Abstract
Familial adult myoclonus epilepsy (FAME) is a genetic epilepsy syndrome that for many years has resisted understanding of its underlying molecular cause. This review covers the history of FAME genetic studies worldwide, starting with linkage and culminating in the discovery of noncoding TTTTA and inserted TTTCA pentanucleotide repeat expansions within six different genes to date (SAMD12, STARD7, MARCHF6, YEATS2, TNRC6A, and RAPGEF2). FAME occurs worldwide; however, repeat expansions in particular genes have regional geographical distributions. FAME repeat expansions are dynamic in nature, changing in length and structure within germline and somatic tissues. This variation poses challenges for molecular diagnosis such that molecular methods used to identify FAME repeat expansions typically require a trade‐off between cost and efficiency. A rigorous evaluation of the sensitivity and specificity of each molecular approach remains to be performed. The origin of FAME repeat expansions and the genetic and environmental factors that modulate repeat variability are not well defined. Longer repeats and particular arrangements of the TTTTA and TTTCA motifs within an expansion are correlated with earlier onset and increased severity of disease. Other factors such as maternal or paternal inheritance, parental age, and repeat length alone have been suggested to influence repeat variation; however, further research is required to confirm this. The history of FAME genetics to the present is a chronicle of perseverance and predominantly collaborative efforts that yielded a successful outcome. The discovery of FAME repeats will spark progress toward a deeper understanding of the molecular pathogenesis of FAME, discovery of new loci, and development of cell and animal models.
Keywords: DNA sequencing, molecular genetics, repeat expansion disorders, seizure
Key Points.
There are six independent genes implicated in FAME
FAME is caused by a noncoding intronic TTTTA and an inserted TTTCA pentamer repeat expansion in one of these six genes
Longer repeat expansions are correlated with earlier disease onset and greater disease severity
FAME repeat expansions show somatic and germline instability
1. INTRODUCTION
Familial adult onset myoclonus epilepsy (FAME) is a rare, autosomal dominant disorder characterized by tremorlike cortical myoclonus in the second to third decade of life and infrequent generalized tonic–clonic and/or myoclonic seizures that usually start later. 1 Cortical myoclonus in FAME is identified by giant somatosensory evoked potentials, long latency reflex, and cortical spikes preceding myoclonus based on jerk‐locked averaging of electrophysiological measures. 1 The definition of FAME as a unique epilepsy syndrome was critical in identifying large families for genetic studies and the eventual discovery of the noncoding TTTTA and inserted TTTCA repeat expansions that cause FAME.
2. EARLY LINKAGE STUDIES AND THE IDENTIFICATION OF MULTIPLE FAME LOCI
A genetic marker is defined as a site in the genome with a known location. These markers are usually sites within the human genome of frequent sequence variation in the general population. Markers that segregate with disease in a family are likely to be physically adjacent to the genetic cause of the disease in the genome. Genetic linkage analysis, where multiple markers are used to hunt down the location of a disease‐causing variant, was instrumental in early genetic investigations of FAME.
The first reported linkage study of FAME (or benign adult familial myoclonus epilepsy) targeted the gene for dentatorubral pallidoluysian atrophy (DRPLA), in which a causative CAG repeat expansion had been discovered, and the GABRB1, GABRB3, and GABRB6 genes. 2 In all cases, there was no linkage to these genes in a large Japanese family, suggesting that FAME was a distinct genetic epilepsy syndrome.
