Abstract
Far‐UVC, from filtered Krypton‐Chloride lamps, is promising for reducing airborne transmission of disease. While significant research has been undertaken to investigate skin safety of these lamps, less work has been undertaken on eye safety. There is limited data on human eye safety or discomfort from the deployment of this germicidal technology. In this pilot study, immediate and delayed eye discomfort were assessed in a simulated office environment with deployment of Krypton‐Chloride lamps, located on the ceiling and directed downwards into the occupied room. Discomfort was assessed immediately postexposure and several days after exposure using validated, Standard Patient Evaluation Eye Dryness (SPEED) and Ocular Surface Disease Index (OSDI) questionnaires. Our results show no significant eye discomfort or adverse effects from the deployment of Far‐UVC in this simulated office environment, even when lamps were operated continuously with participants receiving head exposures of up to 50 mJ cm−2. In addition, a statistically significant reduction in bacteria and fungi of 52% was observed. Far‐UVC in this simulated office environment did not cause any clinically significant eye discomfort and was effective at reducing pathogens in the room. These results contribute an important step to further investigation of the interaction of Far‐UVC with the human eye.
Keywords: 222 nm, eye, Far‐UVC, Krypton‐Chloride
Far‐UVC, from filtered Krypton‐Chloride lamps, is promising for reducing airborne transmission of disease, but there is limited data on eye safety or discomfort from the deployment of this technology. In this pilot study, immediate and delayed eye discomfort were assessed in a simulated office environment with deployment of Krypton‐Chloride lamps, located on the ceiling and directed downwards into the occupied room. Far‐UVC in this study did not cause any clinically significant eye discomfort and was effective at reducing pathogens in the room. These results contribute an important step towards further investigation of the interaction of Far‐UVC with the human eye.

Abbreviations
- ACH
air‐change‐per‐hour
- CFU
colony forming units
- COVID‐19
coronavirus disease 2019
- GUV
germicidal ultraviolet
- KrCl
krypton chloride
- OSDI
Ocular Surface Disease Index
- SARSCoV‐2
severe acute respiratory syndrome coronavirus 2
- SPEED
Standard Patient Evaluation Eye Dryness
- UVC
ultraviolet‐C
INTRODUCTION
Transmission of airborne pathogens, such as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), influenza, measles and tuberculosis, has crucial global implication. The coronavirus disease 2019 (COVID‐19) pandemic, which is caused by SARS‐CoV‐2, highlighted the short‐term and long‐term devastating effects that these pathogens can have on all facets of human life including health, education and economy.
The risk of transmission of airborne pathogens increases in poorly ventilated indoor spaces where groups of people gather. 1 Examples of these environments include educational institutions such as schools and universities, healthcare facilities, offices, public transport hubs, retail and commercial areas. To reduce transmission, 254 nm germicidal ultraviolet (GUV) has been utilized in the past to good effect. 2 , 3 , 4 However, the major challenge of using the conventional 254 nm GUV is that accidental over‐exposure of human skin or eye causes potentially painful sunburn‐type reaction. 5
Far‐UVC, a germicidal ultraviolet‐C radiation with typical wavelength ranging between 200 and 230 nm, can potentially be used to meet this challenge. Filtered Krypton‐Chloride (KrCl) excimer lamps with a primary emission wavelength of 222 nm, and low‐residual emission of other ultraviolet wavelengths is a common source of Far‐UVC. 6 Far‐UVC has been shown to inactivate a range of pathogens, including bacteria and viruses, in laboratory settings. 7 , 8 , 9 , 10 , 11 , 12 It has also been shown to effectively inactivate aerosolized bacteria in a room‐sized chamber. 13
Due to a limited penetration depth of 222 nm in tissue, there are no acute effects observed in skin exposed up to 1500 mJ cm−2 from a KrCl lamp, when it is filtered to minimize longer wavelength emissions. 14 , 15 , 16 There is also evidence suggesting that the induction of non‐melanoma skin cancer is unlikely at current exposure limits, 17 , 18 although other potential long‐term effects need to be explored.
