Abstract
Following infection of mice with lymphocytic choriomeningitis virus (LCMV), virus-neutralizing antibodies appear late, after 30 to 60 days. Such neutralizing antibodies play an important role in protection against reinfection. To analyze whether a neutralizing antibody response which developed earlier could contribute to LCMV clearance during the acute phase of infection, we generated transgenic mice expressing LCMV-neutralizing antibodies. Transgenic mice expressing the immunoglobulin μ heavy chain of the LCMV-neutralizing monoclonal antibody KL25 (H25 transgenic mice) mounted LCMV-neutralizing immunoglobulin M (IgM) serum titers within 8 days after infection. This early inducible LCMV-neutralizing antibody response significantly improved the host’s capacity to clear the infection and did not cause an enhancement of disease after intracerebral (i.c.) LCMV infection. In contrast, mice which had been passively administered LCMV-neutralizing antibodies and transgenic mice exhibiting spontaneous LCMV-neutralizing IgM serum titers (HL25 transgenic mice expressing the immunoglobulin μ heavy and the κ light chain) showed an enhancement of disease after i.c. LCMV infection. Thus, early-inducible LCMV-neutralizing antibodies can contribute to viral clearance in the acute phase of the infection and do not cause antibody-dependent enhancement of disease.
Against many cytopathic viruses such as poliovirus, influenza virus, rabies virus, and vesicular stomatitis virus, protective virus-neutralizing antibodies are generated early, within 1 week after infection (3, 31, 36, 44, 49). In contrast, several noncytopathic viruses (e.g., human immunodeficiency virus and hepatitis viruses B and C in humans or lymphocytic choriomeningitis virus [LCMV] in mice) elicit poor and delayed virus-neutralizing antibody responses (1, 7, 20, 24, 27, 35, 45, 48).
In the mouse, the natural host of LCMV, the acute LCMV infection is predominantly controlled by cytotoxic T lymphocytes (CTLs) in an obligatory perforin-dependent manner (13, 18, 28, 50). In addition to the CTL response, LCMV-specific antibodies are generated. Early after infection (by day 8), a strong antibody response specific for the internal viral nucleoprotein (NP) is mounted (7, 19, 23, 28). These early LCMV NP-specific antibodies exhibit no virus-neutralizing capacity (7, 10). Results from studies of B-cell-depleted mice and B-cell-deficient mice implied that the early LCMV NP-specific antibodies are not involved in the clearance of LCMV (8, 11, 12, 40). Late after infection (between days 30 and day 60), LCMV-neutralizing antibodies develop (7, 19, 22, 28, 33); these antibodies are directed against the surface glycoprotein (GP) of LCMV (9, 10). LCMV-neutralizing antibodies have an important function in protection against reinfection (4, 6, 38, 41, 47).
In some viral infections, subprotective virus-neutralizing antibody titers can enhance disease rather than promote host recovery (i.e., exhibit antibody-dependent enhancement of disease [ADE] [14, 15, 21, 46]). For example, neutralizing antibodies are involved in the resolution of a primary dengue virus infection and in the protection against reinfection. However, if subprotective neutralizing antibody titers are present at the time of reinfection, a severe form of the disease (dengue hemorrhagic fever/dengue shock syndrome [15, 21]), which might be caused by Fc receptor-mediated uptake of virus-antibody complexes leading to an enhanced infection of monocytes (15, 16, 25, 39), can develop. Similarly, an enhancement of disease after intracerebral (i.c.) LCMV infection was observed in mice which had been treated with virus-neutralizing antibodies before the virus challenge (6). ADE in LCMV-infected mice was either due to an enhanced infection of monocytes by Fc receptor-mediated uptake of antibody-virus complexes or due to CTL-mediated immunopathology caused by an imbalanced virus spread and CTL response.
To analyze whether LCMV-neutralizing antibodies generated early after infection improve the host’s capacity to clear the virus or enhance immunopathological disease, immunoglobulin (Ig)-transgenic mice expressing LCMV-neutralizing IgM antibodies were generated. After LCMV infection of transgenic mice expressing the Ig heavy chain (H25 transgenic mice), LCMV-neutralizing serum antibodies were mounted within 8 days, which significantly improved the host’s capacity to eliminate LCMV. H25 transgenic mice did not show any signs of ADE after i.c. LCMV infection.
