Abstract
Entomopoxviruses and baculoviruses are pathogens of insects which replicate in the cytoplasm and nuclei of their host cells, respectively. During the late stages of infection, both groups of viruses produce occlusion bodies which serve to protect virions from the external environment. Immunofluorescence and electron microscopy studies have shown that large bundles of filaments are associated with these occlusion bodies. Entomopoxviruses produce cytoplasmic fibrils which appear to be composed of the filament-associated late protein of entomopoxviruses (FALPE). Baculoviruses, on the other hand, yield filaments in the nuclei and cytoplasm of the infected cell which are composed of a protein called p10. Despite significant differences in their sequences, FALPE and p10 have similar hydrophilicity profiles, and each has a proline-rich stretch of amino acids at its carboxyl terminus. Evidence that FALPE and p10 could produce filaments in the absence of other viral proteins is presented. When FALPE was expressed in insect cells from a recombinant baculovirus, filaments similar to those produced by the wild-type Amsacta moorei entomopoxvirus were observed. In addition, when expression plasmids containing FALPE or p10 genes were transfected into Vero monkey kidney cells, filament structures similar to those found in infected insect cells were produced. The manner in which FALPE and p10 subunits interact to form polymers was investigated through deletion and site-specific mutagenesis in conjunction with immunofluorescence microscopy, yeast two-hybrid protein interaction analysis, and chemical cross-linking of adjacent molecules. These studies indicated that the amino termini of FALPE and p10 were essential for subunit interaction. Although deletion of the carboxy termini did not affect this interaction, it did inhibit filament formation. In addition, modification of several potential sites for phosphorylation also abolished filament assembly. We concluded that although the sequences of FALPE and p10 were different, the structural and functional properties of the two polypeptides appeared to be similar.
Cytoskeletal elements have previously been demonstrated to be involved in several aspects of virus assembly (39, 66). For example, vaccinia virus has been shown to associate with actin during its release from the plasma membrane (15), while adenovirus is transported through the cytoplasm to the nucleus through its interaction with microtubules (17, 38). Actin has been implicated in the transport of baculovirus nucleocapsids to the nucleus (10). Other viruses contain actin in their envelopes along with viral surface glycoproteins, implying some role in the budding process (34, 54, 58). In addition, cytochalasin D, a disruptor of microfilaments, has been shown to impair the assembly of a number of different viruses (18, 42, 45). Most viruses use preexisting microtubule or microfilament proteins derived from host cells in these processes. However, we have recently demonstrated that insect poxviruses establish their own filament network during the later stages of infection, using a protein encoded by the viral genome (2).
Entomopoxviruses (EPVs) are insect pathogens which replicate in the cytoplasm of infected cells and are members of the poxvirus family (reviewed in references 3 to 5 and 22). The genomes of these viruses consist of linear double-stranded DNA molecules which are 130 to 300 kb in length. Amsacta moorei EPV (AmEPV) can be grown in cultured insect cells and is the most studied member of this group of viruses (22–25, 27, 40, 50). AmEPV derives its name from the Indian red army worm, a larva from the Lepidoptera family and the host from which the virus was originally isolated (23, 25, 50). Baculoviruses also infect Lepidoptera larvae but instead replicate in the nuclei of their host cells (44). A number of baculoviruses have been studied, but knowledge of Autographa californica nuclear polyhedrosis virus (AcNPV), which infects a wide variety of larvae including that of the alfalfa leaf hopper, is most extensive (44). This virus is used routinely to produce recombinant proteins in insect virus expression systems (36, 44, 46, 49).
A common property of EPVs and baculoviruses is the formation of large intracellular structures known as occlusion bodies which assemble during the late stages of viral infection. Virions are embedded within these occlusion bodies, and the process serves to protect the virus from the external environment. In the case of baculoviruses, the occlusion bodies are called polyhedra and are composed predominantly of a 31-kDa protein called polyhedrin (52). The occlusion bodies of EPVs are known as spheroids and consist mainly of a 110-kDa protein known as spheroidin (6, 9, 27, 55). Spheroidin and polyhedrin do not appear to exhibit sequence homology (6, 27, 52). A multilamellar envelope also appears to surround both polyhedra and spheroids and may help to stabilize these structures during assembly (2, 53).
During the late phases of AmEPV and baculovirus infections, large bundles of filaments also appear to accumulate in the infected insect cells. In the case of AmEPV, these structures are present in the cytoplasm (2, 22, 23, 40), while those found in cells infected with baculoviruses reside both in the cytoplasm and in the nucleus (1, 14, 57). Baculovirus fibrils are composed primarily of a 10-kDa protein called p10 (47, 59). The p10 gene sequences from AcNPV, Orgyia pseudotsugata nuclear polyhedrosis virus (OpNPV), Bombyx mori nuclear polyhedrosis virus, Perina nuda nuclear polyhedrosis virus, Spodoptera exigua nuclear polyhedrosis virus (SeNPV), and Choristoneura fumiferana nuclear polyhedrosis virus (CfNPV) have been reported (13, 32, 35, 66–68). Although the different p10 protein sequences only exhibit 39 to 51% identity and molecules from different species cannot interact with one another, it is believed that the polypeptides must be structurally and functionally similar (61, 66). Deletion mutagenesis of AcNPV p10 has demonstrated that both the amino- and carboxy-terminal regions of this protein are necessary for the formation of filaments in the infected cell (60). Other studies have assigned an aggregation function to the amino-terminal half of p10 (63, 65), and it has been shown that this region contains a coiled-coil domain which is conserved among the different baculoviruses (66). It is tempting to speculate that p10 aggregation is the result of coiled-coil interaction, but direct evidence for this hypothesis is lacking. The precise role of the carboxy terminus of p10 is still unclear, although it has been proposed to interact with tubulin (11). Deletion of the entire p10 open reading frame (ORF) through homologous recombination produces a mutant virus which is still capable of replication both in vitro and in vivo but produces fragile polyhedra with fragmented polyhedral envelopes (26, 64, 65). The p10 protein has also been implicated in disintegration of the nuclear envelope of the host cell, and this function appears to be associated with the carboxy terminus of this protein (61, 65).
Our laboratory (2) recently demonstrated that the cytoplasmic filaments, which characterize the late stages of infection by AmEPV, are composed primarily of a 156-amino-acid protein called FALPE (filament-associated late protein of EPVs). These filaments are closely associated with the spheroids and their membrane envelopes. FALPE is a phosphoprotein which migrates on sodium dodecyl sulfate (SDS)-polyacrylamide gels as a 25/27-kDa doublet. This protein also contains an unusual proline-glutamic acid repeat region spanning 20 residues in the carboxy terminus of the polypeptide. The ultrastructure and close association of this protein with the occlusion bodies of AmEPV suggested that FALPE and p10 played analogous roles during infections by the respective viruses.
This article addresses the structural and functional similarities between FALPE and p10. These two viral proteins are known to be major components of filamentous structures, but it is not known whether additional viral or cellular proteins cooperate during the polymerization process. In this report, we provide insight into the mechanisms which produce filaments in cells infected with either baculoviruses or EPVs. We demonstrate that p10 and FALPE can produce filaments in the absence of other viral gene products. Using the yeast two-hybrid system and a chemical cross-linking agent, we obtained evidence for self-association of either FALPE or p10. Finally, the polypeptide regions of FALPE and p10 which are required for self-association and subsequent filament formation are mapped.
MATERIALS AND METHODS
Cells and viruses.
Spodoptera frugiperda (Sf9) cells were obtained from Max Summers (Texas A&M University, College Station) and were cultured in Grace’s insect medium (GIBCO/BRL, Gaithersburg, Md.) containing 10% fetal calf serum (WISENT Inc., Ste. Bruno, Quebec, Canada). African green monkey (Vero) cells were maintained in Dulbecco’s modified Eagle medium (GIBCO/BRL) supplemented with 10% fetal calf serum. The original AmEPV stock and BTI-EAA insect cells in which it was grown were obtained from Robert Granados (Boyce Thompson Institute for Plant Research, Ithaca, N.Y.). AmEPV was subsequently adapted to Sf9 cells following several passages in this cell line. AcNPV was obtained from Max Summers and was propagated in Sf9 cells. Amphotericin B (Fungizone; 2.5 mg/ml) and gentamicin (50 mg/ml) were added to all tissue culture growth media to prevent microbial contamination.
Bacterial and yeast strains.
Escherichia coli Top-10 (Invitrogen, San Diego, Calif.) was used for all bacterial plasmid transformations. Saccharomyces cerevisiae Y153 and Y187 were originally obtained from Steve Elledge (Institute for Molecular Genetics, Baylor College of Medicine, Houston, Tex.). Y153 is MATa leu2-3,112 ura3-52 trp-901 his3-Δ200 ade2-101 gal4Δ gal80Δ URA3::GAL-lacZ LYS2::GAL-HIS3, whereas Y187 is MATα leu2-3,112 ura3-52 trp1-901 his3-Δ200 ade2-101 gal4Δ gal180Δ GAL-lacZ. The yeast cells were handled as previously described (8, 30) and grown in YPD medium supplemented with 4% (wt/vol) glucose.
Plasmids.
