Abstract
Objective
To assess the association between maternal asthma and adverse perinatal outcomes in an Australian Indigenous population.
Methods
This prospective cohort study included all Indigenous mother and baby dyads for births from 2001 to 2013 in Western Australia (n = 25 484). Data were linked from Western Australia Births, Deaths, Midwives, Hospital, and Emergency Department collections. Maternal asthma was defined as a self‐reported diagnosis at an antenatal visit or hospitalization or emergency visit for asthma during pregnancy or less than 3 years before pregnancy. Associations with birth, labor, and pregnancy outcomes were assessed using generalized estimating equations. Asthma exacerbation during pregnancy and stratification by remoteness was also assessed.
Results
Maternal asthma was associated with placental abruption (adjusted odds ratio [aOR], 1.59 [95% confidence interval (CI), 1.07–2.35]), threatened preterm labor (aOR, 1.58 [95% CI, 1.39–1.79]), and emergency cesarean sections (aOR, 1.27 [95% CI, 1.13–1.44]). These risks increased further with an asthma exacerbation during pregnancy or if the mother was from a remote area. No associations were found for low birth weight, preterm birth, small for gestational age, or perinatal mortality.
Conclusion
Maternal asthma in Indigenous women is associated with an increased risk of emergency cesarean sections, placental abruption, and threatened preterm labor. These risks may be mitigated by improved management of asthma exacerbations during pregnancy.
Keywords: asthma, birth, exacerbation, indigenous, labor, perinatal, pregnancy
Synopsis
Maternal asthma in Australian Indigenous women is associated with an increased risk in emergency cesarean sections, placental abruption, and threatened preterm labor.
1. INTRODUCTION
Infant mortality, low birth weight, preterm birth, and small for gestational age are more than double the rate in the Australian Indigenous (Aboriginal and Torres Strait Islander people) than the non‐Indigenous population. 1 , 2 Moreover, adverse perinatal outcomes have been shown to have long‐term health consequences in this population. 3
Another discrepant health burden in Indigenous populations is asthma. Australia has one of the highest rates of asthma in the world (10%); however, in the Indigenous community, this rate rises to 18% of women and 13% of men. 4 Additionally, asthma hospitalization and mortality rates attributable to asthma are twice as high in Indigenous Australians. 1 , 5
These discrepancies in perinatal and respiratory outcomes for Australia's Indigenous populations are indicative of broader health inequities attributable in large part to the effects of colonization, marginalization, and racism, including exclusion from socioeconomic opportunities; removal of children from families, communities, culture, and land; and government policies reducing self‐efficacy. 6 These experiences are not unique to Australia's Indigenous population, and the effects on long‐term social and economic consequences leading to higher health burdens have been noted in other Indigenous populations. 6
Despite the fact that a number of studies have shown that maternal asthma in pregnancy is a risk factor for a number of adverse perinatal outcomes including low birth weight, preterm birth, cesarean delivery, and labor complications, 7 , 8 , 9 this relationship has not been tested in Indigenous populations. Given the high burden of asthma in Australian Indigenous women of childbearing age, 2 we hypothesized that maternal asthma may be contributing to the disproportionate burden on adverse perinatal outcomes, hence providing an opportunity for intervention through better asthma control during pregnancy. 7
The objective of this study was to assess the associations between maternal asthma and adverse perinatal outcomes in an Indigenous population.
2. METHODS
The current investigation is a whole‐population, prospective cohort study.
2.1. Study population
The study population included all singleton Indigenous babies ≥20 weeks of gestation born in Western Australia (WA) from 2001 to 2013 to mothers who also identify as Indigenous. 10 Since Australian data sets have historically recorded many Aboriginal people as non‐Aboriginal, an algorithm was applied to a wide range of data sets related to the individual child to give the most robust yet inclusive ascertainment of Indigenous status. Babies were also classified as Aboriginal if a parent or grandparent was classified as Aboriginal following application of the algorithm, since using family data has been shown to improve classification. 11 , 12 Linkage of WA data sets was undertaken by the WA DLB using probabilistic linkage based on birthdate and demographic identifiers as part of a broader project, Defying the Odds. 10 For the current study, data sets included WA Birth Registrations, WA Death Registrations, WA Midwives Notification System (MNS), WA Hospital Morbidity Data Collection, and the WA Emergency Department Data Collection. The MNS was the basis of the linkage since it includes almost every child born in WA and 99.9% of MNS records are able to be linked to other data sets.