Transgenic mice expressing the Ig heavy and light chains (HL25 transgenic mice) exhibited spontaneous LCMV-neutralizing serum antibodies and confirmed the protective role of preexisting LCMV-neutralizing antibodies, even though the neutralizing serum antibodies were of the IgM isotype. Similar to mice which had been treated with LCMV-neutralizing antibodies, HL25 transgenic mice developed an enhanced disease after i.c. LCMV infection, which indicated that ADE was due to an imbalance between virus spread and CTL response. Thus, the early-inducible LCMV-neutralizing antibody response significantly enhanced clearance of the acute infection without any risk of causing ADE.
MATERIALS AND METHODS
Generation of transgenic mice.
Gene segments coding for the Ig heavy-chain V (VH) region and Ig light-chain V (VL) region were cloned from the B-cell hybridoma KL25 (9), which neutralized the LCMV isolate WE. The VH segment contained the autologous promoter, the rearranged VDJ region, and the heavy-chain intron enhancer. It was isolated from a λ ZAP library (Stratagene, La Jolla, Calif.) generated from EcoRI-digested KL25 genomic DNA by using an intron enhancer-specific probe. The EcoRI fragment containing the VH region was ligated into the EcoRI site of a transgene expression vector encoding the genomic Cμ region of mouse IgM allotype a (Cμa) (30).
The VL region of KL25 was PCR amplified from KL25 genomic DNA by using a Vκ4-specific primer (5′- AAA AGA GCT CAA AAT GGA TTT TCA AGT GCA GAT TTT -3′, annealing at the first 23 nucleotides of the Vκ4 leader and introducing a SacI site 5′ of amino acid position −22) and a Jκ4-specific primer (5′- TAT ACT TAC GTT TTA TTT CCA ACT TTG TCC CCG -3′, annealing at the 3′ end of the Jκ4 segment including the sequence of the splice donor signal). The resulting PCR product containing the light-chain leader, the intron, and the rearranged VJ region of the light chain (VL) was cloned into the EcoRV site of pBluescript. After verification of the sequence of the PCR-derived fragment by automated DNA sequencing (Bio-Rad, Hercules, Calif.), the SacI/HindIII fragment containing the VL gene was ligated into the SacI/HindIII sites of a Cκ expression vector encoding the mouse κ light-chain C (Cκ) region (42).
To prepare DNA for microinjection, the μ heavy-chain transgene was excised by using restriction endonucleases AatII and XhoI, and the κ light-chain transgene was linearized at the unique XbaI site.
The two transgene constructs were coinjected into male pronuclei of fertilized oocytes of the mouse strain C57BL/6 LTK. Transgene integrations were screened by PCR and Southern blot analysis. Inbred C57BL/6 (H-2b) mice from the breeding colony of the Institut für Zuchthygiene, Tierspital Zürich, Zürich, Switzerland, were used as donors for fertilized oocytes.
Virus.
LCMV-WE was originally provided by F. Lehmann-Grube, Hamburg, Germany, and was grown on L-929 cells for 48 h in minimal essential medium–5% fetal calf serum after infection with an initial multiplicity of infection of 0.01.
FACS analysis.
Fluorescence-activated cell sorting (FACS) analysis was performed on a FACScan (Becton Dickinson, San Diego, Calif.) according to standard procedures. The following antibodies were used: rat anti-mouse CD45R (B220)–phycoerythrin conjugate (Sigma, St. Louis, Mo.) and mouse anti-mouse IgMa (the constant domain of mouse IgM allotype a [30])–fluorescein isothiocyanate conjugate (PharMingen, San Diego, Calif.). Living cells were gated by using a combination of forward scatter and 90° side scatter.
LCMV infectious focus formation assay.
Viral titers from spleens of infected mice were determined as described previously (5). Briefly, spleen homogenates were serially diluted 10-fold and grown on an MC57G cell monolayer for 48 h under an overlay of 1% methylcellulose in Dulbecco’s modified Eagle’s medium. Cells were fixed with 4% formalin in phosphate-buffered saline, and infectious foci were detected by intracellular LCMV staining of infected cells with the rat anti-LCMV monoclonal antibody (MAb) VL-4 (5).
LCMV neutralization in vitro: infectious focus reduction assay.
LCMV neutralization in vitro was determined with an infectious focus reduction assay as described previously (5). Briefly, serial 2-fold dilutions of 10-fold-prediluted sera were incubated with LCMV for 90 min at 37°C in a 96-well plate. MC57G mouse fibroblasts were added, and after approximately 4 h, when the cells had settled and had absorbed the nonneutralized virus, cells were overlaid with 1% methylcellulose in Dulbecco’s modified Eagle’s medium; 48 h later, cell monolayers were fixed with 4% formalin and remaining infectious foci were detected as in the focus formation assay by intracellular LCMV staining of infected cells with the rat anti-LCMV MAb VL-4. Sera were tested under nonreducing conditions to measure neutralization by total Ig. To obtain values for IgG, sera were reduced prior to the neutralization assay by adding 2-mercaptoethanol at a final concentration of 0.05 M for 90 min at room temperature. Sera were heat inactivated at 56°C for 60 min.