Plasmid pFALPEscript contains the entire coding sequence of FALPE. It was constructed by PCR amplification of FALPE cDNA (2), using primers FALP1 and FALP2, and the amplified DNA was inserted into the SrfI site of pCR-Script SK+ (Stratagene, La Jolla, Calif.). Plasmid pp10script contains the AcNPV baculovirus p10 gene at the SrfI site of pCR-Script SK+. BlueBac2 (pETL) was the expression vector used to generate recombinant baculovirus stocks (33, 48). Yeast expression plasmids pACT II and pAS I contain the GAL4 promoter activation and DNA binding domains, respectively. These vectors were used in yeast two-hybrid protein interaction analysis and were originally obtained from Steve Elledge (19–21). Plasmid pLaminC contains a portion of the human lamin protein fused to the GAL4 promoter DNA binding domain and was used as a negative control in yeast experiments (41). The mammalian cell expression plasmid pRBK was purchased from Invitrogen.
Antibodies and immunoblot analysis.
CLP001 is a monoclonal antibody (MAb) which recognizes the proline-glutamic acid repeat of FALPE and was purchased from Cedar Lane Laboratories (Mississauga, Ontario, Canada). A rabbit polyclonal antibody directed against FALPE was previously generated (2). Rabbit polyclonal antiserum which recognized the p10 protein of AcNPV was supplied by Joyce Wilson and Peter Faulkner (Queens University, Kingston, Ontario, Canada). Immunoblot analysis was performed as previously described (2, 28, 62). Antibody-antigen complexes were detected either with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP-NBT) reagent (Pierce Chemical, Rockford, Ill.) or with an enhanced chemiluminescence kit (Amersham, Little Chalfont, England), using alkaline phosphatase-conjugated or horseradish peroxidase-conjugated antibodies, respectively.
Oligonucleotides.
The oligonucleotides used are listed in Table 1.
TABLE 1.
Oligonucleotides used
| Oligonucleotides | Sequence |
|---|---|
| FALP1 | GCTAGCGCTAGCATCATGGCACCACCAGTAGAT |
| FALP2 | GGATCCTTATTGTTTTCTACGTCC |
| ΔNt45 | GCTAGCGCTAGCATCATGGATCACGATTATTTATCATAT |
| Δ8098 | ACGTTACAATTGAAATAGTCTAGATACAAGATA |
| ΔCt | ATTCCTGGATCCTTACCAAGATTCTACTAG |
| FALPE YACT 5′ | ATTCCTGGATCCGAAATGGCACCACCAGTAGAT |
| FALPE YACT 3′ | GTTACGCTCGAGTTATTGTTTTCTACGTCC |
| p10 YACT 5′ | ATTCCTGGATCCGAATGTCAAAGCCTAACGTT |
| p10 YACT 3′ | GTTACGCTCGAGTTACTTGGAACTGCGTTT |
| p10 YDB 5′ | ATTCCTGCTAGCATGTCAAAGCCTAACGTT |
| p10 YDB 3′ | GTTACGGGATCCTTACTTGGAACTGCGTTT |
| ΔNt.p10 YACT 5′ | ATTCCTGGATCCGAGAAATTCAATCCATATTG |
| ΔCt.p10 YACT 3′ | GTTACGCTCGAGTTATGAGATCTTAGTGTTAAG |
| ΔNt.p10 YDB 5′ | ATTCCTGCTAGCGAGAAATTCAATCCATATTG |
| ΔCt.p10 YDB 3′ | GTTACGGGATCCTTATGAGATCTTAGTGTTAAG |
| Nt.FALPE YACT 3′ | GTTACGCTCGAGTTAATCAGCTAAAGATTTTTT |
| Nt.FALPE YDB 3′ | GTTACGGGATCCTTAATCAGCTAAAGATTTTTT |
| Ct.FALPE YACT 5′ | ATTCCTGGATCCGAGAAGCATACGATGAACTA |
| Ct.FALPE YDB 5′ | ATTCCTGCTAGCGAAGCATACGATGAACTA |
| Δ45 YACT 5′ | ATTCCTGGATCCGAGATCACGATTATTTATCA |
| ΔCt.YACT 3′ | GTTACGCTCGAGTTACCAAGATTCTACTAG |
| Pint | AATAGCTTTCTTTATCAAATC |
| Pmut | GATTTGATAAAGAAAGCTATTAGGGTATTGCTAACATAGATC |
| AAGCTGCAAAAAAAATATTATCCGAAGCTATTAAAAAAGCTTT | |
| AGCTGATCCTAAT |
Construction of baculovirus expression vectors and isolation of recombinant viruses.
The complete FALPE ORF was excised from pFALPEscript by using NheI and BamHI restriction enzymes and subsequently subcloned into pETL cut with the same enzymes (33). To generate a deletion mutant lacking 45 N-terminal amino acids, a portion of FALPE was amplified by PCR using primers ΔNt45 and FALP2. The amplified DNA was ligated into the SrfI site of pCR-Script SK+ and subsequently subcloned into pETL as described above. The internal deletion mutant Δ8098.FALPE lacking amino acids 80 to 98 was constructed in the following manner. A 5′ portion of the FALPE coding sequence was amplified by using primers FALP1 and Δ8098 (which contained the deletion upstream of an MfeI restriction site); the product was subsequently digested with the restriction enzymes NheI and MfeI and ligated between the two same sites in predigested pFALPEscript. The Δ8098 fragment was subsequently inserted between the NheI and BamHI sites of pETL. Another mutant lacking the carboxy-terminal amino acids 123 to 156 of FALPE was constructed by first amplifying a portion of FALPE, using primers FALP1 and ΔCt. This amplified fragment (ΔCt.FALPE) was cloned into the NheI and BamHI sites of pETL. Finally, to generate a mutant in which most of the predicted phosphorylation sites (Thr15, Ser25, Thr26, Ser33, and Ser37) were mutated to alanine, the oligonucleotides Pmut and FALP2 were used to amplify a major part of the FALPE coding sequence. The remaining 5′ end of the coding sequence was synthesized by PCR using primers FALP1 and Pint to yield the shorter terminal fragment. The two preceding PCR products were combined, denatured, annealed, and amplified by PCR using primers FALP1 and FALP2 to yield a full-length FALPE ORF lacking most of the potential phosphorylation sites. This mutated fragment was subsequently inserted into the expression vector pETL. Recombinant baculoviruses were generated following cotransfection of Sf9 insect cells with the expression plasmids and linearized AcNPV DNA (Invitrogen) as previously described (33, 48).
Immunofluorescence microscopy.
Sf9 and Vero cells were grown on the surface of glass microscope slide coverslips. Sf9 cells were infected with AmEPV, AcNPV, recombinant baculoviruses expressing wild-type FALPE, FALPE deletion mutants, or a FALPE mutant deficient in phosphorylation. Four days postinfection, coverslips were removed and washed three times with phosphate-buffered saline solution (PBS). Vero cells were transiently transfected with either pRBK, pRBK-FALPE, or pRBK-p10. DNA was transfected into the cells by using Lipofectamine (GIBCO/BRL). At 72 h posttransfection, the coverslips were removed and washed three times with PBS. Infected Sf9 and transfected Vero cells were fixed and permeabilized with a 1:1 methanol-acetone solution for 5 min. FALPE and most mutant proteins were detected by using CLP001 as a primary antibody. Since ΔCt.FALPE lacked the carboxy-terminal region recognized by CLP001, it was detected with a rabbit polyclonal antibody directed against FALPE. The p10 polypeptide of AcNPV was detected by using specific rabbit polyclonal antibodies. Fluorescein- and rhodamine-conjugated anti-mouse and anti-rabbit antibodies were used to reveal primary antibody-antigen complexes. Finally, nuclear DNA was visualized by treating the cells with a 5-mg/ml solution of Hoescht stain (Sigma Chemical, St. Louis, Mo.) for 2 min. Fluorescent proteins and DNA were viewed with a Leitz fluorescence microscope.
Construction of recombinant plasmids for yeast transformations.
All DNA fragments described below were generated by PCR, using as the template either pFALPEscript or pp10script. We constructed five recombinant pACT II plasmids consisting of the GAL4 activation domain fused in frame with (i) the entire FALPE ORF (pFALPE.ACT), (ii) the 150 5′ nucleotides coding for the N terminus (pNt.FALPE.ACT), (iii) the 150 3′ bp coding for the carboxy terminus (pCt.FALPE ACT), (iv) a deleted form lacking the 135 5′-most nucleotides, which coded for a molecule lacking 45 amino acids at the N terminus (pΔ45N.FALPE.ACT), or (v) another construct missing the 155 3′-most nucleotides, which specified a polypeptide whose carboxy terminus lacked 31 amino acids (pΔCt.FALPE.ACT). In a similar fashion, five versions of pAS I consisting of the GAL4 DNA binding domain fused in frame to the DNA fragments described above were constructed and named pFALPE.DB, pNt.FALPE.DB, pCt.FALPE.DB, pΔ45NFALPE.DB, and pΔCt.FALPE.DB. The combinations of primers used to generate these cloned DNA fragments were as follows: FALPE YACT 5′ and FALPE YACT 3′ for pFALPE.ACT; FALP1 and FALP2 for pFALPE.DB; FALPE YACT 5′ and Nt.FALPE YACT 3′ for pNt.FALPE.ACT; FALPE YDB 5′ and Nt.FALPE YDB 3′ for pNt.FALPE.DB; FALPE YACT 5′ and Ct.FALPE YACT 3′ for pCt.FALPE.ACT; FALP1 and Ct.FALPE YDB 3′ for pCt.FALPE.DB; Δ45 YACT 5′ and FALPE YACT 3′ for pΔ45N.FALPE.ACT; ΔNt45 and FALP2 for pΔ45N.FALPE.DB; FALPE YACT 5′ and ΔCt.YACT 3′ for pΔ33C.FALPE.ACT; and FALP1 and ΔCt for pΔ33C.FALPE.DB. These DNA fragments were inserted between the BamHI and the XhoI sites in the case of pACT II or between the NheI and BamHI sites in the case of pAS I.