2.2. Exposure
Maternal asthma was defined as answering yes to a question about having asthma during a prenatal visit (MNS), or being hospitalized or visiting an urban emergency department (ED) for asthma (principal diagnosis, International Statistical Classification of Diseases, Tenth Revision [ICD‐10] code J45 or J46) during pregnancy or in the 3 years before pregnancy. We included the period of 3 years before pregnancy to increase sensitivity, because although the validity of the MNS for births in WA is extremely high and the specificity for asthma was 99%, the sensitivity was only 44%. 13 Since asthma is a chronic disease, a woman who had an emergency visit or hospitalization for asthma in the 3 years before pregnancy was considered to continue to have asthma during pregnancy. Note that in rural areas of WA, major disease categories are used in EDs rather than ICD‐10 codes. Asthma is incorporated into the major disease category of respiratory diseases and cannot be separated from other respiratory causes.
Asthma exacerbation in pregnancy was defined as hospitalization for asthma during pregnancy OR an urban ED visit for asthma (ICD‐10 code J45/46) during pregnancy OR a rural ED visit for a respiratory condition (Major Diagnostic Category 4) and asthma listed as a medical condition in the MNS.
2.3. Outcomes
Data for birth and labor outcomes were retrieved from the MNS data set. Birth outcomes included birth weight; gestational age; small for gestational age–birth weight lower than the first decile for singleton infants of the same gestational age and sex according to national standards 14 ; stillbirth––birth without evidence of life (>20 gestational weeks); neonatal death––death in the first 30 days of life; perinatal mortality––stillbirths and neonatal deaths combined; and neonatal intensive care unit/special care nursery (NICU/SCN)––hospitalized in the intensive care unit in the first 7 days of life or reported as having being transferred at birth to the special nursery. Maternal pregnancy conditions included gestational diabetes and pre‐eclampsia. Labor and pregnancy outcomes included mode of delivery, placenta previa, placental abruption, preterm rupture of membranes (PROM), and threatened preterm labor (contractions <37 weeks not resulting in preterm birth).
2.4. Covariates
Data for covariates retrieved from the MNS included maternal age, sex of the baby, smoking during pregnancy, parity, maternal pre‐existing medical conditions (including type 2 diabetes and hypertension), and area‐level remoteness of residence at birth derived from the Accessibility/Remoteness Index of Area. 15 Disadvantage at birth in quintiles (Q1 = most disadvantaged areas, Q5 = least disadvantaged areas) based on the Australian Bureau of Statistics Index of Relative Socio‐Economic Disadvantage (IRSD). 16 IRSD is a summary index of variables that indicate relative disadvantage including income, education, employment, occupation, housing, and other miscellaneous indicators. Maternal mental illness was also recorded, which included hospitalization/ED visit for a mood disorder or neurotic stress‐related disorder (ICD‐10 codes F30‐F48) 12 months before birth.
2.5. Statistical analysis
Descriptive statistics for Indigenous mothers with and without asthma were calculated. Logistic regression with a generalized estimating equations approach was used to estimate associations between maternal asthma, asthma exacerbations in pregnancy, and birth and labor outcomes, expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Generalized estimating equations include sandwich estimators allowing the models to account for correlated outcomes for children born to the same mother. A subject‐matter informed directed acyclic graph was used to identify covariates as potential confounders (Figure S1). Based on the directed acyclic graph, adjusted models that included potential confounders were fitted: remoteness, disadvantage, smoking in pregnancy, maternal age, pre‐existing medical conditions, mental illness, and parity. An interaction term for sex and maternal asthma was added to models to assess effect modification by sex.
Investigation of asthma by data source (MNS, hospitalization, or ED) revealed that not only were ED asthma cases under‐represented in rural areas but the proportion of self‐report asthma (MNS) also decreased by remoteness, and yet the hospitalization rate for asthma in infants was constant across regions (Table S1). To reduce the effect of bias from geographic disparity of case definition we conducted several sensitivity analyses. The first included asthma cases based on hospitalization data or self‐report during antenatal visits only (excluding ED visits for asthma). The second sensitivity analysis stratified this analysis further into three geographic location categories: urban, regional (inner and outer regional combined), and remote (remote and very remote combined). We also conducted a bias analysis to assess the potential bias that could be occurring as a result of not having body mass index data (not recorded at antenatal visits in WA). Obesity is a known risk factor for both asthma and adverse perinatal outcomes and therefore could be potentially confounding associations.
Statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc).
The study was approved by the Western Australian Indigenous Health Ethics Committee (RGS 0000002846), the WA Department of Health Human Ethics Committee (Migrated ID DOH‐201530), and the University of Melbourne Medicine and Dentistry Human Ethics Sub‐Committee (#1851158).