Cytotoxicity assay.
The cytolytic activity of spleen cells was tested in a 51Cr release assay as described previously (50). Briefly, MC57G target cells were coated with LCMV-derived peptide GP33-41 (32) or NP396-408 (37) or with an H-2Db-binding adenovirus peptide (26) as a negative control at concentrations of 10−6 M; 104 target cells were incubated in 96-well round-bottom plates with serial threefold dilutions of spleen effector cells starting at an effector-to-target ratio of 70:1 in a final volume of 200 μl. After 5 h of incubation at 37°C, 70 μl of supernatant was harvested and γ irradiation was measured.
RESULTS
Generation of the Ig-transgenic mouse lines H25 and HL25 expressing lymphocytic choriomeningitis virus-specific neutralizing antibodies.
The VH and VL gene segments of the B-cell hybridoma KL25 (9) secreting an LCMV-neutralizing MAb were cloned and ligated into transgene expression vectors encoding IgMa and Cκ (42), respectively (Fig. 1). Both constructs were microinjected into C57BL/6 oocytes. Integration and germ line transmission of the transgenes were monitored by PCR and Southern blot analysis (data not shown). Multiple transgene integrations in one founder led to the establishment of two independent transgenic mouse lines: the transgenic mouse line H25 expressed the transgenic heavy chain, whereas the transgenic mouse line HL25 expressed both the transgenic heavy and light chains.
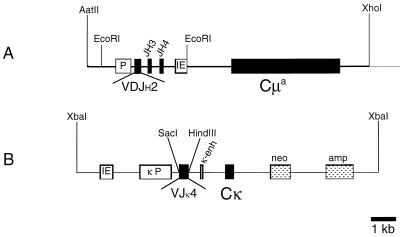
FIG. 1.
Structure of the Ig transgene constructs. (A) μ heavy-chain transgene; (B) κ light-chain transgene. Filled boxes represent Ig exons, hatched boxes represent antibiotic resistance genes, and open boxes, represent cis-acting promoter elements. The heavy- and light-chain constructs were linearized prior to microinjection by using restriction endonucleases AatII and XhoI (heavy chain) and XbaI (light chain). IE, Ig heavy-chain intron enhancer; P, autologous promoter of the cloned VH gene segment; κP, consensus κ light-chain promoter.
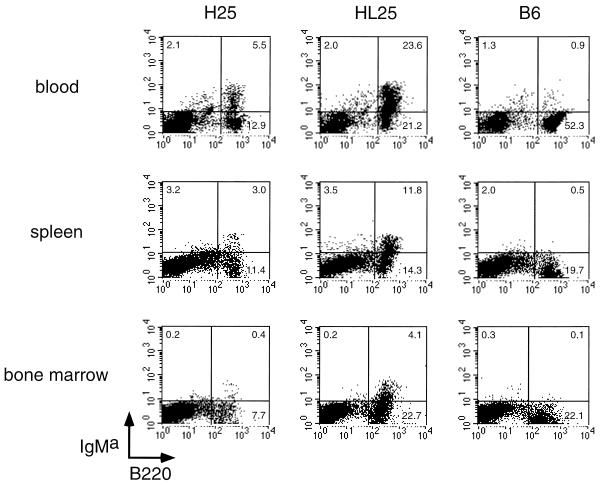
Expression of the transgenic IgMa heavy chain on the surface of B220-positive B cells from peripheral blood lymphocytes, spleen cells, and bone marrow cells of H25 and HL25 transgenic mice was determined by FACS analysis (Fig. 2). The proportion of cells expressing the surface IgMa was lower in H25 transgenic mice than in HL25 transgenic mice. This might be due either to the coexpression of the optimally fitting transgenes encoding the heavy and the light chains in HL25 transgenic mice or to different genomic integration sites of the heavy-chain transgenes in the two different transgenic mouse lines.
FIG. 2.
Surface expression of IgMa on B cells of H25 and HL25 transgenic mice. IgMa and B220 were stained on blood cells, spleen cells, and bone marrow cells of H25 transgenic mice, HL25 transgenic mice, and transgene-negative control mice (B6). Numbers in quadrants indicate percentages of gated living cells.
Expression of early-inducible versus spontaneous LCMV-neutralizing antibodies in H25 and HL25 transgenic mice.
