Key Points
Question
Does targeting a Pao2 of 60 mm Hg vs 90 mm Hg affect the number of days alive without life support in intensive care unit patients with COVID-19 and severe hypoxemia?
Findings
In this randomized trial including 726 patients, targeting a Pao2 of 60 mm Hg resulted in 80.0 days alive without support at 90 days compared with 72.0 days when targeting a Pao2 of 90 mm Hg. This difference was statistically significant.
Meaning
A Pao2 target of 60 mm Hg vs 90 mm Hg resulted in more days alive without life support in intensive care unit patients with COVID-19 and severe hypoxemia.
Abstract
Importance
Supplemental oxygen is ubiquitously used in patients with COVID-19 and severe hypoxemia, but a lower dose may be beneficial.
Objective
To assess the effects of targeting a Pao2 of 60 mm Hg vs 90 mm Hg in patients with COVID-19 and severe hypoxemia in the intensive care unit (ICU).
Design, Setting, and Participants
Multicenter randomized clinical trial including 726 adults with COVID-19 receiving at least 10 L/min of oxygen or mechanical ventilation in 11 ICUs in Europe from August 2020 to March 2023. The trial was prematurely stopped prior to outcome assessment due to slow enrollment. End of 90-day follow-up was June 1, 2023.
Interventions
Patients were randomized 1:1 to a Pao2 of 60 mm Hg (lower oxygenation group; n = 365) or 90 mm Hg (higher oxygenation group; n = 361) for up to 90 days in the ICU.
Main Outcomes and Measures
The primary outcome was the number of days alive without life support (mechanical ventilation, circulatory support, or kidney replacement therapy) at 90 days. Secondary outcomes included mortality, proportion of patients with serious adverse events, and number of days alive and out of hospital, all at 90 days.
Results
Of 726 randomized patients, primary outcome data were available for 697 (351 in the lower oxygenation group and 346 in the higher oxygenation group). Median age was 66 years, and 495 patients (68%) were male. At 90 days, the median number of days alive without life support was 80.0 days (IQR, 9.0-89.0 days) in the lower oxygenation group and 72.0 days (IQR, 2.0-88.0 days) in the higher oxygenation group (P = .009 by van Elteren test; supplemental bootstrapped adjusted mean difference, 5.8 days [95% CI, 0.2-11.5 days]; P = .04). Mortality at 90 days was 30.2% in the lower oxygenation group and 34.7% in the higher oxygenation group (risk ratio, 0.86 [98.6% CI, 0.66-1.13]; P = .18). There were no statistically significant differences in proportion of patients with serious adverse events or in number of days alive and out of hospital.
Conclusion and Relevance
In adult ICU patients with COVID-19 and severe hypoxemia, targeting a Pao2 of 60 mm Hg resulted in more days alive without life support in 90 days than targeting a Pao2 of 90 mm Hg.
Trial Registration
ClinicalTrials.gov Identifier: NCT04425031
This randomized clinical trial assesses the effect of targeting a lower vs higher oxygenation level on 90-day survival without need for life support among intensive care unit patients with COVID-19 and severe hypoxemia.
Introduction
COVID-19 pneumonia may result in hypoxemic respiratory failure requiring high levels of supplemental oxygen and intensive care. From the beginning of the COVID-19 pandemic, the Surviving Sepsis Campaign recommended a peripheral oxygen saturation (Spo2) between 90% and 96%.1 Since then, a subgroup analysis of patients with COVID-19 from the Handling Oxygenation Target in the Intensive Care Unit (HOT-ICU) trial was published,2,3 while other studies of oxygenation targets in the intensive care unit (ICU) excluded4 or included very few5 COVID-19 patients.
Targeted oxygenation in critically ill patients in the ICU has been extensively investigated in recent years, but there remains uncertainty about the effects of higher vs lower oxygenation strategies.6 Prior results may not be transferable to patients with COVID-19 pneumonia, as a distinct pulmonary pathophysiology is described.7 Furthermore, randomized clinical trials primarily included invasively mechanically ventilated patients, but in recent years, noninvasive ventilation and high-flow nasal oxygen are more common tools to correct hypoxemia in the ICU.8,9,10,11 These were widely used during the COVID-19 pandemic,12 but with uncertain evidence.13,14 The post hoc subgroup analysis of 110 patients with COVID-19 and acute hypoxemic respiratory failure in the HOT-ICU trial2 included both mechanically ventilated patients and those treated in open systems, finding that the percentage of days alive without life support was significantly higher when targeting a Pao2 of 60 mm Hg compared with a Pao2 of 90 mm Hg, but with no difference in the primary outcome of mortality at 90 days.3
Establishing a safe oxygenation strategy in COVID-19 patients may ensure that supplemental oxygen is dosed for optimal effect and minimal harm while allowing efficient use of available oxygen supplies, ICU beds, and ventilators. Therefore, the Handling Oxygenation Targets in COVID-19 (HOT-COVID) trial was planned to test the hypothesis that targeting a Pao2 of 60 mm Hg compared with 90 mm Hg would increase the number of days alive without life support in 90 days in ICU patients with COVID-19 and severe hypoxemia.
Methods
Trial Design and Oversight
The trial was an investigator-initiated, multicenter, parallel-group clinical trial using centralized randomization with computer-generated concealed assignment sequences in permuted blocks of varying sizes stratified for trial site. Thirteen ICUs in Denmark, Switzerland, Norway, Iceland, and Wales participated. Due to the nature of the intervention, clinicians, patients, and relatives were not blinded. A detailed protocol with statistical analysis plan was published before inclusion of the last patient (Supplement 1).15 This trial was approved by the Danish Medicines Agency and relevant ethics committees as an amendment to the HOT-ICU trial protocol.2 For all patients, written informed consent was temporarily obtained from a trial-independent physician until patients regained capacity or surrogates became available. In case of consent withdrawal, the intervention was stopped, but permission was requested from the patient or a surrogate to continue data registration and use trial data according to national regulations.
