Abstract
Objective
We undertook this study to evaluate potential predictors of placebo response with intra‐articular (IA) injections for knee/hip osteoarthritis (OA) using individual participant data (IPD) from existing trials.
Methods
Randomized placebo‐controlled trials evaluating IA glucocorticoid or hyaluronic acid published to September 2018 were selected. IPD for disease characteristics and outcome measures were acquired. Potential predictors of placebo response included participant characteristics, pain severity, intervention, and trial design. Placebo response was defined as at least a 20% reduction in baseline pain. Logistic regression models and odds ratios were computed as effect measures to evaluate patient and pain mechanisms and then pooled using a random effects model. Generalized mixed‐effect models were applied to intervention and trial characteristics.
Results
Of 56 eligible trials, 6 shared data, and these were combined with the existing 4 OA Trial Bank studies, yielding 10 studies with IPD of 621 placebo participants for analysis. In the total placebo population, at short‐term follow‐up, the use of local anesthetic and ultrasound guidance were associated with reduced odds of placebo response. At midterm follow‐up, mid‐ to long‐term trial duration was associated with increased odds of placebo response, and worse baseline function scores were associated with reduced odds of a placebo response.
Conclusion
The administration of local anesthetics or ultrasound guidance may reduce IA placebo response at short‐term follow‐up. At midterm follow‐up, participants with worse baseline function scores may be less likely to respond to IA placebo, and mid‐ to long‐term trial duration may enhance the placebo response. Further studies are required to corroborate these potential predictors of IA placebo response.
INTRODUCTION
The majority of pharmacological treatments in osteoarthritis (OA) have failed to demonstrate a minimum clinically important difference over placebo. This directly affects the development of prospective pharmacological innovations and their translation to therapeutic options for this progressively disabling condition. The placebo effect is a well‐recognized phenomenon in OA treatments, with previous meta‐analyses of randomized controlled trials (RCTs) assessing the placebo response across a range of therapies in OA (non‐pharmacological, pharmacological, and surgical treatments), confirming that placebo effect size (ES; standardized mean difference [SMD] between baseline and endpoint) for pain in OA (ES 0.51, 95% confidence interval [CI] 0.46–0.55) is greater than no treatment (ES 0.03, 95% CI −0.13 to 0.18) (1). With invasive therapies, participants’ expectations and beliefs can lead to increased placebo/contextual effects with pain‐relieving effects demonstrated with injections/needles (β = 0.144, P = 0.020) (1). The ES for debridement and lavage was 0.48 (95% CI 0.24–0.71) and from intra‐articular (IA) glucocorticoid and hyaluronic acid (HA) data 0.34 (95% CI 0.11–0.56) for single‐dose placebo injection and 0.63 (95% CI 0.15–1.12) for multiple injections (1). IA placebo injections have been demonstrated to result in a significant improvement in pain reduction in relation to oral placebo (ES = 0.29, credible interval 0.04–0.54) (2, 3). The magnitude of the placebo response in OA clinical trials is significant, with about 75% of the treatment effect being attributable to placebo or contextual effects (4). When considering clinical trial design in IA therapies, the type of placebo, its volume, frequency of injection, concomitant local anesthetic, and radiological guidance use, along with participant disease characteristics, can all be a cause of between‐person heterogeneity within a clinical trial. To date, placebo responses are typically measured as a change in outcome from baseline in the placebo treatment group in comparison with the active treatment group. This method is potentially influenced by spontaneous effects, such as the Hawthorne effect (ie, the effect caused by being observed in trials) (5), natural fluctuation of OA disease, and regression to the mean (1, 6).
SIGNIFICANCE & INNOVATIONS.
This is the first individual participant data meta‐analysis conducted to identify predictors of placebo response, specifically in intra‐articular (IA) injection trials in osteoarthritis (OA).
Identifying potential predictors of placebo in IA interventions in OA may help to provide guidance and modify the design for future IA clinical trials.
Severe baseline function scores, administration of local anesthetic, ultrasound‐guided injections, or joint fluid aspiration attempts may reduce the odds of an IA placebo response, whereas trial durations of 12 to 24 and longer than 24 months may increase the odds of IA placebo response.
A meta‐analysis of OA treatments has shown that the magnitude of placebo response also can vary greatly between individuals (4), but the variations of the treatment or placebo responses across individuals cannot be examined in an aggregate data meta‐analysis. Because the placebo response can be attributed to the individual and factors at the treatment or study level, assessment of the placebo response using individual participant data (IPD) meta‐analysis will give insight into the different predictors of placebo response both at individual and study levels. IPD meta‐analysis is now increasingly used and is considered to provide more robust results, because it facilitates more powerful analyses and the standardization of analyses across different studies and allows derivation of the desired information (7).
The primary aim of this study is to evaluate the potential predictors of placebo response in IA injection trials in OA using IPD from published trials. This IPD meta‐analysis will examine the role of potential placebo response modifiers from the participant level to the intervention and trial design level. For the purpose of this analysis, only placebo‐controlled studies of IA glucocorticoid and HA were included, because these are the more widely studied IA drugs with fewer methodological concerns and less heterogeneity than other IA therapies (8, 9).
PATIENTS AND METHODS
This study was conducted under the umbrella of the OA Trial Bank, an international collaboration endorsed by the Osteoarthritis Research Society International and the European Alliance of Associations for Rheumatology. It brings together IPD data from RCTs (10, 11) to identify specific responsive subgroups for the different OA treatments. The research question and study proposal of this study were approved by the steering committee of the OA Trial Bank before the development of the published study protocol (12). The PROSPERO registration number is CRD42018095188.
Types of study and participants
All randomized placebo‐controlled trials, including crossover trials, evaluating either IA glucocorticoid or IA HA injections were eligible for inclusion. There were no language restrictions. Participants from the identified RCTs, assigned to the placebo group, had to have a diagnosis of knee and hip OA according to the criteria defined by the American College of Rheumatology and the European Alliance of Associations for Rheumatology evidence‐based recommendations for the diagnosis of knee OA (13, 14) or on the basis of defined clinical and/or radiographic information, fulfilling the specified diagnostic criteria of OA of the respective trials.
Outcomes
The minimum criterion for inclusion of RCTs was sufficient participant reporting of pain measures in at least one of the subsequent follow‐up time frames: short‐term (up to 4 weeks), midterm (closest to 12 weeks) or long‐term (closest to 24 weeks). As a minimum, age, sex, and body mass index (BMI) were required. If available, potential placebo response modifier variables were extracted, including disease duration, OA at other joints, radiographic information, stiffness and function scores, signs of effusion and inflammatory features (either by physical examination or by radiographic imaging with ultrasound or magnetic resonance imaging), and intervention and trial design characteristics.
Eligible studies
Literature searches were conducted separately for randomized placebo‐controlled trials of IA glucocorticoid and IA HA. Data were searched from June 2012 to September 2018 for RCTs of IA glucocorticoid versus placebo and then combined with the existing studies from the OA Trial Bank reported in an IPD analysis of subgroup effects of glucocorticoid injections with searches from 1995 until June 2012 (10). The search for IA HA versus placebo was conducted from inception to September 2018 because of the potential availability of earlier studies, hence the broadened search time frame. A systematic literature search was conducted using the following databases: PubMed (Medline), EMBASE, Web of Science, and Cochrane Central. Efforts were made to identify unpublished trials through the International Standard Randomised Controlled Trial Number Registry of clinical trials, ClinicalTrials.gov.au, and the Australian New Zealand Clinical Trials Registry. Reference lists were further searched for identification of published work. The search strategy was developed by the reviewers in consultation with the OA Trial Bank.
Two review authors (SPY and LAD) independently selected citations based on titles and abstracts and assessed full articles that met the eligibility criteria independently before consensus was reached. If a consensus was not reached, the OA Trial Bank members (MvM) were consulted for arbritation.
Data collection and transfer
The corresponding authors of eligible trials were approached, and data sharing was enquired via standardized email initially and subsequently by telephone. If the corresponding authors were uncontactable, communication was attempted with the other authors and/or institutions listed. Three attempts were made to contact the corresponding authors, institutes, and/or study sponsors. IPD data were requested per OA Trial Bank protocol, and terms (15) and data transfer license agreements were signed between both parties. With the existing stored IA glucocorticoid trials in the OA Trial Bank, the corresponding authors were contacted to sign a further data transfer agreement for the purpose of this analysis. All anonymous data were kept in their original versions in a secured server at the University of Sydney and the Erasmus University Medical Centre. All data were checked for consistency with the published papers, and data quality was ensured through independent checking and assessing for data entry mistakes and discrepancies by reproducing the main baseline characteristics and reported changes over time for the available outcomes.