3. RESULTS
The study population included 25 484 births of Indigenous children in WA of Indigenous children with 12 323 Indigenous mothers. Among all pregnancies, the prevalence of asthma was 11.0% (n = 2807), and, of these, 10.3% (n = 289) had an asthma exacerbation during pregnancy (1.3% of all pregnancies). Overall, mothers with reported asthma were more likely to be younger, be from less disadvantaged neighborhoods, and more likely to have pre‐existing medical conditions or mental illness than mothers without asthma (Table 1). The prevalence of smoking during pregnancy was 49% and differed little by asthma status.
TABLE 1.
Description of Indigenous mothers and pregnancies by maternal asthma status
| Pregnancies with asthma, No. (%) | Pregnancies without asthma, No. (%) | |
|---|---|---|
| Whole cohort | 2809 (11.0) | 22 675 (89.0) |
| Maternal age (years) | ||
| ≤19 | 667 (23.8) | 4584 (20.2) |
| 20–24 | 707 (25.2) | 5403 (23.8) |
| 25–29 | 910 (32.4) | 7453 (32.9) |
| ≥30 | 525 (18.7) | 5235 (23.1) |
| Geographic location | ||
| Urban | 1460 (52.0) | 7875 (34.8) |
| Inner regional | 172 (6.1) | 1206 (5.3) |
| Outer regional | 558 (19.9) | 3576 (15.8) |
| Remote | 354 (12.6) | 5004 (22.1) |
| Very remote | 264 (9.4) | 4972 (22.0) |
| Missing | 43 (0.2) | |
| Disadvantage (%) | ||
| 80–100 (highest) | 1254 (45.1) | 11 315 (51.0) |
| 60–80 | 737 (26.5) | 4937 (22.3) |
| 40–60 | 449 (16.2) | 3280 (14.8) |
| 20–40 | 233 (8.4) | 1641 (7.4) |
| 0–20 (lowest) | 106 (3.8) | 1011 (4.6) |
| Missing | 521 (2.0) | |
| Smoking in pregnancy | 1413 (50.3) | 11 032 (48.7) |
| Maternal pre‐existing medical conditions | 1011 (36.0) | 6282 (27.7) |
| Maternal mental illness | 130 (4.6) | 747 (3.3) |
| Sex of child | ||
| Female | 1372 (48.8) | 11 187 (49.3) |
| Male | 1437 (51.2) | 11 487 (50.7) |
Maternal asthma was associated with an increased risk of having an emergency cesarean section (adjusted odds ratio [adjOR], 1.27 [95% confidence interval (CI)], 1.13–1.44), placental abruption (aOR, 1.59 [95% CI, 1.07–2.33]), and threatened preterm labor (aOR, 1.58 [95% CI, 1.39–1.79]) (Table 2). The following risks increased further when there was an asthma exacerbation during pregnancy: emergency cesarean section (aOR, 1.35 [95% CI, 0.95–1.91]), placental abruption (aOR, 2.85 [95% CI, 1.24–6.55]), and threatened preterm labor (aOR, 1.86 [95% CI, 1.37–2.54]) compared with no asthma (Table 3). When compared with mothers who had asthma with no exacerbation, the odds for adverse outcomes in mothers with asthma exacerbations remained increased although CIs included the null (placental abruption [aOR, 2.03; 95% CI, 0.83–4.98] and threatened preterm birth [aOR, 1.25; 95% CI, 0.89–1.74]).
TABLE 2.
Associations between Indigenous maternal asthma and birth and labor outcomes
| Maternal asthma (n = 2809) | No maternal asthma (n = 22 675) | Unadjusted OR (95% CI) | Adjusted OR a (95% CI) | |
|---|---|---|---|---|
| Birth outcomes | ||||
| Birth weight (g) | ||||
| <1500 | 85 (3.0) | 595 (2.6) | 1.19 (0.94–1.49), P = 0.15 | 1.13 (0.89–1.43), P = 0.31 |
| 1500–2499 | 319 (12.0) | 2342 (10.3) | 1.13 (0.99–1.29), P = 0.08 | 1.08 (0.94–1.24), P = 0.30 |
| 2500–4000 | 2170 (77.3) | 17 979 (79.3) | Reference | Reference |
| >4000 | 235 (8.4) | 1759 (7.8) | 1.11 (0.95–1.29), P = 0.19 | 1.08 (0.92–1.26), P = 0.34 |
| Gestational age (week) | ||||