The trial was designed and overseen by a steering committee. An independent data and safety monitoring committee monitored the trial and reviewed the planned interim analysis after 390 patients (50% of the planned sample size) had completed 90-day follow-up (eAppendix 1 in Supplement 2). The data and safety monitoring committee received outcome data for the separate intervention groups without being informed of which group represented the higher and lower oxygenation targets. No outcome data were assessed between the interim analysis and trial termination. The steering committee had no access to the interim data. Trial data collection, storage, approval, and management of consent were externally monitored in accordance with the Good Clinical Practice directive of the European Union. The trial is reported in accordance with the CONSORT 2010 statement on reporting guidelines for parallel-group randomized trials.16
Patients
We included adult patients (≥18 years of age) acutely admitted to a participating ICU with confirmed COVID-19 and severe hypoxemia who were expected to receive supplemental oxygen for at least 24 hours in the ICU and had a functioning arterial line for Pao2 monitoring. An arterial line is standard care in in all participating ICUs. Severe hypoxemia was defined as receiving supplemental oxygen with a flow of at least 10 L/min in an open system or receiving invasive mechanical ventilation, noninvasive ventilation, or continuous positive airway pressure by mask irrespective of the fraction of inspired oxygen (Fio2). Patients who could not undergo randomization within 12 hours of ICU admission, patients for whom consent could not be obtained, and those previously randomized into the HOT-ICU or HOT-COVID trials were excluded. Additional exclusion criteria and detailed descriptions of all inclusion and exclusion criteria are presented in eAppendix 1 in Supplement 2.
Intervention
Patients were randomized 1:1 to receive supplemental oxygen targeting a Pao2 of either 60 mm Hg (lower oxygenation group) or 90 mm Hg (higher oxygenation group). Oxygenation targets were achieved through titration of the administered Fio2 and maintained based on continuous measurement of Spo2 and its correlation with the associated measurement of Pao2. Ventilator settings and choice of oxygen supplementation device were at the discretion of the treating clinicians. Deviations above the allocated oxygenation target were allowed only if Fio2 was 0.21, and deviations below the allocated oxygenation target were allowed only if Fio2 was 1.00. Patients treated with supplementary oxygen in an open system were not to be intubated to reach the allocated oxygenation target if no other criteria for invasive mechanical ventilation were present and if an Fio2 of 1.00 was administrated. Weaning was not protocolized, but neither the allocated oxygenation target nor the specific Fio2 cutoff levels could dictate timing of extubation if all other criteria were present. All sites participated in the HOT-ICU trial and were experienced in handling intubation and extubation in the 2 oxygenation groups. The frequency of Pao2 measurements required to meet the oxygenation target was not protocolized, but at least 4 measurements per day were expected. The intervention was implemented for the entire ICU stay, including readmissions, up to a maximum of 90 days after randomization. During the intervention period, the highest and lowest Pao2 measurements, with concomitant Fio2 and arterial oxygen saturation (Sao2) levels, were recorded in prespecified 12-hour intervals.
Outcome Measures
The primary outcome was the absolute number of days alive without life support in 90 days, defined by the absence of mechanical ventilation (invasive mechanical ventilation, noninvasive ventilation, or nonintermittent continuous positive airway pressure), circulatory support (inotropes or vasopressors), or kidney replacement therapy, with no penalization for death. Extracorporeal membrane oxygenation itself did not count as life support. Patients receiving long-term hemodialysis were included even though these patients had an expected zero days alive without life support in 90 days. All days alive without life support, including days in between episodes of circulatory support or mechanical ventilation, but excluding days in between intermittent kidney replacement therapy, were counted as days alive without life support, regardless of vital status at day 90. In patients transferred to nonparticipating ICUs, data on the primary outcome were obtained through direct contact or use of common electronic patient journal systems depending on local availability. Only patients with full follow-up in all 90 days were included in the analyses of the primary outcome. Secondary outcomes were 90-day all-cause mortality; number of patients with 1 or more serious adverse events in the ICU within 90 days, defined as a new episode of shock, cerebral ischemia, myocardial infarction, or intestinal ischemia; and absolute number of days alive and out of hospital in 90 days. Detailed information regarding outcome measures is provided in eAppendix 1 in Supplement 2.
Statistical Analysis
Based on the data and clinical experience at the beginning of the COVID-19 pandemic in the spring of 2020,3,17 we expected that 40% of patients would die within 90 days while receiving life support, and survivors would receive an average of 14 days of life support, thus averaging 45.6 days alive without life support for the higher oxygenation group. We estimated that the lower oxygenation target would reduce 90-day mortality by a 20% relative reduction and yield a 10% decrease in days receiving life support for survivors, corresponding to an average of 52.6 days alive without life support in the lower oxygenation group. Based on this, we estimated that with a power of 80% and a 2-sided α = .05, 780 patients were needed to detect the absolute between-group difference of 7 days alive without life support.
The statistical analyses were conducted in accordance with the published protocol and statistical analysis plan.15 All statistical analyses, with the exception of the per-protocol sensitivity analyses, were conducted in the intention-to-treat population, corresponding to all randomized patients except those for whom data were unavailable because consent was withdrawn or unobtainable. Analyses of the primary and secondary outcomes were conducted blinded to oxygenation target allocation.
