Abstract
Advanced glycation end‐products (AGEs) are implicated in the pathogenesis of vascular disease. In previous studies we have found increased deposition of N(e)‐(carboxymethyl)lysine (CML) in intramyocardial vasculature in the heart in acute myocardial infarction and myocarditis. It is known that the process of inflammation plays a role in the formation of AGEs. In this study we have explored the presence of CML (a major AGE) in the heart of patients with epicarditis using a monoclonal anti‐CML antibody. Nine patients with epicarditis (n = 9) died and their hearts were used for this study, control were hearts from patients who died from conditions unrelated to heart disease and without signs of myocarditis or epicarditis CML deposition and complement were significantly increased in patients with epicarditis compared to control hearts. Thus epicarditis increases CML depositions in the intramyocardial vasculature.
Keywords: AGE's, epicarditis, intramyocardial vasculature
1. INTRODUCTION
Epicarditis describes the phenomenon of inflammation of the epicardium, the visceral layer of the pericardium, that can occur in the context of myocardial infarction (MI) and myocarditis, but which can also occur without overt additional heart disease. 1 Inflammation in the epi/pericardium can also play a role in the induction of MI as it may exacerbate atherosclerotic plaque vulnerability in epicardial coronary arteries. 2 Moreover, it is known that both MI and myocarditis can induce a pro‐inflammatory status of the intramyocardial vasculature, in which the advanced glycation endproduct (AGE) N(e)‐(carboxymethyl)lysine (CML) plays an important role. 3 Higher plasma levels of protein‐bound AGEs were associated with a higher risk of incident cardiovascular events in individuals with type‐2 diabetes mellitus. 4 In another study AGEs were associated with human rupture‐prone plaques. 5 Microvascular accumulation of AGEs such as CML is an indicator of microvascular inflammation and dysfunction.
We recently found a significant increase in CML accumulation coinciding with markers of increased pro‐inflammatory activation of the intramyocardial vascular endothelium in patients with lethal COVID‐19 that had inflammation both in the myocardium (myocarditis) and epicardium (epicarditis), mainly consisting of lymphocytes and macrophages. 6 , 7
With regard to our findings in these COVID‐19 patients with myo/epicarditis, we wondered whether in patients with epicarditis but not myocarditis the intramyocardial vasculature may also be affected. In this study, therefore, we quantified CML accumulation in the intramyocardial vasculature of deceased non‐COVID patients with epicarditis, but without myocarditis and/or MI, and compared these to control patients.
2. MATERIALS AND METHODS
2.1. Human heart tissue
Transmural left ventricular myocardial specimens were obtained during autopsy. The specimen was fixed in 4% formalin and subsequently embedded in paraffin for (immuno)histochemical analysis. Autopsy was performed within 24 h after death. We included control patients who died of a cause unrelated to heart disease and who did not have epicarditis (n = 11) and patients with histological signs of epicarditis (n = 9; see Figure 1) without signs of other forms of heart disease, including myocardial infarction and/or myocarditis. This study was approved by the ethics committee of the Vrije Universiteit Medical Center, Amsterdam. Use of remaining tissue after the pathological examination has been completed is part of the patient contract in our Hospital.
FIGURE 1.
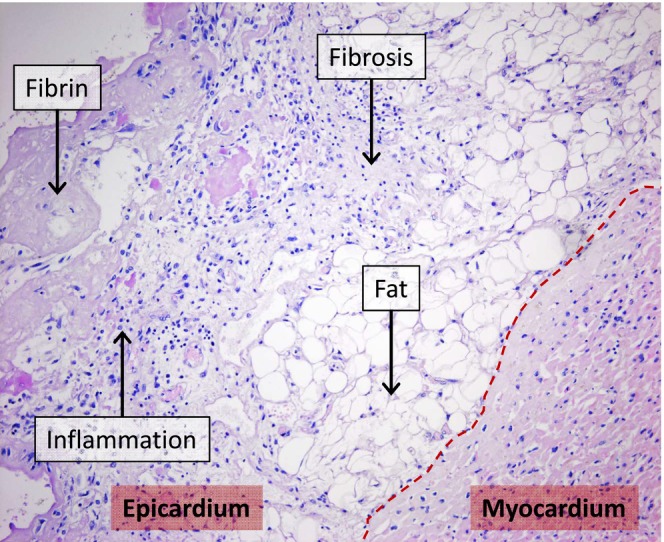
Haematoxylin–Eosin‐stained heart tissue of an epicarditis patient. Visible are the epicardial side of the myocardium and the epicardium with adipose tissue (fat), fibrosis, accumulated inflammatory cells (inflammation) and fibrin‐rich thrombus formation (fibrin). ***p = .0003.
