the EVIDENCE trial, fewer patients developed neutralizing antibodies (NAbs) [57]. The new formulation has demonstrated similar pharmacokinetics and efficacy to the previous formulation, with the potential for a lower incidence of injection-site reactions, though there may be a higher incidence of influenza-like symptoms [57]. This new formulation is approved for use in the European Union (EU) [58].
The new formulation was also evaluated with the Investigating MRI Parameters with Rebif imprOVEd formulation (IMPROVE) study (NCT00441103) [45]. This study tested the short-term efficacy of the new formulation in patients with relatively active RRMS, defined as having at least one clinical event and at least one Gd+ MRI lesion within 6 months before randomization [45]. Patients were randomized to either IFN-β-1a sc 44 µg tiw (n = 120) or placebo (n = 60) for 16 weeks, after which all patients received IFN-β-1a sc 44 µg tiw for 24 weeks. At week 16, the mean number of CUA lesions was significantly lower with IFN-β-1a sc 44 µg tiw than with placebo (p < 0.001; 69% fewer lesions), and 53.3% of patients receiving IFN-β-1a had no CUA lesions compared to 16.7% of those receiving placebo. Post hoc analysis revealed that the mean cumulative number of CUA lesions was lower with IFN-β-1a compared to placebo by week 4 of treatment (p = 0.015) [45].
Initial studies of IFN-β-1a examined lower doses than those which ultimately demonstrated consistent efficacy. Early studies utilized doses starting at only 22 μg qw. Later studies examined both the 22-μg and 44-μg doses given qw and then tiw. Longer-term experience showed the superiority of the higher 44-μg tiw dose over the lower 22 μg tiw dose (Sect. “Extension Studies”).
Extension Studies
The initial PRISMS and EVIDENCE studies were of relatively short duration and, given the chronic nature of the disease, it was clear that research into the longer-term effects of therapy was needed. Through several extensions, PRISMS examined the possibility of sustained treatment benefits and safety as well as the role of early versus delayed treatment for MS [53].
The first extension of the PRISMS study, PRISMS-4, added 2 years of dose-blinded assessment to the original 2 years of observation [46]. Participants who received placebo in the original study were re-randomized to either IFN-β-1a sc 22 µg tiw (n = 85, placebo/22 group) or IFN-β-1a sc 44 µg tiw (n = 87, placebo/44 group), forming two crossover groups. Those who received IFN-β-1a in PRISMS continued into the extension at their originally assigned dose (IFN-β-1a 22 µg, n = 167; IFN-β-1a 44 µg, n = 167) [41]. Each crossover group (from placebo to 22 µg tiw and from placebo to 44 µg tiw) experienced reductions in relapse count, MRI activity, and lesion-burden accumulation with IFN-β-1a compared with their prior 2 year placebo period (p < 0.001 for both doses). Maintenance of the blind for treatment allocation in the first 2 years allowed this extension study to compare the efficacy of delayed treatment (i.e., IFN-β-1a in years 3 and 4 following 2 years on placebo) with earlier treatment (i.e., IFN-β-1a in all 4 years) over the longer term. Relapse rates were calculated as the number of relapses per year for each of the 4 years of the study. Therefore, the impact of delayed treatment initiation could be assessed compared with early treatment. Relapse rates were lower in the groups that received IFN-β-1a sc for 4 years (early treatment) compared with the combined placebo to IFN-β-1a crossover groups (Table 1). New T2 lesion number and lesion burden were also lower, and time to sustained disability was longer, in the IFN early treatment group compared to the delayed treatment group. These findings suggest that patients who started treatment earlier with IFN-β-1a sc 44 µg tiw experienced better outcomes after 4 years than those who delayed starting IFN-β-1a sc 44 µg tiw. This was particularly true with regard to disease progression, where the delayed therapy cohort did not experience the benefits seen in the early treatment group.
The next extension study, PRISMS-7/8, provided up to 8 years of follow-up data on safety, clinical, and MRI outcomes in a subset of patients from the PRISMS study [59]. Of the 68.2% included from the original PRISMS study cohort (n = 382/560), 72.0% (275/382) were still receiving IFN-β-1a sc tiw. Approximately one-third (31.3%, 175/560) of the patients in the original PRISMS study cohort progressed by 2 EDSS points. Progression to an EDSS score of 4.0 occurred in 28.9% (37/128) of patients originally randomized to 22 µg sc tiw (who received the 22-µg dose for at least 4 years), 23.9% (32/134) of those randomized to 44 µg sc tiw (who received the 22-µg dose for at least 4 years), and 27.6% (37/134) of the late treatment group (who received placebo for 2 years, then 22 µg or 44 µg sc tiw for 2 years). Relapse rate was lower for patients originally randomized to 22 µg sc tiw (0.63; rate ratio = 0.81; p < 0.001) or 44 µg sc tiw (0.60; rate ratio = 0.73; p = 0.014) compared to the late treatment group (those originally randomized to placebo, 0.78).
Similarly, relapse-free status at 7–8 years was more common in the group originally randomized to 44 µg sc tiw than 22 µg sc tiw or late treatment (15.4% [21/136], 8.1% [10/123], and 6.5% [8/123] relapse-free, respectively). Finally, median percentage increase in T2 burden of disease was lower at follow-up in those originally randomized to 44 µg sc tiw compared with the late treatment group (5.0 vs 24.5, p = 0.002). Although interpretation of this or any long-term study is limited by potential bias from patient withdrawals, such as differences in treatment response or side effects between patients who return and those who do not, this study supports long-term benefits of early treatment, particularly with the 44 µg sc tiw dose.
The final follow-up, 15 years after the original randomization (PRISMS-15), included 291 of the 560 patients randomized in the PRISMS study. Higher cumulative dose exposure and longer treatment time were associated with better outcomes in ARR, number of relapses, time to EDSS progression, change in EDSS, and time to conversion to SPMS [60]. This study continued to support the benefits of long-term use of the 44 µg sc tiw dose.
Cognition
The negative impact of MS on cognition is difficult for patients and care partners to quantify and is challenging for providers to effectively address in clinical practice. As a consequence, the impact of DMTs on cognitive measures is not well studied compared with measures of physical disability and MRI activity.
The open-label COGnition Impairment in MUltiple Sclerosis patients (COGIMUS) study examined the effect of IFN-β-1a sc (22 μg and 44 μg tiw) on validated neuropsychological measures in patients with RRMS [61]. The proportion of patients with impaired cognitive function, defined as impaired cognitive function on at least three tests, was generally stable over 2 years; 21.4% of patients at baseline and 21.6% of patients at 2 years in the overall study population had impaired cognitive function based on at least three tests. However, the proportion of patients showing impairment on three cognitive tests at 2 years was lower in the 44-μg group (17.0%) compared with the 22-μg group (26.5%; p = 0.034) [61]. Similar results were observed at 5-year follow-up [62].
A 2-year post-authorization observational study evaluated cognition and fatigue in patients with RRMS treated with Rebif® (SKORE study) (NCT01075880) [63]. All patients received IFN-β-1a sc at either 22 μg or 44 μg tiw. Cognition status was assessed with the Paced Auditory Serial Addition Task (PASAT). The proportion of participants with PASAT scores that remained stable or improved was 61.4% at the end of the 2-year follow-up period. Results were similar for fatigue, measured by the Fatigue Descriptive Scale, and disability, measured by the EDSS. In a separate study of patients with early RRMS (diagnosed within 6 months before recruitment), IFN-β-1a sc 44 μg tiw had significant benefits on PASAT scores over 2 years; in baseline Gd+ patients, scores increased from 40.6 at baseline to 46.8 (p = 0.027) [64]. While the data are limited, there is some evidence for a positive effect of IFN-β-1a sc 44 μg tiw on cognitive outcomes and fatigue.
Key Trials with IFN-β-1a as an Active Comparator
As further effective treatments for MS became available, for ethical reasons, placebo-controlled trials were replaced with active-controlled trials, often using IFN-β-1a sc as a “standard of care” comparator because of its long history of use and evidence of efficacy and safety.
The Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis (CARE-MS I) study (NCT00530348) was a randomized, rater-masked, controlled phase 3 trial that examined alemtuzumab 12 mg/day via intravenous (iv) injection (n = 376) for treatment-naïve patients with RRMS compared to IFN-β-1a sc 44 µg tiw (n = 187) [47]. Over 2 years, 22% of the alemtuzumab group and 40% of the IFN-β-1a 44-µg group had at least one relapse. More patients were relapse-free in the alemtuzumab group versus IFN-β-1a (77.6% vs 58.7%, p < 0.0001). However, rates of sustained accumulation of disability and mean EDSS score improvement were not different between the treatment groups.
The second phase 3 trial of alemtuzumab, CARE-MS II (NCT00548405), enrolled patients who had recently relapsed while receiving a standard DMT, either IFN-β or GA [47]. As the entry criteria included at least one relapse while on IFN-β or glatiramer after at least 6 months of treatment, a substantial proportion of the study population may have been partial responders or nonresponders to IFN-β. Eligible participants were initially randomized to alemtuzumab 12 mg (n = 436), alemtuzumab 24 mg (n = 173), or IFN-β-1a sc 44 µg tiw (n = 231); however, the alemtuzumab 24 mg/day group was discontinued so that additional eligible patients could be recruited into the other treatment arms. In this population, rates of relapse and sustained accumulation of disability were lower in the alemtuzumab 12-mg group compared with the IFN-β-1a group (Table 1). Overall, 35% of patients in the alemtuzumab group and 51% of patients in the IFN-β-1a group relapsed (rate ratio 0.51, p < 0.0001), corresponding to a 49.4% improvement with alemtuzumab. A total of 13% of the alemtuzumab group and 20% of the IFN-β-1a group had sustained accumulation of disability (hazard ratio 0.58, p = 0.0084), a 42% improvement in the alemtuzumab group.
The TErifluNomidE and REbif® (TENERE) study (NCT00883337) was a randomized, rater-blinded, controlled phase 3 study of teriflunomide in 324 patients with relapsing MS that included IFN-β-1a sc as an active control [48]. Exclusion criteria included previous use of teriflunomide, IFN-β-1a and other IFNs, and several other DMTs. Time to failure, defined as first occurrence of confirmed relapse or permanent treatment discontinuation for any cause, was not different between the teriflunomide 7-mg or 14-mg and IFN-β-1a 44-µg sc tiw groups (Table 1). ARR also did not differ between the teriflunomide 14-mg and IFN-β-1a groups, although ARR was significantly higher in the teriflunomide 7-mg group compared with the IFN-β-1a group.
The OPERA (ocrelizumab in comparison with interferon-β-1a [Rebif®] in participants with relapsing multiple sclerosis) I (NCT01247324) and II (NCT01412333) studies were identical in design. These phase 3 double-blind, double-dummy, randomized trials compared ocrelizumab 600 mg iv every 24 weeks and IFN-β-1a sc 44 µg tiw for 96 weeks [52]. Exclusion criteria included previous use of some DMTs, including ocrelizumab, as well as intolerance of IFN-β or use of IFN-β within 4 weeks prior to baseline. ARR was lower with ocrelizumab compared with IFN-β-1a in both studies (p < 0.001 for both trials). The percentages of patients with confirmed disability progression at 12 and 24 weeks were lower for ocrelizumab in pooled analyses. Mean numbers of Gd+ lesions per T1-weighted MRI scan and new or newly enlarged hyperintense lesions per T2-weighted MRI scan were lower for ocrelizumab compared with IFN-β-1a in both studies. In OPERA I, patients in the ocrelizumab group experienced a 22.8% lower loss of brain volume than those in the IFN-β-1a group from week 24 to 96 (p = 0.004), while in OPERA II, the ocrelizumab group showed 14.9% less brain volume loss (p = 0.09).
The varied results of these studies may arise from different comparator therapies and from different measures assessed, illustrating the complexity of treating MS. Nonetheless, the proliferation of DMT options since the 1990s has given both patients and clinicians multiple choices for personalizing therapy for a given patient, weighing the potential drawbacks and benefits of each treatment. Factors to take into consideration include those pertaining to the treatment itself, such as effectiveness and tolerability, as well as patient factors such as risk tolerance or aversion and comorbidities.
