Abstract
Background:
Colorectal cancer (CRC) is a leading cause of cancer incidence and mortality. Screening can result in reductions in incidence and mortality, but there are many challenges to uptake and follow up.
Content:
Here, we will review the changing epidemiology of CRC, including increasing trends for early and later onset CRC; evidence to support current and emerging screening strategies, including non-invasive stool and blood based tests; key challenges to ensuring uptake and high quality screening; and the critical role that clinical laboratories can have in supporting health system and public health efforts to reduce the burden of CRC on the population.
Summary:
Clinical laboratories have the opportunity to play a seminal role in optimizing early detection and prevention of CRC.
Keywords: Colorectal cancer screening, fecal immunochemical test, stool DNA-FIT, early onset colorectal cancer, colorectal cancer prevention, colonoscopy
1.0. Introduction
Colorectal cancer (CRC) is the second most common cause of cancer death in the United States, and a leading cause of cancer incidence and mortality worldwide. Incidence and mortality from CRC can be reduced through screening. Several challenges to optimizing CRC prevention and screening exist, including changing epidemiology that includes increasing incidence among younger age groups, as well as suboptimal participation in screening and guideline appropriate follow up. This review provides an overview of these challenges, including the potential roles that clinical laboratories can play in partnering with health systems and public health authorities to reduce the burden of CRC.
2.0. Epidemiology of colorectal cancer
2.1. CRC Burden and Inequities
Colorectal cancer (CRC) is common worldwide, and in the United States specifically it is the third most common incident cancer, second leading cause of cancer death, and is expected to account for 153,020 new cancer cases and 52,550 deaths in 2023 (1). CRC incidence and mortality vary across demographic groups in the US, with higher incidence and mortality observed for Alaska Native, American Indian, and non-Hispanic Black individuals compared to non-Hispanic White individuals. CRC presents at a more advanced stage in non-Hispanic Black, Alaska Native, American Indian, and Hispanic people compared to non-Hispanic White people (1). Five year CRC survival is closely related to stage, at over 90% for localized cancer and less than 15% for distant stage disease, underscoring the key role of early detection in optimizing outcomes (2).
2.2. Changing CRC Trends By Age, Anatomical Location, and Stage
In the US, overall CRC incidence (and mortality) have been decreasing over time (Figure 1, panel A). This decrease is noted across all racial/ethnic populations with incidence decreasing by over 30% in Black and White individuals from 2000 to 2017. However, there are several established and emerging trends of concern. Early onset CRC incidence under age 50 years has been rising for over 20 years (Figure 1, panel B), to the point that incidence among individuals age 45 today is similar to historic incidence among 50 year-olds. This trend is expected to continue, with CRC expected to become the leading cause of cancer death among individuals age 20–49 years by 2030 (3). Furthermore, disparities in early onset CRC mortality exist, with improvements in survival over the last 20 years seen among White individuals, but not among Black, Asian or Hispanic individuals (4). These trends may be linked to social determinants of health such as lack of access to healthcare resources, subsequently leading to delayed CRC diagnosis and advanced disease stages at presentation (5).
Figure 1:
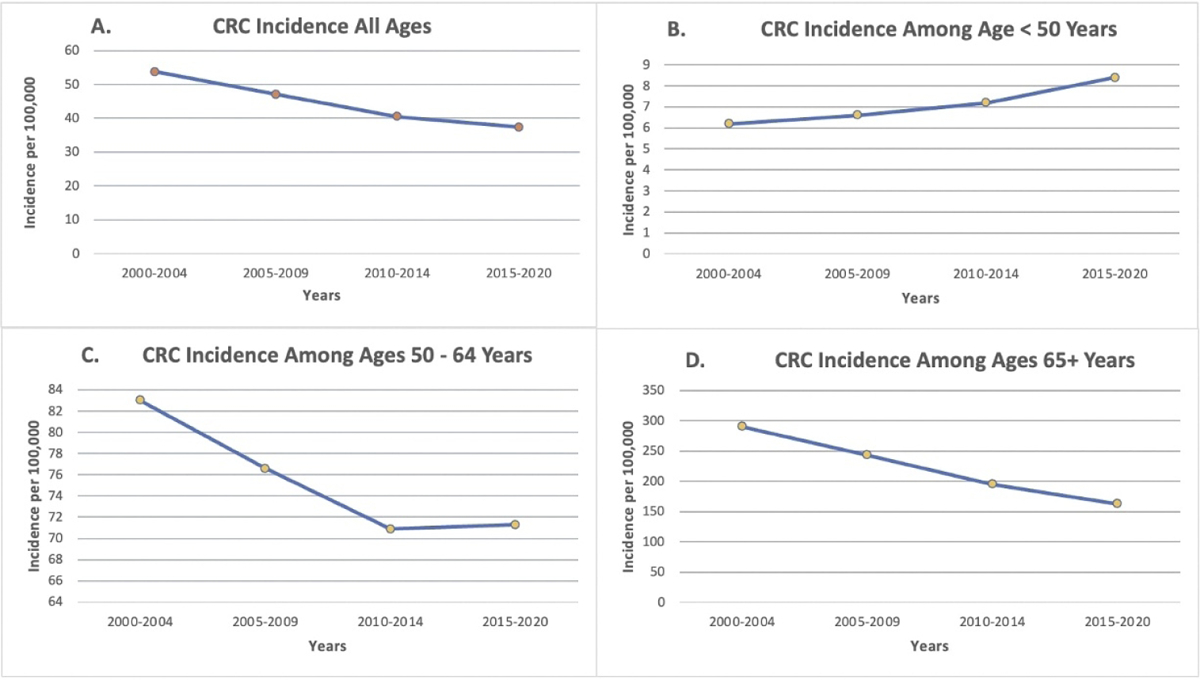
Age- and delay-adjusted trends in Colorectal cancer (CRC) incidence among men and women 2000–2020, Surveillance Epidemiology and End Results Program 22 for all ages combined (Panel A), and stratified by age <50, 50–64, and >64 years (Panels B, C, and D). The data demonstrate that CRC incidence is rising among individuals <50, and that incidence among individuals age 50–64 has been rising since 2013. Data accessed May 30 2023 at https://seer.cancer.gov/statistics-network/explorer/
Worrisome trends regarding later onset CRC above age 50 are also emerging, with an apparent flattening in incidence over time for 50 to 64 year olds (Figure 1, panel C) and some reports showing increases in CRC incidence among 50 to 59 year olds (4–6). Notably, increases in incidence have been mainly attributable to rectal, and to a lesser extent, distal cancer with prominent increases in advanced stage disease (1). Observed trends in the US are also being reported from around the world, with increases in early onset CRC incidence ranging from 1.8% – 3.1% in Denmark, New Zealand, Australia and the United Kingdom (7).
It has been postulated that these trends are attributable to either a birth cohort or time period exposure effect, whereby persons born 1950 and later experience substantially higher CRC risk compared to those born prior to 1950 (1). Features of the postulated birth cohort CRC phenomenon include: increased risk for early onset CRC under age 50; emerging increased risk for CRC among individuals 50 and older; higher risk for rectal cancer, and elevated risk for more advanced stage at diagnosis (Figure 2) (1). Hypothesized reasons for increasing risk include perinatal exposures, such as sulfonamide antibiotics and maternal obesity, exposures during childhood and adolescence, like sugar sweetened beverages and obesity, and adult exposures, such as alcohol, but the full range of risk factors and mechanisms have yet to be defined (Table 1) (8–13).