By initially targeting known genetic loci associated with epilepsy at the time, Mikami et al. 3 serendipitously identified a positive hit on chromosome 8 segregating with FAME in a large Japanese family (Online Mendelian Inheritance in Man [OMIM], FAME1: 601068). They refined the localization of the gene for FAME1 to 8q23.3–q24.11 (Figure 1A). 3 This interval does not include the SAMD12 gene now known to harbor the TTTTA and TTTCA repeat expansions that cause FAME1. 4 In hindsight and based on the remapping of this family, 5 a probable genotyping error in a single individual in the pedigree (II‐18) defined the telomeric end of the linkage interval, which would otherwise have included SAMD12. 3 From a genome‐wide linkage analysis of four Japanese families, Plaster et al. 6 also localized FAME1 to 8q24 (Figure 1A). Based on the knowledge of the position of markers on chromosome 8 at the time, their reported interval also did not include the SAMD12 gene. 6 Reanalysis of these data by Cen et al. 7 15 years later, with the benefit of an available human genome sequence, reassigned the Plaster et al. interval such that it overlapped with intervals identified in other linkage studies and included SAMD12. Three other FAME1 linkage studies involving either Japanese or Chinese families living with FAME followed, which in combination narrowed the chromosome 8 interval to only 4.17 Mbp. 5 , 7 , 8
FIGURE 1.
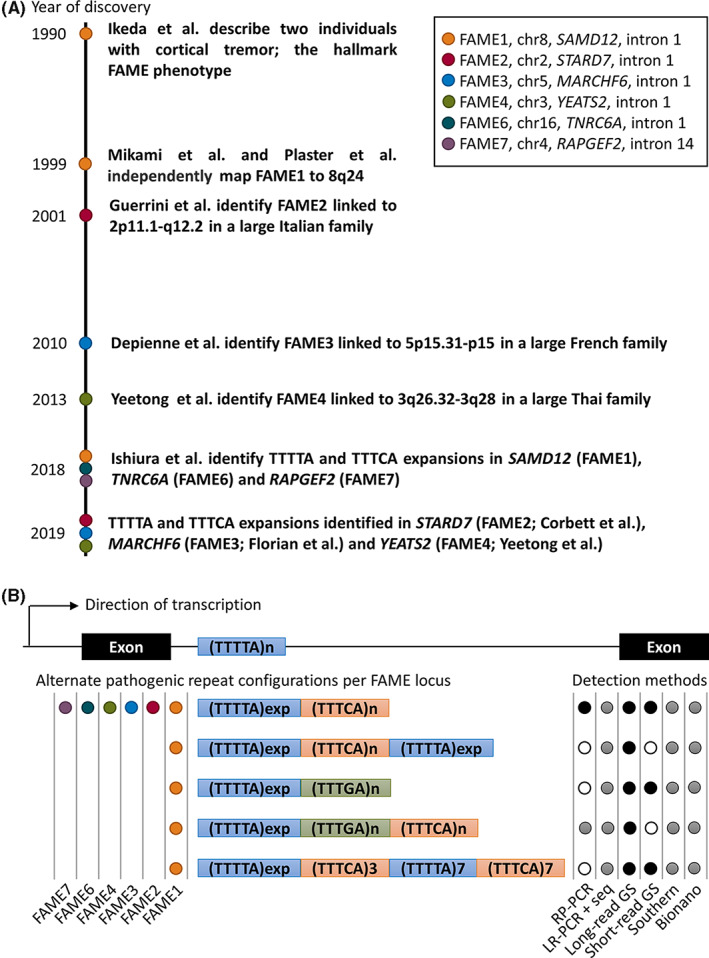
History of familial adult onset myoclonus epilepsy (FAME) genetics and methods for detecting FAME repeat expansions. (A) Timeline depicting years in which key events leading to the discovery of the TTTTA and inserted TTTCA repeat expansions occurred. Colored points on the time line map to events corresponding to particular FAME loci as per the supplied key in the figure. (B) A stylized “FAME gene” indicating the intronic location of the wild‐type TTTTA motif between two exons. For the majority of FAME genes, the expansion occurs in the first intron, except for RAPGEF2 corresponding to FAME7, in which the expansion is in intron 14. Below the stylized gene are representations of the consensus repeat expansion motif configurations that have been reported. Colored dots to the left of each repeat configuration indicate that the configuration has been detected at least once at that FAME locus. To the right of each configuration, the black, gray, or white dots indicate the applicability of the detection method to that configuration. Black dots indicate the method is suitable, gray dots indicate that in some circumstances the method may produce a false negative or false positive result, and white dots indicate the method is highly likely to produce a false negative result. Bionano, Bionano optical genome mapping; GS, genome sequencing; LR‐PCR + seq, long‐range PCR plus sequencing; PCR, polymerase chain reaction; RP‐PCR, repeat‐primed PCR; Southern, Southern blot.
