Summary
Cross-coupling azide and isocyanide have recently gained recognition as ideal methods for efficiently synthesizing asymmetric carbodiimides. This reaction exhibits high reaction rates, efficiency, and favorable atom/step/redox economy. It enables the nitrene-transfer process, facilitating the formation of C-N bonds and providing a direct and cost-effective synthetic strategy for generating diverse carbodiimides. These carbodiimides are highly reactive compounds that can undergo in-situ transformations into various functional groups and organic compounds, including heterocycles. Developing one-pot and tandem processes in this field has significantly contributed to advancements in organic chemistry. Moreover, the demonstrated utility of these architectural motifs extends to areas such as chemical biology and medicinal chemistry, further highlighting their potential in various scientific applications.
Subject areas: Organic chemistry
Graphical abstract
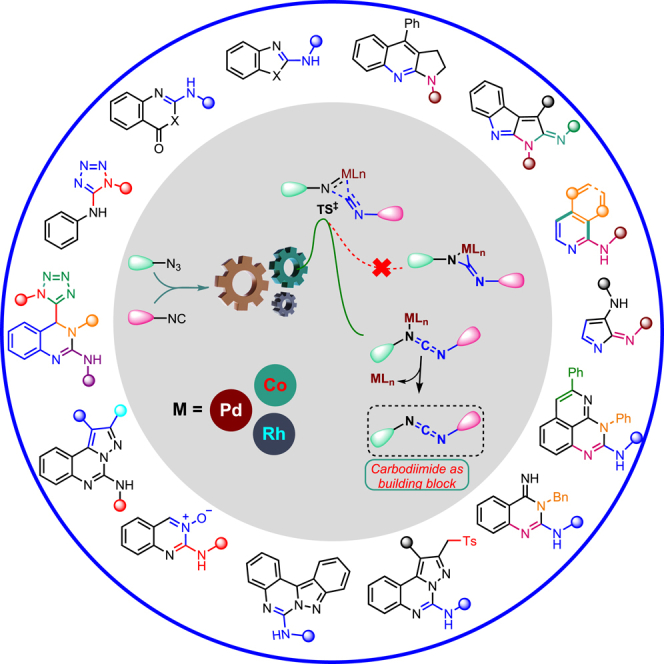
Organic chemistry
Introduction
The nitrene-transfer reaction is of utmost importance for direct C-N bond formation or insertion of amine in various saturated and unsaturated structural frameworks for developing bioactive compounds.1 The history of nitrenes is as old as carbenes. In 1891, Ferdinand Tiemann was the first to report the formation of nitrenes as intermediates in Lossen rearrangement.2 From 1940 to the 1950s, when the click-type reaction was explored, nitrene addition to various bonds gained more attention for synthesizing amines or aziridines. Smith and Brown developed photochemical and thermal conditions for synthesizing carbazoles from azido biphenyls via cyclization.3 Other research groups led by Smolinsky,4 Edwards,5 Breslow,6 Lwowsk,7 etc.,8 reported further advancements in this area. As nitrenes are highly reactive groups, the yield was a vital issue that was solved to some extent by Kwart and Kahn with their first-ever metal (Copper) catalyzed nitrene transfer reaction in 1967.9 This opened a new window for further exploration for critical players in this area.10,11,12 Building upon the historical foundations of nitrene chemistry, recent years have brought forth innovative approaches and catalytic processes, breathing new life into the field and expanding its applications.
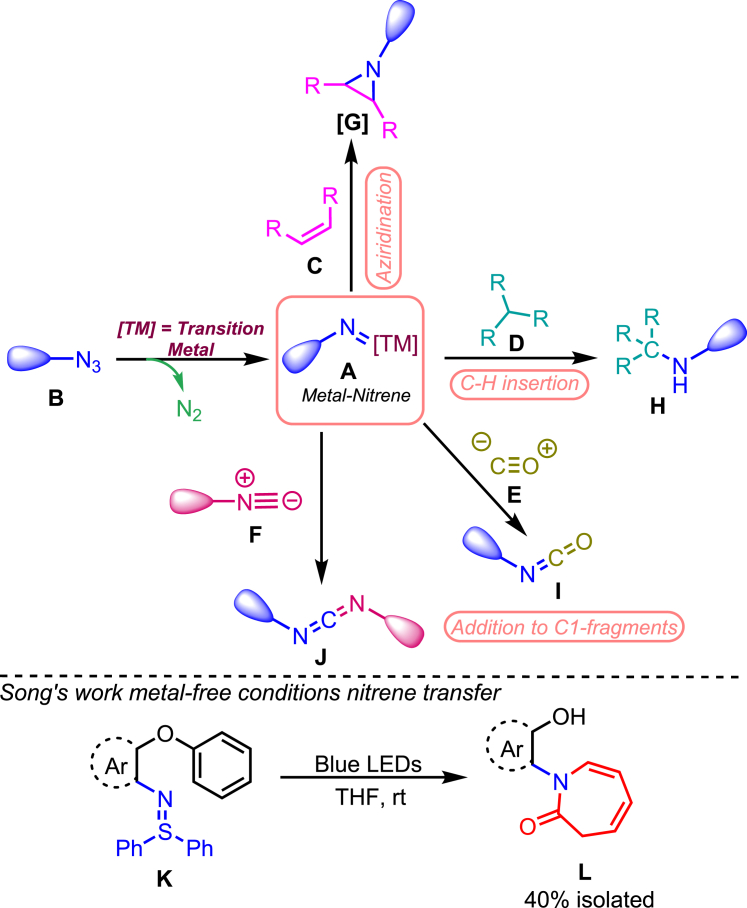
The utility of the nitrene transfer reaction1,13,14 catalyzed by transition metals is well established for developing azaheterocycles. Transition metal (TM) catalysis occupies a central position in enabling the highly efficient in situ generation of nitrene species A, primarily derived from azides B.15 This catalytic approach facilitates a variety of noteworthy transformations, such as the formation of aziridines through their reaction with unsaturated bonds C, and the C-H insertion of nitrenes D, as illustrated in Figure 1.16 The coupling reaction between a nitrene A and CO E or an isocyanide F represents a beneficial approach to obtaining important synthetic intermediates, as depicted in Figure 1. The reaction of a nitrene with CO yields isocyanate I, considered a valuable building block in organic synthesis,17,18 Specifically, the reaction between a nitrene and an isocyanide leads to the formation of a carbodiimide J, which is widely recognized for its utility in synthesizing heterocycles and as a peptide coupling agent.19,20 Hence, the transfer of nitrenes to isocyanides is a promising foundation for developing novel cascade processes, capitalizing on the in situ formation of carbodiimides J as versatile building blocks. Apart from the nitrene transfer reactions, the chemist recently discovered a ‘magical’ technique to allow skeletal editing in the molecule. This method enables the chemist to move, insert, or replace a single atom at a time in the molecule’s core. These reactions have recently emerged as a hot spot of research, which is used for late-stage skeletal modifications and streamlining the chemical synthesis.21 In recent work, Song and his group disclosed an unexpected synthesis of azepinone-based derivatives under metal-free conditions via a photochemical cascade reaction.22 The group was the first to explore 2-aryloxyarylnitrenes to synthesize unprotected carbazoles promoted by blue light.23
Figure 1.
Overview of common transformations involving transition metal nitrene species
Nitrene transfer reactions play a crucial role in the development of azaheterocycles, with transition metal (TM) mediated catalysis being pivotal in their well-established mechanisms. The figure also highlights an exceptional case by Song et al., where they revealed an unexpected synthesis of azepinone-based derivatives. This synthesis occurred under metal-free conditions through a photochemical cascade reaction promoted by blue light, showcasing a unique instance in nitrene transfer reactions.
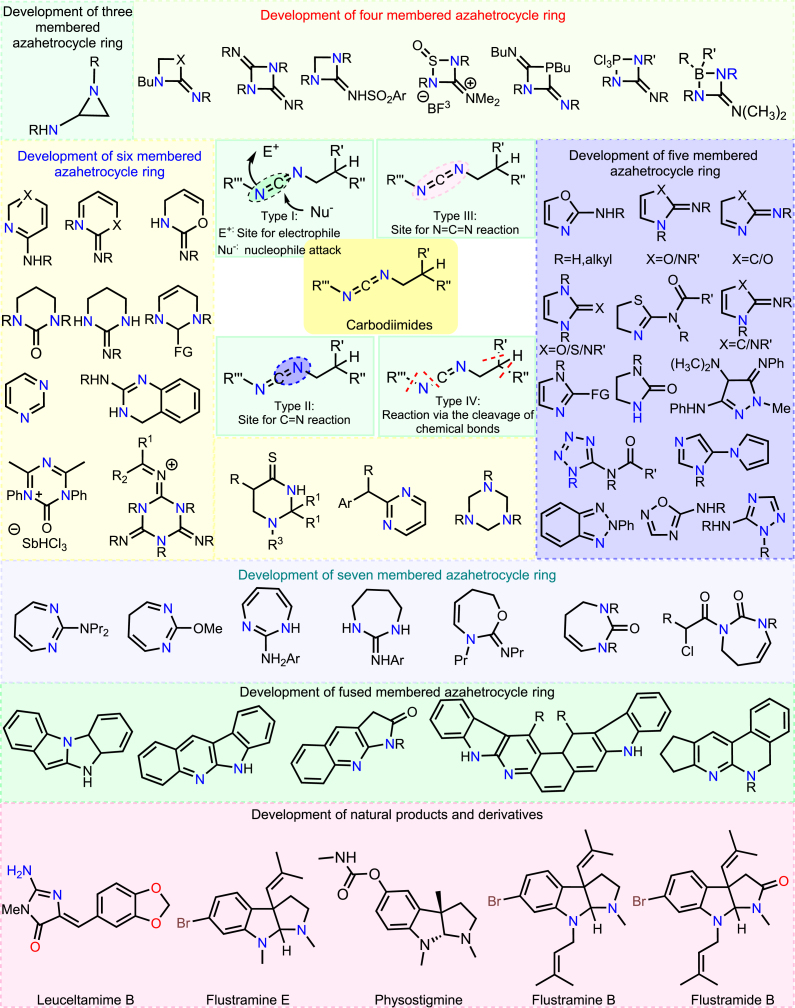
Recently, much emphasis has been placed on forming C-N bonds via the nitrene transfer on the isocyanides toward generating carbodiimides (see the utility of carbodiimides in Figure 2). Compared to the traditional techniques available for synthesizing unsymmetrical carbodiimides, one that involves the nitrene transfer on isocyanides is popular and one of the most efficient alternatives for rapid, atom-economical, and efficient cross-coupling. These synthons are frequently used as precursors in organic synthesis and find an array of utilities in developing polymeric materials, drug synthesis, and the development of dyes and/or dehydrating agents. Xing et al. reported that chemo-selective nitrene transfers to complex natural molecules at a late stage.24 The synthesis of Rapivab (BCX-1812 (RWJ-270201)), an approved anti-influenza drug, is one example that involves nitrene transfer in one of its multi-step synthetic protocols.25
Figure 2.
Reactive sites and utility of carbodiimides for the synthesis of diversified azaheterocycles
This comprehensive figure illustrates the reactive sites and synthetic utility of carbodiimides in the formation of diverse azaheterocycles, including semisynthetic analogues. This underscores their crucial importance, contributing to the expansion of chemical space for applications in organic synthesis, medicinal chemistry, and materials science.
This review aims to delve into the significance of TMs in catalyzing nitrene-transfer reactions on isocyanides and their subsequent post-functionalization. The primary focus of this article is to offer comprehensive insights to medicinal and organic chemists regarding the transfer reaction on isocyanide, which results in the formation of diverse azaheterocycles incorporating alkynes, nitriles, alkenes, allenes, and isocyanides. This review article also covers synthetic and mechanistic applications associated with the described nitrene-transfer reactions. Examining these facets provides a valuable resource for researchers looking to explore the potential of these reactions in their work.
