Abstract
Feline immunodeficiency virus (FIV) provides a model system with which the significance of neutralizing antibody (NA) in immunosuppressive lentivirus infections may be studied. To date, no detailed analysis of the neutralization properties of primary FIV isolates has been reported. In this study, we have conducted the first comprehensive study of the sensitivity to autologous and heterologous neutralization in a lymphoid cell-based assay of 15 primary FIV isolates and, for comparison, of one tissue culture-adapted strain. Primary isolates in general proved highly NA resistant, although there was considerable individual variation. Variation was also observed in the capacity of immune sera to neutralize heterologous FIV isolates. The ability of sera to neutralize isolates or for isolates to be neutralized by sera did not correlate with epidemiological and genetic relatedness or with the quasispecies complexity of the isolates. From the study of specific-pathogen-free cats experimentally infected with viral isolates associated with NA of different breadths, it appears that the development of FIV vaccines cannot rely on the existence of viral strains inherently capable of inducing especially broad NA responses.
Feline immunodeficiency virus (FIV) is a lentivirus that is regarded as the feline counterpart of human immunodeficiency virus (HIV) because it produces persistent infections of domestic cats which, after an incubation period of several years, progress to clinical manifestations of immunodeficiency and neurological damage that closely resemble those observed in HIV-infected humans. FIV is therefore a valuable model for investigating many aspects of AIDS pathobiology and control, including vaccination (4, 11, 39, 56).
Based on DNA phylogenesis, FIV isolates worldwide have been classified into at least five distinct genetic subtypes, designated A to E, with uneven geographical distributions (2). While there is little hope of developing a monovalent vaccine capable of protecting across different FIV subtypes, a more reasonable goal is the development of one or several protective immunogens for each subtype and subsequent selection of the immunogens on the basis of the subtypes prevalent in the area where the vaccine is to be used (56). However, because genetic diversity is also high within a subtype, especially in the env region (2, 42), successful vaccines will have to induce immune responses effective against a wide range of antigenically diverse strains. Mapping the immunological relatedness of FIV strains belonging to the same genetic subtype therefore represents a prerequisite for identifying shared critical protective epitopes and an essential step for ongoing vaccine development efforts. Similar problems exist for HIV vaccine development (33).
Although the humoral and cell-mediated immune responses that will eventually prove important for vaccine-induced protection against lentiviruses are unresolved (3, 7, 17), the ability to evoke a broadly reactive neutralizing-antibody (NA) response would seem to be an advantageous feature of candidate immunogens because it would at least contrast the dissemination of the initial viral inoculum from the site of entry (8, 9). In previous studies, we found that cats immunized with a fixed-cell vaccine were protected against FIV challenge in the apparent absence of NA (27, 28), but it is possible that a detectable NA response could be elicited with improved vaccines, adjuvants, and immunization regimens.
FIV vaccines must be designed to protect against strains of FIV as they circulate in nature. For this reason, it is important to learn more about the immunobiological properties of fresh clinical isolates, including their ability to evoke and interact with NA and their neutralizing determinant(s). Here we report on the sensitivity of 15 FIV isolates subjected to minimal passage in culture to neutralization by autologous and heterologous immune sera. Primary FIV isolates proved only slightly prone to inhibition by immune sera. However, certain isolates were more neutralizable by heterologous sera than others and certain infected cat sera neutralized fairly large numbers of primary isolates. A relationship was also sought between neutralization properties of the isolates and immune sera and a number of factors that theoretically might influence the induction or the activity of cross-reactive NA, including epidemiological and genetic relatedness and quasispecies complexity of the isolates. Finally, to ascertain whether the cross-neutralizing potency of anti-FIV antibody was dependent on properties of the viruses that had induced their formation, we studied the NA response of specific-pathogen-free (SPF) cats inoculated with selected FIV isolates.
MATERIALS AND METHODS
Cells and FIV isolates.
MBM cells are a line of CD3+ CD4− and CD8− T lymphocytes originally established from the peripheral blood mononuclear cells (PBMC) of an FIV-negative and feline leukemia virus-negative cat (26). They are routinely grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 5 μg of concanavalin A, and 20 U of recombinant human interleukin-2 per ml.
The 15 primary isolates studied were designed by an M followed by a number. The M2 and M3 isolates were obtained from SPF cats that had been inoculated with blood from a naturally infected cat and with supernatant of primary MBM cultures, respectively. All the others were derived directly from randomly selected feline leukemia virus-negative, FIV-seropositive domestic cats from two Italian regions. Isolation was performed by standard coculture of the PBMC with MBM cells (13). As summarized in Table 1, eight isolates were obtained from cats living in an open shelter, four were obtained from free-roaming cats living in the same geographical area (maximum distance, 20 miles), and three were obtained from free-roaming cats living in distant areas (minimum distance, 100 miles). The duration of infection for these cats was not known. However, nine were apparently healthy, five presented symptoms compatible with feline AIDS-related complex, and one was in the AIDS stage. The isolates varied considerably with regard to the time the cultures were first positive and to the levels of reverse transcriptase (RT) produced at isolation (Table 1); however, three to five passages in MBM cells were sufficient to expand the virus and accumulate viral stocks of suitable titer. Only one isolate (M88) produced clearly distinguishable syncytia in MBM cells. Isolates M45, M73, and M82 were characterized as belonging to genotype B by sequencing a 308-bp gag segment specifically for this study. The others had been characterized previously and were also of subtype B (42). The Petaluma (Pet) strain, a genotype A virus, consisted of cell-free supernatant from chronically infected FL4 cells (59; kindly provided by J. K. Yamamoto) at their 181st passage.
TABLE 1.
