Abstract
In recent years, brain banks have become valuable resources for examining the molecular underpinnings of various neurological and psychological disorders including Alzheimer disease and Parkinson disease. However, the availability of brain tissue has significantly declined. Proper collection, preparation, and preservation of postmortem autopsy tissue are essential for optimal downstream brain tissue distribution and experimentation. Collaborations between brain banks through larger networks such as NeuroBioBank with centralized sample request mechanisms promote tissue distribution where brain donations are disproportionately lower. Collaborations between brain banking networks also help to standardize the brain donation and sample preparation processes, ensuring proper distribution and experimentation. Ethical brain donation and thorough processing enhances the responsible conduct of scientific studies. Education and outreach programs that foster collaboration between hospitals, nursing homes, neuropathologists, and other research scientists help to alleviate concerns among potential brain donors. Furthermore, ensuring that biorepositories accurately reflect the true demographics of communities will result in research data that reliably represent populations. Implementing these measures will grant scientists improved access to brain tissue, facilitating a deeper understanding of the neurological diseases that impact millions.
Keywords: Alzheimer disease, Brain banking, NeuroBioBank, Parkinson disease, Postmortem
INTRODUCTION
Brain banking is a process that involves collecting postmortem brain tissue samples, a practice that has been ongoing since the end of the 19th century (1). Since its beginning, the collecting and processing of brain tissue has changed drastically and became systematic in the 1960s. It has continued to evolve into what is practiced today in brain banks. Currently, the collecting, processing, and analysis of biospecimens such as brain tissue has transformed into a meticulous process, one that often involves many steps to ensure that the largest amount of optimal tissue is banked for distribution to researchers. Banked human brain tissue is a vital resource, as it provides unique insights into neurological diseases in ways that are not possible with non-invasive measures. As central nervous system (CNS) tissue can generally only be obtained after death (2), postmortem brain tissue serves as one of the only means for comparing changes in the brain with datapoints collected during life using other methodologies (e.g. cognitive assessments, plasma assays), enabling the comprehensive examination of neurological disease processes.
Because neurological disorders are leading causes of disabilities and death worldwide, there is an increasing demand for research on brain diseases (3). To assist research focused on better understanding these complex diseases, brain banks enable researchers to access postmortem human brain tissue from individuals with neurological diseases as well as healthy controls. Nevertheless, we argue that brain banking in the United States needs to become a more collective initiative and more widely utilized in the sciences to better serve the increasing demand for clinically and neuropathologically characterized postmortem tissue samples. Brain banking allows scientists to better understand the mechanistic insights in human neurological and psychiatric diseases (4). While some of the components of these diseases can be observed in other species, replicating complex neurologic diseases in non-human organisms is not possible. To understand the various brain diseases at molecular and cellular levels and advance the development of new therapeutic interventions, studies must be conducted using human antemortem and postmortem tissue (5). Adequate numbers of tissue samples must be available for neuroscientists and neuropathologists for these studies. The current processes for obtaining tissue samples in many institutions are complex and time-consuming, and this limits research productivity. Brain tissue availability is also often not sufficient for the existing scientific demand.
Therefore, enhancing productivity in brain banking operations is essential to improving brain tissue access for scientists. By conducting studies on comprehensively characterized human brain autopsy samples, there will be an ultimate reduction in the existing burden of neurological diseases through the identification and confirmation of effective therapeutic targets in neurological diseases.
BRAIN COLLECTION AND PROCESSING
Several challenges currently exist in brain banking and processing workflows. For one, autopsy rates have continued to decline in recent years from a high of 41.1% in 1964 to less than 5% in many hospitals today (6). This results in challenges for neuropathologists and scientists studying human diseases as there is a declining amount of tissue available for research. Another barrier lies in the significant financial cost involved in collecting human brain tissue. Published cost estimates of brain tissue approximate the cost of collecting a postmortem brain to be $10 000–$30 000 in the United States (4); this varies based on the geographical locations of the brain bank and the brain donor. There is also a large time commitment involved from the time of autopsy when the brain is removed and dissected to eventually having the brain stored properly in the biorepository. Brain bank biorepositories contain several different types of neural tissue samples (Fig. 1); each has its own conditions for long-term storage. The significant cost and time commitment involved in processing and banking postmortem brain tissue for research studies pose challenges for some institutions that lack the required funding and necessary personnel to properly obtain, process, and store postmortem human brain tissue samples. This creates a disparity between institutions and scientists that do not have sufficient access.
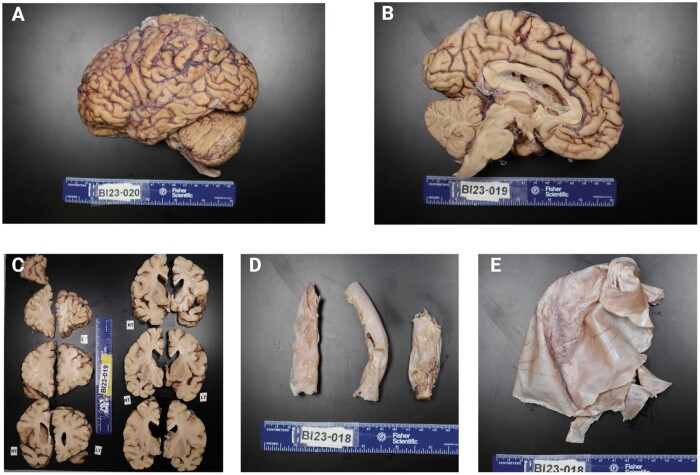
Figure 1.
Representative images of postmortem brain tissue from a formalin-fixed brain donation. (A) Lateral surface of the left hemisphere. (B) Medial surface of the left hemisphere. (C) Coronal sections of left (LT) and right (RT) sides. The top right section is the tip of the right frontal lobe for reference. (D) Sections of the cervical (left), thoracic (middle), and lumbar (right) spinal cord encased in dura mater. (E) Piece of the dura mater.