Figure 2:
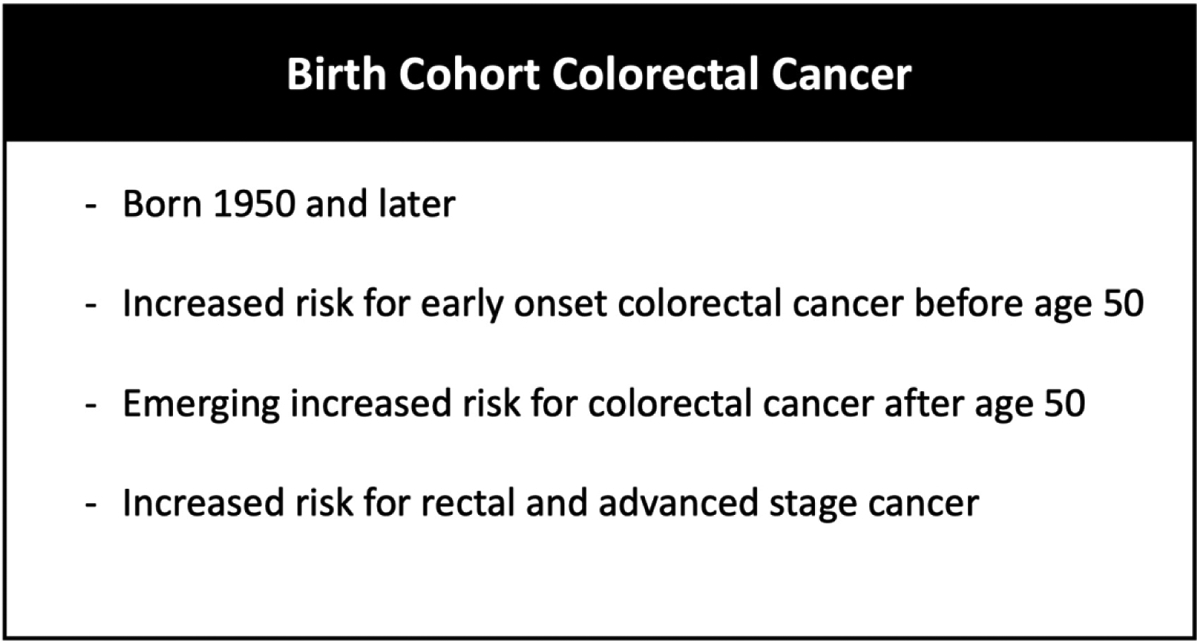
Birth cohort colorectal cancer. The phenomenon of birth cohort Colorectal cancer (CRC) can be conceptualized as including, among persons born 1950 and later: 1) increased risk for early onset CRC before age 50; 2) emerging increased risk for CRC age 50 and older; 3) increased risk for rectal cancer and advanced stage at diagnosis.
Table 1:
Hypothesized and Established Risk Factors for Birth Cohort and Early Onset CRC
| Established Associated Factors | Hypothesized Risk Factors |
|---|---|
| Obesity | Dysbiosis |
| Sedentary lifestyle | Lack of breast feeding |
| Hyperlipidemia | Dietary patterns especially with increases in processed foods, |
| Diabetes | sugar, fat, and refined grains |
| Red or processed meats | High fructose corn syrup |
| Alcohol | Food additives (e.g. synthetic dyes, monosodium glutamate, |
| Tobacco smoking | titanium dioxide) |
| Sugar-sweetened beverages | Food insecurity |
| Maternal weight and weight gain during pregnancy | Elevated birth weight/childhood obesity |
| In utero exposures: | Excessive exposure to insulin and growth hormone in utero |
| Long-acting sulfonamide antibiotic exposure | Environmental toxins (e.g. pesticides, benzene) |
| Maternal obesity | Cesarean delivery |
| Bendectin (doxylamine; pyridoxine; dicyclomine) | Pediatric onset inflammatory bowel disease |
| Periodontal disease | |
| Other antibiotic use: | |
| Ampicillin | |
| Amoxicillin | |
| Gentamycin |
The changing epidemiology of CRC highlights the rationale for recent guideline recommendation changes by the US Preventive Services Task Force (USPSTF), the American Cancer Society, and others to initiate screening at age 45 instead of age 50 (14–17). Notably, some groups, such as the American Association of Family Medicine and the American College of Physicians continue to endorse initiation of screening at 50, citing a need for more evidence to support a lower age and concerns regarding the resources required to screen 45 to 49 year old individuals at a population level (18, 19). Whether initiating screening at age 45 or 50, trends towards increasing CRC risk underscore the importance of ensuring individuals eligible for screening have every opportunity to access either invasive tests such as colonoscopy, or non-invasive, lab-based screening tests such as the fecal immunochemical test (FIT) or stool DNA-FIT (sDNA-FIT).
3.0. Screening for CRC
3.1. Rationale
CRC screening meets widely utilized criteria for appraising screening strategies based on:
Frequency of CRC in the population;
Well understood pathogenesis, including a long lead time between progression from normal mucosa to precancerous polyps and subsequent CRC during which screening can be used for early detection and prevention;
Availability of effective interventions to prevent and treat CRC;
Wide availability of multiple acceptable test strategies for screening; and
Randomized trials, observational studies, and modeling analyses all support the potential for screening to reduce incidence and mortality, and screening is widely endorsed by the USPSTF and others.
3.2. CRC Screening Options
USPSTF recommends a menu of strategies for screening, including annual guaiac fecal occult blood (gFOBT), annual fecal immunochemical test (FIT), stool DNA (sDNA)-FIT (Cologuard) every 1–3 years, computed tomographic colonography (CTC) every 5 years, sigmoidoscopy every 5 years (or every 10 years when combined with annual FIT), and colonoscopy every 10 years for average risk individuals. Additional Food and Drug Administration-approved tests include the colon capsule (Pill-Cam COLON 2; Medtronic Minneapolis, Minnesota, USA), as well as methylated serum septin 9 (Epi proColon, Epigenomics Inc., San Diego, California, USA), but these have not been USPSTF-endorsed, and Epigenomics recently stopped sales of the methylated serum septin 9 test in the US. It should be noted that outside the United States, the most common test offered for population screening is FIT, and that sigmoidoscopy may be offered as a once-only or an every 10 year screen (21).
3.2.a. Options: lab-based tests
The three currently available non-invasive tests take different approaches for screening, all utilizing stool samples taken at home.
3.2.a.i. gFOBT
The gFOBT evaluates for the presence of a peroxidase reaction with heme within a stool sample, and requires 3 samples with manual development and interpretation. Kits may be distributed at point-of-care or by mail, and returned in person or by mail. Challenges with gFOBT include the need for multiple samples, and patient instructions that recommend dietary and medication restrictions such as avoidance of red meat and non-steroidal anti-inflammatory medications, despite mixed evidence regarding the importance of such restrictions (22–24).
3.2.a.ii. FIT
FITs utilize an antibody against the globin moiety of heme to detect bleeding from colorectal neoplasia, and generally require a single stool sample. Available FITs in the US include quantitative FITs as well as qualitative FITs, though all available tests are only currently approved for reporting a qualitative (abnormal/normal) result in the US. Kits may be distributed at point-of-care or by mail, and returned in person or by mail. Quantitative FITs can be batch machine processed, but qualitative FITs require manual development and interpretation. Advantages of FIT over gFOBT include ability to use a single instead of multiple stool samples, manufacturer instructions that do not specify a need for dietary or medication restrictions, and superior test characteristics, to the point that expert societies recommend use of FIT over gFOBT.(25) Substantial within-brand variation in FIT performance has been observed, and thus far the USPSTF has only formally recommended the Polymedco OC Sensor quantitative FIT, and OC Light qualitative FIT for screening (14, 26).
3.2.a.iii. sDNA-FIT
The sDNA-FIT is a multi-marker assay that includes assessment for Kirsten rat sarcoma virus (KRAS) mutations, abnormal Neuregulin 4 (NDRG4) and bone morphogenetic protein 3 (BMP3) methylation, and hemoglobin concentration (27). Using these measurements, a proprietary algorithm is used to generate a qualitative positive/negative result. A single sDNA-FIT is currently available on the market (Cologuard). Test orders are sent to the company, which follows up with patients to mail the testing kit, promote test completion, and assist with test return to a central for processing. The test requires patients to collect a whole spontaneous bowel movement, take a sample for FIT, mix a preservative buffer into the remaining sample, and package all contents for return by a mail courier. Ordering tests and viewing results can either be done entirely through clinical providers accessing a web-based ordering portal, or by working with the company to embed the ordering interface into electronic health record interfaces such as Epic.
3.2.b. Evidence to support use and comparative performance
3.2.b.i. Test Characteristics
Sensitivity, specificity, and test positivity vary widely across tests (Table 2). Sensitivity for CRC and advanced neoplasia (adenomas with villous features, high grade dysplasia, size ≥1cm or CRC) are between 50 – 75% and 7 – 21% for gFOBT, 74 – 81% and 25 – 27% for FIT; 93% and 47% for sDNA-FIT, using colonoscopy as a reference standard. Sensitivity for CRC and advanced neoplasia are estimated to be 95% and 95% for sigmoidoscopy, and 95% and 95% for colonoscopy, respectively (28). Specificity for CRC and advanced neoplasia is between 96 – 98% and 96 – 99% for gFOBT, 93 – 94% and 95 – 96% for FIT; 85 – 89% for sDNA-FIT, and between 86 – 89% for colonoscopy, respectively (2, 26). Specificity for colonoscopy was downgraded for detection and removal of non-adenomatous polyps, and was calculated via the formula:
Table 2.
