Abstract
Introduction
It is unclear which pathophysiological processes initiate and drive dynamic cerebrovascular autoregulation (CA) impairment as seen in traumatic brain injury (TBI). This is not solely attributable to raised intracranial pressure (ICP), but also results from local tissue damage.
Research question
In order to investigate CA disturbing processes, a porcine model is needed that mimics severe TBI as seen in humans. This model requires high amplitude rotational acceleration.
Material and methods
A customized device was built to produce a rotational impulse with high amplitude and short pulse duration. Following preparatory tests on cadaver piglets, six piglets of six weeks old were sedated, ventilated and subjected to rotational impulses of different magnitudes. The impulse was immediately followed by installment of invasive monitoring of ICP, PbO₂, Laser Doppler Flowmetry and arterial blood pressure. TBI was further characterized by magnetic resonance brain imaging.
Results
The current setup enabled to reach sagittal head rotational maximal acceleration magnitudes up to 30 krad/s2. Half of the animals had an increase in ICP, measured shortly after the impulse. It has proved impossible so far to produce a sustained rise in ICP as seen in human severe TBI. MRI showed no anatomical abnormalities which would confirm severe TBI.
Discussion and conclusion
The challenge to build a porcine model in which severe TBI with ICP raise and MRI changes as seen in humans can be reliably reproduced is still ongoing. It might be that higher peak rotational accelerations are needed.
Keywords: Traumatic brain injury, Intracranial pressure, Cerebrovascular autoregulation, Animal model
Highlights
-
•
This preliminary data is being published to provoke a dialogue in the development of a rotational impulse porcine model with the goal to mimic traumatic brain injury, to better understand the underlying physiology of the disturbance of cerebrovascular autoregulation.
-
•
By using a custom-made actuator to deliver a sagittal rotational impulse to the heads of 6 weeks old piglets, we reached a maximum rotational displacement of 125°, velocity of 155 rad/s and acceleration of 30 krad/s2.
-
•
The needed acceleration based on extrapolation of other data was twice as much as reached.
-
•
In half of the animals, there was an increase in ICP directly after the rotational impulse, but we were not able to produce a sustained rise in ICP.
-
•
Magnetic resonance imaging could not withhold any relevant traumatic abnormalities.
-
•
The following steps to optimize the model are to increase rotational acceleration and to eliminate unwanted loss of energy transfer at the connection between the biteplate and the animal's snout.
1. Introduction
Traumatic brain injury (TBI) has a massive global socio-economic impact. Globally 69 million people are estimated to suffer from TBI each year and both young and old people are frequently affected (Dewan et al., 2019; Quaglio et al., 2017). In TBI, mechanically induced primary injury is inevitably followed by a cascade of subsequent pathophysiological events developing in the brain days to weeks after trauma, usually being summarized as secondary injury. Both primary and secondary injury affect prognosis, but secondary injury is still incompletely understood. It has been stated that secondary injury reflects a host response to the primary injury, which probably explains why it differs so much between people. Basically, in this period, a discrepancy between the delivery of energy and the metabolic demands of the brain may develop where the line between hypo- and hyperperfusion is thin. A neuroinflammatory cascade is started, which leads to an increase of the blood-brain-barrier (BBB) permeability and cytotoxic edema, and eventually an increase in intracranial pressure (ICP). ICP secondary to diffuse edema usually increases in the first days following trauma and reaches its highest values within one week (Small et al., 2022; Bramlett and Dietrich, 2015; Maas et al., 2017; Jha and Kochanek, 2018).
Impairment of cerebrovascular autoregulation (CA), which is often seen in TBI, leads to inappropriate alterations of cerebrovascular resistance (CVR), potentially resulting in totally pressure-passive arterioles (Dietvorst et al., 2023). In such scenario, cerebral perfusion pressure (CPP) becomes the main regulator of cerebral blood flow (CBF), with unbalanced negative impact of ICP and cerebral venous pressure (CVP). It is currently unclear which pathophysiological variables initiate and drive dynamic CA impairment as seen in different types of TBI.
Multiple animal models have been developed to investigate different aspects of TBI. Most models use a controlled cortical impact, fluid percussion injury or weight drop injury, executed on rodent brains. Such mechanism, when applied in large animal models, can mimic primary injury, but have been proven insufficient to result in sustained raised ICP (Pareja et al., 2016; de Kegel et al., 2021; Johnson et al., 2015; Hajiaghamemar et al., 2019; Laurer and McIntosh, 1999). A large animal model that did achieve increased ICP and investigated its relation with reduced CPP and impaired CA through transcranial Doppler (TCD), used intracranial balloon inflation (de Lima Oliveira et al., 2018). Another model used hypotonic saline to provoke cerebral edema and investigate the associations between CPP, ICP, partial pressure of brain oxygen (PbO₂) and CVR (Ramirez de Noriega et al., 2018). These models can hardly be considered representative of TBI.