Primary FIV isolates used in this study
| Source and official namea | Shortened name | Health conditions of donor catb | Growth
characteristicsc
|
Genotype | ||
|---|---|---|---|---|---|---|
| D | RT | Syncytia | ||||
| Open-shelter cats | ||||||
| ITTO017PIU | M17 | Asymptomatic | 14 | 21.0 | No | B |
| ITTO019PIU | M19 | Asymptomatic | 15 | 9.9 | No | B |
| ITTO022PIU | M22 | Asymptomatic | 18 | 2.9 | No | B |
| ITTO026PIU | M26 | Asymptomatic | 35 | 4.7 | No | B |
| ITTO045PIU | M45 | FAIDS | 7 | 1.9 | No | B |
| ITTO064PIU | M64 | Asymptomatic | 19 | 0.7 | No | B |
| ITTO073PIU | M73 | Asymptomatic | 35 | 6.3 | No | B |
| ITTO082PIU | M82 | Asymptomatic | 10 | 5.7 | No | B |
| Free-range cats living within 20 miles from shelter | ||||||
| ITTO002PIU | M2 | Asymptomatic | 10 | 3.3 | No | B |
| ITTO003PIU | M3 | Asymptomatic | 33 | 1.5 | No | B |
| ITTO088PIU | M88 | Secondary infections | 10 | 5.0 | Yes | B |
| ITTO089PIU | M89 | Kidney failure | 15 | 11.7 | No | B |
| Free-range cats from distant sites | ||||||
| ITER091PIU | M91 | Stomatitis | 12 | 1.7 | No | B |
| ITER092PIU | M92 | Stomatitis | 33 | 59.2 | No | B |
| ITER097PIU | M97 | Stomatitis | 38 | 2.5 | No | B |
The first two capital letters indicate the country of origin; the next two define the region of origin (TO, Tuscany; ER, Emilia Romagna); the numbers indicate the reference number of the isolate; the last three letters indicate the study source (Pisa University).
At the time of virus isolation.
Growth characteristics in isolation cultures: D, day of first positivity; RT, RT activity when first positive, expressed as 103 counts per minute.
Viral stocks consisted of supernatant fluids clarified at 350 × g for 15 min, stored in 1-ml aliquots in liquid nitrogen until use, and subjected to titer determination at least twice by end-point dilution in quadruplicate in a microtiter MBM cell assay after 1 h at 4°C, i.e., under the same incubation conditions used for the neutralization test. Titers were remarkably similar, ranging between 2 × 102 and 1 × 103 50% tissue culture infective doses (TCID50)/ml.
Serum samples.
Immune sera were obtained from the same donors from which the primary FIV isolates had been obtained, from the same venipuncture as the viral isolates or after an interval of 3 to 12 months, and were given the same identification numbers. Anti FIV-Pet serum was obtained from an SPF cat infected 11.5 months earlier with the supernatant of FL4 cells at their 181st passage. Normal cat serum (NCS) was pooled from 10 adult SPF cats. Before use, all the sera were treated at 56°C for 30 min and checked for infectious FIV by standard culture.
Neutralization assay.
Virus neutralization was performed against 10 TCID50 of FIV by using MBM cells as indicator cells, with quantitation of RT activity in the supernatant as an end point. Each isolate, diluted to 200 TCID50/ml, was incubated with fourfold serial dilutions of sera (as reported in reference 3, plasma could not be used because it is toxic to MBM cells) at 4°C for 1 h and then inoculated (100 μl/well) into 2 to 4 wells of 96-well flat-bottom microplates containing 105 cells in 100 μl. The medium was RPMI 1640 supplemented with 10% fetal bovine serum, 6% NCS, 5 μg of concanavalin A (Sigma, St. Louis, Mo.) per ml, and 20 U of recombinant human interleukin-2 (Boehringer, Mannheim, Germany) per ml. Four days after inoculation, 100 μl of supernatant was removed from each well and replaced with fresh medium (input virus-immune serum mixtures were not washed away from the cells because, as reported previously [3], this step resulted in complete ablation of neutralization). Growth of the cultures was stopped on day 8, when newly produced RT was clearly evident. RT values were chosen rather than p25 antigen values as an indicator of viral replication because the high titer of anti-p25 antibodies in immune sera interfered with p25 determination at the end of the experiment while careful controls showed that residual immune serum did not significantly interfere with RT determination. In each assay, the titer of the input virus was determined in quadruplicate, and if the titer differed by more than threefold, the assay was considered invalid. All experiments were repeated at least twice. In general, the reproducibility was satisfactory since NA titers with a given virus-serum combination exhibited a maximum twofold deviation between experiments, which is within the expected error of neutralization assays with the format used.
Since preliminary experiments had shown that NA titers rarely exceeded 128, immune sera were used diluted 1:8, 1:32, and 1:128 (dilutions before the addition of virus and cells). Despite the presence of 6% NCS in the assay medium, we noticed that FIV preincubation with NCS produced nonspecific effects, occasionally reducing but more often facilitating the growth of FIV. For this reason, each experiment included control cultures receiving virus incubated with fourfold dilutions of NCS, and the percent inhibition of RT production by immune sera was calculated with respect to the corresponding dilution of NCS, using the formula [(mean cpm in cultures inoculated with FIV and immune serum − mean cpm of noninfected cultures)/(mean cpm of cultures inoculated with FIV and the corresponding dilution of NCS − mean cpm of noninfected cultures)] × 100. NA titers were defined as the reciprocal of the serum dilution required to reduce the levels of RT activity produced by ≥50% and calculated by the method of Reed and Muench (44). As recently pointed out by Moore et al. (35), the biological relevance of a twofold reduction in viral infectivity is unclear, but we could not adopt a higher cutoff because it would have almost completely obscured the neutralizing activity of immune sera.
As discussed below, some immune serum-virus combinations showed levels of RT higher than those produced by the control cultures incubated with the corresponding dilution of NCS. This effect was considered significant and scored as virus enhancement when the increase was at least twofold.
SSCP analysis.