Guerrini et al. 9 linked autosomal dominant cortical myoclonic epilepsy (ADCME) in a large pedigree from Northern Italy covering approximately 21.3 Mbp and spanning the centromere on chromosome 2 (Figure 1A). Several linkage studies involving both Northern and Southern Italian families, with individuals who were phenotypically identical to FAME1, also mapped to the chromosome 2 ADCME interval, strongly suggesting ADCME and FAME1 were variants of the same epilepsy syndrome but mapping to different genes (OMIM, FAME2: 607876). 10 , 11 , 12 Madia et al. 13 provided strong evidence for a founder effect in all Italian FAME2 families and narrowed the chromosome 2 interval to 17.1 Mbp. Similar to FAME1, successive linkage studies in a Spanish, 14 an Italian, 15 and a New Zealander/Australian family of European ancestry 16 progressively reduced the boundaries of the FAME2 linkage interval. In a novel approach that took advantage of the known FAME2 founder effect, Henden et al. 17 combined genotyping data from many of the previously analyzed FAME2 families and used identity by descent mapping rather than traditional linkage to narrow the FAME2 interval to 9.78 Mbp.
Two additional FAME loci were discovered by linkage in the period prior to the discovery of the noncoding repeat expansions that cause all forms of FAME. FAME3 (OMIM: 613608) was mapped to a 9.31‐Mbp interval on chromosome 5 in French, 18 Chinese, 19 , 20 and Dutch 21 families and FAME4 (OMIM: 615127) was mapped to a 10‐Mpb interval on chromosome 3 in a large Thai family (Figure 1A). 22
3. THE HUNT FOR THE GENETIC CAUSE OF FAME
A series of novel candidate variants were identified in plausible genes within the mapped FAME intervals. These included NM_173851.3:c.206A > T:p.(Tyr69Phe) in SLC30A8 (FAME1), 7 NM_000682.7:c.675_686delinsGTTTGGCAG in ADRA2B (FAME2), 23 and NM_001332.4:c.3130G > A:p.(Glu1044Lys) in CTNND2 (FAME3). 21 Isolated variants, all of which fell outside known FAME linkage intervals, were also identified: NM_015902.6:c.5720G > A:p.(Arg1907His) in UBR5 in a Chinese family, 24 NM_003560.4:c.475G > A, p.Ala159Thr in PLA2G6 in a Chinese family (who now have a confirmed SAMD12 repeat expansion), 25 , 26 NM_138326.3:c.77G > A:p(Trp26*) in ACMSD in a Spanish family, 27 and NM_003946.7:c.61G > C:p.(Glu21Gln) in NOL3 in a Canadian family with familial cortical myoclonus without epilepsy. 28 Finally, a homozygous variant, NM_005076.5:c.504del (p.Trp168fs) in CNTN2, segregated with autosomal recessive FAME (OMIM, FAME5: 615400) in an Egyptian family. 29 All of these genes have failed to replicate with variants in other similarly mapped families, suggesting they were not the cause. Gene candidate sequencing approaches based on known gene functions (e.g., ion channels, known genes implicated in epilepsy), gene expression patterns, or gene coexpression networks were also unsuccessful. 14 , 17 , 18 The strong suspicion based on the late onset of FAME and apparent absence of plausible coding candidate variants in short read exome and genome sequencing was that FAME was caused by either noncoding single nucleotide variants, structural variants, or repeat expansions.