The collection and processing of brain tissue is complex and time-consuming. It specifically involves a series of well-defined and regulated steps to uphold diagnostic, scientific, and ethical standards. To initiate the brain donation process, a set of preparatory measures must be taken before death of the potential brain donor. Obtaining informed consent is a crucial prerequisite that may come from either the individual themselves directly or from their next of kin.
BRAIN BANKING NETWORKS AND COLLABORATIONS
There are several examples of successful brain biobanking repositories worldwide that have developed and established an organized and collaborative approach to brain banking. These individual brain banks have established comprehensive protocols that help to ensure consistency between brain samples before biospecimens are distributed to researchers.
One such example is the BrainNet Europe, or BNE Consortium, which was composed of 19 established brain banks across Europe. Financial support for BNE's initiatives was provided by the European Commission and the project has concluded its activities (7). It was a collaborative network of institutions founded to define the standard for quality, safety, and ethics regarding obtaining and handling human brain tissue. Furthermore, the overall objective of the BNE consortium was to share and distribute knowledge and information among neuroscientists (7). In addition to the BNE consortium, there are other nationally consolidated brain banks, such as the UK Brain Banks Network and the Australian Brain Bank, which house over 14 000 (8) and 10 000 brains, respectively (9).
Several years after the BNE was founded, the National Institutes of Health (NIH)-funded NeuroBioBank (NBB) was developed in the United States. The NBB is a joint initiative between 6 brain bank repositories from different institutions that have adopted aligned protocols for the collection, processing, and storing of the collected brain samples (Figs 2 and 3) (9). Although the United States has a larger number of brain banks, there is much less integration across US systems than there was for BNE. However, opportunities exist to create a larger collaborative US network in the future via the collaborative integration of new and existing biorepositories. Additionally, more brain tissue samples are becoming available through the consolidated national NBB, as each participating site of NBB collects approximately 100 brain autopsies per year (10). These brain donations have enabled samples from over 450 brain autopsy cases to be distributed to researchers. The NBB has also further collaborated with Autism BrainNet and Track-TBI, which are programs that utilize a collaborative network to transform clinical and research knowledge through the acquisition of human brain tissue samples (11). Collaboration between biorepositories that specialize in researching a variety of neurologic diseases will in turn create a greater sense of collaboration between researchers. For example, sample-derived data from Alzheimer disease (AD) brain autopsy cases may be compared with data from brain autopsy cases from individuals with Parkinson disease or Down syndrome to determine if there are any morphological or microscopic similarities across diseases (12). This can be further expanded in validation studies to stratify study groups of interest to contain large numbers of samples per study group.
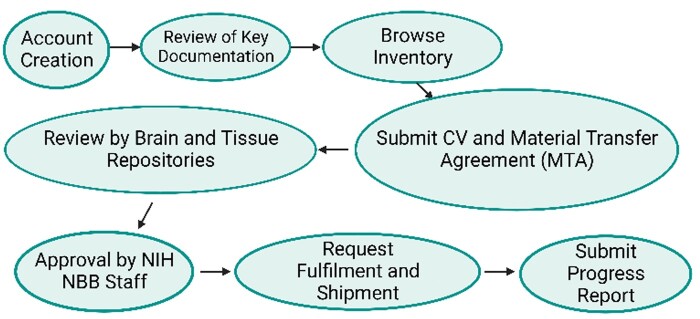
Figure 2.
Process for obtaining tissue from NeuroBioBank (Created with BioRender.com).
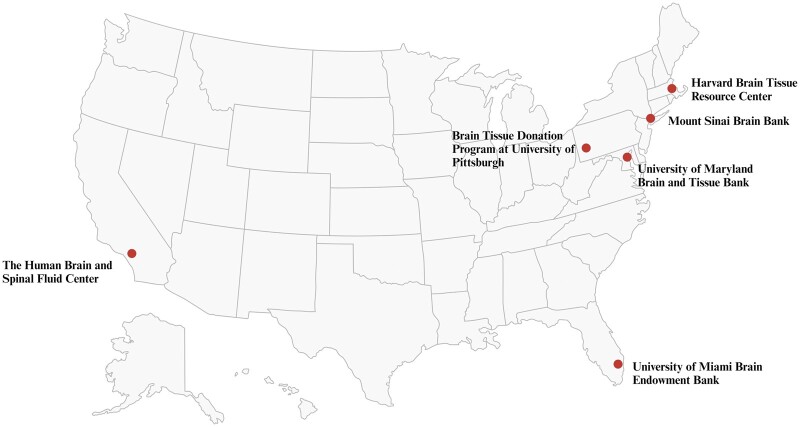
Figure 3.
Locations of participating institutions of the National Institutes of Health NeuroBioBank.
Internationally, there is a great need for collaboration to compile knowledge and promote improved and equitable access to brain specimens and resources to all neuroscience researchers (13). While there are surely legal, ethical, and societal restrictions in some areas regarding brain banking, having a high degree of international collaboration will ensure that the countries that cannot compile large numbers of brain tissue samples will have adequate access to tissue for their research. This will also promote increased scientific output for the global scientific community. Furthermore, international collaboration and increased scientific output will enable researchers to improve the general public’s current awareness and understanding of brain donation and tissue banking processes.
ETHICAL, LEGAL, AND SAFETY CONSIDERATIONS
International brain banks must always adhere to both national and ethical frameworks (14). Ethical and legal restrictions on brain banking may pose a barrier to the success of collecting and banking samples for research purposes. However, if neuropathologists take the appropriate measures to ensure consent, transparency, donor privacy, and proper documentation as defined in the BNE code of conduct (2), ethical and responsible brain banking is possible. While the BNE does not have the legal power to enforce regulations, it does serve as one of the first attempts to define ethical standards specific to brain banking in Europe that can easily be applied to other brain bank networks.