Stoichiometric reaction for nitrene-transfer reaction
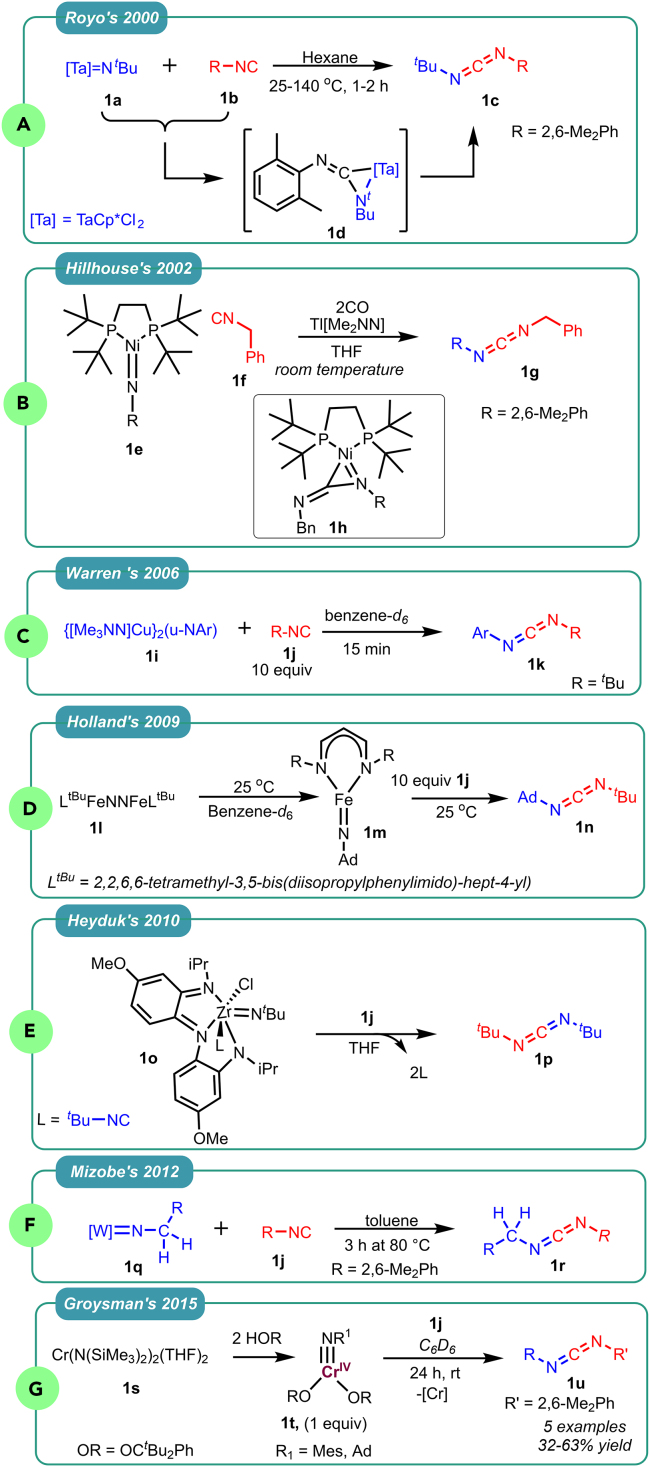
During the late 20th century, researchers employed a range of TM complexes as catalysts in azide-isocyanide cross-coupling reactions to synthesize carbodiimides. In 2000, Royo utilized a tantalum imido complex [TaCp∗Cl2(NtBu)] 1a as a nitrene transfer agent, leading to the synthesis of carbodiimide 1c (Scheme 1A) and proposing the formation of an intermediate three-membered metal-aziridine ring 1d26 In 2002, Hillhouse utilized benzyl isocyanide 1f and the Ni-imido complex 1e to produce carbodiimide 1g (Scheme 1B), suggesting a potential intermediate three-membered metal-aziridine 1h.27 Furthermore, in 2006, Warren and his team developed a protocol to generate asymmetric carbodiimides by reacting an organic azide with [Me3NN]Cu2(toluene) to form the dicopper nitrene [Me3NN]Cu2(-NAr) 1i. They introduced CNtBu 1j and PMe3 as reactants, producing carbodiimide 1k via group transfer through aziridination (Scheme 1C).28 In 2009, Holland reported the synthesis of carbodiimide 1n through the stoichiometric reaction of isocyanide 1j and imido-iron complex 1m (Scheme 1D).29 Similarly, in 2011, Heyduk demonstrated the nitrene-transfer catalyzed reaction of tbutyl isocyanide 1j and zirconium imido complex 1o, generated from tBuN3 and [NNNCat]ZrClL2 (L = THF), for the synthesis of symmetric carbodiimide 1p (Scheme 1E).30 In 2012, Mizobe investigated the isocyanide transfer reaction on low-valent center tungsten imido complexes 1q. Treatment of the tungsten imido complexes with 2,6-dimethyl phenyl isocyanide at 80°C yielded asymmetric carbodiimide 1r in high yield (Scheme 1F).31 In 2015, Groysman et al. reported the nitrene transfer on isocyanides utilizing the Cr-imido complex 1t. They explored the reactivity of trigonal-planar Cr(IV) mono-imido complexes formed by the reaction of Cr(N(SiMe3)2)2(THF)2 with azides bearing bulky substitution. These complexes reacted with various isocyanides, forming corresponding carbodiimides 1u (Scheme 1G).32 It is important to note that all these studies involved the stoichiometric use of imido metal complexes, which limits their application in organic synthesis due to the high cost of metals. In some cases, an excess of isocyanide (up to 10 equivalents) was utilized.
Scheme 1.
Previous stoichiometric reaction for azide-isocyanide cross-coupling reaction
Early reports on the catalytic reaction for the nitrene-to-isocyanide transfer reaction
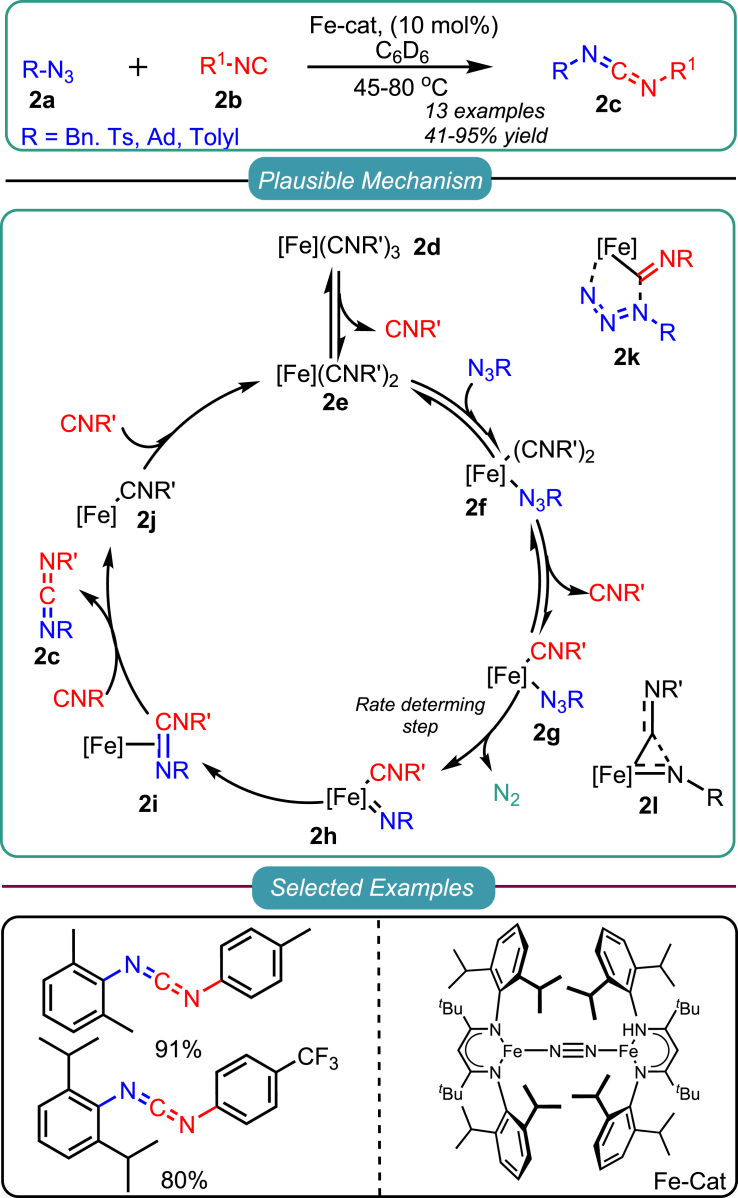
Drawing on their previous work on stoichiometric Fe-catalyzed reactions for carbodiimide synthesis, a catalytic version of the cross-coupling azide-isocyanide response has recently been developed. In 2013, Holland introduced a nitrene transfer approach on isocyanides using low-spin Fe(I) complexes with bulky β-diketiminate ligands to generate asymmetric carbodiimide 2c from azides 2a and isocyanides 2b (Scheme 2).33 Kinetic experiments revealed an inverse second-order dependence on the concentration of isocyanide, indicating the dissociation of two molecules before the rate-determining step. Based on these findings, a plausible mechanism was proposed and illustrated in Scheme 2. Initially, the Fe-complex coordinates with organoazides to form a coordination complex 2f, which then interacts with isocyanide to generate complex 2g. Subsequently, complex 2g eliminates dinitrogen, forming imido iron complex 2h, followed by nitrene transfer to yield carbodiimide 2c. The author proposed the existence of two intermediate species, namely the 5-membered metallacyclic complex 2k and the three-membered metallic-aziridine complex 2L. However, no experimental or DFT studies were provided to support their presence.
Scheme 2.
Holland’s report on Fe-catalyzed cross-coupling reaction
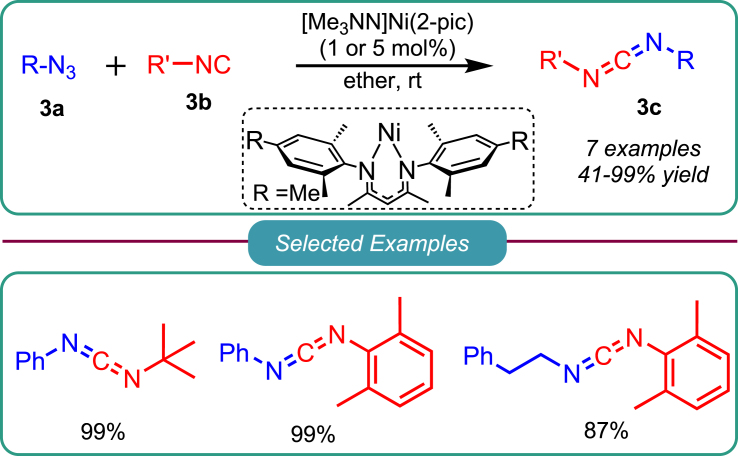
In continuation of early attempts, Warren and the group developed a method for synthesizing asymmetric carbodiimide using a cross-coupling reaction catalyzed by Ni(I) in 2013.34 Variety of aliphatic as well as aromatic azides 3a were investigated with electron-rich partner isocyanides 3b for their ability to produce high yields of corresponding asymmetric carbodiimides 3c, few examples are shown in Scheme 3. However, aromatic isocyanides proceeded slowly and required a more extended period for the reaction.
Scheme 3.
Warren’s work on Ni-catalyzed synthesis of asymmetric carbodiimides
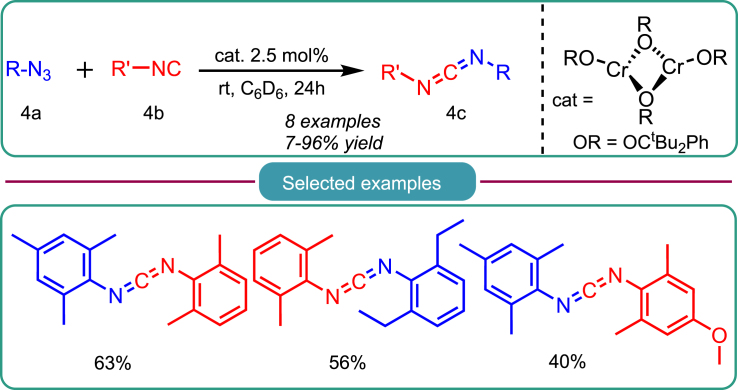
In a parallel manner, a catalytic nitrene transfer reaction is employed to generate carbodiimide using the early TM chromium (Cr). Groysman et al. unveiled a low-coordination Cr(II) complex within this category. They investigated its reactivity in catalyzing nitrene transfer to isocyanide 4b within a bis(alkoxide) ligand environment (as illustrated in Scheme 4).35 Their research team explored the use of less bulky aryl azides, which, however, did not lead to the formation of carbodiimides due to the absence of Cr(IV) imido, a crucial intermediate. Their research deduced that carbodiimide synthesis via nitrene transfer is feasible only in the presence of bulky aryl azides or aryl isocyanides. The reaction does not proceed when aliphatic azides and isocyanides (specifically, adamantyl) are employed to produce the desired carbodiimides 4c.
Scheme 4.
Groysman’s work on the Cr-catalyzed synthesis of carbodiimide
Transition metal catalyzed azide-isocyanide cross-coupling
Palladium-based reactions
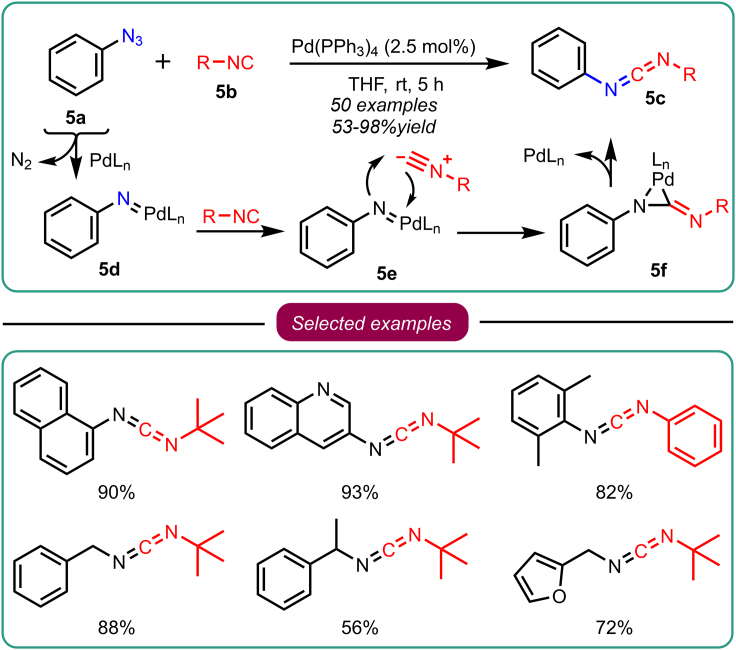
In 2015, Zhang and the group disclosed for the first time that using palladium (0) at a catalytic amount could transform azide-isocyanide cross-coupling. This reaction was mediated via nitrene transfer, which allows the synthesis of asymmetric carbodiimides (Scheme 5).36 The reaction involved aryl azide 5a and isocyanide 5b in THF as a solvent and Pd(PPh3)4 2.5 mol % as a catalyst to accomplish the desired asymmetric carbodiimide 5c at room temperature in 5h. The reaction diversity was further explored by employing different substrates that included aryl azides, inactivated benzyl, and alkyl, leading to the development of numerous asymmetric trisubstituted guanidines in a single pot with a tandem amine insertion. They proposed a 3-membered metallaaziridine ring as an intermediate 5f in the nitrene transfer reaction. It is important to note that most of these reports postulate the involvement of the metal nitrene-transfer reaction. However, none of these reports provided substantial evidence supporting this intermediate.