For the continuous outcomes of absolute numbers of days alive without life support and days alive and out of hospital, both at 90 days, P values were analyzed using the nonparametric van Elteren test with stratification for trial site, as the assumptions of a Poisson or a negative binomial distribution were not met.18 For the primary outcome, the absolute difference adjusted for site and the 95% CI were calculated using a generalized linear model with an identity link and a Gaussian data distribution and were bootstrapped with 10 000 repetitions. A similar approach was applied for a secondary analysis of the primary outcome adjusted for the stratification variable site and for the predefined baseline factors of age, active hematological malignancy, chronic obstructive pulmonary disease, active metastatic cancer, and Sequential Organ Failure Assessment score.19 Furthermore, analysis of the primary outcome was supplemented with 2 post hoc analyses with death penalized to either zero days alive without life support or −1 day alive without life support, as these approaches are prevalent in contemporary randomized clinical trials in ICU patients.20 The dichotomous outcomes of 90-day all-cause mortality and proportion of patients with 1 or more serious adverse event in the ICU were analyzed using a generalized linear model with a log-link and binomial error distribution and stratification for trial site. Results of the secondary outcomes are reported as relative risks and risk differences with multiplicity-adjusted 98.6% CIs.15,21 Statistical significance was indicated by a 2-sided P < .05 for the primary outcome, including interaction tests in subgroup analyses, and by a multiplicity-adjusted P < .014 for the 3 secondary outcomes.15 No adjustments were conducted for multiplicity for the primary outcome.
The analysis of number of days alive without life support was supplemented with stacked bar charts of percentages of patients being deceased, alive receiving life support, or alive without life support, including separate charts for the 3 types of life support. Furthermore, the initiation of mechanical ventilation was visualized using bar charts of the cumulative proportion of patients receiving mechanical ventilation in the intervention period. The analysis of 90-day all-cause mortality was supplemented with crude Kaplan-Meier plots and calculation of a hazard ratio from a Cox proportional hazards model adjusted for site. Heterogeneity of the treatment effect for the primary outcome was assessed in 5 prespecified subgroups that were defined at baseline according to the presence or absence of chronic obstructive pulmonary disease, active hematological malignancy, shock (defined as use of vasopressors or inotropes and serum lactate >2 mmol/L), and invasive mechanical ventilation and according to Pao2:Fio2 ratio. For each subgroup analysis, a bootstrapped 95% CI, adjusted for site, was calculated using 10 000 repetitions. Each subgroup was tested for interaction with the intervention. Sensitivity analyses were conducted to evaluate the primary outcome in 4 prespecified per-protocol populations, excluding patients according to deviations from the allocated oxygenation target (eAppendix 1 in Supplement 2). Additionally, the time from trial initiation to patient randomization was tested for interaction with the intervention effect as a post hoc analysis. All analyses were performed using Stata statistical software, release 17 (StataNordic).
Results
Participants
From August 25, 2020, to March 8, 2023, a total of 726 patients (93%) of the preplanned 780 patients were enrolled in 11 ICUs in Denmark, Switzerland, and Norway, with no patients enrolled in Iceland or Wales; 365 patients were allocated to the lower oxygenation group and 361 patients to the higher oxygenation group (Figure 1). The trial was stopped prematurely after a decision made by the steering committee based on a persistently low enrollment rate in the final year (eFigure 1 in Supplement 2). Data on the primary outcome were available for 697 (96.0%) of 726 patients (Figure 1). Patient characteristics at baseline were largely similar in the intervention groups, except for age and incidence of myocardial infarction (Table 1).
Figure 1. Flow of Participants in the HOT-COVID Trial.
aNumber of exclusions adds to more than total excluded because patients could have more than 1 reason for trial exclusion at screening.
bProvided data at baseline and for the analysis of serious adverse events.
cAll data were deleted on request of patients or next of kin in accordance with national regulations.
Table 1. Baseline Participant Characteristics.
| Characteristics | Lower oxygenation (n = 362)a | Higher oxygenation (n = 358)a |
|---|---|---|
| Age, median (IQR), y | 65 (54-73) | 66 (57-76) |
| Sex, No. (%) | ||
| Female | 109 (30.1) | 116 (32.4) |
| Male | 253 (69.9) | 242 (67.6) |
| Interval between hospital admission and randomization, median (IQR), d | 2 (1-4) | 2 (1-4) |
| Interval between intensive care unit admission and randomization, median (IQR), h | 3 (1-7) | 3 (1-7) |
| Coexisting illness, No. (%) | ||
| Ischemic heart disease | 38 (10.5) | 34 (9.5) |
| Chronic obstructive pulmonary disease | 28 (7.7) | 24 (6.7) |
| Active hematological malignancy | 26 (7.2) | 35 (9.8) |
| Long-term dialysis | 9 (2.5) | 8 (2.2) |
| Active metastatic cancer | 8 (2.2) | 8 (2.2) |
| Type of admission, No. (%) | ||
| Medical | 361 (99.7) | 357 (99.7) |
| Elective surgical | 0 | 0 |
| Emergency surgical | 1 (0.3) | 1 (0.3) |
| Acute illness, No. (%) | ||
| Pneumonia | 265 (73.2) | 258 (72.1) |
| Acute respiratory distress syndrome | 149 (41.2) | 144 (40.2) |
| Myocardial infarction | 7 (1.9) | 1 (0.3) |
| Cardiac arrest | 2 (0.6) | 5 (1.4) |
| Hemorrhagic or ischemic stroke | 1 (0.3) | 2 (0.6) |
| Intestinal ischemia | 2 (0.6) | 0 |
| Multiple trauma | 1 (0.3) | 0 |
| Invasive ventilation | ||
| Patients, No. (%) | 90 (24.9) | 82 (22.9) |
| Tidal volume, median (IQR), mL | 520 (450-608) | 515 (470-595) |
| End-expiratory pressure, median (IQR), cm H2O | 12 (10-14) | 12 (9-12) |
| Peak pressure, median (IQR), cm H2O | 25 (23-28) | 25 (25-28) |
| Noninvasive ventilation or CPAP | ||
| Patients, No. (%) | 41 (11.3) | 52 (14.5) |
| End-expiratory pressure, median (IQR), cm H2O | 8 (6-9) | 8 (6-9) |
| Treatment in an open system, No. (%) | 231 (63.8) | 224 (62.6) |
| Pao2, median (IQR), mm Hg | 70 (62-80) | 71 (62-84) |
| Sao2, median (IQR), % | 94 (91-96) | 94 (91-96) |
| Fio2, median (IQR)b | 0.80 (0.61-1.00) | 0.80 (0.62-1.00) |
| Pao2:Fio2 ratio, median (IQR) | ||
| In all systems | 91 (71-122) | 96 (74-124) |
| In closed systems | 99 (75-148) | 109 (80-149) |
| Lactate level, median (IQR), mmol/L | 1.4 (1-1.9) | 1.4 (1-2) |
| Lowest mean arterial pressure, median (IQR), mm Hgc | 72 (62-84) | 71 (62-83) |
| Use of vasopressors | ||
| Patients, No. (%) | 82 (22.7) | 69 (19.3) |
| Highest dose of norepinephrine, median (IQR), μg/kg/mind | 0.13 (0.07-0.34) | 0.12 (0.06-0.29) |
| SOFA scoree | 3 (2-5) [n = 357] | 3 (2-6) [n = 352] |
Abbreviations: CPAP, continuous positive airway pressure; Sao2, arterial oxygen saturation; Fio2, fraction of inspired oxygen.
