Abstract
Dendritic cells, macrophages, neutrophils, and other antigen-presenting cells express various C-type lectin receptors that function to recognize the glycans associated with pathogens. The dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) binds various pathogens such as HIV glycoprotein 120, the Ebola glycoprotein, hemagglutinin, and the dengue virus glycoprotein in addition to the SARS-CoV-2 spike protein, and also triggers antigen-presenting cell endocytosis and immune escape from systemic infections. Many studies on the binding of SARS-CoV-2 spike protein with glycans have been published, but the underlying mechanism by which intracellular signaling occurs remains unclear. In this study, we report that the S1 spike protein of SARS-CoV-2 induces the phosphorylation of extracellular signal-regulated kinases (ERKs) in THP-1 cells, a DC-SIGN-expressing human monocytic leukemic cell line. On the other hand, the phosphorylation level of NF-κB remained unchanged under the same conditions. These data suggest that the major cell signaling pathway regulated by the S1 spike protein is the ERK pathway, which is superior to the NF-κB pathway in these DC-SIGN-expressing THP-1 cells and may contribute to immune hyperactivation in SARS-CoV-2 infections. Additionally, several glycans such as mannans, mannosylated bovine serum albumin, the serum amyloid beta protein, and intracellular adhesion molecule 3 suppressed ERK phosphorylation, suggesting that these molecules are target molecules for SARS-CoV-2 infection by suppressing immune hyperactivation that occurs in the ERK signaling pathway.
Keywords: SARS-CoV-2 S1, Dendritic cell, DC-SIGN, ERK, NF-κB
Introduction
C-type lectin receptors, which are expressed in innate immune cells, bind to carbohydrates in a calcium-dependent manner.1 More than 1000 proteins are defined as a superfamily of C-type lectin receptors, and these proteins contain C-type lectin-like domains.2 They play critical roles in the innate and adaptive immune systems by recognizing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).3 Upon recognition of PAMPs, DAMPs, etc., C-type lectin receptors enhance their internalization and recruitment to the lysosome where PAMPs-containing and/or DAMPs-containing molecules are digested.
The dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) molecule is one of the C-type lectin receptors expressed in the dendritic cells, tissue-resident macrophages, and B cells.4, 5 DC-SIGN was initially identified through its interaction with glycoprotein 120 on the HIV envelope.6 In addition to glycoprotein 120, the intracellular adhesion molecule 3 (ICAM3),7 and the S1 spike protein of SARS-CoV-2, etc., have been identified as the proteins that carry such carbohydrates, such as Lewis A (Lea), Lewis X (LeX) antigens, and oligo-mannose structure, which are recognized by DC-SIGN.9, 10, 11, 8
It is known that the human angiotensin-converting enzyme 2 interacts with the surface spike (S) protein of SARS-CoV and SARS-CoV-2 and acts as an entry receptor for it.12, 13, 14 In several tissues (the lung epithelial cells), however, the expression of angiotensin-converting enzyme 2 is very low to undetectable.15 This suggests that alternative receptors may exist in case of SARS-CoV-2 entry into an infection of certain human cells. The recognition facilitates their internalization, while also allowing them to transduce cell signals. The immunoreceptor tyrosine-based activation motif and immunoreceptor tyrosine-based inhibitory motif are key amino acid sequences for signal transduction in C-type lectin receptors, but DC-SIGN does not contain such motifs.16 In the macrophages, Khan and co-workers17 reported the crucial role of toll-like receptor 2 against SARS-CoV-2 spike proteins, however, the role of DC-SIGN in dendritic cells is not well understood.
Alternatively, DC-SIGN uses tyrosine kinase, the RAS/RAF pathway, and NF-κB pathway, which are required for an immune response, such as cytokine expression. It has been reported that DC-SIGN-triggered intracellular signals modulate dendritic cell maturations and cause extracellular signal-regulated kinase (ERK), phosphoinositide 3-kinase activation and modulate cytokine proliferation.18 Although the other cell signaling pathways have been analyzed, the integrative signaling pathway generated by DC-SIGN remains unclear. In this regard, we attempted to identify the cell signaling pathways by focusing on the ERK/mitogen-activated protein kinase pathway and attempted to clarify their roles in DC-SIGN-induced human monocytic leukemia cell, THP-1.