As organized brain banking operations are a relatively recent endeavor worldwide, laws are often ambiguous regarding which specific standard operating procedures (SOPs) biorepositories should utilize for the systematic collection, processing, storage, and distribution of tissue to researchers. If new legislation is proposed, it may also modify or restrict current brain bank operations and result in uncertainty for future operational processes (15). Obtaining informed consent from either the brain donor themselves before death or getting appropriate authorization from the next of kin is essential. The brain donation consent process provides a unique opportunity for the brain donor and/or their family members to be educated on what is involved procedurally in the brain donation itself in addition to providing information on any potential outcomes, risks, and benefits of the brain donation (16). Following the autopsy, the banked brain tissue samples are labeled with unique identifiers for de-identification and biospecimen tracking purposes, and clinical and neuropathologic information is linked to banked samples. This process ensures that the privacy of the donor, and their family is maintained while also utilizing necessary brain banking tools to support efficient biobanking procedures. This includes streamlining biospecimen inventory maintenance and enabling efficient laboratory information system sample queries to be performed. Efficient laboratory information system queries promote expedited biospecimen distribution to researchers and generate summary data on sample distributions for annual progress reports over specific periods (e.g. yearly NIH progress reports for AD center P30 grants in Neuropathology and Biospecimen Cores).
Family members of brain donors may proactively maintain their connection with the brain bank after the donation as it maintains a sense of connection to their recently lost loved one. Some brain donor family members may be interested in regularly following upcoming or ongoing research studies that are related to their relative’s neurologic or neuropathologic diagnosis. For example, after losing a loved one, donating their brain, and confirming a neuropathologic diagnosis of frontotemporal lobar degeneration-tau (Pick disease) with a corresponding clinical diagnosis of behavioral variant frontotemporal dementia (bvFTD)—family members may demonstrate increased interest in following studies focused on bvFTD such as the ARTFL-LEFFTDS Longitudinal Frontotemporal Lobar Degeneration study. Sharing neuropathologic diagnoses and clinicopathologic correlations with family members can ultimately help achieve a greater understanding of how impactful a single human brain donation can be. This may also help to increase overall community awareness and engagement in brain banking fundraising endeavors in addition to increasing brain donation advocacy.
Additionally, safety is a priority for brain banks. The banks themselves need to ensure that necessary safety precautions are taken as there are inherent risks involved when working with potentially infectious postmortem tissue in addition to the risks associated with exposures to the specific fixatives (e.g. formalin) used to preserve brain tissue (17). While each respective biorepository will likely have different protocols, universal safety precautions must be followed, as outlined by the Centers for Disease Control and Occupation Safety and Health Administration in the United States (18), or its equivalent agency in other countries. Protective barriers using personal protective equipment are worn at all times when handling biospecimens to minimize the risk of exposure to potentially hazardous materials for all personnel. Proper training of all laboratory personnel on the protocols used when obtaining and storing human brain tissue further ensures a safe working environment while also optimizing brain tissue sample quality (e.g. reducing postmortem interval time).
QUALITY CONTROL AND STANDARD OPERATING PROCEDURES
Quality control and SOPs are fundamental brain banking components. Among several established inter-institutional brain banking consortiums, there are established “best practices” to ensure that minimum biobanking standards are met. While individual protocols and procedures may vary across institutions, quality control must be prioritized. This is best achieved through the implementation of clearly defined SOPs for brain specimen collection, banking, sample characterization (e.g. standardized neuropathologic assessments), sample storage, inventory management, and biospecimen distribution.
The interval between time of death and brain removal/processing, i.e. postmortem interval, is a critical factor that requires careful navigation by brain banks (19). Striking a balance between achieving short postmortem interval times, which are optimal for preserving the biochemical integrity of tissues, and efficiently managing human resources is a complex task. Processing the brain promptly is desirable but maintaining consistent, around-the-clock coverage for brain removal and processing, especially in recently established Alzheimer’s Disease Research Centers (ADRCs), may present limitations. From an institutional standpoint, allocating full-time technical support for a low number of brain donations (e.g. 1–2 brains per month) may not seem financially viable; this requires ADRCs and brain banks to make more financially feasible plans for brain autopsy staff coverage. Additionally, while many brain banks consider postmortem interval time to be a significant indicator of tissue quality (5), some argue that the impact of postmortem interval time delays on biomolecular characteristics is minimal from a biomolecular perspective (20). For example, it has been shown that RNA quality is independent of postmortem interval times up to 36 hours (19). In the effort to establish standardization across institutions, it is essential to address the ongoing need for a clear rationale behind significantly shortened postmortem interval times and reach consensus on an acceptable brain removal timeframe after death to ensure tissues remain viable for molecular biological studies.
Additionally, environmental and internal conditions in which biospecimens are stored long-term must be optimized. Biospecimens should be stored at an optimal pH, temperature, and tissue fixative concentration. These conditions have a direct relationship with the quality of the tissue and can ultimately negatively impact results in later experiments and analyses if these conditions are not optimized for long-term brain tissue storage (21).
Privacy of brain donors when sharing data and biospecimens with researchers is also of utmost importance to protect the identity of the donor and their family members. Biospecimens must be appropriately de-identified prior to distributing brain tissue samples to researchers.