Scheme 5.
Zhang’s work on azide-isocyanide cross-coupling reactions for the generation of asymmetric carbodiimides
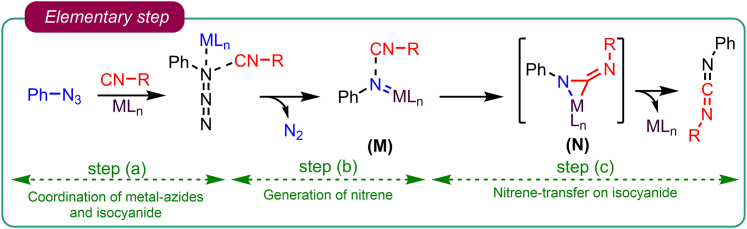
Building upon the groundwork laid by Zhang et al., Sawant et al. embarked on a comprehensive exploration of the azide-isocyanide cross-coupling reaction. Initially, they delved into deciphering the intricacies of this reaction by scrutinizing its fundamental steps. The investigation unveiled that this process unfolds through three pivotal stages: (1) the formation of a coordination complex between the metal and azide as well as isocyanide; (2) subsequent dinitrogen extrusion facilitating the generation of nitrenes; and (3) the transfer of these newly formed nitrenes onto the isocyanides (as illustrated in Figure 3). In a groundbreaking development, they devised a novel tandem Pd-catalyzed cross-coupling azide-isocyanide reaction and their post-cyclization to generate N-containing heterocycles.37
Figure 3.
Elementary step involved in azide-isocyanide cross-couplings
Step (a) Coordination of metal with azide and isocyanide: Involves the coordination of a metal species with azide and isocyanide molecules.
Step (b) Extrusion of dinitrogen to generate nitrenes: In this step, dinitrogen (N2) is eliminated, leading to the formation of reactive species called nitrenes.
Step (c) Transfer of nitrenes onto isocyanides: Nitrenes, the reactive intermediates formed in the previous step, are transferred or react with isocyanide molecules to generate carbodiimide.
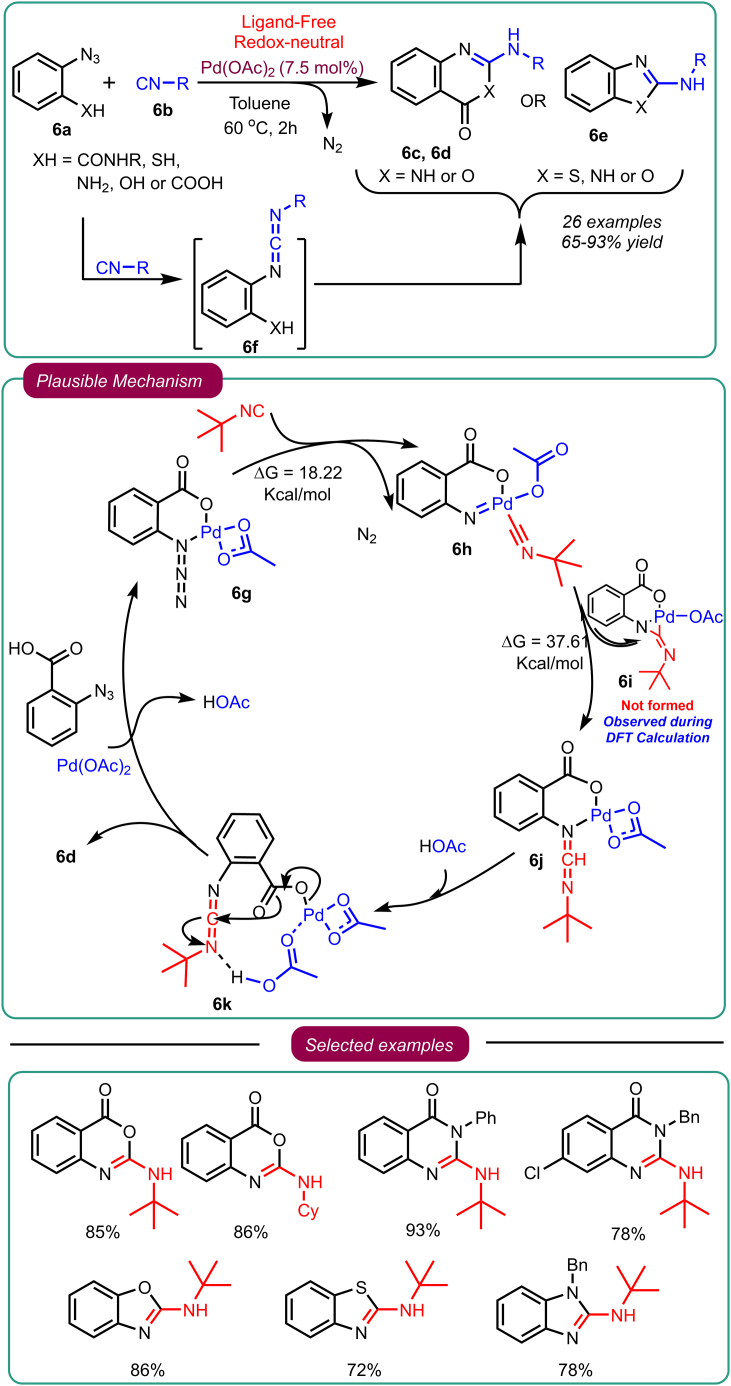
Mechanistically, reaction conditions were optimized by attempting cross-coupling reactions of azide 6a and isocyanide 6b under various solvents, catalysts, and temperatures (Scheme 6). The optimization studies suggested the reaction proceeds with significantly high yields under the catalytic influence of Pd(OAc)2 with toluene as a solvent and a temperature of 60°C. The reaction was diversified and tolerable for numerous substrates that could react with the azide and isocyanide, leading to the development of different azaheterocycles. This includes benzoxazinones 6c, quinazolinones 6d, and benzazoles 6e. Moreover, the established protocol was efficient, rapid, ligand-free, devoid of the prerequisite of a dry condition, and obeyed all the atom/step/redox economy principles. This research concluded that the transfer of nitrene on isocyanide follows a concerted reaction path rather than a sequential one. The research team also disapproved of the formation of metalla-aziridine 6i as an intermediate during the reaction. A series of control experiments and first-principles-based quantum mechanical calculations were carried out to investigate the reaction mechanism. Based on these studies, the proposed catalytic cycle of the tandem reaction commences with the coordination of Pd(OAc)2 with 2-azidobenzoic acid 6a, yielding compound 6g. In the subsequent step, the conversion of 6g into the Pd-imido intermediate 6h occurs, where nitrogen elimination is associated with a Gibbs free energy barrier of 18.22 kcal/mol. Furthermore, chemical kinetic investigations of the tandem cross-coupling/cyclization reaction have revealed that Pd(OAc)2 and tert-butyl isocyanide exhibit first-order kinetics, indicating their participation in the turnover-limiting step. First-principles-based calculations have predicted that the intermolecular nitrene-transfer reaction progresses through a three-membered transition state with an energy barrier of 37.61 kcal/mol. Carbodiimide was exclusively observed instead of the expected palladaaziridine 6i, a surprising outcome contrary to expectations. The intrinsic reaction coordinates (IRC) and potential energy surface (PES) scan demonstrated that the metalla-aziridine intermediate 6i is not stable; instead, a concerted path leads to the formation of 6j without the intermediacy of 6i. Despite a substantial energy barrier of 37.61 kcal/mol, the exceptionally high thermodynamic stability drives the nitrene-transfer reaction, making it the rate-determining step (RDS). Thus, the tandem reaction described in this study is governed by thermodynamics. In the subsequent step, acetic acid coordinates with Pd to form compound 6k. This coordination event ejects carbodiimide from its interaction with the metal, bringing carbodiimide closer to the carboxylate of benzoate. Consequently, a 6-exo-dig cyclization of the carboxylate onto carbodiimide occurs, forming the thermodynamically stable product 6d (accompanied by a favorable energy change of 23.27 kcal/mol). These mechanistic discoveries are pivotal, as the intermediate has been identified as a potent synthon for synthesizing numerous bioactive heterocycles through azide-isocyanide coupling reactions.
Scheme 6.
Sawant’s work on Pd-catalyzed azide-isocyanide cross-coupling reaction
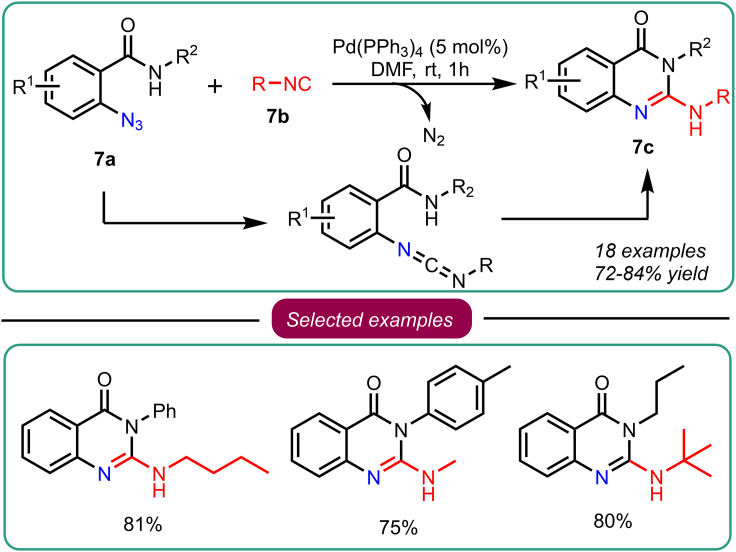
Similarly, in 2018, Ding’s group reported a one-pot Pd-catalyzed domino reaction for synthesizing quinazolin-4(3H)-ones (Scheme 7).38 The reaction involved Pd-catalyzed cross-coupling of 2-azidobenzamides 7a with isocyanides 7b at ambient temperature. The resulting coupled intermediate underwent carbodiimide-mediated cyclization, forming quinazolin-4(3H)-ones 7c. The researchers extended their methodology to synthesize benzoimidazo[2,1-b]quinazolin-12(6H)-ones by employing similar reaction conditions with Cs2CO3 as the base and a reaction temperature of 90°C. This innovative approach offers a convenient and efficient strategy for accessing quinazolin-4(3H)-ones and benzoimidazo[2,1-b]quinazolin-12(6H)-ones, demonstrating the versatility of Pd-catalyzed domino reactions in the synthesis of complex heterocyclic structures.
Scheme 7.