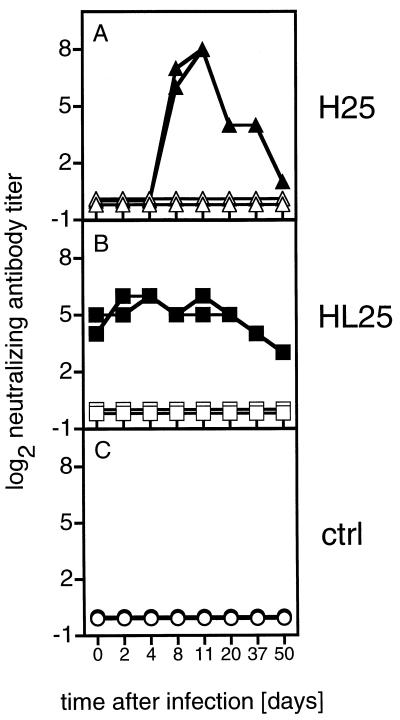
Sera of H25 and HL25 transgenic mice were analyzed for the presence of spontaneous LCMV-neutralizing serum antibodies by the infectious focus reduction assay (5). H25 transgenic mice did not express spontaneous LCMV-neutralizing serum antibodies, whereas HL25 transgenic mice showed LCMV-neutralizing antibody titers before the antigen challenge (Fig. 3A and B, day 0). After intravenous (i.v.) infection with 200 PFU of LCMV-WE, H25 transgenic mice mounted an LCMV-neutralizing Ig response peaking between days 8 and 11 after LCMV infection. In contrast, the spontaneous neutralizing titers of HL25 transgenic mice did not change after infection. Sera of nontransgenic control mice did not show any neutralizing activity within the observation period of 50 days (Fig. 3C). The neutralizing capacity of all sera was abolished when tested under reducing conditions, indicating that the virus neutralization was mediated by IgM antibodies presumably encoded by the transgenes.
FIG. 3.
LCMV-WE-neutralizing antibody titers in the sera of H25 and HL25 transgenic mice. H25 and HL25 transgenic mice and transgene negative control (ctrl) mice were infected i.v. with 200 PFU of LCMV-WE. Sera were collected at the indicated time points and were tested for virus-neutralizing total Ig (nonreducing conditions; closed symbols) and IgG (reducing conditions; open symbols) in an infectious focus reduction assay. Sera were prediluted 10-fold and titrated in 2-fold dilution steps. Values are for individual mice from one representative experiment of five similar experiments.
Enhanced virus elimination in H25 transgenic mice expressing early-inducible neutralizing antibodies.
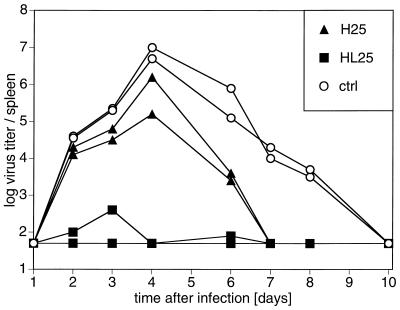
To analyze the influence of early-inducible versus preexisting LCMV-neutralizing antibodies on virus elimination, H25 and HL25 transgenic mice and transgene-negative littermates were infected i.v. with 200 PFU of LCMV-WE. Virus titers in the spleen were monitored from days 1 to 10 after infection. Early-neutralizing serum antibodies induced in H25 transgenic mice did not influence LCMV titers significantly up to day 4 after infection but thereafter reduced virus titers significantly and enhanced virus elimination (Fig. 4). This result demonstrated for the first time that the early generation of LCMV-neutralizing antibodies improved the host’s efficiency in eliminating LCMV.
FIG. 4.
H25 transgenic mice clear LCMV earlier than control mice. H25 and HL25 transgenic mice and transgene-negative control (ctrl) littermates were infected i.v. with 200 PFU of LCMV-WE. At days 1 to 10, spleens were tested for virus titers in an infectious focus formation assay. Virus titers per spleen of individual mice are indicated. The log titer of 1.7 is the detection limit of the assay. Values are for individual mice from one representative experiment of three similar experiments.
Preexisting neutralizing serum antibody titers of HL25 transgenic mice inhibited LCMV replication in the spleen from the day of infection onward. These mice were protected against i.v. infection, even though the transgene-encoded antibodies were of the IgM isotype. This result is in agreement with earlier findings demonstrating the protective capacity of passively administered LCMV-neutralizing antibodies (4, 6, 38, 41, 47).