2.2. Immunohistochemistry
Paraffin‐embedded tissue sections (4 μm) were mounted on microscope slides and were deparaffinized for 10 min in xylene at room temperature and re‐hydrated through descending concentrations of ethanol. The sections were treated with 0.3% H2O2 in methanol for 30 min to block endogenous peroxidase activity, after which they were incubated in pepsin for 30 min. This was followed by washing in phosphate‐buffered saline (PBS) and incubation for 60 min with mouse‐anti‐CML (1:250). 6 After washing in PBS, sections were incubated with Envision at room temperature for 30 min and subsequently washed in PBS. After that, sections were visualized with diaminobenzidine tetrahydrochloride (DAB) for Envision for 3–5 min.
2.3. Immunoscoring
CML staining was quantified using an intensity scoring method, whereby each CML‐positive blood vessel was given an intensity score that was weak (1), moderate (2) or strongly positive (3). To obtain the CML immunohistochemical (IH) score, each intensity score was multiplied by the number of blood vessels positive for this score. These were then added and subsequently divided by the surface area of the analysed tissue, resulting in an IH score per square centimetre. 3 The surface area of the tissue was determined using QPRODIT V.3.2 (Leica Microsystems, Cambridge, UK).
2.4. Statistics
Data analysis was performed with the statistical programs spss and GraphPad. Gender distribution between the groups was analysed with a Pearson chi‐square test. Mann–Whitney tests were used to compare patient age and CML scores between the groups. Statistical significance was defined as a value of p < .05.
3. RESULTS
The patient characteristics are shown in Table 1. Control patients (n = 11; six females and five males) were between 34 and 87 years old and epicarditis patients (n = 9; 2 females and seven males) were between 38 and 71 years old. There were no significant differences in age and gender distribution between the control and epicarditis groups. One of the included epicarditis patients had diabetes. Histopathological changes of the epicardium, including inflammatory cell infiltration of the epicardial fat tissue, fibrosis and fibrin deposition, consistent with epicarditis (Figure 1) were observed in patients of the epicarditis group.
TABLE 1.
Patient characteristics.
| Characteristic | Control (n = 11) | Epicarditis (n = 9) |
|---|---|---|
| Age (mean ± SD) | 67.9 ± 17.6 | 59.7 ± 11.0 |
| Gender (n, %) | ||
| Female | 6 (55%) | 2 (22%) |
| Male | 5 (45%) | 7 (78%) |
| Diabetes mellitus (n, %) | 0 (0%) | 1 (11%) |
| Cause of death (n, %) | ||
| Sepsis | 4 (36%) | 4 (44%) |
| Neuroblastoma | 1 (9%) | 0 (0%) |
| Pulmonary embolism | 1 (9%) | 0 (0%) |
| Pneumonia | 3 (17%) | 3 (33%) |
| Adenocarcinoma | 1 (9%) | 0 (0%) |
| Lung carcinoma | 0 (0%) | 1 (11%) |
| Breast carcinoma | 1 (9%) | 0 (0%) |
| Euthanasia | 2 (18%) | 0 (0%) |
| Car accident | 1 (9%) | 0 (0%) |
| Alcoholic cardiomyopathy | 0 (0%) | 1 (11%) |
CML was found in the endothelium and smooth muscle cells of intramyocardial blood vessels that were distributed diffusely throughout the myocardium. We found a significant increase in the CML IH‐score in epicarditis patients compared with controls (p = .0003) (Figure 2A). This increase in CML was related to significant increases in the numbers of blood vessels with moderate‐ (intensity score 2; p < .0001) and strong‐ (intensity score 3; p = .0001) CML staining in epicarditis patients compared with controls (Figure 2B).