By making a close inspection of single nucleotide polymorphism genotypes within the 4.17‐Mbp interval in six Japanese families, Ishiura et al. 4 identified a shared haplotype of only 134 kbp covering exon 4 and part of intron 4 from the SAMD12 gene. Apparent non‐Mendelian inheritance within a parent–child trio of a variable length TTTTA pentanucleotide repeat located in intron 4 of SAMD12 led to closer inspection of this sequence. Genome sequencing data from an affected individual suggested the insertion of a nonreference TTTCA pentanucleotide repeat at the site of the TTTTA repeat and was the first evidence that the long‐sought noncoding variant causing FAME1 had been identified. 4 In the same study, 47 FAME1 families were confirmed to have TTTTA and inserted TTTCA expansions in SAMD12 and in two families novel loci were identified by discovery of noncoding TTTTA and inserted TTTCA expansions in TNRC6A located on16p21.1 (OMIM, FAME6: 618074) and RAPGEF2 located on 4q32.1 (OMIM, FAME7: 618075), respectively. 4 Within a year, similar noncoding TTTTA and inserted TTTCA repeat expansions were located in the linkage intervals for FAME2: STARD7 intron 1, 30 FAME3: MARCHF6 intron 1, 31 and FAME4: YEATS2 intron 1 (Figure 1A). 32 Individuals in >200 families have now had a confirmed genetic diagnosis of a TTTTA and inserted TTTCA repeat expansion within one of the FAME genes since the genetic cause of FAME1 was discovered. The pathogenic FAME1 repeat expansion has since been discovered in Chinese, 26 , 33 , 34 , 35 , 36 , 37 , 38 Japanese, 39 Indian, 40 , 41 Sri Lankan, 41 and Thai 42 families, all likely arising from one founder. The FAME1 repeat expansion was estimated to be approximately 16 800 years old and predicted to have arrived in Japan approximately 4300 years ago. 41 Expansions within intron 14 of RAPGEF2 of the recently discovered FAME7 locus have been identified in novel families from China and Japan. 35 , 43 There have been no additional genetic studies to date of FAME2, FAME3, FAME4, or FAME6 families since their initial discovery.
4. METHODS OF DETECTING FAME REPEAT EXPANSIONS
At present, there are no facilities that offer a specific molecular test for FAME; however, this could be rapidly addressed by adaptation of existing standard methodologies. Examples of detection methods for FAME repeats that could be adapted for genetic diagnosis of FAME include Southern blotting, repeat‐primed polymerase chain reaction (PCR), molecular combing, long‐range PCR combined with long‐ read sequencing, Cas9 endonuclease target enrichment‐based long‐read sequencing, and Bionano optical genome mapping (Figure 1B). 4 , 30 , 31 , 43 , 44 FAME repeat expansions can be detected from short‐ or long‐read genome sequencing data, and this technique has been shown to have high sensitivity generally for detecting repeat expansion disorders in the setting of a clinical molecular genetics service. 4 , 30 , 31 , 45 Southern blotting, repeat‐primed PCR, and long‐range PCR are routinely used in genetic diagnosis of many repeat expansion disorders and are likely to be the most accessible technologies for established molecular pathology services. The specificity and sensitivity of the current methods for molecular diagnosis of FAME have not been quantified, due to incomplete knowledge about the repeat expansions, so a negative result should be considered with caution. Techniques that only assess the size of an expansion, such as Southern blotting, molecular combing, Bionano optical genome mapping, and long‐range PCR, must be combined with either repeat‐primed PCR or a sequencing technique to ensure the pathogenic TTTCA motif is present before a molecular diagnosis of FAME can be made. Despite having the advantages of requiring low amounts of genomic DNA input and low cost, long‐range PCR may produce a false negative result when the expanded allele is very large. Expansions composed only of TTTTA motifs exist at some loci in unaffected individuals and are not pathogenic. Alternate repeat configurations that contain either novel pentamer insertions (TTTGA), an inserted TTTCA expansion flanked by TTTTA expansions, or a very short TTTCA expansion usually do not provide a diagnostic result by repeat‐primed PCR (Figure 1B); therefore, alternative methods, ideally long‐read genome sequencing, should be applied where there is strong suspicion of FAME and a negative result is obtained with repeat‐primed PCR. 4 , 26 , 34 , 44 , 46 Based on current knowledge, the highest diagnostic yields will be obtained for FAME1, and to a lesser extent FAME7 in patients of Asian ancestry 4 , 35 , 40 and FAME2 and FAME3 in patients of European ancestry. 