In humans and non-human primates, the more severe TBI-related injuries (such as diffuse edema, acute subdural hematoma, and diffuse axonal injury) have a strong relation with the magnitude and pulse duration of head rotational acceleration (Cullen et al., 2016). Several animal models have included a rotational impulse in the induction of TBI, typically as a controllable pulse, while in real life this is a less controllable free rotation secondary to a head impact. Relevant variables in the induction of relative brain-skull motion and brain deformation are the size and weight of the head and brain; the direction of the motion and the brain and skull anatomy; the magnitude of the rotational acceleration, velocity and the pulse duration. Considering these factors, larger brains will sustain higher inertial forces and higher internal shear stresses for a given acceleration; more damage will occur when rotation acts in the same direction as the axons; the falx cerebri results in higher shear stress in the surrounding brain parenchyma in head motions in the coronal plane; and in shorter pulse duration the brain will behave stiffer (Johnson et al., 2015; Gennarelli et al., 1982). It has been demonstrated that the viscoelastic characteristics of the human brain tissue are similar to those of other animals, with porcine and mouse brains being most comparable to the human brain for several regions investigated (MacManus et al., 2020). Both physiologically and in terms of gyrencephalic brain development and anatomy, the porcine brain is close to the human brain and these animals are easily available for research. Cullen et al. developed a porcine trauma model with rotational acceleration produced by a linear actuator, whereby ICP significantly increased following the impact, although the increase did not persist after 4 h (Cullen et al., 2016). We believe that rotational acceleration at head impact is key in producing severe injury including sustained raised ICP and hence, to establish a real representative porcine model of human severe TBI.
2. Material and methods
2.1. Ethical considerations
All animal care and procedures were approved by the Ethics Committee Animal Research Center, KULeuven (Ethical Approval: P065-2021) in compliance with the Belgian Royal Decree (May 29, 2013) and European Directive 2010/63/EU on the protection for animals used for scientific purposes. All animal procedures were conducted under veterinarian supervision according to the guidelines imposed by the Ethical Committee. The goal is to develop a model with sustained increase in ICP.
2.2. Estimation of the required mechanical input and device development
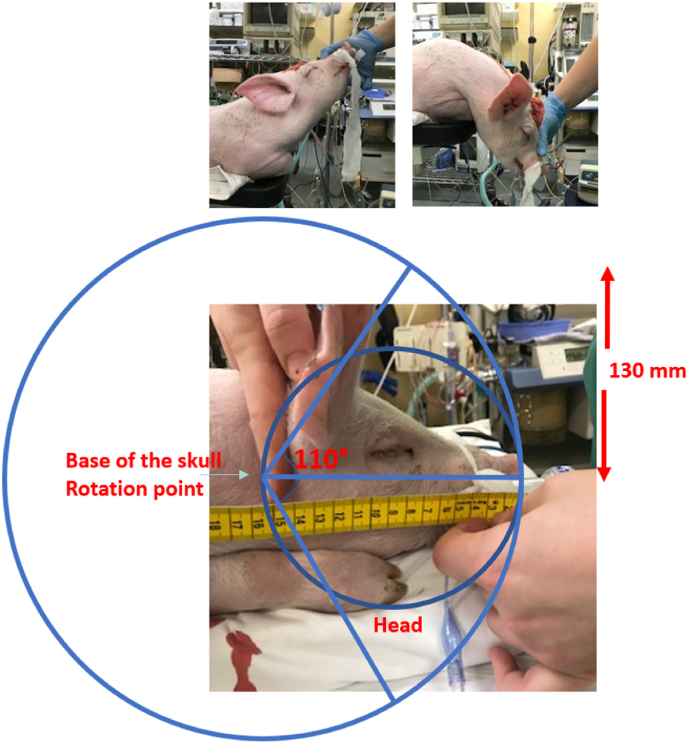
Since we wanted to add severe TBI to the porcine cranial window model developed in our lab for the study of CA, the set-up had to include male domestic swine, approximately 6 weeks old with a weight of 10 kg (Klein et al., 2019). Based on this animal's characteristics, we made an extrapolation of rotational velocity and acceleration used in the HYGE experiments reported by Cullen et al. (2016) to calculate the rotational acceleration and velocity input required to provoke raised ICP in our animal model (Cullen et al., 2016; Atlan et al., 2018; Coats et al., 2017; Hajiaghamemar et al., 2020). When the craniocervical junction is used as the center of rotation; with a radius of 160 mm to the tip of the snout and a range of motion of 110°, the calculated required velocity is 150 rad/s and acceleration 60 krad/s2 (Fig. 1).
