Abstract
Aim
This study aimed to (1) quantify attention and executive functioning in children with developmental coordination disorder (DCD), (2) assess whether some children with DCD are more likely to show attention difficulties, and (3) characterize brain correlates of motor and attention deficits.
Method
Fifty‐three children (36 with DCD and 17 without) aged 8 to 10 years underwent T1‐weighted and diffusion‐weighted magnetic resonance imaging, and standardized attention and motor assessments. Parents completed questionnaires of executive functioning and symptoms of inattention and hyperactivity. We assessed regional cortical thickness and surface area, and cerebellar, callosal, and primary motor tract structure.
Results
Analyses of covariance and one‐sample t‐tests identified impaired attention, non‐motor processing speed, and executive functioning in children with DCD, yet partial Spearman's rank correlation coefficients revealed these were unrelated to one another or the type or severity of the motor deficit. Robust regression analyses revealed that cortical morphology in the posterior cingulate was associated with both gross motor skills and inattentive symptoms in children with DCD, while gross motor skills were also associated with left corticospinal tract (CST) morphology.
Interpretation
Children with DCD may benefit from routine attention and hyperactivity assessments. Alterations in the posterior cingulate and CST may be linked to impaired forward modelling during movements in children with DCD. Overall, alterations in these regions may explain the high rate of non‐motor impairments in children with DCD.
What this paper adds
Children with developmental coordination disorder have difficulties in attention, processing speed, and executive functioning.
Non‐motor impairments were not interrelated or correlated with the type or severity of motor deficit.
Posterior cingulate morphology was associated with gross motor skills and inattention.
Gross motor skills were also associated with left corticospinal tract morphology.
What this paper adds
Children with developmental coordination disorder have difficulties in attention, processing speed, and executive functioning.
Non‐motor impairments were not interrelated or correlated with the type or severity of motor deficit.
Posterior cingulate morphology was associated with gross motor skills and inattention.
Gross motor skills were also associated with left corticospinal tract morphology.
This original article is commented on by Gomez et al. on pages 279–280 of this issue.
Abbreviations
- CCC‐2
Children's Communication Checklist
- CST
corticospinal tract
- DCD
developmental coordination disorder
- FDR
false discovery rate
- TDC
typically developing children
Developmental coordination disorder (DCD) affects approximately 5% of schoolchildren and is characterized by poor motor skills that significantly impact daily life, in the absence of genetic, neurological, or global developmental disorders. 1 , 2 Children with DCD reportedly also show attention, processing speed, and executive functioning impairments, as well as symptoms of inattention and hyperactivity. 3 , 4 Yet few studies have used standardized neuropsychological tests to assess attention abilities in children with DCD, or examined how these abilities relate to motor skills. Research identifying the relationships between motor and attention profiles in DCD is needed to help clinicians tailor the assessment and support for children most at risk of attention difficulties.
Theoretical accounts of DCD postulate that the core deficit is either altered automatization of motor sequence learning, which implicates premotor and superior parietal cortices 5 alongside the basal ganglia, 6 or poor online control of motor skills, driven by anomalies in wider circuits incorporating supplementary and premotor areas, the sensorimotor cortex, cingulate gyrus, parietal lobe, and cerebellum. 7 , 8 Magnetic resonance imaging (MRI) studies of white matter in DCD have inconsistently implicated sensorimotor pathways, including the corticospinal tract (CST), corpus callosum, internal capsule, posterior thalamic radiations, and cerebellar peduncles, as well as tracts outside the sensorimotor system, compared to controls. 3 , 9 , 10 , 11 Poorer overall motor abilities have been associated with lower left posterior cingulate volume, 11 and microstructural changes in a range of tracts including the CST, 12 , 13 posterior thalamic radiations, 12 left superior longitudinal fasciculus, 9 , 13 left internal capsule, and right inferior longitudinal fasciculus. 13 However, there is evidence for unique but overlapping functional brain activation patterns in population norm adults performing fine and gross motor tasks. 14 , 15 , 16 , 17 Indeed, impaired aiming and catching, reflecting gross motor abilities, but not fine motor or balance skills, have been linked with lower volume in the cerebral cortex, primarily the premotor and motor cortices, and the superior cerebellum in a cohort of children with attention‐deficit/hyperactivity disorder (ADHD), DCD, and co‐occurring DCD and ADHD irrespective of symptoms of inattention, hyperactivity, and impulsivity. 18 Considering the heterogenous motor profiles reported in children with DCD 19 , 20 affecting fine motor, gross motor, and balance abilities to different degrees, research is needed to characterize brain–behaviour relationships in children with DCD to inform theoretical models and support the development of interventions that target underlying brain networks.
Despite the high rate of additional impairments in executive functioning, attention abilities, and symptoms of inattention and hyperactivity in children with DCD, 1 , 3 the structural MRI correlates of these abilities are also not well characterized. Primarily, structural MRI studies have investigated the differences between children with DCD alone and DCD with co‐occurring ADHD. In a cohort of 226 children, associations were reported between cerebellar and premotor or motor cortex volumes and gross motor skills in children with ADHD, with DCD and co‐occurring DCD and ADHD, but no differences between groups. 18 In contrast, others reported reduced right temporal pole thickness in children with DCD alone compared to controls, but widespread reductions in the insula, isthmus of the right posterior cingulate gyrus, and the frontal and temporal poles in children with co‐occurring DCD and ADHD. 21 Importantly, thickness in the frontal regions was associated with attention abilities in children with DCD and co‐occurring DCD and ADHD. A study investigating white matter microstructure reported reduced fractional anisotropy in the anterior corpus callosum in children with ADHD, the middle section of the corpus callosum in children with DCD alone, and in both regions in children with concurrent DCD and ADHD compared to controls. 22 While rates of co‐occurrence between ADHD and DCD are around 30% to 50%, 23 up to 80% of children with DCD may have executive functioning, hyperactivity, and attention deficits. 1 , 3 Indeed, relying on co‐occurring categorical diagnoses may not characterize the true nature of additional difficulties in neurodevelopmental disorders or underlying neurobiological correlates. 24 Research is needed to investigate the relationship between MRI metrics and attention difficulties within unselected groups of children with DCD with a spectrum of attention abilities.
The first aim of this study was to assess attention abilities, non‐motor processing speed, executive functioning, and symptoms of inattention and hyperactivity or impulsivity in an ecologically valid group of children with DCD aged 8 to 10 years compared to typically developing children (TDC). The second aim was to determine whether the type or severity of motor or behavioural deficits in children with DCD are associated with co‐occurring attention difficulties. The third aim was to characterize the relationship between the deficits identified as part of the first aim and brain structures of interest (motor control, automatization, and attention networks). We used a transdiagnostic approach and measured brain–behaviour relationships irrespective of additional diagnoses of ADHD or autism spectrum disorder (ASD). 24
We hypothesized that a large proportion of children with DCD would have attention difficulties, irrespective of ADHD profile and the severity or type of motor difficulties, in accordance with previous literature on executive functioning and information processing deficits in children with DCD. 3 , 4 We tested the hypotheses that motor abilities would correlate with premotor and superior parietal cortices, in accordance with the automatization deficit hypothesis, 6 and supplementary and premotor areas, sensorimotor cortex, cingulate gyrus, parietal lobe, and cerebellar networks in accordance with the online motor control deficit hypothesis. 8 Finally, we hypothesized that attention deficits would correlate with anomalies in frontoparietal networks, in line with Posner and Rothbart's neuroanatomical model for attentional orienting, shifting, and executive control, 25 and with regions identified as associated with co‐occurring DCD and ADHD. 18 , 21 , 22
We used an extensive cognitive and motor assessment battery to explore the associations between motor and attention abilities and identify those that are most robust. We chose to assess cortical grey and white matter regions implicated in attention, motor automatization, and motor control networks with corrections for multiple comparisons.
METHOD
The study procedures were approved by the West London Research Ethics Committee (no. 14/LO/00059) from the UK Health Research Authority. Written informed consent and assent were obtained from all parents or guardians and children respectively.
Participants
The recruitment of children with DCD (final sample n = 36) and TDC (final sample n = 17) is summarized in Figure S1. We did not exclude children with DCD and additional diagnoses of developmental disorders that commonly co‐occur. 1 The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) diagnostic criteria for DCD and associated procedures used to assess them in this study are provided in Appendix S1.
Hand dominance was determined by asking parents which hand the child typically used for writing. 26 Maternal years of education after age 14 years were used as a proxy of socioeconomic status. 27 Additional developmental disorders that were either confirmed or under clinical investigation were recorded using a semi‐structured telephone interview with parents.