Data on all baseline characteristics were missing for 3 patients in each group.
Fio2 in open systems was estimated using standardized conversion tables. See eTable 1 in Supplement 2.
Lowest median value of the mean arterial pressure recorded during the 24 hours before randomization.
In patients receiving norepinephrine at baseline.
Scores on the Sequential Organ Failure Assessment (SOFA) range from 0 to 24, with higher scores indicating more severe organ failure. Definitions of SOFA scoring in the HOT-COVID trial and single components of the baseline SOFA score are available in eTables 2 and 3 in Supplement 2.
Trial and Concomitant Interventions
During the 90-day intervention period, Pao2, Sao2, and Fio2 were lower in the lower oxygenation group compared with the higher oxygenation group (Figure 2; eFigures 2-4 and eTable 4 in Supplement 2). Data on the 12-hour highest and lowest Pao2 measurements with corresponding Sao2 and Fio2 values are presented in eFigures 5-7 in Supplement 2. Distributions of daily mean of all registered Pao2 values are presented in eFigure 8 in Supplement 2. Data on the use of mechanical ventilation, prone positioning, inhaled vasodilators, extracorporeal membrane oxygenation, circulatory support, kidney replacement therapy, and blood transfusions during the intervention period, as well as daily ventilator settings at 8 am, are shown in Table 2. A total of 110 (40.4%) of 272 patients in the lower oxygenation group and 133 (48.2%) of 276 patients in the higher oxygenation group who were not invasively mechanically ventilated at baseline received invasive mechanical ventilation in ICU at any time throughout the 90-day intervention period.
Figure 2. Values for Pao2, Sao2, and Fio2 According to Oxygenation Strategy.
Daily medians were calculated from patient-level daily means of the 12-hour lowest and highest Pao2, with concomitant values for arterial oxygen saturation (Sao2) and fraction of inspired oxygen (Fio2). Whiskers represent IQRs. A, For the entire 90-day intervention period, the higher oxygenation group had a median Pao2 of 91 mm Hg (IQR, 84-96 mm Hg), while the lower oxygenation group had a median Pao2 of 71 mm Hg (IQR, 68-75 mm Hg). B, For the entire 90-day intervention period, the higher oxygenation group had a median Sao2 of 95.5% (IQR, 94.3%-96.2%), while the lower oxygenation group had a median Sao2 of 92.9% (IQR, 91.9%-93.9%). C, For the entire 90-day intervention period, the higher oxygenation group had a median Fio2 of 0.68 (IQR, 0.57-0.81), while the lower oxygenation group had a median Fio2 of 0.54 (IQR, 0.45-0.69). Similar presentations of the full 90-day intervention period are presented in eFigures 2-4 and data on the number of patients contributing oxygenation data are presented in eTable 2 in Supplement 2.
Table 2. Primary and Secondary Outcomes and Intensive Care Unit Interventions.
| Outcomes | Lower oxygenation | Higher oxygenation | Bootstrapped mean difference (95% CI) or mean or risk difference (98.6% CI) | Risk ratio (98.6% CI) | P value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Days alive without life support over 90 d, median (IQR)a | 80 (9-89) | 72 (2-88) | .009b | ||
| Supplemental analysis adjusted for the stratification variable | 5.8 (0.2 to 11.5)c | .04c | |||
| Supplemental analysis adjusted for the stratification variable and baseline variablesd | 5.2 (0.0 to 10.4)e | .05e | |||
| Without mechanical ventilation | 71 (1-83) | 62 (0-80) | |||
| Without circulatory support | 86 (21-90) | 84 (11-90) | |||
| Without kidney replacement therapy | 90 (29-90) | 90 (22-90) | |||
| Secondary outcomes | |||||
| Death by day 90, No./total (%), adjusted for the stratification variable | 106/351 (30.2) | 120/346 (34.7) | −4.0 (−13 to 0.5) | 0.86 (0.66-1.13) | .18f |
| Days alive and out of hospital over 90 d, median (IQR), adjusted for the stratification variable | 59 (0-75) | 48 (0-74) | 5.1 (−1.2 to 11.4) | .03f | |
| Serious adverse events, No./total (%), adjusted for the stratification variable | 172/362 (47.5) | 185/358 (51.7) | −3.8 (−12.8 to 5.3) | 0.94 (0.79-1.13) | .43f |
| Shock | 172/362 (47.5) | 184/358 (51.4) | |||
| Myocardial ischemia | 2/362 (0.5) | 1/358 (0.3) | |||
| Ischemic stroke | 1/362 (0.3) | 1/358 (0.3) | |||
| Intestinal ischemia | 3/362 (0.8) | 1/358 (0.3) | |||
| Intensive care unit interventions | |||||
| No. of arterial blood gas samples per d, mean (SD) | 7(2) | 7(2) | |||
| Mechanical ventilation, No./total (%) | 256/362 (70.7) | 282/358 (78.8) | |||
| Invasive ventilation, No./total (%)g | 200/362 (55.2) | 215/358 (60.1) | |||
| Noninvasive ventilation or CPAP, No./total (%)g | 75/362 (20.7) | 84/358 (23.5) | |||
| Prone position, No./total (%) | 114/362 (31.5) | 147/358 (41.1) | |||
| Inhaled vasodilators, No./total (%)h | 23/362 (6.4) | 29/358 (8.1) | |||
| Extracorporeal membrane oxygenation, No./total (%) | 8/362 (2.2) | 8/358 (2.2) | |||
| Vasopressors or inotropes, No./total (%)i | 221/362 (61.0) | 238/358 (66.5) | |||
| Kidney replacement therapy, No./total (%)j | 45/362 (12.4) | 40/358 (11.2) | |||
| Red blood cell transfusion, No./total (%) | 59/362 (16.3) | 82/358 (22.9) | |||
| Volume, median (IQR), mLk | 729 (486-1800) | 936 (486-2100) | |||
| Invasive ventilation | |||||
| End-expiratory pressure, median (IQR), cm H2Og | 11 (9-13) | 12 (10-13) | |||
| Tidal volume, median (IQR), mL/kg of predicted body weightg,l | 7.5 (6.6-8.7) | 7.7 (6.6-8.8) | |||
| Peak pressure, median (IQR), cm H2Og | 24 (20-27) | 24 (21-27) | |||
| Noninvasive ventilation or CPAP | |||||
| End-expiratory pressure, median (IQR), cm H2Og | 8 (8-10) | 9 (7-10) | |||
Abbreviation: CPAP, continuous positive airway pressure.