Fig. 1.
The center of sagittal rotation is the craniocervical junction, as illustrated on a 6-week old piglet. The radius to the snout tip measures 160 mm and the physiological rotational range of motion of the upper cervical spine and craniocervical junction spans 110°.
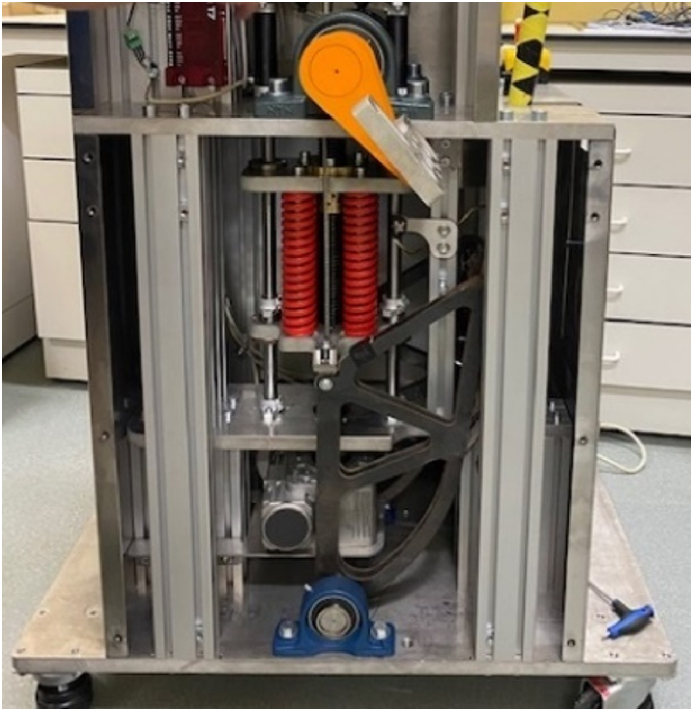
A custom-made device was built by Comate (Leuven, Belgium) that can deliver a rotational impulse with high velocity and short pulse duration through a biteplate. The machine is internally equipped with four parallel springs, which are preloaded after the animal is secured on the table and the biteplate is secured in the mouth. The trigger then initiates the unloading of the springs, which results in sagittal head rotation. The rotational displacement, velocity and acceleration are logged (Fig. 2).
Fig. 2.
The head rotation delivering device is displayed without its covering. The springs are shown in red (four in total, two in the front are visible). The springs can be preloaded to a maximum force of 20 kN and when released the springs cause a rapid rotation of the biteplate (the transmitter of rotation is depicted in orange with the adjacent silver biteplate extending to the side).
2.3. Experimental set up
Animals were sedated by intravenous administration of propofol, pancuronium, midazolam and fentanyl, intubated and mechanically ventilated. An arterial femoral line was placed for continuous ABP monitoring, as well as a saturation probe on their tail and a three-lead ECG. All parameters were displayed on a Philips monitor (Philips Medical Systems, Eindhoven, Netherlands), which connects to ICM+ (Cambridge University, Cambridge, United Kingdom) for data storage.
Next, the animal was placed onto the Comate device. The snout was tightly secured around the bite plate with tie-wraps. A skin incision on top of the skull was already prepared, exposing the coronal suture on the right side. The intracranial catheters were then prepared and zeroed to enable swift placement following head rotation. The intracranial monitors included a laser Doppler flow (LDF) probe (Moor VMS-LDF1 with VP14-CBF probe, Moor Instruments Devon, UK), a PbO₂/ICP/temperature probe (Neurovent-PTO, Raumedic AG Muenchenberg Germany) and an extra ICP probe (Codman Microsensor, Integra Lifesciences, Princeton, New Jersey, United States). The logging device (LJStream, LabJack Corporation, Lakewood, Colorado, United States) was started prior to the impulse to collect time-based rotational displacement, velocity and acceleration of the biteplate.