Eye safety of Far‐UVC has mostly been studied in animal models thus far. Kaidzu et al. 19 demonstrated in rats that even at exposure doses of 600 mJ cm−2, the corneal surface integrity is maintained, which is a surrogate marker of corneal irritation. Furthermore, in mice, rat, rabbit and porcine eyes, the 222 nm appears to only significantly penetrate the corneal epithelium at exposures above 1500 mJ cm−2. 20 The latest work from the same group showed that corneal limbal stem cells are also safe from damage at 600 mJ cm−2 exposure in rat and porcine models, as shown by the absence of cyclobutane pyrimidine dimer formation in the stem cells and the subsequent normal function of the stem cells postirritation demonstrated by normal turnover of the corneal epithelium. 21
Data on the effect of Far‐UVC on human eyes are limited. In one study, where three individuals were exposed to 220 nm produced by an irradiation monochromator with Xenon–Mercury high‐pressure lamp, the threshold before photokeratitis developed was determined to be 10 mJ cm−2, delivered in 256 s with participants staring directly at the source. 22 However in a typical deployment of Far‐UVC, room occupants are unlikely to be subject to direct irradiation of the eye from down‐welling Far‐UVC lamp installations. It has also been argued that the 10 mJ cm−2 is incorrect due to the bandwidth of the monochromator used by Pitts in 1973, and the true threshold may be much higher than noted. 20 , 23 In more recent work on Far‐UVC, six ophthalmologists exposed to filtered 222 nm Far‐UVC in their work environment for more than one year, demonstrated no ill eye effects. 24
The aim of this pilot study was to investigate in a systematic manner whether filtered Krypton‐Chloride lamps at two different exposure levels, when deployed in an office type environment, induce any eye irritation.
MATERIALS AND METHODS
Research ethics
This work was approved by the University of St Andrews Teaching and Ethics Committee (Approval code: MD15737) and adheres to the tenets of the declaration of Helsinki.
Study design
Participants were recruited from the University of St Andrews and surrounding areas through email advertisement and word of mouth. Potential participants who responded to the recruitment were screened by one of the investigators (OK). The exclusion criteria included anyone with a pre‐existing diagnosed eye condition, anyone who may be photosensitive, anyone taking medication or herbal supplements that could induce photosensitivity, anyone who was immunosuppressed or had a history of skin cancer and anyone who could not understand or comply with the protocol requirements, timetables, instructions and protocol‐stated restrictions. A participant information sheet was provided to the potential participants and written informed consent was taken more than 24 h later. Participants were reminded that they could withdraw from the study at any time and without any penalty.
The participants were allocated into one of five groups (A–E) depending on the date they responded to the advert, and the dates they were available to participate. Participants were not randomized to the groups by any criteria but self‐selected their group based on their availability and convenience. Each group participated for a total of 3 days in the study, and each study day was separated by a minimum of 3 days. During a study day, the participants were instructed to remain in an adapted classroom at the University of St Andrews from ten o'clock in the morning to four o'clock in the afternoon. If they wore contact lenses or glasses on the first day of the study, they had to do the same for all the remaining days of the study. At least one of the investigators were present during this time to supervise the study day. While in the classroom the participants were free to undertake tasks as they wished, for example, work on a laptop, read a book, etc. A 15‐min break in the morning, 30‐min lunch break and 15‐min break in the afternoon were provided. Leaving the room at other times was prohibited, except to use the toilet. This design meant the participants spent a minimum of 5 h in the classroom on each study day.
Far‐UVC lamps
Eight filtered KrCl excimer lamps (Biotile, Biocare UV) were installed in the ceiling of the classroom prior to the recruitment process. The location of the lamps within the room is shown in Figure 1, and the spectral emission of the lamps is given in Figure S1. The room has dimensions 12 m × 5.9 m × 3 m and was arranged as shown in Figure 1. As previously described, the room has mechanical ventilation with four air inlets and three open windows for outlets providing a ventilation rate of 6.8 air‐changes‐per‐hour (ACH). 25
FIGURE 1.

(Top) A photograph of the classroom used during the study. The KrCl excimer lamps can be seen secured to the ceiling between the normal room lighting. (Bottom) A schematic of the classroom with the location of the KrCl excimer lamps indicated by purple dots. The available seating positions for the participants are indicated with red circles, and the locations of the agar plates for microbiological sampling are indicated by the letters a–r.
Each study group experienced three different Far‐UVC exposures during the study. The exposures were
NO Far‐UVC Lamps switched on
Far‐UVC Lamps on ALL the time
Far‐UVC Lamps with a DUTY cycle of 30 s on, 270 s off (1:9).
The three scenarios were randomly assigned to study day 1, 2 or 3. Participants were not informed of the Far‐UVC exposure scenario on each study day. However, there was also no attempt to disguise the Far‐UVC exposure scenario, and therefore, for the purposes of study design, the participants were not regarded as blinded. To provide an indication of the Far‐UVC dose received by participants each wore a brimless cloth cap with UVC Dosimeters (UVC 222 Dots, Intellego Technologies) fixed to the top. At the end of each study day, the dosimeters were visually assessed and compared to the included reference chart which provided a dose range, e.g. 0, 0–20, 20–50 mJ cm−2, dependent on the resultant color of the dosimeter.