Assessment of motor skills, IQ, attention, processing speed, and executive function
Motor skills were assessed using the Movement Assessment Battery for Children, Second Edition. 26 Manual dexterity, corresponding to fine motor skills, aiming and catching, corresponding to gross motor skills, balance skills, and total test scaled scores were calculated. The Movement Assessment Battery for Children, Second Edition and Developmental Coordination Disorder Questionnaire were used to detect the impact of motor skill impairments on daily life. 26 , 28
The Wechsler Abbreviated Scales of Intelligence, Second Edition was administered to obtain a full‐scale IQ. 29
The assessment of attention, symptoms of inattention and hyperactivity or impulsivity, and executive functioning is summarized in Table 1. Five subtests of the Test of Everyday Attention for Children 30 were administered to assess sustained attention (Score!), selective attention (Sky Search and Sky Search dual task), and attentional control (creature counting and opposite worlds) abilities. Subtests were chosen because they require verbal responses and calculation of difference scores, and therefore minimized the effect of motor impairment on attentional performance. In addition, the time taken to complete the first trial of the ‘same world’ subtest was taken as a measure of non‐motor processing speed, as previously reported in children born preterm. 31
TABLE 1.
Attention, inattentive and hyperactive symptomatology, and executive functioning assessments undertaken.
| Domain | Task(s) | Procedure | Outcome measure | Variable used for analysis | Categorization of impairment |
|---|---|---|---|---|---|
| Attention: Test of Everyday Attention for Children | |||||
| Selective attention | Sky Search | Circle targets while ignoring distractors on A3 sheet | Time per target circled on a sheet without distractors subtracted from the time per target circled during the test | Age‐ and sex‐specific scaled score (test mean [SD] = 10 [3]) | Scaled score < 6 |
| Sustained attention | Score! | Ten trials of counting the number of tonal sounds heard with varying lengths of silence between them | Accuracy number of correct total tonal counts out of 10 | ||
| Sustained and selective attention dual task | Sky Search dual task | Execute Sky Search and Score! tasks simultaneously | Sky Search dual task time per target minus Sky Search time per target adjusted for the correct number of tones counted | ||
| Attentional control | Creature counting | Seven trials of switching between counting figures on a page upwards (1–3) and downwards (1–3) several times per trial | Accuracy – number of correct totals out of 7 | ||
| Same or opposite worlds | Rapidly naming a string of printed ones and twos either with the correct name (1 say ‘one’– same world condition; 2 trials) or using the opposite name (1 say ‘two’– opposite world condition; 2 trials) | Difference between total time taken on the opposite and same world trials; additionally z‐scored to TDC mean and SD | z‐score > 1.5 | ||
| Non‐motor processing speed | Same world first time 14 | As above | Time taken on the first trial of the same world task, additionally z‐scored to TDC mean and SD 21 |
z‐score > 1.5 |
|
| ADHD symptomatology and executive functioning: Conners 3 | |||||
| Inattention symptoms | DSM‐5 Inattention Scale | Parent‐completed questionnaire related to observed behaviours related to symptoms of inattention and hyperactivity and impaired executive functioning where each question is scored 0–3, where 0 is not true at all, never, and seldom, and 3 is very much true, very often, very frequently | Total score for each scale | Age‐ and sex‐specific T‐score (test mean [SD] = 50 [10]) |
Elevated inattentive symptoms: inattentive T‐score ≥ 65, hyperactive or impulsive < 65 Elevated hyperactive or impulsive: hyperactive or impulsive T‐score ≥ 65, inattentive < 65 Elevated inattentive and hyperactive or impulsive symptoms: ADHD inattentive and hyperactive or impulsive T‐scores both ≥5 |
| Hyperactivity symptoms | DSM‐5 Hyperactivity Scale | ||||
| Executive functioning | Executive Functioning Impairment Scale | T‐score ≥ 65 | |||
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; DSM‐5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; TDC, typically developing children.
Parents completed the Conners 3 full‐length questionnaire 32 to measure inattentive and hyperactive or impulsive symptoms presenting in everyday activities according to the updated DSM‐5 diagnostic criteria, as well as the presence of everyday executive functioning difficulties. The Conners 3 ADHD probability index, reflecting the similarity between a child's scores and children with ADHD assessed with the Conners 3, was calculated.
Parents also completed the Children's Communication Checklist (CCC‐2), 33 which is sensitive to ASD and autistic traits. 34 The Social Interaction Deviance Composite was extracted, representing the presence of social communication impairments that are disproportionate to a child's structural language difficulties. It is calculated by subtracting the total score on ‘inappropriate initiation’, ‘non‐verbal communication’, and ‘social relations and interests’ items from the total score on ‘speech’, ‘syntax’, ‘semantics’, and ‘coherence’ items. Using this composite, children were categorized as having a communication profile consistent with ASD or autistic traits if (1) the global communication score was less than 55 (total score on speech, syntax, semantics, coherence, inappropriate initiation, stereotyped language, use of context, and non‐verbal communication items) and the Social Interaction Deviance Composite was less than 0 or (2) if the Social Interaction Deviance Composite was less than or equal to −15 irrespective of global communication scores. 33
MRI data collection and processing
Participants were scanned on a 3 T Siemens Magnetom Prisma MRI scanner at Great Ormond Street Hospital for Children using a 20‐channel head coil while watching a video of their choice. T1‐weighted magnetization‐prepared rapid acquisition gradient echo (echo time/repetition time = 2.74/2300 ms, voxel size = 1 mm3, field of view = 256 × 256, flip angle = 8 degrees, coronal acquisition) and multi‐shell diffusion‐weighted MRI scans were acquired (b = 1000s/mm2 [n = 60], b = 2200 s/mm2 [n = 60], echo time/repetition time = 60/3050 ms, voxel size = 2 mm3, 13 b = 0 images interspersed, anterior to posterior phase encoding, 1 b = 0 image with negative phase encoding). All T1‐weighted MRI scans were viewed by a consultant paediatric neuroradiologist (KM) and were within normal limits.
Cortical reconstructions were generated from T1‐weighted images using FreeSurfer v5.3 on the Legion High Performance Computing Facility (Legion@UCL) according to the standard automated pipeline. 35 , 36 Cortical surfaces were parcellated into 34 gyral‐based regions of interest based on the Desikan–Killiany atlas. 37 The total surface area and mean cortical thickness for each region and the whole brain were extracted. The total grey and white matter volume was also calculated. Further details on the T1‐weighted image processing are provided in Table S1. One data set from a child with DCD was excluded due to poor surface reconstruction.
Three diffusion‐weighted MRI data sets from children with DCD were excluded due to excessive motion artefact (10% of volumes affected before preprocessing). The remaining data sets were analysed using MRtrix3 (https://www.mrtrix.org/) 38 and pre‐processed using standard methods (https://mrtrix.readthedocs.io/en/3.0_rc3/fixel_based_analysis/mt_fibre_density_cross‐section.html).
Probabilistic tractography was performed in native space for the left and right CST, middle cerebellar peduncles, and corpus callosum (Table S1). Mean fractional anisotropy values for each tract were extracted.
In addition to assessing fractional anisotropy, fixel‐based fibre morphology was used 39 to examine alterations in fibre populations with voxels, known as ‘fixels’. Diffusion‐weighted MRI was processed according to the standard multi‐tissue fixel‐based analysis pipeline. Mean fibre density and fibre cross‐section were extracted for the whole corpus callosum, left and right CST, and bilateral middle cerebellar peduncle. Fibre density is a microstructural measure related to the intra‐axonal volume of fibres in a certain direction. 40 Fibre cross‐section is a macrostructural measure related to fibre bundle diameter; 39 values were log‐transformed to normalize the data as recommended by the MRtrix3 pipeline (log[fold change]). Details of the diffusion‐weighted MRI processing and fixel‐based fibre morphology are provided in Appendix S1.
Fractional anisotropy was reported alongside fixel‐based metrics in accordance with the previous literature. 41 , 42 Fixel‐based metrics were calculated across the whole track level to assess the integrity of the circuits implicated in motor control, learning, and co‐occurring DCD and ADHD, as previously conducted, 43 , 44 including in adults with DCD. 11
We chose whole‐track‐level measures because we did not have a hypothesis regarding focal locations of white matter changes along our tracks of interest.
Statistical analyses
Analyses were performed in R v4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS v27.0 (IBM Corp., Armonk, NY, USA). Participants with missing data were excluded on an analysis‐by‐analysis basis (<8% for all variables; Figure 1).
FIGURE 1.