| <33 | 143 (5.1) | 1013 (4.5) | 1.13 (0.94–1.37), P = 0.19 | 1.05 (0.87–1.27), P = 0.64 |
| 33–36 | 274 (9.8) | 2136 (9.4) | 1.03 (0.89–1.18), P = 0.68 | 0.99 (0.86–1.14), P = 0.86 |
| 37–40 (term) | 2142 (76.3) | 17 193 (75.8) | Reference | Reference |
| >40 | 250 (8.9) | 2333 (10.3) | 0.86 (0.75–0.99), P = 0.04 | 0.92 (0.80–1.07), P = 0.28 |
| SGA | 432 (15.6) | 3744 (16.7) | 0.92 (0.82–1.03), P = 0.15 | 0.96 (0.85–1.08), P = 0.50 |
| Stillbirth | 31 (1.1) | 256 (1.1) | 0.98 (0.68–1.42), P = 0.92 | 0.92 (0.63–1.34), P = 0.66 |
| Neonatal death | 22 (0.8) | 133 (0.6) | 1.02 (0.58–1.78), P = 0.95 | 1.07 (0.61–1.88), P = 0.80 |
| Perinatal mortality | 53 (1.9) | 399 (1.7) | 0.99 (0.73–1.35), P = 0.96 | 0.96 (0.70–1.32), P = 0.82 |
| NICU/SCN | 460 (16.6) | 3112 (13.9) | 1.23 (1.10–1.38), P < 0.001 | 1.05 (0.94–1.18), P = 0.39 |
| Maternal pregnancy conditions | ||||
| Gestational diabetes | 187 (6.7) | 1402 (6.2) | 1.08 (0.92–1.28), P = 0.35 | 1.02 (0.86–1.21), P = 0.83 |
| Pre‐eclampsia | 141 (5.0) | 993 (4.1) | 1.18 (0.99–1.41), P = 0.07 | 1.14 (0.94–1.37), P = 0.17 |
| Pregnancy and labor outcomes | ||||
| Mode of delivery | ||||
| Vaginal birth | 2135 (76.0) | 18 033 (79.5) | Reference | Reference |
| Cesarean section elective | 225 (8.0) | 1703 (7.5) | 1.12 (0.95–1.31), P = 0.19 | 1.08 (0.91–1.28), P = 0.40 |
| Cesarean section emergency | 449 (16.0) | 2939 (13.0) | 1.29 (1.15–1.45), P < 0.001 | 1.27 (1.13–1.44), P < 0.001 |
| Placenta praevia | 20 (0.7) | 114 (0.5) | 1.43 (0.89–2.31), P = 0.14 | 1.27 (0.78–2.06), P = 0.38 |
| Placental abruption | 32 (1.1) | 153 (0.7) | 1.70 (1.16–2.49), P = 0.007 | 1.59 (1.07–2.35), P = 0.02 |
| Preterm rupture of membranes | 322 (11.5) | 2361 (10.4) | 1.11 (0.98–1.26), P = 0.09 | 1.09 (0.96–1.24), P = 0.19 |
| Threatened preterm labor | 394 (14.0) | 2164 (9.5) | 1.55 (1.37–1.75), P < 0.001 | 1.58 (1.39–1.79), P < 0.001 |
Abbreviations: CI, confidence interval; NICU/SCN, neonatal intensive care unit/special care unit; OR, odds ratio; SGA, small for gestational age.
Adjusted for maternal age, smoking in pregnancy, remoteness, disadvantage, maternal mental illness, maternal pre‐existing medical conditions, and parity.
TABLE 3.
Associations between Indigenous maternal exacerbation of asthma during pregnancy and birth and labor outcomes b
| Maternal asthma exacerbations (n = 289) | No maternal asthma (n = 22 675) | Unadjusted OR (95% CI) | Adjusted OR a (95% CI) | |
|---|---|---|---|---|
| Birth outcomes | ||||
| Birth weight (g) | ||||
| <1500 | 7 (2.4) | 595 (2.6) | 0.94 (0.44–1.99), P = 0.87 | 0.93 (0.44–1.96), P = 0.85 |
| 1500–2499 | 28 (9.7) | 2332 (10.3) | 0.96 (0.64–1.44), P = 0.96 | 0.86 (0.56 1.31), P = 0.47 |
| 2500–4000 | 225 (77.9) | 17 979 (79.3) | Reference | Reference |
| >4000 | 29 (10.0) | 1759 (7.8) | 1.32 (0.90–1.93), P = 0.16 | 1.30 (0.88–1.93), P = 0.19 |
| Gestational age (week) | ||||
| <33 | 12 (4.2) | 1013 (4.5) | 0.91 (0.51–1.62), P = 0.79 | 0.93 (0.52–1.65), P = 0.74 |
| 33–36 | 28 (9.7) | 2136 (9.4) | 1.02 (0.69–1.52), P = 0.90 | 0.99 (0.66–1.47), P = 0.95 |
| 37–40 (term) | 220 (76.1) | 17 193 (75.8) | Reference | Reference |
| >40 | 29 (10.0) | 2333 (10.3) | 0.97 (0.66–1.43), P = 0.88 | 0.99 (0.67–1.47), P = 0.98 |
| SGA | 40 (14.0) | 3744 (16.7) | 0.81 (0.58–1.14), P = 0.23 | 0.77 (0.55–1.09), P = 0.14 |
| Perinatal mortality | 6 (2.1) | 367 (1.6) |