Efficacy in Other MS Settings
Clinically Isolated Syndrome
Clinically isolated syndrome (CIS) is a first episode of neurologic symptoms, similar to an MS exacerbation, that lasts at least 24 h and which is caused by inflammation or demyelination in the CNS. Individuals with CIS may or may not go on to be diagnosed with MS, and the criteria for an MS diagnosis continue to be revised to allow clinicians to diagnose MS earlier and more accurately. However, an unintended effect of these revisions is that some patients who entered studies with a diagnosis of CIS under earlier definitions might now be classified as having MS [49, 50, 65–67]. It is likely that some of the participants in the following studies of IFN-β-1a sc in the treatment of CIS would currently be diagnosed with MS rather than CIS.
The Early Treatment of MS (ETOMS) study enrolled patients between 1995 and 1997 [66]. Eligibility criteria included having a first episode of neurological dysfunction suggesting MS, at least one abnormality evident during neurological examination, and a positive brain MRI scan, defined as at least four white matter lesions on T2-weighted scans, or at least three white matter lesions, if at least one was infratentorial or Gd+ [66] (Table 1). Participants were randomized to the low dose of 22 µg IFN-β-1a sc given once per week or placebo. Fewer participants in the IFN group developed clinically definite MS (CDMS) compared with the placebo group. For the primary outcome measure, the conversion to CDMS (defined by the occurrence of a second exacerbation), the time at which 30% of patients had converted to CDMS was longer in patients treated with IFN-β-1a (569 days) compared to placebo (252 days, p = 0.034). In addition, ARR, number of new T2-weighted MRI lesions, and lesion burden were significantly lower in patients treated with IFN-β-1a compared to placebo. The low dose of IFN-β-1a used in this study and the stringent eligibility criteria, which likely included a large proportion of participants who would meet current criteria for MS [65], may have contributed to the comparatively small treatment effects observed in this study.
The phase 3 REbif FLEXible dosing in early MS (REFLEX) study (NCT00404352), conducted from 2006 to 2010, examined participants with a single clinical event suggestive of MS and at least two clinically silent T2 lesions on brain MRI [49]. The REFLEX study compared the serum-free formulation of IFN-β-1a sc 44 μg tiw (n = 171) or qw (n = 175) to placebo (n = 171) for up to 24 months. The 2-year cumulative probability of a diagnosis of MS, as defined by the 2005 McDonald criteria, was lower in patients treated with IFN-β-1a sc tiw compared to placebo [49]. The risk of conversion to MS was lower in the group treated three times per week compared with those who received once weekly treatment [49]. Two-year rates of conversion to CDMS were lower for both IFN-β-1a sc tiw and qw than for placebo [49].
The REFLEX study found that the mean number of MRI CUA lesions was lower per patient in those treated with IFN-β-1a sc compared with those given placebo, with risk reductions of 81% for IFN-β-1a sc tiw vs placebo and 63% for IFN-β-1a sc qw (p < 0.001) [67]. The mean number of CUA lesions per patient was 48% lower in the group treated three times per week compared with those who received once weekly treatment (IFN-β-1a sc 44 μg tiw vs IFN-β-1a sc qw, p = 0.002) [67]. New T2 lesions, T1 hypointense lesions, and Gd+ lesions were also lower in the IFN-β-1a sc groups vs placebo group (p ≤ 0.004) and in the IFN-β-1a sc tiw group vs the IFN-β-1a sc qw group (p ≤ 0.012).
Participants who completed the 24-month double-blind REFLEX study were eligible to enter the REbif FLEXible dosing in early MS extensION (REFLEXION) study [50]. During the REFLEX study, any participant diagnosed with CDMS was switched to open-label IFN-β-1a sc 44 μg tiw. Those in the placebo group of the REFLEX study who did not reach a diagnosis of CDMS received IFN-β-1a sc 44 μg tiw in the REFLEXION study, forming a delayed treatment (DT) group. Those in the IFN-β-1a sc 44-μg tiw or qw groups continued original treatment and comprised the early treatment (ET) group. For this study, all p values are considered nominal as statistical analyses were exploratory. At the end of REFLEXION, the cumulative probability of progression to CDMS was reduced for the IFN-β-1a sc 44-μg tiw ET group compared to the DT group (for IFN-β-1a sc 44 μg qw, nominal p = 0.084 vs DT; for IFN-β-1a sc 44 μg tiw, nominal p = 0.032 vs DT) (Table 1). The cumulative probability of meeting 2005 McDonald criteria was also lower in the IFN-β-1a sc 44-μg tiw group compared with the DT group, as were mean cumulative numbers of new T2, Gd+, and T1 hypointense lesions, as well as T2 and T1 hypointense lesion volume changes (nominal p < 0.05 for all). Further analysis of the REFLEX/REFLEXION studies was performed using the endpoint of no evidence of disease activity (NEDA-3). At 2 years, the group given ET with either sc IFN-β-1a tiw or qw were both more likely to achieve NEDA-3 than the DT group. However, by years 3 and 5, only the IFN-β-1a sc tiw dose group was more likely to achieve NEDA-3 than the DT group, supporting greater efficacy of the 44-μg tiw dose over time [68].
Secondary Progressive Multiple Sclerosis
SPMS is defined by gradual worsening with or without superimposed relapses. Originally considered a separate clinical course from RRMS, SPMS is currently considered part of the continuum of symptomology and neurological damage that defines MS. IFN-β-1b was the first DMT to demonstrate efficacy in the treatment of SPMS [69]. In a European study in which most patients had experienced at least one relapse in the 2 years prior to the study, treatment with IFN-β-1b (8 million IU every other day sc) increased time to confirmed progression of disability, reduced rates of confirmed progression, and had benefits on other clinical and MRI parameters [69]. However, in a second, North American, study of IFN-β-1b in which more than half of the patients had not experienced a relapse in the 2 years prior to the study, there was no significant treatment effect on time to confirmed progression [70]. In line with previous studies in RRMS, however, benefits were observed for the treatment groups compared with the placebo group on some measures related to relapses as well as MRI measures.
Examination of IFN-β-1a in SPMS yielded similar results to those of IFN-β-1b. The Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-beta-1a in MS (SPECTRIMS) study compared IFN-β-1a sc (22 or 44 μg tiw) to placebo in the treatment of SPMS [51]. Eligible patients had clinically definite SPMS, with or without exacerbations, following an initial relapsing–remitting course, with a baseline EDSS of 3.0–6.5 and pyramidal functional score ≥ 2. More than half of the patients in this study (53%) had not experienced a relapse in the 2 years prior to the study. Time to confirmed progression in disability did not differ between the IFN-β-1a sc group and the placebo group (p = 0.146), although relapse rates were reduced for both IFN-β-1a sc dose groups (p < 0.001 for both vs placebo). Exploratory post hoc subanalyses suggested a greater benefit of treatment with IFN-β-1a sc in patients who had experienced at least one exacerbation in the 2 years before the study. Although the interaction between treatment and presence of before-study exacerbations was not significant (p = 0.289, Cox PH), the odds ratio for progression in the IFN-β-1a group, compared with the placebo group, was 0.52 for patients with before-study relapses (95% CI 0.29–0.93; p = 0.027) and 1.07 for those without (95% CI 0.64–1.78, p = 0.802). HR for time to first progression for patients in the IFN-β-1a group with before-study relapses was 0.74 compared with the placebo group (p = 0.055) and 1.01 for those without (p = 0.934); in patients treated with 44-μg IFN-β-1a, HRs for time to progression were 0.76 for relapsing (p = 0.14) and 0.93 for non-relapsing patients (p = 0.69) [51].
A cohort of patients treated with IFN-β-1a sc (22 or 44 μg tiw) in the SPECTRIMS study with regular MRI assessments had lower median numbers of active lesions and less accumulation of disease burden [71]. Additional subgroup analysis found that the treatment benefits were more pronounced in patients who had experienced at least one exacerbation in the 2 years before the study.
Although the SPECTRIMS study did not show a benefit of IFN-β-1a treatment on time to confirmed progression in patients with SPMS overall, there was a treatment benefit for patients with SPMS who were still experiencing relapses. The evidence for both IFN-β-1a and IFN-β-1b in the treatment of SPMS supports a positive impact of IFN-β for patients with SPMS who are experiencing relapses (active SPMS per the Lublin 2013 definition [72]). However, patients with SPMS who are no longer experiencing relapses (non-active SPMS per the Lublin 2013 definition [72]) are less likely to benefit from IFN-β treatment on the basis of these outcome measures. These studies may reflect IFN-β effects on the predominantly inflammatory pathology of relapsing MS versus the predominantly neurodegenerative pathology of non-relapsing SPMS.
Pediatric Populations
MS onset before 18 years of age is relatively rare [73, 74]. The volume of research on pediatric MS is limited compared to adult-onset MS and is often observational [74]. Consequently, the DMTs commonly used for adults are often prescribed for pediatric MS because of clinician familiarity and lack of approved pediatric MS therapies [12, 73]. IFNs and GA, with well-known efficacy and safety profiles, have been preferred in the pediatric MS population [12, 73–76]. A consensus statement from the International Pediatric MS Study Group supports these agents as first-line DMTs [77].
However, clinical trials of other DMTs in pediatric populations are becoming available, including PARADIGMS, which compared fingolimod to im IFN-β-1a [78]; TERIKIDS, which examined teriflunomide [79]; FOCUS and the extension study CONNECTED, which examined dimethyl fumarate [80, 81]; and CONNECT, which compared dimethyl fumarate to IFN-β-1a [82]. Studies of ocrelizumab (NCT04075266) and alemtuzumab (NCT03368664) in children and adolescents with RRMS are also underway. Teriflunomide is approved in the EU for the treatment of both adult and pediatric patients with RRMS aged 10 years and older [83].
The retrospective, multicenter REPLAY study of IFN-β-1a sc (Rebif®) (NCT01207648) examined the health care records of 307 patients who had received at least one injection of IFN-β-1a sc for demyelinating events before the age of 18 years, between 1997 and 2009 [74, 84]. At treatment initiation, most patients were receiving adult doses of IFN-β-1a sc (either 22 µg tiw or 44 µg tiw) [84]. The before-treatment ARR of 1.79 decreased to 0.47 during treatment, and IFN-β-1a sc was generally well tolerated, with a safety profile in younger patients similar to that in adults. However, these data are limited by the study’s retrospective nature and lack of a control group.
The prospective, observational, multicenter quality of liFe in adolescent sUbjecTs affected by mUltiple sclerosis treated with immunomodulatoRy agEnt using self-injecting device (FUTURE) study of IFN-β-1a sc (22 µg tiw) using the RebiSmart® autoinjector assessed self-reported and parent-reported quality of life (QoL) in 50 Italian adolescents (12 to 16 years of age) with RRMS, between 2012 and 2014 [85]. The Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL) was completed by participants and their parents at baseline and at visits throughout the 52-week study. Treatment with IFN-β-1a sc led to increased adolescent self-reported total PedsQL scores and subscale scores, except for the Emotional Functioning subscale. There were significant improvements in parent-reported total PedsQL scores (p = 0.041), Psychosocial Health Summary scores (p = 0.015), and School Functioning subscale scores (p = 0.029) as well. The lack of a control arm and the small number of participants are limitations; however, these results suggest that IFN-β-1a sc and use of the RebiSmart™ autoinjector may be associated with improved QoL among adolescents with RRMS.
In 2018, orally administered fingolimod (Gilenya®) was approved for use in RRMS for individuals under the age of 18 and older than 10 years [74]. In the phase 3 PARADIGMS study (NCT01892722), patients 10 to 17 years of age with relapsing MS were randomized to orally administered fingolimod (0.5 mg per day [0.25 mg/d for patients with body weight ≤ 40 kg]) or IFN-β-1a im (30 μg/week) for up to 2 years [78]. At or before 24 months, the primary endpoint (ARR over the period of active treatment) was lower with fingolimod (0.12) compared with IFN-β-1a im (0.67) (absolute difference, 0.55 relapses; relative difference, 82%; p < 0.001). To date, orally administered fingolimod and teriflunomide are the only DMTs approved in the USA for people with RRMS under the age of 18 [74, 86]. Additional studies are needed to determine the safety and efficacy of other DMTs in this population.