Eye discomfort
Eye and visual discomfort were ascertained by asking the participants to complete validated questionnaires. The Standard Patient Evaluation Eye Dryness (SPEED) questionnaire was completed by participants at the start of each study day, at the end of each study day and the day after each study day. 26 The SPEED questionnaire relates to symptoms which are currently being experienced (Dryness, Soreness, Burning and Eye Fatigue) and provided an indication of any immediate discomfort as a result of Far‐UVC exposure. The SPEED questionnaire produces a score from 0 to 28, with scores of 0–3, 4–6, 7–10, and 10–28 representing normal, mild, moderate and severe ocular surface symptoms respectively. Questionnaire scores were calculated and changes from baseline (start of each study day) were compared for the three different Far‐UVC exposures.
Another questionnaire, the Ocular Surface Disease Index (OSDI) questionnaire, was completed by participants at the start of each study day and one week after the last study day. 27 The OSDI questionnaire provides an indication of discomfort felt over the previous week and, therefore, provided an indication of any delayed effects from the Far‐UVC exposure. The OSDI questionnaire is scored out of 100, with scores of 0–12, 13–22, 23–32, and 33–100 corresponding to normal, mild, moderate and severe ocular surface symptoms, respectively. A similar analysis to the SPEED questionnaire scoring was performed comparing each Far‐UVC exposure to their OSDI score at the start of the study.
Microbiology
While the purpose of this study was to investigate the potential for eye discomfort during typical office‐based Far‐UVC exposure, the opportunity was used to also acquire microbiological data. A total of 18 agar plates were placed at various locations throughout the room between 2 pm and 4 pm on some of the study days (Figure 1). The locations were broadly grouped into table top (a–h), window ledge (i–n) and floor (o–r).
After 2 h exposure the permissive agar plates (BHI agar, Sigma) were collected and incubated at 30°C for 48 h and then 37°C for 24 h. After the 30°C incubation colonies were identified and counted and the plates photographed. After the 37°C incubation, any further colonies were identified and counted. Data were recorded by blinded operators.
Statistical analysis
Statistical analysis of the SPEED, OSDI and Microbiology data was performed using GraphPad Prism 9.4.1 (GraphPad Software LLC).
RESULTS
Demographics of participants
A total of 38 participants were recruited for the study with one failing to attend the first study day. Of the remaining 37 participants, 46% (n = 17) were female. The mean age was 34 years with standard deviation of 14.5 years and a range of 18–68 years. One participant wore contact lenses during the study (3%) and 20 wore glasses (55%)—10 glasses for distance (27%), eight reading glasses (22%) and two wore varifocals (6%). During the study days, the majority of participants sat and undertook tasks on laptops, with a small number reading a book or watching a tablet. Although it was not a requirement to sit in the same location on each study day, most of the participants did. The study scenarios for each group are described in Table 1.
TABLE 1.
Far‐UVC exposure scenarios for each of the study groups A–E.
| Group (no. of participants) | Study day 1 | Study day 2 | Study day 3 |
|---|---|---|---|
| A (8) | NO | DUTY | ALL |
| B (4) | DUTY | ALL | NO |
| C (7) | ALL | NO | DUTY |
| D (10) | DUTY | ALL | NO |
| E (8) | NO | ALL | DUTY |
Note: NO indicates a day when there was no Far‐UVC exposure, DUTY a day when a duty cycle of 30 s on and 270 s off for the KrCl excimer lamps was deployed and ALL when the KrCl excimer lamps were continuously emitting for the full day.
Far‐UVC exposure
Directly under the lamp, at a height of 2 m from the ground, the measured irradiance was 11.5 μW cm−2. At this point in space, a 6 h exposure would result in a UV dose of 248 mJ cm−2 with the lamps on continuously. With the lamps on a duty cycle as indicated previously, there would be 72 cycles within 6 h which would result in a UV dose of 24.8 mJ cm−2.
None of the participants stood directly under a lamp for a 6 h period. The majority of participants sat down during the study. On study days where there were no Far‐UVC lamps switched on, no UV dose was recorded on the UVC dosimeters. On DUTY days, the majority of participants received no UV dose (66%) with 34% receiving a dose between 0 and 20 mJ cm−2. When the lamps were on continuously (ALL), 62% of participants received a dose between 0 and 20 mJ cm−2, 25% between 20 and 50 mJ cm−2 and 13% received no UV dose (Figure 2). Due to the nature of the UVC dosimeters, it was not possible to more accurately define the UV exposure.
FIGURE 2.