Days alive without life support were defined as the absolute number of days alive without use of invasive ventilation, noninvasive ventilation, nonintermittent continuous positive airway pressure, vasopressor or inotropic infusion, or any kidney replacement therapy.
Primary analysis of the primary outcome, calculated using a van Elteren test adjusted for trial site.
Calculated using a bootstrapped general linear model adjusted for trial site.
Baseline variables were age, presence or absence of metastatic cancer, presence or absence of chronic obstructive pulmonary disease, presence or absence of hematological malignancy, and Sequential Organ Failure Assessment score, which ranges from 0 to 24, with higher scores indicating more severe organ failure.
Calculated using a bootstrapped general linear model adjusted for baseline variables.
For death by day 90, days out of hospital over 90 days, and serious adverse events, a multiplicity-adjusted P < .014 was considered statistically significant.
Ventilation parameters recorded once daily at 8 am.
Use of inhaled nitric oxide or inhaled epoprostenol.
Continuous infusion of norepinephrine, epinephrine, dopamine, phenylephrine, vasopressin, dobutamine, milrinone, or levosimendan.
Any continuous or intermittent use of kidney replacement therapy.
In patients receiving red blood cell transfusion.
Predicted body weight was calculated as 50 kg + 0.91 kg/cm × (height − 152.4 cm) for men and 45.5 kg + 0.91 kg/cm × (height − 152.4 cm) for women.
Primary Outcome
In the 90-day period, the median number of days alive without life support was 80.0 days (IQR, 9.0-89.0 days) in the lower oxygenation group and 72.0 days (IQR, 2.0-88.0 days) in the higher oxygenation group (P = .009 by van Elteren test; supplemental bootstrapped site-adjusted mean difference, 5.8 days [95% CI, 0.2-11.5 days]; P = .04) with zero and maximum-inflated distributions for both groups (eFigure 9 in Supplement 2). Similar results were found after adjustment for baseline risk factors (bootstrapped baseline-adjusted mean difference, 5.2 days [95% CI, 0.0-10.4 days]; P = .05) (Table 2). Post hoc analyses of the primary outcome penalized for death and additional post hoc statistical tests showed similar results (eTables 5 and 6 in Supplement 2), as did the preplanned per-protocol sensitivity analyses excluding patients according to deviations from the oxygenation targets (eTable 7 in Supplement 2). Intervention effects on the primary outcome were similar between trial sites (eFigure 15 in Supplement 2). Distributions of days alive without life support, days alive receiving life support, and death are shown in Figure 3A. The difference in numbers of days alive without life support was mainly driven by use of mechanical ventilation (eFigures 10-12 in Supplement 2). Subgroup analyses of the primary outcome showed a significant interaction for patients with shock at baseline with an increased number of days alive without life support in the lower oxygenation group (Figure 3B). The primary outcome was robust throughout the inclusion period (eAppendix 2 in Supplement 2).
Figure 3. Distribution of Use of Life Support and Death and Subgroup Analyses.