Results
DC-SIGN was induced in THP-1cells
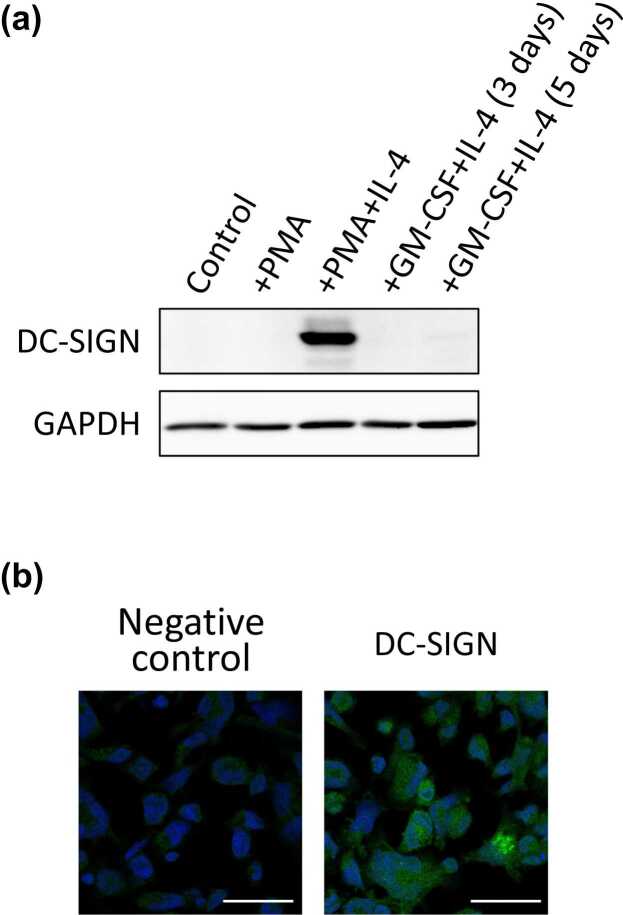
We first analyzed the expression of DC-SIGN in a human monocytic leukemia cell line, THP-1. The cells are differentiated into dendritic cell-like cells by incubation in the presence of phorbol 12-myristate 13-acetate (PMA) and interleukin-4 (IL-4).19, 20 After incubation with PMA or both PMA and IL-4, we analyzed the expression of DC-SIGN by Western blotting. At the same time, we also analyzed the cells that had been incubated with granulocyte-macrophage colony-stimulating factor and IL-4. As shown in Figure 1(a), a strong DC-SIGN expression was observed after incubation with both PMA and IL-4, but not with granulocyte-macrophage colony-stimulating factor. We confirmed the expression of DC-SIGN by immunocytochemical staining (Figure 1(b)). In immunocytochemical staining, we observed the cell surface and intracellular expression of DC-SIGN. These data indicate that both PMA and IL-4 are required for the induction of DC-SIGN expression in THP-1 cells.
Fig. 1.
The expression of DC-SIGN in THP-1 cells. (a) DC-SIGN expression in THP-1 cells was examined by Western blotting. After incubation with only PMA, both PMA and IL-4, or with GM-CSF and IL-4 for 3 days or 5 days, THP-1 cells were lysed and analyzed. GAPDH was used as a loading control. (b) The expression of DC-SIGN was examined by immunocytochemistry. After incubation with PMA and IL-4, the THP-1 cells were stained with an anti-DC-SIGN antibody and an anti-rabbit IgG antibody labeled with Alexa488. Counterstaining with Hoechst 333342 was additionally performed. Bars indicate 50 µm. Abbreviations used: DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-4, interleukin-4; PMA, phorbol 12-myristate 13-acetate.