Risk and quality assessment
The methodological quality of the studies was assessed using the risk of bias assessment tool for randomized trials recommended by the Cochrane Collaboration (16, 17). The domains assessed included randomization of procedure; anonymization of participants, physicians, and treatment allocation; use of intention‐to‐treat analysis; incomplete outcome data; baseline group similarity; reporting bias; and other sources of biases. The risk of bias was scored as “low,” “high,” or “unclear.” Two review authors assessed the risk of bias (SPY and LAD). Any disagreement between the reviewers was resolved by discussion and, if required, input from a third reviewer from the OA Trial Bank.
Data analysis
The primary outcome of the IPD meta‐analysis was change in pain from baseline at short‐, mid‐, and long‐term. Participants were classified as responders if they achieved a clinically important pain reduction, defined as ≥20% reduction in pain score from baseline. This value was chosen to be consistent with the prior study by the OA Trial Bank assessing placebo responders (11) and has been recommended for use in pain and function assessment in rheumatic diseases such as OA (18, 19). The value was also used to define the placebo response, which is equivalent to an ES of 0.8 (20), implying a response was unlikely to be caused by spontaneous effects. The outcomes measured on different scales were standardized. The visual analog scale pain score was preferentially used for the analysis. If unavailable, the pain subscales from the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Knee Injury and Osteoarthritis Outcomes Score, or other Likert pain scores were converted to a 0 to 100 scale as per former OA Trial Bank protocols (15). Supplementary analysis was performed using the absolute change in pain score, defined as a ≥20 points reduction in pain score from baseline on the 0 to 100 scale.
Tests of heterogeneity were conducted using Q statistics, which was distributed as a chi‐square random variable (assumption of the homogeneity of ESs). The between‐study heterogeneity was assessed with τ2 (estimate of between‐study variance) and I 2 (the percentage of total variation due to between‐study variance), with interpretation as follows: I 2 < 25% means no heterogeneity, I 2 < 50% means low heterogeneity, I 2 < 75% means moderate heterogeneity, and I 2 ≥ 75% indicates high heterogeneity (21). Only complete case analysis was performed, as the value of missing observations was <5%.
The study sponsor of one trial requested that their data must be analyzed on a specified secure server. Because of participant confidentiality and data sharing agreement stipulations for the other trials, IPD from those trials were unable to be uploaded to the specified server for a one‐stage approach analysis. Consequently, a two‐stage approach was used.
The trials were grouped for analysis into a total IA placebo population from all available trials, and, to account for potential heterogeneity in trial design between IA therapies, the placebo groups were also separated into IA glucocorticoid/placebo trials and IA HA/placebo trials. Baseline and follow‐up data on outcome measures and putative modifiers from the placebo arm were used to estimate the predictors of the placebo response. The change from baseline pain was determined as the dependent variable, and independent variables were the potential predictors of placebo response. These included patient‐level characteristics and pain mechanisms that were chosen a priori, which were recognized risk factors for OA symptoms:
Participant characteristics: age, sex, BMI, bilateral versus unilateral disease, and disease duration
Pain mechanisms: peripheral pain mechanisms (signs of inflammation, morning stiffness symptoms, and radiographic findings) and central pain mechanisms (OA pain at other joints and pain severity with severe pain, defined as ≥70 on 0–100 scale)
For the first stage univariate analysis, logistic regression models and odds ratios (ORs) were computed as the effect measure to evaluate patient and pain mechanisms. The second stage involved pooling the results using random effects models with restricted maximum likelihood method of estimation. If more than one potential predictor variable was significant in each IA placebo group, multivariate meta‐analysis was planned.
Intervention and trial characteristics were analyzed as follows:
Intervention characteristics: aspiration attempt, frequency of injection, volume of injection, local anesthetic use, and ultrasound‐guided injection
Trial characteristics: dropout rate, role of funder/sponsor, randomization groups, trial duration (≤12, 12–24, and ≥24 weeks), single‐center/multicenter study, or funding/sponsor
A generalized mixed‐effects model using a logit link function accounting for the intrastudy correlations was applied; the study by Chevalier et al (22) was omitted in this analysis because of data server security limitations.
OR effect measures and 95% CIs were generated for each outcome measure. P < 0.05 was considered statistically significant. Statistical analyses were conducted using Stata version 17 (StataCorp).
RESULTS
Study descriptions
The literature search for IA glucocorticoid RCTs resulted in 418 study abstracts. After screening, 30 publications with full text were evaluated, 14 fulfilled the inclusion criteria, and IPD were sought from these studies (Figure 1). Two authors agreed to participate and contributed data (23, 24). The authors/institutions/sponsors of six studies responded positively to the data share request but were subsequently lost to further contact or had data availability/access issues. Contact was unable to be established with six studies.
Figure 1.
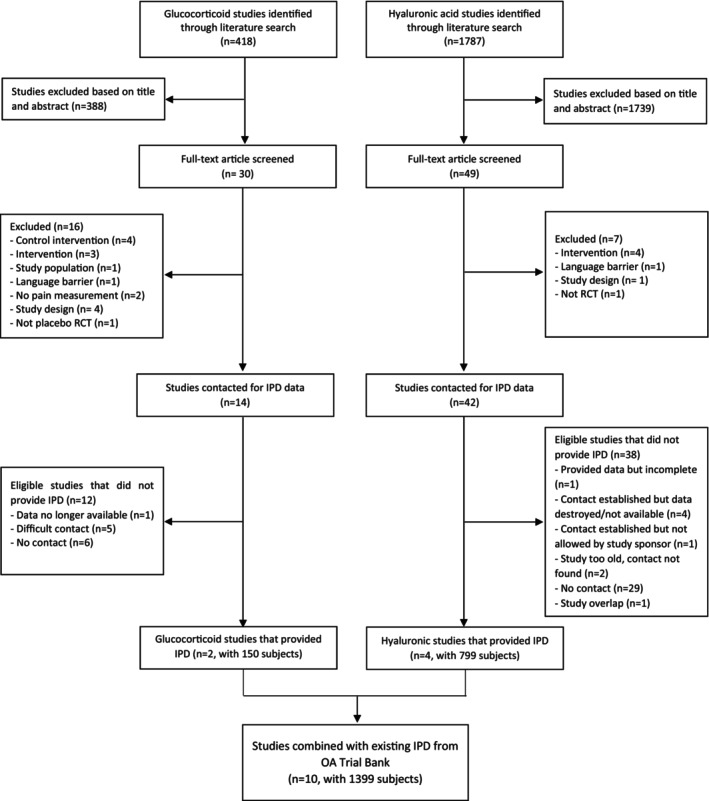
Flow diagram of updated search and included studies. IPD, individual participant data; OA, osteoarthritis.
The literature search for IA HA RCTs resulted in 1,787 abstracts. After screening, 49 studies were evaluated in full text, and 42 studies fulfilled the inclusion criteria and were contacted for IPD for participation (Figure 1). Four studies agreed to participate (22, 25, 26, 27). The authors/institutions/sponsors of six studies did respond to the data share request but were subsequently lost to further contact or had data availability/access issues. Contact was unable to be established with 31 studies. The full list of studies contacted is available in the “Supplementary list of studies contacted.”
The IPD from the six studies (two glucocorticoid and four HA; n = 949) were combined with the existing IPD from the OA Trial Bank (28, 29, 30, 31), yielding 10 studies with 1,399 participants. The characteristics of the included studies are presented in Table 1. A total of six studies compared IA glucocorticoid (n = 190) with placebo (n = 181), and five studies compared IA HA (n = 555) with placebo (n = 458); Atchia et al (28) had both IA glucocorticoid and HA versus placebo groups. Data on 621 placebo participants were available for analysis.
Table 1.