In a similar manner, the entire p10 ORF of AcNPV was fused in frame with either the GAL4 activation domain in pACT II or the GAL4 DNA binding domain in pAS I in order to generate p10.ACT and p10.DB. In addition, the p10 ORF was divided into two segments. Each portion was then cloned into the pACT II and pAS I vectors as described above. The combinations of primers used in these PCRs were as follows: p10 YACT 5′ and p10 YACT 3′ for p10.ACT; p10 YDB 5′ and p10 YDB 3′ for p10.DB; p10 YACT 5′ and ΔCt.p10 YACT 3′ for p10Nt.ACT; p10 YDB 5′ and ΔCt.p10 YDB 3′ for p10Nt.DB; p10 YACT 5′ and Ct.p10 YACT 3′ for p10Ct.ACT; and p10 YDB 5′ and Ct.p10 YDB 3′ for p10Ct.DB. Ligation to either pACT II or pAS I was performed as described above.
Yeast transformation and mating.
Yeast transformations were performed according to previously published procedures, using lithium acetate (8, 12, 30). In our experiments all pACT II-based plasmids were introduced into Y153 cells, whereas Y187 cells were transformed with pAS I-based vectors. Transformed Y153 cells were plated on YPD plates lacking leucine, while Y187 cells were cultured on plates lacking tryptophan. Single colonies were isolated and streaked on similar plates to obtain a good working stock of the desired clone. Different combinations of Y153 and Y187 cells were then allowed to mate for 24 h. Transformed Y153 and Y187 cells were mated by streaking one strain in horizontal rows and the other in vertical columns. Diploid yeast colonies were formed at the intersection of the horizontal and vertical streaks. These plates were replica plated and maintained on agar plates lacking both leucine and tryptophan.
Yeast two-hybrid protein interaction assays using β-galactosidase and histidine reporters.
Two-hybrid protein interaction analyses of recombinant FALPE and p10 proteins and their mutants were performed (19, 21). Diploid cells were transferred onto nitrocellulose membranes and lysed by submersion in a liquid nitrogen bath. Yeast cells which contained both pACT II and pAS I plasmids expressing interactive proteins were capable of synthesizing β-galactosidase and growth in the absence of histidine. β-Galactosidase activity was detected by placing the filters on a solution containing Z buffer (50 mM sodium phosphate, 10 mM KCl, 1 mM magnesium sulfate, 1 mM β-mercaptoethanol [pH 7.0]) which contained 0.2 mg of the color-producing substrate 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside X-Gal; Sigma, St. Louis, Mo.) per ml. Alternatively, growth in the absence of histidine could be detected by culturing yeast colonies on Leu-, Trp-, and His-lacking plates containing 10 mM 3-amino-1,2,4-triazole, a competitive inhibitor of histidine synthetase.
Coimmunoprecipitation of FALPE and ΔCt.FALPE recombinant proteins and cross-linking of FALPE and p10 subunits from infected cell lysates.
Sf9 cells were infected with FALPE and ΔCt.FALPE recombinant baculoviruses and harvested at 96 h postinfection. Cells were washed three times with PBS, lysed with TLC buffer (10 mM Tris-HCl, 25 mM NaCl [pH 8]), and cleared by centrifugation at 10,000 × g for 15 min. The supernatant was incubated overnight at 4°C with MAb CLP001. Antibody-antigen complexes were incubated with protein A-Sepharose beads (Pharmacia, Uppsala, Sweden) and sedimented by centrifugation at 3,000 × g for 30 s. Beads were subsequently washed three times in lysis buffer and washed a final time with 10 mM Tris-HCl (pH 8), and immunoprecipitated proteins were resuspended in protein sample buffer containing 1% (wt/vol) SDS. Following resolution by SDS-polyacrylamide gel electrophoresis under nonreducing conditions, immunoprecipitated proteins were subjected to immunoblot analysis using the polyclonal antibody directed against FALPE.
Chemical cross-linking of proteins was performed on lysates obtained from AmEPV-, AcNPV-, or mock-infected Sf9 cells. Cells were harvested 96 h postinfection, washed twice with PBS, and lysed with radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 μg of aprotinin per ml, 100 μg of phenylmethylsulfonyl fluoride per ml). The cross-linker bis(sulfosuccinimidyl)suberate (BS3) was added to the lysate at a final concentration of 2 mM, and the mixture was incubated for 30 min on ice. Proteins were centrifuged at 12,000 × g for 20 min to yield soluble and insoluble fractions, which were subjected to SDS-polyacrylamide gel electrophoresis and immunoblot analysis with CLP001 or polyclonal antibodies directed against p10.
RESULTS
Secondary structure analysis and sequence comparisons of FALPE and p10 molecules.
Although FALPE and p10 proteins participate in the formation of similar filamentous structures in infected insect cells, superficial examination of their amino acid sequences did not reveal any obvious homology. This is not surprising, since even p10 proteins from different baculoviruses can exhibit as little as 21% identity (61). However, all p10 proteins and FALPE possess similar hydrophilicity profiles consisting of a hydrophobic region lying adjacent to positively charged carboxy-terminal domains (61, 66) (data not shown). It is believed that the functional similarity of FALPE and p10 results from analogous secondary structures. However, upon closer analysis of these proteins, we observed distinct homology between the carboxy terminus of FALPE and that of the p10 protein of OpNPV (Fig. 1). The last three amino acids (Arg, Lys, and Gln) are identical in the two proteins, and residues 62 to 79 of OpNPV p10 are rich in Pro and Glu, similar to the Pro-Glu repeat region near the carboxy terminus of FALPE. In fact, the last 34 amino acids of the p10 protein OpNPV show more homology to the carboxy-terminal regions of FALPE (32.4%) than to those of p10 molecules from AcNPV (14.7%) and SeNPV (26.9%). Secondary structure analysis of FALPE revealed the presence of an amphipathic α-helix spanning residues 5 to 22. Amphipathic α-helices are known to engage in coiled-coil interactions with partners containing a similar motif (37). A similar structural motif was recently shown to exist at the amino termini of baculovirus p10 molecules, and the coiled-coil motif has been implicated in protein-protein interactions (66).
FIG. 1.
Alignment of the carboxy-terminal regions of FALPE and baculovirus p10 proteins from AcNPV, CfNPV, OpNPV, and SeNPV. FALPE was considered to be the consensus sequence, and residues in p10 which match the consensus were aligned. Homology between the carboxy terminus of the p10 molecules from OpNPV and FALPE from AmEPV was particularly evident. The identical carboxyl-terminal amino acids R, K, and Q are boxed. Proline rich repeats are underlined. Analyses were performed by using the Lasergene software package marketed by DNASTAR (Madison, Wis.).
Recombinant FALPE assembles to form cytoplasmic filaments when expressed in Sf9 cells by using the baculovirus system.
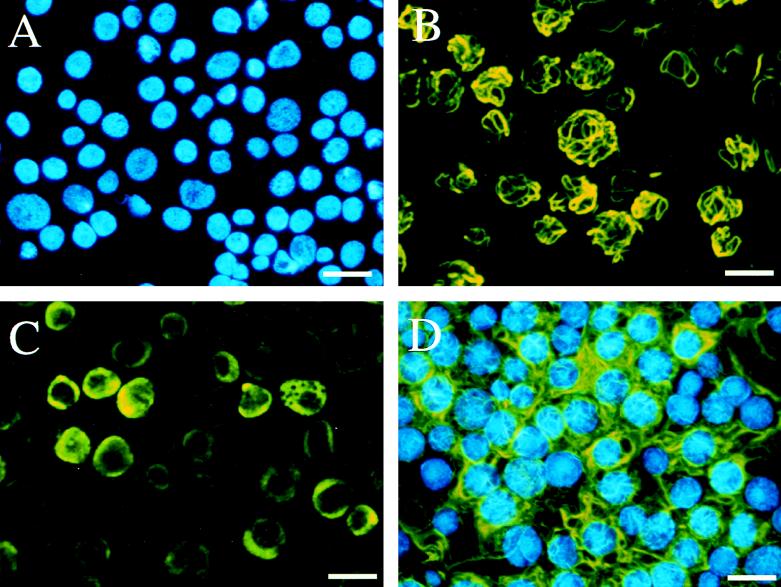
At late times of AmEPV infection, FALPE and spheroidin constitute the major proteins in infected insect cells (2, 7, 27). To demonstrate that additional EPV proteins were not required for filament formation, FALPE was synthesized in Sf9 insect cells by using the baculovirus expression system. Recombinant baculovirus expressing the complete FALPE ORF (471 bp) was prepared by using the BlueBac2 (pETL) expression vector. Sf9 cells were subsequently infected for 72 h with this recombinant virus, and expression was verified by SDS-polyacrylamide electrophoresis followed by immunoblot analysis with MAb CLP001, specific for the Pro-Glu repeat region of FALPE. To determine whether recombinant FALPE assembled to form filament structures, immunofluorescence microscopy was performed with MAb CLP001. Sf9 cells were infected with either AmEPV, wild-type AcNPV, or AcNPV-FALPE recombinant virus (Fig. 2). At 48 h postinfection, distinct cytoplasmic filaments were visible in cells infected with either AmEPV (Fig. 2B) or the FALPE recombinant baculovirus (Fig. 2D). No filament structures were observed in cells infected with wild-type AcNPV (Fig. 2C), but a diffuse nuclear staining surrounding an opaque nucleolus was evident; the latter might be due to the presence of a protein coded for by a recently reported ORF in the genome of this baculovirus. This predicted polypeptide contains a Pro-Glu repeat which may also be recognized by MAb CLP001 (3, 31). The fact that FALPE formed cytoplasmic filaments when expressed in insect cells through use of a recombinant baculovirus suggests that no other EPV proteins cooperate in the formation of these structures. However, at this point we could not rule out the possibility that baculovirus gene products can substitute for missing AmEPV components involved in filament formation.