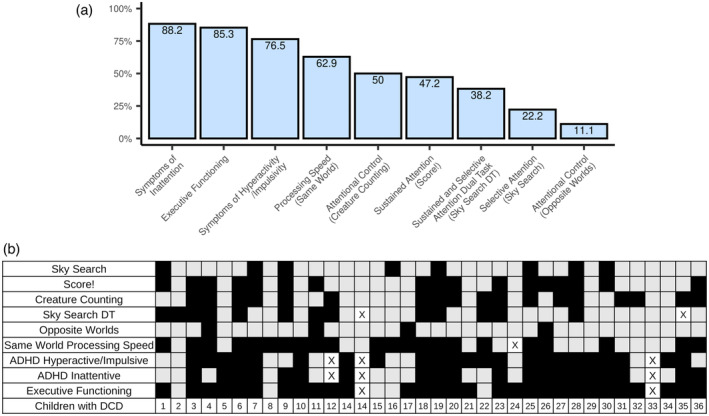
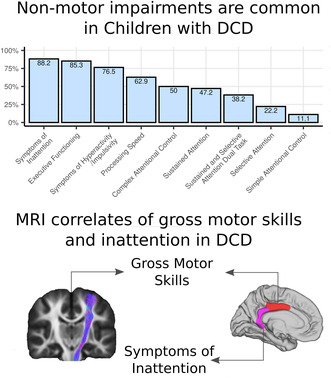
(a) Proportion of children with developmental coordination disorder (DCD) showing significant impairments across attention and processing speed measures, attention‐deficit/hyperactivity disorder (ADHD) symptomatology, and executive functioning. (b) Individual profiles of children with DCD: the black boxes indicate impairment; the grey boxes indicate no impairment; x indicates missing data.
Normality was tested using a Shapiro–Wilk test and skewness and kurtosis values. Differences in demographic variables were tested using t‐tests, and Mann–Whitney U and Fisher's exact tests.
The first aim of the study was assessed by examining differences in motor and non‐motor scores between children with DCD and TDC, as well as the standardized population mean. The second aim was assessed by examining the associations between behavioural scores in children with DCD. The third aim was assessed by characterizing brain–behaviour relationships for abilities found to be impaired in children with DCD in the first aim. All analyses, including statistical tests and covariates, are summarized in Table 2. P‐values underwent false discovery rate (FDR) correction to correct for multiple comparisons (Table 2).
TABLE 2.
Analyses undertaken in this study.
| Analysis | Statistical test | Covariates | Effect size | FDR correction |
|---|---|---|---|---|
| Aim 1: assess behavioural, attention, and motor differences between children with DCD and TDC | ||||
| Analysis 1: differences between children with DCD and TDC | ANCOVA | Full‐scale IQ, maternal education, ADHD probability index, and sex |
Partial η 2 |
Across all analyses: 14 tests |
| Analysis 2: Differences between children with DCD and population test reference mean | One‐sample t‐test | – | – | Across all analyses: 14 tests |
| Aim 2: determine whether different motor or behavioural deficits are associated with co‐occurring attention difficulties in children with DCD | ||||
| Correlations between behavioural measures in children with DCD | Partial Spearman's rank correlation coefficients | Full‐scale IQ, maternal education, sex, and ADHD probability index (post hoc analysis) | Spearman's ρ | Across all analyses: 78 tests |
| Aim 3: characterize brain–behaviour relationships | ||||
| Analysis 1: correlations between cortical grey and white matter metrics and significant measures identified as part of Aim 1 across the whole sample | Spearman's rank correlation coefficients | – |
Spearman's ρ |
Across all measures of a particular brain metric for each behavioural measure separately Motor scores Surface area: 20 tests Cortical thickness: 20 tests Fractional anisotropy: 9 tests Fixel‐based metrics: 8 tests Non‐motor scores Surface area: 18 tests Cortical thickness: 18 tests Fractional anisotropy: 6 tests Fixel‐based metrics: 4 tests |
|
Analysis 2: identify whether brain–behaviour relationships are significant when adjusting for covariates in DCD. When significant in children with DCD, an exploratory analysis was run to determine if the models were also significant in TDC |
Robust regression |
Full‐scale IQ, total grey and white matter volume, sex, hand dominance (lateralized structures), ADHD probability index, and CCC‐2 ASD indication (post hoc analyses) |
Unstandardized coefficients (B) |
Across all brain analyses separately for each behavioural measure |
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; ANCOVA, analysis of covariance; ASD, autism spectrum disorder; CCC‐2, Children's Communication Checklist; DCD, developmental coordination disorder; FDR, false discovery rate; TDC, typically developing children.
Associations between motor, attention and inattention, and hyperactivity symptoms and executive functioning scores and cortical grey matter and white matter structures were hypothesized based on previous theoretical and imaging literature (Table 3). Associations between non‐motor processing speed and white matter volume, mean cortical thickness, and total surface area were also assessed.
TABLE 3.
Brain regions investigated for correlations between brain metrics and motor and attention scores.
| Variable | Motor skills | Attention, inattentive and hyperactive or impulsive symptoms, and executive functioning | ||
|---|---|---|---|---|
| Brain metric | Regions of interest implicated in motor control and automatization of motor skills 5 , 6 , 7 | Regions of interest implicated in motor control 7 | Regions of interest implicated in the literature comparing DCD and DCD with co‐occurring ADHD | Regions of interest implicated in Posner and Rothbart's model of attention 25 |
|
Cortical thickness and surface area |
Caudal middle frontal gyrus (contains premotor cortex) Superior parietal cortex |
Inferior parietal cortex Superior frontal gyrus (contains supplementary motor area) Cingulate gyrus (caudal anterior, posterior, and isthmus of the posterior) Sensorimotor cortex (precentral, paracentral, and postcentral gyri) |
Caudal middle frontal gyrus (contains premotor cortex) 21 Posterior cingulate gyrus 21 Isthmus of the posterior cingulate 21 |
Caudal anterior cingulate gyrus Rostral middle frontal gyrus Superior frontal gyrus Inferior parietal cortex Superior parietal cortex Supramarginal gyrus |
|
Fractional anisotropy |
Whole corpus callosum Callosal fibres connecting the caudal middle frontal gyri (connecting to the premotor cortex) Callosal fibres connecting to the parietal cortex |
CSTs Callosal fibres connecting the precentral gyri Callosal fibres connecting the postcentral gyri Callosal fibres connecting the superior frontal gyri (connecting to the supplementary motor area) Middle cerebellar peduncles |
Whole corpus callosum 22 Callosal fibres connecting the rostral middle frontal gyri 22 Callosal fibres connecting the superior frontal gyri 22 Middle cerebellar peduncles 6 |
|
| Callosal fibres connecting the caudal middle frontal gyri 22 | Callosal fibres connecting the parietal cortices | |||
| Fibre density and cross‐section | Whole corpus callosum |
CSTs Middle cerebellar peduncles |
Whole corpus callosum 22 Middle cerebellar peduncles 6 |
|
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; CST, corticospinal tract; DCD, developmental coordination disorder.
The investigation of brain–behaviour relationships consisted of two analyses. First, non‐parametric correlations assessed the relationship between behavioural measures and brain metrics across the whole group. This maximized statistical power and, by using a non‐parametric correlation method, could detect both linear relationships present across typically developing controls and children with DCD, as well as non‐linear relationships driven by the association with scores in one group. Then, we ran robust regressions (designed to be resistant to outlying observations and non‐normality) 45 in the group with DCD alone, using the brain metrics identified in the previous analysis as predictors and motor and non‐motor skills as the outcome measures, adjusting for appropriate covariates. When a robust regression model was significant in children with DCD, we ran an exploratory analysis to determine if it was also significant in the group of TDC.
RESULTS
Children with DCD and TDC did not differ with regard to age, sex, or hand dominance (see Table 4 for the demographics). The mothers of TDC had significantly higher education than the mothers of children with DCD.
TABLE 4.
Demographics of children with DCD and typically developing peers.
| Children with DCD (n = 36) | Typically developing children (n = 17) | Statistical difference | |
|---|---|---|---|
| Age at assessment, mean (SD), range, years:months | 9:6 (0:9), 8:3–10:11 | 9:4 (0:11), 8:3–10:10 | t (5) = 0.727, p = 0.474 |
| Male, n (%) | 28 (78) | 10 (59) | p = 0.197 |
| Right‐handed, n (%) | 32 (89) | 12 (71) | p = 0.126 |
| Born at fewer than 37 weeks, n (%) | 4 (11) | 0 (0) | p = 0.314 |
| Maternal education in years, median (IQR), range | 7 (3.75–8.0), 2–13 | 8 (7–10), 2–13 | U = 192.5, p = 0.014 |
| Co‐occurring or suspected diagnoses, a n (%) | 23 (67) | 0 (0) | – |
| Number of co‐occurring diagnoses, n (%) | |||
| One | 14 (39) | 0 (0) | – |
| Two | 2 (6) | 0 (0) | – |
| Three | 4 (11) | 0 (0) | – |
| Four | 3 (8) | 0 (0) | – |
| Type of co‐occurring diagnoses, n (%) | |||
| Speech and language disorders | 10 (28) | 0 (0) | – |
| Dyslexia | 10 (28) | 0 (0) | – |
| Autism spectrum disorder or autistic traits | 6 (17) | 0 (0) | – |
| ADHD | 10 (28) b | 0 (0) | – |
| Conners 3 ADHD probability index, median (IQR), range | 99 (91–99), 51–99 | 11 (11–11), 11–56 | – |
| CCC‐2 communication profile indicative of autism spectrum disorder or autistic traits | 19 (53) | 1 (6) | |
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; CCC‐2, Children's Communication Checklist; DCD, developmental coordination disorder; IQR, interquartile range. aReported as either confirmed or under clinical investigation by parents during a semi‐structured telephone interview.