After pre-oxygenation, the ventilation tube and intravenous line to the ear were momentarily disconnected. The piglets then underwent the rotational impulse, immediately followed by the drilling of three boreholes over the right hemisphere (diameter 2 mm). The boreholes were placed 5 mm posterior to the coronal suture, with the first borehole 5 mm lateral to the sagittal suture with a space of 5 mm in between the holes in the lateral direction. The LDF probe was placed the most medially in contact with the dura, with avoidance of large dural or pial vessels. The Neurovent and Microsensor probe were placed 1 cm inside the parenchyma after dural puncture, measured from the inner cortex of the bone. The boreholes were then sealed with bone wax to avoid leakage of cerebrospinal fluid. Physiological monitoring was continued for at least 6 h after the trauma, with extended monitoring till 20 h after the trauma. Next, the animals underwent a cranial 3 T MRI (Siemens, Munich, Germany), with the following sequences: T1, T2, susceptibility-weighted imaging (SWI), magnetization-prepared rapid gradient-echo (MPRAGE), fluid attenuated inversion recovery (FLAIR). After the MRI, they were euthanized subsequently with an overdose of Pentobarbital.
The procedure was first run on 3 piglet cadavers in order to optimize the fixation of the snout on the biteplate and to fine tune the preload spring settings.
3. Results
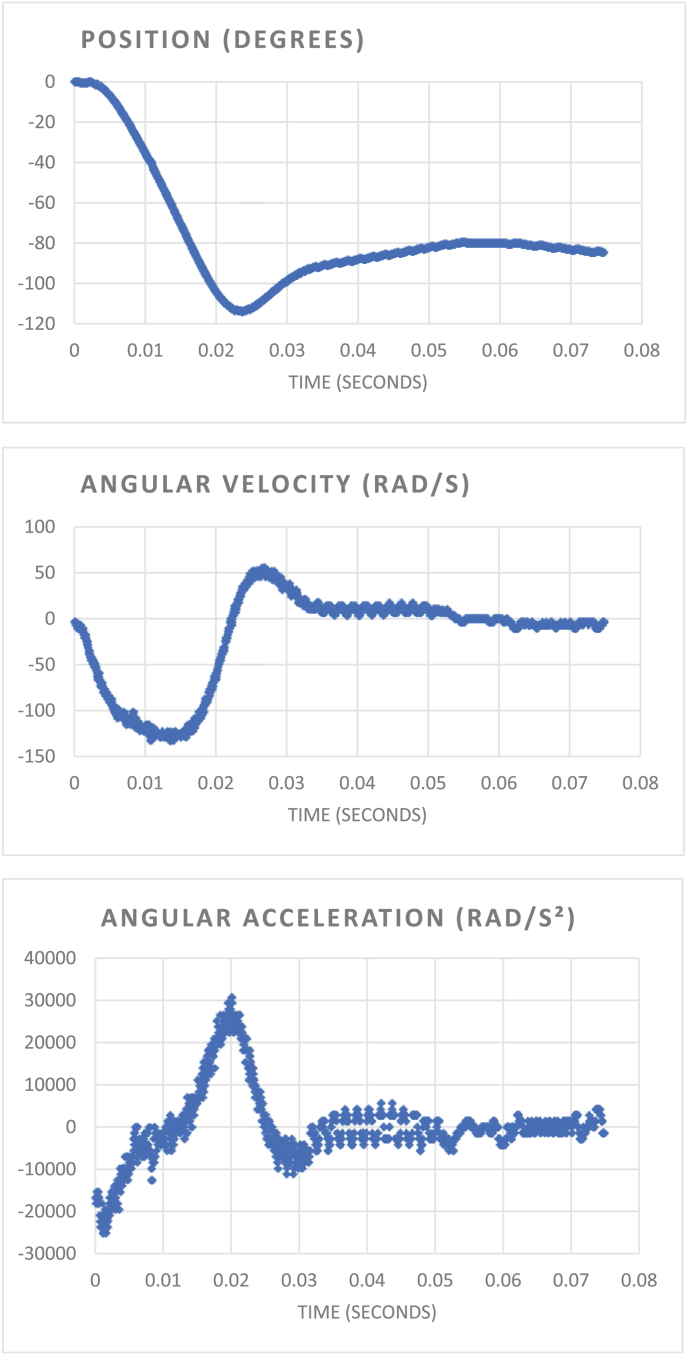
The experiment was performed on six living piglets, the average weight of the animals was 11.2 kg (SD 2.28 kg). Maximum achieved rotational displacement was 125°, maximum velocity 155 rad/s and maximum acceleration 30 krad/s2 (Fig. 3). From the velocity and radius of the circular motion, we can calculate the maximal centripetal force (G force) on the pig's brain. The maximal G force ranges from 24.5 to 122 over the brain, with the highest value measured at the largest radius (in this case the anterior frontal lobes).