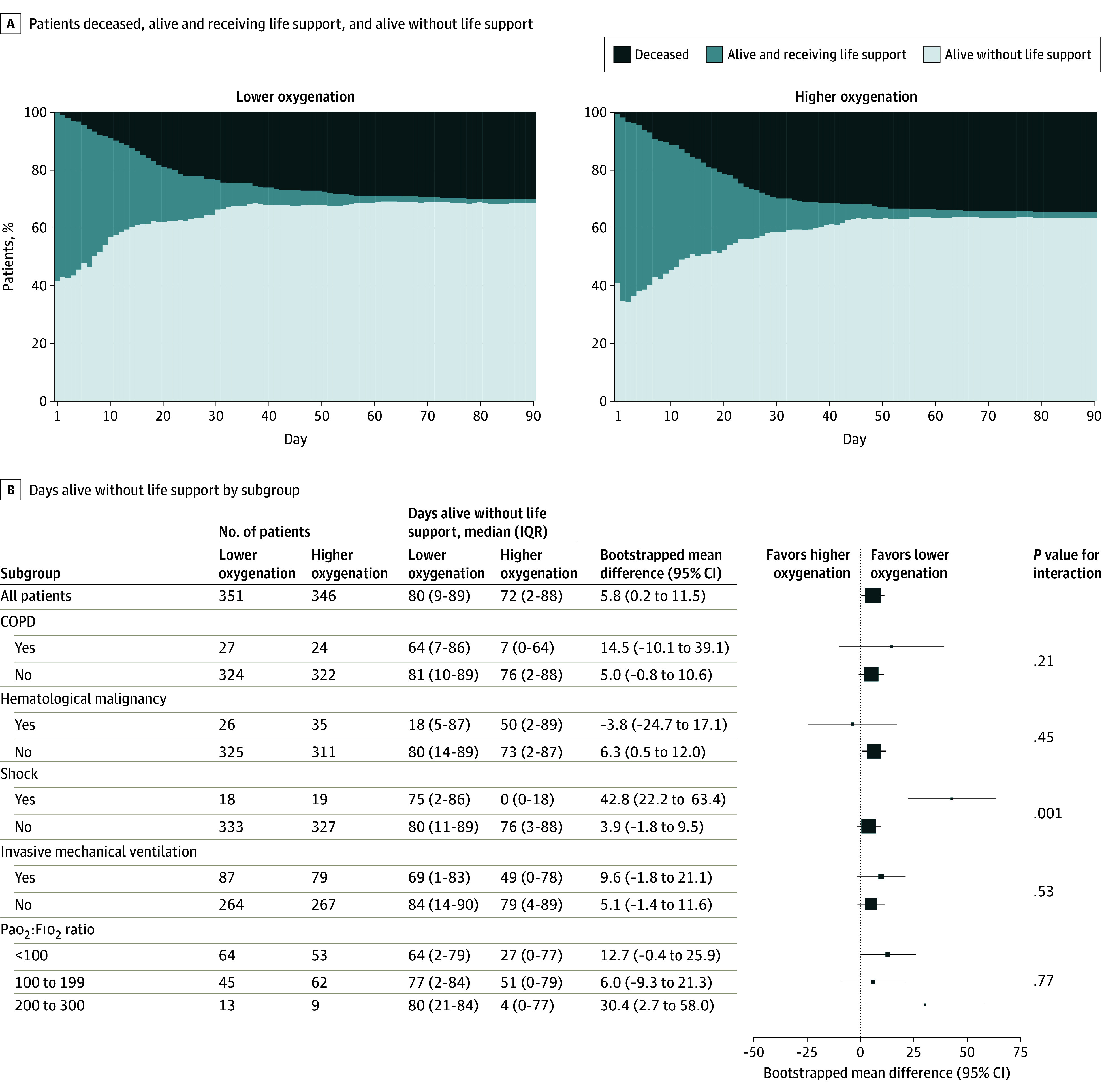
COPD indicates chronic obstructive pulmonary disease; Fio2, fraction of inspired oxygen; Sao2, arterial oxygen saturation. Panel A shows the percentages of patients who were deceased, alive and receiving life support, and alive without life support during the 90-day follow-up period. Panel B shows the results of the primary analysis and subgroup analyses of the primary outcome of days alive without life support over 90 days. All analyses were adjusted for the stratification variable of site. Mean differences and 95% CIs are based on a general linear model with an identity link, bootstrapped with 10 000 repetitions. The size of the data markers corresponds to the number of patients in each subgroup. A statistically significant interaction (P = .001) was found for heterogeneity of treatment effects for patients with or without shock at baseline.
Secondary Outcomes
At 90 days, 106 (30.2%) of 351 patients in the lower oxygenation group had died and 120 (34.7%) of 346 patients in the higher oxygenation group had died (risk ratio, 0.86 [98.6% CI, 0.66-1.13]; P = .18) (Table 2; eFigure 16 in Supplement 2). The numbers of days alive and out of hospital at 90 days and the proportion of patients with 1 or more serious adverse event in the ICU (ie, new episodes of shock, myocardial ischemia, ischemic stroke, or intestinal ischemia) did not differ significantly between the intervention groups (Table 2; eFigure 17 in Supplement 2).
Discussion
In this multicenter, randomized clinical trial in adult patients in the ICU with COVID-19 and severe hypoxemia, we observed more days alive without life support in 90 days in the lower oxygenation group, targeting a Pao2 of 60 mm Hg, compared with the higher oxygenation group, targeting a Pao2 of 90 mm Hg. The point estimates for days alive and out of hospital and mortality at 90 days also both favored the lower oxygenation target group. There was no difference in number of patients with serious adverse events in the ICU between the 2 groups.
More days alive without life support with the lower oxygenation target were similarly observed in the subgroup analysis of patients with COVID-19 in the HOT-ICU trial during the first wave of the pandemic in the spring of 2020,2,3 and the current trial supports these findings. The present result was hypothesized to occur due to more days alive without mechanical ventilation in the lower oxygenation group vs the higher oxygenation group. In contrary, no differences in ventilator-free days in non–COVID-19 patients were found between lower and higher oxygenation targets in the ICU-ROX trial,22 the HOT-ICU trial,2 and the ICONIC trial,5 or among lower, intermediate, and higher oxygenation targets in the PILOT trial.4 Also, no difference in mortality was found between higher and lower oxygenation strategies in adult patients in the ICU.6 The recent Oxy-PICU trial observed a lower duration of life support or incidence of death among pediatric ICU patients with a conservative oxygenation target vs a liberal oxygenation target.23 In a recent systematic review of 19 randomized clinical trials, patients had heterogeneous pathophysiological features of acute illnesses.6 In the present trial, all patients had severe hypoxemia caused by COVID-19 and predominantly single-organ failure at baseline, with the lowest baseline Pao2:Fio2 ratio among all randomized clinical trials investigating targeted oxygenation in the ICU.5,6 This homogeneity of the patients included in the present study may explain the beneficial effect of the lower oxygenation target.