Characteristics of USPSTF-recommended CRC screening strategies and impact on CRC incidence and mortality
| Test Strategy | Approach/Assay | CRC Sensitivity | CRC Specificity | AN Sensitivity | AN Specificity | Positivity Rate | Relative Impact on CRC Incidence vs No Screening | Relative Impact on CRC Mortality vs No Screening | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Guaiac FOBT | Peroxidase reaction with heme in stool | 50 – 75% | 96 – 98% | 7 – 21% | 96 – 99% | 10.1% | 0 to ↓ 20%* | ↓ 9–22% | Risk reduction estimates based on RCT data |
| FIT | Antibody against globin moiety of heme | 74 – 81% | 93 – 94% | 25 – 27% | 95 – 96% | 6.4% | ↓ 10% | ↓ 10% | Risk reduction estimates based on observational data |
| sDNA-FIT | Algorithm using results of testing for 7 DNA markers in occult blood | 93% | 85% | 47% | 89% | 13.5% | NR | NR | -- |
| CT Colonography | CT scan of colon requiring bowel prep and oral contrast | 86 – 100% | 75% | 89% | 94% | 13.4% | NR | NR | -- |
| Sigmoidoscopy | Flexible endoscope used to evaluate rectum and distal colon | 95% | 87% | 95% | 87% | 17.3 – 23.4% | ↓ 22% | ↓ 26% | Risk reduction estimates based on RCT data |
| Colonoscopy | Flexible endoscope used to evaluate entire colon and rectum | 95% | 86 – 89% | 95% | 86 – 89% | 35.3%% | ↓ 47 – 69% | ↓ 50 – 68% | Risk reduction estimates based on observational data and a single RCT |
Data regarding sensitivity, specificity, positivity rate, and relative impact on CRC incidence/mortality pooled from several sources (2, 26, 35, 77–89)
Abbreviations: CRC, colorectal cancer; AN, advanced neoplasia; RR, relative risk; IRR, incidence rate ratio; NR, not reported; HR, hazard ratio; *1 out of 4 large RCTs of gFOBT showed incidence reduction
(Patients correctly identified as not having CRC on colonoscopy) ÷ [(Patients correctly identified as not having CRC) + (patients without CRC incorrectly identified as having CRC on colonoscopy)]. Generally lower performance for sensitivity among non-invasive tests, particularly for advanced neoplasia, is the fundamental reason why non-invasive tests need to be repeated relatively frequently.
3.2.b.ii. Acceptability
Randomized trials have shown that participation rates are substantially higher when non-invasive tests such as FIT, or a choice of tests such as FIT or colonoscopy, are offered compared with offering only colonoscopy (29–32). The highest rates of up-to-date screening have been shown in health systems consistently offering a combination of test strategies, highlighting the important role of lab-based non-invasive tests in optimizing screening participation rates for health systems and at the population level (33).
3.2.b.iii. Impact on Incidence and Mortality (Table 2)
Randomized trials utilizing no screening as a comparator have shown that exposure to gFOBT can reduce mortality, and in one trial, incidence (21, 34). RCTs have shown exposure to sigmoidoscopy substantially reduces CRC incidence and mortality through the detection and removal of precancerous polyps (21). A recently reported RCT has shown that inviting individuals to colonoscopy versus no invitation can reduce CRC incidence; a reduction in CRC mortality was shown on per-protocol analyses among those participating in screening but not on intent-to-screen analysis (35). Observational studies have shown exposure to FIT is associated with reduced risk for incidence and mortality, but RCTs results are not yet available (36). No RCTs or observational studies linking sDNA-FIT or CT colonography to reduced incidence and mortality have been reported. Head-to-head comparisons of various strategies such as FIT, sigmoidoscopy, and colonoscopy are underway, but results comparing the relative impact on cumulative CRC incidence and mortality are not yet available.
Given that the sensitivity and specificity of all available tests are well understood, modeling studies have been utilized to fill evidence gaps regarding the potential comparative effectiveness of available tests on incidence and mortality. These have suggested that similar rates of cancer prevented and deaths averted can be achieved with all strategies currently recommended by the USPSTF, albeit assuming perfect participation in screening and follow up, and optimal quality of testing. While perfect participation is an aspirational goal, the best test is one that is done, and the USPSTF has recommended a “menu of options” to optimize participation (14).
3.2.c. Emerging Lab-Based Non-Invasive Test Strategies
Advances in translational science, as well as novel healthcare policies, promise to usher in a new wave of lab-based, non-invasive strategies for CRC screening. Novel molecular approaches include blood testing for aberrant epigenetic and genetic changes in circulating tumor DNA (ctDNA) and proteomic (including glycoproteomic) patterns, as well as stool testing for epigenetic and genetic changes in tumor and polyp derived DNA, micro-RNA patterns, and protein markers (37). Importantly, in 2021 the Centers for Medicare and Medicaid Services issued a National Coverage Decision in the US supporting coverage of any novel blood-based test that garners FDA approval and has at least 74% sensitivity, and 90% specificity for CRC. This was done in order to provide “a pathway for Medicare coverage to support innovation and to accelerate access to emerging blood-based biomarker screening tests as soon as patient and test criteria are met” (38). This coverage decision has provided key target metrics that are expected to result in development of a new wave of blood-based tests for CRC screening. It may also suggest the Centers for Medicare and Medicaid services will set a similar standard for coverage decisions surrounding novel stool-based CRC screening tests. As a result of evolving science for biomarker detection and recent policy decisions, results of 4-large scale, prospective studies of novel, lab-based screening tests which are near completion among individuals at average risk for CRC undergoing usual care screening colonoscopy are highly anticipated (Table 3). These include one study of a blood-based assay analyzing ctDNA patterns, one study of a blood based assay utilizing ctDNA and proteomic markers, a study of a stool-based test evaluating abnormal expression of 8 RNA biomarkers combined with FIT (sRNA-FIT), and one study of an updated version of the currently approved sDNA-FIT test. Notably, the study of the updated sDNA-FIT also includes assessment of a blood-based assay for genetic and epigenetic alterations. Additional earlier stage studies leveraging ctDNA, proteomic, glycoprotein, and epithelial markers in the blood, as well as DNA, RNA, proteins, and microbiome patterns in the stool, have also been reported and show promise for CRC screening, but performance requires verification in large scale prospective studies (Table 3).
Table 3.