30 , 31 FAME4 and FAME6 have to date only been reported in single families of Asian ancestry. 4 , 32 One family with a FAME1 expansion and European ancestry has been reported; therefore, although ethnicity can guide triaging of tests, all loci should be considered when there is a strong clinical indication of FAME. 44 Family history of FAME is the strongest indicator for a positive molecular diagnosis; very few diagnoses have been made from singleton families. 30 , 39
5. GENOTYPE TO PHENOTYPE RELATIONSHIPS IN FAME
Following the discovery of the TTTTA and inserted TTTCA expansions that cause all autosomal dominant forms of FAME, attention has turned to understanding genotype to phenotype relationships. There appear to be no major phenotypic distinctions between the different genetic forms of FAME. That the genes encompassing the intronic TTTTA and inserted TTTCA repeat expansions have different molecular functions and do not all operate in the same biochemical pathways suggests that the properties of the repeat and not the gene are the important driver of pathogenicity. Longer repeats or homozygosity for the repeats is correlated with an earlier age of disease onset and usually more severe neurological phenotypes. 4 , 31 , 35 , 39 However, it is not possible to make a meaningful prognosis for an individual patient based on the length of their repeat alone, and this suggests there are additional factors that influence symptom onset and severity. Florian et al. 31 suggested that the TTTCA repeat content was more strongly correlated with onset of seizures in FAME3, whereas TTTTA content did not show the same correlation. Pan et al. 26 suggested that individuals with a SAMD12 repeat configuration in which the inserted TTTCA expansion is in between two TTTTA expansions have earlier symptom onset than individuals with the more frequently observed configuration in which the TTTTA expansion is adjacent to the TTTCA expansion. Mizuguchi et al. 43 identified a huge degree of variability in the numbers of copies of TTTTA and TTTCA pentamers in FAME1 repeats between different individuals from different families, including in one individual where only 14 copies of the TTTCA motif were observed. To date, there is no evidence that TTTTA expansions alone are pathogenic. 26
FAME TTTTA and inserted TTTCA repeat expansions exhibit both somatic and generational instability in their repeat copy numbers and structural arrangement of repeat motifs. Anticipation, where age of symptom onset decreases and disease severity increases over successive generations, is a common feature of most repeat expansion disorders and is correlated with intergenerational increases in repeat lengths. Anticipation occurs in FAME families; however, correlation of anticipation with an increase in repeat length between parent and child remains uncertain due to the low numbers of relationships where this has been studied at the molecular level. 31 , 39 , 46 Paternal or maternal inheritance and maternal age have been investigated as potential risk factors; however, these have not shown significant correlation with germline repeat instability to date. 4 , 26 , 39 There are no known genetic modifiers for susceptibility to germline or somatic repeat instability of FAME repeat expansions. A significant enrichment of longer TTTTA repeat alleles in the TNRC6A gene in individuals with FAME1 SAMD12 repeat expansions compared to an unaffected control population has been noted; however, the reason for this remains unclear. 39
Somatic variation of TTTTA and inserted TTTCA repeat expansions has been observed in FAME1, FAME2, and FAME3 both within one tissue and between different tissues from one individual. 4 , 30 , 31 Somatic variability increases with the size of the expansions, with expansions larger than 10 kb showing multiple sizes and configurations within a single tissue. 31 Given that most genotype–phenotype correlation studies to date used genomic DNA extracted from patient blood, repeat lengths and repeat configurations reported may not reflect the dominant repeat configuration in the brain of each individual.
