Abstract
Aims
Buprenorphine is effective at reducing relapse to opioid misuse, morbidity and mortality in opioid‐dependent patients. Urine drug screening (UDS) to assess adherence is used routinely in opioid agonist treatment (OAT). The primary aim of this study was to determine factors which may be associated with a negative qualitative urine drug screen for buprenorphine in OAT patients.
Methods
This prospective pilot study was conducted at a tertiary addiction medicine centre. Twenty participants on stable treatment underwent supervised administration of sublingual buprenorphine. Matched urine and blood samples were collected prior to and 2, 4 and 6 hours after buprenorphine administration. Qualitative urine drug screen results were obtained using gas chromatography–mass spectrometry (GC–MS), while quantitative blood and urine results were obtained using ultra‐performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS).
Results
Qualitative urine assay yielded a negative result for buprenorphine in 57% of tested samples. The median concentration of urinary buprenorphine was 167 mcg/L (range: 2–1730 mcg/L). Thirty percent of all blood samples did not detect buprenorphine (range 0–18 mcg/L). Positive qualitative urine drug screen results were associated with higher urine (343 mcg/L compared with 75 mcg/L; P < .05) and blood (4 mcg/L compared with 2 mcg/L; P < .05) buprenorphine concentrations. Median urine concentrations of buprenorphine were highest at 2 hours and were higher in participants receiving CYP3A4 inhibitors.
Conclusion
Interpretation of qualitative urine drug screens to assess adherence in OAT is complex. Poor adherence with treatment cannot be assumed in patients returning a negative qualitative GC–MS urine drug screen.
Keywords: addiction, mass spectrometry, opioids
What is already known about this subject
Urine drug screens are used to monitor adherence in buprenorphine treatment.
Guidelines recommend confirmatory testing with gas chromatography–mass spectrometry (GC–MS).
There is no standardised assay methodology.
What this study adds
GCMS may have high rates of false negatives.
There is a need for a standardised assay.
Clinicians need to have a clear understanding of limitations of their local assay to inform clinical decision making.
Use of GC–MS for routine UDS is most likely to yield a positive result 2–4 hours after dose administration.
Dose of buprenorphine, CYP3A4 polymorphisms or concurrent use of CYP3A4 interacting drugs do not appear to significantly influence the likelihood of a negative UDS result.
1. INTRODUCTION
Engagement in opioid agonist treatment (OAT) reduces the risk of death for people with opioid dependence by half compared to the out‐of‐treatment population. 1 Adherence to treatment with the low efficacy μ‐opioid agonist buprenorphine (BUP) has been shown to reduce the risk of relapse, morbidity and all‐cause mortality. 2 , 3 Management of opioid dependence with BUP, either alone or in an abuse deterrent combination with naloxone is widely accepted. Sublingual BUP can be provided as directly observed therapy (DOT) with daily attendance or with varying degrees of supervised or unsupervised dosing as treatment progresses.
Adherence with initiation and implementation phases of the medication regimen is critical to ensuring efficacy, while non‐adherence has been linked to increased risk of relapse, overdose and worse HIV treatment outcomes. 4 There are various methods of monitoring adherence to treatment, including pill counts, electronic dosing aids and blood or urine drug screening. In OAT, many jurisdictions require that treatment compliance and outcomes be monitored with regular urine drug screening and the results can influence the assessment of treatment adherence and effectiveness. 5 A urine drug screen (UDS) negative for the prescribed opioid can be interpreted to indicate non‐adherence and/or diversion, 6 the consequences of which can be treatment discontinuation or more restrictive dosing. 5 In addition to clinical decisions, UDS can be used with important consequences in legal and forensic settings where decisions around child custody and sentencing are made. 7 While UDS can be mandated in treatment, patients may find the process stigmatising to perform. 8
Interpretation of drug screening (both plasma and urine) requires an understanding of the pharmacological properties, metabolism and other influencing factors such as drug–drug interactions. Phenoconversion as a result of drug–drug interaction has been shown to influence the plasma concentration and the amount of drug extracted in urine. 9 Further, detection rates are highly dependent on detection thresholds of the assay used. Inadequate interpretation of negative UDS results can be costly for the patient. 10 Practitioner's knowledge of the use and interpretation of UDS in the OAT context has been shown to be critical for treatment individualisation. 11
BUP is extensively metabolised (70–90%) to the primary metabolite norbuprenorphine (Norbup) through cytochrome P450 (CYP) 3A4 12 , 13 , 14 and to a lesser extent by CYP2C8 and CYP3A5. 12 Both BUP and Norbup undergo conjugation to glucuronides via the enzyme UGT1A1/3 15 and subsequently are excreted in bile/faeces and in the urine. Therefore, factors that influence CYP3A4 activity, such as co‐prescribed medications or genetic variations, may impact the conversion of BUP to Norbup 13 , 14 , 16 and influence urine BUP testing.
In a retrospective study, we have previously identified low rates of UDS detection of BUP despite directly observed therapy. Subjects on CYP3A4‐interacting medications were less likely to test positive for BUP. 17 Therefore, the objective of this prospective pilot study was to explore reasons for negative BUP UDS in OAT. We sought to identify the possible impact of timing, plasma concentrations of parent (BUP) and its metabolite (Norbup), BUP dose, polymorphisms in either CYP3A4, CYP3A5, CYP2C8 or UGT1A1 isozymes and drug–drug interactions with medications that were CYP3A4 substrates on detection rates of BUP in urine with routinely used gas chromatography–mass spectrometry (GC–MS) qualitative testing methods.
2. METHODS
2.1. Study participants and sample collection
This study was conducted in two Opioid Treatment Programme clinics in a single Local Health District in inner city Sydney, Australia. Participants were recruited through advertisement displayed in the clinic.