Ding’s work on one-pot Pd(0)-catalyzed domino synthesis of quinazolin-4(3H)-ones
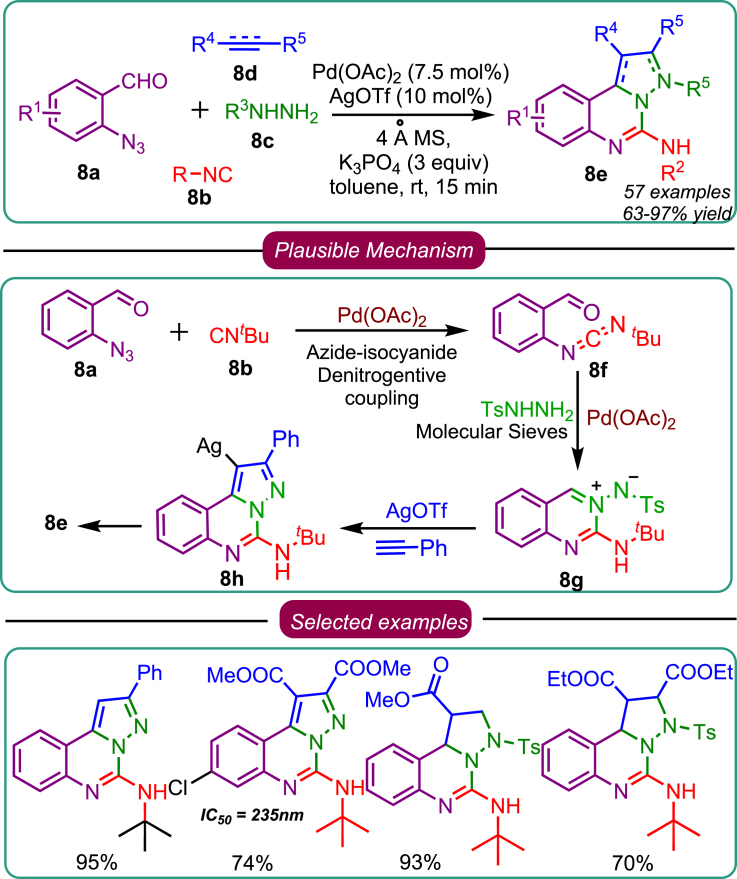
Motivated by the study, as mentioned earlier, Sawant’s research group investigated the rate-determining step of nitrene transfer on isocyanide. To gain a deeper understanding, a relay catalysis strategy was employed, utilizing Pd(II)/Ag(I) as the relay catalyst. The reaction was conducted in a one-pot tricyclic mode, forming pyrazolo[1,5-c]quinazolines 8e (Scheme 8). A remarkable feature of this reaction was the development of 5 new chemical bonds from the readily available precursors. The findings unveiled that the initiation of the reaction involved the azide-isocyanide cross-coupling, which generates carbodiimide 8f. Subsequently, the condensation of tosyl hydrazide 8c led to the formation of azomethine imine 8g, which underwent a base-mediated direct alkynylation to give rise to 8e.39 Further exploration of the substrate scope of this 4-component reaction (4-CR) revealed its extensive applicability. Alkynes, alkenes, and electron-deficient alkenes such as acrylates and acrylonitrile exhibited excellent compatibility, affording the desired product 8e with high yields. Remarkably, the substrate scope study demonstrated the high tolerance of both electronic and steric effects, facilitating the generation of the desired products. Notably, the reaction displayed a single diastereomer in the case of alkynes, and high regioselectivity was observed across all substrates. Biological investigations on the synthesized pyrazolo[1,5-c]quinazolines were conducted. Compound 8e exhibited potent inhibitory activity against kinase EGFR inhibitory activity (IC50 = 235 nM) and displayed significant anticancer potential, as shown in the representative substrate scope. Furthermore, exploring the substrate scope led to the discovery that one of the molecules exhibited twice the potency of the existing drug, erlotinib. This comprehensive study showcases the significance of the developed 4-component reaction and highlights its potential for synthesizing diverse pyrazolo[1,5-c]quinazolines with notable biological activities.
Scheme 8.
Sawant’s work on 4-CR based on nitrene transfer on isocyanide
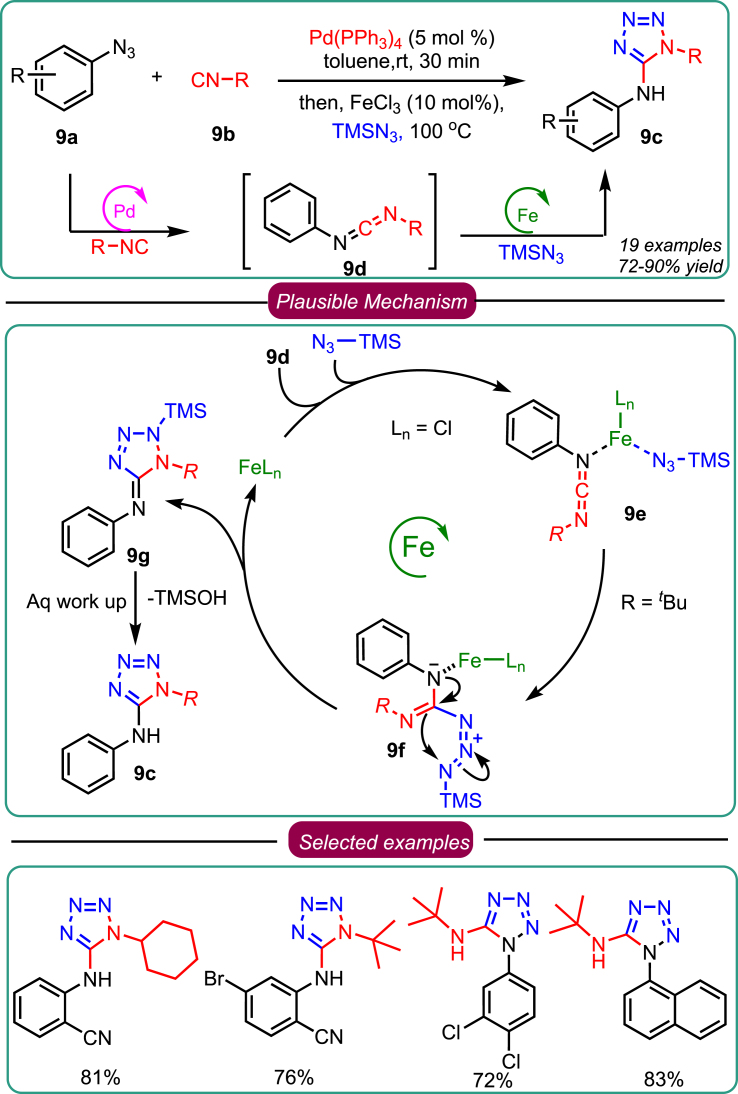
In a similar interest, Sawant’s group, in the year 2018, disclosed the rapid and efficient synthesis of amino tetrazole from 3-CR. The reaction involved sequential dual Pd(0)/Fe(III) catalyzed 3-CR of aryl azides 9a, isocyanides 9b, and TMSN3 in generating amino tetrazole 9c (Scheme 9).40 The optimization study revealed that toluene is the best solvent, and Pd and Fe are the most efficient catalysts to produce the desired product. Based on control experiments, a plausible mechanism was depicted (Scheme 9). The cycle portrays that the transformation involves the development of in-situ asymmetric carbodiimide via cross-coupling of azide-isocyanide denitrogenative coupling reactions catalyzed by Pd. Next, in the presence of FeCl3, intermediate 9d reacts with TMSN3 in a single pot to generate 9e in good to excellent yield. This reaction’s scope (Scheme 9) disclosed that wider varieties of aryl azide derivatives and a range of isocyanides 9b were tolerated well to yield the related 5-amino-1H-tetrazoles 9c. The limitation of this methodology was that aryl isocyanide failed to form a cross-coupled product with aromatic azides. Nevertheless, this methodology has tremendous potential over traditional synthetic approaches and is practical and scalable.
Scheme 9.
Sawant’s works on 3-CR for the synthesis of amino tetrazole
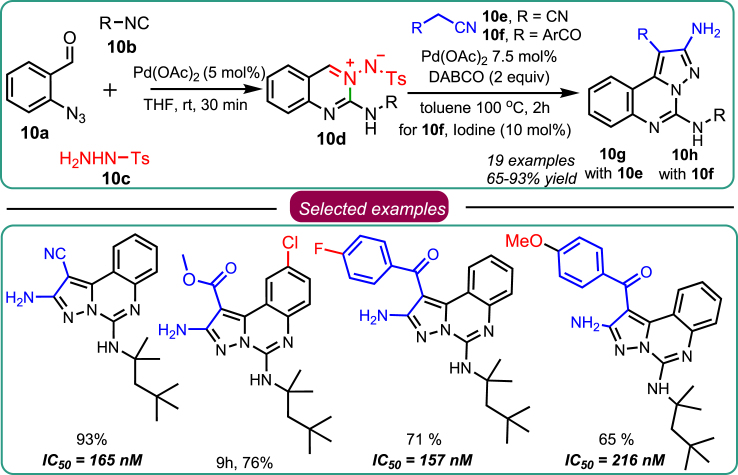
Coming ahead in exploring the competency of established 4-CR presented in Scheme 8, Sawant’s research team developed nitrene-transfer reaction-based 4-CR to synthesize pyrazolo[1,5-c]quinazoline as a bioactive scaffold. The reaction was accomplished under the catalytic influence of Pd with the most readily available starting materials, including 2-azidobenzaldehyde 10a, isocyanide 10b, aryl sulphonyl hydrazide 10c, and acetonitrile 10e, 10f. During the reaction, they found that the desired product was not obtained upon the reaction of β-ketonitrile under optimal conditions. Further, they developed one-pot 4-CR, which typically involves the reaction of the in situ-produced azomethine imine 10d and reacted with the acetonitrile derivative 10g/10h (Scheme 10).41 All the developed molecules were explored for their anti-cancer assets. Among the synthesized leads, two molecules exhibited potent anticancer potential compared to standard kinase inhibitors (erlotinib and gefitinib) and were mechanistically found to inhibit EGFR-mediated kinase activity. The active molecules were found devoid of cardiac cell damage, a common drawback of approved EGFR inhibitors. In a nutshell, the presence of an amino group on the pyrazole ring of pyrazolo[1,5-c]quinazolines made them potent and safe inhibitors of EGFR. The present study validated the Schreiber hypothesis that chemical spacing on small molecules is vital in guiding drug discovery and development.
Scheme 10.
Sawant’s work on 4-CR for synthesizing pyrazolo[1,5-c]quinazoline as an EGFR inhibitor
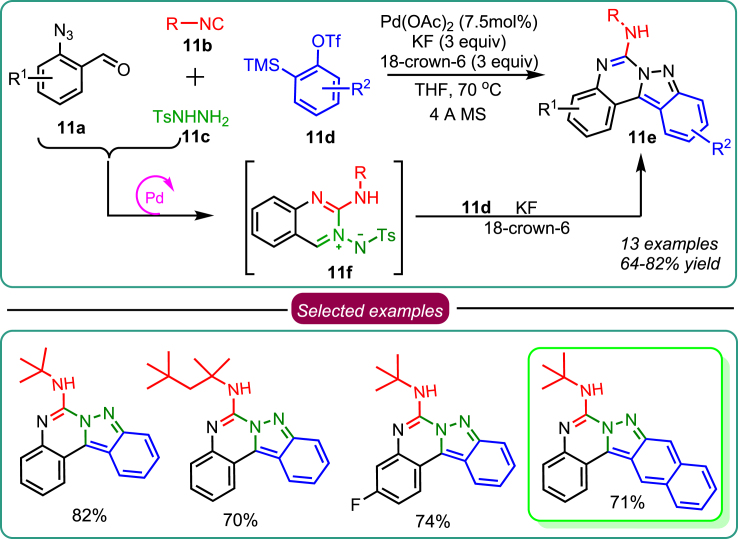
Next, a library of indazolo[2,3-c]quinazolines was synthesized using Pd-catalyzed single-pot sequential 4-CR based on the foundation and concept laid by previous 4-CR research. Five chemical bonds were formed during the sequential reaction of 2-azido benzaldehyde 11a, isocyanide 11b, aryl sulphonyl hydrazide 11c, and aryne precursor 11d, which also involved the concatenation of three simple steps (Scheme 11).42 Green fluorescence was produced under visible light by the synthesized derivative 11e, with a quantum yield of 68%. The compound also exhibited good Stokes shifts of up to 2796 cm−1 in CHCl3 and excellent photostability in DMSO for 30 min. The green dye developed produced a fluorescent effect in cancer cell mitochondria and cytoplasm with superior photo-stability and negligible cytotoxicity. The simplicity with which indazolo[2,3-c]quinazolines 11e have been made available here is a fine example of the great appeal of multicomponent reactions and presents a chance for high-throughput, modulated screening for particular functional molecules.
Scheme 11.