Safety
As a class, IFN-βs have an established safety record and are well tolerated, with mostly mild adverse events (AEs) [38]. Substantial clinical trial and clinical evidence derived from post-marketing or post-approval experience supports the long-term safety and favorable risk–benefit balance of IFN-β-1a sc in approved indications [12, 33]. IFN-β-1a sc has demonstrated a favorable safety profile, with an estimated cumulative exposure of 1,936,801 patient-years in the post-marketing setting as of May 2023 [Data on file, EMD Serono Inc., Rockland, MA USA, an affiliate of Merck KGaA, 2023]. The 20 years of clinical trial experience with IFN-β-1a sc includes the OWIMS, PRISMS, EVIDENCE, REGARD, and SPECTRIMS studies, as well as the extension studies, PRISMS-4, 7/8, and 15 [2, 41, 42, 44, 46, 51, 55, 59, 60].
The most commonly reported AEs observed in clinical trials of IFN-β-1a sc, as listed in the prescribing information, were injection site reactions, influenza-like symptoms, abdominal pain, depression, elevation of liver enzymes, and hematologic abnormalities [2, 28]. The placebo-controlled PRISMS study found that influenza-like symptoms, injection-site reactions, fatigue, myalgia, and fever were reported during the first 3 months of therapy with the interferon and placebo groups, but only injection-site reactions were more common in the IFN-β-1a sc groups compared with placebo. Among laboratory abnormalities reported during the first 3 months of therapy, lymphopenia, increased alanine aminotransferase, leukopenia, and granulocytopenia were more common in the IFN-β-1a sc 44-μg tiw group than the placebo group; no significant differences between the IFN-β-1a sc 22-μg tiw and placebo groups were reported [41]. Similarly, the OWIMS study found that, for the IFN-β-1a sc 44-μg qw dose, influenza-type symptoms, headache, fever, chills, and injection site inflammation were significantly more common in the IFN-β-1a sc 44-μg qw group compared with placebo, but no significant differences in rates of abnormal liver function tests were observed [42]. The EVIDENCE study examined patients who were either continuing on IFN-β-1a sc 44 μg tiw or switching from IFN-β-1a im 30 μg qw to IFN-β-1a sc 44 μg tiw [43]. No significant differences were observed between the post-transition groups in rates of injection-site reactions (inflammation or rash), flu-like symptoms (fever, fatigue, myalgia), liver function abnormalities, ALT elevations, or white blood cell abnormalities. The REGARD study found significantly higher rates of influenza-like illness, headache, myalgia, and increased ALT in the IFN-β-1a sc 44-μg tiw group compared with the glatiramer acetate 20-mg sc od group [44]. Rates of dyspnea, immediate post-injection drug reactions, and injection-site pruritus, swelling, and induration were lower in the IFN-β-1a sc 44-μg tiw group than in the glatiramer acetate sc 20-mg od group.
Depression is more frequent among people with MS, so psychological status is of particular concern during MS treatment [41]. The PRISMS and OWIMS studies found no significant differences in rates of depression between the IFN-β-1a sc study groups and the placebo group [41, 42]. The REGARD study found no significant differences in rates of depression between the IFN-β-1a sc 44-μg tiw and glatiramer acetate sc 20-mg od groups [44].
In general, AEs tend to decrease over time with IFN-β-1a sc treatment [2]. Recent examination of post-approval spontaneously reported AEs found no new safety concerns (cumulative to May 2023) [Data on file] and no increased risk of COVID-19 (cumulative to April 2022) in patients treated with IFN-β-1a sc [87]. IFN-β-1a sc has not been associated with progressive multifocal leukoencephalopathy. Moreover, IFN-β has not been associated with increased malignancy risk [88–90].
Pregnancy and Breastfeeding
MS often manifests during a patient’s reproductive years, impacts more women than men, and is generally treated long-term, leading to concerns about potential treatment effects on pregnancy and breast-feeding. In animal studies, IFN-β in high doses was associated with an increased risk for abortion [26, 27, 29, 91].
Although some small, early studies suggested a potential decrease in birth weight with maternal IFN-β exposure during pregnancy, larger observational studies did not support this finding (Table 2) [28, 92–97]. In addition, these later studies did not show any association with negative pregnancy outcomes, such as spontaneous abortions, major congenital anomalies, or developmental abnormalities [97–101]. Examination of 948 pregnancies with known outcomes reported in the European IFN-beta Pregnancy Registry revealed no evidence that IFN-β exposure affected the rate of congenital anomalies or spontaneous abortions compared to the general population [98]. Most IFN exposures occurred either before conception (13.0%) or during the first trimester (82.6%), with fewer exposures reported in the second (3.3%) or third (1.2%) trimesters.
Table 2.
Key studies of pregnancy outcomes after interferon exposure
| Study and timing | Design and population | Outcomes |
|---|---|---|
| Amato et al. [97] |
Cohort study Study groups: Patients • 87 women (88 pregnancies) who discontinued IFN-β < 4 weeks before conception (exposed) • 311 women (318 pregnancies) who discontinued IFN-β ≥ 4 weeks before conception or who were never treated (not exposed) |
• IFN-β exposure was not related to spontaneous abortion (OR 1.08, 95% CI 0.4 to 2.9, p = 0.88) • IFN-β exposure was associated with lower baby weight (β = − 113.8, 95% CI − 114.1 to − 113.5, p < 0.0001) and length (beta = − 1.102, 95% CI − 1.366 to − 0.839, p < 0.0001) • IFN-β exposure was not associated with an increased risk of birth weight < 2500 g) (OR 1.14, 95% CI 0.41 to 3.15, p = 0.803) |
| Boskovic et al., 2005 [92] |
Longitudinal, cohort study with two control groups Study groups: women who were • Exposed to IFN (to be detrimental 12 women with 21 pregnancies) • Healthy comparative group (18 women with 20 pregnancies) |
• The study group was exposed to IFN-β-1a (Avonex®, Rebif®) or IFN-β-1b (Betaseron®) • Mean birth weight was decreased in the exposed group vs healthy controls (3189 ± 416 g with IFN vs 3783 ± 412 g in controls; p = 0.002) • Women exposed to IFN-β had a higher rate of miscarriages and stillbirths vs healthy controls (39.1% vs 5%, respectively; p = 0.03) |
| Fragoso, et al., 2013 [93] |
Retrospective chart review Mothers with MS (n = 180) • 95 unexposed during pregnancy • 85 exposed to DMTs for ≥ 2 weeks during pregnancy |
Of mothers exposed to DMTs, 39 were exposed to GLAT, 39 to IFN-β (19 IFN-β-1a at 30 µg/week [Avonex®], 10 to IFN-β-1b at 10–250 µg on alternate days [Betaseron®], and 10 to IFN-β-1a at 22 or 44 µg tiw [Rebif®]) • There were also 3 exposures to methylprednisolone, 2 exposures to immunoglobulin, 1 exposure to azathioprine, and 1 exposure to rituximab The authors noted • No pattern of drug-related adverse events or complications in the children whose mothers were exposed to DMTs • No specific long-term adverse events observed in the offspring of women with MS who were exposed to drugs during pregnancy |
| Hellwig et al., 2010 [94] |
Nationwide questionnaire and patients within the authors’ outpatient clinic Children of • MS-fathers with DMT (n = 40) • MS-mothers without DMT (n = 75) • MS-mothers treated with IFN-β at the time of conception (n = 75) • Healthy mothers (n = 75) |
Of pregnancies that were fathered by patients with MS receiving DMTs (32 paternities of 46 pregnancies), the fathers were treated with • 15 IFN-β-1a im • 7 IFN-β-1b sc • 12 under glatiramer acetate • 5 IFN-β-1a im 22 μg • 3 IFN-β-1a im 44 μg • 2 natalizumab • 1 methotrexate • 1 combination of • Azathioprine and IFN-β-1b sc Mean birth weight of newborns from MS fathers treated with DMTs was not significantly reduced vs those from healthy mothers Mean birth weight of newborns of MS mothers was significantly reduced vs MS-fathers whether or not the MS-mothers were treated with IFN-β No statistical difference was observed in birth weight of IFN-β-exposed vs untreated MS-mothers |
| Hellwig et al., 2012 [95] |
Observational, partially retrospective Pregnant women with MS • 78 pregnancies exposed to IFN-β (n = 15 IFN-β-1b, n = 63 IFN-β-1a) • 41 pregnancies exposed to GLAT • 216 non-DMT-exposed pregnancies |
No differences were observed between groups regarding • Birth weight (IFN-β-exposed mothers, 3260 ± 606 g; GLAT-exposed mothers, 3295 ± 688 g; and non–DMT-exposed mothers, 3383 ± 544 g) • Newborn body length (IFN-β-exposed mothers, 51.0 ± 2.3 cm; GLAT-exposed mothers, 51.5 ± 2.7 cm; and non-DMT-exposed mothers 51.4 ± 2.6 cm) • Gestational age (IFN-β-exposed mothers, 38.9 ± 2.4 gw; GLAT-exposed mothers, 39.2 ± 1.7 gw; and non-DMT-exposed mothers, 39.1 ± 2.3 gw) An increased risk for abnormalities in neonates was not observed for DMT-exposed mothers |
| Korjagina et al., 2021 [96] |
Register-based cohort study 2115 pregnancies • Exposed only to IFN-β (n = 718) • Unexposed to any DMTs (n = 1397) |
Serious adverse pregnancy outcomes occurred in • 4.3% (95% CI 1.9–8.3) of pregnancies exposed only to IFN-β 6 months before or during pregnancy • 2.7% (95% CI 1.2–5.0) of unexposed pregnancies The prevalence of serious and other adverse pregnancy outcomes was not significantly different between the exposed and unexposed groups |
DMT disease-modifying therapy, GLAT glatiramer acetate, IFN interferon
A study of Finnish and Swedish (2005–2014) national registry data examined birth weight, length, and head circumference of infants born to women with MS exposed to IFN-β (411 pregnancies in Sweden and 232 in Finland) and those unexposed to any DMT (835 pregnancies in Sweden and 331 in Finland) [99]. There were no significant differences between infants born to women exposed to IFN-β and those unexposed to any DMT. A larger study using the same Finnish (1996–2014) and Swedish (2005–2014) registries examined the prevalence of adverse pregnancy outcomes among pregnant women with MS exposed to IFN-β and those unexposed to any DMT [100]. A study of pregnancy outcomes examined 2831 pregnancies in 1983 women with MS; 797 pregnancies were exposed to IFN-β only, 1647 were not exposed to any DMT, and 328 were exposed exclusively to other DMTs but not to IFN-β. Again, there was no increase in the prevalence of major congenital anomalies, spontaneous abortions, and stillbirths in women exposed to IFN-β. There were insufficient numbers to examine rates by trimester of exposure, and more data are needed to investigate the effects of exposure beyond the first trimester.
Although additional studies are needed, those examining the potential effects of DMT treatment while breastfeeding have supported IFN-β and GA as safe treatment options during pregnancy and breastfeeding in patients requiring MS treatment [102, 103]. The largest real-world study to date on child development and breastfeeding examined 74 infants born to 69 women with MS who breastfed while receiving IFN-β (n = 39), GA (n = 34), or both (n = 1), with data collected during pregnancy and up to 12 months postpartum [104]. The study did not find increased risks of adverse effects on growth, motor and language development, or the proportion of infants hospitalized or requiring systemic antibiotic use.
On the basis of the outcomes of these studies, the EU label for IFN-β-1a sc now specifies that women with relapsing MS can continue treatment with Rebif® during pregnancy if clinically necessary, specifically in the first trimester, and while breastfeeding [29, 100]. Use of IFN-β class drugs in general may now be considered during pregnancy if clinically warranted [100]. In the current US Prescribing Information for Rebif®, the no-longer used ABCDX pregnancy categorization designation has been replaced by data from the Finnish and Swedish register study and other studies to explain the potential risks and benefits of treatment [28]. The prescribing information currently notes that data “have not identified a drug-associated risk of major birth defects with the use of interferon beta during early pregnancy. Findings regarding a potential risk for low birth weight or miscarriage with the use of interferon beta in pregnancy have been inconsistent.”