S1 spike protein of SARS-CoV-2 induced the phosphorylation of ERKs in THP-1 cells
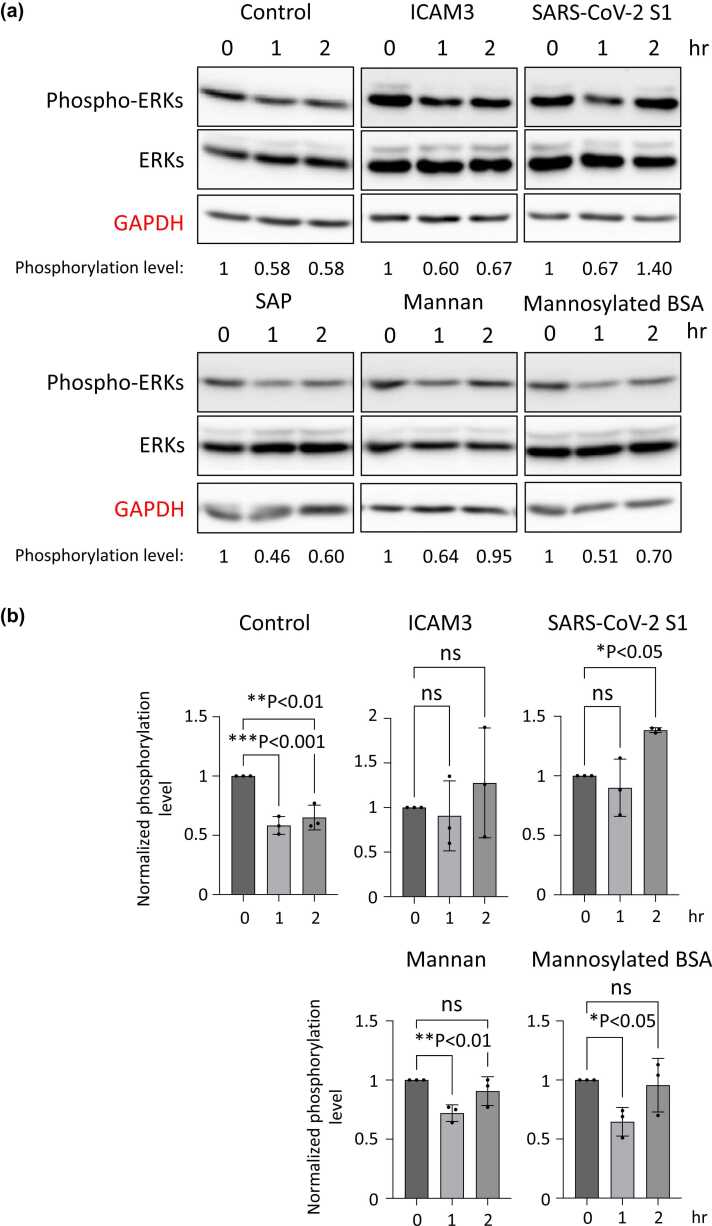
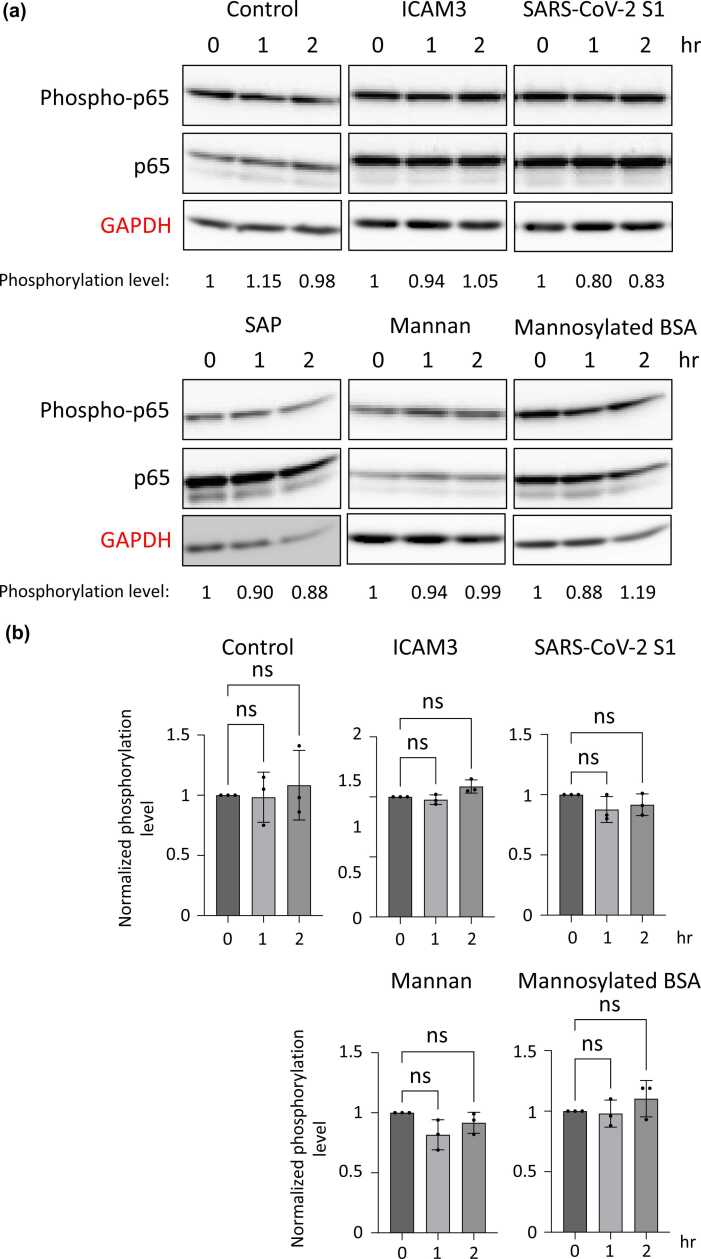
To analyze the cell signaling pathway transduced by DC-SIGN, we focused on the ICAM3,7 S1 spike protein of SARS-CoV-2,21 and serum amyloid P (SAP). Because they have been identified as the binding to and functional molecules of DC-SIGN. It has been speculated that the oligo-mannose type of glycan is also a molecule that is recognized by DC-SIGN.11 Hence, we analyzed the mannan and the bovine serum albumin (BSA) modified with mannoses (mannosylated BSA). As shown in Figure 2, we incubated the cells with these molecules for 0, 1, and 2 h and examined the phosphorylation level of ERKs by Western blotting. To easily analyze the phosphorylation level of ERKs, the concentration of fetal bovine serum (FBS) in the culture media was reduced to 0.2% (v/v) during the incubation with these molecules. Therefore, the phosphorylation level of ERKs was decreased after incubation in the control experiment. After incubation with ICAM3, SAP, mannan, or mannosylated BSA, the phosphorylation level of ERKs decreased to the level of the control. On the other hand, the incubation with S1 spike protein increased the phosphorylation level of ERKs, especially for 2 h (Figure 2(a) and (b)). Notably, the phosphorylation level of NF-κB p65 was unchanged under the same conditions (Figure 3(a) and (b)). The increased phosphorylation level of ERKs by S1 spike protein was partially blocked by treatment with the ERK inhibitor (Supplementary Figure S3). These data suggest that the S1 spike protein allows the phosphorylation level of ERKs to be sustained under the FBS-reducing condition, in other words, it induces the phosphorylation of ERKs in DC-SIGN-expressing THP-1 cells (Figure 4). In the Supplementary Figures S3 and S4, we performed similar experiments using undifferentiated THP-1 cells. The undifferentiated cells do not express DC-SIGN, as shown in Figure 1, and the phosphorylation levels of ERKs, as well as NF-κB p65, were not unregulated by SARS-CoV-2 S1 in these cells. These data provide evidence that the ERK signaling pathway induced by SARS-CoV-2 S1 is dependent on the DC-SIGN expression.