Sawant’s work on 4-CR for synthesizing indazolo[2,3-c]quinazolines as organic fluorophores
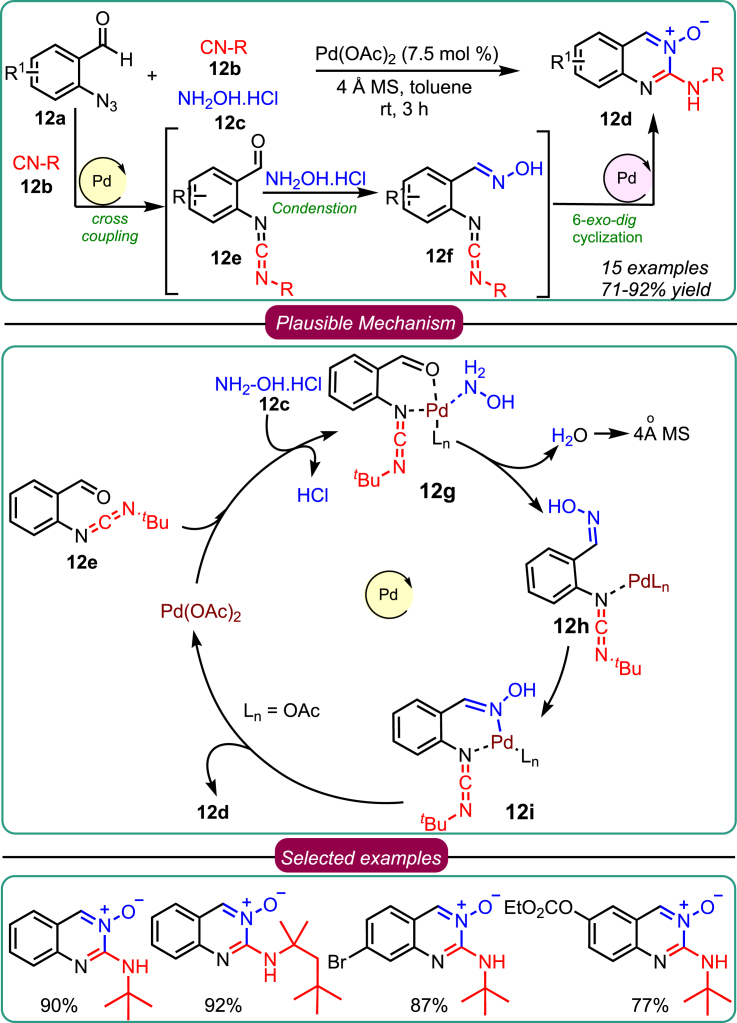
Subsequently, in 2019, the Sawant group embarked on an expedition to develop diversely functionalized quinazoline 3-oxides 12d (Scheme 12) through a novel synthetic process involving 2-azido benzaldehydes 12a, isocyanides 12b, and hydroxylamine hydrochloride 12c in a one-pot three-component reaction.43 The reaction was catalyzed by Pd(II) and facilitated the formation of three new chemical bonds in a regioselective manner. They further explored the versatility of this reaction by varying the substrates and employing different reaction conditions. The reaction exhibited a broad substrate scope and excellent tolerance toward many functional groups, enabling the synthesis of desired products in shorter reaction times with good yields. Mechanistic investigations shed light on the reaction pathways. It was found that the reaction proceeded through three major individual pathways. First, denitrogenative coupling of azide derivatives with the isocyanide occurred, followed by condensation with hydroxylamine to produce complex 12g. Lastly, 12h catalyzed by Pd(II), cyclization occurred in a 6-exo-dig manner with hydrazones on the carbodiimide scaffold, which generated the desired quinazoline 3-oxides. This innovative approach provided the utility for a one-pot, three-component reaction via auto-tandem Pd(II) catalysis, offering a novel method to access quinazoline 3-oxides 12d from readily available substrates with high efficiency and a simple protocol.
Scheme 12.
Sawant’s work on 3-CR for synthesizing quinazoline 3-oxides
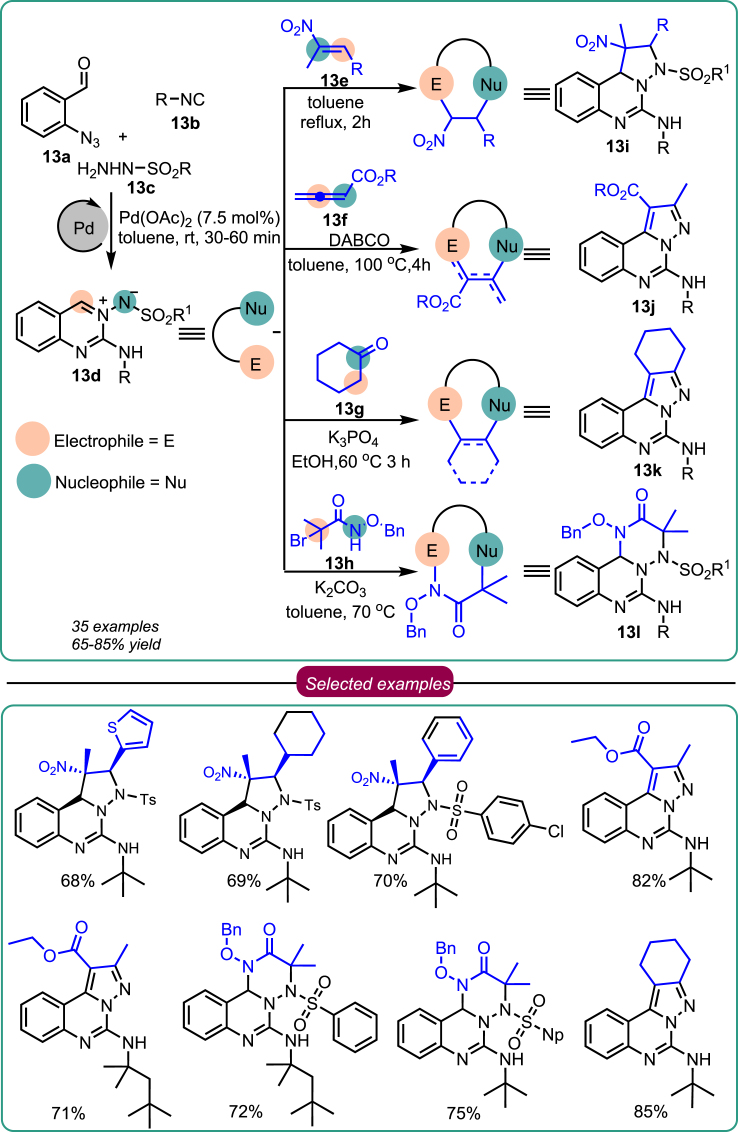
A comprehensive literature search uncovered the significance of azomethine imine as a versatile building block for synthesizing poly-heterocycles. Motivated by this, Sawant’s research group developed a one-pot, three-component reaction for synthesizing azomethine imines, employing an auto-tandem Pd(II) catalysis approach (Scheme 13).44 Encouraged by this result, they envisaged that skeletally diverse compound collections could be generated from the azomethine imines. Incorporating various dipolarophiles through diversity-oriented synthesis enables the generation of a skeletally diverse molecule, facilitating the synthesis of complex heterocycles with potential medicinal applications. To initiate this endeavor, they focused on creating a compound collection centered around azomethine imine. The group accomplished this by reacting 2-azido benzaldehyde 13a, alkyl isocyanide 13b, and aryl sulfonyl hydrazide 13f in toluene as the solvent. Pd(OAc)2 was the catalyst, while 4 Å molecular sieves were employed. The reaction mixture was stirred at room temperature for 30 min, forming azomethine imine 13d in good to excellent yields. Further, azomethine imine 13d was reacted with reagents possessing orthogonal functionality such as nitroolefins, allenoates, cyclic ketone, and α-halo hydroxamates 13e-13h to produce diverse and complex molecules 13i-13l respectively (Scheme 13). Incorporating various dipolarophiles into the skeleton of a molecule through diversity-oriented synthesis was found beneficial in the production of complex heterocycles for medicinal use. The optimal 4-CR protocol facilitated a significant degree of step/atom economy. Excellent regio- and stereoselectivity, as well as functional group tolerance, were observed in the reaction. A collection of compounds with diverse and intricate molecular architectures could play a crucial role in future drug discovery and development.
Scheme 13.
Sawant’s work on diversity-oriented synthesis based on 4-CR using different dipolarophiles to generate complex poly-heterocycles
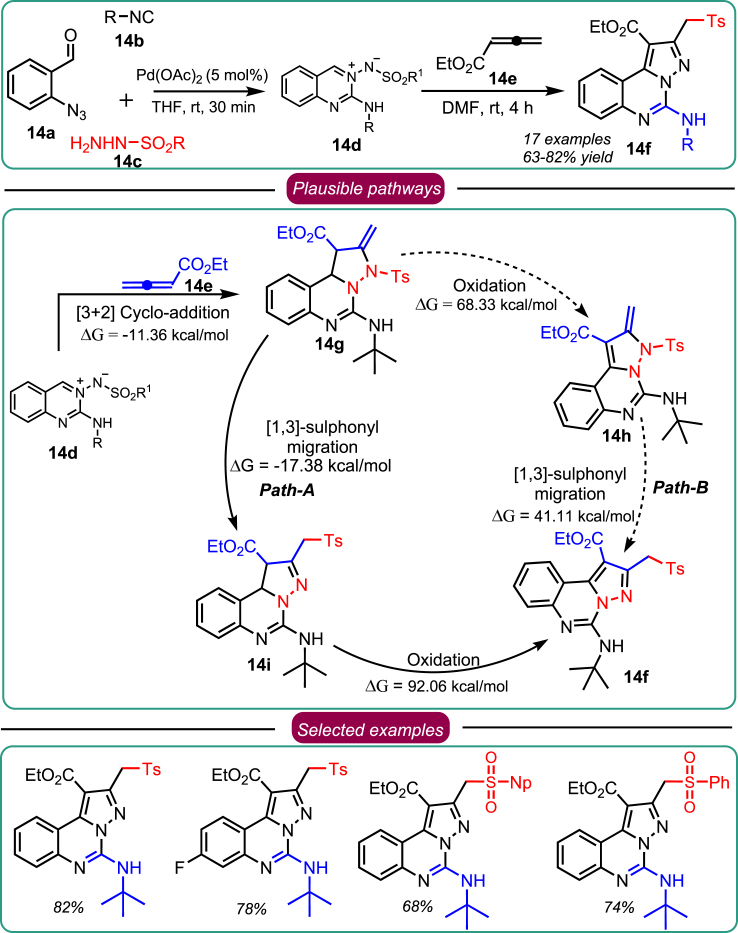
Based on dedicated research efforts, Sawant et al. revealed a novel one-pot synthesis of aryl sulfonyl methyl-substituted pyrazolo[1,5-c]quinazolines 14f from azomethine imine 14d and allenoate 14e. Mechanistic investigations have uncovered an unexpected regioselective sulfonyl migration during the cycloaddition of N-sulfonyl azomethine imine 14d with allenoates 14e, leading to the formation of compound 14f (Scheme 14).45 1,3-sulfonyl migration was further explored using DFT studies in addition to the controlled experiments. The coupling reaction involves oxidative aromatization, which is thermodynamically favored and concerned with the lower activation energy. Energy-wise, mechanistic research indicates that the transition from 14d to 14f is highly favorable and may occur via two pathways: (1) [1,3]-sulfonyl migration followed by oxidation (Path-A) and (2) oxidation followed by [1,3]-sulfonyl migration (Path-B). [1,3]-Sulfonyl migration can be considered a [1,3]-sigmatropic shift. The first pathway includes a concerted [1–3]-sigmatropic shift, allowing the sulfonyl migration with a shallow energy barrier accomplished by a diminished steric cloud in the second step. In controlled experiments, detosylated compound 13j was isolated as a significant product when toluene was used as a solvent. Along Path-B, the oxidation step and migration of ArSO2 occur with an energy gain of 68.3 and 41.1 kcal/mol respectively. This is a spontaneous process, as the transition state for the migration could not be traced along the reaction path. Path-B is probably more favorable because it involves two barrierless reactions, whereas Path-A involves activation energy in the [1,3]-sulfonyl migration step. In a nutshell, DMF was the best solvent for conversion, completely suppressing the side product 13j formation. Thus, it was concluded that an optimized methodology with high product yields could be achieved by stirring the mixture of 14d and 14e in DMF for 4 h at room temperature. The findings thus opened up new avenues for forecasting [1–3]-sulfonyl migration involving two or more components, leading to the synthesis of diverse bioactive compounds.
Scheme 14.
Sawant et.al., work on the synthesis of unprecedented [1,3]-sulfonyl migration
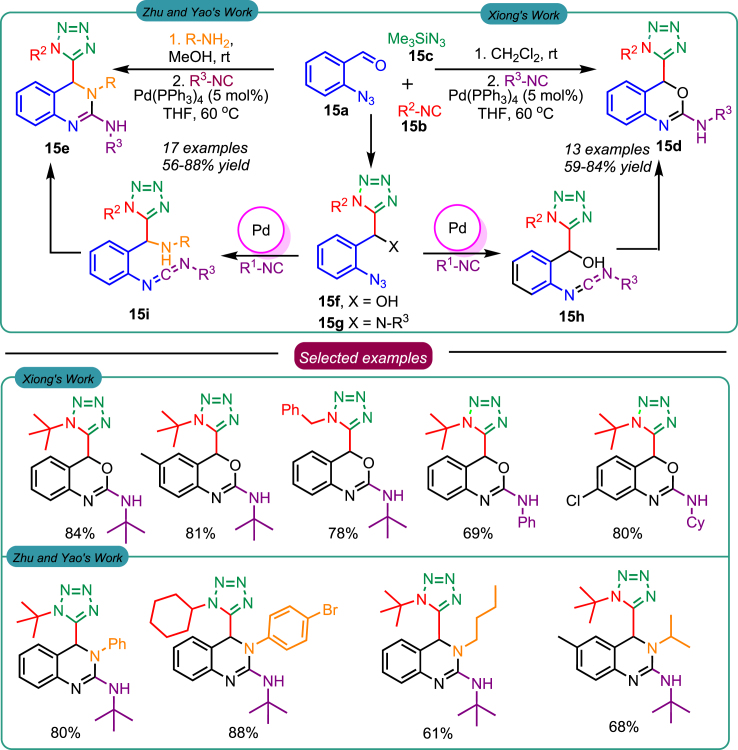
In 2020, Xiong et al. presented their work on the synthesis of 4H-3,1-benzoxazine derivatives using a sequential one-pot, a three-component reaction catalyzed by Pd.46 Initially, the passerine-azide adduct 15f was formed through a three-component reaction involving 2-azido-benzaldehydes 15a, trimethylsilyl azide 15c, and isocyanides 15b. Subsequently, the passerine-azide adduct 15f underwent a tandem palladium-catalyzed transformation to generate the carbodiimide intermediate 15h through an azide-isocyanide cross-coupling process. The 15h was further found to undergo cyclization via 6-exo-dig cyclization (Scheme 15) to afford the desired 4H-3,1-benzoxazine derivatives 15d. In a similar context, Zhu and Yao et al. in 2022 developed the synthetic access route to develop 4-tetrazolyl-3,4-dihydroquinazolines 15e catalyzed by the Pd in one-pot sequential reaction that included the cross-coupling of Ugi-azide with the isocyanide.47 In this Ugi-azide reaction, 4 reactants were involved, including 2-azidobenzaldehydes 15a, amines, trimethylsilyl azide 15c, and isocyanides 15b that yielded the azide-Ugi adduct 15g. Upon reaction with isocyanides, the adduct yielded 4-tetrazolyl-3,4-dihydroquinazoline derivatives 15e (Scheme 15). The derivative of the 15e series was found to possess anticancer potential against breast cancer cells and may possess numerous applications in anticancer drug discovery.