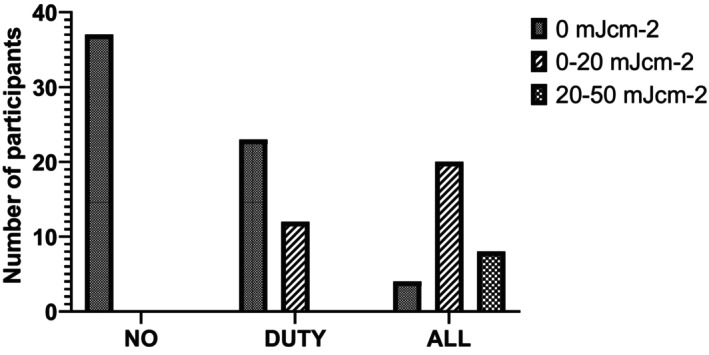
Number of participants and associated Far‐UVC dose for each of the three exposure scenarios (NO, DUTY, ALL) as recorded by UVC Dosimeters (UVC 222 Dots, Intellego Technologies) affixed to the top of brimless cloth caps worn by participants.
Eye discomfort
The mean SPEED score of the participants is given in Table 2. There was no evidence that the mean SPEED score was different at the start of the day, end of the day or the following day for either the NO, DUTY or ALL exposure. There was also no statistically significant difference in the SPEED score when compared to the baseline questionnaire score at the start of the day (Friedman Test). There was no evidence in this study of immediate eye discomfort as a result of the Far‐UVC being deployed.
TABLE 2.
Mean SPEED questionnaire score (95% confidence interval) at the start of the study day, end of study day and the day after the study day for each of the three exposure scenarios (NO/DUTY/ALL).
| Mean (95% CI) | Start of day | End of day | Day after |
|---|---|---|---|
| NO | 1.84 (0.74–2.94) | 1.73 (0.83–2.63) | 1.27 (0.60–1.94) |
| DUTY | 2.14 (1.18–3.09) | 1.62 (0.85–2.39) | 1.46 (0.79–2.12) |
| ALL | 1.95 (0.91–2.98) | 1.68 (0.87–2.48) | 1.27 (0.64–1.90) |
Further data exploration indicated that on the highest exposure day (ALL), there was also no association between those who received the highest UV dose and the SPEED score (Table 3, Kruskal–Wallis test). There was no statistically significant difference in change in SPEED score of those who wore glasses or contact lenses and those who did not (Table 4, Mann–Whitney U test).
TABLE 3.
The difference from Baseline for both the SPEED and OSDI questionnaires was not statistically significant (Friedman Test) between those who received different exposure doses received.
| Mean score difference from Baseline | ||||
|---|---|---|---|---|
| Mean score difference from Baseline | Maximum dose received | p‐Value | ||
| 20–50 mJ cm−2 (n = 9) | 0–20 mJ cm−2 (n = 22) | 0 mJ cm−2 (n = 6) | ||
| End of Day – Baseline SPEED score | −0.78 | −0.05 | −0.17 | 0.537 |
| Next Day – Baseline SPEED score | −0.78 | 0.09 | −2.17 | 0.355 |
| Next Week – Baseline OSDI score | −2.11 | −0.82 | −2.00 | 0.386 |
TABLE 4.
The difference from Baseline for both the SPEED and OSDI questionnaires was not statistically significant (Mann–Whitney U Test) between those who wore glasses or contact lenses and those who did not.
| Mean score difference from Baseline | |||
|---|---|---|---|
| Wearing glasses or contact lenses | p‐Value | ||
| Yes (n = 21) | No (n = 16) | ||
| High exposure day | |||
| End of Day – Baseline SPEED score | −0.37 | −0.11 | 0.682 |
| Next Day – Baseline SPEED score | −0.37 | −0.61 | 0.834 |
| Next Week – Baseline OSDI score | −1.47 | −1.17 | 0.881 |
| Low exposure day | |||
| End of Day – Baseline SPEED score | −0.63 | −0.39 | 0.818 |
| Next Day – Baseline SPEED score | −0.53 | −0.83 | 0.542 |
| Next Week – Baseline OSDI score | −1.05 | −0.89 | 0.741 |
| No exposure day | |||
| End of Day – Baseline SPEED score | −0.37 | 0.167 | 0.603 |
| Next Day – Baseline SPEED score | −0.68 | −0.44 | 0.171 |
| Next Week – Baseline OSDI score | −1.42 | −1.06 | 0.961 |
Similar to the SPEED score, there was no evidence that the OSDI scores after each exposure day were different from the baseline, pre‐study OSDI scores or different from each other (Figure 3). Only one of the OSDI scores was outside the normal range of 0–12, and this result occurred pre‐study. The mean OSDI scores were 2.7 at the start of the study (PRE), 1.4 following no exposure (NO), 1.7 following the study day with duty cycle exposure (DUTY) and 1.4 following the study day with continuous lamp exposure (ALL). As with the SPEED score, there was no association between those who received the highest UV dose or those who wore glasses or contact lenses and the OSDI score (Tables 3 and 4).
FIGURE 3.