Immunogenicity
Recombinant human homologs, including IFN-β, may induce antibodies against self-antigens in some patients over time [16, 105]. The potential resulting NAbs are not known to cause substantial toxicity but remain a significant concern since, in high titers, they have been associated with reduced clinical responses to IFN-β treatments for MS [2, 16, 106].
Estimates of the frequency of NAb development from clinical studies differ because of between-study variables such as differences in IFN-β formulation, route of administration [107], and duration of treatment, as well as NAb measurement techniques and NAb cutoff values that define a positive sample [105]. However, NAbs are more likely to develop with higher and more frequent doses of IFN-β [2, 38]. In addition, NAbs are more likely to develop with IFN-β-1a sc compared with im administration [107–109]. NAbs were most likely to develop in response to IFN-β-1a sc treatment every other day and more likely to develop in response to IFN-β-1a sc tiw than to IFN-β-1a im qw [108].
The clinical picture with regard to the effects of NAbs is complicated in that clinically significant antibodies only develop in a minority of patients and typically appear only after 9–18 months of IFN-β treatment [105]. Conversely, the presence of low-affinity NAbs early in treatment (6–12 months after therapy initiation) may enhance the effect of IFN-β. Therefore, depending on the timing of measurement, the impact of NAbs on IFN-β efficacy is not always predictable or obvious [105]. The effects of low and medium titers are also unclear. Decisions regarding discontinuation of therapy should therefore be made on the basis of clinical activity [105].
No characteristics are known to be reliable predictors of the formation of clinically significant NAbs [105], other than the finding that smokers have a significantly higher risk of developing NAbs to IFN-β-1a than nonsmokers (OR 1.9; 95% CI 1.3–2.8; p = 0.002) [110]. Recent studies have examined potential biomarkers such as serum metabolites and human leukocyte antigen-associated genetic risk factors. These studies found that changes in serum lipids, altered metabolite network associations, and certain HLA haplotypes were all associated with NAb status in patients treated with IFN-β [111, 112].
COVID-19
COVID-19 infection and vaccinations have potential effects on MS and on MS treatment. Importantly, patients receiving IFN-β-1a sc have a reduced risk for serious disease and severe outcomes compared with the general population [113]. Several analyses of people with MS treated with IFN found reduced risks for severe COVID-19 compared with no therapy [114] or compared with pooled DMTs with a moderate/high risk of systemic infection (fingolimod, ocrelizumab, rituximab, cladribine, alemtuzumab) [115]. Reduced rates of hospitalization (compared with population-based controls) or death (in a meta-regression of observational studies) were also observed in patients with MS treated with IFN [114–117].
Regarding vaccination, treatment with IFN-β does not adversely affect the ability to mount an adequate immune response to influenza vaccination [118–120]. Though the numbers of patients examined so far have been small, recent studies have found similar immune responses to COVID-19 vaccination in patients with MS treated with IFN-β and healthy controls [121–124]. The National Multiple Sclerosis Society recommends COVID-19 vaccination for people with MS, including those who are taking DMTs [125]. The MS Society of Canada and the MS International Federation similarly recommend that people with MS consider getting the COVID-19 vaccine in consultation with their health care providers (HCPs) [125, 126]. Those taking IFN-β-1a sc do not need to modify their treatment schedule or delay getting vaccinated against COVID-19.
Some smaller studies have supported IFN-β-1a sc 44 μg tiw as an adjunct treatment for early and late COVID-19 infection [127–129]. However, neither the Adaptive COVID-19 Treatment Trial (ACTT-3, funded by the National Institutes of Health) nor the Solidarity trial (funded by the World Health Organization) found a benefit of IFN-β-1a treatment in patients who were hospitalized for COVID-19 [130, 131]. The phase 3 SPRINTER trial, which used a nebulized formulation of IFN-β, also failed to demonstrate significant benefits in patients hospitalized for COVID-19 [123].
The results of these treatment trials seemingly conflict with the demonstrated real-word protective effects of IFNs. However, many of these trials studied late intervention with interferons, during the hyperinflammatory cytokine storm, instead of early intervention when IFN-β treatment is most likely to be beneficial [128–130]. Given the often unpredictable nature of COVID-19, further testing is needed.
Real-World Evidence
As of 2020, around 25,000 people had been newly diagnosed with MS in the USA each year (incidence rate of 7.9 per 100,000) [132, 133]. Interest in data gained outside of clinical trials, or real-world evidence (RWE), has grown as the number of effective DMTs for MS has increased, and it has become impractical to perform multiple large-scale head-to-head clinical studies. Furthermore, real-world populations include patients who would normally be excluded from clinical trials.
RWE supports the efficacy and safety of IFN-β-1a sc and shows good adherence rates to therapy [134, 135]. The effectiveness of IFN-β-1a sc compared to other DMTs has also been examined. Retrospective cohort studies found no significant differences in ARR and EDSS rates between IFN-β-1a sc, IFN-β-1a im, and GA [136], or in relapse risk or ARR between IFN-β-1a sc and dimethyl fumarate [137, 138]. A propensity score-based comparison of several DMTs found no difference in ARR between IFN-β-1a sc and peginterferon-β-1a [25]. Administrative claims data from 4475 patients showed no differences between IFN-β-1a sc and dimethyl fumarate or fingolimod in terms of risk of relapse [138]. In contrast, some analyses have found greater effectiveness with IFN-β-1a sc than with IFN-β-1a im, IFN-β-1b [139], or teriflunomide [138].
A cohort of 2570 patients with RRMS who had been treated with IFN-β was prospectively followed for up to 7 years in 15 Italian MS centers [140]. Early treatment, defined as starting within 1 year from disease onset, significantly reduced the risk of reaching either a 1-point progression in EDSS score, or reaching an EDSS milestone of 4.0.
A large retrospective US administrative claims study analyzed data from 8107 patients from the Truven MarketScan Commercial and Medicare Supplemental health care claims databases from 2006 to 2012 [141]. The six most common AEs observed with IFN-β-1a sc tiw were influenza-like symptoms, malaise, fatigue, abdominal pain, chest pain, and depression, with incidence rates per 100 patient-years ranging from 15.65 (95% CI 14.96–16.36) for influenza-like symptoms down to 7.75 (95% CI 7.32–8.20) for depression [141]. These real-world findings were in line with the safety profile in the US label for IFN-β-1a sc, based on clinical trial and post-marketing surveillance data [28, 141]. Overall, real-world data have supported the efficacy and safety of IFN-β-1a sc seen in clinical trials.
Autoinjector Devices and Adherence
Adherence to DMTs is a critical factor in ensuring maximum clinical benefit for patients with MS. Poor adherence is associated with worse MS outcomes, including disability progression and relapses [142]. Autoinjector devices reduce injection-site reactions and may thereby improve adherence [143, 144]. The autoinjector devices currently available for dispensing IFN-β-1a sc include the RebiSmart®, Rebiject®, and Rebidose® autoinjectors; RebiSmart® is approved for use in Canada and Europe, Rebiject® is approved for use in the USA, and Rebidose® is approved for use in Europe and the USA. These autoinjectors are associated with good adherence among patients with MS in the SMART and MEASURE studies [142, 145], and the RebiSmart autoinjector is also linked to improved QoL in adolescent patients with RRMS [85].
Current Role of IFN-β-1a SC in MS Treatment
IFN-β has evolved from being the first effective DMT for MS to one within a wide range of treatment options for patients with different clinical and immunological profiles, patient demographics and treatment history, and personal preference.
In clinical studies, IFN-β-1a sc has demonstrated efficacy in relapsing forms of MS, including CIS, RRMS, and active SPMS [41, 43, 49–51, 66, 69, 146]. The tolerability and safety profile of IFN-β make it a good choice for patients who have been unable to tolerate other DMTs, or who are particularly risk averse [12]. Clinical evidence of long-term treatment efficacy and safety suggests that patients with stable disease, plus safety and tolerability during IFN-β treatment, can continue their therapy [12, 33]. IFN-β may also be considered for aging patients in which MS activity declines and long-term immunosuppression associated with some alternative therapies is a concern. Additionally, IFN-β is a viable option in the context of family planning. As mentioned previously, exposure to IFN-β treatment during early pregnancy has demonstrated no negative effects on fetal or maternal outcomes in large registry studies, allowing patients to remain on treatment while trying to conceive. Finally, given the long-term safety and confirmed efficacy data available, IFN-β treatment may be useful as a de-escalation therapy after control of disease activity is established with another agent where long-term toxicity is greater or unknown.
The clinical profile of IFN-β-1a has supported its use in trials as the standard of care/clinically effective comparator for combination therapy with other DMTs and with other potentially beneficial medications and compounds. A study of 118 patients with RRMS, SPMS, or progressive relapsing MS examined teriflunomide (7 or 14 mg) added to ongoing IFN-β treatment [147]. The IFN-β treatment received was not specified; any formulation was acceptable so long as the patient had been on a stable dose. The addition of teriflunomide did not compromise the safety and tolerability of IFN-β treatment and reduced MRI disease activity compared with IFN-β alone. Similarly, a study of patients with active relapsing MS, despite IFN-β treatment, found that the addition of cladribine tablets reduced relapses and MRI lesion activity with a similar safety and tolerability profile to IFN-β alone, except for an increase in lymphopenia [148]. IFN-β, teriflunomide, and cladribine tablets all have different mechanisms of action, suggesting complementary immune effects and clues to the basic immunology of MS.
Vitamin D has been investigated as a potential add-on treatment to IFN-β-1a, as deficiency of this vitamin has been associated with the development of MS and with exacerbations of MS, although the existing data on this have been mixed [149]. The Supplementation of Vigantol Oil versus Placebo Add-on in Patients with RRMS Receiving Rebif Treatment (SOLAR) study examined the effects of high-dose vitamin D (n = 113) compared with placebo (n = 116) [150, 151]. On the primary endpoint, the proportions of patients with NEDA-3 at week 48 were not statistically different between groups. However, there were significant reductions in CUA and changes in volume of T2 lesions in combination with vitamin D3 compared with placebo. Vitamin D was also shown to enhance IFN responses of mononuclear cells from patients with MS in vitro, regardless of whether the patient had previously been treated with IFN-β-1b or was untreated [152]. A subgroup analysis of the Finnish Vitamin D Study showed a statistically significant reduction in T1 lesion numbers, reduced T2 lesion volume growth, and fewer new/enlarging T2 lesions for patients with MS receiving treatment with IFN-β-1b who were also given vitamin D3 versus placebo [153]. Finally, a 1-year, placebo-controlled, double-blind study in 66 patients with MS showed a significant reduction in MRI disease activity in the group given vitamin D3 in addition to IFN-β-1b versus those given placebo and IFN-β-1b [154].
Statins have also been examined as potential add-ons to IFN-β-1a treatment as both statins and IFN-β-1a are thought to have anti-inflammatory effects. Although some studies have found potential benefits of adding statins to IFN-β-1a treatment [155–157], most have found no benefit or have found the combination to be detrimental [3, 158, 159]. It is thought that, although both statins and IFN-β-1a are anti-inflammatory, the mechanisms of actions of these two treatments may interfere, rather than synergize, with each other [3, 149]. It should be noted that the doses of statins used in these studies were lower than those used in the treatment of dyslipidemia [155, 156, 159].
Three decades of research and clinical experience have provided a wealth of data on the long-term safety, efficacy, and real-world effectiveness of IFN-β as well as its mechanism of action. IFN-β-1a sc continues to play a role in research into MS pathology and treatment; it is used as an active comparator to other treatments or as stable background therapy in clinical trials, as reflected in the list of ongoing studies listed in Table 3. IFN-β-1a sc is being employed as an active comparator to the anti-inflammatory neuropeptide combination of metenkefalin and tridecactide (EK-12) (NCT03283397). In the DELIVER-MS and TREAT-MS studies, the efficacy of IFN-β-1a sc as initial therapy in early RRMS will be compared with the efficacy of other treatment regimens (NCT03535298; NCT03500328). Finally, IFN-β-1a sc is part of stable background therapy allowing for the evaluation of BIIB061, an agent that is thought to be neuroprotective and is being investigated as a possible remyelinating agent (NCT04079088).