Emerging Non-Invasive, Lab-Based Strategies for CRC Screening
| Later phase, large-scale prospective studies | |||||
|---|---|---|---|---|---|
| Study | Specimen Source | Assay/Approach | Study Design | Enrolled subjects (n) | Test Characteristics |
| Evaluation of the ctDNA LUNAR Test in an Average Patient Screening Episode (ECLIPSE)(90) | Blood | Measurement of ctDNA | Observational study of average risk patients ages 45–84 years old, undergoing routine CRC screening | 22,877 | CRC sensitivity: 83%, CRC Specificity: 90%, AA Sensitivity: 13%* |
| Prevention of Colorectal Cancer Through Multiomics Blood Testing (PREEMPT CRC)(92) | Blood | Measurement of tumor and non-tumor derived signals from ctDNA, epigenetic and protein biomarkers | Observational study of average risk patients ages 45–85 | >30,000 | Not published yet, prior data from earlier trial shows CRC sensitivity: 94%, CRC Specificity: 94%* |
| Colorectal Cancer and Pre-Cancerous Adenoma Non-Invasive Detection Test Study (CRC-PREVENT)(40) | Stool | Multi-target stool RNA test plus a FIT | Interventional study of average risk individuals >45 years of age | 8,289 | CRC sensitivity: 94%, AA sensitivity: 45%, Specificity: 88% |
| Clinical Validation of An Optimized Multi-target Stool DNA (Mt-sDNA 2.0) Test, for Colorectal Cancer Screening “BLUE-C”(93) | Stool | Mt-sDNA 2.0 screening test | Observational study including patients age 40 and older who are enrolled in screening colonoscopy and will also complete mt-sDNA 2.0 test and FIT | 23,494 | CRC sensitivity: 94%, CRC Specificity: 91%, AA Sensitivity: 43%* |
| Early phase studies | |||||
| Sample Collection Study for the CellMax Life Circulating Tumor Cell and Circulating Tumor DNA Platforms for the Early Detection of Colorectal Cancer and Adenomas (91) | Blood | Evaluation of aberrations in ctDNA via NGS, detection of circulating epithelial cells | Observational study of average risk patients ages 45–80 | 1,038 | CRC sensitivity: 92.1%, AA sensitivity: 54.5%, AA specificity: 91%* |
| Collection of Samples USOPTIVAL Study (96) | Blood | Evaluation of cell-free DNA methylation and fragmentation characteristics, tumor-derived signal deduction and machine learning algorithm | Observational study of patients between the ages of 45 and 84 who were either average risk or had suspected AA or newly diagnosed CRC that has not been resected | 997 | CRC sensitivity: 93%, AA sensitivity 54%, AA specificity: 92%* |
| Non-invasive Identification of Colorectal Cancer and Adenomas in Early Stages (NICE)(95) | Blood | InterVenn Glycoprotein test | Prospective, multi-site study using glycoproteomic testing for early detection of advanced adenoma and colorectal cancer for average-risk patients undergoing routine screening colonoscopy | 575 | Not available |
3.2.c.i. Early results from large trials of non-invasive tests
Results for the Guardant ctDNA blood-based Shield test have been reported in abstract form for a study that included 65 CRC cases among an age/sex stratified random sample of 7,796 cancer-free controls (39). The test was reported to have 83% sensitivity for CRC (54/65, 95% CI 72–90%), 13% sensitivity for advanced precancerous lesions (147/1,116; 95% CI 11–15%), and 90% specificity for the absence of CRC or advanced precancerous lesions (5,982/6,680; 95% CI 89–90%). Whereby advanced adenoma is defined as any adenoma with greater than or equal to 10 mm in size or with greater than 25% villous histology or high-grade dysplasia. Results for the Geneoscopy sRNA-FIT study report 94% sensitivity for CRC, 45% sensitivity for advanced adenoma, and 88% specificity for their cohort of 8,289 enrolled subjects (40). Also in press release form, the study of a second generation sDNA-FIT has reported 94% sensitivity for CRC, 43% sensitivity for advanced precancerous lesions, and 91% specificity for their cohort of over 20,000 subjects (https://www.exactsciences.com/newsroom/press-releases/next-generation-cologuard-test-demonstrates-94-percent-sensitivity, accessed October 2023). Results from the other large prospective studies are expected to be made available within the next two years. Available results may herald an opportunity to make these and other non-invasive blood and stool-based CRC screening tests available for clinical use in the near future. Novel FDA approved, CMS-covered tests could have the potential to optimize screening through better acceptability, availability, detection of neoplasia, or all 3 relative to 1 or more currently available tests.
4.0. Opportunities for clinical laboratories to address current challenges to CRC screening
While the rationale and evidence to support CRC screening are robust, outcomes of CRC screening remain suboptimal due to multiple challenges. Clinical labs can play a crucial role in helping optimize CRC screening and prevention by taking advantage of opportunities to address many current challenges (Table 4).
Table 4.
Clinical Lab Opportunities for Optimizing Colorectal Cancer Screening and Outcomes
| Opportunity | Challenge | Intervention |
|---|---|---|
| Optimize screening participation | Suboptimal screening rates | Establish infrastructure to implement mailed outreach programs |
| Implement embedded EHR tools to allow for standing orders at point of lab care for screening tests such as FIT for patients not up to date (“Lab-FIT”) | ||
| Optimize screening effectiveness | Subpar patient instructions | Distribute instructions following best practices for literacy, with consideration for wordless instructions, multiple translations, and links to instructional videos |
| Distribution of low performance stool tests | Phase out gFOBT and more broadly adopt FIT | |
| Select FIT brands with consistently good performance characteristics | ||
| Discarding of irreplaceable FITs due to missing collection dates | Result FITs missing collection dates or out of window from collection to processing and report abnormal results, and advise repeat testing for normal results | |
| Non-reporting of quantitative FIT values that correlate closely with probability of neoplasia | Support implementation of quantitative FIT reporting Report quantitative fecal hemoglobin values | |
| Low rates of colonoscopy follow up after an abnormal non-invasive CRC screening test | Flag abnormal results as critical values, provide education on test result interpretation, partner to create registries of patients with abnormal lab-based CRC screening test results | |
| Socioeconomic variability in access | Promote access to FDA/CMS approved testing | |
| Reduce inappropriate screening test exposure | High rates of FIT overuse/misuse in emergency department and inpatient settings | Eliminate or restrict emergency department and inpatient use of CRC screening tests such as FIT and guaiac FOBT |
4.1. Optimize screening participation
Screening participation rates remain suboptimal, and as of 2021 just 59% of the US population was up to date in terms of a CRC screening test, far short of the 80% target set by the National Colorectal Cancer Round Table (1, 41). A complicating factor is that screening suffered a setback as a result of the COVID-19 pandemic and estimates suggest the negative impacts on CRC-incidence and CRC-mortality could last for years (42). Modeling studies suggest pandemic-related disruptions to screening can be mitigated through expansion of stool-based testing (43). Improving overall screening rates will likely rely heavily on lab-based stool testing, and potentially in the near future blood tests, underscoring the role that clinical labs will have in facilitating optimal participation.
4.1.a. Partner to support mailed FIT outreach
Mailed outreach is one of several evidence-based strategies for increasing CRC screening completion along with visit-based FIT distribution, patient navigation, patient reminders and patient education (44, 45). Among all strategies for promoting CRC screening completion, mailed outreach appears to be the most effective, based on indirect comparisons. Meta-analyses have shown that offering mailed outreach can result in an absolute 28% increase in screening completion compared to usual, health-system visit-based offers for screening (46). Real world examples of implementation have shown impressive results. Kaiser Permanente established a mailed-FIT program for nearly 700,000 eligible screening-aged adults in 2008 that led to dramatic increases in proportion of patients up to date from 38.9% in 2000 to 82.7% in 2015, along with concurrent decreases in CRC incidence and mortality, and elimination of disparities in CRC incidence and mortality between Black and Non-Hispanic White individuals (33, 47). Mailed FIT approach is the standard approach to population-based CRC screening in over 15 countries around the world (21). Clinical laboratories can partner with providers to establish necessary infrastructure to successfully implement a mailed outreach program and significantly increase rates of up to date CRC screening utilizing guides available from the Centers for Disease Control, National Association of Chronic Disease Directors, National Colorectal Cancer Roundtable, and others (48–50).
4.1.b. Collaborate to allow for standing orders for FIT
A policy of having standing orders for FIT that can be acted on by different members of the healthcare team at time of usual healthcare visits, including primary care, specialty or nurse visits, has been shown to facilitate screening completion (51). We have observed some centers leverage this approach to a concept known as “Lab-FIT”, in which lab personnel, as part of patient interaction during lab visits, make note of open/overdue healthcare reminders, and, in the case of patients not-up-to-date with CRC screening, offer to distribute FIT kits to promote screening completion. Implementing Lab-FIT, and perhaps in the future even allowing for standing orders for blood-based CRC screening tests to patients not up-to-date at the time of usual care blood draws, could be one opportunity for clinical labs to help promote screening participation. Lab-FIT could lead to inappropriate testing among those with severe comorbidities. As such any implementation of this strategy should include multidisciplinary discussion with primary care clinicians and other stakeholders to set up clear parameters for consideration for this strategy.
4.2. Optimize screening effectiveness
Screening effectiveness can be impacted by problems with high quality test completion, selection of tests, and systems barriers to test ordering and reporting as well as follow up to colonoscopy completion after an abnormal non-invasive test. Clinical laboratories can play a large role in overcoming these barriers.
4.2.a. Distribute Optimal Patient Instructions for Stool-Based Tests
Reliance on patients for sample collection and handling naturally leads to increased variability in sample quality, and highlights the importance of effective patient instruction. Instructions deemed complex and burdensome by patients are a barrier to screening adherence, and patient literacy and language must be addressed to enhance comprehension (52). Effective interventions include utilization of low-literacy instructions, which led to decreased rates of mishandled FIT samples, and wordless instructions, which were unanimously preferred over written instructions in one cohort (53, 54). Manufacturer instructions for FIT often need to be supplemented with additional instructions to optimize proper test completion, for example by using strategies recommended at https://research.kpchr.org/mailed-fit/ (date accessed October, 2023). Resources available include wordless instructions, multiple translations, and QR codes with links to videos on test completion. By ensuring testing instructions are clear and easy to comprehend, clinical laboratories can help improve screening participation across the populations they serve, and improve lab efficiency by reducing work burden of following up on unsatisfactory samples.