DATA COLLECTION, STANDARDIZATION, AND SHARING
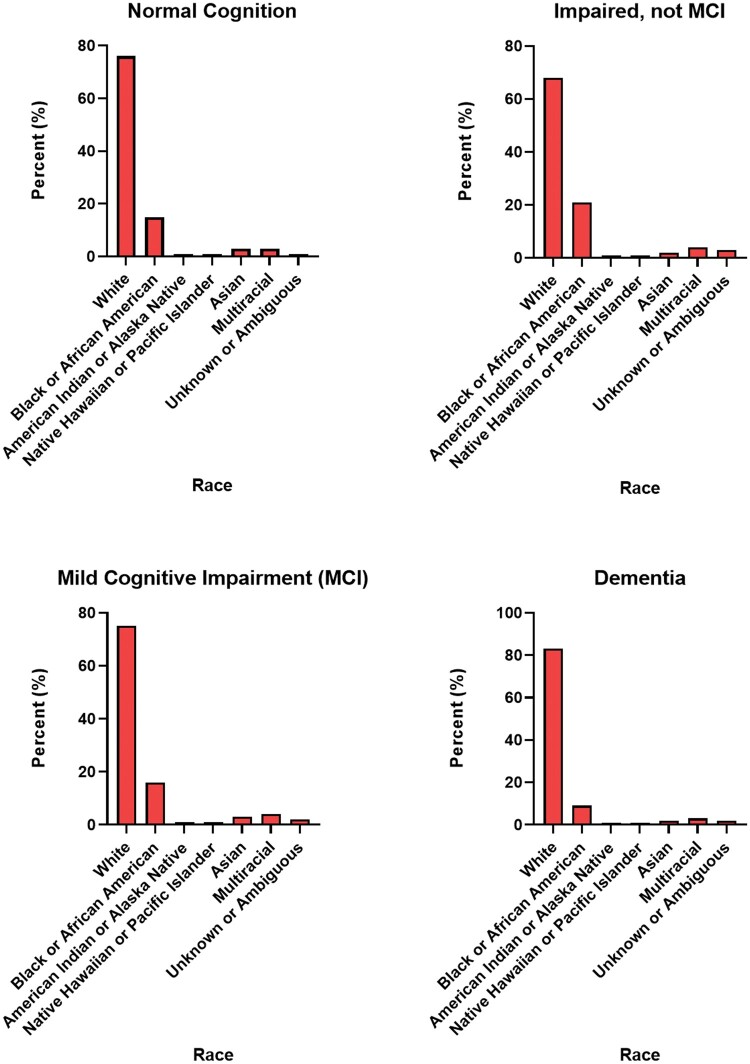
Several organizations have begun larger-scale collaborative data-sharing initiatives. For example, the National Alzheimer’s Coordinating Center (NACC) is an organization coordinated by the National Institute of Aging (NIA) that specifically aims to distribute data from multiple brain banks. NACC data have been collected from over 47 000 research participants at 33 Alzheimer’s Disease Centers (ADCs) and 4 exploratory ADCs (22). NACC houses and maintains valuable data including neuropathology information and clinical data in the Uniform Data Set (UDS). The UDS contains longitudinal clinical data from thousands of clinical research participants; a subset of deceased research participants who donated their brains also has neuropathologic data available. Data collected from research participants that are housed by NACC are from individuals with normal cognition, mild cognitive impairment, and dementias. This dataset also contains clinical variables that were generated from over 174 000 clinical assessments (1–17 clinical assessments with a median of 3 clinical assessments per research participant) (23). Moreover, the NACC dataset specifically contains neuropathologic variables generated from over 7340 standardized neuropathologic assessments of postmortem human brains (58% of the total deceased research participants). NACC also stores magnetic resonance imaging (MRI) and positron emission tomography (PET) neuroimaging data. One potential limitation of the NACC, however, is that the data are not representative of the demographics of the US population. Rather, the sampling is based on referral or volunteer donation and does not reflect the prevalence of these diseases in the general population (Fig. 4).
Figure 4.
Percent of tissue in National Alzheimer’s Coordinating Center data by race.
NACC additionally partners with several other organizations to further provide data and specimens to researchers. The National Centralized Repository for Alzheimer’s Disease and Related Dementias provides biological specimens, and the NIA Genetics of Alzheimer's Disease Data Storage Site provides genetic data. These three organizations partner together with ADRCs nationally to compile a large amount of data for scientists to utilize in analyses. Researchers interested in tissue samples or data can make requests using the respective applications that are available online; multiple types of data collected from a single individual are often available (22).
However, while many institutional brain banks understand the value of collaboration and data standardization, practical implementation of these principles remains deficient. In a study examining brain banking operation and characterization practices, only 8 out of the 24 brain banks had data standardization practices in place (5). Additionally, in this study, it was found that defining an ideal non-diseased control was a significant challenge for brain banks, further emphasizing the crucial need for the standardization of clinically and neuropathologically classifying control brains.
TECHNOLOGICAL INNOVATIONS
While novel approaches to study the diseases of the brain are becoming more common, clinically and neuropathologically characterized postmortem human brain samples are still one of the most informative resources for researchers to utilize when studying neurological disorders and healthy aging. Furthermore, many of these novel models are only available at a limited number of institutions, which creates a disparity in scientists’ ability to conduct their studies.
As biochemical “Omic” techniques such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, and neuroimaging are becoming more common and readily available, it is essential that brain banking become a more widely used practice to generate the necessary biospecimens to support these types of experiments (23). To ensure that the samples obtained from different brain regions of a single subject can be used for diverse research applications, brain banks can employ a range of preservation and processing techniques. An example is the use of formalin fixation, cryoprotection freezing, and fresh freezing for the storage of tissue samples collected from each brain autopsy.
Omic technologies identify susceptibility genes, relevant disease-specific pathways, and novel biomarkers for neurological diseases (Fig. 5). Detection of dysregulated pathways that are not present in brain samples obtained from cognitively normal individuals enables the identification of new therapeutic targets while also contributing to a personalized medicine approach to dementia prevention and intervention (23). Comparisons can be made between regional quantitative postmortem neuropathological data derived from postmortem brain autopsies and antemortem data collected from neuropsychological assessments, neurological examinations, neuroimaging studies, and fluid biomarkers (e.g. CSF or plasma tau). Making such comparisons to identify significant correlations enables scientists to further delineate pathogenic mechanisms underlying neurological diseases (23).