Inclusion criteria were stability of treatment with BUP, defined by at least 2 weeks of a stable prescribed dose of BUP or combination BUP and naloxone, and able to provide written informed consent. Exclusion criteria were age less than 18 and if participants were unable or unwilling to commit to the 6 hour study protocol. Subjects were provided a $20 grocery voucher on completion of the study protocol.
After screening for inclusion criteria and written informed consent, on the study day, urine and blood samples were collected (time = 0), and participants were then administered their usual dose and formulation of BUP. Both UDS and plasma samples were collected every 2 hours for 6 hours at time = 2, 4, 6 hours. Two individual urine and one blood sample were collected at each time point. Blood and one urine sample underwent quantitative analysis, while the second urine sample underwent qualitative analysis.
Baseline demographic data including age, sex, height, weight, BUP dose, indication for BUP, other opioid use, comorbidities and medication use were recorded. Drug use history was collected and assessed using the Australian Treatment Outcomes Profile (ATOP), 18 on the study day. Baseline biochemistry was also performed on each participant, including full blood count, liver function, urea, electrolytes and blood creatinine concentrations.
2.2. Toxicological testing
Urine drug screening was carried out through two independent National Association of Testing Authorities (NATA) accredited laboratories. NATA accreditation requires laboratories to adhere to strict Australian standards on equipment, including selection, checks and maintenance, validation and verification. 19 Both laboratories are part of the state health department and have external governance by New South Wales (NSW) Health Pathology.
Laboratory 1 performed qualitative assay for detection of BUP with GC–MS. Laboratory 2 carried out quantitative testing for both BUP and Norbup in plasma and urine with ultra‐performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS).
2.2.1. Qualitative testing
Urine was stored at 2–8°C prior to transport for testing, and transported to the laboratory within 24 hours of collection. Urine testing was a two‐step process.
In the first step, 5 mL of urine underwent GC–MS for detection of compounds other than BUP. In the second step, 40 μL of lipid‐β‐glucuronidase was added to a further 5 mL of the same urine sample. The enzyme‐impregnated sample was hydrolysed overnight at a temperature of 40–42°C prior to GC–MS confirmatory BUP testing. The presence of BUP was confirmed on samples with an ion match greater than 70% using software analysis against a pre‐categorised drug library reference. There was no lower limit of detection for BUP reported for this laboratory.
2.2.2. Quantitative testing
This was performed on both plasma and urine. Samples were analysed for BUP and Norbup using UPLC–MS/MS in multiple reaction monitoring (MRM) mode. Certified reference standards of BUP and Norbup were prepared in drug‐free matrices and extracted as matrix‐matched calibrators. Drug concentrations were quantitated based on the ratio of calibrator to deuterated internal standard responses using calibration curves calculated by the instrument software.
Plasma samples were prepared for analysis by protein precipitation of 100 μL of plasma followed by mixed mode solid phase extraction (SPE). Isotopically labelled internal standards, D4‐BUP and D3‐Norbup, were used to normalise extraction efficiencies in plasma. Urine samples (1 mL sample) were extracted using mixed mode SPE after enzymatic hydrolysis with BG100 β‐glucuronidase. D4‐BUP was used as internal standard to normalise extraction efficiencies in urine.
Analytical methods were locally validated in accordance with NATA requirements for validation and verification of quantitative and qualitative methods. Limits of detection for BUP are 1.00 μg/L in plasma and 0.03 μg/L in urine. Limits of detection for Norbup are 2.00 μg/L in plasma and 0.30 μg/L in urine.
2.3. Genetic testing for CYP and UGT1A1 polymorphisms
DNA was isolated from blood using a QIAcube automated extraction instrument (Qiagen, Hilden, Germany). Genotyping was performed using TaqMan SNP genotyping assays (Thermo Fisher Scientific, Waltham, MA, USA) for three single nucleotide polymorphisms (SNPs) across CYP3A4 (CYP3A4*13: rs4986909, CYP3A4*15A: rs4986907 and CYP3A4*22: rs35599367), one SNP in CYP3A5 (CYP3A5*3: rs776746) and three SNPs across CYP2C8 (CYP2C8*3: rs11572080 and rs10509681, CYP2C8*4: rs1058930). Briefly, polymerase chain reaction (PCR) was performed using the following parameters: 60°C for 30 seconds (data collection), 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute (data collection), and finally 60°C for 30 seconds (data collection). Genotypes were read using the TaqMan Genotyping Software (v3.1) (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). A 2 base‐pair (bp) deletion in the promoter region of UGT1A1 (UGT1A1*28: rs3064744) was genotyped using Sanger DNA sequencing. A 184 bp product was amplified using the following parameters: 94°C for 2 minutes (1 cycle), 94°C for 15 seconds, 59°C for 15 seconds and 68°C for 50 seconds (35 cycles), and finally 68°C for 7 minutes. Primers used for amplification were: Forward 5′‐TCCCTGCTACCTTTGTGGAC‐3′ and reverse 5′‐AGCAGGCCCAGGACAAGT‐3′. Sequencing PCR was performed using the Dye Terminator Cycle Sequencing Kit 3.1 (Applied Biosystems) and sequenced on 3730 DNA Analyzer (Applied Biosystems).
2.4. Ethics
Approval was granted by the Sydney Local Health District Research Ethics and Governance Office, New South Wales Health, reference: X19–0266 and 2019/ETH11851, and consent included biobank storage for subsequent genomic analysis.
2.5. Data analysis
Outcomes of qualitative analysis (QLA) of UDS, in particular a negative result, were explored against factors including dose, timing of UDS, urine concentrations of BUP on quantitative (QNA) analysis, plasma concentrations of BUP, BUP:Norbup, use of CYP450 interacting medications and polymorphisms in CYP and UGT1A1. Demographic characteristics and data from ATOP, while collected, were not explored in relationship to the UDS result.