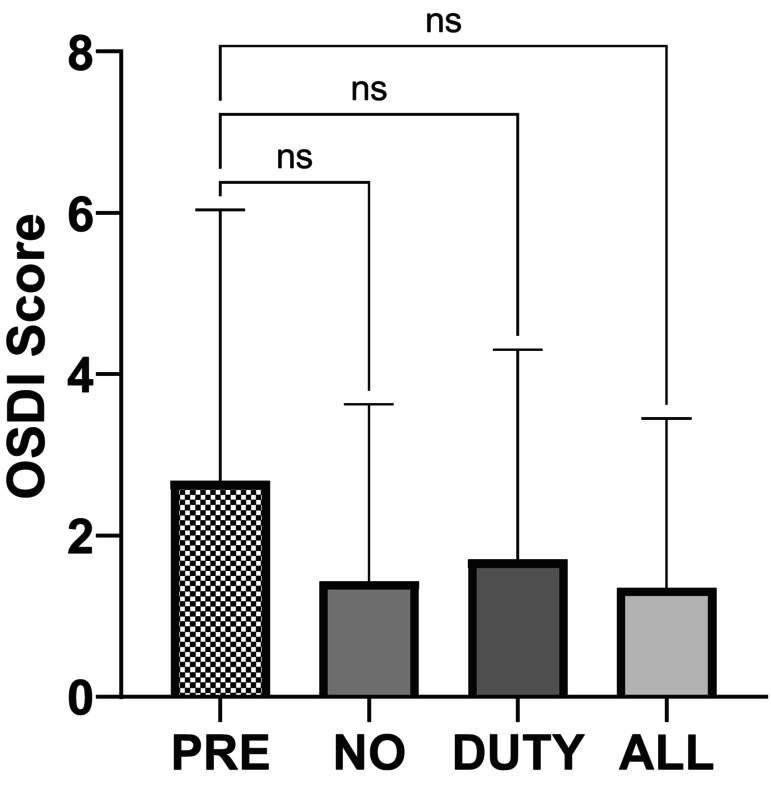
OSDI scores for pre‐study, following NO exposure, DUTY cycle exposure and ALL exposure. Columns represent the mean OSDI score with lines representing the standard deviation. There is no statistically significant difference in the OSDI scores for any exposure scenario (Friedman test).
Adverse events
During the study, one participant reported to develop a non‐infected chalazion on the lower eyelid of the left eye a week after their first study day. As their first day was NO exposure, it was not believed that this adverse event was as a result of the study.
Microbiology
The only statistically significant difference in CFU was from the agar plates on the tables (Figure 4), between the NO exposure day and the ALL exposure day, with mean CFU of 14.5 and 6.9, respectively, providing an average reduction in CFU of 52% (Ordinary One‐Way ANOVA). A mean reduction in CFU of 22% (mean CFU 11.3) was observed in the data from the tables between the NO exposure and DUTY cycle exposure days, but this was not statistically significant.
FIGURE 4.
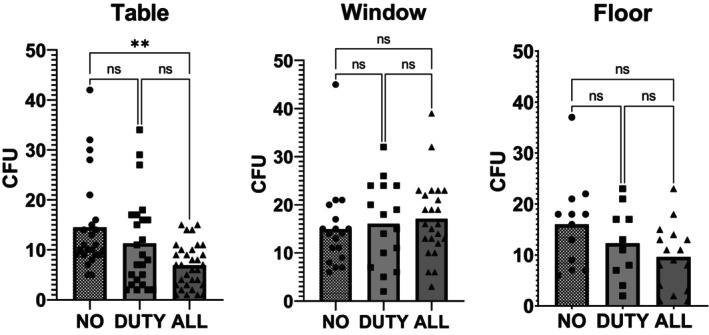
Number of colony forming units (CFU) counted on agar plates for each of the exposure scenarios (NO/DUTY/ALL). Individual data points are plotted on the graphs along with the mean number of CFU (column). Data are grouped for the agar plates located on the tables (left), window ledges (middle) and floor (right). Data from the tables and the floor agar plates show a general trend for lower colony forming units during Far‐UVC exposure, however, only the table data show a statistically significant difference between the no exposure scenario and the lamps on continuously. A lack of data points may be the reason for not reaching statistical significance or the differences seen could be due to chance.
The data from the sample plates on the floor showed a mean reduction from the NO exposure day (16.0 CFU) of 23% (12.3 CFU) and 40% (9.6 CFU) for the DUTY cycle and ALL exposure scenarios, respectively. The data from the agar plates on the window ledges showed a 7.5% and 14.3% increase when comparing DUTY cycle and ALL exposure to the NO exposure day (no exposure = 14.9 CFU, duty cycle exposure = 16.1 CFU and continuous exposure = 17.1 CFU). None of these reductions or increases was statistically significant.