Table 3.
Active clinical studies including IFN-β-1a sc
| Study title | ClinicalTrials.gov identifier/link | Role of IFN-β-1a sc |
|---|---|---|
| Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS) | NCT03535298 | Possible initial step in escalation therapy |
| Traditional Versus Early Aggressive Therapy for Multiple Sclerosis Trial (TREAT-MS) | NCT03500328 | Active comparator: traditional therapy |
| Effect of Disease Modifying Therapy on Antibody Response to COVID19 Vaccination in Multiple Sclerosis | NCT04834401 | Stable background therapy |
| A Phase IIIb, Multicenter, International Study to Evaluate the Efficacy, Safety and Tolerability of EK-12 in Patients With RRMS | NCT03283397 | Active comparator |
| Study to Evaluate Oral BIIB061 Added to Interferon-beta1 (IFN-β1) or Glatiramer Acetate in Relapsing Multiple Sclerosis (RMS) | NCT04079088 | Stable background therapy |
IFN interferon, MS multiple sclerosis, RRMS relapsing–remitting MS, sc subcutaneous
New Research and Future Perspectives
Owing to the number of DMTs currently approved for treating RRMS and the continuing development of new agents, it is becoming important to identify patient factors that influence the acceptability of a treatment and improve long-term adherence. Achieving this goal requires more than amassing and evaluating clinical evidence. Factors that should be considered when selecting the right treatment include patient comfort and risk tolerance/aversion, comorbidities, perceptions of a medication’s efficacy, as well as consideration of other treatment options, including generic medications. Factors that enhance adherence for a particular patient, including cost, dosing frequency, administration method (e.g., pills vs injections), and impact on family planning should also be factored into treatment decisions. Effectiveness in real-world settings may be of particular interest to HCPs, while tolerability, particularly as reported by patients, may carry more weight for people with MS.
For a disease such as MS, with a highly variable course and few predictors of treatment response, biomarkers are of particular interest to help determine safe and effective therapies for individual patients, although the same variables that make MS difficult to treat also complicate the identification of biomarkers [160]. Studies have examined and identified potential biomarkers that reflect the effects of IFN-β treatment, though further research is needed to establish reliable and accessible biomarkers of clinically meaningful treatment response to IFN-β [13, 33, 161–166].
Serum neurofilament light chains (sNfL), growth differentiation factor 15 (GDF-15), and autoantibody signatures have been studied in a subset of patients from the REFLEX study, in order to investigate if these factors may contribute to MS pathology, disease course, disease prognosis, and therapeutic response [162, 167–169]. In patients with a first clinically demyelinating event, higher baseline sNfL levels have been associated with a greater likelihood of conversion to MS, although conversion to MS was delayed in patients treated with IFN-β-1a sc regardless of higher or lower sNfL levels [168]. Higher baseline sNfL levels also moderately correlated with increased MRI lesion load and with lesion count in patients given placebo but correlated only weakly with lesion counts in patients treated with IFN-β-1a sc, who demonstrated reduced lesion counts compared to patients receiving placebo [167]. Conversely, GDF-15 levels increased with IFN-β-1a sc treatment, and GDF-15 levels also increased in those patients who did not convert to MS [169]. Finally, antigenome technology is being used to identify autoantibody targets in the human genome, helping to identify potential biomarkers for IFN-β-1a treatment response in patients with MS and to determine which patients may progress to CDMS [162].
Despite ongoing research to identify more sensitive biomarkers, the inflammation and tissue damage seen on MRI is considered the best available biomarker of disease and contributes to diagnosis and assessment of disease progression and treatment efficacy [170]. NfL levels and other soluble markers are promising as biomarkers of disease progression and treatment effects, but their relevance to clinical practice and ability to predict treatment response remains an area of continued study [170].
Research into the effects of IFN-β on gene expression may ultimately yield information both about the mechanism of action of IFN-β therapies as well as the pathophysiology of MS [35]. However, evidence so far has been mixed regarding genes with polymorphisms related to IFN-β response. Candidate genes have included those that encode the common receptor for type I IFNs, IFN response-element sequences, IFN regulatory transcription factors, and other cytokine genes as well as a host of other alleles unrelated to cytokines [160]. Pharmacogenetic biomarkers may become a vital part of enabling MS treatment choices in the future but are not yet fully developed for clinical use.
Conclusions
The state of MS treatment has vastly improved over the last 30 years. Through this evolving clinical landscape, IFN-β-1a sc has remained a mainstay of therapy, with an established efficacy and safety profile with an estimated cumulative exposure of 1,936,801 patient-years in the post-marketing setting as of May 2023 [Data on file, EMD Serono Inc., Rockland, MA USA, an affiliate of Merck KGaA, 2023]. IFN-β-1a sc has become an important standard-of-care comparator for studies of newer treatments and combination therapies, as well as providing a framework for research into the mechanisms by which MS begins and progresses. Research is ongoing in the hopes of refining treatment to improve the lives and health of people with MS.
Acknowledgements
Medical Writing/Editorial Assistance.
Writing and editorial support for the development of this publication was provided by Jennifer Steeber, PhD, and Kathleen Blake, PhD, of Ashfield MedComms (New York, NY, USA), an Inizio Health company, and was funded by the manuscript sponsor.
Author Contributions
Mark S. Freedman, Patricia K. Coyle, Kerstin Hellwig, Barry Singer, Daniel Wynn, Bianca Weinstock-Guttman, Silva Markovic-Plese, Andrew Galazka, Fernando Dangond, Julie Korich, Anthony T. Reder participated sufficiently in the work to take public responsibility for the entire content of the manuscript.
Funding
This work, including the journal’s Rapid Service Fee, was supported by EMD Serono, Rockland, MA, USA (CrossRef Funder ID: 10.13039/100004755).
Declarations
Conflict of Interest
Mark S. Freedman has received honoraria or consultation fees from Actelion (Janssen/J&J), Alexion/Astra Zeneca, Biogen, Celgene (BMS), Find Therapeutics, Hoffmann La Roche, Horizon Therapeutics, Merck and Co, Novartis, Sandoz, Sanofi-Genzyme, and Teva Canada Innovation; has received research support unrelated to this study from Sanofi-Genzyme Canada, Hoffman-La Roche, and Merck Healthcare KGaA, Darmstadt, Germany; was a member of a company advisory board, board of directors, or other similar group for Actelion (Janssen/J&J), Alexion/Astra Zeneca, Atara Biotherapeutics, Bayer Healthcare, Biogen Idec, Celgene (BMS), Horizon Therapeutics, Hoffman La-Roche, Merck Healthcare KGaA, Darmstadt, Germany, Novartis, Sanofi-Genzyme, and Teva Canada Innovation; and has been a participant in a company sponsored speaker’s bureau for Sanofi-Genzyme and Merck Healthcare KGaA, Darmstadt, Germany). Patricia K. Coyle declares receiving consulting & nonbranded speaker fees from Accordant, Biogen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Horizon Therapeutics, Janssen, Mylan, Novartis, Sanofi Genzyme, TG Therapeutics, and Viela Bio; and research support from Actelion, Alkermes, Celgene, CorEvitas LLC, Genentech/Roche, MedDay, NINDS, Novartis, and Sanofi Genzyme. Kerstin Hellwig has received consulting fees, fees for non-CME services, and speaker’s bureau from Bayer, Biogen, Merck and Co, Novartis, Roche, Sanofi, and Teva. Barry Singer has received research support from AbbVie, Biogen, Greenwich Biosciences, Novartis, and Sanofi Genzyme; and consulting and/or speaking fees from AbbVie, Alexion, Biogen, Bristol Myers Squibb, Cigna, Merck Healthcare KGaA, Darmstadt, Germany, Janssen, Genentech, Greenwich Biosciences, Horizon, Novartis, Octave Bioscience, Roche, Sanofi Genzyme, and TG Therapeutics. Daniel Wynn – employer has received research funding, speaker fees, or served as expert witness for AbbVie, Adamas, Allergan, ANI Pharma, Avanir, Banner Life, Biogen, Bristol Myers Squibb, Chugai, Eli Lilly, Merck Healthcare KGaA, Darmstadt, Germany, Genentech, GW Therapeutics, Immunic, InnoCare, Janssen, Jazz Pharmaceuticals, Mallinckrodt, MAPI Therapeutics, Mylan, National MS Society, Novartis, SanBio, Sanofi Genzyme, UCB Biopharma, Viela Bio, Teva Pharmaceuticals, and TG Therapeutics. Bianca Weinstock-Guttman has participated in speaker’s bureaus and/or served as a consultant for Bayer, Biogen, Celgene/Bristol Meyers Squibb, Merck Healthcare KGaA, Genentech, Janssen and Horizon, Novartis, and Sanofi Genzyme; has received grant/research support from the agencies listed in the previous sentence; and serves in the editorial board for BMJ Neurology, Children, CNS Drugs, Frontiers Epidemiology, and MS International. Silva Markovic-Plese has received grant research support from Bristol Myers Squibb and honoraria from Genentech and Janssen Pharmaceuticals for participation in editorial boards. Andrew Galazka has received consulting fees from the healthcare business of Merck Healthcare KGaA, Darmstadt, Germany. Fernando Dangond was formerly an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, and is currently an employee of Bristol Myers Squibb. Julie Korich is an employee of EMD Serono Inc., Rockland, MA, USA, an affiliate of Merck KGaA. Anthony T. Reder has received consulting fees from Acorda, Bayer, Biogen, BMS, Merck Healthcare KGaA, Genentech, Genzyme, Novartis, Pfizer, Mallinckrodt (formerly Questcor), Sanofi, and Teva Pharmaceuticals.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Fernando Dangond was at EMD Serono Research & Development Institute Inc. at the time of manuscript development.
References
- 1.Klineova S, Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(9):a028928. doi: 10.1101/cshperspect.a028928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manfredonia F, Pasquali L, Dardano A, Iudice A, Murri L, Monzani F. Review of the clinical evidence for interferon beta 1a (Rebif) in the treatment of multiple sclerosis. Neuropsychiatr Dis Treat. 2008;4(2):321–336. doi: 10.2147/ndt.s476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reder AT, Feng X. How type I interferons work in multiple sclerosis and other diseases: some unexpected mechanisms. J Interferon Cytokine Res. 2014;34(8):589–599. doi: 10.1089/jir.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhlmann T, Moccia M, Coetzee T, et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 2023;22(1):78–88. doi: 10.1016/S1474-4422(22)00289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Multiple Sclerosis Society of America. History of multiple sclerosis: mymsaa.org; [updated February 26, 2020]. https://mymsaa.org/ms-information/overview/history/. Accessed Nov 30, 2021.