1.29 (0.57–2.91) P = 0.54 |
1.03 (0.42–2.50) P = 0.95 |
| NICU/SCN | 38 (13.3) | 3112 (13.9) | 0.95 (0.67–1.34), P = 0.77 | 0.97 (0.68–1.38), P = 0.85 |
| Maternal pregnancy conditions | ||||
| Gestational diabetes | 26 (9.0) | 1402 (6.2) | 1.50 (1.00–2.23), P = 0.05 | 1.20 (0.77–1.86), P = 0.41 |
| Pre‐eclampsia | 17 (5.9) | 1111 (4.9) | 1.21 (0.74–1.99), P = 0.44 | 1.20 (0.72–1.99), P = 0.49 |
| Pregnancy and labor outcomes | ||||
| Mode of delivery | ||||
| Vaginal birth | 216 (74.7) | 18 033 (79.5) | Reference | Reference |
| Cesarean section elective | 25 (8.7) | 1703 (7.5) | 1.23 (0.80–1.88), P = 0.35 | 1.18 (0.76–1.82), P = 0.46 |
| Cesarean section emergency | 48 (16.6) | 2939 (13.0) | 1.36 (0.97–1.91), P = 0.07 | 1.35 (0.95–1.91), P = 0.09 |
| Placental abruption | 6 (2.1) | 153 (0.7) | 3.12 (1.37–7.12), P = 0.007 | 2.85 (1.24–6.55), P = 0.01 |
| Preterm rupture of membranes | 34 (11.8) | 2361 (10.4) | 1.15 (0.80–1.64), P = 0.45 | 1.08 (0.75–1.57), P = 0.68 |
| Threatened preterm labor | 52 (18.0) | 2164 (9.5) | 2.08 (1.54–2.82), P < 0.001 | 1.86 (1.37–2.54), P < 0.001 |
Abbreviations: CI, confidence interval; NICU/SCN, neonatal intensive care unit/special care unit; OR, odds ratio; SGA, small for gestational age.
Adjusted for maternal age, smoking in pregnancy, remoteness, disadvantage, maternal mental illness, maternal pre‐existing medical conditions, and parity.
Stillbirth, neonatal death and placental praevia not shown as the cell sizes for maternal asthma exacerbations were <5.
There were no observed associations of maternal asthma or maternal asthma exacerbations with lower birth weight, preterm birth, small for gestational age, stillbirth, neonatal death, perinatal mortality, gestational diabetes, NICU/SCN admission, or elective cesarean sections (Tables 2 and 3). The associations between maternal asthma and pre‐eclampsia, placenta previa, and PROM were raised; however, it is not possible to make definitive interpretations since the 95% CIs included one (Table 2).
Tests for interaction by the baby's sex did not suggest that associations differed for male and female babies except for the association with pre‐eclampsia, which found that asthma was associated with pre‐eclampsia (aOR, 1.39 [95% CI, 1.09–1.78] in pregnancies with female babies, but not with male babies [aOR, 0.97; 95% CI, 0.74–1.27]).
Findings from sensitivity analysis including only hospitalizations and self‐reported asthma showed similar results to the main analysis (Table S2). The sensitivity analysis stratifying the analysis by remoteness of maternal residence revealed that the asthma prevalence in Indigenous mothers decreased by remoteness: 15.3% in urban areas, 13.1% in regional areas, and 5.8% in remote areas. Maternal asthma was associated with threatened preterm labor among all remoteness areas (Table S3 and Figure 1), and risk of placental abruptions and emergency cesarean sections were increased for all areas but were not statistically significant, except for emergency cesarean sections in remote areas (odds ratio, 1.66 [95% CI, 1.32–2.10]). Other perinatal outcomes were found to have an increased risk with maternal asthma in remote but not urban or regional areas: NICU/SCN admissions (aOR, 1.34 [95% CI, 1.05–1.72]), elective cesarean sections (aOR, 1.55 [95% CI, 1.13–2.13]), and pre‐eclampsia (aOR, 1.76 [95% CI, 1.22–2.55]).
FIGURE 1.