Scheme 15.
Azide-isocyanide cross-coupling reaction-based synthesis of tetrazole-containing heterocyclic scaffolds
Rhodium-based reaction
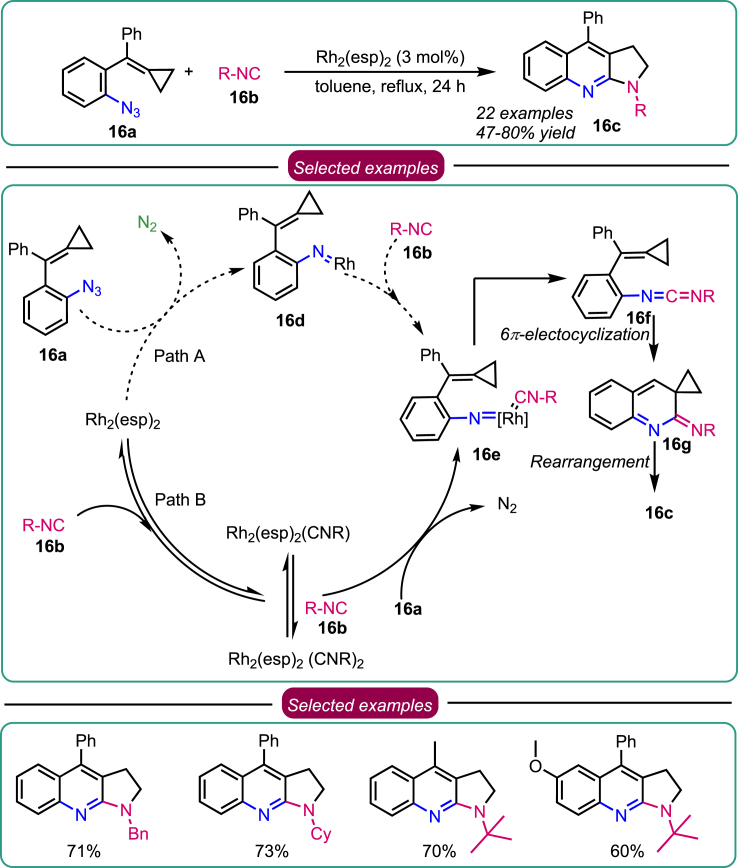
Based on their previous report, Shi et al. designed azide-tethered methylenecyclopropanes (MCPs) 16a, which proceeded via an intermolecular cyclization that involved the reaction of isonitriles 16b by a Rh(II) complex via carbodiimide intermediates 16f to afford the pyrrole-fused quinoline skeletons 16c (Scheme 16).48 Initially, the reaction condition was investigated, and Shi’s group found Rh2(esp)2 was the catalyst and toluene was the solvent. Next, examining the substrate scope electronic parameter on the aromatic ring did not alter the outcome of the product. tBuNC was found to be best toward the reactivity to test the other substrate. Based on control experiments, two plausible paths were depicted. In path A, the extrusion of nitrogen generates the Rh-nitrene complex 16d, followed by the formation of complex isocyanide 16e. Alternatively, another proposed path of this reaction (path B) involves the reaction of the Rh2(esp)2 with tBuNC, allowing the formation of an unstable intermediate, Rh2(esp)2(CNtBu)2, that further decomposes readily to yield stable Rh2(esp)2(CNtBu). Path A is more favorable because of its stability and Rh-nitrene intermediate formation. After the renaissance of the rhodium catalyst, nitrene was transferred to isocyanide to generate carbodiimide 16f. A sequential 6-electrocyclization affords the intermediate 16g, which then undergoes a thermally induced rearrangement to yield the product 16c. In addition, synthetic applications of these compounds for constructing structurally new and diversified bioactive heterocycles were explored.
Scheme 16.
Shi’s reports on the synthesis of pyrrolo[2,3-b]quinolines
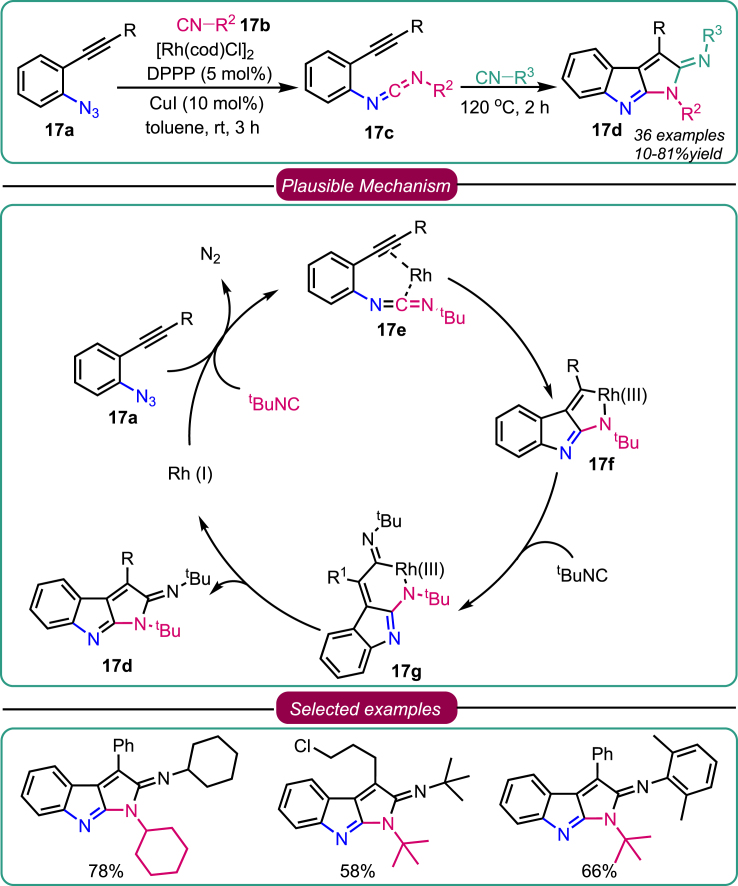
In 2016, building upon the concept of nitrene transfer to isocyanides, Zhang et al. introduced a straightforward methodology for synthesizing pyrrolo[2,3-b]indole scaffold 17d. This reaction relied on rhodium (I) as a catalyst, enabling the self-relay reaction of tandem nitrene transformations. The alkyne-azides 17a underwent aza-Pauson-Khand cyclization with ligand isonitrile 17b, possessing both electron-donating and electron-withdrawing groups, sequentially forming a poly-fused heterocycle system (Scheme 17).49 During the optimization of the reaction conditions, [Rh(cod)Cl]2 was identified as the catalyst, with 1,3-bis(diphenylphosphine)propane (DPPP) serving as the ligand. The inclusion of CuI as an additive and toluene as the solvent further enhanced the reaction efficiency. Diversified electron-donating and electron-withdrawing groups on the aromatic ring of intermediate 1-azido-2-(phenylethynyl)benzenes 17a were tolerated, resulting in high yields of the desired product 17d. Additionally, various isonitriles were found to provide excellent results (Scheme 17). The mechanistic insights suggest two plausible routes for forming pyrrolo[2,3-b]indoles. The first route involves the cross-coupling reaction of azides 17a with isonitriles 17b, catalyzed by Rh(I). The second route involves an aza-Pauson-Khand cyclization mechanism. Initially, nitrene transfer to the isocyanide leads to the formation of alkynyl carbodiimide intermediate 17c through the expulsion of nitrogen. Complexation of 17e with Rh(I), followed by oxidative cyclometallation, results in the formation of a rhodacycle species 17f. Subsequent isonitrile insertion into the Rh-C bond of species 17f generates complex 17g, which, upon reductive elimination, affords the pyrrolo[2,3-b]indole derivatives 17d while regenerating the active rhodium (I) catalyst.
Scheme 17.
Zhang’s work on Rh-catalyzed synthesis of pyrrolo[2,3-b]indoles
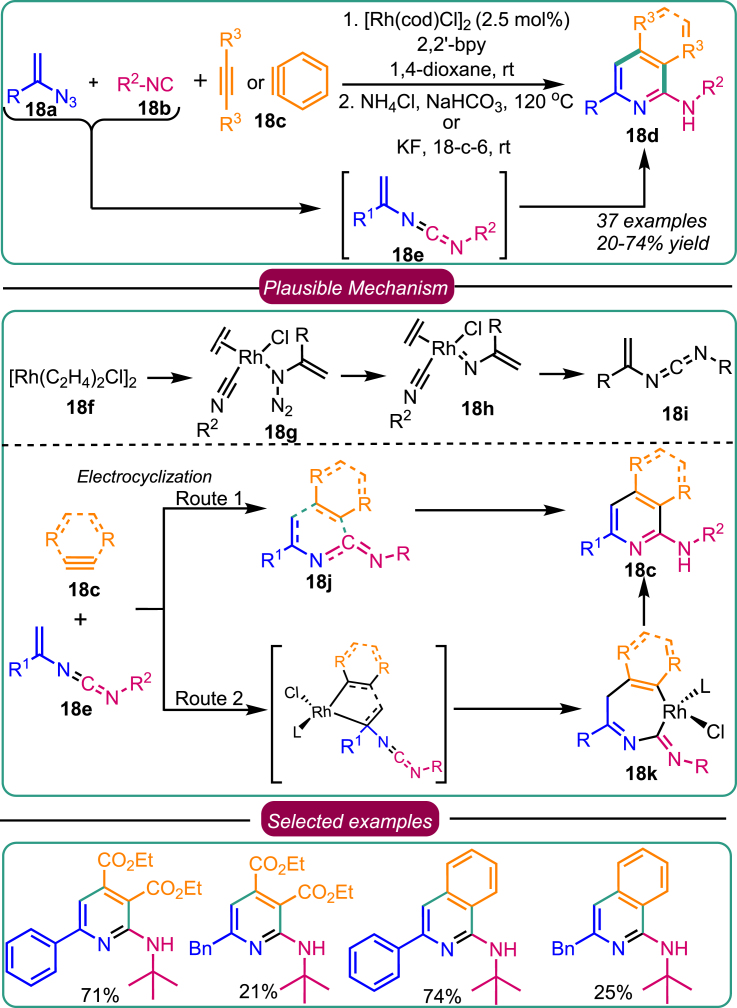
In 2018, Zhang et al. developed a coupling reaction for the formation of diversified azaheterocycles 18d via the Rh(I)-catalyzed reaction of vinyl azides 18a with isonitriles 18b and alkynes/benzynes 18c (Scheme 18).50 Initially, azide moiety and isonitrile form active vinyl carbodiimide intermediates 18e through the involvement of Rh-nitrene species. This was supported by using control experiments and DFT calculations. The process initiated with the Rh(I) dimer catalyst (18f) undergoing dissociation into a monomeric active catalyst, which coordinated with the isonitrile and vinyl azide to generate the Rh-complex 18g. In a subsequent step, species 18h released N2, creating nitrene intermediate 18i through an exergonic transition state with an energy of 25.6 kcal/mol. The nitrene moiety of 18i then engaged in a coupling reaction with the isonitrile, forming the vinyl carbodiimide. Ultimately, a ligand exchange event yielded the free vinyl carbodiimide, accompanied by a decrease in Gibbs energy (ΔG = 21.3 kcal/mol). This observation supports that the coupling step necessitated the involvement of a Rh-nitrene intermediate. Following the successful formation of the vinyl carbodiimide, intermediate 18e engaged in a tandem cyclization process with unsaturated compounds, including alkynes and benzynes 18c. A comprehensive exploration of this synthetic route unveiled two distinct pathways: direct electrocyclization and oxidative cyclization reductive elimination, both facilitated by Rh(I), ultimately yielding the desired azaheterocycles 18d. Further investigation into the substrate scope revealed that electronic influences had a negligible impact and did not compromise the desired product’s outcome. Remarkably, alkyne and benzyne substrates exhibited robust reactivity, resulting in the desired products with excellent yields.
Scheme 18.