4.2.b. Replace gFOBT with FIT
With a single sample, FIT is more sensitive and similarly specific for colonic bleeding due to neoplasia, can be done effectively with one sample instead of three samples as required by gFOBT, and, in the case of some FITs, be performed with automated reading (25). Replacing FIT with gFOBT has also been shown to increase screening completion (55). Clinical laboratories can work to improve overall screening effectiveness by phasing out gFOBT as a screening test and more broadly adopting FIT.
4.2.c. Use a High Quality FIT
Commercially available FITs vary in test performance, number of samples required, and cutoff of hemoglobin concentration to determine an abnormal/positive result. Among the nearly 65 FITs commercially available in the US, significant heterogeneity in terms of sensitivity and specificity exist, with sensitivity for advanced neoplasia and CRC ranging from 2–66% and 50–97% respectively, and specificity for AN ranging between 60–99% (26). In their evidence review of available FIT tests in the United States, the USPSTF found sufficient evidence to recommend the Polymedco OC-Sensor and OC-Light tests due to their consistently good performance characteristics, and notably did not find sufficient evidence to support use of many of the other commercially available FIT tests in the United States (26). A comparative effectiveness study comparing 5 different brands of FIT is underway, and may help to clarify relative performance of FIT brands (56). In the meantime, clinical labs have an opportunity to optimize detection and prevention of CRC by careful selection of which FIT brand is offered to their patients.
4.2.d. Reduce Rates of Ineffective FIT
Another challenge seen with FIT is user error and mishandling, which can lead to invalid tests. In routine practice, patients have been observed to provide FITs with missing collection dates, too much or too little fecal sample, a sample taken with a kit that is past the manufacturer expiration date, or kits returned at a time outside of the recommended window between sample collection and lab processing. In a study by Wang et al., among 1,871 patients, 19.8% of tests were mishandled, most commonly due to missing collection dates (53). In many instances these tests would be discarded, but as their study notes, rates of positivity were similar between the correctly labeled group and mishandled group. Time at room temperature, as well as variability in ambient air temperature can impact rates of hemoglobin degradation within FIT samples, as well as positivity rates, and refrigeration can mitigate degradation (57–60).
These data highlight the importance of promoting prompt return and proper storage of samples, underscoring the importance of research showing that providing a deadline can promote timely FIT return (61). Importance of time from sample collection to processing also supports current laboratory practice of monitoring collection dates, and provides a rationale for why some labs routinely discard FITs for which the collection date cannot be established. However, we view the practice of discarding FITs with missing collection dates or out of window with regard to collection date as a major missed opportunity to support patients when stool specimens are otherwise adequate. As has been discussed, screening participation in the population is suboptimal, and patients often collect and submit stool samples with great effort. Further, cancers intermittently bleed, such that a positive test on one date might be negative if repeated even at a short interval on another date. As such, these samples should be viewed as irreplaceable.
A patient and public-health centric approach would allow for a standard lab policy of processing FITs missing collection dates or out of the time window from collection. Results with an abnormal or positive result can be reliably interpreted as true positive results, while normal/negative results can be interpreted as uncertain, with a recommendation to repeat the test. This strategy could reduce the risk of missing the opportunity to identify a patient with an abnormal, mishandled sample who might not return a repeat test or might have a repeat test that is normal due to the intermittent nature of bleeding from colonic neoplasia. This scenario has important clinical implications, since 1 in 20 with abnormal FIT have CRC, and failure to follow up with colonoscopy after an abnormal FIT confers 2.5 fold increased risk for CRC-related mortality (62–64). As such, many clinical labs may be discarding irreplaceable abnormal FITs where patients may not submit a repeat sample or submit a subsequent negative sample, and thus miss an opportunity for early detection of CRC that could come from reporting results for the original mishandled sample.
4.2.e. Report Quantitative FIT Results
For patients who have had a quantitative FIT with an abnormal result, the absolute hemoglobin concentration is measurable, and research has definitively shown that increasing hemoglobin concentration is directly proportional to the positive predictive value for finding advanced adenomas and CRC at follow up colonoscopy. For example, the most commonly used quantitative FIT in the US is reported as abnormal/normal based on reaching a hemoglobin concentration of 100 ng hemoglobin/mL buffer, but the positive predictive value for advanced neoplasia increases from 25.9% to 31.0% for a 100 vs 150 ng hemoglobin/mL buffer threshold (65). Just as absolute values of white blood cell counts and other continuous lab measures are used to guide urgency of clinical care, absolute hemoglobin concentrations among patients with an abnormal FIT could also be used to guide urgency of colonoscopy follow up, particularly in settings with long wait times for colonoscopy. This issue is particularly salient at this moment given worldwide backlogs in colonoscopy associated with the COVID-19 pandemic (66). As such, clinical labs could have a role in advocating for reporting of qualitative and quantitative FIT results as a strategy for improving clinical care.
4.2.f. Optimize Result Reporting and Messaging
Non-invasive CRC screening can only be effective if individuals with abnormal tests complete the screening process with a colonoscopy. Yet, colonoscopy follow up rates for abnormal FIT tests range from 18–65%, and are estimated at 66% after an abnormal sDNA-FIT (67). Furthermore, colonoscopy completion rates are lower among people of lower socioeconomic station and minority groups (68, 69). In identifying reasons for poor follow up, common reasons include lack of patient knowledge of the result, lack of knowledge of the significance of the result, and provider failure to respond to a result (70).
We postulate clinical laboratories can play a vital role in optimizing messaging for both patients and providers. Abnormal results should be flagged as critical values which may optimize recognition by both patients and providers, potentially increasing rates of follow up. Furthermore, laboratories may be able to optimize patient understanding of results by providing information regarding risk of cancer and advanced neoplasia with positive results, and also reminding patients and providers that cancers intermediately bleed, making colonoscopy referral conditional on a second abnormal result clinically inappropriate. For example, an abnormal FIT result can be accompanied by a result comment, which we have employed at UC San Diego:
“Follow up colonoscopy is recommended to exclude colorectal cancer. Among individuals with abnormal FIT, 1 in 20 have colorectal cancer and 1 in 4 have an advanced polyp. Repeat testing after an abnormal result is not recommended because cancers can intermittently bleed.” (62).
Clinical labs can also potentially spearhead quality improvement projects aimed at increasing colonoscopy follow up after an abnormal test, with one way being through creation of weekly lists or a registry of patients with abnormal results. Leveraging lists and registries of abnormal test patients has been successfully implemented and proven to improve colonoscopy follow up in several health systems (71). We suggest clinical labs, in partnership with primary clinicians and patient navigators, can work towards improving identification of patients who require follow up, and ultimately improving outreach to those patients.
4.2.g. Improve Screening Access
Screening rates vary across the US population, with 51% of Hispanic individuals, 48% of Asian individuals, 52% of Native American/Alaska Native individuals up-to-date compared to 60% non-Hispanic White individuals, and lower screening rates among those with lower socioeconomic status, Medicaid insurance, and the uninsured (1). Non-invasive tests such as FIT have been shown to result in higher screening uptake among underserved populations (29). As such, addressing screening inequities will rely heavily on offering non-invasive stool tests. Clinical labs can play a role in addressing screening inequities by promoting access to all FDA and CMS approved testing for people of all socioeconomic and racial backgrounds.
4.3. Reduce inappropriate screening test exposure
While suboptimal CRC screening rates remain a main challenge, overuse and misuse of non-invasive lab-based tests are also a challenge with clinical and resource implications. Examples of overuse include FIT after exposure to prior colonoscopy, and misuse includes repeating FIT after a prior abnormal result and utilization of FIT for non-screening indications such as in the inpatient or emergency room setting among patients presenting with signs or symptoms of GI disease.
Inappropriate use is common, with rates ranging from 26 – 51% of ordered FITs (72, 73). Overuse contributed significantly, with one study finding that 28% of inappropriate FIT tests ordered were within the recommended surveillance interval from previous colonoscopy (72). One way clinical laboratories could reduce overuse is through an EHR intervention whereby orders considered inappropriate would be flagged and prompt a best practice alert or trigger a clinical decision support system (CDSS). Implementation of CDSSs have been shown to increase guideline adherence among ordering providers, as well as a reduce unnecessary testing (74).