H25 transgenic mice develop normal CTL activity.
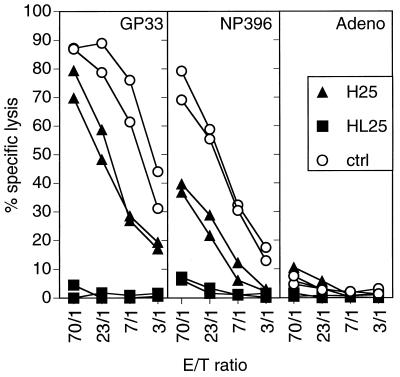
To analyze whether the enhanced LCMV elimination was due to the early LCMV-neutralizing antibody response or due to an enhanced CTL activity, H25 and HL25 transgenic mice and transgene-negative littermates were infected i.v. with 200 PFU of LCMV-WE. Eight days later, spleen cells were tested for cytolytic activity in a primary ex vivo 51Cr release assay. H25 transgenic mice showed an almost normal or even slightly reduced CTL activity compared to transgene negative littermates (Fig. 5). HL25 transgenic mice exhibited no measurable CTL activity. This finding indicated that the complete antiviral protection in HL25 transgenic mice and the enhanced clearance of LCMV in H25 transgenic mice were mediated by the transgene-encoded antibodies and were not due to an enhanced CTL activity.
FIG. 5.
CTL activity in H25 and HL25 transgenic mice. H25 and HL25 transgenic mice and transgene-negative control (ctrl) littermates were infected i.v. with 200 PFU of LCMV-WE. Eight days later, spleen cells were tested for cytolytic activity in a 5-h 51Cr release assay. MC57G target cells were labeled with the LCMV-specific peptide GP33-41 or NP396-408 or an irrelevant, H-2Db-binding adenovirus (Adeno) peptide. Percentages of specific lysis by splenic effectors of individual mice are plotted at the indicated effector/target (E/T) ratios. Spontaneous release was below 17%. Values are for individual mice from one representative experiment of three similar experiments.
ADE by preexisting LCMV-neutralizing IgM antibodies.
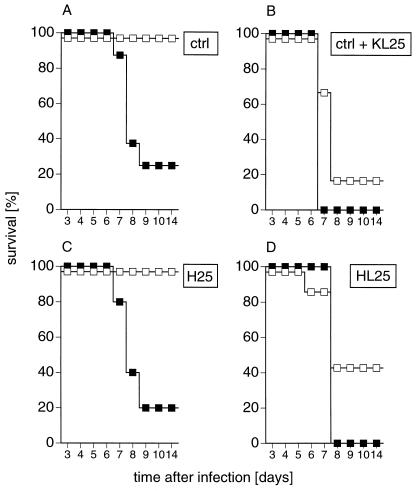
Within 7 to 8 days, mice succumbed to a low-dose (30-PFU) i.c. infection with LCMV-WE caused by CTL-mediated immunopathology. However, they survived a high-dose (105-PFU) i.c. infection because LCMV-specific CTLs were exhausted (17, 29) (Fig. 6A). If 200 μg of the LCMV-neutralizing IgG1 MAb KL25 was administered intraperitoneally 4 h before i.c. virus challenge, mice died after low and high doses of infection (6) (Fig. 6B). To test whether such an ADE after high-dose i.c. infection also occurred in H25 and HL25 transgenic mice, Ig-transgenic mice were infected i.c. with low- or high-dose LCMV-WE. As expected, the majority of H25 transgenic mice infected i.c. with low-dose LCMV-WE died. However, all H25 transgenic mice infected i.c. with high-dose LCMV-WE survived, indicating the absence of ADE (Fig. 6C). In contrast, 100 and 60% of HL25 transgenic mice infected i.c. with low-dose or high-dose LCMV-WE, respectively, died, indicating the presence of ADE (Fig. 6D). Thus, ADE was observed in mice treated with MAb KL25 and in HL25 transgenic mice exhibiting spontaneous LCMV-neutralizing antibody titers. In constrast, ADE was absent in normal mice which do not generate LCMV-neutralizing antibodies before day 30 and in H25 transgenic mice which mount an inducible LCMV-neutralizing antibody response after i.c. infection by day 8, similar to what was observed after i.v. infection (data not shown). Since after LCMV infection neutralizing IgM antibodies are observed in H25 and HL25 transgenic mice, enhancement of disease via binding to polymeric Fc receptor (2, 34, 43) should have occurred in both Ig-transgenic mice. Therefore, our results indicate that ADE of lymphocytic choriomeningitis presumably is caused by an antibody-influenced shift of the balance between virus spread and the CTL response.