Trial characteristics*
| Study | Origin | Joint | OA diagnosis | N in intervention | N in Placebo | Interventions | Injection technique | Outcome | Inflammation | Follow‐up | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atchia et al (2011) (28) | UK | Hip | Clinical diagnosis of knee OA + radiographic evidence | n = 19 steroid; n = 19 hyaluronic acid | 29 (n= 19, placebo; n= 20, standard care [no injection control group]) | Methylprednisolone acetate 3 ml/120 mg vs placebo (3 mg saline) vs standard care vs hyaluronic acid (Durolane) | U/S guided; LA used; aspiration attempted | Pain (NRS); WOMAC pain, physical function, stiffness, total | Presence of synovitis >7 mm on ultrasound | 1, 4, 8, and 16 weeks | Government institution and funding agency |
| Chao et al (2010) (29) | USA | Knee | ACR criteria for knee OA (1986) + radiograph within 1 year of enrollment | 40 | 39 | Triamcinolone acetonide 40 mg vs placebo (1 ml 0.9% saline) | No U/S guidance; no LA use; aspiration not attempted | Pain (VAS); WOMAC pain and total | Pathologic effusion of ≥5 mm present on ultrasound | 4 and 12 weeks | Government institution and funding agency |
| Chevalier et al (2010) (22) | France | Knee | ACR criteria for knee OA (knee pain for most days of the previous month, and osteophyte(s) at joint margins visible on x‐ray and measurable joint space) | 124 | 129 | Hylan G‐F 20 vs placebo (6 ml, buffered physiological sodium chloride solution) | U/S guided; LA used; aspiration attempted | WOMAC pain, physical function, stiffness; patient global assessment; clinical observer global assessment | Presence of effusion by clinical assessment | 1, 4, 8, 12, 18, and 26 weeks | Funding agency |
| Dahlberg et al (1994) (25) | Sweden | Knee | Cartilage abnormality on arthroscopy (Outerbridge score ≥2 in 1 joint surface) | 28 | 24 | Hyaluronan 2.5 ml, 10 mg/ml vs placebo (2.5 ml sodium chloride, dibasic sodium phosphate, sodium dihydrogen, and phosphate dihydrate) | No U/S guidance; LA used; aspiration attempted | Pain (VAS); function (VAS); activity (VAS); mobility (VAS); Lysholm score | ‐ | 2, 4, 13, 26, and 52 weeks | Government institution |
| Hall et al (2014) (24) | UK | Knee | Clinical diagnosis of painful knee OA + KL grade ≥2 | 25 | 25 | Methylprednisolone 40 mg vs placebo (1 ml, 0.9% saline) | No U/S guidance; LA use – N/A; aspiration attempted | Pain (VAS); WOMAC pain, physical function, stiffness | Presence of effusion/synovial hypertrophy on ultrasound | 1 week | Government institution |
| Henriksen et al (2015) (23) | Denmark | Knee | ACR criteria for knee OA (1986) + radiographic confirmation | 50 | 50 | Methylprednisolone acetate 40 mg + 4 ml lidocaine hydrochloride (10 mg/ml) vs placebo (1 ml isotonic saline + 4 ml lidocaine hydrochloride [10 mg/ml]). Followed by 12‐week exercise program starting at week 2 | U/S guided; LA used; aspiration attempted | KOOS pain, ADL, QOL, function in sports or recreation, symptoms | Presence of effusion/synovitis on MRI imaging | 2, 14, and 26 weeks | Government institution |
| Lambert et al (2007) (31) | Canada | Hip | ACR criteria for hip OA (1991) + radiologic evidence of OA | 31 | 21 | Triamcinolone 40 mg + 10 mg bupivacaine vs placebo (10 mg bupivacaine + 2 ml saline) | U/S guided; LA used; aspiration attempted | WOMAC pain, physical function, stiffness, total; global assessment | ‐ | 1, 2, 3, and 6 months | Funding agency |
| Ravaud et al (1999) (30) | France | Knee | ACR criteria for knee OA (1986) + inclusion criteria of KL grade ≥2 | 25 | 73 (placebo n = 28; joint lavage plus placebo n = 21; joint lavage plus corticosteroid, n = 24) | Cortivazol 3.75 mg in 1.5 ml vs placebo (1.5 ml 0.9% saline) vs joint lavage plus IA placebo vs joint lavage plus IA steroid | No U/S guidance; no LA used; aspiration not attempted | Pain (VAS); global status (VAS) | Evidence of effusion by clinical assessment (present or not) | 1, 4, 12, and 24 weeks | Government institution and funding agency |
| Strand et al (2012) (27) | USA; Japan | Knee | Pain in affected knee of ≥4 weeks while standing or walking with radiological evidence of OA with KL grade 1‐3 | 251 | 128 | Gel‐200 (30 mg in 3.0 ml) vs placebo (3.0 ml, phosphate buffered saline) | No U/S guidance; no LA used; aspiration attempted | Pain (WOMAC pain subscore ‐ VAS); WOMAC total, physical function, stiffness; physician and patient global | Evidence of effusion by clinical assessment (present or not) | 1,3, 6, 9, and 19 weeks | Funding agency |
| Takamura et al (2019) (26) | USA; Japan | Knee | Clinical diagnosis of knee OA with radiological evidence of OA with KL grade 1–3 | 152 | 159 | Gel‐200 (30 mg in 3.0 ml) vs placebo (3.0 ml, phosphate buffered saline) | U/S guided; LA used; aspiration attempted | Pain (WOMAC pain subscore ‐ VAS); WOMAC total, physical function, stiffness; physician and patient global | Evidence of effusion by clinical assessment (present or not) | 3, 6, 12, 18, and 26 weeks | Funding agency |
ACR, American College of Rheumatology; ADL, activity of daily living; KL, Kellgren‐Lawrence; KOOS, Knee Injury and Osteoarthritis Outcome Score; LA, local anesthetic; MRI, magnetic resonance imaging; NRS, Numerical Rating Scale; OA, osteoarthritis; QOL, quality of life; U/S, ultrasound guided; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
The risk of bias scores of the studies are presented in Supplementary Table 1 (available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25212). All studies were deemed to be of low bias in relation to randomization, compliance, timing, and selective outcome reporting.
Table 2 details the baseline characteristics of the study participants for all studies and the placebo comparison groups. For the total placebo participants, the average age was 62 years, and 58% were women. Severe pain was reported in 29% of the placebo participants.
Table 2.
Baseline characteristics of participant*
| Characteristic | Total population, N = 1399 | Placebo‐only population, n = 621 | Placebo only (glucocorticoid trials group), n = 181 | Placebo only (hyaluronic acid trials group), n = 458 |
|---|---|---|---|---|
| Age, y, mean (SD) | 62.66 (10.57) | 62.07 (10.82) | 65.08 (10.77) | 60.90 (10.65) |
| Female sex, n (%) | 842 (60.1) | 361 (58.1) | 89 (49.2) | 284 (62.0) |
| BMI, kg/m2, mean (SD) | 28.88 (4.52)† | 29.27 (4.54)‡ | 29.38 (4.56)§ | 29.03 (4.08) |
| Duration of symptoms, mean (SD), mon | 44.49 (71.66)¶ | 78.07 (79.59)# | 85.29 (106.87)** | 66.79 (36.23)†† |
| KL grade, n % | ||||
| 0 | 2 (0.1) | 1 (0.2) | 1 (0.6) | 0 (0.0) |
| 1 | 60 (4.3) | 22 (3.5) | 4 (2.2) | 21 (4.6) |
| 2 | 513 (36.7) | 217 (34.9) | 27 (14.9) | 194 (42.4) |
| 3 | 657 (47.0) | 283 (45.5) | 75 (41.4) | 218 (47.6) |
| 4 | 83 (5.9) | 35 (5.6) | 34 (18.8) | 1 (0.2) |
| Missing | 84 (6.0) | 64 (10.3) | 40 (22.1) | 24 (5.2) |
| Inflammation, n (%) | 481 (51.2)§§ | 259 (45.2)¶¶ | 113 (71.97)## | 88 (28.9)*** |
| Effusion, n (%) | 306 (35.5)††† | 168 (30.4)‡‡‡ | 96 (70.07)§§§ | 40 (13.9)¶¶¶ |
| Pain (0–100), mean (SD) | 60.32 (18.30) | 61.85 (17.04) | 55.78 (19.86) | 65.53 (14.25) |
| Severe pain (≥70 points on VAS), n (%) | 293 (28.1)### | 179 (29.0)**** | 49 (27.53)†††† | 137 (29.9) |
| OA other joints, n (%) | 373 (58.5)‡‡‡‡ | 275 (57.9)§§§§ | 6 (21.43)¶¶¶¶ | 194 (67.6)#### |
BMI, body mass index; KL, Kellgren‐Lawrence; OA, osteoarthritis; VAS, visual analog scale.
N = 1,162 (not available for Lambert and Chao).
N = 561 (not available for Lambert and Chao).
N = 121 (not available for Lambert and Chao).
N = 1,223 (not available for Henriksen, Hall, Dahlberg).
N = 433.
N = 101.
N = 350.
N = 939 (not available for Lambert and Dahlberg and there are only placebo data for inflammation for Strand and Takamura).
N = 537.
N = 157.
N = 305 (not available for Dahlberg).
N = 861 (not available for Lambert and Dahlberg and there are only placebo data for inflammation for Strand and Takamura).
N = 553.
N = 137.
N = 287.
N = 1,043 (only have placebo data for Strand and Takamura).
N = 618.
N = 178.
N = 638 (not available for Lambert, Hall, Henriksen, Dahlberg, Chao, or Atchia).
N = 444 (not available for Lambert, Hall, Henriksen, Dahlberg, Chao, or Atchia).
N = 28 (not available for Lambert, Hall, Henriksen, Chao, or Atchia).
N = 287.
Placebo response
At short‐term follow‐up, 45.5% of placebo participants were placebo responders reporting ≥20% pain reduction from baseline, 52.6% were responders at midterm follow‐up, and 54.7% were responders at long‐term follow‐up. Of the 262 nonresponders at short‐term follow‐up, 107 participants became responders at midterm follow‐up and 82 participants at long‐term follow‐up. Of the 219 responders at short‐term follow‐up, 53 became nonresponders at midterm follow‐up and 32 at long‐term follow‐up.