Zhang’s work on Rh-catalyzed synthesis of azaheterocycles
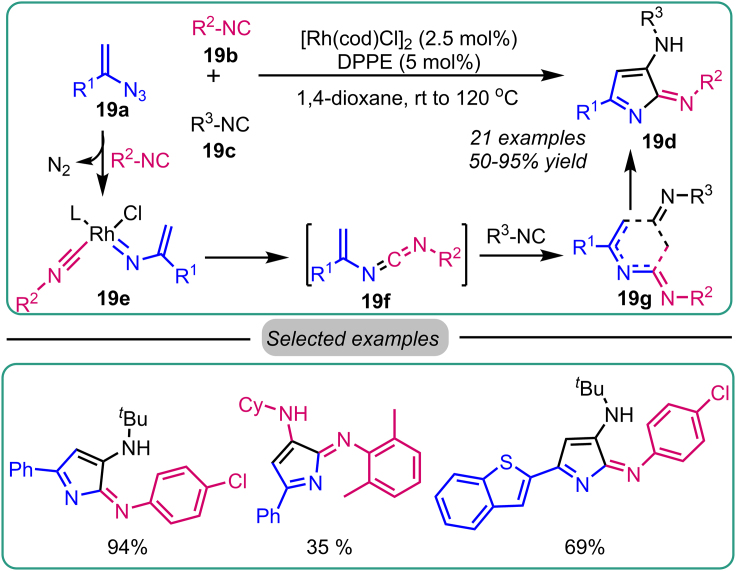
In 2019, Zhang et al. developed another Rh-catalyzed reaction to develop 2H- pyrrol-2-imine 19d (Scheme 19) in high yields. The mechanistically mediated reaction involves the cross-coupling of azide with isocyanide.51 The reaction presents broader substrate tolerance, possessing diversified functionality and electronic surroundings. Mechanistically, the in situ generated Rh(I) complex 19e initiates the reaction from vinyl azide 19a with isocyanide 19b to generate vinyl carbodiimide intermediate 19f via nitrene transfer on isocyanide, which consequently undergoes thermal cyclization with the second isocyanide 19c to yield the desired product, 2H-pyrrol-2-imine 19d. During the evaluation of the substrate scope, electron-donating aryl isocyanides participated well, whereas electron-withdrawing aryl isocyanides produced moderate yields, providing access to 2H-Pyrrol-2-imine. The second isocyanide was an aliphatic, and aromatic isocyanide participated well in producing the proposed product with good yields (Scheme 18).
Scheme 19.
Zhang’s work on Rh-catalyzed synthesis of 2H-Pyrrol-2-imine
Cobalt-based reaction
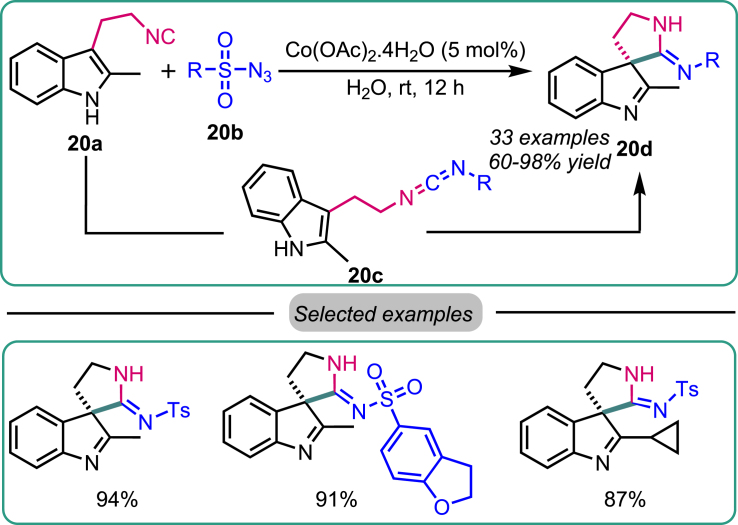
In 2021, Ji et al. reported the synthesis of spiroindolenine 20d through a Co-catalyzed cross-coupling reaction of tryptamine-derived isocyanides 20a with sulfonyl azides 20b (Scheme 20).52 The reaction was carried out in pure water at room temperature for 12 h using an aqueous system that proved to be highly efficient. The recyclability of the cobalt catalyst was also investigated, revealing that the same aqueous system could be used with the catalyst for approximately 10 cycles without a significant decrease in catalytic efficiency in mediating the cross-coupling reaction. The plausible mechanism proposed involves cross-coupling the azide derivative with the isocyanide derivative under the catalytic influence of cobalt, leading to the generation of a Co(III)-nitrene intermediate. This intermediate was further converted to the carbodiimide intermediate 20c upon nitrogen (N2) release. Subsequently, the nucleophilic C3 position of the indole undergoes spiro cyclization, followed by an isomerization process, ultimately yielding the desired product 20d.
Scheme 20.
Ji’s work on Co-catalyzed synthesis of spiroindolenine via nitrene-transfer reaction
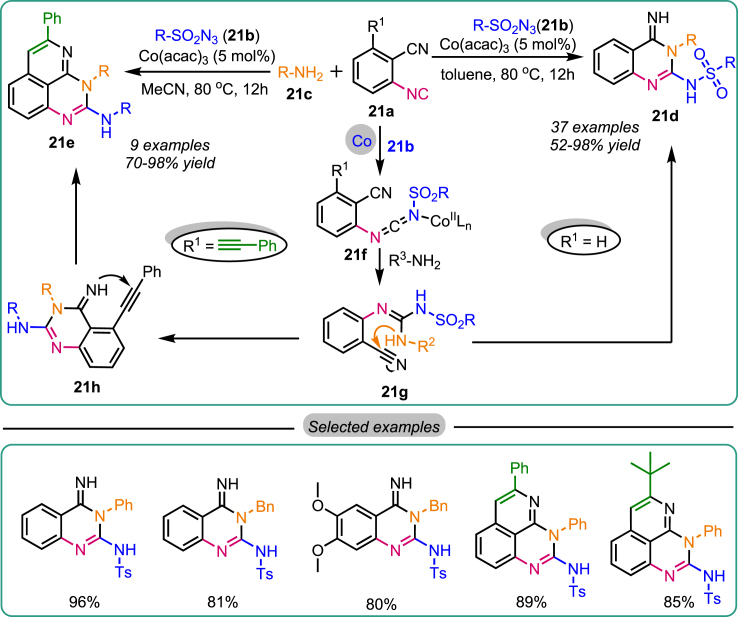
The same research group developed a Co-catalyzed, highly efficient, and cost-effective method for synthesizing various quinazoline derivatives. The methodology involved a three-component reaction comprising 2-isocyanobenzonitriles 21a, sulfonyl azides 21b, and amines 21c under Co-catalysts’ influence. This approach led to the formation of two distinct heterocycles: quinazoline-4(H)-imines 21d and pyrido[2,3,4-de]-quinazolines 21e (using alkyne) in good to excellent yields, exhibiting a broad substrate scope (Scheme 21).53 Through control experiments, a plausible mechanism was proposed. The reaction initiates with the Co-catalysts, facilitating the utilization of azide and isocyanide to form the carbodiimide intermediate 21f. Subsequently, the nucleophilic addition of an amine to the carbodiimide leads to the formation of the guanidine product 21g. The nitrogen atom from the amine further attacks the cyano group, resulting in the desired product 21d. Additionally, intermediate 21h undergoes intramolecular cross-coupling to form a C-N bond, particularly in the alkynyl and iminyl groups in the alkyne substituent, ultimately affording fused quinazolines 21e.
Scheme 21.
Co-catalyzed synthesis of quinazolin-4(H)-imines and pyrido[2,3,4-de]-quinazolines
Conclusion
In summary, the cross-coupling reaction of azides with isocyanides through nitrene transfer is a robust, efficient, and adaptable method for synthesizing diverse carbodiimides. The evolution of TM complexes, notably employing stable metals like Pd, Rh, and Co, has considerably broadened the scope and practicality of this approach. Additionally, the conversion of carbodiimides into valuable compounds via relay transition-metal-catalyzed cascade reactions has yielded a plethora of unique structures, enriching the utility of the azide-isocyanide coupling technique.
While significant strides have been made, these strategies remain necessary for simplification and cost-effectiveness. Exploring earth-abundant metals such as Fe, Ni, and Cu for cascade reactions, with cobalt being a notable exception, presents an exciting avenue for further research. Investigating the compatibility and feasibility of heterogeneously catalyzed cascade reactions holds promise. Furthermore, the future of this field is likely to witness innovative approaches, including electrochemical or visible-light-induced models, aligning with the principles of green and sustainable chemistry.
Looking forward, the organic chemistry community is poised to discover an increasing array of consequential consecutive reactions. These discoveries will enrich the arsenal of synthetic methodologies and redefine the limits of chemical synthesis. As researchers delve deeper into unexplored realms, the horizon of possibilities in chemical synthesis is boundless, promising a future of even more sophisticated and environmentally conscious approaches.
Note:The alphanumeric designations (1b,1f,1j,2b,3b,4b,5b,6b,7b,8b,9b,10b,11b,12b,13b,14b,15b,16b,17b,18b,19b,19c,20a, and21a) exclusively represent the isocyanides in the manuscript. However, these represented isocyanides may vary in their derivatization or functional groups, a distinction that is clearly delineated within the manuscript.
Acknowledgments
The authors thank CURaj for the facilities, D.M.S. thanks SERB-DST, New Delhi for the grant (CRG/2022/001303) and CURaj for funding. A.J.A. thanks ICMR for a Senior research fellowship, and G.J. thanks Department of Biotechnology, New Delhi, for providing a grant as extramural project no. PR47642/CMD/150/19/2023.
Author contributions
D.M.S., G.J., and A.J.A. collected the references. A.J.A. and G.J. conceived the project and prepared this manuscript. A.J.A. and D.M.S. overall supervised the project.
Declaration of interests
The authors declare no competing interests.