6. SIGNIFICANCE OF THE DISCOVERY OF FAME REPEAT EXPANSIONS AND FUTURE DIRECTIONS
Unlocking the genetic cause of FAME was a breakthrough discovery in human disease genetics. The discovery places FAME broadly within the family of >50 repeat expansion disorders. 47 Within these, FAME now accounts for the majority of a special category of transcribed but noncoding adenine and thymine (AT)‐rich pentamer expansions. There are now multiple options available to provide individuals with a genetic diagnosis based on the detection of TTTCA repeat expansions at any of the currently known loci, providing greater certainty than a test based on linkage, but these are yet to be translated to molecular pathology services beyond the sphere of laboratories specializing in FAME genetics research. There remain families in which known loci were excluded by linkage, or direct molecular tests for the known repeat expansions, suggesting additional FAME genes are yet to be discovered. 28 , 39 , 43 , 48 , 49 , 50 The questions of what modifies the correlation between repeat length and disease onset and which factors influence germline and somatic instability also remain open and will require new cell and animal preclinical models to uncover. 51
AUTHOR CONTRIBUTIONS
Mark A. Corbett drafted the original manuscript. All remaining authors contributed revisions and had intellectual input into the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors wish to acknowledge the following for funding support: National Health and Medical Research Council Synergy Grant 2010562 and Senior Principal Research Fellowship 1155224 to J.G.; Deutsche Forschungsgemeinschaft (project number 455314768) to C.D.; and the Italian Ministry of Health (ricerca corrente) to F.B. Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Corbett MA, Depienne C, Veneziano L, Klein KM, Brancati F, Guerrini R, et al. Genetics of familial adult myoclonus epilepsy: From linkage studies to noncoding repeat expansions. Epilepsia. 2023;64:S14–S21. 10.1111/epi.17610
REFERENCES
- 1. van den Ende T, Sharifi S, van der Salm SMA, van Rootselaar A‐F. Familial cortical myoclonic tremor and epilepsy, an enigmatic disorder: from phenotypes to pathophysiology and genetics. a systematic review. Tremor Other Hyperkinet Mov (N Y). 2018;8:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuwano A, Takakubo F, Morimoto Y, Uyama E, Uchino M, Ando M, et al. Benign adult familial myoclonus epilepsy (BAFME): an autosomal dominant form not linked to the dentatorubral pallidoluysian atrophy (DRPLA) gene. J Med Genet. 1996;33(1):80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mikami M, Yasuda T, Terao A, Nakamura M, Ueno S, Tanabe H, et al. Localization of a gene for benign adult familial myoclonic epilepsy to chromosome 8q23.3‐q24.1. Am J Hum Genet. 1999;65(3):745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishiura H, Doi K, Mitsui J, Yoshimura J, Matsukawa MK, Fujiyama A, et al. Expansions of intronic TTTCA and TTTTA repeats in benign adult familial myoclonic epilepsy. Nat Genet. 2018;50(4):581–90. [DOI] [PubMed] [Google Scholar]
- 5. Mori S, Nakamura M, Yasuda T, Ueno S‐I, Kaneko S, Sano A. Remapping and mutation analysis of benign adult familial myoclonic epilepsy in a Japanese pedigree. J Hum Genet. 2011;56(10):742–7. [DOI] [PubMed] [Google Scholar]
- 6. Plaster NM, Uyama E, Uchino M, Ikeda T, Flanigan KM, Kondo I, et al. Genetic localization of the familial adult myoclonic epilepsy (FAME) gene to chromosome 8q24. Neurology. 1999;53(6):1180–3. [DOI] [PubMed] [Google Scholar]