Fig. 3.
Example of the rotational impulse and its calculations as measured by the automatic logging device in one animal (experiment 2). Here, the maximal rotation was 114°, with a velocity of 130 rad/s and acceleration of 30 krad/s2.
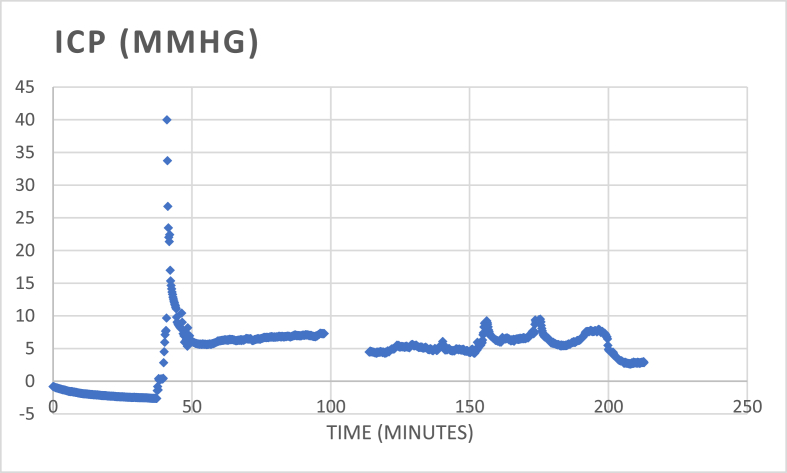
One pig died early, presumably due to hemorrhagic shock. In three pigs, there was a short rise of ICP immediately following the trauma (when the measurement was started) and returning to baseline within 30 min (Fig. 4). No ICP, PbO₂ nor LDF events were registered in the following 20 h of monitoring. The rotation was repeated in two different animals, but this did not lead to sustained ICP rise. In one pig, there was a gradual increase of ICP till 70 mmHg, and till 100 mmHg after two more hours, measured through the Neurovent probe, while the Codman microsensor displayed stable values between 10 and 15 mmHg (Table 1). Beside this experiment, the values of the Neurovent and Microsensor probes were comparable. No posttraumatic intracranial abnormalities were displayed on any of the MR images of the animals. Half of the animals had a jaw fracture on T2-images.
Fig. 4.
Overview of the time-curve of ICP of the Neurovent-probe in one animal (experiment 3). At time zero, the catheter is zeroed while holding still in water. The catheter is then removed from the water and exposed to the air, which shows a small drift of the value to −2.5 mmHg. Meanwhile, the skin is opened, and the skull is exposed. The impulse is then performed while the skull is intact. Directly after the impulse, the three boreholes are made. The LDF probe is placed in the medial borehole on intact dura, the dura of the two other boreholes is opened to place the Neurovent- and Codman-catheters in the parenchyma. The measurement catheters are in place within 10 min after the impulse, at that time the rapid decrease of high levels of ICP is seen. The highest measured ICP in this experiment measured 40 mmHg. For the remaining time of monitoring, ICP remained stable between 5 and 10 mmHg.
Table 1.
Overview of the results of six experiments on living animals. After the rotational impulse, burr holes were made in the skull and the intraparenchymal catheters were placed. In three animals, there was still an increase in ICP after the catheters were placed. The maximal rotation, velocity and acceleration of each animal are depicted. In two animals, there is missing data of the rotational impulse due to malfunctioning of the logging device. na = not applicable.