Statistical analysis was performed using Graphpad Prism® version 9.0 (GraphPad Software Inc., La Jolla, CA, USA). Descriptive statistics were used for demographic and categorical data. Non‐parametric testing (Mann–Whitney U‐test) was performed to assess differences between groups (positive vs negative UDS, higher dose vs lower dose) for individual factors hypothesised to impact the UDS result. EMERGE guidelines were followed in reporting this study. 20
3. RESULTS
3.1. Demographic data
Of 20 participants, 16 (80%) were male, with a mean age of 51 years (range 32–67). The median dose of BUP was 14 mg (range 0.4–32). Forty percent had co‐existing mental health diagnosis, and only 25% had a history of hepatitis C (Table 1).
TABLE 1.
Demographic data of patients enrolled in study
| Characteristics | n | N (%) |
|---|---|---|
| Number of participants, n (%) | 20 | |
| Sex, n | 20 | |
| Male | 16 (80.00) | |
| Female | 4 (20.00) | |
| Age, years, median (IQR) a | 20 | 50 (43.50–59.30) |
| Body mass index (BMI), median (IQR) a | 20 | 28 (24–32) |
| Indication for buprenorphine treatment | 20 | |
| Pain | 5 (25.00) | |
| Opioid dependence | 15 (75.00) | |
| Dose of buprenorphine, mg, median (IQR) a | 20 | 14 (4–24) |
| Duration of treatment on current dose of buprenorphine, days, median (IQR) a | 217 (77–364) | |
| Dosing site | 20 | |
| Pharmacy | 9 (45.00) | |
| Outpatient hospital clinic | 11 (55.00) | |
| Number of days out of 28 observed dosing | 20 | |
| <7 | 10 (50.00) | |
| 7–14 | 0 (0.00) | |
| 14–28 | 10 (50.00) | |
| Organ dysfunction (%) | 20 | |
| Liver impairment b | 3 (15.00) | |
| Kidney impairment c | 2 (10.00) | |
| Pre‐existing mental health diagnosis | 20 | |
| Yes | 8 (40.00) | |
| No | 12 (60.00) | |
| Co‐prescription of CYP3A4 interacting medication, number of participants | 20 | |
| Inducer | 1 (5.00) | |
| Inhibitor | 4 (20.00) | |
| Substrate | 8 (40.00) | |
| Prior history of blood‐borne virus | 20 | |
| HCV | 4 (20.00) | |
| HBV | 0 (0.00) | |
| HIV | 0 (0.00) | |
| Co‐infection with HCV, HBV, HIV | 1 (5.00) |
IQR; interquartile range.
Liver impairment defined as presence or absence of documented cirrhosis.
Kidney impairment defined as estimated glomerular filtration rate (eGFR) less than the lower limit of normal for age.
Fifteen participants were on BUP treatment for previous opioid dependence, including heroin (n = 10), codeine (n = 3), oxycodone (n = 1) and morphine (n = 1). Five participants had BUP initiated as a part of chronic pain treatment. Only one participant was on concurrent opioid treatment (transdermal fentanyl) in the 4 weeks prior to study.
3.2. ATOP summary
Data from the Australian Treatment Outcome Profile demonstrate low rates of other drug or alcohol use, with the majority of participants reporting infrequent use, that is, on less than 7 days over the previous 28 days. Twenty percent of participants used alcohol, and 30% used benzodiazepines on seven or more days over the previous 28 day period. Approximately 45% of participants smoked tobacco products on a daily basis (Table S1 in the Supporting Information).
3.3. Samples
Blood samples were provided by all 20 participants at each of the four time points. Ninteen subjects provided UDS at all four time points and one subject provided three samples at time t = 0, 2 and 6 hours.
3.4. Toxicological testing
3.4.1. Urine analysis, dose and timing
Thirty‐four (43% of 79) UDS samples that underwent QLA detected BUP, and 45 (57%) did not detect BUP, despite DOT. In addition, in participants (n = 3) taking daily BUP doses of 0.8 mg or less, BUP was not detected in QLA at any time point. Dose of buprenorphine did not appear to significantly impact on the likelihood of negative UDS result. Of 45 UDS that failed to detect buprenorphine, 21 (47%) were from participants receiving ≤ 8 mg buprenorphine per day while 24 (53%) were from participants receiving > 8 mg buprenorphine per day.
All quantitative UDS (QNA) samples detected both BUP and Norbup, including in those participants on BUP doses of 0.8 mg or less. The median concentration of urinary BUP in the quantitative analysis was 167 (mean 330) mcg/L (range: 2–1730 mcg/L, Figure 1A) and Norbup was 366 (mean 743) mcg/L (range: 5–4322 mcg/L, Figure 1B).
FIGURE 1.