The response rate was 56.3% in women and 47.5% in men at short‐term (P = 0.02), 61.4% in women and 54.7% in men at midterm (P = 0.10), and 61.2% in women and 54.7% in men at long‐term follow‐up (P = 0.17).
The mean (±SD) age was 60.8 years (±10.5 years) and the mean (±SD) BMI was 29.2 kg/m2 (± 4.0 kg/m2) for responders, and it was 61.3 years (± 8.9 years) with a BMI of 29.0 kg/m2 (± 4.2 kg/m2) for nonresponders at short‐term follow‐up. The mean (±SD) age was 61.3 years (± 10.1 years) with a BMI of 29.0 kg/m2 (± 3.8 kg/m2) for responders and 62.0 years (± 11.01 years) with a BMI of 29.2 kg/m2 (± 4.3 kg/m2) for nonresponders at midterm follow‐up. The mean (±SD) age was 61.3 years (± 10.4 years) with a BMI of 28.9 kg/m2 (± 3.8 kg/m2) for responders and 60.9 years (± 11.0 years) with a BMI of 29.4 kg/m2 (± 4.0 kg/m2) for nonresponders at long‐term follow‐up.
Potential predictors of placebo response
There were no associations with placebo response at the short‐term follow‐up. At the midterm follow‐up, participants with a severe baseline pain score (≥70 on a 100‐point scale) compared with those with a less severe baseline pain score in the IA glucocorticoid/placebo population had increased odds of a response to placebo (OR 2.98, 95% CI 1.01–8.82). The absolute pain reduction using SMD was 3.84 (95% CI −5.57 to 13.25). When analyzed using absolute change in pain, increased odds of placebo response was not demonstrated (Supplementary Table 2).
Worse baseline function score (WOMAC) at midterm follow‐up was significantly associated with a reduction in placebo response in the total placebo population (OR 0.98, 95% CI 0.96–1.00) with SMD 0.05 (95% CI −0.11 to 0.22) and in the IA HA/placebo group (OR 0.98, 95% CI 0.96–0.99) with SMD 0.03 (95% CI −0.14 to 0.20). With absolute change analysis, the OR was 0.99 (95% CI 0.97–1.00) in the total placebo population and 0.98 (95% CI 0.97–1.00) in the IA HA/placebo group.
There was no difference in placebo response at mid‐ or long‐term follow‐up according to age, sex, BMI, disease duration, baseline pain level, OA in other joints, radiographic severity, signs of inflammation (with or without imaging detection), or symptoms of stiffness (WOMAC) (Table 3).
Table 3.
Predictors of placebo response: participant and disease characteristics at short‐, mid‐, and long‐term*
| Short‐term | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential predictors | Placebo‐only population, n = 621 in ten studies | Placebo only (glucocorticoid trials group), n = 181 in six studies | Placebo only (hyaluronic acid trials group), n = 458 in five studies | |||||||||||||||
| n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | |
| Age | 609 | 0.991 (0.97–1.01) | 0.346 | 6.19 (0.721) | 0.0 | 0.000 | 174 | 0.992 (0.95–1.03) | 0.694 | 5.62 (0.345) | 11.0 | 0.000 | 453 | 0.989 (0.97–1.01) | 0.280 | 1.96 (0.743) | 0.0 | 0.000 |
| Sex | 609 | 1.075 (0.61–1.91) | 0.806 | 15.46 (0.051) | 48.2 | 0.316 | 174 | 0.855 (0.25–2.91) | 0.801 | 8.98 (0.062) | 55.5 | 1.060 | 453 | 1.024 (0.53–1.98) | 0.945 | 8.72 (0.068) | 54.2 | 0.274 |
| BMI | 554 | 1.004 (0.96–1.05) | 0.884 | 8.49 (0.291) | 17.6 | 0.001 | 120 | 1.056 (0.96–1.56) | 0.257 | 3.32 (0.345) | 9.6 | 0.001 | 452 | 0.992 (0.95–1.04) | 0.747 | 4.28 (0.369) | 6.6 | 0.000 |
| Disease duration | 427 | 0.999 (0.99–1.00) | 0.721 | 4.97 (0.548) | 0.0 | 0.000 | 98 | 1.000 (0.99–1.01) | 0.912 | 3.47 (0.325) | 13.5 | 0.000 | 347 | 1.001 (1.00–1.01) | 0.825 | 5.32 (0.150) | 43.6 | 0.000 |
| OA other joints | 439 | 0.824 (0.41–1.64) | 0.582 | 7.47 (0.058) | 59.8 | 0.280 | – | N/A | – | – | – | – | 411 | 0.752 (0.35–1.62) | 0.466 | 6.68 (0.035) | 70.1 | 0.323 |
| Pain at baseline | 609 | 1.007 (0.99–1.02) | 0.329 | 11.44 (0.247) | 21.3 | 0.000 | 174 | 1.010 (0.99–1.03) | 0.299 | 3.49 (0.625) | 0.0 | 0.000 | 453 | 1.006 (0.98–1.03) | 0.618 | 7.72 (0.103) | 48.2 | 0.000 |
| Severe pain (≥70) | 609 | 0.987 (0.66–1.47) | 0.947 | 6.37 (0.606) | 0.0 | 0.000 | 168 | 1.072 (0.40–2.87) | 0.891 | 4.93 (0.295) | 18.9 | 0.241 | 453 | 0.913 (0.59–1.41) | 0.685 | 2.15 (0.707) | 0.0 | 0.000 |
| KL grade | 551 | 1.054 (0.79–1.40) | 0.718 | 6.24 (0.512) | 0.0 | 0.000 | 140 | 1.117 (0.64–1.94) | 0.693 | 4.55 (0.337) | 12.0 | 0.049 | 428 | 0.954 (0.66–1.38) | 0.804 | 3.46 (0.326) | 13.4 | 0.020 |
| Patient global | 437 | 1.003 (0.99–1.01) | 0.549 | 2.89 (0.409) | 0.0 | 0.000 | – | – | – | – | – | – | 409 | 1.002 (0.99–1.02) | 0.740 | 2.84 (0.242) | 29.6 | 0.000 |
| Effusion | 511 | 0.712 (0.45–1.13) | 0.151 | 3.86 (0.696) | 0.0 | 0.000 | 133 | 0.512 (0.19–1.36) | 0.178 | 1.58 (0.664) | 0.0 | 0.000 | 411 | 0.785 (0.46–1.33) | 0.367 | 1.71 (0.425) | 0.0 | 0.000 |
| Inflammation | 530 | 0.893 (0.60–1.33) | 0.575 | 5.59 (0.470) | 0.0 | 0.000 | 153 | 0.420 (0.16–1.14) | 0.088 | 2.24 (0.524) | 0.0 | 0.000 | 429 | 1.160 (0.75–1.79) | 0.502 | 1.83 (0.401) | 0.0 | 0.000 |
| Inflammation detected by imaging | 153 | 0.388 (0.10–1.53) | 0.177 | 2.22 (0.329) | 10.0 | 0.150 | 153 | 0.388 (0.10–1.53) | 0.177 | 2.22 (0.329) | 10.0 | 0.150 | – | – | – | – | – | – |
| Stiffness | 476 | 0.992 (0.98–1.00) | 0.186 | 4.37 (0.498) | 0.0 | 0.000 | 65 | 0.985 (0.96–1.01) | 0.287 | 0.65 (0.723) | 0.0 | 0.000 | 429 | 0.993 (0.98–1.01) | 0.331 | 4.07 (0.254) | 26.2 | 0.000 |
| Function | 476 | 0.990 (0.98–1.00) | 0.150 | 3.65 (0.601) | 0.0 | 0.000 | 65 | 1.008 (0.97–1.04) | 0.660 | 2.07 (0.355) | 3.4 | 0.000 | 429 | 0.987 (0.97–1.00) | 0.087 | 0.47 (0.925) | 0.0 | 0.000 |
| Midterm | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo‐only population, n = 545 | Placebo only (Glucocorticoid trials group), n = 129 | Placebo only (Hyaluronic acid trials group), n = 434 | ||||||||||||||||
| n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | |
| Age | 545 | 0.997 (0.98–1.02) | 0.773 | 7.63 (0.470) | 0.0 | 0.000 | 129 | 1.013 (0.96–1.07) | 0.625 | 5.99 (0.200) | 33.2 | 0.001 | 434 | 0.994 (0.97–1.02) | 0.582 | 1.22 (0.747) | 0.0 | 0.000 |
| Sex | 545 | 1.163 (0.70–1.93) | 0.558 | 10.14 (0.181) | 31.0 | 0.149 | 129 | 2.446 (0.98–6.08) | 0.054 | 1.97 (0.579) | 0.0 | 0.000 | 434 | 0.903 (0.54–1.51) | 0.697 | 4.25 (0.236) | 29.3 | 0.080 |
| BMI | 498 | 0.997 (0.95–1.04) | 0.882 | 2.53 (0.865) | 0.0 | 0.000 | 83 | 0.992 (0.95–1.03) | 0.694 | 5.62 (0.345) | 11.0 | 0.000 | 433 | 0.997 (0.95–1.04) | 0.906 | 1.96 (0.744) | 0.0 | 0.000 |
| Disease duration | 394 | 1.001 (1.00–1.01) | 0.762 | 6.37 (0.383) | 5.8 | 0.000 | 82 | 0.998 (0.99–1.01) | 0.570 | 3.09 (0.379) | 2.8 | 0.000 | 330 | 1.001 (1.00–1.01) | 0.564 | 2.70 (0.440) | 0.0 | 0.000 |
| OA other joints | 412 | 0.809 (0.46–1.42) | 0.461 | 4.74 (0.192) | 36.7 | 0.118 | – | – | – | – | – | – | 393 | 0.848 (0.46–1.58) | 0.603 | 4.21 (0.122) | 52.5 | 0.159 |
| Pain at baseline | 545 | 1.006 (0.98–1.03) | 0.595 | 16.35 (0.038) | 51.1 | 0.001 | 129 | 1.019 (0.98–1.06) | 0.382 | 7.86 (0.097) | 49.1 | 0.001 | 434 | 0.998 (0.97–1.03) | 0.895 | 7.17 (0.067) | 58.2 | 0.000 |
| Severe pain (>70) | 545 | 0.987 (0.61–1.60) | 0.957 | 8.15 (0.320) | 14.1 | 0.069 | 129 | 2.980 (1.01–8.82) | 0.049 | 0.12 (0.989) | 0.0 | 0.000 | 434 | 0.752 (0.48–1.18) | 0.214 | 2.74 (0.433) | 0.0 | 0.000 |
| KL grade | 492 | 0.863 (0.63–1.18) | 0.357 | 2.76 (0.737) | 0.0 | 0.000 | 99 | 0.709 (0.34–1.46) | 0.352 | 2.25 (0.325) | 11.1 | 0.046 | 410 | 0.911 (0.64–1.30) | 0.606 | 0.08 (0.962) | 0.0 | 0.000 |
| Patient global | 411 | 0.998 (0.98–1.02) | 0.855 | 7.06 (0.070) | 57.5 | 0.000 | – | – | – | – | – | – | 392 | 0.993 (0.98–1.01) | 0.234 | 2.24 (0.326) | 10.7 | 0.000 |
| Effusion | 486 | 0.904 (0.55–1.49) | 0.693 | 2.06 (0.841) | 0.0 | 0.000 | 93 | 0.573 (0.16–2.00) | 0.383 | 0.83 (0.661) | 0.0 | 0.000 | 393 | 1.010 (0.99–1.03) | 0.420 | 0.37 (0.830) | 0.0 | 0.000 |
| Inflammation | 505 | 0.930 (0.52–1.66) | 0.804 | 7.57 (0.181) | 34.0 | 0.163 | 112 | 0.573 (0.16–2.00) | 0.383 | 0.83 (0.661) | 0.0 | 0.000 | 411 | 1.033 (0.48–2.24) | 0.934 | 5.92 (0.052) | 66.2 | 0.309 |
| Inflammation detected by imaging | 112 | 0.462 (0.09–2.48) | 0.367 | 0.68 (0.408) | 0.0 | 0.000 | 112 | 0.462 (0.09–2.48) | 0.367 | 0.68 (0.408) | 0.0 | 0.000 | – | – | – | – | – | – |
| Stiffness | 429 | 0.991 (0.98–1.00) | 0.161 | 4.18 (0.382) | 4.4 | 0.000 | 36 | 0.967 (0.92–1.02) | 0.210 | 0.12 (0.726) | 0.0 | 0.000 | 411 | 0.992 (0.98–1.01) | 0.320 | 3.94 (0.268) | 23.9 | 0.000 |
| Function | 429 | 0.979 (0.96–1.00) | 0.010 | 3.86 (0.425) | 0.0 | 0.000 | 36 | 0.974 (0.85–1.12) | 0.706 | 1.98 (0.159) | 49.5 | 0.006 | 411 | 0.978 (0.96–0.99) | 0.006 | 1.15 (0.564) | 0.0 | 0.000 |
| Long‐term | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo‐only population, n = 349 | Placebo only (glucocorticoid trials group), n = 72 | Placebo only (hyaluronic acid trials group), n = 277 | ||||||||||||||||
| n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | n | OR (95% CI) | P‐value | Cochran's Q (P‐value) | I 2, % | τ2 | |
| Age | 349 | 1.021 (0.98–1.06) | 0.271 | 8.32 (0.140) | 39.9 | 0.001 | 72 | 1.037 (0.95–1.13) | 0.408 | 3.91 (0.141) | 48.9 | 0.003 | 277 | 0.997 (0.97–1.02) | 0.807 | 1.54 (0.462) | 0.0 | 0.000 |
| Sex | 349 | 1.408 (0.82–2.42) | 0.214 | 0.42 (0.981) | 0.0 | 0.000 | 72 | 1.484 (0.54–4.09) | 0.446 | 0.19 (0.908) | 0.0 | 0.000 | 277 | 1.140 (0.70–1.87) | 0.604 | 1.09 (0.581) | 0.0 | 0.000 |
| BMI | 337 | 0.980 (0.93–1.03) | 0.429 | 2.16 (0.706) | 0.0 | 0.000 | 60 | 0.996 (0.82–1.21) | 0.969 | 1.53 (0.217) | 34.5 | 0.007 | 277 | 0.977 (0.93–1.03) | 0.395 | 0.54 (0.764) | 0.0 | 0.000 |
| Disease duration | 283 | 0.999 (1.00–1.01) | 0.672 | 0.12 (0.989) | 0.0 | 0.000 | 29 | 1.001 (0.99–1.02) | 0.866 | 0.05 (0.828) | 0.0 | 0.000 | 254 | 0.999 (0.99–1.00) | 0.636 | 0.00 (0.997) | 0.0 | 0.000 |
| OA other joints | 272 | 0.896 (0.54–1.50) | 0.678 | 1.12 (0.570) | 0.0 | 0.000 | – | – | – | – | – | – | 255 | 0.896 (0.54–1.50) | 0.678 | 1.12 (0.570) | 0.0 | 0.000 |
| Pain at baseline | 349 | 1.007 (0.99–1.03) | 0.441 | 5.19 (0.393) | 3.6 | 0.000 | 72 | 1.018 (0.98–1.06) | 0.375 | 2.56 (0.277) | 22.0 | 0.000 | 277 | 1.004 (0.98–1.03) | 0.759 | 2.29 (0.318) | 12.6 | 0.000 |
| Severe pain (>70) | 349 | 0.889 (0.44–1.79) | 0.741 | 6.55 (0.257) | 23.6 | 0.179 | 72 | 1.275 (0.24–6.69) | 0.774 | 3.51 (0.173) | 43.0 | 0.926 | 277 | 0.755 (0.36–1.58) | 0.456 | 2.50 (0.287) | 19.9 | 0.095 |
| KL grade | 327 | 0.873 (0.52–1.46) | 0.603 | 5.20 (0.268) | 23.0 | 0.079 | 72 | 0.793 (0.24–2.61) | 0.702 | 4.08 (0.130) | 50.9 | 0.567 | 255 | 0.947 (0.58–1.56) | 0.830 | 0.79 (0.373) | 0.0 | 0.000 |
| Patient global | 272 | 1.00 (0.98–1.02) | 0.879 | 3.76 (0.153) | 46.8 | 0.000 | – | – | – | – | – | – | 255 | 0.992 (0.98–1.01) | 0.331 | 0.91 (0.340) | 0.0 | 0.000 |
| Effusion | 315 | 0.593 (0.21–1.66) | 0.321 | 3.81 (0.149) | 47.5 | 0.390 | – | – | – | – | – | – | 255 | 0.881 (0.44–1.76) | 0.720 | 0.54 (0.461) | 0.0 | 0.000 |
| Inflammation | 315 | 0.854 (0.30–2.42) | 0.766 | 5.60 (0.061) | 64.3 | 0.504 | – | – | – | – | – | – | 255 | 1.295 (0.74–2.26) | 0.364 | 0.94 (0.333) | 0.0 | 0.000 |
| Inflammation detected by imaging | – | N/A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Stiffness | 267 | 0.990 (0.97–1.01) | 0.310 | 2.51 (0.286) | 20.2 | 0.000 | – | – | – | – | – | – | 255 | 0.993 (0.98–1.01) | 0.380 | 0.58 (0.447) | 0.0 | 0.000 |
| Function | 267 | 0.978 (0.95–1.01) | 0.194 | 3.59 (0.166) | 44.2 | 0.000 | – | – | – | – | – | – | 255 | 0.981 (0.94–1.03) | 0.402 | 3.44 (0.064) | 70.9 | 0.001 |
Participant and disease characteristics were adjusted for age, sex, and BMI. BMI, body mass index; CI, confidence interval; KL, Kellgren‐Lawrence; N/A, not available; OA, osteoarthritis; OR, odds ratio.