- 6.Poser CM. Multiple sclerosis. Med Clin North Am. 1979;63(4):729–743. doi: 10.1016/S0025-7125(16)31671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodkin DE, Ransohoff RM, Rudick RA. Experimental therapies for multiple sclerosis: current status. Cleve Clin J Med. 1992;59(1):63–74. doi: 10.3949/ccjm.59.1.63. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y. Fifty years of interference. Nat Immunol. 2004;5(12):1193. doi: 10.1038/ni1204-1193. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 10.Taylor MW. Interferons. Viruses and man: a history of interactions. 1. Cham: Springer; 2014. [Google Scholar]
- 11.Borden EC, Sen GC, Uze G, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6(12):975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldschmidt CH, Hua LH. Re-evaluating the use of IFN-beta and relapsing multiple sclerosis: safety, efficacy and place in therapy. Degener Neurol Neuromuscul Dis. 2020;10:29–38. doi: 10.2147/DNND.S224912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasper LH, Reder AT. Immunomodulatory activity of interferon-beta. Ann Clin Transl Neurol. 2014;1(8):622–631. doi: 10.1002/acn3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virtanen JO, Jacobson S. Viruses and multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11(5):528–544. doi: 10.2174/187152712801661220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao Y, Zhang X, Chopra M, et al. The role of endogenous IFN-beta in the regulation of Th17 responses in patients with relapsing-remitting multiple sclerosis. J Immunol. 2014;192(12):5610–5617. doi: 10.4049/jimmunol.1302580. [DOI] [PubMed] [Google Scholar]
- 16.Tovey MG, Lallemand C. Safety, tolerability, and immunogenicity of interferons. Pharmaceuticals (Basel) 2010;3(4):1162–1186. doi: 10.3390/ph3041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagano Y, Kojima Y. Pouvoir immunisant du virus vaccinal inactivé par des rayons ultraviolets [Immunizing property of vaccinia virus inactivated by ultraviolets rays] C R Seances Soc Biol Fil. 1954;148(19–20):1700–1702. [PubMed] [Google Scholar]
- 18.Browder JF, Araujo OE, Myer NA, Flowers FP. The interferons and their use in condyloma acuminata. Ann Pharmacother. 1992;26(1):42–45. doi: 10.1177/106002809202600111. [DOI] [PubMed] [Google Scholar]
- 19.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1(8538):893–895. doi: 10.1016/S0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs L, O'Malley J, Freeman A, Ekes R. Intrathecal interferon reduces exacerbations of multiple sclerosis. Science (New York, NY) 1981;214(4524):1026–1028. doi: 10.1126/science.6171035. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs L, O'Malley J, Freeman A, Murawski J, Ekes R. Intrathecal interferon in multiple sclerosis. Arch Neurol. 1982;39(10):609–615. doi: 10.1001/archneur.1982.00510220007002. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs L, O'Malley JA, Freeman A, Ekes R, Reese PA. Intrathecal interferon in the treatment of multiple sclerosis. Patient follow-up. Arch Neurol. 1985;42(9):841–847. doi: 10.1001/archneur.1985.04060080019009. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs L, Johnson KP. A brief history of the use of interferons as treatment of multiple sclerosis. Arch Neurol. 1994;51(12):1245–1252. doi: 10.1001/archneur.1994.00540240089022. [DOI] [PubMed] [Google Scholar]
- 24.Rivera VM, Macias MA. Access and barriers to MS care in Latin America. Mult Scler J Exp Transl Clin. 2017;3(1):2055217317700668. doi: 10.1177/2055217317700668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reder AT, Arndt N, Roman C, et al. Real-world propensity score comparison of treatment effectiveness of peginterferon beta-1a vs. subcutaneous interferon beta-1a, glatiramer acetate, and teriflunomide in patients with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2021;51:102935. doi: 10.1016/j.msard.2021.102935. [DOI] [PubMed] [Google Scholar]
- 26.Bayer HealthCare Pharmaceuticals. Betaseron [package insert]. Whippany, NJ: 2018.
- 27.Biogen Inc. Avonex [package insert]. Cambridge, MA: 2016.
- 28.EMD Serono, Inc. Rebif [package insert]. Rockland, MA: 2021.
- 29.Merck Europe B.V. Rebif [summary of product characteristics]. Amsterdam, the Netherlands: 2021.
- 30.Kunkl M, Frascolla S, Amormino C, Volpe E, Tuosto L. T helper cells: the modulators of inflammation in multiple sclerosis. Cells. 2020;9(2):482. doi: 10.3390/cells9020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann-Horn K, Kronsbein HC, Weber MS. Targeting B cells in the treatment of multiple sclerosis: recent advances and remaining challenges. Ther Adv Neurol Disord. 2013;6(3):161–173. doi: 10.1177/1756285612474333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011;25(6):491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Jakimovski D, Kolb C, Ramanathan M, Zivadinov R, Weinstock-Guttman B. Interferon beta for multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(11):a032003. doi: 10.1101/cshperspect.a032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rommer PS, Milo R, Han MH, et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019;10:1564. doi: 10.3389/fimmu.2019.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng X, Bao R, Li L, Deisenhammer F, Arnason BGW, Reder AT. Interferon-beta corrects massive gene dysregulation in multiple sclerosis: short-term and long-term effects on immune regulation and neuroprotection. EBioMedicine. 2019;49:269–283. doi: 10.1016/j.ebiom.2019.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Jin J, Tang Y, Speer D, Sujkowska D, Markovic-Plese S. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182(6):3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- 37.Ramgolam VS, Sha Y, Marcus KL, et al. B cells as a therapeutic target for IFN-beta in relapsing-remitting multiple sclerosis. J Immunol. 2011;186(7):4518–4526. doi: 10.4049/jimmunol.1000271. [DOI] [PubMed] [Google Scholar]
- 38.Hart FM, Bainbridge J. Current and emerging treatment of multiple sclerosis. Am J Manag Care. 2016;22(6 Suppl):s159–s170. [PubMed] [Google Scholar]
- 39.Sedaghat N, Etemadifar M. Revisiting the antiviral theory to explain interferon-beta's effectiveness for relapsing multiple sclerosis. Mult Scler Relat Disord. 2022;67:104155. doi: 10.1016/j.msard.2022.104155. [DOI] [PubMed] [Google Scholar]
- 40.Bellucci G, Albanese A, Rizzi C, Rinaldi V, Salvetti M, Ristori G. The value of Interferon beta in multiple sclerosis and novel opportunities for its anti-viral activity: a narrative literature review. Front Immunol. 2023;14:1161849. doi: 10.3389/fimmu.2023.1161849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–504. [PubMed]
- 42.The Once Weekly Interferon for MS Study Group. Evidence of interferon beta-1a dose response in relapsing-remitting MS: the OWIMS Study. Neurology. 1999;53(4):679–86. [DOI] [PubMed]
- 43.Schwid SR, Panitch HS. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther. 2007;29(9):2031–2048. doi: 10.1016/j.clinthera.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–914. doi: 10.1016/S1474-4422(08)70200-X. [DOI] [PubMed] [Google Scholar]
- 45.De Stefano N, Curtin F, Stubinski B, et al. Rapid benefits of a new formulation of subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis. Mult Scler. 2010;16(7):888–892. doi: 10.1177/1352458510362442. [DOI] [PubMed] [Google Scholar]
- 46.PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group. PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology. 2001;56(12):1628–36. [DOI] [PubMed]
- 47.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 48.Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler. 2014;20(6):705–716. doi: 10.1177/1352458513507821. [DOI] [PubMed] [Google Scholar]
- 49.Comi G, De Stefano N, Freedman MS, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol. 2012;11(1):33–41. doi: 10.1016/S1474-4422(11)70262-9. [DOI] [PubMed] [Google Scholar]
- 50.Comi G, De Stefano N, Freedman MS, et al. Subcutaneous interferon beta-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry. 2017;88(4):285–294. doi: 10.1136/jnnp-2016-314843. [DOI] [PubMed] [Google Scholar]
- 51.Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon- beta-1a in secondary progressive MS: clinical results. Neurology. 2001;56(11):1496–504. [DOI] [PubMed]
- 52.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 53.Cohen BA, Rivera VM. PRISMS: the story of a pivotal clinical trial series in multiple sclerosis. Curr Med Res Opin. 2010;26(4):827–838. doi: 10.1185/03007991003604018. [DOI] [PubMed] [Google Scholar]
- 54.Li DK, Paty DW. Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing-remitting multiple sclerosis. Prevention of Relapses and Disability by Interferon-beta1a Subcutaneously in Multiple Sclerosis. Ann Neurol. 1999;46(2):197–206. doi: 10.1002/1531-8249(199908)46:2<197::AID-ANA9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 55.Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology. 2002;59(10):1496–1506. doi: 10.1212/01.WNL.0000034080.43681.DA. [DOI] [PubMed] [Google Scholar]
- 56.Jaber A, Driebergen R, Giovannoni G, Schellekens H, Simsarian J, Antonelli M. The Rebif new formulation story: it's not trials and error. Drugs R D. 2007;8(6):335–348. doi: 10.2165/00126839-200708060-00002. [DOI] [PubMed] [Google Scholar]
- 57.McKeage K, Wagstaff AJ. Subcutaneous interferon-beta-1a: new formulation. CNS Drugs. 2007;21(10):871–876. doi: 10.2165/00023210-200721100-00006. [DOI] [PubMed] [Google Scholar]
- 58.Portaccio E, Amato MP. Improving compliance with interferon-beta therapy in patients with multiple sclerosis. CNS Drugs. 2009;23(6):453–462. doi: 10.2165/00023210-200923060-00001. [DOI] [PubMed] [Google Scholar]
- 59.Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67(6):944–953. doi: 10.1212/01.wnl.0000237994.95410.ce. [DOI] [PubMed] [Google Scholar]
- 60.Kappos L, Kuhle J, Multanen J, et al. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry. 2015;86(11):1202–1207. doi: 10.1136/jnnp-2014-310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patti F, Amato MP, Bastianello S, et al. Subcutaneous interferon beta-1a has a positive effect on cognitive performance in mildly disabled patients with relapsing-remitting multiple sclerosis: 2-year results from the COGIMUS study. Ther Adv Neurol Disord. 2009;2(2):67–77. doi: 10.1177/1756285608101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patti F, Morra VB, Amato MP, et al. Subcutaneous interferon beta-1a may protect against cognitive impairment in patients with relapsing-remitting multiple sclerosis: 5-year follow-up of the COGIMUS study. PLoS ONE. 2013;8(8):e74111. doi: 10.1371/journal.pone.0074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benesova Y, Tvaroh A. Cognition and fatigue in patients with relapsing multiple sclerosis treated by subcutaneous interferon beta-1a: an observational study SKORE. Ther Adv Neurol Disord. 2017;10(1):18–32. doi: 10.1177/1756285616671882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mori F, Kusayanagi H, Buttari F, et al. Early treatment with high-dose interferon beta-1a reverses cognitive and cortical plasticity deficits in multiple sclerosis. Funct Neurol. 2012;27(3):163–168. [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 66.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357(9268):1576–1582. doi: 10.1016/S0140-6736(00)04725-5. [DOI] [PubMed] [Google Scholar]
- 67.De Stefano N, Comi G, Kappos L, et al. Efficacy of subcutaneous interferon beta-1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry. 2014;85(6):647–653. doi: 10.1136/jnnp-2013-306289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freedman MS, Comi G, Coyle PK, et al. No evidence of disease activity status in patients treated with early vs. delayed subcutaneous interferon beta-1a. Mult Scler Relat Disord. 2019;39:101891. doi: 10.1016/j.msard.2019.101891. [DOI] [PubMed] [Google Scholar]
- 69.Kappos L. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352(9139):1491–1497. doi: 10.1016/S0140-6736(98)10039-9. [DOI] [PubMed] [Google Scholar]
- 70.Panitch H, Miller A, Paty D, Weinshenker B. North American Study Group on Interferon beta-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63(10):1788–1795. doi: 10.1212/01.WNL.0000146958.77317.3E. [DOI] [PubMed] [Google Scholar]
- 71.Li DK, Zhao GJ, Paty DW. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: MRI results. Neurology. 2001;56(11):1505–1513. doi: 10.1212/WNL.56.11.1505. [DOI] [PubMed] [Google Scholar]
- 72.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 73.Frahm N, Peters M, Batzing J, et al. Treatment patterns in pediatric patients with multiple sclerosis in Germany-a nationwide claim-based analysis. Ther Adv Neurol Disord. 2021;14:17562864211048336. doi: 10.1177/17562864211048336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brola W, Steinborn B. Pediatric multiple sclerosis - current status of epidemiology, diagnosis and treatment. Neurol Neurochir Pol. 2020;54(6):508–517. doi: 10.5603/PJNNS.a2020.0069. [DOI] [PubMed] [Google Scholar]
- 75.Alroughani R, Boyko A. Pediatric multiple sclerosis: a review. BMC Neurol. 2018;18(1):27. doi: 10.1186/s12883-018-1026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghezzi A, Amato MP, Makhani N, Shreiner T, Gartner J, Tenembaum S. Pediatric multiple sclerosis: conventional first-line treatment and general management. Neurology. 2016;87(9 Suppl 2):S97–S102. doi: 10.1212/WNL.0000000000002823. [DOI] [PubMed] [Google Scholar]
- 77.Chitnis T, Tenembaum S, Banwell B, et al. Consensus statement: evaluation of new and existing therapeutics for pediatric multiple sclerosis. Mult Scler. 2012;18(1):116–127. doi: 10.1177/1352458511430704. [DOI] [PubMed] [Google Scholar]
- 78.Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med. 2018;379(11):1017–1027. doi: 10.1056/NEJMoa1800149. [DOI] [PubMed] [Google Scholar]
- 79.Chitnis T, Banwell B, Kappos L, et al. Safety and efficacy of teriflunomide in paediatric multiple sclerosis (TERIKIDS): a multicentre, double-blind, phase 3, randomised, placebo-controlled trial. Lancet Neurol. 2021;20(12):1001–1011. doi: 10.1016/S1474-4422(21)00364-1. [DOI] [PubMed] [Google Scholar]
- 80.Alroughani R, Das R, Penner N, Pultz J, Taylor C, Eraly S. Safety and efficacy of delayed-release dimethyl fumarate in pediatric patients with relapsing multiple sclerosis (FOCUS) Pediatr Neurol. 2018;83:19–24. doi: 10.1016/j.pediatrneurol.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Alroughani R, Huppke P, Mazurkiewicz-Beldzinska M, et al. Delayed-release dimethyl fumarate safety and efficacy in pediatric patients with relapsing-remitting multiple sclerosis. Front Neurol. 2020;11:606418. doi: 10.3389/fneur.2020.606418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vermersch P, Scaramozza M, Levin S, et al. Effect of dimethyl fumarate vs interferon beta-1a in patients with pediatric-onset multiple sclerosis: The CONNECT Randomized Clinical Trial. JAMA Netw Open. 2022;5(9):e2230439. doi: 10.1001/jamanetworkopen.2022.30439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aubagio [package insert]. Cambridge, MA: Genzyme Corporation; 2021.