RNA extracted from aliquots of the viral stocks was reverse transcribed and amplified for 35 cycles (denaturation at 94°C for 30 s, annealing at 52°C for 30 s, and primer extension at 72°C for 45 s) with outer primers (TGTTATGTAGACAGAGTAGAT, positions 7113 to 7133, and TGCAAGACCAATTTCCAGCA, positions 7847 to 7866) covering a 753-bp fragment encompassing the V3 and V4 regions of the env gene. Two second-round PCRs were then performed for each sample, one with a V3-specific antisense primer (CTTGAACTTCTCATTGCAAA, positions 7535 to 7554) and the other with a V4-specific sense primer (AACCTTTGCAATGAGAAGTT, positions 7532 to 7551), to obtain 441- and 334-bp fragments of the V3 and V4 regions, respectively. The resulting amplicons were separated on a 1.5% agarose gel, extracted from the gel, and purified with the QIAquick gel extraction kit (Qiagen, Chatsworth, Calif.). Asymmetric PCRs were then carried out with 5 μl of the purified product with the same parameters and conditions as above, except for the use of the inner antisense primers only. Single-strand conformation polymorphism (SSCP) analysis was performed essentially as described previously (22). Briefly, 1 volume of the single-stranded DNA products was mixed with 2 volumes of formamide loading dye (95% deionized formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol) and electrophoresed in a 10% nondenaturing polyacrylamide gel containing 0.5× TBE buffer, 1.5% N,N′-methylenebisacrylamide, and 5% glycerol. Electrophoresis was carried out immediately after loading at 23°C at 40 mA for 4 to 5 h. Finally, the gels were visualized by silver staining and dried on Whatmann 3MM paper. The electrophoretic mobility of the products was identified with a GDS 7500 gel documentation system (UVP, Cambridge, England), and the numbers of SSCP bands were analyzed with the GelBase Pro software program (UVP).
Infection of SPF cats.
SPF cats were infected with selected FIV isolates by intravenous inoculation of 105 infected autologous cells. The inocula were prepared by culturing the PBMC of each individual animal in concanavalin A for 3 days, infecting 14 × 106 to 26 × 106 cells with 6 × 102 TCID50 of the viral isolates under scrutiny, and harvesting after an additional 8 days of incubation, when 55 to 80% of the cells were FIV positive by surface immunofluorescence.
RESULTS
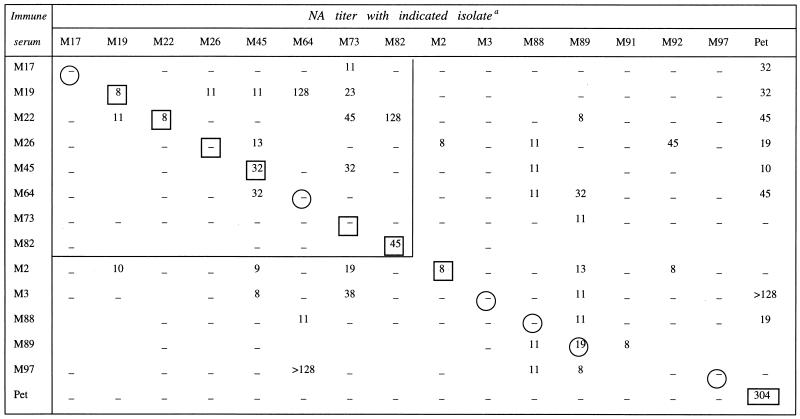
Overall, primary FIV isolates proved weakly sensitive to neutralization. Throughout the experiments, 90% neutralization was observed only with one virus-serum combination and, as shown in Table 2, which summarizes the mean NA titers obtained with the primary isolates and the immune sera in at least two independent checkboard analyses, even 50% inhibition was sporadic and produced NA titers that remained frequently of borderline significance.
TABLE 2.
Results of checkboard neutralization assays performed with 15 primary FIV isolates and autologous and heterologous immune sera and results with the tissue culture-adapted strain FIV-Pet (for comparison)
Values shown are the geometric means of the titers obtained in two or three independent assays. Dashes indicate that no neutralizing activity was detected; mean titers of <8 were not considered significant and scored as negative. The large box encloses the virus-immune serum sets obtained from cats living in the same shelter. Autologous virus-immune serum combinations are enclosed in a circle (sera obtained before or contemporaneously with virus isolation) or in a small box (sera obtained 3 to 12 months after virus isolation).
Neutralization by autologous immune sera.
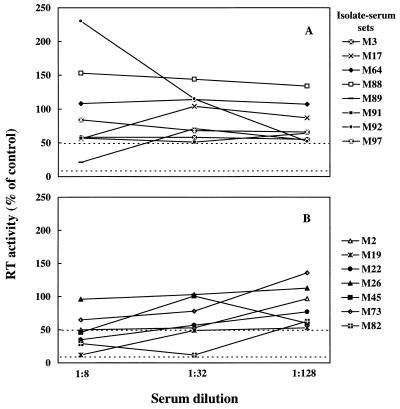
We could test autologous sera for all 15 primary isolates. As depicted in Fig. 1, despite the use of fourfold dilutions, neutralization curves were often irregular in shape. Of the eight isolates reacted with serum collected before or at the same time as virus isolation (Fig. 1A), only M89 was significantly (>50%) neutralized, whereas of the seven isolates reacted with serum taken 3 to 12 months after virus isolation, five were neutralized (Fig. 1B). The maximum mean NA titer observed with primary isolates was 45. In contrast, the tissue-culture-adapted FIV-Pet yielded a mean titer of 304 (Table 2), thus confirming its pronounced susceptibility to homologous NA (3).
FIG. 1.
Neutralization curves of 15 primary FIV isolates reacted with autologous sera. Sera were obtained before or contemporaneously with (A) or 3 to 12 months after (B) virus isolation. Dashed lines represent the 50 and 90% neutralization levels.
Neutralization by heterologous immune sera.
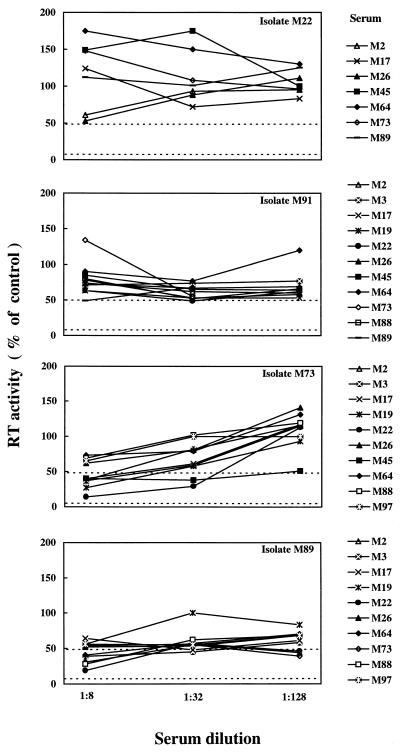
Sera M91 and M92 could not be tested with heterologous viruses due to insufficient volumes. Figure 2, which depicts representative data, shows that heterologous neutralization curves were again irregular in shape and frequently plateaued between 1:32 and 1:8, without reaching the 50% neutralization level. Significant cross-neutralization was observed in 34 of 142 of the virus-serum combinations tested (24%), and in general the mean NA titers were in the same range as those observed in autologous neutralizations (Table 2). However, individual primary isolates varied considerably in their sensitivity to heterologous neutralization. Thus, certain isolates (M45, M73, M88, and M89) were inhibited by multiple sera, others were highly resistant (M3, M17, M22, M91, and M97), and still others showed intermediate sensitivity. As shown in Table 2, FIV-Pet exhibited a diffuse and relatively high sensitivity to cross-neutralization.