In the present trial, less than 25% of patients received invasive mechanical ventilation at baseline compared with approximately 60% of the entire study population in the HOT-ICU trial.2 However, the use of invasive mechanical ventilation more than doubled during ICU stay for up to a maximum of 90 days but remained lower compared with the HOT-ICU trial. During the first wave of the COVID-19 pandemic, concerns for aerosolization with high-flow nasal oxygen probably led to more patients being mechanically ventilated,24 while a shift toward the use of open systems, including high-flow nasal oxygen, was observed in later waves.25 Consistently, the overall mortality at 90 days was more than 10 percentage points lower in patients enrolled in the present trial compared with those included in the first-wave COVID-19 subgroup analysis of the HOT-ICU trial.3 A finding also supported by a newly published systematic review.26 Importantly, although COVID-19 therapy and, possibly, virus subgroup virulence changed throughout the pandemic, in the post hoc interaction analysis we found no effect modification of date of inclusion on the primary outcome.
This trial has several strengths. It was a multicenter trial, and all participating ICUs had experience with handling the 2 oxygenation targets because of their participation in the HOT-ICU trial. Clear between-group separation in Pao2, Sao2, and Fio2 levels was achieved, and data were largely complete. Patients undergoing invasive mechanical ventilation, those undergoing noninvasive ventilation, and those treated in open systems at baseline were included, thus representing the clinical setting in the ICU.
Limitations
Our trial has several limitations. First, the trial was stopped prematurely due to slow enrollment, as patients with severe hypoxemia from COVID-19 toward the end of the pandemic were no longer commonly encountered in the ICU. However, the anticipated difference in days alive without life support based on the HOT-ICU subgroup analysis was achieved, and the data analyses were performed after the decision to stop the trial had been made. Second, clinicians were not blinded to the interventions, and there were no specific protocols for intubation or weaning from mechanical ventilation. However, it was stressed that the decision to intubate, wean, or extubate should not be dictated by the allocated oxygenation target but should be based on a clinical judgment of overall patient condition, including all factors relevant to the timing of intubation or extubation. The proportions of patients in whom invasive mechanical ventilation was initiated on day 1 or 2 were similar in the 2 oxygenation groups, suggesting that clinicians did not immediately intubate patients to obtain the higher oxygenation target. However, we acknowledge that oxygenation-related parameters affected by the allocation may have altered clinicians’ decisions, but in a pragmatic trial this should be considered as a consequence of the allocated intervention. Third, no adjustments for multiplicity on the basis of the conducted interim analysis were included; however, in the light of the results of the primary outcome analysis, the risk of a type I statistical error is low. Fourth, we do not have any information about the devices used for supplemental oxygen therapy in open systems nor any data on COVID-19 virus variants or COVID-19–specific treatments.
Conclusions
In adult patients in the ICU with COVID-19 and severe hypoxemia, targeting a Pao2 of 60 mm Hg resulted in more days alive without life support over 90 days than targeting a Pao2 of 90 mm Hg.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial Protocol
eAppendix 1. Supplemental Methods
eAppendix 2. Supplemental Results
eFigure 1. Trial Recruitment
eFigure 2. Pao2 by Treatment Group Over 90 Days
eFigure 3. Sao2 by Treatment Group Over 90 Days
eFigure 4. Fio2 by Treatment Group Over 90 Days
eFigure 5. Highest and Lowest Pao2 by Treatment Group
eFigure 6. Corresponding Sao2 by Treatment Group
eFigure 7. Corresponding Fio2 by Treatment Group
eFigure 8. Pao2 Measurements by Intervention Group
eFigure 9. Distribution of the Primary Outcome
eFigure 10. Difference in Mechanical Ventilation Over Time
eFigure 11. Difference in Circulatory Support Over Time
eFigure 12. Difference in Renal Replacement Therapy Over Time
eFigure 13. Cumulative Proportion of Invasive Mechanical Ventilation Over Time
eFigure 14. Cumulative Proportion of Any Type of Mechanical Ventilation Over Time
eFigure 15. Intervention Effects on the Primary Outcome by Trial Site
eFigure 16. Kaplan Meier Plots of Survival at 90 Days
eFigure 17. Distribution of Days Alive Out of Hospital in 90 Days
eTable 1. Fraction of Inspired Oxygen (Fio2) Conversion Tables for Open Systems
eTable 2. Sequential Organ Failure Assessment (SOFA) Scoring in the HOT-COVID Trial
eTable 3. Single Components of the Baseline SOFA Score
eTable 4. Number of Patients Contributing With Data on Pao2, Fio2, and Sao2
eTable 5. Days Alive Without Life Support in 90 Days Penalized by Death
eTable 6. Supplemental Mann-Whitney U Test and Proportional Odds Model
eTable 7. Per-Protocol Analyses of the Primary Outcome
eReferences
Nonauthor Collaborators. HOT-COVID Trial Group
Data Sharing Statement
References
- 1.Alhazzani W, Evans L, Alshamsi F, et al. Surviving Sepsis Campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219-e234. doi: 10.1097/CCM.0000000000004899 [DOI] [PubMed] [Google Scholar]