- 84.Tenembaum SN, Banwell B, Pohl D, et al. Subcutaneous interferon beta-1a in pediatric multiple sclerosis: a retrospective study. J Child Neurol. 2013;28(7):849–856. doi: 10.1177/0883073813488828. [DOI] [PubMed] [Google Scholar]
- 85.Ghezzi A, Bianchi A, Baroncini D, et al. A multicenter, observational, prospective study of self- and parent-reported quality of life in adolescent multiple sclerosis patients self-administering interferon-beta1a using RebiSmart-the FUTURE study. Neurol Sci. 2017;38(11):1999–2005. doi: 10.1007/s10072-017-3091-6. [DOI] [PubMed] [Google Scholar]
- 86.Jakimovski D, Awan S, Eckert SP, Farooq O, Weinstock-Guttman B. Multiple sclerosis in children: differential diagnosis, prognosis, and disease-modifying treatment. CNS Drugs. 2022;36(1):45–59. doi: 10.1007/s40263-021-00887-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Freedman MS, Todorović M, Murgašová Z, Jack D, Korich J. Post-approval safety of subcutaneous interferon β-1a in the treatment of multiple sclerosis, with particular reference to respiratory viral infections. Consortium of Multiple Sclerosis (CMSC); National Harbor, MD, USA: DMT40; 2022.
- 88.Bloomgren G, Sperling B, Cushing K, Wenten M. Assessment of malignancy risk in patients with multiple sclerosis treated with intramuscular interferon beta-1a: retrospective evaluation using a health insurance claims database and postmarketing surveillance data. Ther Clin Risk Manag. 2012;8:313–321. doi: 10.2147/TCRM.S31347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kingwell E, Evans C, Zhu F, Oger J, Hashimoto S, Tremlett H. Assessment of cancer risk with beta-interferon treatment for multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(10):1096–1102. doi: 10.1136/jnnp-2013-307238. [DOI] [PubMed] [Google Scholar]
- 90.Stamatellos VP, Siafis S, Papazisis G. Disease-modifying agents for multiple sclerosis and the risk for reporting cancer: a disproportionality analysis using the US Food and Drug Administration Adverse Event Reporting System database. Br J Clin Pharmacol. 2021;87(12):4769–4779. doi: 10.1111/bcp.14916. [DOI] [PubMed] [Google Scholar]
- 91.Biogen Inc. Plegridy [package insert]. Cambridge, MA: 2014.
- 92.Boskovic R, Wide R, Wolpin J, Bauer DJ, Koren G. The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology. 2005;65(6):807–811. doi: 10.1212/01.wnl.0000180575.77021.c4. [DOI] [PubMed] [Google Scholar]
- 93.Fragoso YD, Adoni T, Alves-Leon SV, et al. Long-term effects of exposure to disease-modifying drugs in the offspring of mothers with multiple sclerosis: a retrospective chart review. CNS Drugs. 2013;27(11):955–961. doi: 10.1007/s40263-013-0113-7. [DOI] [PubMed] [Google Scholar]
- 94.Hellwig K, Haghikia A, Gold R. Parenthood and immunomodulation in patients with multiple sclerosis. J Neurol. 2010;257(4):580–583. doi: 10.1007/s00415-009-5376-z. [DOI] [PubMed] [Google Scholar]
- 95.Hellwig K, Haghikia A, Rockhoff M, Gold R. Multiple sclerosis and pregnancy: experience from a nationwide database in Germany. Ther Adv Neurol Disord. 2012;5(5):247–253. doi: 10.1177/1756285612453192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korjagina M, Hakkarainen KM, Burkill S, et al. Prevalence of adverse pregnancy outcomes after exposure to interferon beta prior to or during pregnancy in women with MS: stratification by maternal and newborn characteristics in a register-based cohort study in Finland and Sweden. Mult Scler Relat Disord. 2021;48:102694. doi: 10.1016/j.msard.2020.102694. [DOI] [PubMed] [Google Scholar]
- 97.Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology. 2010;75(20):1794–1802. doi: 10.1212/WNL.0b013e3181fd62bb. [DOI] [PubMed] [Google Scholar]
- 98.Hellwig K, Geissbuehler Y, Sabido M, et al. Pregnancy outcomes in interferon-beta-exposed patients with multiple sclerosis: results from the European Interferon-beta Pregnancy Registry. J Neurol. 2020;267(6):1715–1723. doi: 10.1007/s00415-020-09762-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burkill S, Vattulainen P, Geissbuehler Y, et al. The association between exposure to interferon-beta during pregnancy and birth measurements in offspring of women with multiple sclerosis. PLoS ONE. 2019;14(12):e0227120. doi: 10.1371/journal.pone.0227120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hakkarainen KM, Juuti R, Burkill S, et al. Pregnancy outcomes after exposure to interferon beta: a register-based cohort study among women with MS in Finland and Sweden. Ther Adv Neurol Disord. 2020;13:1756286420951072. doi: 10.1177/1756286420951072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weber-Schoendorfer C, Schaefer C. Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler. 2009;15(9):1037–1042. doi: 10.1177/1352458509106543. [DOI] [PubMed] [Google Scholar]
- 102.Varyte G, Arlauskiene A, Ramasauskaite D. Pregnancy and multiple sclerosis: an update. Curr Opin Obstet Gynecol. 2021;33(5):378–383. doi: 10.1097/GCO.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krysko KM, Bove R, Dobson R, Jokubaitis V, Hellwig K. Treatment of women withmultiple sclerosis planning pregnancy. Curr Treat Options Neurol. 2021;23(4):11. doi: 10.1007/s11940-021-00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ciplea AI, Langer-Gould A, Stahl A, et al. Safety of potential breast milk exposure to IFN-beta or glatiramer acetate: one-year infant outcomes. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e757. doi: 10.1212/NXI.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sorensen PS. Neutralizing antibodies against interferon-beta. Ther Adv Neurol Disord. 2008;1(2):125–141. doi: 10.1177/1756285608095144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dunn N, Fogdell-Hahn A, Hillert J, Spelman T. Long-term consequences of high titer neutralizing antibodies to interferon-beta in multiple sclerosis. Front Immunol. 2020;11:583560. doi: 10.3389/fimmu.2020.583560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bertolotto A, Deisenhammer F, Gallo P, Solberg SP. Immunogenicity of interferon beta: differences among products. J Neurol. 2004;251(2):II15–II24. doi: 10.1007/s00415-004-1204-7. [DOI] [PubMed] [Google Scholar]
- 108.Bachelet D, Hassler S, Mbogning C, et al. Occurrence of anti-drug antibodies against interferon-beta and natalizumab in multiple sclerosis: a collaborative cohort analysis. PLoS ONE. 2016;11(11):e0162752. doi: 10.1371/journal.pone.0162752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ross C, Clemmesen KM, Svenson M, et al. Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann Neurol. 2000;48(5):706–712. doi: 10.1002/1531-8249(200011)48:5<706::AID-ANA3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 110.Hedstrom AK, Ryner M, Fink K, et al. Smoking and risk of treatment-induced neutralizing antibodies to interferon beta-1a. Mult Scler. 2014;20(4):445–450. doi: 10.1177/1352458513498635. [DOI] [PubMed] [Google Scholar]
- 111.Waddington KE, Papadaki A, Coelewij L, et al. Using serum metabolomics to predict development of anti-drug antibodies in multiple sclerosis patients treated with IFN-beta. Front Immunol. 2020;11:1527. doi: 10.3389/fimmu.2020.01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andlauer TFM, Link J, Martin D, et al. Treatment- and population-specific genetic risk factors for anti-drug antibodies against interferon-beta: a GWAS. BMC Med. 2020;18(1):298. doi: 10.1186/s12916-020-01769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freedman MS, Jack D, Murgasova Z, Todorovic M, Seitzinger A. Outcomes of COVID-19 among patients treated with subcutaneous interferon beta-1a for multiple sclerosis. Mult Scler Relat Disord. 2021;56:103283. doi: 10.1016/j.msard.2021.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sormani MP, Schiavetti I, Carmisciano L, et al. COVID-19 severity in multiple sclerosis: putting data into context. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1105. doi: 10.1212/NXI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prosperini L, Tortorella C, Haggiag S, Ruggieri S, Galgani S, Gasperini C. Determinants of COVID-19-related lethality in multiple sclerosis: a meta-regression of observational studies. J Neurol. 2022;269(5):2275–2285. doi: 10.1007/s00415-021-10951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult Scler Relat Disord. 2020;45:102439. doi: 10.1016/j.msard.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwid SR, Decker MD, Lopez-Bresnahan M. Rebif-Influenza Vaccine Study I. Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. Neurology. 2005;65(12):1964–1966. doi: 10.1212/01.wnl.0000188901.12700.e0. [DOI] [PubMed] [Google Scholar]
- 120.Olberg HK, Eide GE, Cox RJ, et al. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur J Neurol. 2018;25(3):527–534. doi: 10.1111/ene.13537. [DOI] [PubMed] [Google Scholar]
- 121.Brill L, Rechtman A, Zveik O, et al. Effect of cladribine on COVID-19 serology responses following 2 doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. ECTRIMS; Virtual: 2021. [DOI] [PMC free article] [PubMed]
- 122.Brill L, Rechtman A, Zveik O, et al. Effect of cladribine on COVID-19 serology responses following two doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;57:103343. doi: 10.1016/j.msard.2021.103343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maniscalco GT, Manzo V, Ferrara AL, et al. Interferon beta-1a treatment promotes SARS-CoV-2 mRNA vaccine response in multiple sclerosis subjects. Mult Scler Relat Disord. 2022;58:103455. doi: 10.1016/j.msard.2021.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98(5):e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS 2021 [updated September 15, 2021]. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance. Accessed Oct 24, 2021.
- 126.MS Canada. COVID-19 vaccine guidance for people living with MS 2021 [updated December 7, 2021]. https://mssociety.ca/resources/news/article/covid-19-vaccine-guidance-for-people-living-with-ms?force_lang=en_CA. Accessed Dec 7, 2021.