- 7. Cen Z‐D, Xie F, Lou D‐N, Lu X‐J, Ouyang Z‐Y, Liu L, et al. Fine mapping and whole‐exome sequencing of a familial cortical myoclonic tremor with epilepsy family. Am J Med Genet B Neuropsychiatr Genet. 2015;168(7):595–9. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki T. Familial essential myoclonus and epilepsy (FEME). Niigata Igakkai Zasshi. 2002;116(11):535–45. [Google Scholar]
- 9. Guerrini R, Bonanni P, Patrignani A, Brown P, Parmeggiani L, Grosse P, et al. Autosomal dominant cortical myoclonus and epilepsy (ADCME) with complex partial and generalized seizures: a newly recognized epilepsy syndrome with linkage to chromosome 2p11.1‐q12.2. Brain. 2001;124(Pt 12):2459–75. [DOI] [PubMed] [Google Scholar]
- 10. de Falco FA, Striano P, de Falco A, Striano S, Santangelo R, Perretti A, et al. Benign adult familial myoclonic epilepsy: genetic heterogeneity and allelism with ADCME. Neurology. 2003;60(8):1381–5. [DOI] [PubMed] [Google Scholar]
- 11. Striano P, Chifari R, Striano S, Fusco MD, Elia M, Guerrini R, et al. A new benign adult familial myoclonic epilepsy (BAFME) pedigree suggesting linkage to chromosome 2p11.1‐q12.2. Epilepsia. 2004;45(2):190–2. [DOI] [PubMed] [Google Scholar]
- 12. Striano P, Madia F, Minetti C, Striano S, Zara F. Electroclinical and genetic findings in a family with cortical tremor, myoclonus, and epilepsy. Epilepsia. 2005;46(12):1993–5. [DOI] [PubMed] [Google Scholar]
- 13. Madia F, Striano P, Di Bonaventura C, de Falco A, de Falco FA, Manfredi M, et al. Benign adult familial myoclonic epilepsy (BAFME): evidence of an extended founder haplotype on chromosome 2p11.1‐q12.2 in five Italian families. Neurogenetics. 2008;9(2):139–42. [DOI] [PubMed] [Google Scholar]
- 14. Saint‐Martin C, Bouteiller D, Stevanin G, Popescu C, Charon C, Ruberg M, et al. Refinement of the 2p11.1‐q12.2 locus responsible for cortical tremor associated with epilepsy and exclusion of candidate genes. Neurogenetics. 2008;9(1):69–71. [DOI] [PubMed] [Google Scholar]
- 15. Licchetta L, Pippucci T, Bisulli F, Cantalupo G, Magini P, Alvisi L, et al. A novel pedigree with familial cortical myoclonic tremor and epilepsy (FCMTE): clinical characterization, refinement of the FCMTE2 locus, and confirmation of a founder haplotype. Epilepsia. 2013;54(7):1298–306. [DOI] [PubMed] [Google Scholar]
- 16. Crompton DE, Sadleir LG, Bromhead CJ, Bahlo M, Bellows ST, Arsov T, et al. Familial adult myoclonic epilepsy: recognition of mild phenotypes and refinement of the 2q locus. Arch Neurol. 2012;69(4):474–81. [DOI] [PubMed] [Google Scholar]
- 17. Henden L, Freytag S, Afawi Z, Baldassari S, Berkovic SF, Bisulli F, et al. Identity by descent fine mapping of familial adult myoclonus epilepsy (FAME) to 2p11.2‐2q11.2. Hum Genet. 2016;135(10):1117–25. [DOI] [PubMed] [Google Scholar]
- 18. Depienne C, Magnin E, Bouteiller D, Stevanin G, Saint‐Martin C, Vidailhet M, et al. Familial cortical myoclonic tremor with epilepsy: the third locus (FCMTE3) maps to 5p. Neurology. 2010;74(24):2000–3. [DOI] [PubMed] [Google Scholar]
- 19. Li J, Hu X, Chen Q, Zhang Y, Zhang Y, Hu G. A Chinese benign adult familial myoclonic epilepsy pedigree suggesting linkage to chromosome 5p15.31–p15.1. Cell Biochem Biophys. 2014;69(3):627–31. [DOI] [PubMed] [Google Scholar]
- 20. Liu C, Sun W, Chen Q, Li J, Hu G. Genetic analysis of a Chinese family provides further evidence for linkage of familial cortical myoclonic tremor with epilepsy to 5p15.31‐p15. Neurol India. 2015;63(2):215–9. [DOI] [PubMed] [Google Scholar]