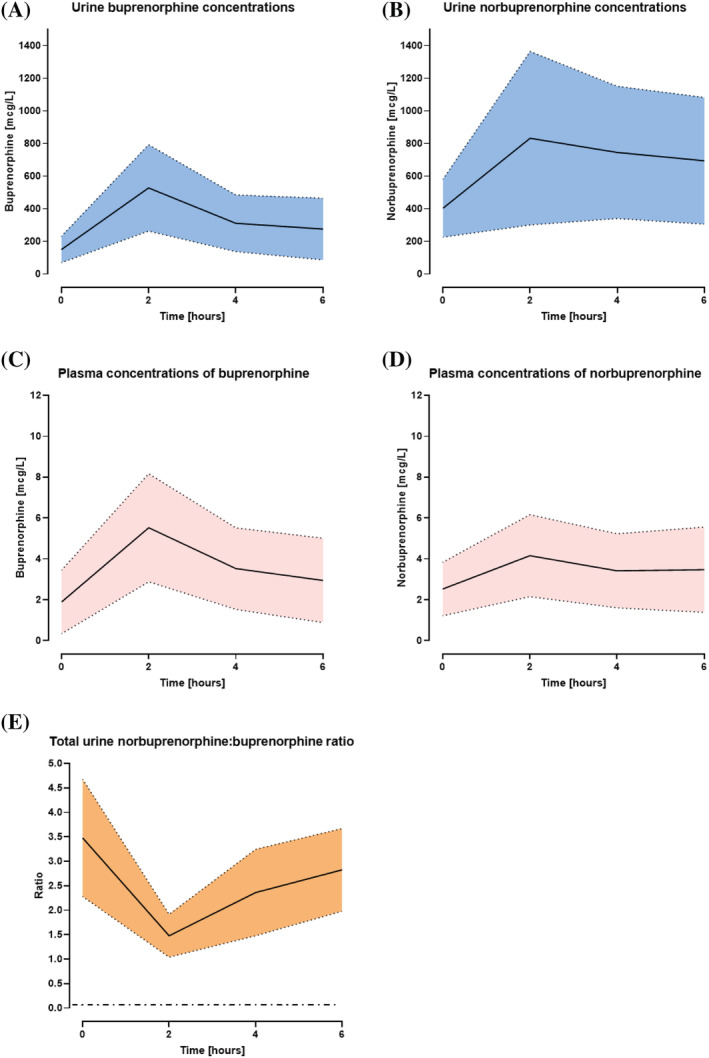
Urine (A & B) and plasma (C& D) concentrations of BUP and Norbup at t = 0, 2, 4, 6 hours. (E) Norbuprenorphine:buprenorphine urine ratio vs time at all time points. Horizontal dash‐dot black line denotes ratio proposed to indicate adulteration (ratio less than 0.26). Solid line represents median. Dotted lines represent interquartile range
A negative QLA UDS was observed in 65% of tested samples at t = 0 and 60% of samples tested at t = 6 hours. Quantitative plasma and urine concentrations of buprenorphine were lowest at t = 0 (Figure 1) compared with t = 2, t = 4 or t = 6 hours (Mann–Whitney U‐test; P < .05). The likelihood of a positive QLA UDS was highest at 2 and 4 hours after administration of buprenorphine, with 50% of samples testing positive at each of these time points. Peak plasma concentration of buprenorphine was observed at t = 2 hours.
3.4.2. Blood analysis
The range of plasma BUP was 0–18 mcg/L and Norbup was 0–16 mcg/L.
Thirty percent (n = 24) of plasma samples did not detect (ND) BUP and 49% (n = 31) did not detect Norbup. BUP samples were ND most commonly in plasma at trough levels (t = 0; n = 9) and t = 6 hours (n = 7). Highest concentration of both plasma and urine BUP and Norbup were detected at t = 2 hours post buprenorphine administration (Figure 1).
Lower urine (Figure 2A) and plasma (Figure 2B) concentrations of buprenorphine detected via quantitative methods were associated with negative qualitative UDS results (Mann–Whitney U‐test; P < .05).
FIGURE 2.
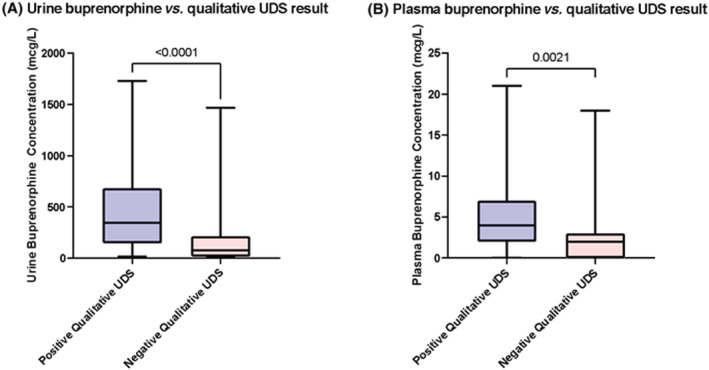
Comparison of combined buprenorphine urine (A) or plasma (B) concentrations at all time points (n = 80) vs qualitative urine drug screen results (n = 79). Detection of buprenorphine on qualitative UDS was associated with higher urine and plasma concentrations in quantitative testing (P < .0001, P = .0021, respectively). Solid lines represent the median. Error bars represent range
3.5. Urinary Norbup:BUP ratios
The mean ratio was 3, median was 2, and mode was 1.76 with a range of ratios spanning 0.36 to 9.73. There was no association between Norbup:BUP ratios in QNA and likelihood of detection on QLA (Figure 3). This was consistent across all individual time points.
FIGURE 3.
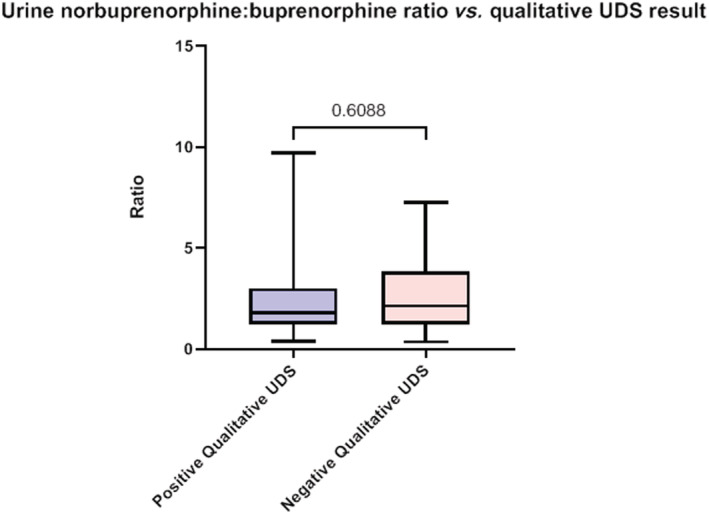
Comparison of combined norbuprenorphine:buprenorphine urine concentrations at all time points compared to qualitative UDS results. Solid lines represent median. Error bars represent range
There was a correlation of urinary ratios with the dose of BUP. Participants on doses of BUP greater than or equal to 8 mg had a higher urinary Norbup:BUP ratio (Mann–Whitney U‐test; P < .05, Figure 4).