FIG. 2.
Immunofluorescence microscopy showing filaments associated with FALPE in Sf9 insect cells infected with AmEPV and a FALPE recombinant baculovirus. Sf9 cells were mock infected (A), infected with AmEPV (B), infected with wild-type AcNPV (C), or inoculated with a recombinant AcNPV expressing the FALPE gene (D). At 72 h postinfection, cells were incubated with MAb CLP001, and bound antibody was detected with goat antimouse antibody conjugated to fluorescein. Labeled proteins were visualized with a Leitz fluorescence microscope. Nuclear DNA in panels A and D was also stained with Hoescht dye, and the two panels represent double exposures from the fluorescein and Hoescht signals. Panels B and D show the filaments formed by FALPE when expressed by AmEPV and AcNPV, respectively, while panel C illustrates background fluorescence found in the nuclei of cells infected with wild-type AcNPV. The bar represents 20 μm.
Transient expression of AcNPV p10 and AmEPV FALPE in African green monkey kidney (Vero) cells yields formation of cytoplasmic filaments.
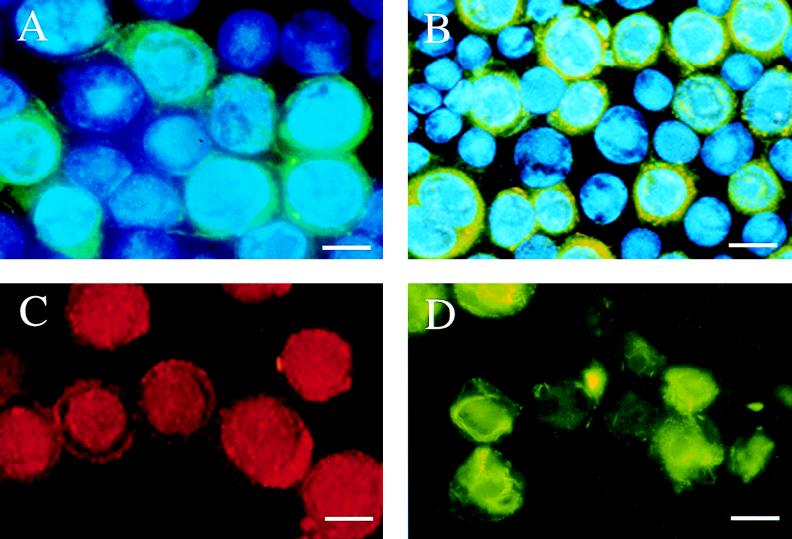
To demonstrate that no other viral proteins were required for the formation of these filamentous structures, we introduced the FALPE gene (under control of the Rous sarcoma virus long terminal repeat promoter) into mammalian cells through liposome-mediated transfection. The baculovirus p10 gene was also transfected into the same Vero monkey kidney cells. At 72 h posttransfection, cells were fixed, permeabilized, and stained with either rabbit polyclonal anti-p10 antibody or monoclonal anti-FALPE antibody (CLP001). Antibody-filament complexes were visualized with fluorescein-coupled secondary antibodies, and nuclei were stained with Hoescht dye (Fig. 3). Cytoplasmic filaments were evident in cells transfected with p10 or FALPE genes but not in cells transfected with the expression plasmid (pRBK) by itself. It is clear that both p10 and FALPE are capable of forming filaments in the absence of other viral proteins.
FIG. 3.
Immunofluorescence microscopy of Vero monkey kidney cells transfected with a FALPE or p10 expression plasmid. Vero cells were transiently transfected with either the expression plasmid pRBK (A), plasmids expressing the p10 gene of AcNPV (B and D), or a plasmid expressing FALPE (C). FALPE was visualized by using MAb CLP001 (C), while p10 was detected with a rabbit polyclonal antibody directed against the p10 protein of AcNPV (B and D). Control cells in panel A were stained with the p10 polyclonal antibody; nuclei in panel D were also stained with Hoescht dye. Clearly both p10 and FALPE formed filament networks following transient transfections of their genes into mammalian cells. The bar in each panel represents 15 μm.
Expression and immunofluorescence microscopy of FALPE mutants in insect cells.
Deletions in FALPE were made in regions which were most likely to be exposed on the surface of the molecule. It seemed likely that these stretches of amino acids participate in the protein-protein interaction required for filament formation. FALPE mutants lacking the 45 N-terminal amino acids (ΔN45.FALPE/Ac), the 32 C-terminal amino acids (ΔCt.FALPE/Ac), or internal amino acids 80 to 98 (Δ8098.FALPE/Ac) were expressed by using recombinant baculoviruses (Fig. 4A). We constructed an additional mutant lacking some of the predicted phosphorylation sites through mutation of residues 15, 25, 26, 33, and 37 to alanine (Pmut.FALPE/Ac). Expression of the mutant forms of FALPE in Sf9 cells was verified by SDS-polyacrylamide electrophoresis and immunoblot analysis. The rabbit polyclonal antibody directed against FALPE recognized all the mutant proteins (Fig. 4B). We had previously demonstrated that FALPE migrated on SDS-polyacrylamide gels as a doublet with a molecular masses of 25 and 27 kDa, and this finding is confirmed in Fig. 4B, lane 1. The faster-migrating 25-kDa species of FALPE was shown to be phosphorylated and could be converted to a slower-migrating dephosphorylated protein by treatment with alkaline phosphatase (2). The ΔN45.FALPE/Ac mutant produced a single polypeptide band of 20 kDa where most of the potential phosphorylation sites were deleted (Fig. 4B, lane 4). The internal deletion mutant Δ8098.FALPE/Ac still produced a protein doublet, indicating that some phosphorylation still occurred. When five potential phosphorylation sites at positions 15, 25, 26, 33, and 37 were mutated to alanine (Pmut.FALPE/Ac), the mutant polypeptide migrated predominantly as a single 27-kDa band. However, other fainter bands at 25 kDa and lower indicated that some phosphorylation still occurred. The exact sites of phosphorylation will ultimately have to be derived from peptide mapping studies and more thorough site-specific mutagenesis studies. It also remains to be determined whether filament formation is disrupted by defective phosphorylation or through alterations in the amphipathic α-helix which is believed to be involved in protein interactions. Sf9 cells were also infected with wild-type AcNPV as a control (Fig. 4B, lane 2). A faint band due to nonspecific binding of antibodies to the abundant polyhedrin protein was evident at 30 kDa. This protein is not present in cells infected with the recombinant baculoviruses.
FIG. 4.
Expression of FALPE deletion and phosphorylation mutants in Sf9 insect cells, using recombinant baculoviruses. (A) Schematic representation of FALPE deletion mutants inserted into baculovirus expression vectors. Numbers at the top indicate the positions of amino acid residues in the sequence. (B) Immunoblot of the total proteins from infected insect cells following electrophoresis on SDS-polyacrylamide gels. Cells were infected with FALPE/Ac recombinant virus (lane 1), wild-type AcNPV baculovirus (lane 2), the phosphorylation mutant Pmut.FALPE/Ac (lane 3), the N-terminal deletion mutant ΔN45.FALPE/Ac (lane 4), an internal deletion mutant Δ8098.FALPE/Ac (lane 5), or a carboxy-terminal deletion mutant ΔCt.FALPE/Ac (lane 6). Polypeptides were probed with a rabbit polyclonal antibody directed against FALPE and detected with alkaline phosphatase-conjugated secondary antibody and BCIP-NBT reagent. Numbers on the left indicate positions of molecular weight standards in kilodaltons. FALPE is normally present in the infected cell as a 25/27-kDa phosphoprotein. The abundant polyhedrin protein is present in Sf9 cells infected with wild-type AcNPV but not those infected with recombinant baculoviruses. The faint band (30 kDa) in lane 2 is due to nonspecific recognition of polyhedrin by the antibodies used for immunoblot detection.