| Experiment | ICP after rotation | Max rotation | Max velocity | Max acceleration | Note |
|---|---|---|---|---|---|
| Exp 1 | 9,1 mmHg | 100° | 80 rad/s | 21 krad/s2 | Repeated rotation: ICP 35 mmHg |
| Exp 2 | 25,4 mmHg | 114° | 130 rad/s | 30 krad/s2 | Repeated rotation: ICP 9,5 mmHg |
| Exp 3 | 40 mmHg | 121° | 150 rad/s | 20 krad/s2 | |
| Exp 4 | 94 mmHg | na | na | na | ICP till 100 mmHg, after initial decrease; discrepancy Neurovent-Codman values |
| Exp 5 | 0,1 mmHg | na | na | na | |
| Exp 6 | 10,0 mmHg | 125° | 155 rad/s | 22 krad/s2 | Died early |
4. Discussion
The aim of the current project is to establish a piglet model of severe TBI resembling human TBI and including the occurrence of secondary insults such as raised ICP. Much higher amplitudes of rotational velocity and acceleration are required in a piglet head to achieve similar intracranial strains as in humans due to its smaller size and thus smaller inertial forces (Johnson et al., 2015). In the current set of experiments, nor on MR imaging nor by physiological monitoring, hallmarks of severe TBI as known from humans were observed. A maximum rotational velocity of 155 rad/s and a maximum rotational acceleration of 30 krad/s2 was logged on the rotation device and these values were consistent throughout the experiments. We know from the work by Gennarelli et al. and by Cullen et al. that human-like severe TBI resulting in diffuse axonal injury, acute subdural hematoma and raised ICP can be produced by high amplitude rotational acceleration in non-human large animals (Cullen et al., 2016; Gennarelli et al., 1982). A possible explanation for not achieving this in the current set of experiments may be that the amplitudes of rotational velocity and rotation reached in the current study are still not high enough. Part of the problem may lie in the inaccurate transfer of energy from the biteplate to the animal since we saw a jaw fracture in half of the animals. Both will be optimized in future experiments. We still think that the biteplate is the best method to displace the head around the center of rotation (in this case, the craniocervical junction). The first step would be to optimize the biteplate by making the piece in the snout longer. When combining this with a better fixation on the outside of the snout, by adding a more proximal fixation band, the stability of the biteplate will increase. If possible, an accelerometer on the head of the pig will guarantee the correct measurements and transfer of energy to the pig. In this regard, this is a report on work in progress.
The rotational velocity and acceleration required for piglets of 6 weeks was estimated from reports on neonatal and adult pigs assuming a linear relationship (Johnson et al., 2015; Ramirez de Noriega et al., 2018; Atlan et al., 2018). However, skull growth rate is not linear, and this may explain an underestimation. The machine can be set at higher preload to deliver higher amplitudes of rotational velocity and acceleration, but this will be associated with an increase in rotational angle as well, potentially leading to high cervical spine injuries. As this has not been observed until now, there still seems to be space for an increase in output by the current rotational device.
There are aspects in the current experiments that differ from real life human TBI. The impulse that is given is purely rotational in one direction, while in real life it is most often a combination of an impact and the rotational (and inertial) forces induced by the impact. However, such a combination is much harder to control in an experimental setting than a pure impulse and would imply a more complex device to deliver this impact. We opted for rotation in the sagittal plane, since the goal of this project is to develop an animal model that mimics severe human TBI, with development of contusions and hematoma together with a persistent increase in ICP. In previous pediatric swine models, it has been shown that the sagittal rotation was associated with the largest increase in intracranial pressure and with decrease of CBF, compared to axial and coronal rotation (Eucker et al., 2011). In swine, the sagittal plane is in line with the body and can reach the largest angle of rotation, which is proportional to acceleration. Previous research has shown that rotation in the same direction as the axons is more likely to provoke DAI. DAI in the corpus callosum, which is linked to worse outcomes in TBI, is known to occur more frequently with rotation in the coronal plane (Gennarelli et al., 1982; van Eijck et al., 2018). Since we wanted to develop a clinical model to understand the impact of TBI on the physiology CA, we opted for the sagittal rotation.
One may argue that the pigs were already under anesthesia during the impulse, and that the anesthetics may have provided a neuroprotective effect. Such effect has been described for propofol and midazolam, which we both used (Dominguini et al., 2021; Tanguy et al., 2012; Gu et al., 2014; Adembri et al., 2007). Further, real life head impacts may result in apnea, airway obstruction and hypoventilation contributing to later secondary injury. In the current set up, the piglets were already intubated and pre-oxygenated during the impulse. Due to ethical concerns, it is not possible to withdraw from this protocol of sedation and ventilation. The monitoring following the trauma consisted of a period of approximately 20 h, which is quite short compared to clinical series. In real life, raising ICP is usually observed later than day 1. The phase of edema, adding to increased ICP, is most distinct from day 3 to day 5 after TBI, but can extend to more than one week after TBI (Jha and Kochanek, 2018). Since the animals that we use are quite small, with a higher heart rate compared to humans, the TBI might evolve faster timewise compared to humans, although this is also a speculation. Although logistically very difficult, extending the monitoring duration may be another point of optimization.
Lastly, we did not really find preexisting literature on regular MR imaging in piglet TBI on day 1 before we started the experiments. Shin et al. were able to identify white matter changes in a 4 week piglet controlled cortical impact model by diffusion tensor imaging, but such sequences were not used in the current protocol (Shin et al., 2023). Still, one would expect DAI-like changes to be visible shortly after the trauma (Janas et al., 2022; Benjamini et al., 2021). This may again be an argument for the mechanical input variables and resulting shear strains in the brain still having been insufficient under the current settings.