FIG. 6.
Early-inducible LCMV-neutralizing antibodies in H25 mice do not enhance lethal choriomeningitis. H25 and HL25 transgenic mice were infected i.c. with 30 PFU (closed symbols) or 105 PFU (open symbols) LCMV-WE. Control mice were either passively treated intraperitoneally with 200 μg of MAb KL25 4 h prior to infection or left untreated and were i.c. infected as were the Ig-transgenic mice. Survival was monitored from days 1 to 14. Each group consisted of 6 to 10 mice. Shown are results of one of two similar experiments. ctrl, control.
DISCUSSION
In this present study, we cloned the gene segments encoding the VH and VL regions of the LCMV-neutralizing MAb KL25 and expressed them as μ heavy and κ light chains in transgenic mice. We established two independent mouse lines expressing the transgenic heavy chain (H25 transgenic mice) or both the transgenic heavy and light chains (HL25 transgenic mice). H25 transgenic mice developed LCMV-neutralizing IgM serum titers early after infection, which augmented elimination of the virus. These experiments demonstrated that early-inducible LCMV-neutralizing antibody titers supported the control of a noncytopathic virus during the acute phase of the infection and indicated a potentially important role of LCMV-neutralizing antibodies in clearance of the virus.
The HL25 transgenic mice exhibited spontaneous LCMV-neutralizing antibody serum titers which protected against i.v. infection, even though the transgene-encoded antibodies were of the IgM isotype. This finding is in accordance with previous reports showing the protective capacity of passively administered IgG antibodies (4, 6, 38, 41, 47).
The enhanced capacity of the H25 and HL25 transgenic mice to eliminate LCMV was not due to an enhanced CTL activity. Ex vivo CTL activity was always lower in H25 transgenic mice than in transgene-negative littermates and was below detection limits in HL25 transgenic mice. Obviously, the early-developing LCMV-neutralizing antibodies in H25 transgenic mice allowed an almost normal priming of CTLs, whereas the spontaneous titers of LCMV-neutralizing antibodies in HL25 transgenic mice neutralized LCMV quantitatively and prevented induction of CTLs. Therefore, the improvement of LCMV clearance in H25 and HL25 transgenic mice was mediated by the transgene-encoded antibodies.
Earlier studies suggested some role of the antibody-Fc part for in vivo protection, since LCMV-neutralizing MAbs of the IgG2a isotype protected from lethal lymphocytic choriomeningitis, whereas MAbs of the IgG1 isotype did not (4). This was further supported by the finding that the F(ab′)2 fragment generated proteolytically from one protective IgG2a MAb did not protect (4). The LCMV-neutralizing MAb KL25, which is of the IgG1 subclass, was protective against i.v. LCMV infection in an in vivo passive immunization experiment (38). After transferring the LCMV GP1 specificity of MAb KL25 to the IgM isotype, the transgenic IgM retained the in vitro neutralizing capacity and was protective in vivo. These results indicated that the isotype dependence of in vivo antiviral protection against LCMV infection is related to the antibody specificity analyzed.
Mice passively immunized with LCMV-neutralizing antibodies showed ADE of choriomeningitis after i.c. infection with a high dose (105 PFU) of LCMV-WE (6). This was due either to enhanced infection of monocytes with virus-antibody complexes via their Fc receptors or to an antibody-mediated shift of the balance between virus spread and the CTL response leading to CTL-mediated immunopathology (6). In our experiments, the transferred MAb KL25 and the preexisting LCMV-neutralizing antibodies in HL25 transgenic mice led to ADE after high-dose i.c. infection, whereas the inducible LCMV-neutralizing antibodies in H25 transgenic mice did not. Since LCMV-neutralizing IgM antibodies are generated after infection of H25 transgenic mice, an enhanced infection of monocytes should have occurred in H25 transgenic mice as well as in HL25 transgenic mice via the binding to the polymeric Fc receptor (2, 34, 43). Obviously, the inducible virus-neutralizing serum antibodies are generated too late to influence the balance between virus spread and CTL response. Therefore, ADE of lymphocytic choriomeningitis presumably is due to an imbalance between virus spread and CTL response mediated by preexisting neutralizing antibody titers.
In conclusion, we demonstrated that early-generated LCMV-neutralizing antibodies enhanced clearance of LCMV after i.v. infection without the risk for ADE after i.c. infection. Therefore, vaccination strategies accelerating virus-neutralizing antibody responses may enhance clearance of noncytopathic viruses without the risk of causing ADE.