FIGURE 4.
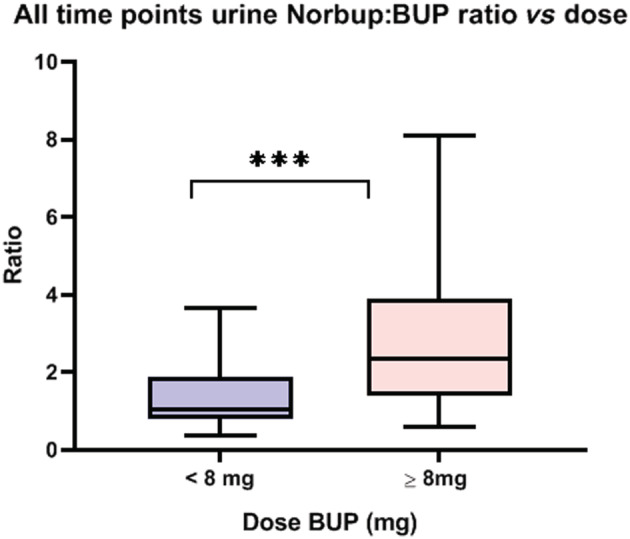
Comparison of urine norbuprenorphine:buprenorphine ratios of participants on low (< 8 mg) vs high (> 8 mg) BUP dose. Comparison with Mann–Whitney test, *** P = .0002
3.6. CYP3A4 medications and UDS results
There were four participants who reported taking a CYP3A4 inhibitor (20%). Three were male and one female, two were prescribed BUP for pain. The CYP3A4 inhibitors included esomeprazole, tacrolimus and duloxetine. All four tested positive on qualitative UDS assays at all time points (Figure S1 in the Supporting Information), irrespective of dose (range 8–32 mg). There was a single participant on the CYP3A4 inducer topiramate, who tested negative on QLA at all time points (BUP dose 12 mg).
Genetic testing for CYP3A4*13, CYP3A4*15A, CYP3A4*22, CYP3A5*3, CYP2C8*3, CYP2C8*4 and UGT1A1*28 polymorphisms were carried out; however, no correlation was observed between those who had a polymorphism and their UDS result, likely due to small sample size.
4. DISCUSSION
Traditionally, urine drug testing in OAT has been used as a part of routine clinical practice for two reasons: to monitor adherence to prescribed therapy 21 , 22 and the use of non‐prescribed illicit substances (treatment effectiveness). 23 , 24 Recent work demonstrating that up to 43% of BUP UDS can test negative in this setting 17 has challenged the use of UDS as a definitive indicator of non‐adherence. Therefore, we prospectively evaluated the accuracy of BUP UDS, compared to QNA testing in a treatment programme of sublingual BUP.
In the current study, urinary concentrations of BUP in QNA ranged between 2 and 1730 mcg/L and for Norbup between 5 and 4322 mcg/L. These are consistent with previous studies 25 , 26 which reported concentration ranges of 28–1458 mcg/L for BUP and 28–2050 mcg/L for Norbup. Overall, Norbup concentrations were higher in urine compared to both BUP and Norbup in plasma. This is in keeping with previously published literature that has also demonstrated urine Norbup concentrations to be greater than BUP concentrations 2 hours post‐dosing. 25 , 27 , 28 , 29
However, in our study, of the 79 UDS results obtained, 57% of samples did not detect BUP with qualitative GC–MS testing. When QLA did not detect BUP, the QNA urine and plasma BUP concentration were lower than those QLA samples when BUP was detected. Therefore in this study, the routine UDS utilising GC–MS testing for BUP detected the majority of participants with a high concentration of BUP, but failed to detect those who had lower urinary or plasma concentrations, indicating the test may lack sensitivity.
Plasma analysis indicated that BUP and Norbup rates of detection are time dependent. This was consistent with routine QLA testing, where BUP was most likely to be detected at t = 2 and t = 4 hours. Further, testing at t = 0 or t = 6 hours is least likely to yield a positive result on QLA UDS. Given these findings of higher 2 and 4 hour concentrations of urinary Norbup and BUP, our study suggests that measuring urine concentrations at 2–4 hours post BUP administration may be the most sensitive way to assess adherence to treatment. Random or trough measurements may result in high rates of false negative results with qualitative analyses. Further, assaying for Norbup rather than, or as well as, BUP may increase test sensitivity. However, testing within a 2 to 4 hour window post dosing may be impracticable for both participants and clinicians, and likely not consistent with efficient clinical workflow.
The decision over which UDS methodology to use and how frequently to monitor is complex. A number of guidelines recommend a two‐step process of UDS: an initial immunoassay which can be undertaken as a point of care test, followed by confirmatory testing if an irregularity is identified on immunoassay. Both GC–MS and LC–MS/MS are identified as confirmatory assays. 30 , 31 This two‐step process may provide little clinical utility given the often low sensitivity and high specificity of immunoassay tests. In our study, GC–MS had low sensitivity in detecting BUP. Moreover, there is no Australian laboratory standard for BUP assays. Developing a national standard for assays of BUP and Norbup could improve reliability of UDS analysis.
Cost and availability may drive assay choice. In the jurisdiction in which the authors work, UPLC–MS/MS UDS can cost up to three times that of GC–MS. Further, there is limited data and variability in recommendations about frequency of UDS. Some Canadian states recommend weekly urine drug screening during medication initiation, then monthly in later implementation, while others recommend three‐monthly testing. 32 Regardless of whether the cost is borne by the individual or the health service, with frequent testing, the cost of UDS can easily become onerous and unsustainable.