Another significant contributor to inappropriate ordering is misuse, particularly in emergency department (ED) and inpatient settings. Bhatti et al conducted a study of FIT use specifically in the inpatient and ED setting and found that 99.5% of tests ordered were inappropriate, with the vast majority being for indications such as anemia or overt GI bleeding (75). This study led to FITs being removed from inpatient and emergency department ordering menus, a process termed “disinvesting”. Disinvesting from inpatient stool-test use has been adopted by several institutions, and in one health system led to a 98% reduction in inpatient use and cost-savings of approximately $40,000 per year (76). Clinical labs can consider similar methods of disinvestment in inpatient FIT by flagging or restricting use in the inpatient and ED settings, which can ultimately have downstream consequences such as delivery of higher-value care and significant cost-savings.
6.0. Conclusion
CRC is a highly prevalent disease, with increasing incidence among persons born 1950 and later due to a birth cohort risk effect. System-level strategies for preventing inappropriate use of FIT for screening purposes will need to be carefully managed in context of emerging enthusiasm for use of quantitative FIT (in combination with age) to help triage patients with GI symptoms such as change in bowel habit to fast track evaluation, as recommended by UK NICE guidelines (97). Screening for CRC can reduce incidence and mortality. Multiple options for screening exist, including invasive tests such as colonoscopy, and non-invasive, lab-based tests such as FIT and sDNA-FIT, with more non-invasive lab options, including blood-based options, on the horizon. Challenges to optimizing CRC screening outcomes include suboptimal screening rates (with variation by race/ethnicity and socioeconomic status), suboptimal quality of testing due to process issues, and low rates of follow up of abnormal non-invasive test results. Clinical labs can have a major role in improving CRC outcomes by partnering with stakeholders, including patients, clinicians, and health systems, to improve access to non-invasive tests, implement strategies to promote completion such as mailed FIT outreach, put in place processes and infrastructure that promote high quality non-invasive screening and abnormal test follow up. As such, clinical laboratories are poised to play a significant role in decreasing CRC morbidity and mortality throughout the populations they serve.
Conflict of Interest Statement:
Dr. Gupta reports receiving personal fees from Guardant Health, InterVenn Biosciences, Geneoscopy, and Universal Diagnostics; receiving stock options from CellMax LLC, and serving as a local site investigator for Freenome and Epigenomics. Drs. Toth and Trivedi have no relevant conflicts of interest to report.
Conflict of Interest:
Samir Gupta is a consultant/Advisor for Geneoscopy; InterVenn; Universal Diagnostics; CellMax Life
Samir Gupta has received grant/research support from: National Institutes of Health, National Cancer Institute; Epigenomics; Freenome
List of abbreviations:
- CRC
colorectal cancer
- US
United States
- USPSTF
United States Preventative Services Task Force
- FIT
fecal immunochemical test
- sDNA-FIT
stool DNA-FIT
- gFOBT
guiac fecal occult blood test
- CTC
computed tomographic colonography
- RCT
randomized control trial
- ctDNA
circulating tumor DNA
- CMS
center for Medicare & Medicaid Services
- FDA
food and drug administration
- COVID-19
coronavirus disease 19
- AN
advanced neoplasia
- EHR
electronic health record
- CDSS
clinical decision support system
- ED
emergency department
- GI
gastrointestinal
- RR
relative risk
- IRR
incidence rate ratio
- NR
not reported
- HR
hazard ratio
Contributor Information
Joseph F. Toth, III, Department of Internal Medicine, University of California San Diego Health, 200 W Arbor Dr, San Diego, CA 92103, USA..
Mehul Trivedi, Department of Internal Medicine, University of California San Diego Health, 200 W Arbor Dr, San Diego, CA 92103, USA..
Samir Gupta, Department of Gastroenterology and Hepatology, University of California San Diego Health, 200 W Arbor Dr, San Diego, CA 92103, USA..
References:
- 1.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–54. Epub 20230301. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S Screening for Colorectal Cancer. Hematol Oncol Clin North Am. 2022;36(3):393–414. Epub 20220430. doi: 10.1016/j.hoc.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open. 2021;4(4):e214708. Epub 20210401. doi: 10.1001/jamanetworkopen.2021.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaki TA, Singal AG, May FP, Murphy CC. Increasing Incidence Rates of Colorectal Cancer at Ages 50–54 Years. Gastroenterology. 2022;162(3):964–5.e3. Epub 20211029. doi: 10.1053/j.gastro.2021.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho MY, Siegel DA, Demb J, Richardson LC, Gupta S. Increasing Colorectal Cancer Incidence Before and After Age 50: Implications for Screening Initiation and Promotion of “On-Time” Screening. Dig Dis Sci. 2022;67(8):4086–91. Epub 20210905. doi: 10.1007/s10620-021-07213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CC, Lee JK, Liang PS, May FP, Zaki TA. Declines in colorectal cancer incidence and mortality rates slow among older adults. Clin Gastroenterol Hepatol. 2023. Epub 20230610. doi: 10.1016/j.cgh.2023.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;4(7):511–8. Epub 20190516. doi: 10.1016/S2468-1253(19)30147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, et al. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20(6):1229–40.e5. Epub 20210129. doi: 10.1016/j.cgh.2021.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Hur J, Otegbeye E, Joh HK, Nimptsch K, Ng K, Ogino S, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. 2021;70(12):2330–6. Epub 20210506. doi: 10.1136/gutjnl-2020-323450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy CC, Cirillo PM, Krigbaum NY, Singal AG, Lee M, Zaki T, et al. Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer. Gut. 2022;71(7):1332–9. Epub 20210824. doi: 10.1136/gutjnl-2021-325001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy CC, Cirillo PM, Krigbaum NY, Singal AG, Jones DP, Zaki T, et al. In-utero exposure to antibiotics and risk of colorectal cancer in a prospective cohort of 18 000 adult offspring. Int J Epidemiol. 2023. Epub 20230124. doi: 10.1093/ije/dyad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352–64. Epub 20200221. doi: 10.1038/s41575-019-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020;158(2):341–53. Epub 20190805. doi: 10.1053/j.gastro.2019.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965–77. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 15.Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–81. Epub 20180530. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 16.Patel SG, May FP, Anderson JC, Burke CA, Dominitz JA, Gross SA, et al. Updates on Age to Start and Stop Colorectal Cancer Screening: Recommendations From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022;162(1):285–99. Epub 20211115. doi: 10.1053/j.gastro.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Ness R Updates in Screening Recommendations for Colorectal Cancer. JNCCN 2022. [Google Scholar]
- 18.Clinical Preventive Service RecommendationColorectal Cancer Screening, Adults. American Academy of Family Physicians Clinical Preventive Service Recommendation: American Academy of Family Physicians; 2021. [Google Scholar]
- 19.Qaseem A, Harrod CS, Crandall CJ, Wilt TJ, Balk EM, Cooney TG, et al. Screening for Colorectal Cancer in Asymptomatic Average-Risk Adults: A Guidance Statement From the American College of Physicians (Version 2). Ann Intern Med. 2023;176(8):1092–100. Epub 20230801. doi: 10.7326/M23-0779. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JM, Jungner YG. [Principles and practice of mass screening for disease]. Bol Oficina Sanit Panam. 1968;65(4):281–393. [PubMed] [Google Scholar]
- 21.Lauby-Secretan B, Vilahur N, Bianchini F. IARC Handbook of Cancer Prevention Vol. 17 – Colorectal Cancer Screening. IARC Handbooks of Cancer Prevention: IARC Handbooks of Cancer Prevention; 2019. [Google Scholar]
- 22.Konrad G, Katz A. Are medication restrictions before FOBT necessary?: practical advice based on a systematic review of the literature. Can Fam Physician. 2012;58(9):939–48. [PMC free article] [PubMed] [Google Scholar]
- 23.Konrad G Dietary interventions for fecal occult blood test screening: systematic review of the literature. Can Fam Physician. 2010;56(3):229–38. [PMC free article] [PubMed] [Google Scholar]