Figure 5.
Illustration of the role of brain banking in advancing Multiomic technologies. [1] Study Design involves stratifying disease groups while aiming to minimize confounding factors. [2] Samples from selected individuals are collected and processed depending on the intended detection method for the tissue or extraction. [3] Unbiased statistical analysis is carried out on generated and established Multiomic data allowing for integration and biological interpretation. [4] Disease subtyping and biomarker prediction are achieved by correlating stratified clinical data with Multiomic profiles which include expression data, copy number variation data, gene mutations, as well as pathway/network activation information (created with BioRender.com).
In amyotrophic lateral sclerosis (ALS), much of the research that has been done over the past 20 years has predominantly used in vivo imaging studies such as PET or MRI to identify the various neuropathological changes (24–26). Despite many technological advances, brain imaging studies continue to lack the necessary resolution to be able to comprehensively assess the cellular and molecular structural changes in disease states in the brain and spinal cord (24). Without access to sufficient clinically and neuropathologically characterized postmortem biospecimens from brain banks, scientists are unable to perform necessary experiments to validate in vivo methods in the human CNS.
Digital pathology (DP) has also been increasingly used in recent years. A PubMed search for “digital pathology” yielded results showing that the numbers of publications nearly doubled from 2013 to 2022, i.e. from 1267 to 2396. Technological advances have enabled objective and in-depth comparisons to be made through the quantification of neuropathological features from whole slide images generated from microscopic slides. With more powerful computing and improved quantitative DP algorithms, large-scale analyses can now be performed on whole slide images that were previously not feasible.
With increased DP use across the research community, brain banks may also compile large numbers of whole slide images that can be used for multiple different types of quantitative neuropathological analyses. Whole slide images are a powerful research tool for many reasons. Whole slide imaging files are created by scanning microscope slides with stained tissue sections. Although physical microscope slides develop faded staining over time, this does not occur with whole slide image files for the optimal preservation of the sample for future teaching or research purposes. Additionally, whole slide images can be shared an unlimited number of times without having to worry about prioritizing research requests in an attempt to avoid exhausting a non-renewable tissue resource (e.g. hippocampal tissue requests).
Despite the promising potential of DP, its incorporation into institutions may face constraints due to infrastructure challenges. A survey conducted in 33 ADCs within the NIH revealed that the majority had access to a digital slide scanner, with Aperio/Leica being the most commonly mentioned brand (27). However, some Centers noted associated fees for scanner use. Less than half reported no support from their ADCs regarding DP and machine learning (ML) resources and only half received institutional support for slide scanner purchases. There was uncertainty about the file size of the scanned images and the storage space occupied by the scans. A significant percentage were aware of other departments utilizing machine learning. It was concluded that a substantial number of ADCs had the opportunity to use digital slide scanners. Further investigations are warranted to better comprehend the obstacles hindering the implementation of DP and ML workflows (27). In comparison to a pathological workflow that involves just brain tissue, implementing digital DP into existing/novel workflows requires additional equipment, properly trained staff, and well-defined quality control procedures (28). Above all, while DP has the potential to be greatly beneficial, it cannot be a replacement for physical brain tissue (29).
Distributing stained or unstained slides containing tissue sections of interest is easier than distributing whole brain or partial brain samples to researchers. The distribution of unstained brain slides enables researchers to perform staining experiments while reducing the equipment and materials needed to carry out the research study of interest. For example, researchers do not need to each have their own microtome to cut their tissue sections. Distributing physical slides to scientists provides the potential additional benefit to brain banks of having the opportunity to collaborate with the receiving scientist to obtain whole slide images from their stained slides for long-term storage, redistribution, and future analyses. Over time, brain banks can easily collect numerous whole slide images for integration into their brain bank. In summary, “digital brain banking” is an emerging area with substantial research potential for brain banks and other tissue banks. While DP does require large amounts of data storage and computer processing power, the potential benefits far outweigh the drawbacks. For example, whole slide images can be shared with requesting scientists in a matter of minutes versus taking days to months to distribute physical brain tissue samples. This is because exhaustible tissue resources require more extensive committee review prior to distribution. Taken together, DP reduces the timeline of acquiring necessary research materials to increase research efficiency.
In response to the diverse techniques employed in research projects, laser capture dissection has been examined within brain banks, including BrainNet Europe. This method has been confirmed as a viable approach for sampling, but its limitations are still being assessed (30). Ongoing research seeks to determine the most effective laser capture dissection method, as well as its optimal integration with freezing techniques and preservation methods, to yield the most valuable cellular-level data (31).
Utilizing computer technology biorepository management software has been designed for precise sample tracking, effective study/trial management, and comprehensive freezer inventory control. This software is being employed by several brain banks, globally.
NEURODEGENERATIVE DISEASES AND BRAIN BANKING
Neurodegenerative diseases such as AD are leading causes of death worldwide (32). Brain banking has been crucial for elucidating the molecular mechanisms and etiology of these diseases. Because CNS tissue samples are generally limited to being obtained after death, the need for brain banks continues to grow to be able to feasibly provide adequate tissue samples to neurodegenerative disease researchers (2). Despite technological innovations such as MRI, genetic, and biomarker studies, brain banking offers researchers an essential validation method through histology, cellular, biochemical, and molecular techniques that can only be achieved by analyzing clinically and neuropathologically characterized postmortem human brain tissue (33).