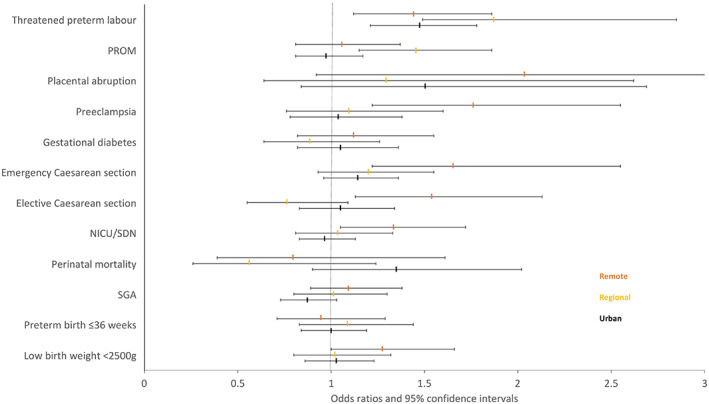
Sensitivity analysis of maternal asthma and perinatal outcomes in Indigenous women by remoteness. Adjusted odds ratios and 95% confidence intervals. Abbreviations: NICU/SCN, neonatal intensive care unit/special care unit; PROM, preterm rupture of membranes; SGA, small for gestational age. Orange indicates remote; yellow, regional; and black, urban.
Bias analyses for unmeasured obesity suggest that it is unlikely that there is confounding by obesity for the association between maternal asthma and placental abruption and threatened preterm labor. However, the association with emergency cesarean section may include some bias, particularly if the obesity rates in this population are high (see Supplement, Bias Analysis and Table S4).
4. DISCUSSION
This study of Indigenous mothers and babies found that maternal asthma is associated with an increase in pregnancy complications including emergency cesarean sections, placental abruption, and threatened preterm labor, which were further increased for those with an exacerbation during pregnancy.
Our findings confirm those from other studies, which found associations between maternal asthma and each of placental abruption, preterm labor, and emergency cesarean sections. 7 , 8 In addition, a study based in Sweden found an increased risk of emergency cesarean sections with asthma exacerbations. 17 Exacerbations in pregnancy can reduce oxygen and increase alkalosis of maternal blood plasma impeding uterine blood flow and fetal oxygenation leading to complications. 18 A recent Australian study showed that women with severe asthma and asthma exacerbations before pregnancy are most at risk of an exacerbation during pregnancy, highlighting the need for careful monitoring of existing asthma during pregnancy. 19
Counter to other cohort studies, 7 , 8 this study did not find associations between maternal asthma and low birth weight, preterm birth, or small for gestational age. One explanation is that the majority of previous cohort studies were observed in Western White–majority populations with different demographics regarding smoking rates, maternal age, and level of disadvantage. The cohort in the current study represents a whole population of Indigenous mothers and babies in WA and is largely representative of Australian Indigenous mothers, having higher smoking rates, younger maternal age, more disadvantage, and rural residency than the non‐Indigenous maternal population. 1 Of note, the rate of smoking in pregnant women in this study was high, at 50%, consistent with national reporting for Australian Indigenous women. 1 Given that smoking in particular is a large driver of preterm birth, low birth weight, and small for gestational age in Indigenous populations, 20 , 21 it is possible that the high rates of smoking, which were similar between women with asthma (50%) and women without asthma (49%), concealed or even removed any additive effect from maternal asthma on birth outcomes. Interestingly, research in the United States on maternal asthma in black American women with a similar demographic profile regarding smoking, dy mass index, lower socioeconomic status, and increased comorbidities, also did not find any increase in preterm births, low birth weight, small for gestational age, or perinatal mortality. 22
Our results indicate that pregnant women with asthma in remote areas are at greater risk of adverse perinatal outcomes. However, our analysis of asthma reported by data source suggests that there may be under‐reporting at antenatal appointments in remote areas rather than a true lower prevalence of asthma in these areas, as hospitalization rates for asthma were similar by geographic region, and, in national data, have actually been shown to increase with remoteness. 23 If this is the case, these findings may indicate that there are a number of undiagnosed maternal asthma cases in remote areas and that the cases identified represent more severe and uncontrolled asthma, explaining the observed increased associations for adverse perinatal outcomes in remote areas. Nonetheless, consideration should be given to Indigenous perinatal care in women living remotely with asthma, providing integrated, holistic maternity care such as the Birthing on Country and continuity of midwifery care models that have demonstrated significant improvements in Indigenous perinatal outcomes and reduction in smoking rates. 24 , 25
A major strength of this study was having access to more than a decade of data on Indigenous women and their babies across a whole state. Not only did this provide a large sample size for a population that is usually under‐represented in research but, by using administrative‐linked data, the potential for selection bias caused by lack of participation or loss to follow‐up was minimized. Using a range of different data sources and previously developed algorithms, we were able to maximize the identification of Indigenous status and minimize missing data for some variables such as geographical location, date of birth, and identification of mothers and fathers. 11 , 12 Another strength was having coauthorship by two senior Aboriginal academics, which promoted the Indigenous gaze and fulfilled the ethical obligations of research with Aboriginal and Torres Strait Islander Peoples in Australia (DH‐ Kamilaroi Nation descendent, and SE‐ Noongar Nation woman).