FIG. 2.
Representative neutralization curves of primary FIV isolates reacted with heterologous immune sera. The viral isolates are indicated in the right upper corner of each panel; immune sera are shown outside the panels. Dashed lines represent the 50% and 90% neutralization levels.
The cross-neutralizing capabilities of immune sera also exhibited a wide spectrum. Some sera had a relatively broad neutralizing activity, inhibiting up to 40% of the isolates, while others showed few or no cross-neutralizing effects. Among the most cross-neutralizing sera were M2, M19, M26, and M89, while among the least neutralizing were M17, M73, and M82. No correlation was noted between the sensitivity of a virus isolate to cross-neutralization and the ability of the corresponding serum to cross-neutralize. For example, isolates M73 and M89 were neutralized by several sera, but the corresponding sera inhibited 7 and 40% of the heterologous isolates tested, respectively. Conversely, isolates M17 and M97 appeared refractory to cross-neutralization and the corresponding sera showed widely divergent neutralizing breadth. Importantly, cross-neutralization was generally unidirectional, since reciprocal reactivity was seen with two isolate-serum pairs only. Also of interest is that serum FIV-Pet, which neutralized the homologous virus at high titer, lacked detectable cross-neutralizing activity for the heterologous fresh isolates.
Infection enhancement.
Facilitation of infection was infrequent, since only 5 of the 546 virus-immune serum dilutions tested yielded a reproducible ≥2-fold increase of viral replication over the corresponding NCS control that could be scored as enhancement (Fig. 1A), with no differences in the frequency or magnitude of the phenomenon between autologous and heterologous virus-serum combinations. Moreover, the effect was often seen with a given serum dilution but not with the other dilutions of the same serum (data not shown).
Cross-neutralization and relatedness of the isolates.
All the primary isolates included in this study had been characterized as belonging to genotype B, based on sequencing of a specific segment of the gag gene. The gag segment considered is most probably not involved in serum neutralization but provides a reliable estimate of the genetic divergence between FIV strains (42); therefore, we used the gag sequence data to evaluate whether there was a relationship between cross-neutralization and genetic relatedness of the isolates. The average genetic distance of the cross-neutralizing combinations, determined by the DNADIST program of the PHYLIP software, version 3.5c (12), was 0.0474 ± 0.0205, whereas it was 0.0478 ± 0.0230 in the nonneutralizing combinations. Also, no correlation between cross-neutralization and genetic distance was found by using the CLIQUE program of the PHYLIP package (this program assumes that each character and different lineages evolve independently and uses the compatibility method for two-state characters to produce unrooted trees; input data consisted of a compatibility matrix created with the FACTOR program and containing the genetic distances evaluated by the DNADIST program and a series of two-state characters for the neutralization data; characters 1, 0, and ? were assigned to the neutralizing combinations, nonneutralizing combinations, and untested combinations, respectively).
Eight isolate-serum sets were obtained from cats living in an open shelter, and the others were obtained at random from cats living at more or less distant locations. There was, however, no obvious correlation between epidemiological linkages and cross-neutralization. For example, a preferential cross-neutralization among the isolates from cats living in the shelter would have been indicated by a higher frequency of neutralization within the boxed area compared to the rest of Table 2.
Cross-neutralization and viral quasispecies complexity.
We also compared the genetic polymorphism of the FIV strains under scrutiny. RNA samples extracted from the culture fluids used as the test virus in the NA assays were reverse transcribed and amplified in the V3 and V4 regions of the env gene, and the resulting amplicons were examined by SSCP analysis. As expected from previous results (20), all the isolates were in the form of a mixture of genetic variants (quasispecies) and therefore produced SSCP bands which varied in number (Table 3) as well as in relative intensities and positions (data not shown). The quasispecies complexity of the viral isolates and NA breadth of the corresponding immune sera did not appear to correlate. As shown in Table 3, a difference was observed between sensitivity to cross-neutralization and quasispecies complexity, in that isolates neutralized by at least 30% of the heterologous sera tested exhibited larger numbers of variants than did isolates neutralized by 10% or less of the sera; however, the difference was modest and limited to the V3 region. Moreover, the highly NA-sensitive, tissue-culture-adapted strain FIV-Pet showed the lowest quasispecies complexity among the strains examined.
TABLE 3.
Quasispecies composition in the V3 and V4 regions of the env gene of the 15 primary FIV isolates examined, grouped by sensitivity to cross-neutralization, and the genetic polymorphism of the tissue culture-adapted FIV-Pet
| Category of virus | Sensitivity to cross-neutralization | Isolate | No. of
variantsa
|
|
|---|---|---|---|---|
| V3 region | V4 region | |||
| Primary | High (neutralized by ⩾30% of test sera) | M19 | 11 | 3 |
| M45 | 10 | 4 | ||
| M64 | 11 | 8 | ||
| M73 | 10 | 8 | ||
| M88 | 11 | 3 | ||
| M89 | 10 | 2 | ||
| Mean ± SD | 10.5 ± 0.54b | 4.6 ± 2.65 | ||
| Low (neutralized by ⩽10% of test sera) | M2 | 7 | 9 | |
| M3 | 6 | 23 | ||
| M17 | 8 | 7 | ||
| M22 | 13 | 9 | ||
| M26 | 9 | 8 | ||
| M82 | 6 | 6 | ||
| M91 | 4 | 7 | ||
| M92 | 5 | NDc | ||
| M97 | 6 | 2 | ||
| Mean ± SD | 7.1 ± 2.66 | 8.8 ± 6.12 | ||
| Tissue-culture adapted | High | Pet | 2 | 3 |
Variants detected in at least two independent assays.