Fig. 2.
The phosphorylation level of ERKs in THP-1 cells after incubation with DC-SIGN-targeting molecules. (a) Representative results of Western blotting indicating the phosphorylation level of ERKs. After incubation with PMA and IL-4, THP-1 cells were subsequently incubated with recombinant ICAM3, SARS-CoV-2 S1, SAP, mannan, or mannosylated BSA for 1 and 2 h. Then, the cells were lysed, and the 20 µg of total proteins were analyzed. The band intensities of phospho-ERKs relative to total-ERKs were measured, and they were normalized to the control results. GAPDH is a loading control. (b) The phosphorylation levels of ERKs in three independent experiments are summarized. The band images of two additional times of Western blotting are shown in Supplementary Figure S1. *P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations used: BSA, bovine serum albumin; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; ERK, extracellular signal-regulated kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ICAM3, intracellular adhesion molecule 3; IL-4, interleukin-4; ns, not significant; PMA, phorbol 12-myristate 13-acetate; SAP, serum amyloid P.
Fig. 3.
The phosphorylation level of NF-κB p65 in THP-1 cells after incubation with DC-SIGN-targeting molecules. (a) Representative results of Western blotting indicating the phosphorylation level of NF-κB p65. The band intensities of phospho-p65 relative to total-p65 were measured, and were normalized to the control results. GAPDH is a loading control. (b) The phosphorylation levels of NF-κB p65 in three independent experiments are summarized. The band images of two additional times of Western blotting are shown in Supplementary Figure S2. Abbreviations used: BSA, bovine serum albumin; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ICAM3, intracellular adhesion molecule 3; ns, not significant; SAP, serum amyloid P.