The analysis of the intervention and trial characteristics at short‐term follow‐up (Table 4) for the total placebo population revealed that participants who had ultrasound‐guided injections were significantly less likely to respond to placebo (OR 0.42, 95% CI 0.23–0.76; SMD −10.03, 95% CI −19.88 to −0.19). Those who received local anesthetics compared with those who did not receive local anesthetics were less likely to respond to placebo (OR 0.54, 95% CI 0.32–0.91; SMD −8.84, 95% CI −18.45 to 0.77). Similarly, participants who had joint fluid aspiration attempted compared with those those who did not have aspiration attempt were less likely to respond to placebo (OR 0.65, 95% CI 0.44–0.96; SMD −3.32, 95% CI −9.33 to 2.69). Participants of trials done at single centers compared with those who were in multicenter trials were less likely to respond to placebo (OR 0.62, 95% CI 0.39–0.99; SMD −8.75, 95% CI −16.94 to −0.57). Of the trials included in this study, all the single‐center trials (n = 6) were government funded, and the multicenter trials (n = 4) were commercially funded; thus, further analysis of funding sources was not conducted. With absolute change analysis, only ultrasound‐guided injections (OR 2.4, 95% CI 0.11–0.53) and local anesthetics administration (OR 0.40, 95% CI 0.17–0.94) were associated with reduced odds of placeo response (Supplementary Table 3).
Table 4.
Potential predictors of response: intervention and trial characteristics*
| Potential predictors of response | Placebo‐only population, n = 492 (exclude Chevalier trial) | Placebo only (glucocorticoid trials group), n = 181 | Placebo only (hyaluronic acid trials group), n = 329 (exclude Chevalier trial) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | P‐value | 95% CI | OR | P‐value | 95% CI | OR | P‐value | 95% CI | ||
| Short‐term | Volume of injection | 0.877 | 0.314 | 0.68–1.13 | 0.909 | 0.447 | 0.71–1.16 | 0.921 | 0.391 | 0.12–7.04 |
| Ultrasound‐guided injection | 0.415 | 0.004 | 0.23–0.76 | 0.690 | 0.363 | 0.31–1.53 | 0.376 | 0.076 | 0.13–1.11 | |
| Local anesthetics | 0.539 | 0.021 | 0.32–0.91 | 0.699 | 0.480 | 0.26–1.89 | 0.648 | 0.221 | 0.32–1.30 | |
| Aspiration attempted | 0.645 | 0.029 | 0.44–0.96 | N/A | – | – | 0.791 | 0.345 | 0.49–1.29 | |
| Injection frequency | 1.165 | 0.290 | 0.89–1.55 | 1.460 | 0.457 | 0.54–3.96 | 1.010 | 0.937 | 0.78–1.30 | |
| Randomization groups | 1.431 | 0.224 | 0.80–2.55 | 0.683 | 0.504 | 0.22–2.09 | 1.276 | 0.326 | 0.78–2.07 | |
| Single/multicenter | 0.620 | 0.046 | 0.39–0.99 | 0.825 | 0.694 | 0.32–2.15 | 0.648 | 0.221 | 0.32–1.30 | |
| Dropout rate | 0.979 | 0.585 | 0.91–1.06 | 0.976 | 0.591 | 0.89–1.07 | 0.969 | 0.439 | 0.89–1.05 | |
| Trial duration | 1.870 | 0.130 | 0.83–4.21 | – | – | – | 2.552 | 0.082 | 0.88–7.34 | |
| Potential predictors of response | Placebo‐only population, n = 422 (exclude Chevalier trial) | Placebo only (glucocorticoid trials group), n = 129 | Placebo only (hyaluronic acid trials group), n = 311 (exclude Chevalier trial) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Midterm | Volume of injection | 1.378 | 0.136 | 0.90–2.11 | 1.537 | 0.163 | 0.84–2.81 | 0.522 | 0.788 | 0.01–59.57 |
| Ultrasound‐guided injection | 0.630 | 0.442 | 0.19–2.04 | 1.037 | 0.979 | 0.07–14.83 | 0.047 | 0.003 | 0.01–0.36 | |
| Local anesthetics | 0.696 | 0.492 | 0.25–1.95 | 1.037 | 0.979 | 0.07–14.83 | 0.279 | 0.001 | 0.13–0.60 | |
| Aspiration attempted | 0.685 | 0.193 | 0.39–1.21 | – | – | – | 0.529 | 0.013 | 0.32–0.87 | |
| Injection frequency | 1.016 | 0.940 | 0.68–1.52 | – | – | – | 1.085 | 0.788 | 0.60–1.96 | |
| Randomization groups | 1.003 | 0.990 | 0.33–1.61 | 0.101 | 0.015 | 0.02–1.03 | 1.028 | 0.913 | 0.63–1.68 | |
| Single/multicenter | 0.696 | 0.492 | 0.25–1.95 | 1.037 | 0.979 | 0.07–14.83 | 0.279 | 0.001 | 0.13–0.60 | |
| Dropout rate | 1.027 | 0.814 | 0.82–1.28 | 1.419 | 0.355 | 0.68–2.98 | 1.057 | 0.779 | 0.72–1.55 | |
| Trial duration | 10.33 | 0.003 | 2.25–47.35 | – | – | – | 10.68 | 0.002 | 2.32–49.25 | |
| Potential predictors of response | Placebo‐only population, n = 232 | Placebo only (glucocorticoid trials group), n = 72 | Placebo only (hyaluronic acid trials group), n = 160 (exclude Chevalier trial) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Long‐term | Volume of injection | 1.301 | 0.109 | 0.94–1.80 | 1.388 | 0.123 | 0.91–2.10 | 0.625 | 0.705 | 0.06–7.11 |
| Ultrasound‐guided injection | 1.853 | 0.131 | 0.83–4.13 | 3.15 | 0.123 | 0.73–13.52 | – | – | – | |
| Local anesthetics | 2.137 | 0.038 | 1.04–4.37 | 3.15 | 0.123 | 0.73–13.52 | 1.264 | 0.705 | 0.37–4.26 | |
| Aspiration attempted | 1.190 | 0.564 | 0.66–2.16 | – | – | – | 0.815 | 0.656 | 0.33–2.01 | |
| Injection frequency | 1.178 | 0.243 | 0.89–1.55 | – | – | – | 1.061 | 0.704 | 0.78–1.44 | |
| Randomization groups | – | – | – | – | – | – | – | – | – | |
| Single/multicenter | 2.137 | 0.038 | 1.04–4.37 | 3.15 | 0.123 | 0.73–13.52 | 1.264 | 0.705 | 0.37–4.26 | |
| Dropout rate | 0.815 | 0.060 | 0.66–1.01 | 0.564 | 0.123 | 0.27–1.17 | – | – | – | |
| Trial duration | – | – | – | – | – | – | – | – | – | |
Trial characteristics were adjusted for age, sex, and body mass index. CI, confidence interval; N/A, not available; OR, odds ratio.
At midterm follow‐up, comparable findings were seen in participants who received local anesthetics, received ultrasound‐guided injections, had joint aspiration attempted, were in single‐center studies, or were seen in the IA HA/placebo group with reduced odds of placebo response. Trial duration of 12 to 24 weeks and ≥24 weeks in both the total placebo population and the IA HA/placebo comparison group were associated with increased odds of placebo response OR 10.33 (95% CI 2.25–47.35), OR 10.29 (95% CI 2.29–46.31, P = 0.002) respectively for 12 to 24 weeks and OR 10.68 (95% CI 2.32–49.27), OR 10.40 (95% CI 2.29–47.24, P = 0.002), respectively for ≥24 weeks. When analyzed using absolute change, these intervention and trial characteristics were still significantly associated with reduced/increased odds of placebo response.
There was no association of placebo response with volume of injection, randomization ratio, or dropout rate. No significant associations were seen at long‐term follow‐up.
DISCUSSION
To our knowledge, this is the first IPD meta‐analysis conducted to identify predictors of placebo response, specifically in IA injection trials in OA.
The finding of this IPD meta‐analysis demonstrated that participants with severe baseline pain may be more likely to respond to IA placebo in the IA glucocorticoid/placebo group at midterm follow‐up. However, this association was not demonstrated in the other placebo comparison groups at any other follow‐up time frame, and supplementary analysis using absolute change to avoid small changes from a smaller baseline value did not reveal any significance. Increased placebo ES and response have been associated with higher baseline pain scores (1, 3), but one study has negated this (4). This can potentially reflect regression to the mean, and this analysis did not find overall baseline pain to be a predictor of placebo response.