Significantly different from the low-sensitivity group (P < 0.01, Student’s t test).
ND, not done.
NA response of SPF cats infected with selected FIV isolates.
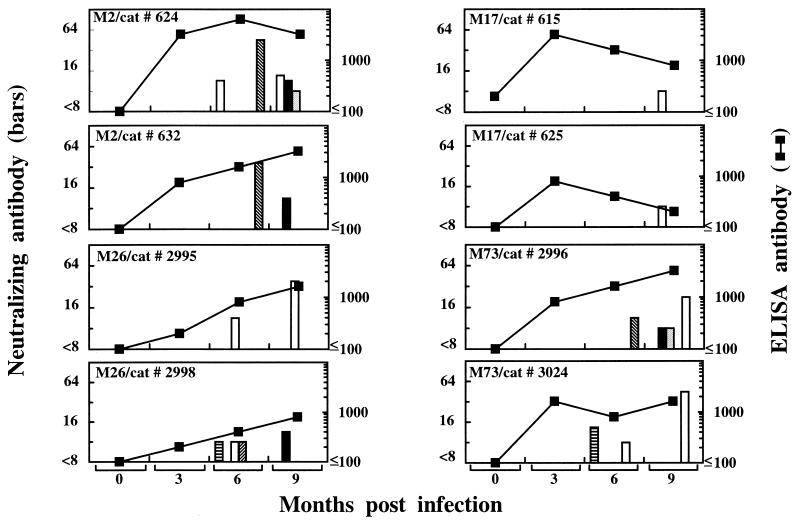
In these studies, we investigated whether FIV isolates obtained from cats whose sera showed different cross-neutralizing potencies differed in the ability to induce the production of cross-neutralizing antibodies when injected into naive cats. To this end, isolates M2 and M26, obtained from animals whose sera neutralized 36 and 33% of the isolates, respectively, and isolates M17 and M73, obtained from animals whose sera neutralized 8 and 7% of the FIV isolates tested, respectively, were used to infect SPF cats (two animals per isolate). To obviate the possible influence of any host cell-dependent epigenetic factor on the subsequent NA assays to be carried out with the sera of these animals, the inocula consisted of cultured autologous PBMC infected and incubated in vitro for 8 days and diluted to contain 105 FIV-positive cells as determined by immunofluorescence. All the cats became infected, as determined by virus reisolation and enzyme-linked immunosorbent assay serology, and all developed some NA activity (Fig. 3). Homologous NA were generally first detected in the 6-month sera, and their titers remained low. Cross-reactivity developed after a similar delay and was sporadic, also low in titer, and often directed to different viruses even in sera collected from one cat at different time points. Most importantly, cross-reactive NA were detected in three of four cats inoculated with isolates obtained from animals with broadly cross-reactive NA (Fig. 3, left) versus two of four cats inoculated with isolates obtained from cats with poorly cross-reactive NA (Fig. 3, right). It should be noted that among the latter cats, those infected with M17, for reasons that have remained unclear, showed an abnormally weak immune response, since after the third month the postinfection ELISA titers declined instead of increasing as in the other animals.
FIG. 3.
Development of NA in cats infected with selected FIV isolates. Groups of two SPF cats were infected with isolates obtained from animals with broadly (left panels) or poorly (right panels) cross-reactive NA. At the times indicated, injected cats were examined for anti-FIV enzyme-linked immunosorbent assay (ELISA) antibodies with gradient-purified, disrupted whole M2 virus as the antigen (▪) and for NA antibodies to the homologous virus (open columns) and to selected heterologous isolates (filled columns: ▤, M2; ▪, M17; ░⃞, M26; ▨, M45; ▧, M92); cross-reactive NA to M73 tested constantly negative.
DISCUSSION
To date, most studies on FIV neutralization have been carried out with tissue culture-adapted strains of the virus and fibroblastoid Crandell feline kidney (CrFK) cells or lymphoid cells as substrates. The results have shown that NA assessed in lymphoid cells are considerably more isolate specific, lower in titer, and less targeted to the V3 region of the surface glycoprotein than NA measured in CrFK cells (3, 21, 37, 45, 50). While the reasons for these discrepancies remain unclear, it is likely that NA assays performed with lymphoid cells are especially relevant to immunity in vivo, as suggested by the fact that protection conferred by experimental FIV vaccines also tends to be isolate specific (18, 60). By using a lymphoid cell-based assay, in this study we have examined the neutralization properties of 15 primary FIV isolates subjected to minimal passage in culture and therefore as close as possible to in vivo strains.
In general, primary FIV isolates proved hard to neutralize, as shown by the virtual absence of 90% inhibition of viral replication and by the low NA titers obtained with the less stringent 50% end point. Similar results have been reported for primary isolates of HIV-1 and have raised considerable concern (19, 35, 36, 43) despite speculations that current assays for functional NA may not be particularly sensitive (14, 32, 41, 61, 62). Of note, only one of eight fresh FIV isolates tested against autologous sera collected before or at the time of virus isolation were neutralized, in contrast to five of seven reacted with autologous sera taken 3 or more months after virus isolation, thus indicating that FIV NA are specific to virus circulating earlier in the course of infection rather than to the virus coexisting with the NA. This is in agreement with findings showing that NA to contemporaneous, autologous HIV-1 isolates are generally absent or present only in low titer (31, 51, 53, 57) and suggests that FIV persistence in vivo occurs at least partly via the generation of neutralization escape mutants.
Consistent neutralization of primary FIV isolates by heterologous immune sera was observed in about one-fourth of the virus-serum combinations tested. This is a significant frequency, especially if one considers that cross-neutralizing titers were in the same range as in autologous reactions, and supports the concept that conserved epitopes that elicit and are target for effective NA exist at least in some isolates. However, in our checkboard analyses, cross-neutralization was predominantly unidirectional and showed no recognizable pattern. Moreover, the cross-neutralization frequency did not correlate with the epidemiological and genetic relatedness of the isolates examined or with the extent of viral glycoprotein binding measured by a recently described method (46) at the corresponding virus-serum combinations (data not shown). This indicates that delineation of conserved neutralization epitopes of FIV and antigenic grouping of FIV may not be realistic tasks at present and will probably require more refined reagents such as neutralizing and subtype-restricted monoclonal antibodies. Studies with patient sera have shown that neutralization clusters of HIV-1 are recognizable but coincide with genetic subtypes only partially, if at all (19, 23, 35, 52, 55).