- 24.Coulter B Hemoccult II Sensa. Beckman Coulter; 2012. [Google Scholar]
- 25.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112(1):37–53. Epub 20161018. doi: 10.1038/ajg.2016.492. [DOI] [PubMed] [Google Scholar]
- 26.Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978–98. doi: 10.1001/jama.2021.4417. [DOI] [PubMed] [Google Scholar]
- 27.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–97. Epub 20140319. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 28.Lin JS, Piper MA, Perdue LA, Rutter C, Webber EM, O’Connor E, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. 2016. [PubMed]
- 29.Gupta S, Halm EA, Rockey DC, Hammons M, Koch M, Carter E, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–32. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singal AG, Gupta S, Tiro JA, Skinner CS, McCallister K, Sanders JM, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer. 2016;122(3):456–63. Epub 20151104. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singal AG, Gupta S, Skinner CS, Ahn C, Santini NO, Agrawal D, et al. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial. JAMA. 2017;318(9):806–15. doi: 10.1001/jama.2017.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–82. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology. 2018;155(5):1383–91.e5. Epub 20180719. doi: 10.1053/j.gastro.2018.07.017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 35.Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387(17):1547–56. Epub 20221009. doi: 10.1056/NEJMoa2208375. [DOI] [PubMed] [Google Scholar]
- 36.Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325(19):1998–2011. doi: 10.1001/jama.2021.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna M, Dey N, Grady WM. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023;21(3):604–16. Epub 20221217. doi: 10.1016/j.cgh.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen T, Chin J, Evans M, Ashby L, Li C, Long K. National Coverage Determination for Screening for Colorectal Cancer-Blood-Based Biomarker Tests: Centers for Medicare & Medicaid Services; 2021. [cited 2023 June 1]. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=299. [Google Scholar]
- 39.Chung D, Gray D, Greenson J, Gupta S, Eagle C, Hu S, et al. Clinical Validation for a Cell-free DNA Blood Based Test for Colorectal Cancer Screening in an Average Risk Population. Digestive Diseases Weekly; Chicago: American Gastroenterological Association; 2023. [Google Scholar]
- 40.Barnell EK, Wurtzler EM, La Rocca J, Fitzgerald T, Petrone J, Hao Y, et al. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023. Epub 20231023. doi: 10.1001/jama.2023.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NCCN Roundtable Tackles Disparities in Cancer Care. J Natl Compr Canc Netw. 2017;15(5S):671–5. doi: 10.6004/jnccn.2017.0069. [DOI] [PubMed] [Google Scholar]
- 42.de Jonge L, Worthington J, van Wifferen F, Iragorri N, Peterse EFP, Lew JB, et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatol. 2021;6(4):304–14. Epub 20210203. doi: 10.1016/S2468-1253(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Issaka RB, Taylor P, Baxi A, Inadomi JM, Ramsey SD, Roth J. Model-Based Estimation of Colorectal Cancer Screening and Outcomes During the COVID-19 Pandemic. JAMA Netw Open. 2021;4(4):e216454. Epub 20210401. doi: 10.1001/jamanetworkopen.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Issaka RB, Avila P, Whitaker E, Bent S, Somsouk M. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: A systematic review. Prev Med. 2019;118:113–21. Epub 20181024. doi: 10.1016/j.ypmed.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, et al. Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018;178(12):1645–58. doi: 10.1001/jamainternmed.2018.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jager M, Demb J, Asghar A, Selby K, Mello EM, Heskett KM, et al. Mailed Outreach Is Superior to Usual Care Alone for Colorectal Cancer Screening in the USA: A Systematic Review and Meta-analysis. Dig Dis Sci. 2019;64(9):2489–96. Epub 20190326. doi: 10.1007/s10620-019-05587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doubeni CA, Corley DA, Zhao W, Lau Y, Jensen CD, Levin TR. Association between Improved Colorectal Screening and Racial Disparities. N Engl J Med. 2022;386(8):796–8. doi: 10.1056/NEJMc2112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prevention CfDCa. Mailed Fecal Immunochemical Test (FIT) Online Courses Division of Cancer Control and Prevention, Centers for Disease Control and Prevention: Centers for Disease Control and Prevention; 2023. [cited 2023 6/9/2023]. Available from: https://www.cdc.gov/cancer/colorectal/mailed-fit.htm. [Google Scholar]
- 49.Directors NAoCD. Using the Mail to Help Save Lives! National Association of Chronic Disease Directors: National Association of Chronic Disease Directors. Available from: https://chronicdisease.org/using-the-mail-to-help-save-lives/. [Google Scholar]
- 50.Roundtable NCC. Mailed FIT Implementation Guide: American Cancer Society; 2022. [cited 2023 June 9]. Available from: nccrt.org/resource/mailed-fit-implementation-guide/.
- 51.Coronado GD, Petrik AF, Vollmer WM, Taplin SH, Keast EM, Fields S, et al. Effectiveness of a Mailed Colorectal Cancer Screening Outreach Program in Community Health Clinics: The STOP CRC Cluster Randomized Clinical Trial. JAMA Intern Med. 2018;178(9):1174–81. doi: 10.1001/jamainternmed.2018.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bapuji SB, Lobchuk MM, McClement SE, Sisler JJ, Katz A, Martens P. Fecal occult blood testing instructions and impact on patient adherence. Cancer Epidemiol. 2012;36(4):e258–64. Epub 20120413. doi: 10.1016/j.canep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Wang A, Rachocki C, Shapiro JA, Issaka RB, Somsouk M. Low Literacy Level Instructions and Reminder Calls Improve Patient Handling of Fecal Immunochemical Test Samples. Clin Gastroenterol Hepatol. 2019;17(9):1822–8. Epub 20181129. doi: 10.1016/j.cgh.2018.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coronado GD, Sanchez J, Petrik A, Kapka T, DeVoe J, Green B. Advantages of wordless instructions on how to complete a fecal immunochemical test: lessons from patient advisory council members of a federally qualified health center. J Cancer Educ. 2014;29(1):86–90. doi: 10.1007/s13187-013-0551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akram A, Juang D, Bustamante R, Liu L, Earles A, Ho SB, et al. Replacing the Guaiac Fecal Occult Blood Test With the Fecal Immunochemical Test Increases Proportion of Individuals Screened in a Large Healthcare Setting. Clin Gastroenterol Hepatol. 2017;15(8):1265–70.e1. Epub 20170204. doi: 10.1016/j.cgh.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 56.Levy BT, Daly JM, Xu Y, Crockett SD, Hoffman RM, Dawson JD, et al. Comparative effectiveness of five fecal immunochemical tests using colonoscopy as the gold standard: study protocol. Contemp Clin Trials. 2021;106:106430. Epub 20210508. doi: 10.1016/j.cct.2021.106430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Roon AH, Hol L, van Vuuren AJ, Francke J, Ouwendijk M, Heijens A, et al. Are fecal immunochemical test characteristics influenced by sample return time? A population-based colorectal cancer screening trial. Am J Gastroenterol. 2012;107(1):99–107. Epub 20111122. doi: 10.1038/ajg.2011.396. [DOI] [PubMed] [Google Scholar]