- 2.Schjørring OL, Klitgaard TL, Perner A, et al. ; HOT-ICU Investigators . Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384(14):1301-1311. doi: 10.1056/NEJMoa2032510 [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen BS, Klitgaard TL, Perner A, et al. Oxygenation targets in ICU patients with COVID-19: a post hoc subgroup analysis of the HOT-ICU trial. Acta Anaesthesiol Scand. 2022;66(1):76-84. doi: 10.1111/aas.13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semler MW, Casey JD, Lloyd BD, et al. ; PILOT Investigators and the Pragmatic Critical Care Research Group . Oxygen-saturation targets for critically ill adults receiving mechanical ventilation. N Engl J Med. 2022;387(19):1759-1769. doi: 10.1056/NEJMoa2208415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Wal LI, Grim CCA, Del Prado MR, et al. ; ICONIC Investigators . Conservative versus liberal oxygenation targets in intensive care unit patients (ICONIC): a randomized clinical trial. Am J Respir Crit Care Med. 2023;208(7):770-779. doi: 10.1164/rccm.202303-0560OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klitgaard TL, Schjørring OL, Nielsen FM, et al. Higher versus lower fractions of inspired oxygen or targets of arterial oxygenation for adults admitted to the intensive care unit. Cochrane Database Syst Rev. 2023;9(9):CD012631. doi: 10.1002/14651858.CD012631.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9(6):622-642. doi: 10.1016/S2213-2600(21)00218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563-572. doi: 10.1007/s00134-019-05590-5 [DOI] [PubMed] [Google Scholar]
- 9.Aswanetmanee P, Limsuwat C, Maneechotesuwan K, Wongsurakiat P. Noninvasive ventilation in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2023;13(1):8283. doi: 10.1038/s41598-023-35323-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226-2237. doi: 10.1007/s00134-020-06312-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 12.Calligaro GL, Lalla U, Audley G, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine. 2020;28:100570. doi: 10.1016/j.eclinm.2020.100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. ; HiFLo-Covid Investigators . Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(21):2161-2171. doi: 10.1001/jama.2021.20714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frat JP, Quenot JP, Badie J, et al. ; SOHO-COVID Study Group and the REVA Network . Effect of high-flow nasal cannula oxygen vs standard oxygen therapy on mortality in patients with respiratory failure due to COVID-19: the SOHO-COVID randomized clinical trial. JAMA. 2022;328(12):1212-1222. doi: 10.1001/jama.2022.15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mølgaard Nielsen F, Lass Klitgaard T, Crescioli E, et al. Handling oxygenation targets in ICU patients with COVID-19: protocol and statistical analysis plan in the HOT-COVID trial. Acta Anaesthesiol Scand. 2021;65(10):1497-1504. doi: 10.1111/aas.13956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Hopewell S, Schulz KF, et al. ; Consolidated Standards of Reporting Trials Group . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):e1-e37. doi: 10.1016/j.jclinepi.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 17.Haase N, Plovsing R, Christensen S, et al. Characteristics, interventions, and longer term outcomes of COVID-19 ICU patients in Denmark—a nationwide, observational study. Acta Anaesthesiol Scand. 2021;65(1):68-75. doi: 10.1111/aas.13701 [DOI] [PubMed] [Google Scholar]
- 18.Jakobsen JC, Tamborrino M, Winkel P, et al. Count data analysis in randomised clinical trials. J Biom Biostat. 2015;6(1):2155-6180. [Google Scholar]
- 19.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754-1758. doi: 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 20.Granholm A, Anthon CT, Kjær MN, et al. Patient-important outcomes other than mortality in contemporary ICU trials: a scoping review. Crit Care Med. 2022;50(10):e759-e771. doi: 10.1097/CCM.0000000000005637 [DOI] [PubMed] [Google Scholar]
- 21.Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol. 2014;14:120. doi: 10.1186/1471-2288-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackle D, Bellomo R, Bailey M, et al. ; ICU-ROX Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group . Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382(11):989-998. doi: 10.1056/NEJMoa1903297 [DOI] [PubMed] [Google Scholar]
- 23.Peters MJ, Gould DW, Ray S, et al. ; Oxy-PICU Investigators of the Paediatric Critical Care Society Study Group (PCCS-SG) . Conservative versus liberal oxygenation targets in critically ill children (Oxy-PICU): a UK multicentre, open, parallel-group, randomised clinical trial. Lancet. 2024;403(10424):355-364. doi: 10.1016/S0140-6736(23)01968-2 [DOI] [PubMed] [Google Scholar]
- 24.Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020;323(18):1839-1841. doi: 10.1001/jama.2020.4914 [DOI] [PubMed] [Google Scholar]
- 25.Haase N, Plovsing R, Christensen S, et al. Changes over time in characteristics, resource use and outcomes among ICU patients with COVID-19—a nationwide, observational study in Denmark. Acta Anaesthesiol Scand. 2022;66(8):987-995. doi: 10.1111/aas.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandel A, Leazer S, Alcover KC, et al. Intensive care and organ support related mortality in patients with COVID-19: a systematic review and meta-analysis. Crit Care Explor. 2023;5(3):e0876. doi: 10.1097/CCE.0000000000000876 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Supplemental Methods
eAppendix 2. Supplemental Results
eFigure 1. Trial Recruitment
eFigure 2. Pao2 by Treatment Group Over 90 Days
eFigure 3. Sao2 by Treatment Group Over 90 Days
eFigure 4. Fio2 by Treatment Group Over 90 Days
eFigure 5. Highest and Lowest Pao2 by Treatment Group
eFigure 6. Corresponding Sao2 by Treatment Group
eFigure 7. Corresponding Fio2 by Treatment Group
eFigure 8. Pao2 Measurements by Intervention Group
eFigure 9. Distribution of the Primary Outcome
eFigure 10. Difference in Mechanical Ventilation Over Time
eFigure 11. Difference in Circulatory Support Over Time
eFigure 12. Difference in Renal Replacement Therapy Over Time
eFigure 13. Cumulative Proportion of Invasive Mechanical Ventilation Over Time
eFigure 14. Cumulative Proportion of Any Type of Mechanical Ventilation Over Time
eFigure 15. Intervention Effects on the Primary Outcome by Trial Site
eFigure 16. Kaplan Meier Plots of Survival at 90 Days
eFigure 17. Distribution of Days Alive Out of Hospital in 90 Days
eTable 1. Fraction of Inspired Oxygen (Fio2) Conversion Tables for Open Systems
eTable 2. Sequential Organ Failure Assessment (SOFA) Scoring in the HOT-COVID Trial
eTable 3. Single Components of the Baseline SOFA Score
eTable 4. Number of Patients Contributing With Data on Pao2, Fio2, and Sao2
eTable 5. Days Alive Without Life Support in 90 Days Penalized by Death
eTable 6. Supplemental Mann-Whitney U Test and Proportional Odds Model
eTable 7. Per-Protocol Analyses of the Primary Outcome
eReferences
Nonauthor Collaborators. HOT-COVID Trial Group
Data Sharing Statement