Infection-enhancing antibodies have been observed in FIV-infected or vaccinated cats (3, 48) as well as in HIV-1-infected and vaccinated people (29). In the present study, antibody-dependent enhancement was an infrequent finding. This is probably due to our assay conditions (for example, the use of an NCS control for each immune serum dilution may have reduced possible artifacts due to nonspecific effects of sera on virus replication [19, 58]), as well as to the stringent criterion adopted to define an increase of FIV replication as enhancement (≥twofold increase of RT production). Although enhancement is often seen at subneutralizing concentrations of test sera (49), we exclude the possibility that our immune sera were tested too concentrated to observe the phenomenon, since on repeated occasions they were diluted further with no evidence of more frequent or potent enhancement (data not shown). It has been suggested that lentivirus-infected subjects produce mixed populations of antibodies exerting contrasting functions (19, 48). However, from the present results, it would seem that the net effect of antibodies produced during natural infections is to more often inhibit than enhance FIV replication. Interestingly, one isolate (M88) proved unusually susceptible to enhancement, since its replication was augmented by three immune sera whereas we found no immune sera that increased the infection of multiple isolates. Thus, enhancement appears to be dependent on some inherent property of the FIV isolate under study more than on the characteristics of the immune sera. It has been suggested that in humans, HIV variants with increased susceptibility to enhancing antibodies are selected over time from infection and eventually may become predominant in the host (16).
Individual primary isolates of FIV varied considerably in their sensitivity to neutralization by heterologous immune sera: while certain isolates appeared refractory to inhibition or were inhibited infrequently, others were inhibited by one-third of the immune sera tested or more. As for HIV-1 (35, 55, 57), the reasons for this diversity are not clear. In an SSCP analysis of the V3 and V4 regions, i.e., two env domains that have been implicated in FIV neutralization (5, 10, 21, 25, 38, 47, 54), we found no relationships between FIV quasispecies complexity and sensitivity to cross-neutralization, except that the variants detected in V3 were slightly more numerous in the cross-neutralization-sensitive than in the nonsensitive isolates. Thus, NA susceptibility did not appear to be a function of a low degree of genetic polymorphism in the env regions examined, at least in primary isolates. In contrast to most primary isolates, FIV-Pet, which had an extensive passage history in culture, was neutralized by many heterologous sera even though they were from animals infected with another subtype. This indicates that tissue-culture adapted FIV strains can be abnormally sensitive not only to homologous NA (3; see above) but also to heterologous NA, thus underlining the need to exert great caution in interpreting the significance of NA results obtained with these strains (24), and implies that the failure of heterologous sera to neutralize the primary isolates was not due to a lack of potentially inhibitory antibodies but to the absence or inaccessibility of appropriate epitopes on the surface of primary isolates (33).
Considerable variation was also observed in the ability of individual immune sera to neutralize heterologous strains, thus again reproducing what was observed with HIV (55, 57). Possible reasons include a peculiar immune responsiveness or repeated exposure to multiple viruses of the donor cats and a particularly long duration of infection at the time of serum collection. The first explanation seems unlikely because the cross-neutralizing potency did not correlate with the extent of binding to whole FIV antigen and to an immunodominant peptide of the transmembrane glycoprotein (1) that we used as a measure of the general reactivity of sera (data not shown). The selection pressure exerted by the immune system is considered a major force leading to genetic heterogeneity of persistent viruses, and quasispecies complexity can therefore be viewed as an indirect measure of the host immune responsiveness. Thus, the observation that FIV isolates had similar quasispecies complexities regardless of the cross-neutralizing breadth of the corresponding sera also argues against marked differences in the immune responsiveness of donor cats. The risk that donor cats were exposed to multiple viruses exists, especially if one considers that several lived in a shelter with a high FIV infection rate. However, in sequencing the gag region for subtyping the isolates, we noted no ambiguities suggestive of the existence of dual infections. The duration of infection was not known for most of the cats, but the sera of animals that were symptomatic at the time of serum collection were among the more cross-neutralizing, suggesting that NA can indeed increase in breadth with evolution of infection. However, our study was not designed to address this issue, and prospective long-term follow-up studies of larger numbers of animals are needed to clarify this aspect. Although not a constant finding (15), NA of HIV-1-infected long-term nonprogressors had broader reactivity with primary HIV isolates than did NA from patients with standard clinical progression (6, 30).
We finally considered the possibility that the varying breadth of the NA present in different immune sera was generated by differences in the isolates that had induced their production. This was a compelling question because identifying FIV strains capable of evoking broad NA responses would be valuable in the present struggle to develop vaccines. We therefore compared the NA-inducing properties of the viral isolates obtained from two animals with broadly reactive NA and from two animals with poorly cross-reactive NA, by infection of SPF cats and subsequent analysis of the NA response elicited for a period of 9 months. Similar to what was observed in primary HIV infections (32, 34, 40), NA were detected after a delay of several months. The strength and breadth of the NA response showed no major differences among the isolates, provided that these induced normal antibody responses, and also varied considerably between cats infected with the same isolate, suggesting that they do not depend on special properties of the infecting virus.
In summary, these data show that the neutralization properties of primary FIV isolates are very similar to those reported for primary HIV isolates. Thus, their study may offer unique opportunities for understanding the scope of humoral immune responses in infections by immunosuppressive lentiviruses and for directing vaccine efforts appropriately. If the findings of this study can be extrapolated to HIV, there would appear to be little hope of identifying naturally occurring HIV strains that are inherently capable of consistently inducing broadly reactive NA within time limits exploitable for vaccine development. As recently suggested (33), engineering viruses in such a way as to expose conserved NA epitopes might be more fruitful.
ACKNOWLEDGMENTS
We thank Giuliana Castellani for lovingly taking care of the animals and Marco Franchi for valuable technical help.
This work was supported by grants from Ministero della Sanità—Istituto Superiore di Sanità, “Progetto Allestimento Modelli Animali per l’AIDS,” and the Ministero della Università e Ricerca Tecnologica, Rome, Italy.