To examine the abilities of the various FALPE mutant proteins to form filaments, we infected Sf9 cells with each of the recombinant baculoviruses. Immunofluorescence microscopy was performed at 48 h postinfection by using FALPE-specific antibodies. Deletion of either the amino or the carboxy terminus or the internal region (residues 80 to 98) abolished the protein’s ability to assemble and form cytoplasmic filaments (Fig. 5A to C). However, the mutants displayed different patterns of immunofluorescence within the infected cell. Protein synthesized by the amino-terminal deletion mutant ΔN45.FALPE/Ac was found mostly as cytoplasmic aggregates (Fig. 5A). The internal deletion mutant Δ8098.FALPE/Ac yielded a diffuse distribution of protein in the cytoplasm of the infected insect cell (Fig. 5B). ΔCt.FALPE/Ac produced abundant amounts of recombinant protein in infected Sf9 cells, and this was verified by SDS-polyacrylamide gel electrophoresis followed by Coomassie blue staining of total cell proteins and immunoblot analysis. However, it clearly evident that ΔCt.FALPE/Ac could not produce filament structures in infected insect cells (Fig. 5C). Finally, changing the potential phosphorylation sites (T15, S25, T26, S33, and S37) to alanine also abolished filament formation in infected insect cells (Fig. 5D). Based on the preceding results, it appears that the amino terminus, a hydrophilic internal domain, the carboxy terminus, and phosphorylation sites are all important for polymerization of FALPE molecules to form filament structures. These deletion studies and the mutation of five potential phosphorylation sites to alanine serve as a prelude to more exact experiments designed to implicate particular residues in the polymerization process.
FIG. 5.
Immunofluorescence microscopy showing intracellular localization of FALPE mutant proteins produced by recombinant baculoviruses. Sf9 insect cells were infected with baculoviruses expressing ΔN45.FALPE (A), Δ8098.FALPE (B), ΔCt.FALPE (C), or the phosphorylation mutant P Mut.FALPE (D) as described for Fig. 2. MAb CLP001 and fluorescein isothiocyanate-coupled goat anti-mouse antibody were used to detect FALPE variants in panels A, B, and D. The mutant ΔCt.FALPE was detected by using a rabbit polyclonal primary antibody directed against FALPE and rhodamine-coupled goat anti-rabbit secondary antibody. Nuclear DNA in panels A and B was also stained with Hoescht dye, and the photographs represent double exposures of fluorescein and DNA signals. All mutations abolished the ability of FALPE to produce filaments in cells infected with the different recombinant baculoviruses. The bars in panels A and C represent 10 μm, while those in panels B and D indicate 15 μm.
Analysis of AcNPV p10 and AmEPV FALPE self-association by using the yeast two-hybrid system.
To show that p10 and FALPE could associate with other p10 and FALPE molecules, we proposed to analyze this protein interaction by using the yeast two-hybrid system (19). A similar approach was recently used to investigate the interactions between intermediate filament proteins (43). The complete ORFs coding for FALPE and p10 were cloned into the yeast protein expression plasmids pACT II and pAS I. pACT II expresses proteins as fusions with the yeast GAL4 transcription activation domain, while pAS I produces fusions with the GAL4 DNA binding domain of the transactivator. The pACT II plasmids were introduced into yeast strain Y153, while pAS I vectors were transformed into strain Y187. The two transformed yeast strains were mated, and diploid cells containing both activation and DNA binding plasmids were isolated. Diploid cells, in which interaction between FALPE/p10 GAL4 activation domain and FALPE/p10 GAL4 DNA binding fusions occurred, were identified by the ability to induce transcription of LacZ/His3 reporter genes under the control of the GAL4-inducible promoter. Activation of the promoter can be measured by the production of blue yeast colonies in the presence of the β-galactosidase substrate X-Gal or by the growth of yeast in the absence of histidine in the media. Using this test, we assessed the ability of FALPE and p10 to interact with themselves, with each other, or with the lamin C control molecule. Results from both the β-Galactosidase–X-Gal assay and growth in the absence of histidine indicated that FALPE and p10 can self-associate (Fig. 6), but the proteins cannot interact with each other. In addition, the two polypeptides failed to interact with the control consisting of lamin C fused to the GAL4 DNA binding domain or with the GAL4 activation and GAL4 DNA binding domains alone.
FIG. 6.
Analysis of the ability of FALPE and p10 to self-associate in the yeast two-hybrid system. (A) Results of the FALPE self-association assay; (B) results of the AcNPV p10 self-association assay. Yeast cells were transformed with plasmids pACT II and pAS I containing FALPE or p10. Colonies were assayed for β-galactosidase activity (top strips) or for the ability to grow in the absence of added histidine in the growth medium (culture plates 2). As a control, diploid cells were streaked on the surface of petri dishes containing growth medium supplemented with histidine (culture plates 1). The different combinations of GAL4 activation domain (pACT II) and GAL4 DNA binding domain (pAS I) fusion proteins expressed in these cells were as follows: (A) pFALPE.ACT II and pAS I (sample 1), pACT II and pFALPE.AS I (sample 2), pFALPE.ACT II and pLamin.AS I (sample 3), p10.ACT II and pFALPE.AS I (sample 4); and pFALPE.ACT II and pFALPE.AS I (sample 5); (B) p10.ACT II and pAS I (sample 1), pACT II and p10.AS I (sample 2), p10.ACT II and pLaminC.AS I (sample 3), pFALPE.ACT II and p10.AS I (sample 4), and p10.ACT II and p10.AS I (sample 5). These assays proved that FALPE could interact with itself and that p10 could do the same. However, FALPE and p10 could not interact with each other.
Mapping the self-association domains of FALPE and p10 by using the yeast two-hybrid system.
To dissect the self-association domains of FALPE and p10, we again used the yeast two-hybrid system to assess protein-protein interactions between mutant forms of FALPE and p10. Various truncated versions of FALPE and p10 were inserted into the pACT II and pAS I yeast expression plasmids. Four classes of FALPE deletion mutants were generated: (i) Nt.FALPE, consisting of only the 45 N-terminal amino acids; (ii) Ct.FALPE, coding for the 33 C-terminal amino acids; (iii) Δ33C.FALPE, lacking the 33 amino acids at the carboxyl terminus; and (iv) Δ45N.FALPE, missing 45 amino acids from the amino terminus of wild-type FALPE. The coding regions for each of the deletion mutants were expressed in both pACT II and pAS I expression vectors. Diploid yeast cells containing both expression plasmids were generated and assayed for induction of β-galactosidase and growth in the absence of histidine as described above. Different combinations of haploid yeast cells containing the various FALPE hybrid proteins were mated, and the results of the protein-protein interactions are presented in Fig. 7. In addition to being able to interact with full-length FALPE, Δ33C.FALPE can self-associate to yield β-galactosidase activity and produce yeast colonies which grow in the absence of histidine (Fig. 7B). However, Δ33C.FALPE cannot interact with Ct.FALPE or Δ45N.FALPE. Nt.FALPE and Δ45N.FALPE also failed to interact with full-length FALPE. One interesting anomaly observed in these studies is that Δ45N.FALPE when fused to GAL4 DNA binding domain can act as a very strong transactivator even in the absence of a GAL4 activating partner. This nonspecific transactivation was not observed when Δ45N.FALPE was fused to the GAL4 DNA activation domain (Fig. 7B). Taken together, these results suggest that the amino-terminal domain of FALPE is necessary for self-association and polymerization during the process of filament formation.
FIG. 7.
Mapping the FALPE and AcNPV p10 self-association domains by using deletion mutagenesis and the yeast two-hybrid system. FALPE and p10 deletion mutants were cloned into pACT II and pAS I yeast expression vectors. Schematic representations of the GAL4 activation (ACT) and DNA binding (DB) domains fused with either the FALPE (A) or the p10 (C) deletion mutants are presented. Haploid yeast colonies containing GAL4 ACT constructs (vertical columns) were mated with haploid yeast colonies transformed with GAL4 DB constructs (horizontal lines), and the results of the matings for FALPE and p10 deletions mutants are presented in panels B and D, respectively. Each square of the matrix represents a potential diploid yeast colony. Diploid cells which express β-galactosidase and can grow in the absence of histidine indicate the ability of fusion proteins to interact and activate the reporter genes. Reporter gene activation and protein interaction is represented by filled squares. Deletions of the amino termini from either FALPE or p10 abolished protein interaction, while similar mutations at the carboxy termini had no effect. The Δ45N mutant of FALPE and Ct mutant of p10 when fused with the DB domain acted as nonspecific transactivators of the GAL4 promoter.