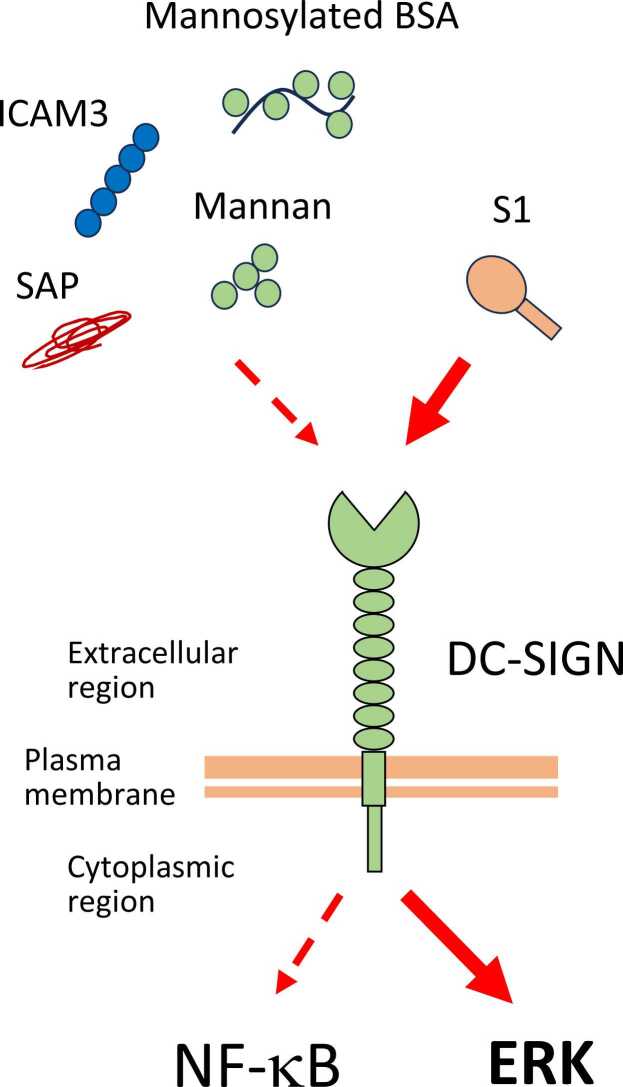
Fig. 4.
The S1 spike protein induces ERK pathway, which is superior to the NF-κB pathway in the DC-SIGN-expressing THP-1 cells. Abbreviations used: BSA, bovine serum albumin; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; ERK, extracellular signal-regulated kinase; ICAM3, intracellular adhesion molecule 3; SAP, serum amyloid P.
Discussion
The extracellular events switch individual cell signaling through the recognition by, or the activation of plasma membrane proteins. Individual cell signaling, usually cross-talk, converges on the downstream mediators that modulate gene expression patterns and define cell behavior.22 It is also noteworthy that dendritic cells are known to play a role in the balance between tolerance and the induction of immunity and help to distinguish harmless “self-antigens” from pathogens.
Three types of mitogen-activated protein kinase have been identified, such as ERK, c-Jun N-terminal kinase, and p38.23 Among them, ERK has been targeted for cancer therapy because the abnormal activation of ERK is frequently observed in many types of cancer and its activation could facilitate faster cell growth by upregulating genes involved in the driving the cell cycle.24 On the other hand, ERK also plays an important role in dendritic cells. For example, the ERK signaling mediated by the C-C chemokine receptor type 7 is required for the survival of human mature dendritic cells.25 ERK signaling is also involved in the production of tumor necrosis factor α and interleukin-10 in myeloid dendritic cells.18 In addition, Groft and co-workers26 reported that the toll-like receptor 2 transduces ERK signaling and mediates the induction of interleukin-12, which may promote the priming of Th1 responses in dendritic cells. Knowledge of DC-SIGN-mediated ERK signaling is currently accumulating,27 although these reports suggest that ERK signaling has an essential role in the immune responses of dendritic cells.