5. Conclusion
By using a custom-made actuator to deliver a sagittal rotational impulse to the heads of 6 weeks old piglets, we reached a maximum rotational displacement of 125°, velocity of 155 rad/s and acceleration of 30 krad/s2. In half of the animals, there was an increase in ICP directly after the rotational impulse, but we were not able to produce a sustained rise in ICP. Magnetic resonance imaging could not withhold any relevant traumatic abnormalities after prolonged monitoring for 20 h. The steps to optimize next consist of increasing the device preload to aim at higher amplitudes of rotational acceleration and to eliminate unwanted loss of energy transfer at the connection between the biteplate and the animal's snout.
Author contribution statement
SD and BD designed the study concept. SD and AV developed the protocol and performed all the experiments. SD analyzed the data and wrote the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Bart Depreitere holds a Medtronic research grant (EFD-LSTBI1-O2010).
Acknowledgements
Sincere thanks to the laboratory of Biomechanics of KULeuven to develop the setup of the device. Sincere thanks to Comate, for the development of the device and the help with the practical installation.
Sincere thanks to Ronald Peeters, to optimize the protocol of MRI to acquire the images after the rotational impact, and to Johannes Devos, Neuroradiologist at UZLeuven, for analysis of the MRIs of the animals.
Handling editor: W Peul
References
- Adembri C., Venturi L., Pellegrini-Giampietro D.E. Neuroprotective effects of propofol in acute cerebral injury. CNS Drug Rev. 2007;13(3):333–351. doi: 10.1111/j.1527-3458.2007.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlan L.S., Smith C., Margulies S.S. Improved prediction of direction-dependent, acute axonal injury in piglets. J. Neurosci. Res. 2018;96(4):536–544. doi: 10.1002/jnr.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini D., Iacono D., Komlosh M.E., Perl D.P., Brody D.L., Basser P.J. Diffuse axonal injury has a characteristic multidimensional MRI signature in the human brain. Brain. 2021;144(3):800–816. doi: 10.1093/brain/awaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett H.M., Dietrich W.D. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J. Neurotrauma. 2015;32(23):1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats B., Binenbaum G., Smith C., Peiffer R.L., Christian C.W., Duhaime A.C., et al. Cyclic head rotations produce modest brain injury in infant piglets. J. Neurotrauma. 2017;34(1):235–247. doi: 10.1089/neu.2015.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen D., Harris J., Browne K., Wolf J., Duda J., Meaney D., et al. A porcine model of traumatic brain injury via head rotational acceleration. Methods Mol. Biol. 2016;1462:289–324. doi: 10.1007/978-1-4939-3816-2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kegel D., Musigazi G.U., Menichetti A., Hellings P.W., Sciot R., Demaerel P., et al. Investigation of tissue level tolerance for cerebral contusion in a controlled cortical impact porcine model. Traffic Inj. Prev. 2021;22(8):616–622. doi: 10.1080/15389588.2021.1957856. [DOI] [PubMed] [Google Scholar]
- de Lima Oliveira M., Salinet A.M., Nogueira R. de C., Belon A.R., Paiva W.S., Jeng B.C.P., et al. The effects of induction and treatment of intracranial hypertension on cerebral autoregulation: an experimental study. Neurol Res Int. 2018:1–8. doi: 10.1155/2018/7053932. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan M., Rattani A., Gupta S., Baticulon R. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019;130:1080–1097. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- Dietvorst S., Depreitere B., Meyfroidt G. Beyond intracranial pressure: monitoring cerebral perfusion and autoregulation in severe traumatic brain injury. Curr. Opin. Crit. Care. 2023;29(2):85–88. doi: 10.1097/MCC.0000000000001026. [DOI] [PubMed] [Google Scholar]
- Dominguini D., Steckert A.V., Michels M., Borges M.S., Ritter C., Barichello T., et al. The effects of anaesthetics and sedatives on brain inflammation. Neurosci. Biobehav. Rev. 2021;127:504–513. doi: 10.1016/j.neubiorev.2021.05.009. [DOI] [PubMed] [Google Scholar]