Fifty percent of the subjects in our study had BUP dosing supervision on less than 7 days out of 28 and thus adherence with medication implementation may be questioned. In a study of BUP‐naive healthy volunteers administered a single dose of 0.4 mg BUP, 33 the Norbup:BUP ratio increased progressively with time for 24 hours after ingestion. A ratio greater than 1 indicated that UDS was taken within 7 hours after dose administration, while a ratio less than 0.5 indicated very recent BUP use (within 2–3 hours). In our study, the majority of participants had a ratio of Norbup:BUP of >1, with the highest ratios occurring immediately prior to dosing (t = 0), and the lowest ratios occurring t = 2 hours after ingestion of BUP. These findings are in some contrast to those of Kronstrand et al. 33 Our results suggest a ratio of 1.5 as being more representative of recent administration (within 2 hours) of buprenorphine. In our study, the nadir of Norbup:BUP ratios occurs shortly after BUP ingestion, with ratios increasing thereafter and peaking prior to next BUP ingestion. The discrepancies in ratio likely reflect differences in study population (steady state maintenance treatment vs single dose BUP‐naïve) and dose of BUP (variable vs 0.4 mg) in our study compared to Kronstrand et al. 33
In the present study, our participants were observed by the study investigators to self‐administer their BUP dose on the study day, and the results consistently demonstrated Norbup:BUP ratios greater than 1. However, unlike George et al., 26 we found a dose correlation between metabolite/parent ratios, where doses ≥8 mg had a greater ratio than those on less than 8 mg. This is most likely due to the low maximum daily dose of 10 mg in the George et al. 26 study. In our study population, the maximum dose was 32 mg with 11 subjects on doses greater than 10 mg.
Urine Norbup:BUP ratios have previously been reported to be more reliable indicators of ingestion 29 , 33 , 34 with some studies suggesting urine Norbup:BUP ratios of <0.02 being indicative of adulteration. 27 , 35 , 36 The urinary Norbup:BUP ratios in the present study were well above those previously reported in the literature, 27 supporting published data that <0.02 is likely indicative of adulteration. Moreover, Norbup urinary concentrations were consistently higher than BUP concentrations. Ensuring that assays measure Norbup either alone or in addition to BUP could potentially improve sensitivity. Warrington et al. 37 recently demonstrated that measuring naloxone concentrations in conjunction with Norbup:BUP can assist with identifying adulterated samples more effectively. Future work could also incorporate urinary naloxone assay.
Polymorphisms of CYP3A4 have been shown to be associated with failure of treatment in OAT setting, 38 and with increased metabolic activity of BUP, resulting in negative UDS. 39 Further, the UGT1A1*28 polymorphism has been shown to phenotypically result in a 28% decrease of BUP glucuronidation. 15 , 16 Analysis for both polymorphisms in our patient population were inconclusive, most likely due to a limited sample size. A correlation may be possible with larger sample size. However, CYP3A4 drug–drug interactions were noted to contribute to UDS status of patients. As expected, inhibitors increased BUP urinary concentrations with a positive QLA UDS seen at all time points, and inducers decreased levels with negative UDS at all time points. This is consistent with a previous case report where co‐administration of prednisolone (a CYP3A4 inducer) resulted in a negative BUP UDS. 36
Study limitations include a lack of measurement of urine creatinine (UCr) and specific gravity to control for dilution, adulteration and tampering. UCr has been shown to be a marker of dilution, with lower urinary creatinine a potential marker of sample tampering. 9 , 40 There is also a potential that in order to produce timed urines, patients ingested large volumes of water and inadvertently diluted urine samples below the detection threshold of testing. A lack of UCr in this study makes this hard to exclude. Out of keeping with this is the fact that all quantitative urine results had a detectable level of BUP and Norbup. Given that the primary goal of the study was to assess UDS detection for BUP after supervised dosing of BUP, self‐reported medication adherence was not elicited.
As a pilot study, sample size was small and therefore significant associations may not have been detected, which further limits our findings. Future studies should include a larger sample size, with sufficient power, to enable detection of significant differences that are likely to translate into clinical practice. The increase in power would decrease the chance of a false negative (a type II error) because it would be more likely to reject a null hypothesis (no difference between the groups) that is false. Further, reducing frequency of UDS to t = 0, and t = 3 hours would improve study feasibility, participant tolerability and ease of recruitment. The impact of assaying for both BUP and Norbup in QLA should be evaluated. Finally, clinician understanding of the limitations of UDS and the understanding of laboratory or testing staff of the application of UDS should be explored.
This study highlights that the use of UDS in BUP treatment as a means of adherence monitoring has limitations. Interpretation of urine drug results should be carried out cautiously and within the clinical context of dose and timing of dose relative to sampling. Practitioners should understand the limitations of their local laboratory testing methodology, particularly as BUP treatment moves away from specialist settings and into general practice. Communication between laboratory staff and clinicians about assay sensitivity and limitations could assist practitioners in interpreting UDS. Non‐adherence to treatment cannot be assumed when UDS does not detect BUP. In summary, this study reinforces the need for understanding UDS methodologies and drug metabolism in order to correctly interpret and use UDS. Standardisation of urine drug assays would improve the clinical application of this testing.
COMPETING INTERESTS
The authors declare no conflicts of interest and do not have any financial disclosures.
CONTRIBUTORS
N.J. conceived and designed the project, recruited participants, analysed the results and drafted and revised the manuscript. A.A. assisted in the project design, and was involved in participant recruitment, data collection and analysis, and extensively revised the manuscript. C.T. assisted in participant recruitment and data collection and revised the manuscript. B.M. assisted in conceiving and designing the project, data analysis and interpretation and extensively revised the manuscript. M.J. contributed to project design, data analysis and extensively revised the manuscript. C.M., S.B. and S.V. analysed the quantitative blood and urine samples, wrote the corresponding methods and revised the manuscript. N.L. performed the genetic studies and wrote the corresponding methods and revised the manuscript.