REFERENCES
- 1.Avrameas A, Strosberg A D, Moraillon A, Sonigo P, Pancino G. Serological diagnosis of feline immunodeficiency virus infection based on synthetic peptides from Env glycoproteins. Res Virol. 1993;144:209–218. doi: 10.1016/s0923-2516(06)80031-2. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M H, Mathiason-Dubard C, Learn G H, Rodrigo A G, Sodora D L, Mazzetti P, Hoover E A, Mullins J I. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cammarota G, Matteucci D, Pistello M, Nicoletti E, Giannecchini S, Bendinelli M. Reduced sensitivity to strain-specific neutralization of laboratory-adapted feline immunodeficiency virus after one passage in vivo: association with amino acid substitutions in the V4 region of the surface glycoprotein. AIDS Res Hum Retroviruses. 1996;12:173–175. doi: 10.1089/aid.1996.12.173. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Qin L, Zhang L, Safrid J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 7.Conley A J, Kessler II J A, Boots L J, McKenna P M, Schleif W A, Emini E A, Mark III G E, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimmock N J. Neutralisation of animal viruses. Curr Top Microbiol Immunol. 1993;183:1–149. doi: 10.1007/978-3-642-77849-0. [DOI] [PubMed] [Google Scholar]
- 9.Dimmock N J. Update on the neutralization of animal viruses. Rev Med Virol. 1995;5:165–179. [Google Scholar]
- 10.Egberink H, Keldermans L, Schuurman N, Stam J, Hesselink W, van Vliet A, Verschoor E, Horzinek M, de Ronde A. Monoclonal antibodies to immunodominant and neutralizing domains of the envelope surface protein of feline immunodeficiency virus. J Gen Virol. 1994;75:889–893. doi: 10.1099/0022-1317-75-4-889. [DOI] [PubMed] [Google Scholar]
- 11.Elder J H, Phillips T R. Feline immunodeficiency virus as a model for development of molecular approaches to intervention strategies against lentivirus infections. Adv Virus Res. 1995;45:225–247. doi: 10.1016/s0065-3527(08)60062-7. [DOI] [PubMed] [Google Scholar]
- 12.Felsestein J. PHYLIP (phylogeny interference package), version 3.5c. Seattle: University of Washington; 1993. [Google Scholar]
- 13.Giannecchini S, Matteucci D, Mazzetti P, Bendinelli M. Incubation time for feline immunodeficiency virus cultures. J Clin Microbiol. 1996;34:2036–2038. doi: 10.1128/jcm.34.8.2036-2038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golding H, D’Souza M P, Bradac J, Mathieson B, Fast P. Neutralization of HIV-1. AIDS Res Hum Retroviruses. 1994;10:633–643. doi: 10.1089/aid.1994.10.633. [DOI] [PubMed] [Google Scholar]
- 15.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, Johnson R P, Buchbinder S P, Walker B D. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 16.Homsy J, Meyer M, Levy J A. Serum enhancement of human immunodeficiency virus (HIV) infection correlates with disease in HIV-infected individuals. J Virol. 1990;64:1437–1440. doi: 10.1128/jvi.64.4.1437-1440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosie M J, Osborne R, Yamamoto J K, Neil J C, Jarrett O. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J Virol. 1995;69:1253–1255. doi: 10.1128/jvi.69.2.1253-1255.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyaw-Tanner M T, Robinson W F. Quasispecies and naturally occurring superinfection in feline immunodeficiency virus infection. Arch Virol. 1996;141:1703–1713. doi: 10.1007/BF01718293. [DOI] [PubMed] [Google Scholar]
- 21.Lombardi S, Garzelli C, La Rosa C, Zaccaro L, Specter S, Malvaldi G, Tozzini F, Esposito F, Bendinelli M. Identification of a linear neutralization site within the third variable region of feline immunodeficiency virus envelope. J Virol. 1993;67:4742–4749. doi: 10.1128/jvi.67.8.4742-4749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggi F, Fornai C, Vatteroni M L, Giorgi M, Morrica A, Pistello M, Cammarota G, Marchi S, Ciccorossi P, Bionda A, Bendinelli M. Differences in hepatitis C virus quasispecies composition between liver, peripheral blood mononuclear cells and plasma. J Gen Virol. 1997;78:1521–1525. doi: 10.1099/0022-1317-78-7-1521. [DOI] [PubMed] [Google Scholar]
- 23.Mascola J R, Louwagie J, McCutcham F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 24.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 25.Massi C, Lombardi S, Indino E, Matteucci D, La Rosa C, Esposito F, Garzelli C, Bendinelli M. Most potential linear B-cell epitopes of Env glycoproteins of feline immunodeficiency virus are immunologically silent in mice. AIDS Res Hum Retroviruses. 1997;13:1121–1129. doi: 10.1089/aid.1997.13.1121. [DOI] [PubMed] [Google Scholar]
- 26.Matteucci D, Mazzetti P, Baldinotti F, Zaccaro L, Bendinelli M. The feline lymphoid cell line MBM and its use for feline immunodeficiency virus isolation and quantitation. Vet Immunol Immunopathol. 1995;46:71–82. doi: 10.1016/0165-2427(94)07007-t. [DOI] [PubMed] [Google Scholar]
- 27.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Lonetti I, Zaccaro L, Pollera C, Specter S, Bendinelli M. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J Virol. 1997;71:8368–8376. doi: 10.1128/jvi.71.11.8368-8376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Zaccaro L, Bandecchi P, Tozzini F, Bendinelli M. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J Virol. 1996;70:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montefiori D C. In vitro correlates of HIV and SIV humoral immunity and infection enhancement. AIDS Res Rev. 1993;3:161–183. [Google Scholar]
- 30.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressor. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 31.Montefiori D C, Zhou J, Barnes B, et al. Homotypic antibody responses to fresh clinical isolates of human immunodeficiency virus. Virology. 1991;182:635–643. doi: 10.1016/0042-6822(91)90604-a. [DOI] [PubMed] [Google Scholar]
- 32.Moog C, Spenlehauer C, Fleury H, Heshmati F, Saragosti S, Letournenr F, Kirn A, Aubertin A M. Neutralization of primary human immunodeficiency virus type 1 isolates: a study of parameters implicated in neutralization in vitro. AIDS Res Hum Retroviruses. 1997;13:19–27. doi: 10.1089/aid.1997.13.19. [DOI] [PubMed] [Google Scholar]