- 58.Gies A, Cuk K, Schrotz-King P, Brenner H. Direct comparison of ten quantitative fecal immunochemical tests for hemoglobin stability in colorectal cancer screening. Clin Transl Gastroenterol. 2018;9(7):168. Epub 20180706. doi: 10.1038/s41424-018-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reid MS, Paul HA, Mostoufi A, Robinson JL, Sadrzadeh SMH. Evaluation of the stability of fecal immunochemical test specimens. Clin Biochem. 2023;115:92–6. Epub 20221202. doi: 10.1016/j.clinbiochem.2022.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Doubeni CA, Jensen CD, Fedewa SA, Quinn VP, Zauber AG, Schottinger JE, et al. Fecal Immunochemical Test (FIT) for Colon Cancer Screening: Variable Performance with Ambient Temperature. J Am Board Fam Med. 2016;29(6):672–81. doi: 10.3122/jabfm.2016.06.160060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lieberman A, Gneezy A, Berry E, Miller S, Koch M, Argenbright KE, et al. The effect of deadlines on cancer screening completion: a randomized controlled trial. Sci Rep. 2021;11(1):13876. Epub 20210706. doi: 10.1038/s41598-021-93334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohan BP, Khan SR, Daugherty E, Chandan S, Ponnada S, Facciorusso A, et al. Pooled rates of adenoma detection by colonoscopy in asymptomatic average-risk individuals with positive fecal immunochemical test: a systematic review and meta-analysis. Gastrointest Endosc. 2022;96(2):208–22.e14. Epub 20220409. doi: 10.1016/j.gie.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 63.San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to Colonoscopy After Abnormal Stool-Based Screening and Risk for Colorectal Cancer Incidence and Mortality. Gastroenterology. 2021;160(6):1997–2005.e3. Epub 20210202. doi: 10.1053/j.gastro.2021.01.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, Yueh-Hsia Chiu S, Ching-Yuan Fann J, Chuang SL, et al. Association Between Colorectal Cancer Mortality and Gradient Fecal Hemoglobin Concentration in Colonoscopy Noncompliers. J Natl Cancer Inst. 2017;109(5). doi: 10.1093/jnci/djw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liles EG, Perrin N, Rosales AG, Smith DH, Feldstein AC, Mosen DM, et al. Performance of a quantitative fecal immunochemical test for detecting advanced colorectal neoplasia: a prospective cohort study. BMC Cancer. 2018;18(1):509. Epub 20180502. doi: 10.1186/s12885-018-4402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta S, Lieberman D. Screening and Surveillance Colonoscopy and COVID-19: Avoiding More Casualties. Gastroenterology. 2020;159(4):1205–8. Epub 20200716. doi: 10.1053/j.gastro.2020.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohl JT, Ciemins EL, Miller-Wilson LA, Gillen A, Luo R, Colangelo F. Rates of Follow-up Colonoscopy After a Positive Stool-Based Screening Test Result for Colorectal Cancer Among Health Care Organizations in the US, 2017–2020. JAMA Netw Open. 2023;6(1):e2251384. Epub 20230103. doi: 10.1001/jamanetworkopen.2022.51384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Issaka RB, Singh MH, Oshima SM, Laleau VJ, Rachocki CD, Chen EH, et al. Inadequate Utilization of Diagnostic Colonoscopy Following Abnormal FIT Results in an Integrated Safety-Net System. Am J Gastroenterol. 2017;112(2):375–82. Epub 20161213. doi: 10.1038/ajg.2016.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bharti B, May FFP, Nodora J, Martínez ME, Moyano K, Davis SL, et al. Diagnostic colonoscopy completion after abnormal fecal immunochemical testing and quality of tests used at 8 Federally Qualified Health Centers in Southern California: Opportunities for improving screening outcomes. Cancer. 2019;125(23):4203–9. Epub 20190903. doi: 10.1002/cncr.32440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cusumano VT, Corona E, Partida D, Yang L, Yu C, May FP. Patients without colonoscopic follow-up after abnormal fecal immunochemical tests are often unaware of the abnormal result and report several barriers to colonoscopy. BMC Gastroenterol. 2020;20(1):115. Epub 20200419. doi: 10.1186/s12876-020-01262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selby K, Jensen CD, Zhao WK, Lee JK, Slam A, Schottinger JE, et al. Strategies to Improve Follow-up After Positive Fecal Immunochemical Tests in a Community-Based Setting: A Mixed-Methods Study. Clin Transl Gastroenterol. 2019;10(2):e00010. doi: 10.14309/ctg.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masood U, Bernshteyn M, Pavelock N, Singh K, Schad LA, Morley CP, et al. Appropriateness of fecal immunochemical testing utilization for colorectal cancer screening at an academic center. Proc (Bayl Univ Med Cent). 2023;36(1):20–3. Epub 20221006. doi: 10.1080/08998280.2022.2123667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015;30(6):732–41. Epub 20150121. doi: 10.1007/s11606-014-3163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zare S, Meidani Z, Shirdeli M, Nabovati E. Laboratory test ordering in inpatient hospitals: a systematic review on the effects and features of clinical decision support systems. BMC Med Inform Decis Mak. 2021;21(1):20. Epub 20210118. doi: 10.1186/s12911-020-01384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhatti U, Jansson-Knodell C, Saito A, Han A, Krajicek E, Han Y, et al. Not FIT for Use: Fecal Immunochemical Testing in the Inpatient and Emergency Settings. Am J Med. 2022;135(1):76–81. Epub 20210909. doi: 10.1016/j.amjmed.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Gupta A, Tang Z, Agrawal D. Eliminating In-Hospital Fecal Occult Blood Testing: Our Experience with Disinvestment. Am J Med. 2018;131(7):760–3. Epub 20180327. doi: 10.1016/j.amjmed.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462–70. Epub 20070925. doi: 10.1093/jnci/djm150. [DOI] [PubMed] [Google Scholar]
- 79.Levin B, Hess K, Johnson C. Screening for colorectal cancer. A comparison of 3 fecal occult blood tests. Arch Intern Med. 1997;157(9):970–6. [PubMed] [Google Scholar]
- 80.Chiu HM, Chen SL, Yen AM, Chiu SY, Fann JC, Lee YC, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121(18):3221–9. Epub 20150520. doi: 10.1002/cncr.29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kooyker AI, Toes-Zoutendijk E, Opstal-van Winden AWJ, Spaander MCW, Buskermolen M, van Vuuren HJ, et al. The second round of the Dutch colorectal cancer screening program: Impact of an increased fecal immunochemical test cut-off level on yield of screening. Int J Cancer. 2020;147(4):1098–106. Epub 20200109. doi: 10.1002/ijc.32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anand S, Liang PS. A Practical Overview of the Stool DNA Test for Colorectal Cancer Screening. Clin Transl Gastroenterol. 2022;13(4):e00464. Epub 20220401. doi: 10.14309/ctg.0000000000000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357(14):1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 84.Weissfeld JL, Schoen RE, Pinsky PF, Bresalier RS, Church T, Yurgalevitch S, et al. Flexible sigmoidoscopy in the PLCO cancer screening trial: results from the baseline screening examination of a randomized trial. J Natl Cancer Inst. 2005;97(13):989–97. doi: 10.1093/jnci/dji175. [DOI] [PubMed] [Google Scholar]
- 85.Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann Intern Med. 2018;168(11):775–82. Epub 20180424. doi: 10.7326/M17-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corley DA, Jensen CD, Marks AR, Zhao WK, de Boer J, Levin TR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11(2):172–80. Epub 20120914. doi: 10.1016/j.cgh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cooper GS, Chak A, Koroukian S. The polyp detection rate of colonoscopy: a national study of Medicare beneficiaries. Am J Med. 2005;118(12):1413. doi: 10.1016/j.amjmed.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 88.Giorgi Rossi P, Vicentini M, Sacchettini C, Di Felice E, Caroli S, Ferrari F, et al. Impact of Screening Program on Incidence of Colorectal Cancer: A Cohort Study in Italy. Am J Gastroenterol. 2015;110(9):1359–66. Epub 20150825. doi: 10.1038/ajg.2015.240. [DOI] [PubMed] [Google Scholar]
- 89.Hassan C, Piovani D, Spadaccini M, Parigi T, Khalaf K, Facciorusso A, et al. Variability in adenoma detection rate in control groups of randomized colonoscopy trials: a systematic review and meta-analysis. Gastrointest Endosc. 2023;97(2):212–25.e7. Epub 20221013. doi: 10.1016/j.gie.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 90.Raymond VM, Higashi L, Marino E, Lang K. Evaluation of the ctDNA LUNAR-2 Test In an Average Patient Screening Episode (ECLIPSE). Journal of Clinical Oncology 2021. p. TPS142–TPS. [Google Scholar]
- 91.Friedland S, Watson D, Rex DK, Nimgaonkar A, Zhang Z, Atkins A, et al. Clinical performance of a multimodal screening blood test for advanced adenomas and CRC in an average-risk cohort of 1,038 participants. Journal of Clinical Oncology 2023. p. 75.35867951 [Google Scholar]
- 92.Putcha G, Xu C, Aasma Shaukat M, MPH, Levin TR. Prevention of colorectal cancer through multiomics blood testing: The PREEMPT CRC study. Journal of Clinical Oncology 2022. p. TPS208–TPS. [Google Scholar]
- 93.Imperiale T, Porter K, Zella J, Gagrat Z, Olson M, Statz S, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening: A Prospective Clinical Validation Study (BLUE-C) [Abstract] American Journal of Gastroenterology 2023 [Google Scholar]
- 94.Imperiale T. Clinical Validation of An Optimized MultiTarget Stool DNA (Mt-sDNA 2.0) Test, for Colorectal Cancer Screening “BLUE-C.” Identifier NCT04144738. (2019, November -).
- 95.Hommes D. Non-invasive Identification of Colorectal Cancer and Adenomas in Early Stages (NICE). Identifier NCT05445570. (2022, July -).
- 96.Diagnostics U. Collection of Samples USOPTIVAL Study. Identifier NCT04792684. (2021, March -).
- 97.Quantitative faecal immunochemical testing to guide colorectal cancer pathway referral in primary care, NICE diagnostic guidance, https://www.nice.org.uk/guidance/dg56, accessed October, 2023