Treated with medication at the time of the assessment, n = 3.
Children with DCD scored significantly worse than both the group of TDC and the test reference mean on selective attention (Sky Search), sustained attention (Score!), attentional control (creature counting), and non‐motor processing speed (same world time). Children with DCD also displayed significantly more symptoms of inattention and hyperactivity and executive function impairments than TDC (Table 5). The results were not different when excluding children with DCD who were taking medication for ADHD at the time of the assessment (Table S2). Impairments outside the motor domain were highly prevalent in children with DCD (Figure 1). More than 50% of children with DCD were categorized as impaired on sustained attention (Score!), attentional control (creature counting), and non‐motor processing speed (same world time); 58% to 86% of children with DCD had symptoms of inattention or hyperactivity, or executive functioning difficulties (Figure 1a). There was significant heterogeneity in attention impairments in children with DCD (Figure 1b).
TABLE 5.
Motor and non‐motor scores in children with DCD and typically developing peers.
| Variable | Children with DCD | Typically developing children | Group difference | Partial η 2 | One‐sample t‐test |
|---|---|---|---|---|---|
| Full‐scale IQ a | 98.03 (19.1), 62–136 | 111.12 (9.39), 92–127 | F (1, 47) = 10.4, p FDR = 0.003 | 0.19 | t (35) = − 0.620, p FDR = 0.539 |
| Motor skills | |||||
| Total motor test score b | 4 (3), 1–8 | 8.5 (2.75), 5–11 | F (1, 43) = 99.6, p FDR <0.001 | 0.70 | t (34) = − 22, p FDR <0.001 |
| Manual dexterity b | 4 (1.25–6.75), 2–10 | 8 (5–11), 3–13 | F (1, 45) = 39.1, p FDR <0.001 | 0.46 | t (35) = − 17.8, p FDR <0.001 |
| Aiming and catching a | 6.64 (3), 2–13 | 9.75 (2.11), 6–14 | F (1, 44) = 12.8, p FDR = 0.001 | 0.23 | t (35) = − 6.66, p FDR <0.001 |
| Balance b | 5 (3–7), 1–10 | 9 (6–12), 5–14 | F (1, 45) = 52.5, p FDR <0.001 | 0.54 | t (34) = − 14.2, p FDR <0.001 |
| Attention | |||||
| Sky Search b | 8 (6–9), 1–16 | 11 (9–12), 4–13 | F (1, 45) = 10.2, p FDR = 0.003 | 0.18 | t (35) = − 3.73, p FDR <0.001 |
| Score! b | 6 (3–10), 1–14 | 10 (8–13), 5–14 | F (1, 45) = 15.0, p FDR <0.001 | 0.25 | t (35) = − 6.58, p FDR <0.001 |
| Creature counting b | 5.5 (4–10), 1–13 | 12 (9–14), 8–14 | F (1, 45) = 43.5, p FDR <0.001 | 0.49 | t (35) = − 6.47, p FDR <0.001 |
| Sky Search dual task b | 7 (1–8.75), 1–10 | 8 (8–11), 3–13 | F (1, 43) = 10.22, p FDR = 0.003 | 0.19 | t (33) = − 6.95, p FDR <0.001 |
| Opposite worlds time difference b | 9.01 (5.56–14.36), 0.92–24.71 | 7.30 (5.40–8.45), 3.05–13.55 | F (2, 43) = 1.82, p FDR = 0.185 | 0.04 | – |
| Non‐motor processing speed | |||||
| Same world time taken b | 14.35 (12.74–16.52), 10.15–23.09 | 10.82 (10.37–11.67), 9.37–16.05 | F (1, 43) = 27.3, p FDR <0.001 | 0.39 | – |
| ADHD symptomatology and executive functioning difficulties | |||||
| DSM‐5 ADHD Inattention Scale a | 78.3 (10.68), 58–90 | 48.3 (6.59), 38–64 | F (1, 44) = 123, p FDR <0.001 | 0.73 | t (35) = 17.2, p FDR <0.001 |
| DSM‐5 ADHD Hyperactivity Scale a | 75.4 (12.0), 48–90 | 46.5 (5.84), 39–61 | F (1, 45) = 90.9, p FDR <0.001 | 0.67 | t (35) = 13.0, p FDR <0.001 |
| Executive functioning difficulties a | 75.4 (10.72), 57–90 | 46.3 (5.62), 38–57 | F (1, 45) = 122, p FDR <0.001 | 0.72 | t (35) = 14.5, p FDR <0.001 |
Note: Full‐scale IQ mean (SD) = 100 (15), motor and attention measures mean (SD) = 10 (3); inattentive and hyperactive or impulsive symptomatology and executive functioning measures mean (SD) = 50 (10); significant results are indicated in bold. p FDR, false discovery rate (FDR)‐corrected p‐value. Covariates: full‐scale IQ (excluding full‐scale IQ analysis), maternal years of education, sex, ADHD probability index, and CCC‐2 communication profile indicative of autism spectrum disorder or autistic traits (yes/no).
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; CCC‐2, Children's Communication Checklist; DCD, developmental coordination disorder; DSM‐5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; IQR, interquartile range.
Mean (SD), range.
Median (IQR), range.
Relationship between motor, attention, hyperactivity or impulsivity, and executive functioning skills in children with DCD
Attention, hyperactivity or impulsivity, and executive functioning skills were unrelated to type or degree of impairment in motor skills after adjusting for full‐scale IQ, maternal education, and sex (Figure 2a). Impaired executive functioning was associated with poorer sustained attention (Score!) abilities and more symptoms of inattention in children with DCD. A post hoc analysis revealed that adding the ADHD probability index and CCC‐2 ASD or autistic traits as covariates did not alter the associations between impaired executive functioning, sustained attention (Score!), and symptoms of inattention (Figure 2b–d). When the data of children who were medicated for ADHD were removed, impaired executive functioning remained associated with impaired sustained attention (Score!; ρ = −0.534, p = 0.002) and symptoms of inattention (ρ = 0.714, p < 0.001). Inattentive and hyperactive or impulsive symptoms were highly correlated after adjusting for full‐scale IQ, maternal education, and sex; however, when including the ADHD probability index and CCC‐2 ASD or autistic traits as covariates, this correlation was no longer significant.
FIGURE 2.
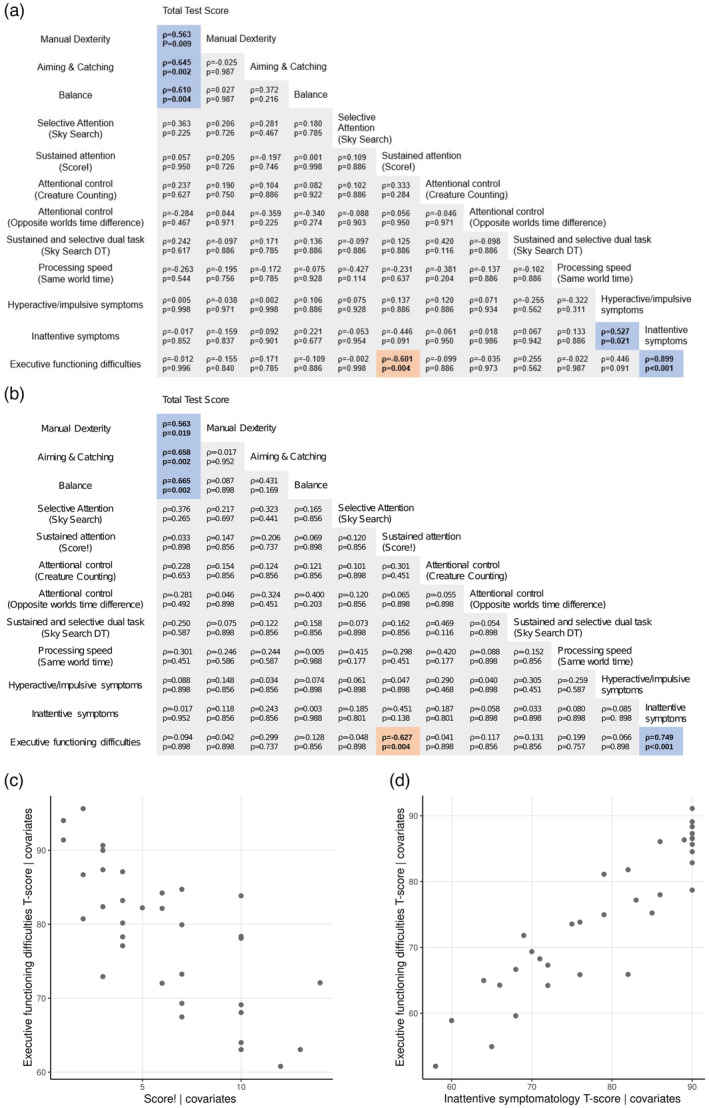
(a) Partial Spearman's rank correlation coefficients between motor function, attention, processing speed, symptoms of inattention and hyperactivity, and executive functioning impairments in children with developmental coordination disorder correcting for full‐scale IQ, sex, and maternal education. (b) Partial Spearman's rank correlation coefficients in (a) with additional covariates of attention‐deficit/hyperactivity disorder (ADHD) probability index and Children's Communication Checklist (CCC‐2) autism spectrum disorder (ASD) or autistic traits. Blue indicates positive correlations, red indicates negative correlations. (c) Partial plot showing the correlation between difficulties in executive functioning T‐score and sustained attention (Score!), adjusting for all covariates including ADHD probability index and CCC‐2 ASD or autistic traits. (d) Partial plot showing the correlation between difficulties in executive functioning T‐score and inattentive symptoms T‐score, adjusting for all covariates including ADHD probability index and CCC‐2 ASD or autistic traits.