One of the limitations in this study was the lack of body mass index measurements, which, at the time of this study, were not recorded in WA. A bias analysis (Supplement) for obesity suggests that there may be some bias in the maternal asthma and emergency cesarean section associations, particularly if the association between obesity and emergency cesarean section in this population is high. However, the bias and E‐value analyses suggest that bias is unlikely because of obesity in the maternal asthma and placental abruption or threatened preterm labor analyses. Another limitation was that we were missing asthma diagnoses in the ED data collections for rural areas. However, the sensitivity analysis restricting the analysis to only asthma hospitalizations and self‐report to midwives had similar results to the main analysis (see Supplement).
5. CONCLUSION
Maternal asthma experienced by Indigenous women in pregnancy is associated with an increased risk of emergency cesarean sections, placental abruptions, and threatened preterm labor. Women with asthma exacerbations and women living remotely are particularly at risk for adverse outcomes and could benefit from careful monitoring and management of their asthma during pregnancy. Consideration should also be given to maternity models of care that have a demonstrated impact in improving Indigenous perinatal outcomes.
AUTHOR CONTRIBUTIONS
B.B. designed the study, performed the literature review and statistical analysis, and wrote the first draft; she is also the guarantor of the paper. A.G., B.M., G.M., and S.E. contributed to the analytical design of the study. All of the authors contributed to the interpretation of the results and revising manuscript drafts, as well as approving the final version for publication. D.H. and S.E. contributed specific Indigenous experience and knowledge. B.M., A.G., and S.E. organized the acquisition of data and data management. L.M. and G.C. provided expertise on perinatal outcomes. All of the authors agree to be accountable for all aspects of the work.
FUNDING INFORMATION
This work was supported by the National Health and Medical Research Council of Australia (NHMRC project grant 1078214). This project is also part of the NHMRC Centre for Research Excellence in Indigenous Adolescent and Child Health (GNT 1135273). Both awarded grants included external peer review for scientific quality and priority assessment requiring a scientific panel. The funder played no role in conducting the research or preparing the manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Supporting information
Figure S1
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
We acknowledge and thank the WA Data Linkage Branch, the WA Department of Health, and the data custodians of WA Midwives Notification System, WA Hospital Morbidity Data Collection, WA Emergency Department Data Collection, WA Birth Registrations, and WA Death Registrations; WA Infant, Child and Youth Mortality Database (Telethon Kids Institute); and WA Electoral Roll records for their advice and assistance in providing the linked data for the study. Thanks also to Dr Awad Smew and Dr Lottie Phillips, Karolinska Institute, who provided support for the Qualitative Bias Analysis. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Brew BK, Gibberd A, Marks GB, et al. Maternal asthma in Australian indigenous women and perinatal outcomes: A whole population–linked study. Int J Gynecol Obstet. 2023;160:653‐660. doi: 10.1002/ijgo.14363
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Australian Institute of Health and Welfare & National Indigenous Australians Agency . Aboriginal and Torres Strait islander health performance Framework. 2020. Accessed August 16, 2021. https://www.indigenoushpf.gov.au/
- 2. Diouf I, Gubhaju L, Chamberlain C, et al. Trends in maternal and newborn health characteristics and obstetric interventions among aboriginal and Torres Strait islander mothers in Western Australia from 1986 to 2009. Aust N Z J Obstet Gynaecol. 2016;56(3):245‐251. [DOI] [PubMed] [Google Scholar]
- 3. Westrupp EM, D'Esposito F, Freemantle J, Mensah FK, Nicholson JM. Health outcomes for Australian aboriginal and Torres Strait islander children born preterm, low birthweight or small for gestational age: a nationwide cohort study. PLoS One. 2019;14(2):e0212130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Australian Bureau of Statistics . National Aboriginal and Torres Strait Islander Health Survey 2018–2019. Cat.no. 4715.0. 2019. Accessed August 16, 2021. https://www.abs.gov.au/statistics/people/aboriginal‐and‐torres‐strait‐islander‐peoples/national‐aboriginal‐and‐torres‐strait‐islander‐health‐survey/2018‐19#media‐releases