There are large numbers of studies of neurodegenerative diseases using animal models as there are several advantages to utilizing animal models alongside characterized human brain samples. Animal models allow for the manipulability of certain traits or aspects of diseases. For example, mutations in APP, PSEN1, and PSEN2 can be replicated in mouse models to better understand mechanisms of AD (34). However, although they have the ability to recapitulate the proteinopathy and/or pathological features of diseases of interest, they are only able to replicate a small portion of the complex human disease processes (34).
PSYCHIATRIC DISORDERS AND BRAIN BANKING
As with dementing diseases such as AD and FTD, analysis of postmortem tissue enables researchers of psychiatric diseases to understand conditions such as schizophrenia, bipolar disorder, and depression. It has been previously noted that both the etiology and pathophysiology of psychiatric disorders are not fully understood (14). Because of this, there is an increasing demand for postmortem tissue samples from neuropsychiatric brain banks for the application of modern techniques to identify changes at cellular and molecular levels.
In this regard, in the United States, three brain banks have followed the models of brain banking that have been developed in Europe. The NIMH Brain Tissue Collection, the Mt. Sinai School of Medicine Alzheimer's Disease Brain Bank, the Schizophrenia Brain Bank, and Harvard Brain Tissue Resource Center are pioneering the acquisition, banking, and distribution of postmortem brains for psychiatric research. Importantly, two of these institutions are part of the NBB, which houses thousands more brains for other neurological diseases. While there may not be as many psychiatric brains currently available in brain banks, having these cases housed within these large repositories allows for easier distribution of such biospecimens through an already established network. One major advantage of having large brain banks as opposed to many small institutional biorepositories, is the standardization of clinical characterization, neuropathology characterization, toxicology, and brain dissection and slide preparation protocols (35). These larger institutions have established a larger presence through their donations, and establishing several smaller individual biorepositories instead results in more challenges when providing adequate samples to outside researchers.
OTHER DISEASE-FOCUSED BRAIN BANKS
The UNITE brain bank centered in Boston is the largest tissue repository in the world devoted to traumatic brain injury and chronic traumatic encephalopathy. It houses over 1400 brains in addition to spinal cords and eyes. It is known for studying the brains of high-profile football players and is presently focused on clinical, pathological, and molecular research (36).
The National Posttraumatic Stress Disorder (PTSD) Brain Bank led by the Veterans Association (VA) is part of the VA’s National Center for PTSD. It is based in Vermont, with reception, assessment, and research sites elsewhere. It receives brains from veterans and non-veterans and accepts non-military-related PTSD cases. In addition to PTSD, it also collects and preserves CNS tissues related to non-diseased donors, ALS, and disorders of Veterans of the 1990–1991 Gulf War (37).
EDUCATION AND OUTREACH
Collaboration between different fields is a necessary component of successful brain banking. Collaboration between clinicians, hospitals, nursing homes, pathologists, autopsy technicians, and scientists will promote necessary outreach and education initiatives to promote brain donation in various populations (13). To meet the growing demand for CNS tissue in brain banking, comprehensive and personalized public education and outreach measures are essential. Collaboration between scientific professionals and neuropathologists ensures that potential donors are well-versed in how their tissue will make an invaluable impact on the scientific community. For example, the NBB has an affiliated outreach program that serves to educate the general public about the benefits of brain donation. The Brain Donor Project (BDP) outlines the main procedure to donate a brain, from signing up to the autopsy itself (38). It is also possible that brain donation may also be included as one of the options for organ donation for individuals who opt to join national organ donation registries (39). In 2022 alone, there were a total of 14 903 individuals who became deceased organ donors marking 12 consecutive years of increased organ donation (40). Had these individuals donated their brains as well, there would be thousands more tissue samples available for research.
Additionally, there is another source of brain tissue that could aid in providing more brains for biorepositories in future years. Medical examiners (MEs) and coroner's offices are often overlooked in their potential for easing the ever-decreasing rates of hospital brains available for research. These offices provide a plentiful supply of potential brain donations. ME populations of are additionally racially diverse and are generally thought to be representative of the demographics of the area of the particular ME (41). Being able to bank adequate samples that are closely representative of a particular population reduces the donation disparities within the brain bank. However, there are often times restriction placed upon MEs that prevent them from being able to readily share their biospecimens with brain banks and other biorepositories. In the United States, there is not a centralized standard operating procedure on the national level for the donation of postmortem brain tissue from MEs. Rather, this varies between county-based, district-based, and centralized state ME offices (42). In the future, MEs may have a crucial role with the medical and research communities’ shared ethical responsibility to promote brain donation to meet the ever-increasing demand for tissue in brain banks. They have the unique opportunity to work as liaisons between the government and biorepositories in order to reduce the medico-legal restrictions that currently hinder donation (43).
In addition to the donation of diseased brain tissue, the donation of non-diseased “control” brain tissue is also essential. These brains are primarily used as a control to compare the data from the diseased brain. Control brains are also advantageous to help highlight age-related processes that occur in the healthy brain. In return, this allows researchers to deduce which specific aspects of age-related decline are associated with diseases such as AD and which specific aspects are associated with normal age-related neurodegeneration (44).
One ongoing challenge that continues to impact brain banks worldwide is the fact that certain populations are underrepresented in the number of available banked biospecimens. According to the NACC dataset, most brain donors to date have been Caucasian. This is true across all neurological classifications as illustrated in Figure 4. This is a common problem posing a risk to generating generalized data and conclusions, i.e. there is limited availability of biospecimens that accurately represent the entire population. To overcome this, there have been increasing numbers of specialized outreach and education programs nationally that aim to increase the donations of specific underrepresented populations. Outreach and education programs can develop the most effective materials for each unique community to be able to best educate individuals. Tailored comprehensive outreach programs for brain banking will ultimately increase diversity within brain banks moving forward. It is important to have diversity within a large cohort, as some conditions may be more prevalent in a particular group.