Associations between brain measures and motor skills
In the first brain–behaviour analysis (correlations across the whole sample), lower aiming and catching scores (reflecting gross motor skills) were associated with reduced surface area in the bilateral posterior cingulate cortex (left ρ = 0.397; right ρ = 0.436), left inferior parietal cortex (ρ = 0.393), left superior frontal gyrus (ρ = 0.384), and right caudal middle frontal gyrus (ρ = 0.410; all p FDR = 0.034). Lower aiming and catching scores correlated with lower log(fold change) in the corpus callosum (ρ = 0.397, p FDR = 0.034) and left CST (ρ = 0.421; p FDR = 0.034), and lower fractional anisotropy in the whole corpus callosum (ρ = 0.394; p FDR = 0.034) and the parietal (ρ = 0.392; p FDR = 0.034) and postcentral callosal fibres (ρ = 0.373; p FDR = 0.048) (Figure 3). There were no other significant correlations between brain measures and motor skills.
FIGURE 3.
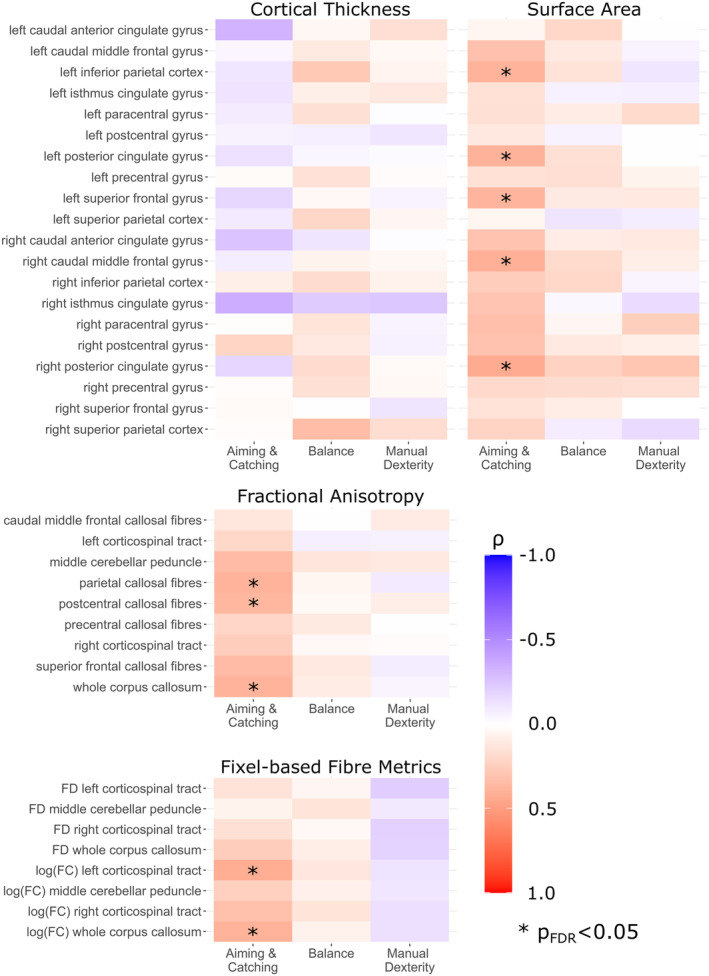
Heatmaps showing Spearman's rank correlation coefficients between motor scores and grey and white matter measures across the whole sample.
In the second brain–behaviour analysis (brain predictors of behavioural scores in children with DCD), the surface area of the left posterior cingulate gyrus and log(fold change) in the left CST predicted aiming and catching scores, corresponding to gross motor abilities, in children with DCD when controlling for full‐scale IQ, sex, grey and white matter volume, and hand dominance (Table 6; Figure 4). Adding the ADHD probability index (left posterior cingulate gyrus, p = 0.012; left CST log[fold change], p = 0.018) or CCC‐2 ASD or autistic traits (left posterior cingulate gyrus, p = 0.008; left CST log[fold change], p = 0.026), and removing children who were medicated for ADHD (left posterior cingulate gyrus, p = 0.048; left CST log[fold change], p = 0.008) did not alter these results. These brain measures were not significant predictors of aiming and catching in TDC.
TABLE 6.
Robust regression analyses of predictors of gross motor skills
| Region | Children with DCD | Typically developing children | ||||||
|---|---|---|---|---|---|---|---|---|
| B | t | p FDR | R 2 | B | t | p | R 2 | |
| Left CST log(fold change) | 28.3 | 2.96 | 0.032 | 0.38 | 12.1 | 0.848 | 0.416 | 0.27 |
| Left posterior cingulate gyrus surface area | 0.0105 | 3.13 | 0.032 | 0.35 | <0.001 | 0.032 | 0.975 | 0.15 |
| Postcentral callosal fibre fractional anisotropy | 40.3 | 1.83 | 0.260 | 0.24 | ||||
| Parietal callosal fibre fractional anisotropy | 25.4 | 0.887 | 0.424 | 0.16 | ||||
| Whole corpus callosum fractional anisotropy | 39.8 | 1.6 | 0.300 | 0.22 | ||||
| Corpus callosum log(fold change) | 15.9 | 1.39 | 0.348 | 0.20 | ||||
| Right posterior cingulate gyrus surface area | 0.005 | 1.16 | 0.364 | 0.16 | ||||
| Right caudal middle frontal gyrus surface area | 0.001 | 0.765 | 0.704 | 0.13 | ||||
| Left superior frontal gyrus surface area | 0.0009 | 0.996 | 0.410 | 0.15 | ||||
| Left inferior parietal cortex surface area | 0.002 | 1.28 | 0.352 | 0.13 | ||||
Abbreviations: CST, corticospinal tract; DCD, developmental coordination disorder.
FIGURE 4.
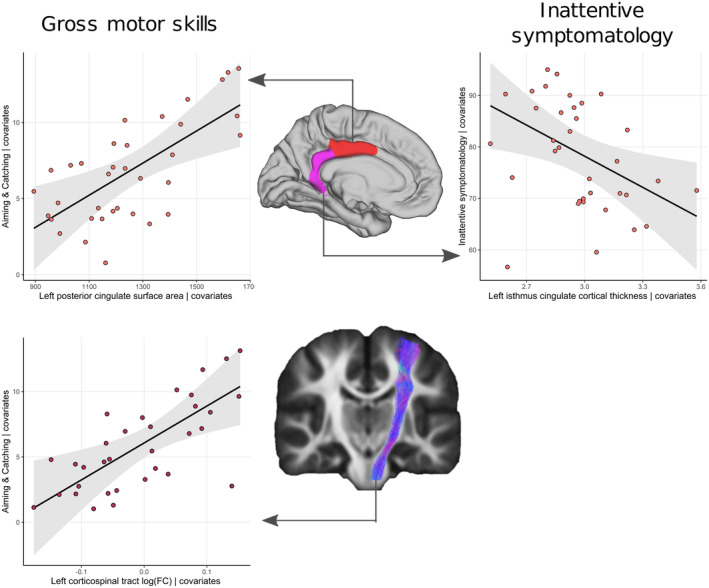
Relationship between gross motor abilities (aiming and catching) and left posterior cingulate surface area, and log(fold change) in the left corticospinal tract, and inattentive symptoms and cortical thickness in the left isthmus of the posterior cingulate in children with developmental coordination disorder covarying for sex, hand dominance, full‐scale IQ, and grey and white matter volume.
No other brain metrics identified in the first analysis predicted aiming and catching scores in children with DCD when adjusting for covariates.
Associations between brain measures and attention, hyperactivity or impulsivity, processing speed, and executive functioning
In the first brain–behaviour analysis, increased inattentive symptoms were associated with thinning in the isthmus of the left posterior cingulate cortex across the whole sample (ρ = −0.451; p FDR = 0.040; Figure 5). Non‐motor processing speed (same world time) was negatively correlated with total cortical surface area (ρ = −0.342, p FDR = 0.049), but not mean cortical thickness (ρ = 0.075, p FDR = 0.609) or white matter volume (ρ = −0.211, p FDR = 0.195). There were no other significant correlations between brain measures and skills outside the motor domain (Figures S2 and 5).