FIGURE 2.
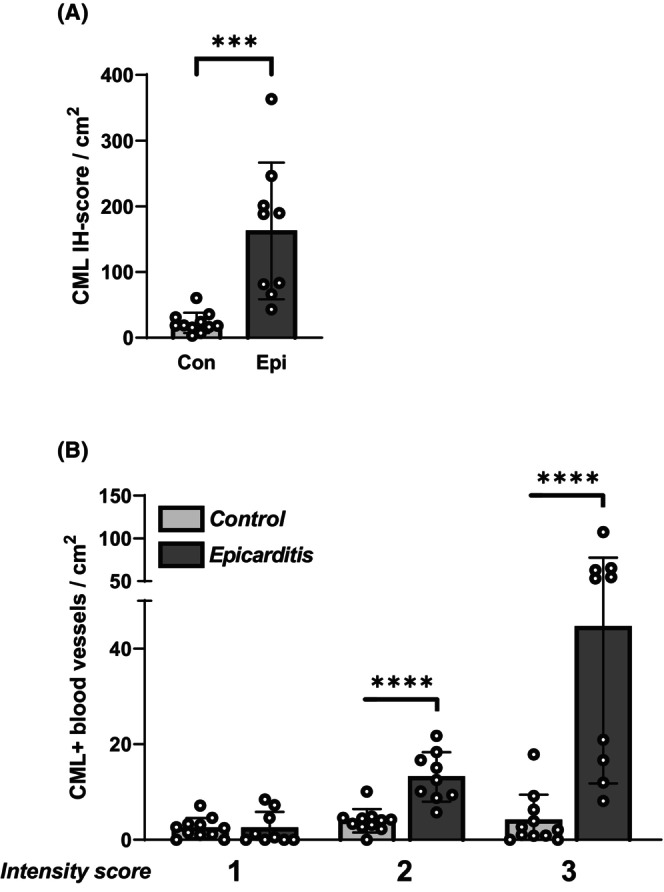
Quantification of N(e)‐(carboxymethyl)lysine (CML) in the intramyocardial vasculature. Shown are (A): the CML‐immunohistochemical (IH) score, as well as (B): the numbers of intramyocardial blood vessels with weak‐ (intensity score 1); moderate‐ (intensity score 2); and strong‐ (intensity score 3) CML staining. ***p = .0001, ****p < .0001.
4. DISCUSSION
Increased depositions of CML, coinciding with other markers of microvascular dysfunction, were found previously in the cardiac microvasculature of deceased COVID‐19 patients with both myocarditis and epicarditis. 8 We now found significantly increased CML accumulation in the intramyocardial vasculature and also in patients with sole epicarditis and without COVID‐19.
Epi/pericarditis can be idiopathic but can also be induced by viral‐, bacterial‐ and fungal infections, MI, trauma, surgery, neoplasms, radiation and uraemia due to renal failure. 8 Patients with epicarditis have an increased cardiovascular risk that is said to be most probably related to its effect on the vulnerability of atherosclerotic plaques in the coronary arteries within the epicardium. 2 Our study now indicates that this increased cardiovascular risk may also be attributable to a pro‐inflammatory change in intramyocardial vasculature.
The vascular accumulation of AGEs such as CML often coincides with increased oxidative stress and a pro‐inflammatory activation of the vasculature, 9 as we have shown in the hearts and brains of diabetic rats and MI patients. 3 , 10 , 11 In addition, AGE accumulation has been shown to play a pathophysiological role in vascular dysfunction, including impaired vasodilation and increased vascular permeability. 12 , 13 As such it is considered an indicator of vascular dysfunction. Our current findings thus indicate the occurrence of coronary microvascular dysfunction in patients with sole epicarditis, predominantly without diabetes, that may contribute to an increased cardiovascular risk.