DISCUSSION
This study has assessed, in a controlled and standardized manner, self‐reported eye discomfort when filtered Krypton‐Chloride lamps are deployed in a typical office/classroom environment using a validated standardized questionnaire. The results indicate that there is no clinically significant immediate or delayed discomfort experienced when Far‐UVC is deployed as described.
Moreover, eye symptoms were monitored with repeated administration of OSDI questionnaire for the duration of the study in each participant without any worsening symptoms. By administering the SPEED questionnaire before, immediately after, and 24 h after exposure it was ensured to detect any delayed discomfort. Importantly, one of the major potential confounders, i.e. wearing glasses or contact lenses did not have any effect on the development of eye symptoms, as individuals who did not wear refractive correction aids remained symptom free. In addition, the self‐reported eye scores of individuals with higher maximum exposure were no different from individuals with zero exposure as per dosimeter indicator. In all groups, the self‐report eye scores did not change in a statistically significant way from the baseline.
One historical study has reported eye irritation after 10 mJ cm−2 of 220 nm FAR‐UVC exposure but was potentially flawed in its design due to bandwidth of the irradiation monochromator used in the study. 23
Although we measured the Far‐UVC exposure to the top of the head, we cannot know the actual eye exposure of our participants. The relationship between Far‐UVC exposure to the top of the head and the eye is highly variable and will depend upon multiple factors, including distance from and angle to the lamps throughout the exposure duration. Duncan et al. demonstrated in a mannequin study that the eye received, on average, 5.8% of the dose measured from the top of the head. However, the variability in recorded measurements was very large, including several measurements where the eye received no UV dose despite significant exposure on the top of the head. 28 Similar variability was observed in a study by First et al., 29 which found variation in participant eye dose of between 3% and 37% compared to a calculated dose. We can, therefore, conclude that while we do not know the exact eye exposures of our participants, it is unlikely that eye exposure will have been higher than the recorded top of head exposure and could have been just a few percent or less of this measurement. As such our study should not be regarded as a defining exposure level study but more a representation of what may be experienced in typical deployment of Far‐UVC.
Our study is the largest human study to date that evaluates eye discomfort effects of Far‐UVC when deployed in a simulated real‐world environment. Although formal sample size was not carried out in this study, this seminal work can pave the way for an appropriately powered study designed to ascertain more definitively whether eye irritation occurs due to Far‐UVC exposure. Given the avant‐garde nature of this work, we restricted participants to individuals with healthy eyes. This limits the applicability of this work to individuals with already compromised ocular surface. Indeed, exposing individuals with ocular surface disease to Far‐UVC will require careful consideration of interaction of the Far‐UVC with diseased eye. This work does not evaluate the cumulative effects of repeated Far‐UVC exposure without a washout period on eye irritation over a longer time, which could potentially be the case if this technology is deployed in public spaces.
Thus far, most animal studies have investigated the effect of Far‐UVC on the different layers of the cornea and its penetration potential into the deeper ocular tissues. There is one study investigating the health of corneal limbal stem cells in animal models. Investigating the structure and function of the limbal stem cells will be crucial in determining if Far‐UVC will have ill effect on the cornea in the long run. There are no reports of the effect of Far‐UVC on conjunctival stem cells in the published literature. Studying these two populations of stem cells will ascertain carcinogenic potential of Far‐UVC in ocular surface. In addition, potentially non‐carcinogenic effects of the Far‐UVC on the ocular surface including its interaction with tear film layer, effects on the conjunctival cells such as goblet cells and long‐term fibrogenic properties of Far‐UVC in causing conditions such as pterygium need to be elucidated.
While this study was not designed to investigate the inactivation of pathogens, it was encouraging to observe a reduction in sampled bacteria and fungi as the quantity of Far‐UVC increased (Figure 4). Of particular interest were results from the agar plates placed on the floor of the room, which demonstrated a trend toward lower colonies at higher levels of Far‐UVC. As these plates were distant from direct Far‐UVC irradiation, as shown in Figure 1, they could be representative of a reduction in circulating pathogen within the room when Far‐UVC was deployed.
The settle plate method, as used in this study, is practical but has its limitations in accurately determining the bactericidal effect of the Far‐UVC lamps. Settle plates are not a representation of inactivated airborne particles but are instead inactive or active microbes which fall out of the air and are deposited on the plate. The number of microbes deposited is likely to vary greatly each day, depending on a number of environmental factors and how individuals move around the room. This is observed in high coefficients‐of‐variation in the data; 54% (Floor NO exposure), 59% (Window NO exposure) and 65% (Table NO exposure). A sample size estimation indicates that to observe a 30% statistically significant change from the NO exposure would have required between 52 and 74 samples. There were, therefore, not enough samples acquired in this study to provide statistical significance for most comparisons. There was, however, an indication that CFU on the settle plates were reduced for both the table and floor samples.