It was shown that participants with decreased baseline function may be less likely to respond to placebo in both the whole placebo population and the IA HA/placebo group at midterm follow‐up. It is likely that participants who have heightened functional limitations may have more severe disease and are thus less prone to placebo response, especially at longer follow‐up time points.
Other participant‐related factors were not shown to be predictors of response at any time frame in this study. This may be in part because of the nature of the RCTs included with adequate randomization of participants in the placebo arm, leading to a wide spread of participant characteristics. A recent study assessing proportional contextual effects (PCEs) in RCTs across different conditions and treatments found that blinded outcome assessor, allocation concealment, lower mean age, higher proportion of women, larger placebo effect, and nonchronic condition trials led to increased PCEs (32). But the sensitivity analysis revealed outcome assessor blinding being the only significant factor. Although this factor was not assessed, our study findings of no association with participant baseline characteristics is concordant with the sensitivity analysis. A crucial difference between this study and the PCE study is that this is an IPD analysis assessing a specific pharmacological intervention in a single disease condition using pain as the primary outcome. Both studies are in accordance that some important contextual factors are unable to be measured, including participants’ conceptual beliefs in the intervention, physician characteristics, and attitude and participant–physician interactions—all of which could impact the ability to define predictors of placebo response (33).
We have assessed to what extent specific treatment and trial characteristics affect the placebo response and demonstrated that, at short‐term follow‐up, participants in the total placebo population who had local anesthetic, aspiration attempt, ultrasound guidance or enrollment in single‐center trials were less likely to demonstrate a placebo response. The significance of the characteristics of ultrasound guidance and use of local anesthetic were substantiated using the absolute change analysis. Participant beliefs and knowledge of high‐tech equipment has been reported to influence placebo response (34). Based on studies comparing sonograph‐guided injections with blinded knee injections with active therapies, participants who had sonograph‐guided injections reported increased improvements in pain (35, 36). Therefore, there is the expectation that ultrasound‐guided placebo injection will lead to increased placebo response, but our study showed the inverse of this. One hypothetical rationale would be that ultrasound guidedance leads to more localization of the placebo injected and therefore can minimize any potential systemic effect of the placebo itself.
There has been no literature in relation to local anesthetic and placebo response in OA. Given the analgesic effect of local anesthetic, there is the assumption that it can impact the placebo response. However, given their short duration of action, the rebound in pain may lead to less placebo response.
Pain and poor function have been related to synovitis in OA (37, 38), and there is the hypothesis that aspiration of synovial fluid before injection may reduce the pro‐inflammatory cytokines in the joint, leading to a heightened placebo effect (3, 39). Conversely, in patients with OA who have sufficient synovial fluid to aspirate, baseline synovial inflammation volume may be elevated, thus the reduction in cytokines due to aspiration may be transient and the synovial fluid could re‐accumulate, possibly leading to reduced placebo response over time. In this study, the aspiration attempt was specified as part of the intervention protocol with each study, but there were limited data on whether synovial fluid was aspirated or the volume.
At midterm follow‐up, in the IA HA/placebo group, the same trial characteristics were demonstrated to have reduced odds of placebo response, but this was not seen in the total placebo or IA glucocorticoid/placebo groups. Even though absolute change analysis demonstrated significance, these findings may reflect multiplicity and need to be interpreted with care. Compared with meta‐analyses of placebo‐controlled HA trials (40, 41), only a very limited number of trials were obtained for this analysis. From the prior meta‐analyses, viscosupplementation compared with placebo did not reach the minimum clinically important between‐group difference, and in the subgroup analysis characteristics in which the SMD favored that of placebo or had clinical equivalence smaller than the minimal clinically important difference included large trial size (≥100), >3 injections (40), trials of 3 to 6 months and >6 months duration, and nonindustry sponsorship (40, 41). In our relative change analysis, participants in the single‐center, government‐funded trials had reduced odds of placebo response. Studies have shown that sample size was associated with the placebo response (1, 4, 42). Clinical trials with large participant numbers are commonly multicenter trials and can result in larger placebo effects because of the challenges of ensuring homogeneity between centers with outcome assessments (42).
At midterm follow‐up, participants in trials of moderate‐to‐long duration had increased odds of placebo response. Studies analyzing predictors of placebo response in various diseases have found variable associations with study duration (32, 43). With the added number of responders at mid‐ to long‐term follow‐up, the placebo effect may relate to regression to the mean or symptom fluctuation with the natural history of OA.
This IPD meta‐analysis showed that the placebo response persisted well into long‐term follow‐up, with more than 50% of participants being responders, which is concordant with recent meta‐analyses of IA saline placebo injections with therapeutic benefits seen beyond 6 months (39, 44, 45). This raises the ongoing debate of whether IA saline should be considered as a “pure” placebo, having potential biomechanical effects, leading to a therapeutic effect because of a possible dilutional effect on the inflammatory elements in the joint (39, 44). This IPD meta‐analysis showed no influence in relation to the frequency or volume of the injections on the placebo response to support this hypothesis. Given the clinical impact of saline placebo injections, further studies are required to explore this issue, and the significant placebo effect should be taken into consideration when planning for new IA therapy trials.
The use of IPD meta‐analysis allowed for an increased study sample size, thus reducing the issues with inadequate power often seen in subgroup analyses of traditional meta‐analysis. However, there are several limitations to the study. Overall, the authors of 56 potentially eligible IA glucocorticoid and HA publications were approached, and only the IPD of 6 studies were obtained and combined with existing OA Trial Bank studies for a total of 10 studies. Only a subset of eligible studies was analyzed; therefore, this analysis may be subject to selection bias and data availability bias.
The potential predictors of placebo response, including participant characteristics, pain mechanisms, treatment, and trial characteristics were chosen theoretically by the authors; thus, the analysis of potential predictors should be considered as investigative. There were some deviations from the study protocol because of the data availability of factors including unilateral/bilateral disease, comorbidities, morning stiffness (total WOMAC stiffness scores were available), aspirate volume, and IA injection approach (lateral vs medial).
To harmonize the data, participant outcome scores were standardized from their original scores to a 0 to 100 scale, despite potentially having different measurement sensitivities. This reflects the evolution of outcome measures use over time and the introduction of recommended Osteoarthritis Research Society International and Outcome Measures in Rheumatology core outcome measures. The inconsistencies across different trials with outcome measures and clinical signs call for more rigorous outcome measure recommendations by governing associations and measurement groups to enable higher precision in meta‐analysis.
In summary, this study demonstrated that participants who are administered local anesthetics or have ultrasound guidance may be less likely to respond to IA placebo at short‐term follow‐up. At midterm follow‐up, participants with worse baseline function scores may be less likely to respond to IA placebo, and those in trials of moderate‐to‐long duration are more likely to respond to IA placebo. These findings need to be corroborated by further studies before they can be identified as true contextual factors predictive of IA placebo response.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article and revising it critically for important intellectual content, and all authors approved the final version to be puslished. Dr. Yu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Yu, van Middelkoop, Ferreira, Bierma‐Zeinstra, Hunter.
Acquisition of data
Yu, van Middelkoop, Bierma‐Zeinstra, Zhang, Atchia, Birrell, Hunter.
Analysis and interpretation of data
Yu, Deveza, Bierma‐Zeinstra, Zhang, Bhagavath, Hunter.
Supporting information
Disclosure Form
Supplementary table 1 Risk of bias assessment
Supplementary Table 2 Predictors of placebo response – participant and disease characteristics at short, mid, and long‐term (absolute change in pain ≥20 points on 100 points scale)
Supplementary Table 3 Potential predictors of response – Intervention and trial characteristics (absolute difference in pain ≥ 20 points on 100 points scale)
List of studies contacted for individual patient data.
ACKNOWLEDGMENTS
All data contributors are to be acknowledged for the provision of their data to the OA Trial Bank. Thank you to Seikagaku Corporation for the contribution of their data. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
PROSPERO registration number: CRD42018095188.
This publication includes research using data from data contributors Sanofi that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
The OA Trial Bank is supported by the Dutch Arthritis Society. Dr. Shirley Yu's work was supported by a University of Sydney Postgraduate Scholarship (Merck PhD Scholarship in Medicine). Dr. Ferreira's work was supported by an National Health and Medical Research Council Investigator Fellowship. Dr. Bierma‐Zeinstra's work was supported by grants from the European Union, The Netherlands Organization for Health Research and Development, and the Dutch Arthritis Foundation. Dr. Zhang's work was supported by grants from Versus Arthritis UK, NIHR, and the Foundation for Research in Rheumatology. Dr. Fraser Birrell's work was supported by grants from the Sir Jules Thorn Trust, the MRC Versus Arthritis Centre for Integrated Research into Musculoskeletal Ageing, and the NIHR. Dr. Hunter's work was supported by a a National Health and Medical Research Council Investigator Fellowship. The funders were not involved in the study design, data collection, and interpretation.