Similar protein-protein interaction studies were performed with two deletion mutants of AcNPV p10. We constructed mutants (Fig. 7C) in which either the carboxy-terminal half of p10 (p10Nt) or the amino-terminal half of p10 (p10Ct) was deleted. The truncated versions of p10 were cloned into pACT II and pAS I yeast expression plasmids. Diploid yeast colonies were isolated and assayed for β-galactosidase activity and the ability to grow in the absence of histidine (Fig. 7D). Deletion of the carboxy terminus had no effect on the ability of p10 to self-associate since p10Nt could interact with full-length p10 as well as itself. However, when p10Ct was fused to the GAL4 activation domain, it did not interact with either full-length p10 or p10Nt fused to the GAL4 DNA binding domain. As was the case with Δ45N.FALPE, p10Ct fused to the DNA GAL4 binding domain was a strong nonspecific transactivator of the GAL4 promoter (Fig. 7D). This anomaly also suggested that there are distinct similarities between the carboxy-terminal structures of p10 and FALPE. Again, the preceding results suggest that the amino-terminal domain of p10 is necessary for self-association during filament formation.
Chemical cross-linking and coimmunoprecipitation studies with FALPE and p10 proteins.
To confirm by more direct means that FALPE and p10 proteins are capable of self-associating to form filaments composed of multiple protein subunits, we proposed to chemically cross-link components of these filaments by using the cross-linker BS3. This compound was selected due to its resistance to SDS and reducing agents. Sf9 cells were infected with AmEPV or AcNPV and collected 4 days postinfection. The cells were subsequently lysed in radioimmunoprecipitation assay buffer. Proteins in the lysate were treated with BS3 and resolved by SDS-polyacrylamide gel electrophoresis. Cross-linked proteins were analyzed by immunoblot analysis using the FALPE-specific monoclonal antibody CLP001 or polyclonal p10 antibodies. A ladder of protein bands was observed from each infected cell preparation (Fig. 8). From cells infected with AmEPV, multiple bands migrating with molecular masses of 25, 50, 75, 100, and 130 kDa were observed (Fig. 8A). These multimers are consistent with the unit masses of 25 and 27 kDa for FALPE. No other proteins appeared to be cross-linked to the FALPE subunits. AcNPV-infected cells produced a ladder on SDS-polyacrylamide gels corresponding to 24, 36, 48, and 60 kDa (Fig. 8B). The multimers formed by cross-linking p10 appeared to be consistent with the unit mass of 12 kDa for p10. Again no other proteins appeared to be cross-linked to p10. Cross-linking of each protein appeared to yield dimers, trimers, tetramers, and pentamers in addition to higher-molecular-weight bands at the top of the gels. These multiple bands were absent in the uninfected Sf9 control cells.
FIG. 8.
Chemical cross-linking of FALPE and AcNPV p10 subunits and coimmunoprecipitation studies with a FALPE deletion mutant. (A) Sf9 insect cells were infected with AmEPV for 48 h, lysed, cross-linked with BS3 for 30 min, and divided into soluble and insoluble fractions by centrifugation. Proteins were resolved by SDS-polyacrylamide electrophoresis and analyzed by immunoblotting using a MAb directed against FALPE and chemiluminescence detection. Lanes 1 and 2 represent soluble and insoluble protein fractions, respectively, from mock-infected cells; lanes 3 and 4 represent soluble and insoluble proteins from AmEPV-infected cells. (B) Insect cells were infected with AcNPV for 48 h, lysed, cross-linked with BS3 for 30 min, and divided into soluble and insoluble protein fractions following centrifugation. Cross-linked proteins were again resolved by SDS-polyacrylamide electrophoresis and subjected to immunoblot analysis using a rabbit polyclonal antibody directed against p10. Lane 1 represents the soluble fraction from mock-infected cells; lanes 2 and 3 represent soluble and insoluble proteins, respectively, from AcNPV-infected cells. (C) Coimmunoprecipitation of full-length FALPE with the ΔCt.FALPE deletion mutant. Sf9 cells were infected for 48 h with either the baculovirus ΔCt.FALPE/Ac alone (lane 1) or together with another recombinant baculovirus (FALPE/Ac) which expressed the whole protein (lane 2). Cells were harvested and lysed, and MAb CLP001 was used to immunoprecipitate proteins in the coinfected cells (lane 2). Proteins were resolved by SDS-polyacrylamide electrophoresis under nonreducing conditions, transferred onto nitrocellulose membranes, and probed with rabbit polyclonal antibody directed against FALPE. Immune detection was performed by chemiluminescence using horseradish peroxidase-conjugated secondary antibodies. A control cell lysate derived from cells infected with the recombinant ΔCt.FALPE/Ac was subjected to electrophoresis and also probed with the polyclonal antibody specific for FALPE (lane 1). MAb CLP001 recognizes the proline-glutamic acid repeat in the carboxy terminus of FALPE. Immunoprecipitations with this antibody coprecipitated ΔCt.FALPE as a complex with the whole protein, FALPE. A 150-kDa band in lane 2 of panel C represents nonreduced primary antibody which reacted with the secondary antibody used for detection. Sizes are indicated in kilodaltons.
Results from the yeast two-hybrid system analysis suggested that self-association of FALPE did not require its carboxy-terminal domain but instead was mediated through its amino terminus. To confirm the fact that the carboxy terminus of FALPE was not required for protein interaction, we proposed to coimmunoprecipitate full-length FALPE with the truncated molecule Δ33C.FALPE. Sf9 cells were coinfected with FALPE/Ac and ΔCt.FALPE/Ac baculovirus recombinants for a period of 120 h, and the infected cells were lysed under mild conditions. Proteins were immunoprecipitated with MAb CLP001, which recognizes the proline-glutamic acid repeat in the FALPE carboxy terminus. This region is missing in the Δ33C.FALPE truncated protein. Immunoprecipitated protein was resolved by SDS-polyacrylamide gel electrophoresis under nonreducing conditions, and proteins were subjected to immunoblot analysis with a rabbit polyclonal antibody directed against FALPE (Fig. 8C). Both 25- and a 14-kDa proteins, corresponding to FALPE and Δ33C.FALPE, respectively, were recognized by the polyclonal antibody following the coimmunoprecipitation. A band migrating with a molecular mass of 150 kDa (Fig. 8C, lane 2) represents undenatured MAb which was used in the immunoprecipitation. This antibody was recognized by the secondary antibody (goat anti-rabbit antibody) in the subsequent immunoblot analyses. Recognition of the truncated form of FALPE by the polyclonal antibody is routinely fainter due to the deletion of the very antigenic carboxy terminus, and reactions between MAb CLP001 and the entire FALPE molecule during immunoblot analysis are usually more sensitive. The poor detection of truncated FALPE by the polyclonal antibody was also evident when solubilized proteins from Sf9 cells infected with ΔCt.FALPE/Ac baculovirus were subjected to immunoblot analysis (Fig. 8C, lane 1). From these studies, we concluded that FALPE, with the deletion of its carboxy terminus, could interact with the full-length parent molecule.
DISCUSSION
Late infections of insect cells by AmEPV and AcNPV are characterized by the production of large bundles of filaments in the infected cell. These filaments are closely associated with occlusion body envelopes and may participate in the morphogenesis of these structures. Immunofluorescence microscopy and immunogold electron microscopy have revealed that these filaments are composed predominantly of FALPE, in the case of EPVs, and p10, in the instance of baculoviruses (2, 65). Little is known concerning the function of these filaments or how they are assembled. In this study, we used various techniques to investigate the process by which the subunits of these structures associate. FALPE and p10 contain similar structural features at their amino-terminal and carboxy-terminal domains which help determine their functional roles. An attractive hypothesis is that these two different proteins originated from a common molecular ancestor. This precursor could originally have been a cellular gene that was subsequently captured and inserted into the viral genome. Poxviruses have been noted to exhibit this behavior (56). However, the identity of a potential cellular homolog to FALPE and p10 is not known. Throughout evolution, several substitutions and deletions of amino acids appear to have taken place in the precursor to FALPE and p10, leaving only important structural domains which are required for filament formation and occlusion body morphogenesis.
The large quantities of FALPE and p10 which are produced in infected cells suggested that these proteins constituted either the major or sole components of the fibrils produced by the virus. Our experiments supported this view since we were able to generate filaments specified by the EPV FALPE protein by using a baculovirus expression vector. This result indicated that other EPV proteins were not required for filament formation. In addition, fibrils were produced in mammalian cells when they were transfected with plasmids containing FALPE and p10 genes under control of the Rous sarcoma virus long terminal repeat promoter. We were able to conclude that no other viral proteins were required for FALPE or p10 filament formation. However, it is possible that some cytoskeletal proteins which are conserved between insect and mammalian cells can also participate in EPV or baculovirus filament formation. A number of other viruses have previously been shown to encode proteins which interact with the cellular skeleton. For example, the E4 proteins of human papillomaviruses have been shown to interact with cytokeratin networks in epidermal cells (51), tobacco mosaic virus movement protein associates with actin in plant cells (29), and the p10 protein of baculovirus may associate with tubulin (11). However, in our hands, FALPE and p10 expressed as glutathione S-transferase fusions failed to bind any cellular proteins following in vitro incubation with insect cell lysates (data not shown). In addition, we previously demonstrated, using confocal microscopy and double-label fluorescence, that AmEPV cytoplasmic filaments are distinct from the actin present in infected insect cells (2). Nevertheless, more experiments are required to completely rule out the involvement of cellular proteins in EPV and baculovirus filament formation.