Therefore, while not definitive, this study does provide good preliminary evidence that acute eye discomfort is not experienced by individuals when filtered KrCl lamps are deployed in a real‐world environment at intensity levels sufficient to reduce circulating pathogen load. Further study is warranted to expand the inclusion criteria and number of participants and to determine the received ocular dose of Far‐UVC.
Supporting information
Figure S1. The spectral irradiance of the Far‐UVC lamps used in this study (Biotile, Biocare UV, Warrington, United Kingdom) with both a linear (top) and logarithmic (bottom) y‐axis.
ACKNOWLEDGMENTS
This work was funded by the Science and Technology Facilities Council (STFC) Impact Accelerator Account (IAA) at the University of St Andrews. We would like to thank Andrew Bunting, Buildings, Safety and Technical Services Manager at the University of St Andrews for enabling the installation. We would also like to thank Mike Humphreys, Adrian Leatherland and BiocareUV for the loan and installation of the filtered Krypton‐Chloride lamps and to thank Intellego Technologies for the UVC dosimeters. The preceding individuals had no contribution to the conception, design, execution or analysis of this study.
Kousha O, O’Mahoney P, Hammond R, Wood K, Eadie E. 222 nm Far‐UVC from filtered Krypton‐Chloride excimer lamps does not cause eye irritation when deployed in a simulated office environment. Photochem Photobiol. 2024;100:137‐145. doi: 10.1111/php.13805
REFERENCES
- 1. Miller SL, Nazaroff WW, Jimenez JL, et al. Transmission of SARS‐CoV‐2 by inhalation of respiratory aerosol in the Skagit Valley chorale superspreading event. Indoor Air. 2021;31:314‐323. doi: 10.1111/INA.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wells WF, Wells MW, Wilder TS. The environmental control of epidemic contagion: I. An epidemiologic study of radiant disinfection of air in day schools. Am J Epidemiol. 1942;35:97‐121. doi: 10.1093/oxfordjournals.aje.a118789 [DOI] [Google Scholar]
- 3. Mphaphlele M, Dharmadhikari AS, Jensen PA, et al. Institutional tuberculosis transmission: controlled trial of upper room ultraviolet air disinfection: a basis for new dosing guidelines. Am J Respir Crit Care Med. 2015;192:477‐484. doi: 10.1164/rccm.201501-0060OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Escombe AR, Moore DAJ, Gilman RH, et al. Upper‐room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e1000043. doi: 10.1371/journal.pmed.1000043.g001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliver H, Moseley H, Ferguson J, Forsyth A. Clustered outbreak of skin and eye complaints among catering staff. Occup Med (Chic Ill). 2005;55:149‐153. doi: 10.1093/occmed/kqi021 [DOI] [PubMed] [Google Scholar]
- 6. Barnard IRM, Eadie E, Wood K. Further evidence that far‐UVC for disinfection is unlikely to cause erythema or pre‐mutagenic DNA lesions in skin. Photodermatol Photoimmunol Photomed. 2020;36:476‐477. doi: 10.1111/phpp.12580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buonanno M, Ponnaiya B, Welch D, et al. Germicidal efficacy and mammalian skin safety of 222‐nm UV light. Radiat Res. 2017;187:493‐501. doi: 10.1667/rr0010cc.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buonanno M, Welch D, Shuryak I, Brenner DJ. Far‐UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci Rep. 2020;10:1‐8. doi: 10.1038/s41598-020-67211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welch D, Buonanno M, Shuryak I, Randers‐Pehrson G, Spotnitz HM, Brenner DJ. Effect of far ultraviolet light emitted from ann optical diffuser on methicillin‐resistant Staphylococcus aureus in vitro. PLoS One. 2018;13:e0202275. doi: 10.1371/journal.pone.0202275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Narita K, Asano K, Naito K, et al. Ultraviolet C light with wavelength of 222 nm inactivates a wide spectrum of microbial pathogens. J Hosp Infect. 2020;105:459‐467. doi: 10.1016/j.jhin.2020.03.030 [DOI] [PubMed] [Google Scholar]
- 11. Ma B, Gundy PM, Gerba CP, Sobsey MD, Linden KG. UV inactivation of SARS‐CoV‐2 across the UVC spectrum: KrCl* excimer, mercury‐vapor, and light‐emitting‐diode (LED) sources. Appl Environ Microbiol. 2021;87:e0153221. doi: 10.1128/AEM.01532-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma B, Linden YS, Gundy PM, Gerba CP, Sobsey MD, Linden KG. Inactivation of coronaviruses and phage Phi6 from irradiation across UVC wavelengths. Environ Sci Technol Lett. 2021;8:425‐430. doi: 10.1021/acs.estlett.1c00178 [DOI] [PubMed] [Google Scholar]