References
- 1.Dequirez G., Pons V., Dauban P. Nitrene chemistry in organic synthesis: Still in its infancy? Angew. Chem. Int. Ed. Engl. 2012;51:7384–7395. doi: 10.1002/anie.201201945. [DOI] [PubMed] [Google Scholar]
- 2.Tiemann F. Ueber die Einwirkung von Benzolsulfonsäurechlorid auf Amidoxime. Ber. Dtsch. Chem. Ges. 1891;24:4162–4167. doi: 10.1002/cber.189102402316. [DOI] [Google Scholar]
- 3.Smith P.A.S., Boyer J.H. The Synthesis of Heterocyclic Compounds from Aryl Azides. II. Carbolines and Thienoindole. J. Am. Chem. Soc. 1951;73:2626–2629. doi: 10.1021/ja01150a061. [DOI] [Google Scholar]
- 4.Dodd D., Johnson M.D. Electrophilic substitution at a saturated carbon atom by mercury(I) J. Chem. Soc. D. 1970;82:460a–4719a. doi: 10.1039/C2970000460a. [DOI] [Google Scholar]
- 5.ApSimon J.W., Edwards O.E. a New Photochemical Reaction: the Structure and Absolute Stereochemistry of Atisine. Can. J. Chem. 1962;40:896–902. doi: 10.1139/v62-136. [DOI] [Google Scholar]
- 6.Sundberg R., Smith Jr R., Bloor J., Renfrow W.B. Thermal Reactions of Sulfonyl Azides. J. Am. Chem. Soc. 1969;91:5697. doi: 10.1021/ja01048a610. [DOI] [Google Scholar]
- 7.Iredale T. The photodecomposition of ethyl iodide. J. Phys. Chem. 1929;33:290–295. doi: 10.1021/j150296a011. [DOI] [Google Scholar]
- 8.Anastassiou A.G., Simmons H.E., Marsh F.D. Cyanonitrene. Reaction with Saturated Hydrocarbons. J. Am. Chem. Soc. 1965;87:2296–2297. doi: 10.1021/ja01088a043. [DOI] [Google Scholar]
- 9.Kwart H., Kahn A.A. Copper-Catalyzed Decomposition of Benzenesulfonyl Azide in Hydroxylic Media. J. Am. Chem. Soc. 1967;89:1950–1951. doi: 10.1021/ja00984a034. [DOI] [Google Scholar]
- 10.Breslow R., Gellman S.H. Intramolecular Nitrene C-H Insertions Mediated by Transition-Metal Complexes as Nitrogen Analogues of Cytochrome P-450 Reactions. J. Am. Chem. Soc. 1983;105:6728–6729. doi: 10.1021/ja00360a039. [DOI] [Google Scholar]
- 11.Mansuy D., Mahy J.P., Dureault A., Bedi G., Battioni P. Iron- and manganese-porphyrin catalysed aziridination of alkenes by tosyl- and acyl-iminoiodobenzene. J. Chem. Soc. Chem. Commun. 1984:1161–1163. doi: 10.1039/c39840001161. [DOI] [Google Scholar]
- 12.Evans D.A., Bilodeau M.T., Faul M.M. Development of the Copper-Catalyzed Olefin Aziridination Reaction. J. Am. Chem. Soc. 1994;116:2742–2753. doi: 10.1021/ja00086a007. [DOI] [Google Scholar]
- 13.Luo Y., Zhang X., Xia Y. Recent advances in transition-metal catalyzed nitrene transfer reactions with carbamates. Chin. Chem. Lett. 2024;35 doi: 10.1016/j.cclet.2023.108778. [DOI] [Google Scholar]
- 14.Hayashi H., Uchida T. Nitrene Transfer Reactions for Asymmetric C–H Amination: Recent Development. Eur. J. Org. Chem. 2020;2020:909–916. doi: 10.1002/ejoc.201901562. [DOI] [Google Scholar]
- 15.Wu X.-F., Neumann H., Beller M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2013;113:1–35. doi: 10.1021/cr300100s. [DOI] [PubMed] [Google Scholar]
- 16.Roose T.R., Verdoorn D.S., Mampuys P., Ruijter E., Maes B.U.W., Orru R.V.A. Transition metal-catalysed carbene- and nitrene transfer to carbon monoxide and isocyanides. Chem. Soc. Rev. 2022;51:5842–5877. doi: 10.1039/d1cs00305d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozaki S. Recent advances in isocyanate chemistry. Chem. Rev. 1972;72:457–496. doi: 10.1021/cr60279a002. [DOI] [Google Scholar]
- 18.Kreye O., Mutlu H., Meier M.A.R. Sustainable routes to polyurethane precursors. Green Chem. 2013;15:1431–1455. doi: 10.1039/c3gc40440d. [DOI] [Google Scholar]
- 19.Williams A., Ibrahim I.T. Carbodiimide chemistry: recent advances. Chem. Rev. 1981;81:589–636. [Google Scholar]
- 20.Wang Y., Zhang W.X., Xi Z. Carbodiimide-based synthesis of N-heterocycles: Moving from two classical reactive sites to chemical bond breaking/forming reaction. Chem. Soc. Rev. 2020;49:5810–5849. doi: 10.1039/c9cs00478e. [DOI] [PubMed] [Google Scholar]
- 21.Peplow M., Thompson B. Audio long read: ‘Almost magical’ — chemists can now move single atoms in and out of a molecule’s core. Nature. 2023;618:21–24. doi: 10.1038/d41586-023-02081-y. [DOI] [PubMed] [Google Scholar]
- 22.Song L., Tian X., Farshadfar K., Shiri F., Rominger F., Ariafard A., Hashmi A.S.K. An unexpected synthesis of azepinone derivatives through a metal-free photochemical cascade reaction. Nat. Commun. 2023;14:831. doi: 10.1038/s41467-023-36190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian X., Song L., Hashmi A.S.K. α-Imino Gold Carbene Intermediates from Readily Accessible Sulfilimines: Intermolecular Access to Structural Diversity. Chem. Eur J. 2020;26:3197–3204. doi: 10.1002/chem.201904869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Q., Jiang D., Zhang J., Guan L., Li T., Zhao Y., Di M., Chen H., Che C., Zhu Z. Combining visible-light induction and copper catalysis for chemo-selective nitrene transfer for late-stage amination of natural products. Commun. Chem. 2022;5:79. doi: 10.1038/s42004-022-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babu Y.S., Chand P., Bantia S., Kotian P., Dehghani A., El-Kattan Y., Lin T.H., Hutchison T.L., Elliott A.J., Parker C.D., et al. BCX-1812 (RWJ-270201): Discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design [3] J. Med. Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 26.Royo P., Sánchez-Nieves J. Oxo and imido/imido exchange and C-H activation reactions based on pentamethylcylopentadienyl imido tantalum complexes. J. Organomet. Chem. 2000;597:61–68. doi: 10.1016/S0022-328X(99)00593-8. [DOI] [Google Scholar]
- 27.Mindiola D.J., Hillhouse G.L. Isocyanate and carbodiimide synthesis by nitrene-group-transfer from a nickel(II) imido complex. Chem. Commun. 2002:1840–1841. doi: 10.1039/b204846a. [DOI] [PubMed] [Google Scholar]
- 28.Badiei Y.M., Krishnaswamy A., Melzer M.M., Warren T.H. Transient terminal Cu-nitrene intermediates from discrete dicopper nitrenes. J. Am. Chem. Soc. 2006;128:15056–15057. doi: 10.1021/ja065299l. [DOI] [PubMed] [Google Scholar]
- 29.Cowley R.E., Eckert N.A., Elhaïk J., Holland P.L. Catalytic nitrene transfer from an imidoiron (III) complex to form carbodiimides and isocyanates. Chem. Commun. 2009:1760–1762. doi: 10.1039/B820620A. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen A.I., Zarkesh R.A., Lacy D.C., Thorson M.K., Heyduk A.F. Catalytic nitrene transfer by a zirconium(IV) redox-active ligand complex. Chem. Sci. 2011;2:166–169. doi: 10.1039/c0sc00414f. [DOI] [Google Scholar]
- 31.Dai Q.X., Seino H., Mizobe Y. Tungsten(II) alkylimido complexes from insertion of nitriles into tungsten hydride: Alkylideneamido intermediate stage and nitrene group transfer to isocyanide. Organometallics. 2012;31:4933–4936. doi: 10.1021/om3004338. [DOI] [Google Scholar]
- 32.Yousif M., Tjapkes D.J., Lord R.L., Groysman S. Catalytic Formation of Asymmetric Carbodiimides at Mononuclear Chromium(II/IV) Bis(alkoxide) Complexes. Organometallics. 2015;34:5119–5128. doi: 10.1021/acs.organomet.5b00703. [DOI] [Google Scholar]
- 33.Cowley R.E., Golder M.R., Eckert N.A., Al-Afyouni M.H., Holland P.L. Mechanism of catalytic nitrene transfer using Iron(I)-isocyanide complexes. Organometallics. 2013;32:5289–5298. doi: 10.1021/om400379p. [DOI] [Google Scholar]
- 34.Wiese S., Aguila M.J.B., Kogut E., Warren T.H. Β-Diketiminato Nickel Imides in Catalytic Nitrene Transfer To Isocyanides. Organometallics. 2013;32:2300–2308. doi: 10.1021/om300909n. [DOI] [Google Scholar]
- 35.Kurup S.S., Staples R.J., Lord R.L., Groysman S. Synthesis of chromium(II) complexes with chelating bis(alkoxide) ligand and their reactions with organoazides and diazoalkanes. Molecules. 2020;25:273. doi: 10.3390/molecules25020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z., Li Z., Fu B., Zhang Z. Palladium-catalyzed cross-coupling reaction of azides with isocyanides. Chem. Commun. 2015;51:16312–16315. doi: 10.1039/c5cc05981j. [DOI] [PubMed] [Google Scholar]
- 37.Ansari A.J., Pathare R.S., Maurya A.K., Agnihotri V.K., Khan S., Roy T.K., Sawant D.M., Pardasani R.T. Synthesis of Diverse Nitrogen Heterocycles via Palladium-Catalyzed Tandem Azide–Isocyanide Cross-Coupling/Cyclization: Mechanistic Insight using Experimental and Theoretical Studies. Adv. Synth. Catal. 2018;360:290–297. doi: 10.1002/adsc.201700928. [DOI] [Google Scholar]
- 38.Ren Z.L., Kong H.H., Lu W.T., Sun M., Ding M.W. One-pot synthesis of quinazolin-4(3H)-ones and fused quinazolinones by a palladium-catalyzed domino process. Tetrahedron. 2018;74:184–193. doi: 10.1016/j.tet.2017.11.060. [DOI] [Google Scholar]
- 39.Sawant D.M., Sharma S., Pathare R.S., Joshi G., Kalra S., Sukanya S., Maurya A.K., Metre R.K., Agnihotri V.K., Khan S., et al. Relay tricyclic Pd(ii)/Ag(i) catalysis: Design of a four-component reaction driven by nitrene-transfer on isocyanide yields inhibitors of EGFR. Chem. Commun. 2018;54:11530–11533. doi: 10.1039/c8cc05845h. [DOI] [PubMed] [Google Scholar]
- 40.Pathare R.S., Ansari A.J., Verma S., Maurya A., Maurya A.K., Agnihotri V.K., Sharon A., Pardasani R.T., Sawant D.M. Sequential Pd(0)/Fe(III) Catalyzed Azide-Isocyanide Coupling/Cyclization Reaction: One-Pot Synthesis of Aminotetrazoles. J. Org. Chem. 2018;83:9530–9537. doi: 10.1021/acs.joc.8b01261. [DOI] [PubMed] [Google Scholar]
- 41.Ansari A.J., Joshi G., Yadav U.P., Maurya A.K., Agnihotri V.K., Kalra S., Kumar R., Singh S., Sawant D.M. Exploration of Pd-catalysed four-component tandem reaction for one-pot assembly of pyrazolo[1,5-c]quinazolines as potential EGFR inhibitors. Bioorg. Chem. 2019;93 doi: 10.1016/j.bioorg.2019.103314. [DOI] [PubMed] [Google Scholar]
- 42.Ansari A.J., Joshi G., Sharma P., Maurya A.K., Metre R.K., Agnihotri V.K., Chandaluri C.G., Kumar R., Singh S., Sawant D.M. Pd-Catalyzed Four-Component Sequential Reaction Delivers a Modular Fluorophore Platform for Cell Imaging. J. Org. Chem. 2019;84:3817–3825. doi: 10.1021/acs.joc.8b02845. [DOI] [PubMed] [Google Scholar]
- 43.Pathare R.S., Maurya A.K., Kumari A., Agnihotri V.K., Verma V.P., Sawant D.M. Synthesis of quinazoline-3-oxides via a Pd (II) catalyzed azide–isocyanide coupling/cyclocondensation reaction. Org. Biomol. Chem. 2019;17:363–368. doi: 10.1039/c8ob02627k. [DOI] [PubMed] [Google Scholar]
- 44.Ansari A.J., Pathare R.S., Kumawat A., Maurya A.K., Verma S., Agnihotri V.K., Joshi R., Metre R.K., Sharon A., Pardasani R.T., Sawant D.M. A diversity-oriented synthesis of polyheterocycles: Via the cyclocondensation of azomethine imine. New J. Chem. 2019;43:13721–13724. doi: 10.1039/c9nj02874a. [DOI] [Google Scholar]
- 45.Ansari A.J., Wani A.A., Maurya A.K., Verma S., Agnihotri V.K., Sharon A., Bharatam P.V., Sawant D.M. An unprecedented: N-to C-sulfonyl migration in the reaction of azomethine amine and allenoates: Access to arylsulfonylmethyl substituted pyrazolo[1,5-c] quinazoline and mechanistic studies. Chem. Commun. 2019;55:14825–14828. doi: 10.1039/c9cc06751e. [DOI] [PubMed] [Google Scholar]
- 46.Xiong J., Feng Q.X., Mu Z.Y., Yao G., Zhang J.A., He H.T., Pang Y.L. Efficient Synthesis of 4H-3, 1-Benzoxazine Derivatives via One-Pot Sequential Passerini-Azide/Palladium-Catalyzed Azide–Isocyanide Coupling/Cyclization Reaction. Synlett. 2020;31:1003–1006. [Google Scholar]
- 47.Xiong J., He H.T., Yang H.Y., Zeng Z.G., Zhong C.R., Shi H., Ouyang M.L., Tao Y.Y., Pang Y.L., Zhang Y.H., et al. Synthesis of 4-Tetrazolyl-Substituted 3,4-Dihydroquinazoline Derivatives with Anticancer Activity via a One-Pot Sequential Ugi-Azide/Palladium-Catalyzed Azide-Isocyanide Cross-Coupling/Cyclization Reaction. J. Org. Chem. 2022;87:9488–9496. doi: 10.1021/acs.joc.2c00382. [DOI] [PubMed] [Google Scholar]
- 48.Chen K., Tang X.-Y., Shi M. Rh (II)-Catalyzed formation of pyrrolo [2, 3-b] quinolines from azide-methylenecyclopropanes and isonitriles. Chem. Commun. 2016;52:1967–1970. doi: 10.1039/c5cc09236a. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z., Xiao F., Huang B., Hu J., Fu B., Zhang Z. Cyclization of Alkyne-Azide with Isonitrile/CO via Self-Relay Rhodium Catalysis. Org. Lett. 2016;18:908–911. doi: 10.1021/acs.orglett.5b03570. [DOI] [PubMed] [Google Scholar]
- 50.Li Z., Huo T., Li L., Feng S., Wang Q., Zhang Z., Pang S., Zhang Z., Wang P., Zhang Z. Rh-Catalyzed Reaction of Vinyl Azides with Isonitriles and Alkynes. Org. Lett. 2018;20:7762–7766. doi: 10.1021/acs.orglett.8b03115. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Li Z., Zhao H., Zhang Z. Rhodium-Catalyzed Double Isocyanide Insertion via a Vinylcarbodiimide Intermediate for the Synthesis of 2H-Pyrrol-2-imines. Synth. Met. 2019;51:3250–3258. doi: 10.1055/s-0037-1611830. [DOI] [Google Scholar]
- 52.Jiang S., Cao W.B., Li H.Y., Xu X.P., Ji S.J. Convenient synthesis of spiroindolenines from tryptamine-derived isocyanides and organic azides by cobalt catalysis in pure water. Green Chem. 2021;23:2619–2623. doi: 10.1039/d1gc00270h. [DOI] [Google Scholar]
- 53.Jiang S., Cao W.B., Xu X.P., Ji S.J. Cobalt-Catalyzed Isocyanide-Based Three-Component Cascade for the Synthesis of Quinazolines. Org. Lett. 2021;23:6740–6744. doi: 10.1021/acs.orglett.1c02316. [DOI] [PubMed] [Google Scholar]