- 33.Moore J, Trkola A. HIV type 1 coreceptors, neutralization serotypes, and vaccine development. AIDS Res Hum Retroviruses. 1997;13:733–736. doi: 10.1089/aid.1997.13.733. [DOI] [PubMed] [Google Scholar]
- 34.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 37.Osborne R, Rigby M, Siebelink K, Neil J C, Jarrett O. Virus neutralization reveals antigenic variation among feline immunodeficiency virus isolates. J Gen Virol. 1994;75:3641–3645. doi: 10.1099/0022-1317-75-12-3641. [DOI] [PubMed] [Google Scholar]
- 38.Pancino G, Fossati I, Chappey C, Castelot S, Hurtrel B, Moraillon A, Klatzmann D, Sonigo P. Structure and variations of feline immunodeficiency virus envelope glycoproteins. Virology. 1993;192:659–662. doi: 10.1006/viro.1993.1083. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen N C. Feline immunodeficiency virus infection. In: Levy J A, editor. The Retroviruses. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 181–228. [Google Scholar]
- 40.Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin J-L, Ragnaud J-M, Bernard N, Fleury H J A. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquired Immune Defic Syndr. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Pincus S H, Cole R, Ireland R, McAtee F, Fujisawa R, Portis J. Protective efficacy of nonneutralizing monoclonal antibodies in acute infection with murine leukemia virus. J Virol. 1995;69:7152–7158. doi: 10.1128/jvi.69.11.7152-7158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pistello M, Cammarota G, Nicoletti E, Matteucci D, Curcio M, Del Mauro D, Bendinelli M. Analysis of genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates high prevalence and heterogeneity of subtype B. J Gen Virol. 1997;78:2247–2257. doi: 10.1099/0022-1317-78-9-2247. [DOI] [PubMed] [Google Scholar]
- 43.Poignard P, Klasse P J, Sattentau Q J. Antibody neutralization of HIV-1. Immunol Today. 1996;17:239–246. doi: 10.1016/0167-5699(96)10007-4. [DOI] [PubMed] [Google Scholar]
- 44.Reed L J, Muench H A. A simple method for estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 45.Richardson J, Fossati I, Moraillon A, Castelot S, Sonigo P, Pancino G. Neutralization sensitivity and accessibility of continuous B cell epitopes of the feline immunodeficiency virus envelope. J Gen Virol. 1996;77:759–777. doi: 10.1099/0022-1317-77-4-759. [DOI] [PubMed] [Google Scholar]
- 46.Sibille P, Avraméas A, Moraillon A, Richardson J, Sonigo P, Pancino G, Strosberg A D. Comparison of serological tests for the diagnosis of feline immunodeficiency virus infection of cats. Vet Microbiol. 1995;45:259–267. doi: 10.1016/0378-1135(94)00128-j. [DOI] [PubMed] [Google Scholar]
- 47.Siebelink K H J, Huisman W, Karlas J A, Rimmelzwaan G F, Bosch M L, Osterhaus A D M E. Neutralization of feline immunodeficiency virus by polyclonal feline antibody: simultaneous involvement of hypervariable regions 4 and 5 of the surface glycoprotein. J Virol. 1995;69:5124–5127. doi: 10.1128/jvi.69.8.5124-5127.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siebelink K H J, Tijhaar E, Huisman R C, Huisman W, deRonde A, Darby I H, Francis M J, Rimmelzwaan G F, Osterhaus A D M E. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J Virol. 1995;69:3704–3711. doi: 10.1128/jvi.69.6.3704-3711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell-line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tozzini F, Matteucci D, Bandecchi P, Baldinotti F, Siebelink K, Osterhaus A, Bendinelli M. Neutralizing antibodies in cats infected with feline immunodeficiency virus. J Clin Microbiol. 1993;31:1626–1629. doi: 10.1128/jcm.31.6.1626-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tremblay M, Wainberg M A. Neutralization of multiple HIV-1 isolates from a single subject by autologous sequential sera. J Infect Dis. 1990;162:735–737. doi: 10.1093/infdis/162.3.735. [DOI] [PubMed] [Google Scholar]
- 52.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-immunoglobulin G. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsang M L, Evans L A, McQueen P, Hurren L, Byrne C, Penny R, Tindall B, Cooper D A. Neutralizing antibodies against sequential autologous human immunodeficiency virus type 1 isolates after seroconversion. J Infect Dis. 1994;170:1141–1147. doi: 10.1093/infdis/170.5.1141. [DOI] [PubMed] [Google Scholar]
- 54.Verschoor E J, Willemse M J, Stam J G, van Vliet A L W, Pouwels H, Chalmers S K, Horzinek M C, Sondermeijer P J A, Hesselink W, de Ronde A. Evaluation of subunit vaccines against feline immunodeficiency virus infection. Vaccine. 1996;14:285–289. doi: 10.1016/0264-410x(95)00205-f. [DOI] [PubMed] [Google Scholar]
- 55.Weber J, Fenyö E-M, Beddows S, Kaleebu P, Björndal Å WHO Network for HIV Isolation and Characterization. Neutralization serotypes of human immunodeficiency virus type 1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 57.Wrin T, Crawford L, Sawyer L, Weber P, Sheppard H W, Hanson C V. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J AIDS. 1994;7:211–219. [PubMed] [Google Scholar]
- 58.Wu S-C, Spouge J L, Conley S R, Tsai W-P, Merges M J, Nara P L. Human plasma enhances the infectivity of primary human immunodeficiency virus type 1 isolates in peripheral blood mononuclear cells and monocyte-derived macrophages. J Virol. 1995;69:6054–6062. doi: 10.1128/jvi.69.10.6054-6062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto J K, Ackley C D, Zochlinski H, Louie H, Pembroke E, Torten M, Hansen H, Munn R, Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32:361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto J K, Hohdatsu T, Holmsted R A, Pu R, Louie H, Zochlinski H A, Acevedo V, Johnson H M, Soulds G A, Gardner M B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993;67:601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zolla-Pazner S, O’Leary J, Burda S, Gorny M K, Kim M, Mascola J, McCutchan F. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J Virol. 1995;69:3807–3815. doi: 10.1128/jvi.69.6.3807-3815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]