- 13. Eadie E, Hiwar W, Fletcher L, et al. Far‐UVC (222 nm) efficiently inactivates an airborne pathogen in a room‐sized chamber. Sci Rep. 2022;121(12):1‐9. doi: 10.1038/s41598-022-08462-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finlayson L, Barnard IRM, McMillan L, et al. Depth penetration of light into skin as a function of wavelength from 200 to 1000 nm. Photochem Photobiol. 2022;98:974‐981. doi: 10.1111/php.13550 [DOI] [PubMed] [Google Scholar]
- 15. Eadie E, Barnard IMR, Ibbotson SH, Wood K. Extreme exposure to filtered far‐UVC: a case study. Photochem Photobiol. 2021;97:527‐531. doi: 10.1111/php.13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukui T, Niikura T, Oda T, et al. Exploratory clinical trial on the safety and bactericidal effect of 222‐nm ultraviolet C irradiation in healthy humans. PLoS One. 2020;15:e0235948. doi: 10.1371/journal.pone.0235948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Welch D, Kleiman NJ, Arden PC, et al. No evidence of induced skin cancer or other skin abnormalities after long‐term (66 week) chronic exposure to 222‐nm far‐UVC radiation. Photochem Photobiol. 2022;99:168‐175. doi: 10.1111/php.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamano N, Kunisada M, Kaidzu S, et al. Long‐term effects of 222 nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation. Photochem Photobiol. 2020;94:853‐862. doi: 10.1111/php.13269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaidzu S, Sugihara K, Sasaki M, Nishiaki A, Igarashi T, Tanito M. Evaluation of acute corneal damage induced by 222‐nm and 254‐nm ultraviolet light in Sprague–Dawley rats. Free Radic Res. 2019;53:611‐617. doi: 10.1080/10715762.2019.1603378 [DOI] [PubMed] [Google Scholar]
- 20. Kaidzu S, Sugihara K, Sasaki M, et al. Re‐evaluation of rat corneal damage by short‐wavelength UV revealed extremely less hazardous property of far‐UV‐C†. Photochem Photobiol. 2021;97:505‐516. doi: 10.1111/PHP.13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaidzu S, Sugihara K, Sasaki M, et al. Safety evaluation of Far‐UVC irradiation to epithelial basal cells in corneal limbus. In 41st American Society for Photobiology Biennial Meeting. Albuquerque. 2022.
- 22. Pitts DG. The ocular ultraviolet action spectrum and protection criteria. Health Phys. 1973;25:559‐566. doi: 10.1097/00004032-197312000-00002 [DOI] [PubMed] [Google Scholar]
- 23. Chaney EK, Sliney DH. Re‐evaluation of the ultraviolet hazard action spectrum – the impact of spectral bandwidth. Health Phys. 2005;89:322‐332. doi: 10.1097/01.HP.0000164650.96261.9d [DOI] [PubMed] [Google Scholar]
- 24. Sugihara K, Kaidzu S, Sasaki M, et al. One‐year ocular safety observation of workers and estimations of microorganism inactivation efficacy in the room irradiated with 222‐nm far ultraviolet‐C lamps. Photochem Photobiol. 2022. doi: 10.1111/php.13710 [DOI] [PubMed] [Google Scholar]
- 25. Wood K, Wood A, Peñaloza C, Eadie E. Turn up the lights, leave them on and Shine them all around—numerical simulations point the way to more efficient use of far‐UVC lights for the inactivation of airborne coronavirus. Photochem Photobiol. 2022;98:471‐483. doi: 10.1111/php.13523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013;32:1204‐1210. doi: 10.1097/ICO.0B013E318294B0C0 [DOI] [PubMed] [Google Scholar]
- 27. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615‐621. doi: 10.1001/ARCHOPHT.118.5.615 [DOI] [PubMed] [Google Scholar]
- 28. Duncan MA, Welch D, Shuryak I, Brenner DJ. Ocular and facial far‐UVC doses from ceiling‐mounted 222 nm far‐UVC fixtures. Photochem Photobiol. 2022;99:160‐167. doi: 10.1111/php.13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. First MW, Weker RA, Yasui S, Nardell EA. Monitoring human exposures to upper‐room germicidal ultraviolet irradiation. J Occup Environ Hyg. 2005;2:285‐292. doi: 10.1080/15459620590952224 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The spectral irradiance of the Far‐UVC lamps used in this study (Biotile, Biocare UV, Warrington, United Kingdom) with both a linear (top) and logarithmic (bottom) y‐axis.