This is therefore an observation of potential clinical importance. For instance, dysfunction of the coronary microvasculature can, among others, be an underlying factor in the development of stable ischemic heart disease, but is also considered a potential cause of MI in the absence of obstructive coronary artery disease. 14 Our previous observation of increased cardiac microvascular CML levels in patients very soon, that is within 6 h, after the onset of MI, suggests that in these patients the cardiac microvasculature may have been dysfunctional prior to the onset of MI. 3
In conclusion, we found increased CML levels in the intramyocardial vasculature of patients with sole epicarditis, which could point to a novel risk factor for further cardiac complications.
CONFLICT OF INTEREST STATEMENT
None.
ACKNOWLEDGMENTS
None.
Baylan U, Baidoshvili A, Simsek S, Schalkwijk CG, Niessen H, Krijnen P. Increased accumulation of the advanced glycation endproduct Ne(carboxymethyl) lysine in the intramyocardial vasculature in patients with epicarditis. Int J Exp Path. 2024;105:48‐51. doi: 10.1111/iep.12499
REFERENCES
- 1. Tingle LE, Molina D, Calvert CW. Acute pericarditis. Am Fam Physician. 2007;76(10):1509‐1514. [PubMed] [Google Scholar]
- 2. Konishi M, Sugiyama S, Sato Y, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213(2):649‐655. doi: 10.1016/j.atherosclerosis.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 3. Baidoshvili A, Krijnen PA, Kupreishvili K, et al. N(epsilon)‐(carboxymethyl)lysine depositions in intramyocardial blood vessels in human and rat acute myocardial infarction: a predictor or reflection of infarction? Arterioscler Thromb Vasc Biol. 2006;26(11):2497‐2503. doi: 10.1161/01.ATV.0000245794.45804.ab [DOI] [PubMed] [Google Scholar]
- 4. Nordin MJ, Hanssen NM, Beulens JW, et al. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: a case‐cohort study with amedian follow‐up of 10 years. Diabetes. 2015;64(1):257‐265. doi: 10.2337/db13-1864 [DOI] [PubMed] [Google Scholar]
- 5. Hanssen NM, Wouters K, Huijberts MS, et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture‐prone phenotype. Eur Heart J. 2014;35(17):1137‐1146. doi: 10.1093/eurheartj/eht402 [DOI] [PubMed] [Google Scholar]
- 6. Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID‐19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290‐e299. doi: 10.1016/S2666-5247(20)30144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu L, Baylan U, van der Leeden B, et al. Cardiac inflammation and microvascular procoagulant changes are decreased in second wave compared to first wave deceased COVID‐19 patients. Int J Cardiol. 2022;349:157‐165. doi: 10.1016/j.ijcard.2021.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramasamy V, Mayosi BM, Sturrock ED, Ntsekhe M. Established and novel pathophysiological mechanisms of pericardial injury and constrictive pericarditis. World J Cardiol. 2018;10(9):87‐96. doi: 10.4330/wjc.v10.i9.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Picchi A, Capobianco S, Qiu T, et al. Coronary microvascular dysfunction in diabetes mellitus: a review. World J Cardiol. 2010;2(11):377‐390. doi: 10.4330/wjc.v2.i11.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baylan U, Korn A, Emmens RW, et al. Liraglutide treatment attenuates inflammation markers in the cardiac, cerebral and renal microvasculature in streptozotocin‐induced diabetic rats. Eur J Clin Invest. 2022;52(9):e13807. doi: 10.1111/eci.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korn A, Baylan U, Simsek S, Schalkwijk CG, Niessen HWM, Krijnen PAJ. Myocardial infarction coincides with increased NOX2 and N(epsilon)‐(carboxymethyl) lysine expression in the cerebral microvasculature. Open Heart. 2021;8(2):e001842. doi: 10.1136/openhrt-2021-001842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582‐592. doi: 10.1016/j.cardiores.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 13. Zhang WJ, Li PX, Guo XH, Huang QB. Role of moesin, Src, and ROS in advanced glycation end product‐induced vascular endothelial dysfunction. Microcirculation. 2017;24(3):e12358. doi: 10.1111/micc.12358 [DOI] [PubMed] [Google Scholar]
- 14. Del Buono MG, Montone RA, Camilli M, et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;78(13):1352‐1371. doi: 10.1016/j.jacc.2021.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]