Subunit interaction in the formation of FALPE and p10 filaments was studied through the effects of deletion mutagenesis. Hydrophilic regions of FALPE at the carboxy terminus or at an internal region (residues 80 to 98), or amino acids at the amino terminus, were found to be important for filament formation when deletion mutants were analyzed in insect cells infected with recombinant baculoviruses. Similar sorts of studies revealed the importance of both the amino and carboxy termini of p10 (60, 63, 65). Aggregation phenomena can be mapped to the amino terminus and the internal regions of FALPE by immunofluorescence microscopy. In addition, mutation of five potential phosphorylation sites in the amino terminus to alanine abolished filament formation. Finally, we further implicated the amino termini of FALPE and p10, using these deletion mutants in a yeast two-hybrid protein interaction system. The deletions made in these studies could also affect other regions in the FALPE and p10 molecules which could also be involved in subunit association. Thus, fine mapping of the FALPE self-association region through site-specific amino acid mutagenesis is required to refine the binding sites.
Chemical cross-linking studies performed on FALPE and p10 with BS3 reagent rely on the availability of lysine residues on adjacent molecules. Resolution of cross-linked species on SDS-polyacrylamide gels yielded dimers, trimers, tetramers, pentamers, and higher-molecular-weight species at the top of the gel. Again it seems likely that FALPE and p10 are the major components of the polymeric structures. There is the possibility that small quantities of cellular protein were associated with FALPE and p10 but were not detected in our experiments. An additional study involving in vitro production of FALPE or p10 filaments from monomeric subunits should eventually resolve this issue.
Other types of viruses such as mammalian poxviruses and adenoviruses (16, 39) replicate in close association with the cellular cytoskeletal system. However, EPVs and baculoviruses are unique in their ability to generate web-like filament structures by using proteins encoded by their own genomes. It is not known whether these filament proteins were originally pirated from cellular proteins of similar function and modified through an evolutionary process. Further studies regarding FALPE and p10 filament formation will also yield important knowledge concerning protein-protein interactions and clarify the role of these structures in occlusion body formation and viral replication.
ACKNOWLEDGMENTS
We thank Jane McGlade and Ralph Salvino, Amgen Research Institute, Toronto, Ontario, Canada, for assistance with the yeast two-hybrid system.
This work was funded by Medical Research Council of Canada operating grant MA10638. M.H.A.-I. was partially supported by an F. C. Harrison graduate student fellowship from McGill University and a Canadian International Development Agency scholarship.
REFERENCES
- 1.Adams J R, McClintock J T. Baculoviridae. Nuclear polyhedrosis viruses. Part 1. Nuclear polyhedrosis viruses of insects. In: Adams J R, Bonami J R, editors. Atlas of invertebrate viruses. Boca Raton, Fla: CRC Press; 1991. pp. 187–204. [Google Scholar]
- 2.Alaoui-Ismaili M H, Richardson C D. Identification and characterization of a filament associated protein encoded by the Amsacta moorei entomopoxvirus. J Virol. 1996;70:2697–2705. doi: 10.1128/jvi.70.5.2697-2705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arif B M, Kurstak E. The entomopoviruses. In: Kurstak E, editor. Viruses of invertebrates. New York, N.Y: Marcel Dekker Publishing; 1991. pp. 179–195. [Google Scholar]
- 4.Arif B M. The entomopoxviruses. Adv Virus Res. 1984;29:195–213. doi: 10.1016/s0065-3527(08)60409-1. [DOI] [PubMed] [Google Scholar]
- 5.Arif B M. Recent advances in the molecular biology of entomopoxviruses. J Gen Virol. 1995;76:1–13. doi: 10.1099/0022-1317-76-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Ayres M D, Howard S C, Kuzio J, Lopez-Ferber M, Possee R D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- 7.Banville M, Dumas F, Trifiro S, Arif B, Richardson C D. The predicted amino acid sequence of the spheroidin protein form Amsacta moorei entomopoxvirus: lack of homology between the major occlusion body proteins of different poxviruses. J Gen Virol. 1992;73:559–566. doi: 10.1099/0022-1317-73-3-559. [DOI] [PubMed] [Google Scholar]
- 8.Becker D M, Lundblad V. Manipulations of yeast genes. Introduction of DNA into yeast cells. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons Inc.; 1994. pp. 13.7.1–13.7.10. [Google Scholar]
- 9.Bilimoria S L, Arif B M. Subunit protein and alkaline protease of entomopoxvirus spheroids. Virology. 1979;96:596–603. doi: 10.1016/0042-6822(79)90115-6. [DOI] [PubMed] [Google Scholar]
- 10.Charlton C A, Volkman L E. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf21 cells induces actin cable formation. Virology. 1993;197:245–254. doi: 10.1006/viro.1993.1585. [DOI] [PubMed] [Google Scholar]
- 11.Cheley S, Kosik K S, Paskevich P, Bakalis S, Bayley H. Phosphorylated baculovirus p10 is a heat-stable microtubule associated protein associated with process formation in Sf9 cells. J Cell Sci. 1992;102:739–752. doi: 10.1242/jcs.102.4.739. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Yang B. One step transformation of yeast in stationary phase. Curr Genet. 1992;21:83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- 13.Chou J M, Lo C F, Huang C J, Wang C H. Proceedings of the XIX International Congress of Entomology, Beijing, China. 1992. Isolation and nucleotide sequence of the Perina nuda multinucleocapsid nuclear polyhedrosis virus (PnMNPV) two late genes: polyhedrin and p10 gene. GenBank accession no. U50411. [Google Scholar]
- 14.Chung K L, Brown M, Faulkner P. Studies on the morphogenesis of polyhedral inclusion bodies of a baculovirus Autographa californica NPV. J Gen Virol. 1980;46:335–347. [Google Scholar]
- 15.Cudmore S, Reckmann I, Griffiths S G, Way M. Vaccinia virus: a model system for actin-membrane interactions. J Cell Sci. 1996;109:1739–1747. doi: 10.1242/jcs.109.7.1739. [DOI] [PubMed] [Google Scholar]
- 16.Cudmore S, Reckmann I, Way M. Viral manipulations of the actin cytoskeleton. Trends Microbiol. 1997;5:142–148. doi: 10.1016/S0966-842X(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 17.Dales S, Chardonnet Y. Early events in the interaction of adenovirus with HeLa cells. IV. Association with microtubules and the nuclear core complex during vectorial movement in the inoculum. Virology. 1973;56:465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- 18.Damsky C H, Sheffield J B, Tuszynski G P, Warren L. Is there a role for actin in virus budding? J Cell Biol. 1977;75:593–605. doi: 10.1083/jcb.75.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–247. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 20.Finley R L, Brent R. Interaction mating reveals binary and ternary connections between Drosophila cell cycle regulators. Proc Natl Acad Sci USA. 1994;91:12980–12984. doi: 10.1073/pnas.91.26.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golemis E A, Gyuris J, Brent R. Interaction trap/two-hybrid system to identify interacting proteins. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols In molecular biology. New York, N.Y: John Wiley & Sons Inc.; 1994. pp. 13.14.1–13.14.17. [Google Scholar]
- 22.Goodwin R H, Milner R J, Beaton C D. Entomopoxvirinae. In: Adams J R, Bonami J R, editors. Atlas of invertebrate viruses. Boca Raton, Fla: CRC Press; 1991. pp. 259–285. [Google Scholar]
- 23.Granados R R. Insect poxviruses: pathology, morphology and development. Misc Publ Entomol Soc Am. 1973;9:73–94. [Google Scholar]
- 24.Granados R R. Entomopoxvirus infections in insects. In: Davidson E W, editor. Pathogenesis of invertebrate microbial disease. Totowa, N.J: Allenheld Press; 1981. pp. 102–126. [Google Scholar]
- 25.Granados R R, Roberts D W. Electron microscopy of a poxlike virus infecting an invertebrate host. Virology. 1970;40:230–243. doi: 10.1016/0042-6822(70)90398-3. [DOI] [PubMed] [Google Scholar]
- 26.Gross C H, Russell R L Q, Rohrmann G F. Orgyia pseudotsugata baculovirus p10 and polyhedron envelope protein genes: analysis of their relative expression levels and role in polyhedron structure. J Gen Virol. 1994;75:1115–1123. doi: 10.1099/0022-1317-75-5-1115. [DOI] [PubMed] [Google Scholar]
- 27.Hall R L, Moyer R M. Identification, cloning, and sequencing of a fragment of Amsacta moorei entomopoxvirus DNA containing the spheroidin gene and three vaccinia virus-related open reading frames. J Virol. 1991;65:6516–6527. doi: 10.1128/jvi.65.12.6516-6527.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harlow E, Lane E. Antibodies. A laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 29.Heinlein M, Epel B L, Padgett H S, Beachy R N. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. A Cold Spring Harbor Laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 31.Kool M, Broer R, Zuidema D, Golbach R W, Vlak J M. Nucleotide sequence and genetic organization of 7.3 kb regions (map unit 47 to 52.5) of Autographa californica nuclear polyhedrosis virus fragment EcoR I-C. J Gen Virol. 1994;75:487–494. doi: 10.1099/0022-1317-75-3-487. [DOI] [PubMed] [Google Scholar]