ACKNOWLEDGMENTS
We thank H. Gram and A. Traunecker for providing the Ig transgene expression vectors, G. Christiansen and D. Zimmerman for synthesis of the oligonucleotides, and K. Karlsson, S. Walser-Förster, J. Brecher, and K. Riem for excellent and always patient technical support. Special thanks go to P. Aichele, B. M. Senn, T. Uhr, and the entire institute for very helpful discussions.
This work was supported by Swiss National Science Foundation grants 31.32179.91 and 31.50884.97 and by the Kanton Zürich, Switzerland.
REFERENCES
- 1.Alberti A, Pontisso P, Tagariello G, Cavalletto D, Chemello L, Belussi F. Antibody response to pre-S2 and hepatitis B virus induced liver damage. Lancet. 1988;25:1421–1424. doi: 10.1016/s0140-6736(88)92237-4. [DOI] [PubMed] [Google Scholar]
- 2.Andersson B, Skoglund A C, Ronnholm M, Lindsten T, Lamon E W, Collisson E W, Walia A S. Functional aspects of IgM and IgG Fc receptors on murine T lymphocytes. Immunol Rev. 1981;56:5–50. doi: 10.1111/j.1600-065x.1981.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 3.Baer G M, Bellini W J, Fishbein D B. Rhabdoviruses. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 883–930. [Google Scholar]
- 4.Baldridge J R, Buchmeier M J. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J Virol. 1992;66:4252–4257. doi: 10.1128/jvi.66.7.4252-4257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel R M. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 or 96 well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 6.Battegay M, Kyburz D, Hengartner H, Zinkernagel R M. Enhancement of disease by neutralizing antiviral antibodies in the absence of primed antiviral cytotoxic T cells. Eur J Immunol. 1993;23:3236–3241. doi: 10.1002/eji.1830231229. [DOI] [PubMed] [Google Scholar]
- 7.Battegay M, Moskophidis D, Waldner H, Bründler M A, Fung-Leung W P, Mak T W, Hengartner H, Zinkernagel R M. Impairment and delay of neutralizing antiviral antibody responses by virus specific cytotoxic T cells. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 8.Bründler M-A, Aichele P, Bachmann M F, Kitamura D, Rajewsky K, Zinkernagel R M. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur J Immunol. 1996;26:2257–2262. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 9.Bruns M, Cihak J, Müller G, Lehmann-Grube F. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology. 1983;130:247–251. doi: 10.1016/0042-6822(83)90135-6. [DOI] [PubMed] [Google Scholar]
- 10.Buchmeier M J, Lewicki H A, Tomori O, Oldstone M B A. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981;113:73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- 11.Cerny A, Hügin A W, Sutter S, Bazin H, Hengartner H, Zinkernagel R M. Immunity to lymphocytic choriomeningitis virus in B cell-depleted mice: evidence for B cell and antibody independent protection by memory T cells. Eur J Immunol. 1986;16:913–917. doi: 10.1002/eji.1830160807. [DOI] [PubMed] [Google Scholar]
- 12.Cerny A, Sutter S, Bazin H, Hengartner H, Zinkernagel R M. Clearance of lymphocytic choriomeningitis virus in antibody- and B-cell-deprived mice. J Virol. 1988;62:1803–1807. doi: 10.1128/jvi.62.5.1803-1807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole G A, Nathanson N, Prendergast R A. Requirement for thetabearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972;238:335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- 14.Corapi W V, Olsen C W, Scott F W. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J Virol. 1992;66:6695–6705. doi: 10.1128/jvi.66.11.6695-6705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 16.Homsy J, Meyer M, Tateno T, Clarkson S, Levy J A. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science. 1989;244:1357–1360. doi: 10.1126/science.2786647. [DOI] [PubMed] [Google Scholar]
- 17.Hotchin J. The biology of lymphocytic choriomeningitis infection: virus induced immune disease. Cold Spring Harbor Symp Quant Biol. 1962;27:479–499. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- 18.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 19.Kimmig W, Lehmann-Grube F. The immune response of the mouse to lymphocytic choriomeningitis virus. I. Circulating antibodies. J Gen Virol. 1979;45:703–710. doi: 10.1099/0022-1317-45-3-703. [DOI] [PubMed] [Google Scholar]
- 20.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurane I, Ennis F E. Immunity and immunopathology in dengue virus infections. Semin Immunol. 1992;4:121–127. [PubMed] [Google Scholar]
- 22.Larsen J H. Development of humoral and cell-mediated immunity to lymphocytic choriomeningitis virus in the mouse. J Immunol. 1969;102:941–946. [PubMed] [Google Scholar]
- 23.Lehmann-Grube F. Lymphocytic choriomeningitis virus. Virol Monogr. 1971;10:1–173. [Google Scholar]