The S1 spike protein of SARS-CoV-2 is a highly glycosylated protein.28 The S1 protein region carries a total of 16 N-glycans, which are mixed oligo-mannose types and hybrid types. ICAM3 is also a highly glycosylated protein with at least five N-glycans in its N-terminal domain,29 however, we did not detect an increase in the phosphorylation level of ERKs after incubation with ICAM3. These results suggest that the glycan itself is not sufficient for the induction of ERK signaling, implying that the amino acid sequence of the non-self pathogen would also be required for the transduction of ERK signaling in DC-SIGN-expressing THP-1 cells. In addition, incubation with mannose or mannosylated BSA failed to induce the phosphorylation of ERKs in these cells. This also indicates that the oligo-mannose type of N-glycan is not sufficient for the induction of ERK signaling, suggesting that the hybrid type of N-glycan is required to induce the ERK signaling.
NF-κB signaling is the central pathway for immune responses in dendritic cells.30 However, our data also demonstrate the regulation of ERK signaling as an alternative pathway. Further studies will be required to uncover the recognition and transduction machinery by DC-SGIN, although these data provide new insights into our understanding of the immune responses against SARS-CoV-2 infection.
Materials and methods
Cells and cell culture
Human monocytic leukemia cell line: THP-1, was obtained from the RIKEN BioResource Research Center (Tsukuba, Japan). THP-1 cells were cultured in RPMI-1640 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) supplemented with 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin (Thermo Fisher Scientific, Waltham, MA) at 37 ℃ under 5% CO2.
Differentiation of THP-1 cells
THP-1 cells (2 × 106) were initially incubated with PMA (10 ng/mL) (Sigma-Aldrich, St. Louis, MO) for 24 h. Th cells were then incubated with fresh PMA (10 ng/mL) and recombinant human IL-4 (20 ng/mL) (R&D systems, Minneapolis, MN) for an additional 3 days.
Immunocytochemical staining
Cells were washed three times with ice-cold phosphate-buffered saline (PBS) and fixed with 4% (w/v) paraformaldehyde in PBS for 10 min. After washing three times with PBS, the cells were incubated with 0.5% (v/v) TritonX-100 in PBS for 10 min at room temperature for permeabilization. After washing three times with 0.1% (v/v) TritonX-100 in PBS (PBST), the cells were blocked by incubation with 5% (w/v) BSA in 0.3% (v/v) TritonX-100 in PBS for 1 h at room temperature. After washing three times with PBST, cells were incubated with an anti-DC-SIGN antibody (Cell Signaling Technology, Danvers, MA) and an anti-rabbit IgG antibody labeled with Alexa488 (Thermo Fisher Scientific) sequentially. Counterstaining with Hoechst33342 was also performed, and the resulting cells were mounted with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific). The staining pattern was analyzed by fluorescent microscopy, BZ-X710 (Keyence Corporation, Osaka, Japan).
Modification of BSA with mannose
Mannosylated BSA was prepared as previously described.31 Briefly, a freshly prepared solution of 4-(a-D-mannopyranosyl)phenyl isothiocyanate (1.5 mg in 100 µL DMSO) (Sigma-Aldrich) was added to a BSA-fluorescein solution (2.6 mg in 400 µL 50 mM NaHCO3 buffer (pH 9.0)) (Sigma-Aldrich). Then the mixture was rotated at 4 °C for 24 h. After the coupling reaction, excess 4-(a-D-mannopyranosyl) phenyl isothiocyanate was washed four times with 10 mM NaHCO3 buffer (pH 9.0) with Amicon Ultra-15 centrifugal filter devices (Merck Millipore, Burlington, MA). The concentration of mannosylated BSA was quantified with a BCA protein assay kit (BioRad Laboratories, Hercules, CA).
Preparation of cell lysates
After incubation with PMA and IL-4, the resulting cells were washed with PBS twice and incubated with ICAM3 (1 µg/mL, PeproTech, Cranbury, NJ), SARS-CoV-2 S1 (1 µg/mL, Abcam, Cambridge, UK), SAP (1 µg/mL, Abcam), Mannan (10 µg/mL, Nacalai Tesque, Inc., Kyoto, Japan), or mannosylated BSA (20 µg/mL) in the culture media containing 0.2% (v/v) of FBS. After incubation for 0, 1, or 2 h, the cells were washed three times with ice-cold PBS and lysed by treatment with RIPA buffer (50 mM Tris-HCl (pH 8.0), 150 mM sodium chloride, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) sodium dodecyl sulfate, 1% (w/v) NP-40) containing cOmplete (Roche, Basel, Switzerland) and PhosSTOP phosphatase inhibitor (Roche). The lysates were sonicated briefly and centrifuged at 17,900 g at 4 ℃ for 20 min to remove the insoluble materials. The protein concentration of the lysates was determined with a BCA protein assay kit (BioRad Laboratories).