FIGURE 5.
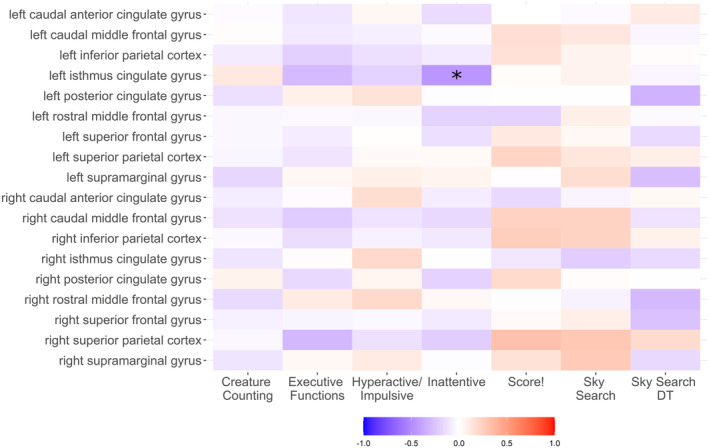
Heatmap showing Spearman's rank correlation coefficients between attention scores, symptoms of inattention and hyperactivity, executive functioning, and regional cortical thickness. *p FDR <0.05.
In the second brain–behaviour analysis, cortical thickness in the left isthmus of the posterior cingulate cortex significantly predicted symptoms of inattention in children with DCD (B = −20.1, t = −2.48, p = 0.019; R 2 = 0.23; Figure 4) when correcting for full‐scale IQ, sex, and hand dominance (Table 6). When adding the ADHD probability index to the model, the relationship was no longer significant in children with DCD (B = −9.46, t = −1.88, p = 0.071; R 2 = 0.72). Removing children with DCD medicated for ADHD did not alter this result (p = 0.618). The left isthmus of the posterior cingulate cortex did not significantly predict symptoms of inattention in TDC (B = 5.88, t = 1.331, p = 0.212; R 2 = 0.67).
The cortical surface area did not predict non‐motor processing speed in children with DCD (B < −0.001, t = −1.58, p = 0.124; R 2 = 0.27) when correcting for sex and full‐scale IQ. Removing children with DCD medicated for ADHD did not alter this result (p = 0.208).
DISCUSSION
In this study, we identified impaired attention and non‐motor processing speed, and reported higher rates of executive function impairments and symptoms of inattention and hyperactivity in children with DCD than in age‐matched TDC. We did not find any association between motor and attentional measures. Reduced area of the left posterior cingulate gyrus and altered morphology of the left CST were implicated in gross motor skills. More inattentive symptoms in children with DCD were associated with increased thickness of the left isthmus of the posterior cingulate gyrus; however, this was not significant when correcting for the probability of ADHD.
Children with DCD had impaired executive functioning and information processing abilities, and lower attention scores, when adjusting for probability of ADHD, IQ, and socioeconomic status, with large effect sizes in auditory sustained attention and attentional control. A recent meta‐analysis reported moderate‐to‐large deficits in executive functioning in individuals with DCD, including in those without co‐occurring ADHD. 3 Deficits were identified in multiple aspects of executive functioning, including working memory, inhibitory control, and executive attention. 3 Previous studies also identified impaired information processing on standardized clinical assessments 46 and across experimental modalities, including those without a motor component. 4 It is important to note that the creature counting task requires counting up and down between 1 and a maximum of 15, and there is evidence that children with DCD show counting impairments; 47 therefore, we cannot discount the influence of mathematical abilities on these results. Nevertheless, our results contribute to the literature, which demonstrates that impaired executive functioning, attention, and information processing are a common feature in school‐aged children with DCD, irrespective of ADHD features or the severity and type of motor deficit.
Executive functioning is a set of abilities that enable a person to plan and execute goal‐directed activities and support other cognitive functions. 48 A key part of the diagnostic criteria for DCD is a significant impact of poor motor skills on daily living, such as self‐care, schooling, and social life. It is possible that impaired executive functioning hinders the ability of children with poor motor skills to compensate for the motor impairment, resulting in a significant detrimental impact on daily living. Indeed, our results highlight the need to examine the impact of attention and executive function impairments on motor intervention strategies for children with DCD. Of particular interest are task‐based approaches, such as the Cognitive Orientation to daily Occupational Performance approach, designed to enable skill acquisition through the use of cognitive strategies. 49 In this intervention, typically a child follows a ‘think–plan–do–check’ model to achieve a skill of their choice. Interestingly, presence of ADHD or ASD has no reported impact on the effectiveness of this intervention; 19 however, additional impairments in attention and executive functioning may have an impact on efficacy. Assessment by a psychology professional may be necessary to fully characterize the impairments in a child with DCD. Indeed, we demonstrated that children with DCD are at high risk of attention and executive function impairments that probably impact their functioning while at school. 48
Although only 10 participants had a diagnosis or suspected diagnosis of ADHD, the Conners 3 questionnaire indicated the prevalence of symptoms of inattention and hyperactivity or impulsivity in nearly 80% of our sample, with over 50% having significantly elevated symptoms of both inattention and hyperactivity. We were not able to determine if all children in the elevated range would meet the DSM‐5 criteria for ADHD; however, when adjusting for probability of ADHD, scores were still significantly higher than in TDC. This may reflect a bias in study participation towards children with additional deficits. Alternatively, our results may reflect the presence of inattention and hyperactivity in children with developmental disorders who are not diagnosed with ADHD. Quantitative measures of symptoms of inattention and hyperactivity capture information on individual variability that is not available from diagnostic categories. Indeed, a clustering analysis of the Conners 3 rating scores in a transdiagnostic sample of children with difficulties at school found that while diagnoses of ADHD are overrepresented in children with inattention, hyperactivity, and executive functioning impairments, this profile was not specific to ADHD. 50 Further large‐scale studies of motor skills in children with ADHD and DCD are needed to characterize the motor and attention phenotypes related to each diagnosis.
Poorer attention, executive functioning, processing speed, and symptoms of inattention and hyperactivity were not associated with the type or severity of motor impairment, even when adjusting for the ADHD probability index. In addition, attention impairments were highly heterogenous with no correlation between scores. Our results suggest that all children with DCD are at risk of additional impairments in attention and executive functioning, rather than those with a particular subtype or severity of DCD. Impaired performance on standardized tests of attention is not diagnostic for ADHD. 51 Interestingly, deficits in executive functioning and processing speed have been identified in other developmental disorders that co‐occur with DCD. 52 , 53 Information processing and executive function deficits may explain overlapping behavioural phenotypes with other developmental disorders.
Lower gross motor skills (assessed using an aiming and catching task) were associated with reduced surface area in the left posterior cingulate, while cortical thinning in the adjacent isthmus was associated with higher inattentive symptomatology. A previous study reported that decreased grey matter concentration in a region of the left posterior cingulate and precuneus was associated with poorer overall motor abilities in children. 11 We refined this finding to suggest that surface area but not cortical thickness in the posterior cingulate is specifically related to gross motor abilities. Resting state functional connectivity between the posterior cingulate and sensorimotor networks is also reportedly reduced in children with DCD compared to TDC. 54 When correcting for the ADHD probability index, the association between cortical thickness of the isthmus of the posterior cingulate and inattention was no longer significant, suggesting that alterations in this area may be a correlate of co‐occurring ADHD. In accordance with this suggestion, Langevin and colleagues 21 reported differences in cortical thickness of the right isthmus of the posterior cingulate in children with DCD with co‐occurring ADHD compared to controls. Altered structure and function of the posterior cingulate cortex has been reported in individuals with ADHD and ASD. 55 It is possible that altered structure and function of the posterior cingulate is a feature common to multiple developmental disorders, reflecting altered cortical maturation; the nature of these alterations may account for co‐occurrence between disorders.
In typical brain development, thickness increases rapidly to a peak before 2 years of age, before decreasing across childhood and adulthood, while cortical surface area increases over childhood to a peak at 11 to 12 years before decreasing in adolescence and adulthood. 56 Wenger and colleagues 57 suggested that learning and skill acquisition expands cortical thickness before thickness reduces as inefficient and redundant connections are pruned. In contrast, a higher cortical surface area may reflect more cortical columns, functional units in the cortex, available for information processing. 58 The associations with cortical features in this study may reflect delayed or altered maturation. We speculate that reduced surface area of the posterior cingulate gyrus may reflect fewer cortical columns, while increased thickness may reflect impaired cortical pruning.
The posterior cingulate may be implicated in detecting changes in the environment requiring a behavioural response. 55 The prevailing theoretical account suggests that poor online control of motor skills underlies DCD and a recent review cited evidence of deficits in internal modelling of motor commands across a range of behavioural paradigms. 3 According to key models of motor control, 59 copies of motor commands sent to the musculoskeletal system (‘efference copies’) are used in the brain to create predictions of the functional, sensory, and positional outcome of motor commands. The actual outcome of movement is estimated based on sensory input and compared to the prediction. If a discrepancy between predicted and actual movement is detected, errors are used to correct motor execution and update stored motor programmes. Children with DCD may have impaired error detection resulting in poor motor control and unrefined motor programmes. 60 In this study, we propose that the altered detection of environmental changes by the posterior cingulate impairs the estimation of motor outcomes and online motor control in children with DCD.