- 24.Lemon S M, Thomas D L. Vaccines to prevent viral hepatitis. N Engl J Med. 1997;336:196–204. doi: 10.1056/NEJM199701163360307. [DOI] [PubMed] [Google Scholar]
- 25.Lewis R M, Cosgriff T M, Griffin B Y, Rhoderick J, Jahrling P B. Immune serum increases arenavirus replication in monocytes. J Gen Virol. 1988;69:1735–1739. doi: 10.1099/0022-1317-69-7-1735. [DOI] [PubMed] [Google Scholar]
- 26.Luescher I F, Loez J A, Malissen B, Cerottini J C. Interaction of antigenic peptides with MHC class I molecules on living cells studied by photoaffinity labeling. J Immunol. 1992;148:1003–1011. [PubMed] [Google Scholar]
- 27.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskophidis D, Cobbold S P, Waldmann H, Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987;61:1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskophidis D, Lechner F, Pircher H P, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 30.Nemazee D A, Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 31.Ogra P L, Fishaut M, Gallagher M R. Viral vaccination via the mucosal routes. Rev Infect Dis. 1980;2:352–369. doi: 10.1093/clinids/2.3.352. [DOI] [PubMed] [Google Scholar]
- 32.Pircher H P, Moskophidis D, Rohrer U, Bürki K, Hengartner H, Zinkernagel R M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 33.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Specific cytotoxic T cells eliminate cells producing neutralizing antibodies. Nature. 1996;382:726–728. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 34.Raghavan M, Bjorkman P J. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181–220. doi: 10.1146/annurev.cellbio.12.1.181. [DOI] [PubMed] [Google Scholar]
- 35.Robert G M, Brown M, Gallo R C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985;316:72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- 36.Sabin A B. Paralytic poliomyelitis: old dogmas and new perspectives. Rev Infect Dis. 1981;3:543–564. doi: 10.1093/clinids/3.3.543. [DOI] [PubMed] [Google Scholar]
- 37.Schulz M, Aichele P, Vollenweider M, Bobe F W, Cardinaux F, Hengartner H, Zinkernagel R M. MHC dependent T cell epitopes of LCMV nucleoprotein and their protective capacity against viral disease. Eur J Immunol. 1989;19:1657–1667. doi: 10.1002/eji.1830190921. [DOI] [PubMed] [Google Scholar]
- 38.Seiler, P., M.-A. Bründler, C. Zimmerman, D. Weibel, M. Bruns, H. Hengartner, and R. M. Zinkernagel. Induction of protective cytotoxic T cell responses in the presence of high titers of virus-neutralizing antibodies: implications for passive and active immunization. J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 39.Takeda A, Tuazon C U, Ennis F A. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988;242:580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- 40.Thomsen A R, Johansen J, Marker O, Christensen J P. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 41.Thomsen A R, Marker O. The complementary roles of cellular and humoral immunity in resistance to re-infection with LCM virus. Immunology. 1988;65:9–15. [PMC free article] [PubMed] [Google Scholar]
- 42.Traunecker A, Luke W, Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988;331:84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- 43.Uher F, Dobronyi I, Gergel J. IgM-Fc receptor-mediated phagocytosis of rat macrophages. Immunology. 1981;42:419–425. [PMC free article] [PubMed] [Google Scholar]
- 44.Webster R G, Rott R. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell. 1987;50:665–666. doi: 10.1016/0092-8674(87)90321-7. [DOI] [PubMed] [Google Scholar]
- 45.Weiss R A, Clapham P R, Cheingsong P R, Dalgleish A G, Carne C A, Weller I, Tedder R S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985;316:69–71. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- 46.Weiss R C, Dodds W J, Scott F W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparison with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis. 1981;4:175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright K E, Buchmeier M J. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J Virol. 1991;65:3001–3006. doi: 10.1128/jvi.65.6.3001-3006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright T L, Lau J Y N. Clinical aspects of hepatitis B virus infection. Lancet. 1993;342:1340–1344. doi: 10.1016/0140-6736(93)92250-w. [DOI] [PubMed] [Google Scholar]
- 49.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel R M, Leist T P, Hengartner H, Althage A. Susceptibility to lymphocytic choriomeningitis virus isolates correlates directly with early and high cytotoxic T cell activity, as well as with footpad swelling reaction, and all three are regulated by H-2D. J Exp Med. 1985;162:2125–2141. doi: 10.1084/jem.162.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]