Western blotting
Cell lysates were separated by SDS-PAGE using 10% (w/v) acrylamide gels. After transferring to a nitrocellulose membrane (Merck Millipore), the membrane was blocked with 5% (w/v) BSA (Nacalai Tesque, Inc.) or 5% (w/v) skim milk (Nacalai Tesque, Inc.) in TBST (0.1% (v/v) Tween 20 in TBS). The membrane was then incubated with an anti-DC-SIGN antibody (Cell Signaling Technology), an anti-glyceraldehyde-3-phosphate dehydrogenase antibody (Merck Millipore), an anti-phospho-ERK antibody (Cell Signaling Technology), an anti-ERKs antibody (Cell Signaling Technology), an anti-phospho-p65 antibody (Cell Signaling Technology), or an anti-p65 antibody (Cell Signaling Technology) diluted in the blocking buffer. After washing four times with TBST, the membrane was then incubated with an anti-rabbit IgG, or an anti-mouse IgG labeled with horseradish peroxidase (GE Healthcare, Chicago, IL). To visualize the bands, the membrane was analyzed with Amersham ECL Prime Western Blotting Detection Reagents (GE Healthcare) and ImageQuant LAS4000 mini (GE Healthcare). The band intensities were measured by using the ImageJ software.
Statistical analysis
All data are presented as the mean ± standard deviation. For comparison between multiple groups, the one-way ANOVA test or the two-way ANOVA test was performed. The Dunnett’s multiple comparisons test was used to compare control and test samples.
Conclusion
The major cell signaling pathway regulated by S1 spike protein was found to be the ERK pathway, which is superior to the NFkB pathway in the DC-SIGN-expressing THP-1 cells. This fact may contribute to immune hyperactivation in SARS-COV-2 infection. These activations were suggested by inhibiting ERK signaling by several glycans such as mannan, mannosylated BSA, serum amyloid beta protein, and ICAM3 suggesting that these molecules are target molecules for the SARS-CoV-2 infection.
Funding and support
This study was supported by the JST SPRING program (Support for Pioneering Research Initiated the Next Generation) (JPMJSP2138 to E.L.J.). This study was also supported by Grants-in-Aid for Scientific Research (23K06734 to N.T., 23K06420 to Y.O., 22H02967/A22Z029670 to E.M.), the public grants from Osaka Prefecture (5200009052000093), and the personal contribution (20211201).
Declarations of interest
The authors declare no conflicts of interest associated with this study.
Acknowledgments
The authors wish to thank Dr Yoichiro Harada in the Department of Glyco-Oncology and Medical Biochemistry and Dr Shigeki Higashiyama, Dr Yusuke Imagawa, Dr Toru Hiratsuka, and Dr Yosuke Matsuoka in the Department of Oncogenesis and Growth regulation at Osaka International Cancer Institute for their generous supports. The authors also wish to thank Dr Milton S. Feather for English editing.
Author contribution
E.L.J., Y.O., and N.T. designed this study. E.L.J., Y.O., N.K., R.F., and T.K. performed experiments. E.L.J. and Y.O. drafted the manuscript. E.M. and N.T. revised the manuscript. All authors agreed with the submission of this manuscript.
Footnotes
Supplementary data associated with this article can be found online at doi:10.1016/j.cstres.2024.03.002.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Data availability statement
Data will be made available on request.