Poorer gross motor skills in children with DCD were associated with reduced left CST cross‐section. Our results are in contrast with Hyde and colleagues, 9 who reported no differences in fibre density or log(fold change) in adults with DCD compared to TDC, whereas Brown‐Lum and colleagues 10 reported reduced fractional anisotropy and axial diffusivity in the right but not left CST. Interestingly, neither of these studies investigated whether the type of motor deficit was associated with tract structure. Our results suggest that altered fibre morphology but not microstructure in the left CST may be a specific feature of DCD with gross motor impairments in childhood. Several functional MRI studies in children with DCD reported reduced activation in the left primary motor cortex and reduced functional connectivity between the left sensorimotor cortex and the rest of the brain (see Subara‐Zukic et al. 3 for a review); however, these studies did not relate the MRI findings to the severity or type of motor skill impairments. Altogether, our finding of an altered CST morphology points to the importance of sensorimotor tracts in motor skills and is consistent with impaired motor control in children with DCD. 3 , 7 , 59
Limitations
Our sample could be considered heterogeneous because we included children with a wide range of co‐occurring disorders, as well as children taking medication for ADHD, which may have influenced the MRI findings. We also did not exclude children who had received intervention to improve motor skills. Nevertheless, the strength of this study was precisely that our results are generalizable to the wider population with DCD with co‐occurring difficulties and those with differing support levels. While our group of TDC was not large, our primary aim was to characterize non‐motor abilities in children with DCD.
In this study, we investigated how an ecologically valid cohort of children with DCD differed from a sample of typically developing controls. However, as our group of TDC was a convenience sample, we were not able to investigate how children with DCD differ from children with ADHD and other attention and executive functioning impairments. Future research with larger population samples would further our understanding of the relationship between neurodevelopmental disorders in the wider paediatric population.
Conclusions
Impaired attention, processing speed, and executive functioning were features of DCD in our ecologically valid cohort, regardless of the type or severity of motor impairment or probability of ADHD, and a communication profile indicative of ASD or autistic traits. Altered cortical morphology in the posterior cingulate was associated with both gross motor skills and inattentive symptoms, suggesting a possible feature of co‐occurring disorders. Gross motor skills were also associated with fibre morphology in the left CST. These regions may be implicated in online motor control and environmental change detection, deficits in which are thought to underlie DCD.
Supporting information
Appendix S1: Supplementary methods
Table S1: Tractography generation methods
Table S2: Differences in motor and non‐motor scores in children with DCD and TDC
Figure S1: Recruitment process for children with DCD and TDC.
Figure S2: Heatmaps showing Spearman's rank correlation coefficients between attention scores, ADHD symptomatology, executive functioning, regional surface area, white matter fractional anisotropy and fixel‐based fibre morphology.
ACKNOWLEDGMENTS
We thank the participants and their families for taking part in the study. This work was supported by a Child Development Grant from The Waterloo Foundation awarded to FJL, DG, and CAC. AFB was supported by a Child Health Research C.I.O PhD studentship. FJL, CAC, and AFB were also supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health and Care Research or the Department of Health and Social Care. ATM was supported by a National Health and Medical Research Council (NHMRC) Practitioner Fellowship (no. 1105008) and the NHMRC Centre of Research Excellence in Speech and Language (no. 1116976).
Bonthrone AF, Green D, Morgan AT, Mankad K, Clark CA, Liégeois FJ. Attention and motor profiles in children with developmental coordination disorder: A neuropsychological and neuroimaging investigation. Dev Med Child Neurol. 2024;66:362–378. 10.1111/dmcn.15745
This original article is commented on by Gomez et al. on pages 279–280 of this issue.
DATA AVAILABILITY STATEMENT
Processed data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Blank R, Barnett AL, Cairney J, et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev Med Child Neurol 2019; 61: 242–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lingam R, Hunt L, Golding J, Jongmans M, Emond A. Prevalence of developmental coordination disorder using the DSM‐IV at 7 years of age: a UK population‐based study. Pediatrics 2009; 123: e693‐700. [DOI] [PubMed] [Google Scholar]
- 3. Subara‐Zukic E, Cole MH, McGuckian TB, et al. Behavioral and Neuroimaging Research on Developmental Coordination Disorder (DCD): A Combined Systematic Review and Meta‐Analysis of Recent Findings. Front. Psychol. 2022; 13. https://www.frontiersin.org/article/10.3389/fpsyg.2022.809455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piek JP, Dyck MJ, Francis M, Conwell A. Working memory, processing speed, and set‐shifting in children with developmental coordination disorder and attention‐deficit‐hyperactivity disorder. Dev Med Child Neurol 2007; 49: 678–83. [DOI] [PubMed] [Google Scholar]
- 5. Berlot E, Popp NJ, Diedrichsen J. A critical re‐evaluation of fMRI signatures of motor sequence learning. Elife. 2020. May 13;9:e55241. 10.7554/eLife.55241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nicolson RI, Fawcett AJ. Procedural learning difficulties: reuniting the developmental disorders? Trends Neurosci. 2007. Apr;30(4):135–41. 10.1016/j.tins.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 7. Battaglia‐Mayer A, Caminiti R. Corticocortical Systems Underlying High‐Order Motor Control. J Neurosci. 2019. Jun 5;39(23):4404–4421. 10.1523/JNEUROSCI.2094-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams IL, Lust JM, Wilson PH, Steenbergen B. Compromised motor control in children with DCD: a deficit in the internal model?—A systematic review. Neurosci Biobehav Rev. 2014. Nov;47:225–44. 10.1016/j.neubiorev.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 9. Hyde C, Fuelscher I, Enticott PG, et al. White matter organization in developmental coordination disorder: A pilot study exploring the added value of constrained spherical deconvolution. NeuroImage Clin 2019; 21: 101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown‐Lum M, Izadi‐Najafabadi S, Oberlander TF, Rauscher A, Zwicker JG. Differences in White Matter Microstructure Among Children With Developmental Coordination Disorder. JAMA Netw open 2020; 3: e201184–e201184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reynolds JE, Licari MK, Reid SL, et al. Reduced relative volume in motor and attention regions in developmental coordination disorder: A voxel‐based morphometry study. Int J Dev Neurosci 2017; 58: 59–64. [DOI] [PubMed] [Google Scholar]
- 12. Zwicker JG, Missiuna C, Harris SR, Boyd LA. Developmental coordination disorder: a pilot diffusion tensor imaging study. Pediatr Neurol. 2012. Mar;46(3):162–7. [DOI] [PubMed] [Google Scholar]
- 13. Williams J, Kashuk SR, Wilson PH, Thorpe G, Egan GF. White matter alterations in adults with probable developmental coordination disorder: an MRI diffusion tensor imaging study. Neuroreport. 2017. Jan 18;28(2):87–92. 10.1097/WNR.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 14. Karim HT, Sparto PJ, Aizenstein HJ, Furman JM, Huppert TJ, Erickson KI, Loughlin PJ. Functional MR imaging of a simulated balance task. Brain Res. 2014. Mar 25;1555:20–7. 10.1016/j.brainres.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taube W, Mouthon M, Leukel C, Hoogewoud H, Annoni J, Keller M. Brain activity during observation and motor imagery of different balance tasks: An fMRI study. Cortex 2015;64:102–114. [DOI] [PubMed] [Google Scholar]
- 16. Mayhew SD, Porcaro C, Tecchio F, Bagshaw AP. fMRI characterisation of widespread brain networks relevant for behavioural variability in fine hand motor control with and without visual feedback. Neuroimage. 2018;148:330–342. [DOI] [PubMed] [Google Scholar]
- 17. Fautrelle L, Pichat C., Ricolfi F., Peyrin C., Bonnetblanc F.. Catching falling objects: the role of the cerebellum in processing sensory‐motor errors that may influence updating of feedforward commands. An fMRI study. Neuroscience. 2011;190: 135–144 [DOI] [PubMed] [Google Scholar]
- 18. Shaw P, Weingart D, Bonner T, Watson B, Park MT, Sharp W, Lerch JP, Chakravarty MM. Defining the neuroanatomic basis of motor coordination in children and its relationship with symptoms of attention‐deficit/hyperactivity disorder. Psychol Med. 2016. Aug;46(11):2363–73. [DOI] [PubMed] [Google Scholar]