- Eucker S.A., Smith C., Ralston J., Friess S.H., Margulies S.S. Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp. Neurol. 2011;227(1):79–88. doi: 10.1016/j.expneurol.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli T.A., Thibault L.E., Adams J.H., Graham D.I., Thompson C.J., Marcincin R.P. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982;12(6):564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- Gu J wen, Yang T., Kuang Y qin, Huang H dong, Kong B., Shu H feng, et al. Comparison of the safety and efficacy of propofol with midazolam for sedation of patients with severe traumatic brain injury: a meta-analysis. J. Crit. Care. 2014;29(2):287–290. doi: 10.1016/j.jcrc.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Hajiaghamemar M., Seidi M., Oeur R.A., Margulies S.S. Toward development of clinically translatable diagnostic and prognostic metrics of traumatic brain injury using animal models: a review and a look forward. Exp. Neurol. 2019;318:101–123. doi: 10.1016/j.expneurol.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiaghamemar M., Wu T., Panzer M.B., Margulies S.S. Embedded axonal fiber tracts improve finite element model predictions of traumatic brain injury. Biomech. Model. Mechanobiol. 2020;19(3):1109–1130. doi: 10.1007/s10237-019-01273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas A.M., Qin F., Hamilton S., Jiang B., Baier N., Wintermark M., et al. Diffuse axonal injury grade on early MRI is associated with worse outcome in children with moderate-severe traumatic brain injury. Neurocritical Care. 2022;36(2):492–503. doi: 10.1007/s12028-021-01336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R.M., Kochanek P.M. A precision medicine approach to cerebral edema and intracranial hypertension after severe traumatic brain injury: quo vadis? Curr. Neurol. Neurosci. Rep. 2018;18(12):105. doi: 10.1007/s11910-018-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V.E., Meaney D.F., Cullen D.K., Smith D.H. 2015. Animal Models of Traumatic Brain Injury; pp. 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.P., De Sloovere V., Meyfroidt G., Depreitere B. Autoregulation assessment by direct visualisation of pial arterial blood flow in the piglet brain. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-50046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurer H.L., McIntosh T.K. Experimental models of brain trauma. Curr. Opin. Neurol. 1999;12(6):715–721. doi: 10.1097/00019052-199912000-00010. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- MacManus D.B., Menichetti A., Depreitere B., Famaey N., vander Sloten J., Gilchrist M. Towards animal surrogates for characterising large strain dynamic mechanical properties of human brain tissue. Brain Multiphys. 2020;1 [Google Scholar]
- Pareja J.C.M., Keeley K., Duhaime A.C., Dodge C.P. 2016. Modeling Pediatric Brain Trauma: Piglet Model of Controlled Cortical Impact; pp. 345–356. [DOI] [PubMed] [Google Scholar]
- Quaglio G., Gallucci M., Brand H., Dawood A., Cobello F. Traumatic brain injury: a priority for public health policy. Lancet Neurol. 2017;16(12):951–952. doi: 10.1016/S1474-4422(17)30370-8. [DOI] [PubMed] [Google Scholar]
- Ramirez de Noriega F., Manley G.T., Moscovici S., Itshayek E., Tamir I., Fellig Y., et al. A swine model of intracellular cerebral edema – cerebral physiology and intracranial compliance. J. Clin. Neurosci. 2018;58:192–199. doi: 10.1016/j.jocn.2018.10.051. [DOI] [PubMed] [Google Scholar]
- Shin S.S., Chawla S., Jang D.H., Mazandi V.M., Weeks M.K., Kilbaugh T.J. Imaging of white matter injury correlates with plasma and tissue biomarkers in pediatric porcine model of traumatic brain injury. J. Neurotrauma. 2023;40(1–2):74–85. doi: 10.1089/neu.2022.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small C., Lucke-Wold B., Patel C., Abou-Al-Shaar H., Moor R., Mehkri Y., et al. What are we measuring? A refined look at the process of disrupted autoregulation and the limitations of cerebral perfusion pressure in preventing secondary injury after traumatic brain injury. Clin. Neurol. Neurosurg. 2022;221 doi: 10.1016/j.clineuro.2022.107389. [DOI] [PubMed] [Google Scholar]
- Tanguy M., Seguin P., Laviolle B., Bleichner J.P., Morandi X., Malledant Y. Cerebral microdialysis effects of propofol versus midazolam in severe traumatic brain injury. J. Neurotrauma. 2012;29(6):1105–1110. doi: 10.1089/neu.2011.1817. [DOI] [PubMed] [Google Scholar]
- van Eijck M.M., Schoonman G.G., van der Naalt J., de Vries J., Roks G. Diffuse axonal injury after traumatic brain injury is a prognostic factor for functional outcome: a systematic review and meta-analysis. Brain Inj. 2018;32(4):395–402. doi: 10.1080/02699052.2018.1429018. [DOI] [PubMed] [Google Scholar]