- 5. Australian Institute of Health and Welfare . Aboriginal and Torres Strait Islander Health Performance Framework 2014 Report: Detailed Analyses. Cat. No. IHW 167. AIHW; 2015. [Google Scholar]
- 6. Griffiths K, Coleman C, Lee V, Madden R. How colonisation determines social justice and indigenous health—a review of the literature. J Pop Res. 2016;33(1):9‐30. [Google Scholar]
- 7. Murphy VE, Namazy JA, Powell H, et al. A meta‐analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118(11):1314‐1323. [DOI] [PubMed] [Google Scholar]
- 8. Rejno G, Lundholm C, Gong T, Larsson K, Saltvedt S, Almqvist C. Asthma during pregnancy in a population‐based study‐‐pregnancy complications and adverse perinatal outcomes. PLoS One. 2014;9(8):e104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yland JJ, Bateman BT, Huybrechts KF, et al. Perinatal outcomes associated with maternal asthma and its severity and control during pregnancy. J Allergy Clin Immunol Pract. 2020;8(6):1928‐1937e1923. [DOI] [PubMed] [Google Scholar]
- 10. McNamara B, Gubhaju L, Jorm L, Preen D., Jones J., Joshy G., Shepherd C., McAullay D., Eades S., Defying the Odds project investigators . Exploring factors impacting early childhood health among aboriginal and Torres Strait islander families and communities: protocol for a population‐based cohort study using data linkage (the 'Defying the Odds' study). BMJ Open 2018;8(3):e021236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McNamara BJ, Jones J, Shepherd CCJ, et al. Identifying young aboriginal and Torres Strait islander children in linked administrative data: a comparison of methods. Int J Popul Data Sci. 2020;5(1): 1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gibberd AJ, Simpson JM, Eades SJ. Use of family relationships improved consistency of identification of aboriginal people in linked administrative data. J Clin Epidemiol. 2017;90:144‐155. [DOI] [PubMed] [Google Scholar]
- 13. Downey F. A Validation Study of the Western Australian Midwives' Notification System. 2005 Data. Department of Health, Western Australia; 2007. [Google Scholar]
- 14. Dobbins TA, Sullivan EA, Roberts CL, Simpson JM. Australian national birthweight percentiles by sex and gestational age, 1998‐2007. Med J Aust. 2012;197(5):291‐294. [DOI] [PubMed] [Google Scholar]
- 15. Australian Bureau of Statistics . Australian statistical geography standard (ASGS) volume 5‐ remoteness structure. Catno 1270055005 . 2018. Accessed June 16, 2021. https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/1270.0.55.005Main+Features1July%202016?OpenDocument
- 16. Australian Bureau of Statistics . Socio‐economic indexes for areas 2018. Accessed June 16, 2021. https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa
- 17. Robijn AL, Brew BK, Jensen ME, et al. Effect of maternal asthma exacerbations on perinatal outcomes: a population‐based study. ERJ Open Res. 2020;6:00295‐02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meakin AS, Saif Z, Jones AR, Aviles PFV, Clifton VL. Review: placental adaptations to the presence of maternal asthma during pregnancy. Placenta. 2017;54:17‐23. [DOI] [PubMed] [Google Scholar]
- 19. Bokern M, Robijn A, Jensen ME, et al. Factors associated with asthma exacerbations during pregnancy. J Allergy Clin Immunol Pract. 2021;9:4343‐4352. [DOI] [PubMed] [Google Scholar]
- 20. Eades S, Read AW, Stanley FJ, Eades FN, McCaullay D, Williamson A. Bibbulung Gnarneep ('solid kid'): causal pathways to poor birth outcomes in an urban aboriginal birth cohort. J Paediatr Child Health. 2008;44(6):342‐346. [DOI] [PubMed] [Google Scholar]
- 21. Gibberd AJ, Simpson JM, Jones J, Williams R, Stanley F, Eades SJ. A large proportion of poor birth outcomes among aboriginal Western Australians are attributable to smoking, alcohol and substance misuse, and assault. BMC Pregnancy Childbirth. 2019;19(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flores KF, Robledo CA, Hwang BS, Leishear K, Laughon Grantz K, Mendola P. Does maternal asthma contribute to racial/ethnic disparities in obstetrical and neonatal complications? Ann Epidemiol 2015;25(6):392–397 e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Australian Centre for Asthma Monitoring . Asthma in Australia 2011. AIHW Asthma Series No. 4. Cat. No. ACM 22. AIHW; 2011. [Google Scholar]
- 24. Kildea S, Gao Y, Hickey S, et al. Effect of a birthing on country service redesign on maternal and neonatal health outcomes for first nations Australians: a prospective, non‐randomised, interventional trial. Lancet, Global Health. 2021;9:e651‐e659. [DOI] [PubMed] [Google Scholar]
- 25. Hartz DL, Blain J, Caplice S, et al. Evaluation of an Australian aboriginal model of maternity care: the Malabar community midwifery link service. Women Birth. 2019;32(5):427‐436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
Research data are not shared.