Additional supplementary information cited in this article can be found online in the Supporting Information section (http://onlinelibrary.wiley.com/doi/10.1002/acr.25212).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr.25212.
References
- 1. Zhang W, Robertson J, Jones AC, et al. The placebo effect and its determinants in osteoarthritis: meta‐analysis of randomised controlled trials. Ann Rheum Dis 2008;67:1716–23. [DOI] [PubMed] [Google Scholar]
- 2. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta‐analysis. Ann Intern Med 2015;162:46–54. [DOI] [PubMed] [Google Scholar]
- 3. Bannuru RR, McAlindon TE, Sullivan MC, et al. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta‐analysis of osteoarthritis trials. Ann Intern Med 2015;163:365–72. [DOI] [PubMed] [Google Scholar]
- 4. Zou K, Wong J, Abdullah N, et al. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta‐analysis of randomised controlled trials. Ann Rheum Dis 2016;75:1964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W. The powerful placebo effect in osteoarthritis. Clin Exp Rheumatol 2019;37 Suppl 120:118–23. [PubMed] [Google Scholar]
- 6. Abhishek A, Doherty M. Mechanisms of the placebo response in pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1229–35. [DOI] [PubMed] [Google Scholar]
- 7. Riley RD, Lambert PC, Abo‐Zaid G. Meta‐analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 8. Bennell KL, Hunter DJ, Paterson KL. Platelet‐rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep 2017;19:24. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez‐Garcia SC, Castellanos‐Moreira R, Uson J, et al. Efficacy and safety of intra‐articular therapies in rheumatic and musculoskeletal diseases: an overview of systematic reviews. RMD Open 2021;7:e001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Middelkoop M, Arden NK, Atchia I, et al. The OA Trial Bank: meta‐analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra‐articular glucocorticoids. Osteoarthritis Cartilage 2016;24:1143–52. [DOI] [PubMed] [Google Scholar]
- 11. Fu Y, Persson MS, Bhattacharya A, et al. Identifying placebo responders and predictors of response in osteoarthritis: a protocol for individual patient data meta‐analysis. Syst Rev 2016;5:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu SP, Ferreira ML, van Middelkoop M, et al. Predictors of placebo response to local (intra‐articular) therapy in osteoarthritis: an individual patient data meta‐analysis protocol. BMJ Open 2019;9:e027372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 14. Zhang W, Doherty M, Peat G, et al. EULAR evidence‐based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis 2010;69:483–9. [DOI] [PubMed] [Google Scholar]
- 15. van Middelkoop M, Dziedzic KS, Doherty M, et al. Individual patient data meta‐analysis of trials investigating the effectiveness of intra‐articular glucocorticoid injections in patients with knee or hip osteoarthritis: an OA Trial Bank protocol for a systematic review. Syst Rev 2013;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration ; 2011. [Google Scholar]
- 18. Groenwold RH, Donders AR, van der Heijden GJ, et al. Confounding of subgroup analyses in randomized data. Arch Intern Med 2009;169:1532–4. [DOI] [PubMed] [Google Scholar]
- 19. Tubach F, Ravaud P, Martin‐Mola E, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res (Hoboken) 2012;64:1699–707. [DOI] [PubMed] [Google Scholar]
- 20. Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta‐analysis. BMJ 2010;341:c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 22. Chevalier X, Jerosch J, Goupille P, et al. Single, intra‐articular treatment with 6 ml hylan G‐F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double‐blind, placebo controlled trial. Ann Rheum Dis 2010;69:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henriksen M, Christensen R, Klokker L, et al. Evaluation of the benefit of corticosteroid injection before exercise therapy in patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Intern Med 2015;175:923–30. [DOI] [PubMed] [Google Scholar]
- 24. Hall M, Doherty S, Courtney P, et al. Ultrasound detected synovial change and pain response following intra‐articular injection of corticosteroid and a placebo in symptomatic osteoarthritic knees: a pilot study. Ann Rheum Dis 2014;73:1590–1. [DOI] [PubMed] [Google Scholar]
- 25. Dahlberg L, Lohmander LS, Ryd L. Intraarticular injections of hyaluronan in patients with cartilage abnormalities and knee pain. A one‐year double‐blind, placebo‐controlled study. Arthritis Rheum 1994;37:521–8. [DOI] [PubMed] [Google Scholar]
- 26. Takamura J, Seo T, Strand V. A single intra‐articular injection of Gel‐200 for treatment of symptomatic osteoarthritis of the knee is more effective than phosphate buffered saline at 6 months: a subgroup analysis of a multicenter, randomized controlled trial. Cartilage 2019;10:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strand V, Baraf HSB, Lavin PT, et al. A multicenter, randomized controlled trial comparing a single intra‐articular injection of Gel‐200, a new cross‐linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 2012;20:350–6. [DOI] [PubMed] [Google Scholar]
- 28. Atchia I, Kane D, Reed MR, et al. Efficacy of a single ultrasound‐guided injection for the treatment of hip osteoarthritis. Ann Rheum Dis 2011;70:110–6. [DOI] [PubMed] [Google Scholar]
- 29. Chao J, Wu C, Sun B, et al. Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol 2010;37:650–5. [DOI] [PubMed] [Google Scholar]
- 30. Ravaud P, Moulinier L, Giraudeau B, et al. Effects of joint lavage and steroid injection in patients with osteoarthritis of the knee: results of a multicenter, randomized, controlled trial. Arthritis Rheum 1999;42:475–82. [DOI] [PubMed] [Google Scholar]
- 31. Lambert RG, Hutchings EJ, Grace MG, et al. Steroid injection for osteoarthritis of the hip: a randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 2007;56:2278–87. [DOI] [PubMed] [Google Scholar]
- 32. Hafliethadottir SH, Juhl CB, Nielsen SM, et al. Placebo response and effect in randomized clinical trials: meta‐research with focus on contextual effects. Trials 2021;22:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015;16:403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richardson C, Plaas A, Block JA. Intra‐articular hyaluronan therapy for symptomatic knee osteoarthritis. Rheum Dis Clin North Am 2019;45:439–51. [DOI] [PubMed] [Google Scholar]
- 35. Sibbitt WL Jr, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol 2012;41:66–72. [DOI] [PubMed] [Google Scholar]
- 36. Kianmehr N, Hasanzadeh A, Naderi F, et al. A randomized blinded comparative study of clinical response to surface anatomy guided injection versus sonography guided injection of hyaloronic acid in patients with primary knee osteoarthritis. Int J Rheum Dis 2018;21:134–9. [DOI] [PubMed] [Google Scholar]
- 37. Garnero P, Piperno M, Gineyts E, et al. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis 2001;60:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Jin X, Han W, et al. Cross‐sectional and longitudinal associations between knee joint effusion synovitis and knee pain in older adults. J Rheumatol 2016;43:121–30. [DOI] [PubMed] [Google Scholar]
- 39. Altman RD, Devji T, Bhandari M, et al. Clinical benefit of intra‐articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta‐analysis of randomized trials. Semin Arthritis Rheum 2016;46:151–9. [DOI] [PubMed] [Google Scholar]
- 40. Rutjes AW, Juni P, da Costa BR, et al. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta‐analysis. Ann Intern Med 2012;157:180–91. [DOI] [PubMed] [Google Scholar]
- 41. Pereira TV, Juni P, Saadat P, et al. Viscosupplementation for knee osteoarthritis: systematic review and meta‐analysis. BMJ 2022;378:e069722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wen X, Luo J, Mai Y, et al. Placebo response to oral administration in osteoarthritis clinical trials and its associated factors: a model‐based meta‐analysis. JAMA Netw Open 2022;5:e2235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walach H, Sadaghiani C, Dehm C, et al. The therapeutic effect of clinical trials: understanding placebo response rates in clinical trials–a secondary analysis. BMC Med Res Methodol 2005;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saltzman BM, Leroux T, Meyer MA, et al. The therapeutic effect of intra‐articular normal saline injections for knee osteoarthritis: a meta‐analysis of evidence level 1 studies. Am J Sports Med 2017;45:2647–53. [DOI] [PubMed] [Google Scholar]
- 45. Previtali D, Merli G, Di Laura Frattura G, et al. The long‐lasting effects of “placebo injections” in knee osteoarthritis: a meta‐analysis. Cartilage 2021;13:185S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary table 1 Risk of bias assessment
Supplementary Table 2 Predictors of placebo response – participant and disease characteristics at short, mid, and long‐term (absolute change in pain ≥20 points on 100 points scale)
Supplementary Table 3 Potential predictors of response – Intervention and trial characteristics (absolute difference in pain ≥ 20 points on 100 points scale)
List of studies contacted for individual patient data.