References
- 1.Osorio F., Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–664. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Brown G.D., Willment J.A., Whitehead L. C-type lectins in immunity and homeostasis. Nat Rev Immunol. 2018;18:374–389. doi: 10.1038/s41577-018-0004-8. [DOI] [PubMed] [Google Scholar]
- 3.Tang D., Kang R., Coyne C.B., et al. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koppel E.A., Van Gisbergen K.P.J.M., Geijtenbeek T.B.H., Van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 5.Rappocciolo G., Piazza P., Fuller C.L., et al. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLOS Pathogens. 2006;2 doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong P.W.-P., Flummerfelt K.B., de Parseval A., et al. Human immunodeficiency virus envelope (gp120) binding to DC-SIGN and primary dendritic cells is carbohydrate dependent but does not involve 2G12 or cyanovirin binding sites: implications for structural analyses of gp120-DC-SIGN binding. J Virol. 2002;76:12855–12865. doi: 10.1128/JVI.76.24.12855-12865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geijtenbeek T.B.H., Torensma R., Vliet S.J. van, et al. Identification of DC-SIGN, a novel dendritic cell–specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 8.Geurtsen J, Driessen NN, Appelmelk BJ. Chapter 34 - Mannose–fucose recognition by DC-SIGN ScienceDirect. Microbial GlycobiologyS tructures, Relevance and Applications. 2010;673-695. 10.1016/B978-0-12-374546-0.00034-1. [DOI]
- 9.Nonaka M., Ma B.Y., Murai R., et al. Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells1. J Immunol. 2008;180:3347–3356. doi: 10.4049/jimmunol.180.5.3347. [DOI] [PubMed] [Google Scholar]
- 10.Pederson K., Mitchell D.A., Prestegard J.H. Structural characterization of the DC-SIGN–LewisX complex. Biochemistry. 2014;53:5700–5709. doi: 10.1021/bi5005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Liempt E., Bank C.M.C., Mehta P., et al. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W., Moore M.J., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hikmet F., Méar L., Edvinsson Å., et al. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Fresno C., Iborra S., Saz-Leal P., et al. Flexible signaling of myeloid C-type lectin receptors in immunity and inflammation. Front Immunol. 2018;9:804. doi: 10.3389/fimmu.2018.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan S., Shafiei M.S., Longoria C., et al. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife. 2021;10 doi: 10.7554/eLife.68563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caparrós E., Munoz P., Sierra-Filardi E., et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 19.Jin C., Wu L., Li J., et al. Multiple signaling pathways are involved in the interleukine-4 regulated expression of DC-SIGN in THP-1 cell line. BioMed Res Int. 2012;2012 doi: 10.1155/2012/357060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puig-Kröger A., Serrano-Gómez D., Caparrós E., et al. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem. 2004;279:25680–25688. doi: 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- 21.Thépaut M., Luczkowiak J., Vivès C., et al. DC/L-SIGN recognition of spike glycoprotein promotes SARS-CoV-2 trans-infection and can be inhibited by a glycomimetic antagonist. PLOS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vert G., Chory J. Crosstalk in cellular signaling: background noise or the real thing? Dev Cell. 2011;21:985–991. doi: 10.1016/j.devcel.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta - Mol Cell Res. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Chambard J.-C., Lefloch R., Pouysségur J., Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta - Mol Cell Res. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 25.López-Cotarelo P., Escribano-Díaz C., González-Bethencourt I.L., et al. A novel MEK-ERK-AMPK signaling axis controls chemokine receptor CCR7-dependent survival in human mature dendritic cells. J Biol Chem. 2015;290:827–840. doi: 10.1074/jbc.M114.596551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groft S.G., Nagy N., Boom W.H., Harding C.V. Toll-like receptor 2-Tpl2-dependent ERK signaling drives inverse interleukin 12 regulation in dendritic cells and macrophages. Infect Immunity. 2020;89 doi: 10.1128/iai.00323-20. PMID: 33077627; PMCID: PMC7927937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L.-J., Wang W., Ren H., Qi Z.-T. ERK signaling is triggered by hepatitis C virus E2 protein through DC-SIGN. Cell Stress Chaperones. 2013;18:495–501. doi: 10.1007/s12192-013-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe Y., Allen J.D., Wrapp D., et al. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song G., Yang Y., Liu J., et al. An atomic resolution view of ICAM recognition in a complex between the binding domains of ICAM-3 and integrin αLβ2. Proc Natl Acad Sci. 2005;102:3366–3371. doi: 10.1073/pnas.0500200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Sig Transduct Target Ther. 2017;2:1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y.-P., Park S., Oh E., et al. On-chip detection of protein glycosylation based on energy transfer between nanoparticles. Biosens Bioelectron. 2009;24:1189–1194. doi: 10.1016/j.bios.2008.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Data Availability Statement
Data will be made available on request.