Supporting information
Table S1. Characteristics of Australian Treatment Outcomes Profile (ATOP) responses
Figure S1. Evaluating possible drug–drug interactions. Urine drug screen results in participants taking CYP3A4 inhibitors, inducers or substrates
ACKNOWLEDGEMENTS
The authors would like to thank Professor Andrew Dawson and NHMRC grants for funding this project.
This project was funded by NHMRC APP 1059542. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Jamshidi N, Athavale A, Tremonti C, et al. Evaluation of adherence monitoring in buprenorphine treatment: A pilot study using timed drug assays to determine accuracy of testing. Br J Clin Pharmacol. 2023;89(7):1938‐1947. doi: 10.1111/bcp.15318
The authors confirm that the Principal Investigator for this paper is Nazila Jamshidi and that she had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor‐Barriuso R Mortality risk during and after opioid substitution treatment: systematic review and meta‐analysis of cohort studies. BMJ 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, Bruneau J, Altice FL, Henderson G, Rahimi‐Movaghar A, Larney S Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet 2019;394(10208):1560–1579. doi: 10.1016/S0140-6736(19)32229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ronquest N, Willson T, Montejano L, Nadipelli V, Wollschlaeger B. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil 2018; 9:59–78. doi: 10.2147/SAR.S150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warden D, Subramaniam GA, Carmody T, Woody GE, Minhajuddin A, Poole SA, Potter J, Fishman M, Bogenschutz M, Patkar A, Trivedi MH Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addict Behav 2012;37(9):1046–1053. doi: 10.1016/j.addbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elarabi HF, Shawky M, Mustafa N, Radwan D, Elarasheed A, Yousif Ali A, Osman M, Kashmar A, al Kathiri H, Gawad T, Kodera A, al Jneibi M, Adem A, Lee AJ, Marsden J Effectiveness of incentivised adherence and abstinence monitoring in buprenorphine maintenance: a pragmatic, randomised controlled trial. Addiction 2021;116(9):2398–2408. doi: 10.1111/add.15394. [DOI] [PubMed] [Google Scholar]
- 6. Pesce A, West C, Egan City K, Strickland J. Interpretation of urine drug testing in pain patients. Pain Med 2012;13(7):868–885. doi: 10.1111/j.1526-4637.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- 7. Wood E, Mattick RP, Burns L, Shakeshaft A. The Costs and Utility of Parental Drug‐Testing in Child Protection: a Review of the Available Literature and Commentary. NDARC Technical Report No. 242; 2006.
- 8. Lancaster K, Seear K, Ritter A. Reducing stigma and discrimination for people experiencing problematic alcohol and other drug use. Drug Policy Modelling Program Monograph Series No. 26; 2017.
- 9. Kapur BM, Aleksa K. What the lab can and cannot do: clinical interpretation of drug testing results. Crit Rev Clin Lab Sci. 2020;2020(8):548‐585. [DOI] [PubMed] [Google Scholar]
- 10. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol 2012;8(4):408–416. doi: 10.1007/s13181-012-0274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barthwell AG. Clinical and public health considerations in urine drug testing to identify and treat substance use. Subst Use Misuse 2016;51(6):700–710. doi: 10.3109/10826084.2015.1135953. [DOI] [PubMed] [Google Scholar]
- 12. Cone EJ, Gorodetzky CW, Yousefnejad D, Buchwald WF, Johnson RE. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577‐581. [PubMed] [Google Scholar]
- 13. Chang Y, Moody DE, McCance‐Katz EF. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos 2006;34(3):440–448. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi K, Yamamoto T, Chiba K, et al. Human buprenorphine N‐dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab Dispos. 1998;26(8):818‐821. [PubMed] [Google Scholar]
- 15. Rouguieg K, Picard N, Sauvage FL, Gaulier JM, Marquet P. Contribution of the different UDP‐glucuronosyltransferase (UGT) isoforms to buprenorphine and norbuprenorphine metabolism and relationship with the main UGT polymorphisms in a bank of human liver microsomes. Drug Metab Dispos 2010;38(1):40–45. doi: 10.1124/dmd.109.029546. [DOI] [PubMed] [Google Scholar]
- 16. Seguí HA, Melin K, Quiñones DS, Duconge J. A review of the pharmacogenomics of buprenorphine for the treatment of opioid use disorder. J Transl Genet Genomics 2020;4:263–277. doi: 10.20517/jtgg.2020.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jamshidi N, Athavale A, Murnion B. Buprenorphine not detected on urine drug screening in supervised treatment. J Opioid Manag 2021;17(7):69–76. doi: 10.5055/jom.2021.0644. [DOI] [PubMed] [Google Scholar]
- 18. Lintzeris N, Monds LA, Rivas G, Leung S, Withall A, Draper B. The Australian Treatment Outcomes Profile instrument as a clinical tool for older alcohol and other drug clients: a validation study. Drug Alcohol Rev 2016;35(6):673–677. doi: 10.1111/dar.12393. [DOI] [PubMed] [Google Scholar]
- 19. NATA . Human Pathology Accreditation Criteria Publications Checklist. https://nata.com.au/accreditation/medical-laboratory-accreditation-iso-15189/ Accessed October 26, 2021.