- 127.Alavi Darazam I, Hatami F, Rabiei MM, et al. An investigation into the beneficial effects of high-dose interferon beta 1-a, compared to low-dose interferon beta 1-a (the base therapeutic regimen) in moderate to severe COVID-19: a structured summary of a study protocol for a randomized controlled l trial. Trials. 2020;21(1):880. doi: 10.1186/s13063-020-04812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davoudi-Monfared E, Rahmani H, Khalili H, et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64(9):e01061-20. doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dastan F, Nadji SA, Saffaei A, et al. Subcutaneous administration of interferon beta-1a for COVID-19: a non-controlled prospective trial. Int Immunopharmacol. 2020;85:106688. doi: 10.1016/j.intimp.2020.106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kalil AC, Mehta AK, Patterson TF, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9(12):1365–1376. doi: 10.1016/S2213-2600(21)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.WHO Solidarity Trial Consortium. Pan H, Peto R, et al. Repurposed antiviral drugs for COVI-19 - interim WHO Solidarity Trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.MS International Federation. Number of people newly diagnosed with MS each year 2022. https://www.atlasofms.org/map/united-states-of-america/epidemiology/number-of-people-newly-diagnosed-with-ms#about. Accessed June 10, 2022.
- 133.MS International Federation. Atlas of MS 3rd edition 2020. https://www.msif.org/wp-content/uploads/2020/10/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf. Accessed June 10, 2022.
- 134.Hupperts R, Ghazi-Visser L, Martins Silva A, et al. The STAR Study: a real-world, international, observational study of the safety and tolerability of, and adherence to, serum-free subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis. Clin Ther. 2014;36(12):1946–1957. doi: 10.1016/j.clinthera.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Allignol A, Boutmy E, Sabido Espin M, Marhardt K, Vermersch P. Effectiveness, healthcare resource utilization and adherence to subcutaneous interferon beta-1a according to age in patients with multiple sclerosis: a cohort study using a US claims database. Front Neurol. 2021;12:676585. doi: 10.3389/fneur.2021.676585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gobbi C, Zecca C, Linnebank M, et al. Swiss analysis of multiple sclerosis: a multicenter, non-interventional, retrospective cohort study of disease-modifying therapies. Eur Neurol. 2013;70(1–2):35–41. doi: 10.1159/000346761. [DOI] [PubMed] [Google Scholar]
- 137.Ernst FR, Barr P, Elmor R, Wong SL. Relapse outcomes, safety, and treatment patterns in patients diagnosed with relapsing-remitting multiple sclerosis and initiated on subcutaneous interferon beta-1a or dimethyl fumarate: a real-world study. Curr Med Res Opin. 2017;33(12):2099–2106. doi: 10.1080/03007995.2017.1380616. [DOI] [PubMed] [Google Scholar]
- 138.Bowen JD, Kozma CM, Grosso MM, Phillips AL. A real-world comparison of relapse rates, healthcare costs and resource use among patients with multiple sclerosis newly initiating subcutaneous interferon beta-1a versus oral disease-modifying drugs. Mult Scler J Exp Transl Clin. 2018;4(4):2055217318819031. doi: 10.1177/2055217318819031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalincik T, Jokubaitis V, Izquierdo G, et al. Comparative effectiveness of glatiramer acetate and interferon beta formulations in relapsing-remitting multiple sclerosis. Mult Scler. 2015;21(9):1159–1171. doi: 10.1177/1352458514559865. [DOI] [PubMed] [Google Scholar]
- 140.Trojano M, Pellegrini F, Paolicelli D, et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol. 2009;66(4):513–520. doi: 10.1002/ana.21757. [DOI] [PubMed] [Google Scholar]
- 141.Smith MY, Sabido-Espin M, Trochanov A, et al. Postmarketing safety profile of subcutaneous interferon beta-1a given 3 times weekly: a retrospective administrative claims analysis. J Manag Care Spec Pharm. 2015;21(8):650–660. doi: 10.18553/jmcp.2015.21.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bayas A, Ouallet JC, Kallmann B, et al. Adherence to, and effectiveness of, subcutaneous interferon beta-1a administered by RebiSmart® in patients with relapsing multiple sclerosis: results of the 1-year, observational SMART study. Expert Opin Drug Deliv. 2015;12(8):1239–1250. doi: 10.1517/17425247.2015.1057567. [DOI] [PubMed] [Google Scholar]
- 143.Mikol D, Lopez-Bresnahan M, Taraskiewicz S, Chang P, Rangnow J, Rebiject Study Group A randomized, multicentre, open-label, parallel-group trial of the tolerability of interferon beta-1a (Rebif) administered by autoinjection or manual injection in relapsing-remitting multiple sclerosis. Mult Scler. 2005;11(5):585–591. doi: 10.1191/1352458505ms1197oa. [DOI] [PubMed] [Google Scholar]
- 144.Stewart TM, Tran ZV. Injectable multiple sclerosis medications: a patient survey of factors associated with injection-site reactions. Int J MS Care. 2012;14(1):46–53. doi: 10.7224/1537-2073-14.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Devonshire VA, Feinstein A, Moriarty P. Adherence to interferon beta-1a therapy using an electronic self-injector in multiple sclerosis: a multicentre, single-arm, observational, phase IV study. BMC Res Notes. 2016;9(1):148. doi: 10.1186/s13104-016-1948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Freedman MS. Evidence for the efficacy of interferon beta-1b in delaying the onset of clinically definite multiple sclerosis in individuals with clinically isolated syndrome. Ther Adv Neurol Disord. 2014;7(6):279–288. doi: 10.1177/1756285614549554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Freedman MS, Wolinsky JS, Wamil B, et al. Teriflunomide added to interferon-beta in relapsing multiple sclerosis: a randomized phase II trial. Neurology. 2012;78(23):1877–1885. doi: 10.1212/WNL.0b013e318258f7d4. [DOI] [PubMed] [Google Scholar]
- 148.Montalban X, Leist TP, Cohen BA, et al. Cladribine tablets added to IFN-beta in active relapsing MS: the ONWARD study. Neurol Neuroimmunol Neuroinflamm. 2018;5(5):e477. doi: 10.1212/NXI.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reder AT, Feng X. Aberrant type I interferon regulation in autoimmunity: opposite directions in MS and SLE, shaped by evolution and body ecology. Front Immunol. 2013;4:281. doi: 10.3389/fimmu.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hupperts R, Smolders J, Vieth R, et al. Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon beta-1a. Neurology. 2019;93(20):e1906–e1916. doi: 10.1212/WNL.0000000000008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smolders J, Hupperts R, Barkhof F, et al. Efficacy of vitamin D3 as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: a phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011;311(1–2):44–49. doi: 10.1016/j.jns.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 152.Feng X, Wang Z, Howlett-Prieto Q, Einhorn N, Causevic S, Reder AT. Vitamin D enhances responses to interferon-beta in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e622. doi: 10.1212/NXI.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aivo J, Lindsrom BM, Soilu-Hanninen M. A randomised, double-blind, placebo-controlled trial with vitamin D3 in MS: subgroup analysis of patients with baseline disease activity despite interferon treatment. Mult Scler Int. 2012;2012:802796. doi: 10.1155/2012/802796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Soilu-Hanninen M, Aivo J, Lindstrom BM, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon beta-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(5):565–571. doi: 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 155.Ghasami K, Faraji F, Fazeli M, Ghazavi A, Mosayebi G. Interferon beta-1a and atorvastatin in the treatment of multiple sclerosis. Iran J Immunol. 2016;13(1):16–26. [PubMed] [Google Scholar]
- 156.Lanzillo R, Orefice G, Quarantelli M, et al. Atorvastatin combined to interferon to verify the efficacy (ACTIVE) in relapsing-remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Mult Scler. 2010;16(4):450–454. doi: 10.1177/1352458509358909. [DOI] [PubMed] [Google Scholar]
- 157.Markovic-Plese S, Jewells V, Speer D. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology. 2009;72(22):1965. doi: 10.1212/01.wnl.0000349667.27301.c8. [DOI] [PubMed] [Google Scholar]
- 158.Bhardwaj S, Coleman CI, Sobieraj DM. Efficacy of statins in combination with interferon therapy in multiple sclerosis: a meta-analysis. Am J Health Syst Pharm. 2012;69(17):1494–1499. doi: 10.2146/ajhp110675. [DOI] [PubMed] [Google Scholar]
- 159.Kamm CP, El-Koussy M, Humpert S, et al. Atorvastatin added to interferon beta for relapsing multiple sclerosis: 12-month treatment extension of the randomized multicenter SWABIMS trial. PLoS ONE. 2014;9(1):e86663. doi: 10.1371/journal.pone.0086663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Coyle PK. Pharmacogenetic biomarkers to predict treatment response in multiple sclerosis: current and future perspectives. Mult Scler Int. 2017;2017:6198530. doi: 10.1155/2017/6198530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Freedman MS, Wojcik J, Holmberg KH, et al. Pharmacodynamic biomarkers of long-term interferon beta-1a therapy in REFLEX and REFLEXION. J Neuroimmunol. 2021;360:577715. doi: 10.1016/j.jneuroim.2021.577715. [DOI] [PubMed] [Google Scholar]
- 162.Dicillo EB, Kountikov E, Zhu M, et al. Genome-wide mapping of patient autoantibody targets to understand and predict multiple sclerosis pathogenesis and patient responses to interferon beta-1a therapy. 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); Switzerland: 2021.
- 163.Hecker M, Hartmann C, Kandulski O, et al. Interferon-beta therapy in multiple sclerosis: the short-term and long-term effects on the patients' individual gene expression in peripheral blood. Mol Neurobiol. 2013;48(3):737–756. doi: 10.1007/s12035-013-8463-1. [DOI] [PubMed] [Google Scholar]
- 164.Hecker M, Thamilarasan M, Koczan D, et al. MicroRNA expression changes during interferon-beta treatment in the peripheral blood of multiple sclerosis patients. Int J Mol Sci. 2013;14(8):16087–16110. doi: 10.3390/ijms140816087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martinez-Aguilar L, Perez-Ramirez C, Maldonado-Montoro MDM, et al. Effect of genetic polymorphisms on therapeutic response in multiple sclerosis relapsing-remitting patients treated with interferon-beta. Mutat Res Rev Mutat Res. 2020;785:108322. doi: 10.1016/j.mrrev.2020.108322. [DOI] [PubMed] [Google Scholar]
- 166.Ayatollahi SA, Ghafouri-Fard S, Taheri M, Noroozi R. The efficacy of interferon-beta therapy in multiple sclerosis patients: investigation of the RORA gene as a predictive biomarker. Pharmacogenomics J. 2020;20(2):271–276. doi: 10.1038/s41397-019-0114-0. [DOI] [PubMed] [Google Scholar]
- 167.Kuhle J, Leppert D, Comi G, et al. Baseline serum neurofilament light chain levels predict conversion to McDonald 2005 multiple sclerosis (MS) within 2 years of a first clinical demyelinating event in patients with MS. AAN 2021 Virtual Congress; April 17–22, 2021. Program number P15.001
- 168.Kuhle J, Leppert D, Comi G, et al. Investigating the correlation between serum neurofilament light chain (sNfL) concentration and magnetic resonance imaging (MRI)-outcomes in patients with a first clinical demyelinating Event in the REFLEX Trial. AAN 2021 Virtual Congress; April 17–22, 2021. Program number P15.005
- 169.Coray M, Seitzinger A, Roy S, et al. Exploratory analysis of serum GDF-15 levels in patients receiving subcutaneous interferon β-1a in the REFLEX Trial. ECTRIMS 2021 Virtual Congress October 13–15, 2021. P674.
- 170.Berger T, Adamczyk-Sowa M, Csepany T, et al. Factors influencing daily treatment choices in multiple sclerosis: practice guidelines, biomarkers and burden of disease. Ther Adv Neurol Disord. 2020;13:1756286420975223. doi: 10.1177/1756286420975223. [DOI] [PMC free article] [PubMed] [Google Scholar]