Common arguments used against brain donation are often associated with the high cost, religious compatibility concerns, and how the donation might affect funeral arrangements. In the case of the NBB, several measures are taken to ensure that these concerns are proactively addressed. For example, to address the known significant costs that are associated with brain donation, in NBB-there is no cost to the donor or family of the donor. At the time of autopsy, the NBB has specialists that will remove the brain via the posterior aspect of the head to ensure that there is no disfigurement (45). Additionally, a neuropathological summary of the diseased brain can be provided to the family of the donor upon request.
CONCLUSION
In the coming years, as neurodegenerative diseases continue to pose physical and financial burdens on society, brain banking will continue to offer a multitude of opportunities to effectively reduce the impact of these diseases through research and discovery. Collaboration between intra-institutional brain banks, inter-institutional brain banks, and other biorepositories will further help to reduce the existing inequality of access to postmortem human brain tissue for research studies. With the advent of novel technology growing rapidly in the coming years, brain banking as a traditional approach can continue to be utilized to validate and support emerging novel quantitative scientific methods. Taken together, we have summarized the importance of collective action to increase the donations and storage of brains in brain banks to better understand neurological diseases that are a burden to millions of people worldwide.
Contributor Information
Benjamin Danner, Biggs Institute, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA.
Angelique D Gonzalez, Biggs Institute, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA.
William Cole Corbett, Biggs Institute, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA.
Mohammad Alhneif, Biggs Institute, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA.
Shahroo Etemadmoghadam, Biggs Institute, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA.
Julie Parker-Garza, Biggs Institute, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA.
Margaret E Flanagan, Biggs Institute, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA; Department of Pathology, University of Texas Health Science Center San Antonio, San Antonio, Texas, USA.
FUNDING
This work was supported by grants K08AG065463 (PI Flanagan), U24NS133945 (PIs Nelson, Flanagan, Bumgardner), RF1AG072080 (PIs Flanagan, Popko), R01AG082118 (PIs Cheng, Flanagan, Pieper), and P30AG066546 (PIs Seshadri, Maestra) from the National Institutes of Health. Additionally, this work was supported by funding from the Owen’s Foundation Grant (PI Flanagan) and Baptist Foundation of San Antonio Endowment (Flanagan).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1. Kretzschmar H. Brain banking: Opportunities, challenges and meaning for the future. Nat Rev Neurosci 2009;10:70–8 [DOI] [PubMed] [Google Scholar]
- 2. Klioueva NM, Rademaker MC, Dexter DT, et al. BrainNet Europe’s Code of Conduct for brain banking. J Neural Transm (Vienna) 2015;122:937–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feigin VL, Vos T, Nichols E, et al. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol 2020;19:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hulette CM. Brain banking in the United States. J Neuropathol Exp Neurol 2003;62:715–22 [DOI] [PubMed] [Google Scholar]
- 5. Palmer-Aronsten B, Sheedy D, McCrossin T, et al. An international survey of brain banking operation and characterization practices. Biopreserv Biobank 2016;14:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasson J, Schneiderman H.. Autopsy training programs. To right a wrong. Arch Pathol Lab Med 1995;119:289–91 [PubMed] [Google Scholar]
- 7. Bank NB. Netherlands Brain Bank. Brain Net Europe. Available at: https://www.brainbank.nl/about-us/brain-net-europe/. Accessed August 31, 2023
- 8. Gilbert V. World MS Day. Brain banks: Advancing neurological research worldwide. 2018. Available at: https://worldmsday.org/brain-banks-advancing-neurological-research-worldwide/. Accessed September 21, 2023
- 9. Europe Leads the Way Toward Standardization and Brain Bank Networks | ALZFORUM [Internet]. Available at: https://www.alzforum.org/news/community-news/europe-leads-way-toward-standardization-and-brain-bank-networks. Accessed August 31, 2023
- 10. National Institute of Neurological Disorders and Stroke [Internet]. The NIH NeuroBioBank: Addressing the Urgent Need for Brain Donation. 2017. Available at: https://www.ninds.nih.gov/news-events/directors-messages/all-directors-messages/nih-neurobiobank-addressing-urgent-need-brain-donation. Accessed September 21, 2023
- 11. Zielke HR, Mash DC, Chapter 6 - A review of brain biorepository management and operations. In: Huitinga I, Webster MJ, eds. Handbook of Clinical Neurology, vol. 150. 2018:83-92 [DOI] [PubMed] [Google Scholar]
- 12. Mullins D, Daly E, Simmons A, et al. Dementia in Down’s syndrome: An MRI comparison with Alzheimer’s disease in the general population. J Neurodev Disord 2013;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravid R, Park YM.. Brain banking in the twenty-first century: Creative solutions and ongoing challenges. Bsam 2014;2:17–27 [Google Scholar]
- 14. Schmitt A, Parlapani E, Bauer M, et al. Is brain banking of psychiatric cases valuable for neurobiological research? Clinics (Sao Paulo) 2008;63:255–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huitinga I, de Goeij M, Klioueva N.. Legal and ethical issues in brain banking. Neurosci Bull 2018;35:267–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah P, Thornton I, Turrin D, et al. Informed consent. In: StatPearls [Internet]. Treasure Island (FL: ): StatPearls Publishing; 2023. Available at: http://www.ncbi.nlm.nih.gov/books/NBK430827/. Accessed September 28, 2023 [Google Scholar]