- 20. de Geest S., Zullig LL, Dunbar‐Jacob J, Helmy R, Hughes DA, Wilson IB, Vrijens B ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med 2018;169(1):30–35. doi: 10.7326/M18-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Melanson SEF, Ptolemy AS, Wasan AD. Optimizing urine drug testing for monitoring medication compliance in pain management. Pain Med 2013;14(12):1813–1820. doi: 10.1111/pme.12207. [DOI] [PubMed] [Google Scholar]
- 22. Tkacz J, Severt J, Cacciola J, Ruetsch C. Compliance with buprenorphine medication‐assisted treatment and relapse to opioid use. Am J Addict 2012;21(1):55–62. doi: 10.1111/j.1521-0391.2011.00186.x. [DOI] [PubMed] [Google Scholar]
- 23. Gaither JR, Goulet JL, Becker WC, Crystal S, Edelman EJ, Gordon K, Kerns RD, Rimland D, Skanderson M, Justice AC, Fiellin DA The association between receipt of guideline‐concordant long‐term opioid therapy and all‐cause mortality. J Gen Intern Med 2016;31(5):492–501. doi: 10.1007/s11606-015-3571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ko M, Smith T. (227) Medication adherence monitoring in patients prescribed buprenorphine/naloxone. J Pain 2017;18(4):S32. doi: 10.1016/j.jpain.2017.02.119. [DOI] [Google Scholar]
- 25. Kronstrand R, Selden TG, Josefsson M. Analysis of buprenorphine, norbuprenorphine, and their glucuronides in urine by liquid chromatography–mass spectrometry. J Anal Toxicol 2003;27, 7, 464, 470. doi: 10.1093/jat/27.7.464. [DOI] [PubMed] [Google Scholar]
- 26. George S, George C, Chauhan M. The development and application of a rapid gas chromatography–mass spectrometry method to monitor buprenorphine withdrawal protocols. Forensic Sci Int 2004;143(2–3):121–125. doi: 10.1016/j.forsciint.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 27. Hull MJ, Bierer MF, Griggs DA, Long WH, Nixon AL, Flood JG. Urinary buprenorphine concentrations in patients treated with Suboxone® as determined by liquid chromatography–mass spectrometry and CEDIA immunoassay. J Anal Toxicol 2008;32(7):516–521. doi: 10.1093/jat/32.7.516. [DOI] [PubMed] [Google Scholar]
- 28. McMillin GA, Slawson MH, Marin SJ, Johnson‐Davis KL. Demystifying analytical approaches for urine drug testing to evaluate medication adherence in chronic pain management. J Pain Palliat Care Pharmacother 2013;27(4):322–339. doi: 10.3109/15360288.2013.847889. [DOI] [PubMed] [Google Scholar]
- 29. Böttcher M, Beck O. Evaluation of buprenorphine CEDIA assay versus GC–MS and ELISA using urine samples from patients in substitution treatment. J Anal Toxicol 2005;29(8):769–776. doi: 10.1093/jat/29.8.769. [DOI] [PubMed] [Google Scholar]
- 30. Arthur JA. Urine drug testing in cancer pain management. Oncologist 2020;25(2):99–104. doi: 10.1634/theoncologist.2019-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Argoff CE, Alford DP, Fudin J, Adler JA, Bair MJ, Dart RC, Gandolfi R, McCarberg BH, Stanos SP, Gudin JA, Polomano RC, Webster LR Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med 2018;19(1):97–117. doi: 10.1093/pm/pnx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moss E, McEachern J, Adye‐White L, Priest KC, Gorfinkel L, Wood E, Cullen W, Klimas J Large variation in provincial guidelines for urine drug screening during opioid agonist treatment in Canada. Can J Addict 2018;9(2):6–9. doi: 10.1097/CXA.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kronstrand R, Nystrom I, Andersson M, Gunnarsson L, Hagg S, Josefsson M, Ahlner J Urinary detection times and metabolite/parent compound ratios after a single dose of buprenorphine. J Anal Toxicol 2008;32(8):586–593. doi: 10.1093/jat/32.8.586. [DOI] [PubMed] [Google Scholar]
- 34. Donroe JH, Holt SR, O'Connor PG, Sukumar N, Tetrault JM. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office‐based clinical practice. Drug Alcohol Depend 2017;180:46–51. doi: 10.1016/j.drugalcdep.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 35. McMillin GA, Davis R, Carlisle H, Clark C, Marin SJ, Moody DE. Patterns of free (unconjugated) buprenorphine, norbuprenorphine, and their glucuronides in urine using liquid chromatography–tandem mass spectrometry. J Anal Toxicol 2012;36(2):81–87. doi: 10.1093/jat/bkr020. [DOI] [PubMed] [Google Scholar]
- 36. Sethi R, Petrakis I. Differential diagnosis for a stable patient maintained on buprenorphine who gives a urine toxicology screen negative for buprenorphine. Am J Addict 2014;23(3):318–319. doi: 10.1111/j.1521-0391.2014.12087.x. [DOI] [PubMed] [Google Scholar]
- 37. Warrington JS, Warrington GS, Francis‐Fath S, Brooklyn J. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med 2020;14(6):e344–e349. doi: 10.1097/ADM.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 38. Crist RC, Li J, Doyle GA, Gilbert A, Dechairo BM, Berrettini WH. Pharmacogenetic analysis of opioid dependence treatment dose and dropout rate. Am J Drug Alcohol Abuse 2018;44(4):431–440. doi: 10.1080/00952990.2017.1420795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meaden CW, Mozeika A, Asri R, Santos CD. A review of the existing literature on buprenorphine pharmacogenomics. Pharmacogenomics J 2021;21(2):128–139. doi: 10.1038/s41397-020-00198-1. [DOI] [PubMed] [Google Scholar]
- 40. Raouf M, Bettinger JJ, Fudin J. A practical guide to urine drug monitoring. Fed Pract. 2018;35(4):38‐44. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Australian Treatment Outcomes Profile (ATOP) responses
Figure S1. Evaluating possible drug–drug interactions. Urine drug screen results in participants taking CYP3A4 inhibitors, inducers or substrates
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