- 32.Kuzio J, Rohel D Z, Curry C J, Krebs A, Carstens E B, Faulkner P. Nucleotide sequence of the p10 polypeptide gene of Autographa californica nuclear polyhedrosis virus. Virology. 1984;139:414–418. doi: 10.1016/0042-6822(84)90388-x. [DOI] [PubMed] [Google Scholar]
- 33.Lalumiére M, Richardson C D. Production of recombinant baculoviruses using rapid screening vectors that contain the gene for β-galactosidase. In: Richardson C D, editor. Baculovirus expression protocols. Totowa, N.J: Humana Press; 1995. pp. 161–177. [DOI] [PubMed] [Google Scholar]
- 34.Lamb R A, Mahy B W J, Choppin P W. The synthesis of Sendai virus polypeptides in infected cells. Virology. 1976;69:116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- 35.Leisy D, Rohrmann G F, Nesson M, Beudreau G. Nucleotide sequencing and transcriptional mapping of the Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus p10 gene. Virology. 1986;153:157–167. doi: 10.1016/0042-6822(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 36.Luckow V A, Summers M D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989;170:31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- 37.Luftig R B, Weihing R R. Adenovirus binds to rat brain microtubules in vitro. J Virol. 1975;16:696–706. doi: 10.1128/jvi.16.3.696-706.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luftig R B, Lupo L D. Viral interactions with the host-cell cytoskeleton: the role of retroviral proteases. Trends Microbiol. 1994;2:178–182. doi: 10.1016/0966-842x(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 39.Lupas A. Coiled coils: new structures and new functions. Trends Biol Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 40.Marlow S A, Palmer C P, King L A. Cytopathic effects of Amsacta moorei entomopoxvirus infection on the cytoskeletion of Estigmene acrea cells. Virus Res. 1992;26:41–55. [Google Scholar]
- 41.McKeon F D, Kirschner M W, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;316:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 42.Melamed I, Stein L, Roifman C M. Epstein-Barr virus induces actin polymerization in human B cells. J Immunol. 1994;153:1998–2003. [PubMed] [Google Scholar]
- 43.Meng J J, Khan S, Ip W. Intermediate filament protein domain interactions as revealed by two-hybrid screens. J Biol Chem. 1996;271:1599–1604. doi: 10.1074/jbc.271.3.1599. [DOI] [PubMed] [Google Scholar]
- 44.Miller L K. Insect viruses. In: Fields B N, Knipe D M, Howley P M, Channock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. New York, N.Y: Lippincott-Raven Publishers; 1996. pp. 533–556. [Google Scholar]
- 45.Mousa G Y, Trevithick J R, Bechberger J, Blair D G. Cytochalasin D induces the capping of both leukaemia viral proteins and actin in infected cells. Nature. 1978;274:808–809. doi: 10.1038/274808a0. [DOI] [PubMed] [Google Scholar]
- 46.O’Reilley D R, Miller L K, Lukow V A. Baculovirus expression vectors. A laboratory manual. W. H. New York, N.Y: Freeman and Co.; 1992. [Google Scholar]
- 47.Quant-Russell R L, Pearson M N, Rohrmann G F. Characterization of baculovirus p10 protein synthesis using monoclonal antibodies. Virology. 1987;160:9–19. doi: 10.1016/0042-6822(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 48.Richardson C D, Banville M, Lalumiére M, Vialard J, Meighen E A. Bacterial luciferase produced with rapid screening baculovirus vectors is a sensitive reporter for infection of insect cells and larvae. Intervirology. 1992;34:213–227. doi: 10.1159/000150285. [DOI] [PubMed] [Google Scholar]
- 49.Richardson C D, editor. Baculovirus expression protocols. Totowa, N.J: Humana Press; 1995. [Google Scholar]
- 50.Roberts D W, Granados R R. A poxlike virus from the Amsacta moorei (Lepidoptera: Arctiidae) J Invertebr Pathol. 1968;12:141–143. [Google Scholar]
- 51.Roberts S, Ashmole I, Johnson G D, Kreider J W, Gallimore P H. Cutaneous and mucosal human papillomavirus E4 proteins form intermediate filament-like structures in epithelial cells. Virology. 1993;197:176–187. doi: 10.1006/viro.1993.1578. [DOI] [PubMed] [Google Scholar]
- 52.Rohrmann G F. Polyhedrin structure. J Gen Virol. 1986;67:1499–1513. doi: 10.1099/0022-1317-67-8-1499. [DOI] [PubMed] [Google Scholar]
- 53.Russell R L Q, Rohrmann G F. A baculovirus polyhedron envelope protein:immunogold localization in infected cells and mature polyhedra. Virology. 1990;174:177–184. doi: 10.1016/0042-6822(90)90066-z. [DOI] [PubMed] [Google Scholar]
- 54.Sasaki H, Nakamura M, Ohno T, Matsuda Y, Yuda Y, Nonomura Y. Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc Natl Acad Sci USA. 1995;92:2026–2030. doi: 10.1073/pnas.92.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanz P, Veyrunes J C, Cousserans F, Bergoin M. Cloning and sequencing of the spherulin gene, the occlusion body major polypeptide from the Melolontha melolontha entomopoxvirus (MmEPV) Virology. 1994;202:449–457. doi: 10.1006/viro.1994.1361. [DOI] [PubMed] [Google Scholar]
- 56.Smith G L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994;2:81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 57.Summers M D, Arnott H J. Ultrastructural studies on occlusion formation and virus occlusion in nuclear polyhedrosis and granulosis-infected cells of Trichoplusia ni (Hubner) J Ultrastruct Res. 1969;28:462–480. doi: 10.1016/s0022-5320(69)80034-1. [DOI] [PubMed] [Google Scholar]
- 58.Tyrrell D L J, Ehrnst A. Transmembrane communication in cells chronically infected with measles virus. J Cell Biol. 1979;81:396–402. doi: 10.1083/jcb.81.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van der Wilk F, Van Lent J W M, Vlak J M. Immunogold detection of polyhedrin, p10 and virion antigens in Autographa californica nuclear polyhedrosis virus infected Spodoptera frugiperda cells. J Gen Virol. 1987;68:2615–2623. [Google Scholar]
- 60.Van Oers M M, Flipsen J T M, Reusken C B E M, Sliwinsky E L, Goldbach R W, Vlak J M. Functional domains of the p10 protein of Autographa californica nuclear polyhedrosis virus. J Gen Virol. 1993;74:563–574. doi: 10.1099/0022-1317-74-4-563. [DOI] [PubMed] [Google Scholar]
- 61.Van Oers M M, Flipsen J T M, Reusken C B F M, Vlak J M. Specificity of baculovirus p10 functions. Virology. 1994;200:513–523. doi: 10.1006/viro.1994.1214. [DOI] [PubMed] [Google Scholar]
- 62.Vialard J E, Richardson C D. The 1,629-nucleotide open reading frame located downstream of the Autographa californica nuclear polyhedrosis virus polyhedrin gene encodes a nucleocapsid-associated phosphoprotein. J Virol. 1993;67:5859–5866. doi: 10.1128/jvi.67.10.5859-5866.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlak J M, Klinkenberg F A, Zaal K J M, Usmany M, Klinge-Roode E C, Geervliet J B F, Rousien J, van Lent J W M. Functional studies on the p10 gene of Autographa californica nuclear polyhedrosis virus using a recombinant expressing a p10-β-galactosidase fusion gene. J Gen Virol. 1988;69:765–776. doi: 10.1099/0022-1317-69-4-765. [DOI] [PubMed] [Google Scholar]
- 64.Vlak J M, Schouten A, Usmany M, Belsham G J, Klingeroode E C, Maule A J, Van Lent J W M, Zuidema D. Expression of cauliflower mosaic virus gene I using a baculovirus vactor based upon the p10 gene and a novel selection method. Virology. 1990;179:312–320. doi: 10.1016/0042-6822(90)90299-7. [DOI] [PubMed] [Google Scholar]
- 65.Williams G V, Rohel D Z, Kuzio J, Faulkner P. A cytopathological investigation of Autographa californica nuclear polyedrosis virus p10 gene function using insertion/deletion mutants. J Gen Virol. 1989;70:187–202. doi: 10.1099/0022-1317-70-1-187. [DOI] [PubMed] [Google Scholar]
- 66.Wilson J A, Hill J E, Kuzio J, Faulkner P. Characterization of the baculovirus Choristoneura fumiferana multicapsid nuclear polyhedrosis virus p10 gene indicates that the polypeptide contains a coiled-coil domain. J Gen Virol. 1995;76:2923–2932. doi: 10.1099/0022-1317-76-12-2923. [DOI] [PubMed] [Google Scholar]
- 67.Yaozhou Z. Studies on the p10 gene of Bombyx mori nuclear polyhedrosis virus. Ph.D. thesis. Shanghai, People’s Republic of China: Graduate School of Chinese Academy of Agricultural Sciences, Shanghai Institute of Biochemistry Academia Sinica; 1992. [Google Scholar]
- 68.Zuidema D, Van Oers M M, Van Strien E A, Caballero P C, Klok E J, Goldback R W, Vlak J M. Nucleotide sequence and transcriptional analysis of the p10 gene of Spodoptera exigua nuclear polyhedrosis. J Gen Virol. 1993;74:1017–1024. doi: 10.1099/0022-1317-74-6-1017. [DOI] [PubMed] [Google Scholar]