- 17. Ervin JF. Banking tissue for neurodegenerative research. In: Alzate O, ed. Neuroproteomics [Internet]. Boca Raton, FL: CRC Press/Taylor & Francis; 2010, Chapter 2. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56014/ [PubMed] [Google Scholar]
- 18. Perspectives in Disease Prevention and Health Promotion Update: Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and Other Bloodborne Pathogens in Health-Care Settings [Internet]. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00000039.htm. Accessed September 28, 2023
- 19. White K, Yang P, Li L, et al. Effect of postmortem interval and years in storage on RNA quality of tissue at a repository of the NIH NeuroBioBank. Biopreserv Biobank 2018;16:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ervin JF, Heinzen EL, Cronin KD, et al. Postmortem delay has minimal effect on brain RNA integrity. J Neuropathol Exp Neurol 2007;66:1093–9 [DOI] [PubMed] [Google Scholar]
- 21. Vonsattel JPG, Amaya M, del P, et al. 21st Century Brain Banking Practical prerequisites and lessons from the past: The experience of New York Brain Bank—Taub Institute—Columbia University. Cell Tissue Bank 2008;9:247–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NACC’s partnerships in genetics and imaging | National Alzheimer’s Coordinating Center [Internet]. Available at: https://naccdata.org/nacc-collaborations/partnerships#genotypicData. Accessed September 28, 2023
- 23. Carlos AF, Poloni TE, Medici V, et al. From brain collections to modern brain banks: A historical perspective. Alzheimers Dement (N Y) 2019;5:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazumder S, Kiernan MC, Halliday GM, et al. The contribution of brain banks to knowledge discovery in amyotrophic lateral sclerosis: A systematic review. Neuropathol Appl Neurobiol 2022;48:e12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esopenko C, Levine B.. Aging, neurodegenerative disease, and traumatic brain injury: The role of neuroimaging. J Neurotrauma 2015;32:209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veldsman M, Egorova N.. Advances in neuroimaging for neurodegenerative disease. Adv Neurobiol 2017;15:451–478 [DOI] [PubMed] [Google Scholar]
- 27. Scalco R, Hamsafar Y, White CL III, et al. The status of digital pathology and associated infrastructure within Alzheimer’s Disease Centers. J Neuropathol Exp Neurol 2023;82:202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aeffner F, Zarella MD, Buchbinder N, et al. Introduction to digital image analysis in whole-slide imaging: A white paper from the Digital Pathology Association. J Pathol Inform 2019;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The challenges of digital pathology [Internet]. Available at: https://healthcare-in-europe.com/en/news/healthcare-in-europe.com/en/news/the-challenges-of-digital-pathology.html. Accessed cited December 28, 2023
- 30. Almeida D, Turecki G.. Profiling cell-type specific gene expression in post-mortem human brain samples through laser capture microdissection. Methods 2022;207:3–10 [DOI] [PubMed] [Google Scholar]
- 31. Meyronet D, Dorey A, Massoma P, et al. The workflow from post-mortem human brain sampling to cell microdissection: a Brain Net Europe study. J Neural Transm (Vienna) 2015;122:975–91 [DOI] [PubMed] [Google Scholar]
- 32. The top 10 causes of death [Internet]. Available at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed September 14, 2023
- 33. Lin CY, Sawa A, Jaaro-Peled H.. Better understanding of mechanisms of schizophrenia and bipolar disorder: From human gene expression profiles to mouse models. Neurobiol Dis 2012;45:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dawson TM, Golde TE, Tourenne CL.. Animal models of neurodegenerative diseases. Nat Neurosci 2018;21:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Psychiatric Brain Banking: Three Perspectives on Current Trends and Future Directions—PMC [Internet]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3105380/. Accessed August 31, 2023 [DOI] [PMC free article] [PubMed]
- 36. UNITE Brain Bank | CTE Center [Internet]. Available at: https://www.bu.edu/cte/brain-donation-registry/brain-bank/. Accessed October 30, 2023
- 37. The Department of Veterans Affairs Biorepository Brain Bank [Internet]. Available at: https://www.research.va.gov/programs/specimen_biobanking.cfm. Accessed October 30, 2023
- 38. Brain Donor Project [Internet]. What To Expect. Available at: https://braindonorproject.org/what-to-expect/. Accessed September 28, 2023
- 39. The Lancet Neurology. Brain banking: More effective strategies needed. Lancet Neurol 2013;12:1035. [DOI] [PubMed] [Google Scholar]
- 40. 2022 organ transplants again set annual records; organ donation from deceased donors continues 12-year record-setting trend—OPTN [Internet]. Available at: https://optn.transplant.hrsa.gov/news/2022-organ-transplants-again-set-annual-records-organ-donation-from-deceased-donors-continues-12-year-record-setting-trend/. Accessed September 28, 2023
- 41. Mighdoll MI, Hyde TM, Chapter 11 - Brain donation at autopsy: Clinical characterization and toxicologic analyses. In: Huitinga I, Webster MJ, eds. Handbook of Clinical Neurology [Internet]. Elsevier; 2018:143–54 (Brain Banking; vol. 150). Available at: https://www.sciencedirect.com/science/article/pii/B9780444636393000116. Accessed December 28, 2023 [DOI] [PubMed] [Google Scholar]
- 42. Becker M, Berry M, Boyd J.. Guide to medical examiner & coroner cases. American Association of Tissue Banks; 2018:1–36. Available at: https://giftofhope.org/wp-content/uploads/2021/03/2018-AATB-MECoronerGuide.pdf [Google Scholar]
- 43. Shafer TJ, Schkade LL, Siminoff LA, et al. Ethical analysis of organ recovery denials by medical examiners, coroners, and justices of the peace. J Transpl Coord 1999;9:232–49 [DOI] [PubMed] [Google Scholar]
- 44. National Institute on Aging [Internet]. Brain Donation Resources for ADRCs. Available at: https://www.nia.nih.gov/health/brain-donation-resources-adrcs. Accessed September 24, 2023
- 45. Brain Donor Project [Internet]. Science Needs You. Available at: https://braindonorproject.org/. Accessed September 24, 2023