- 19. Green D, Chambers ME, Sugden DA. Does subtype of developmental coordination disorder count: is there a differential effect on outcome following intervention? Hum Mov Sci. 2008. Apr;27(2):363–82. [DOI] [PubMed] [Google Scholar]
- 20. Lust JM, Steenbergen B, Diepstraten JAEM, Wilson PH, Schoemaker MM, Poelma MJ. The subtypes of developmental coordination disorder. Dev Med Child Neurol. 2022. Nov;64(11):1366–1374. 10.1111/dmcn.15260. Epub 2022 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langevin LM, MacMaster FP, Dewey D. Distinct patterns of cortical thinning in concurrent motor and attention disorders. Dev Med Child Neurol, 2015;57: 257–264. 10.1111/dmcn.12561 [DOI] [PubMed] [Google Scholar]
- 22. Langevin LM, Macmaster FP, Crawford S, Lebel C, Dewey D. Common white matter microstructure alterations in pediatric motor and attention disorders. J Pediatr. 2014. May;164(5):1157‐1164.e1. 10.1016/j.jpeds.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 23. Kadesjo B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school‐age children. J Child Psychol Psychiatry 2001; 42: 487–92. [PubMed] [Google Scholar]
- 24. Astle DE, Holmes J, Kievit R, Gathercole SE. Annual Research Review: The transdiagnostic revolution in neurodevelopmental disorders. J Child Psychol Psychiatr 2022; 63: 397–417. 10.1111/jcpp.13481 [DOI] [PubMed] [Google Scholar]
- 25. Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. [DOI] [PubMed] [Google Scholar]
- 26. Henderson S, Sugden D, Barnett A. Movement Assessment Battery for Children‐ Second Edition. London: Pearson, 2007. [Google Scholar]
- 27. Linsell L, Johnson S, Wolke D, O'Reilly H, Morris JK, Kurinczuk JJ, Marlow N. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population‐based cohort study. Arch Dis Child. 2018. Apr;103(4):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson BN, Crawford SG, Green D, Roberts G, Aylott A, Kaplan B. Psychometric properties of the revised developmental coordination disorder questionnaire. Phys Occup Ther Paediatr 2009; 29: 182–202. [DOI] [PubMed] [Google Scholar]
- 29. Wechsler D. Wechsler Abbreviated Scale of Intelligence‐ Second Edition. Bloomington, MN: Pearson, 2011. [Google Scholar]
- 30. Manly T, Anderson V, Nimmo‐Smith I, Turner A, Watson P, Robertson IH. The Differential Assessment of Children's Attention: The Test of Everyday Attention for Children (TEA‐Ch), Normative Sample and ADHD Performance. J Child Psychol Psychiatry 2001; 42: 1065–81. [DOI] [PubMed] [Google Scholar]
- 31. Mulder H, Pitchford NJ, Marlow N. Processing speed mediates executive function difficulties in very preterm children in middle childhood. J Int Neuropsychol Soc 2011; 17: 445–54. [DOI] [PubMed] [Google Scholar]
- 32. Conners KC. Conner's Third Edition. Pearson, 2008. [Google Scholar]
- 33. Bishop DVM. The Children's Communication Checklist‐2. Pearson Psychological Corporation, 2003. [Google Scholar]
- 34. Norbury CF, Nash M, Baird G, Bishop, DVM . Using a parental checklist to identify diagnostic groups in children with communication impairment: a validation of the Children's Communication Checklist‐2. International Journal of Language & Communication Disorders 2004; 39: 345–364. 10.1080/13682820410001654883 [DOI] [PubMed] [Google Scholar]
- 35. Dale A, Fischl B, Sereno MI. Cortical Surface‐Based Analysis: I. Segmentation and Surface Reconstruction. Neuroimage 1999; 9: 179–94. [DOI] [PubMed] [Google Scholar]
- 36. Fischl B, Sereno MI, Tootell RBH, Dale AM. High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 1999; 8: 272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- 38. Tournier J‐D, Smith RE, Raffelt DA, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019; 202: 116137. [DOI] [PubMed] [Google Scholar]
- 39. Raffelt DA, Tournier J‐DD, Smith RE, et al. Investigating white matter fibre density and morphology using fixel‐based analysis. Neuroimage 2017; 144: 58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raffelt D, Tournier JD, Rose S, Ridgway GR, Henderson R, Crozier S, Salvado O, Connelly A. Apparent Fibre Density: a novel measure for the analysis of diffusion‐weighted magnetic resonance images. Neuroimage. 2012. Feb 15;59(4):3976–94. [DOI] [PubMed] [Google Scholar]
- 41. Lautarescu A, Bonthrone AF, Pietsch M, Batalle D, Cordero‐Grande L, Tournier JD, et al. Maternal depressive symptoms, neonatal white matter, and toddler social‐emotional development. Transl Psychiatry. 2022. Aug 9;12(1):323. 10.1038/s41398-022-02073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier JD, Salvado O, et al. Fibre‐specific white matter reductions in Alzheimer's disease and mild cognitive impairment. Brain. 2018. Mar 1;141(3):888–902. 10.1093/brain/awx355. PMID: 29309541. [DOI] [PubMed] [Google Scholar]
- 43. Thomson P, Vijayakumar N, Fuelscher I, Malpas CB, Hazell P, Silk TJ. White matter and sustained attention in children with attention/deficit‐hyperactivity disorder: A longitudinal fixel‐based analysis. Cortex. 2022. Dec;157:129–141. 10.1016/j.cortex.2022.09.006. Epub 2022 Oct 7. PMID: 36283135. [DOI] [PubMed] [Google Scholar]
- 44. Genc S, Malpas CB, Ball G, Silk TJ, Seal ML. Age, sex, and puberty related development of the corpus callosum: a multi‐technique diffusion MRI study. Brain Struct Funct. 2018. Jul;223(6):2753–2765. 10.1007/s00429-018-1658-5. Epub 2018 Apr 5. PMID: 29623479. [DOI] [PubMed] [Google Scholar]
- 45. Lourenço VM, Pires AM, Kirst M. Robust linear regression methods in association studies. Bioinformatics 2011; 27: 815–21. [DOI] [PubMed] [Google Scholar]
- 46. Sumner E, Pratt ML, Hill EL. Examining the cognitive profile of children with Developmental Coordination Disorder. Research in Developmental Disabilities, 2016; 56, 253: 10–17 [DOI] [PubMed] [Google Scholar]
- 47. Gomez A, Huron C. Subitizing and counting impairments in children with developmental coordination disorder. Res Dev Disabil. 2020;104: 103717 [DOI] [PubMed] [Google Scholar]
- 48. Amso D, Scerif G. The attentive brain: Insights from developmental cognitive neuroscience. Nat Rev Neurosci 2015; 16: 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Missiuna C, Mandich AD, Polatajko HJ, Malloy‐Miller T. Cognitive orientation to daily occupational performance (CO‐OP): part I‐‐theoretical foundations. Phys Occup Ther Pediatr 2001; 20: 69–81. [PubMed] [Google Scholar]
- 50. Bathelt J, Holmes J, Astle DE; Centre for Attention Learning and Memory (CALM) Team . Data‐Driven Subtyping of Executive Function‐Related Behavioral Problems in Children. J Am Acad Child Adolesc Psychiatry. 2018. Apr;57(4):252‐262.e4. 10.1016/j.jaac.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. The World Federation of ADHD International Consensus Statement: 208 Evidence‐based conclusions about the disorder. Neurosci Biobehav Rev. 2021. Sep;128:789–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pievsky MA, McGrath RE. The Neurocognitive Profile of Attention‐Deficit/Hyperactivity Disorder: A Review of Meta‐Analyses. Arch Clin Neuropsychol 2017; 33: 143–57. [DOI] [PubMed] [Google Scholar]
- 53. Fossum IN, Andersen PN, Øie MG, Skogli EW. Development of executive functioning from childhood to young adulthood in autism spectrum disorder and attention‐deficit/hyperactivity disorder: A 10‐year longitudinal study. Neuropsychology 2021; 35: 809–21. [DOI] [PubMed] [Google Scholar]
- 54. Rinat S, Izadi‐Najafabadi S, Zwicker JG. Children with developmental coordination disorder show altered functional connectivity compared to peers. NeuroImage Clin 2020; 27: 102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137: 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature. 2022. Apr;604(7906):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wenger E, Brozzoli C, Lindenberger U, Lövdén M. Expansion and renormalization of human brain structure during skill acquisition. Trends in cognitive sciences. 2017. Dec 1;21(12):930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rakic P. Confusing cortical columns. Proceedings of the National Academy of Sciences. 2008. Aug 26;105(34):12099–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 2008; 185: 359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smits‐Engelsman BCM, Wilson PH. Noise, variability, and motor performance in developmental coordination disorder. Dev Med Child Neurol 2013; 55: 69–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary methods
Table S1: Tractography generation methods
Table S2: Differences in motor and non‐motor scores in children with DCD and TDC
Figure S1: Recruitment process for children with DCD and TDC.
Figure S2: Heatmaps showing Spearman's rank correlation coefficients between attention scores, ADHD symptomatology, executive functioning, regional surface area, white matter fractional anisotropy and fixel‐based fibre morphology.
Data Availability Statement
Processed data are available from the corresponding author upon reasonable request.