- 21. van Rootselaar A‐F, Groffen AJ, de Vries B, Callenbach PMC, Santen GWE, Koelewijn S, et al. δ‐Catenin (CTNND2) missense mutation in familial cortical myoclonic tremor and epilepsy. Neurology. 2017;89(23):2341–50. [DOI] [PubMed] [Google Scholar]
- 22. Yeetong P, Ausavarat S, Bhidayasiri R, Piravej K, Pasutharnchat N, Desudchit T, et al. A newly identified locus for benign adult familial myoclonic epilepsy on chromosome 3q26.32‐3q28. Eur J Hum Genet. 2013;21(2):225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Fusco M, Vago R, Striano P, Di Bonaventura C, Zara F, Mei D, et al. The α2B ‐adrenergic receptor is mutant in cortical myoclonus and epilepsy. Ann Neurol. 2013;75(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato T, Tamiya G, Koyama S, Nakamura T, Makino S, Arawaka S, et al. UBR5 gene mutation is associated with familial adult myoclonic epilepsy in a Japanese family. ISRN Neurol. 2012;2012:508308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao L, Li L, Ye J, Zhu X, Shen N, Zhang X, et al. Identification of a novel mutation in PLA2G6 gene in a Chinese pedigree with familial cortical myoclonic tremor with epilepsy. Seizure. 2016;41:81–5. [DOI] [PubMed] [Google Scholar]
- 26. Pan S, Li X, Li L, Lin H, Wang D, Zhang X, et al. Comprehensive genetic, clinical and electrophysiological studies of familial cortical myoclonic tremor with epilepsy 1 highlight the role of gene configurations. Seizure. 2021;87:69–74. [DOI] [PubMed] [Google Scholar]
- 27. Martí‐Massó JF, Bergareche A, Makarov V, Ruiz‐Martinez J, Gorostidi A, de Munain AL, et al. The ACMSD gene, involved in tryptophan metabolism, is mutated in a family with cortical myoclonus, epilepsy, and parkinsonism. J Mol Med (Berl). 2013;91(12):1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Russell JF, Steckley JL, Coppola G, Hahn AFG, Howard MA, Kornberg Z, et al. Familial cortical myoclonus with a mutation in NOL3. Ann Neurol. 2012;72(2):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stogmann E, Reinthaler E, Eltawil S, El Etribi MA, Hemeda M, El Nahhas N, et al. Autosomal recessive cortical myoclonic tremor and epilepsy: association with a mutation in the potassium channel associated gene CNTN2. Brain. 2013;136(Pt 4):1155–60. [DOI] [PubMed] [Google Scholar]
- 30. Corbett MA, Kroes T, Veneziano L, Bennett MF, Florian R, Schneider AL, et al. Intronic ATTTC repeat expansions in STARD7 in familial adult myoclonic epilepsy linked to chromosome 2. Nat Commun. 2019;10(1):4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Florian RT, Kraft F, Leitão E, Kaya S, Klebe S, Magnin E, et al. Unstable TTTTA/TTTCA expansions in MARCH6 are associated with familial adult myoclonic epilepsy type 3. Nat Commun. 2019;10(1):4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeetong P, Pongpanich M, Srichomthong C, Assawapitaksakul A, Shotelersuk V, Tantirukdham N, et al. TTTCA repeat insertions in an intron of YEATS2 in benign adult familial myoclonic epilepsy type 4. Brain. 2019;142:3360–6. [DOI] [PubMed] [Google Scholar]
- 33. Cen Z, Jiang Z, Chen Y, Zheng X, Xie F, Yang X, et al. Intronic pentanucleotide TTTCA repeat insertion in the SAMD12 gene causes familial cortical myoclonic tremor with epilepsy type 1. Brain. 2018;141(8):2280–8. [DOI] [PubMed] [Google Scholar]
- 34. Cen Z, Chen Y, Yang D, Zhu Q, Chen S, Chen X, et al. Intronic (TTTGA)n insertion in SAMD12 also causes familial cortical myoclonic tremor with epilepsy. Mov Disord. 2019;34(10):1571–6. [DOI] [PubMed] [Google Scholar]
- 35. Lei XX, Liu Q, Lu Q, Huang Y, Zhou XQ, Sun HY, et al. TTTCA repeat expansion causes familial cortical myoclonic tremor with epilepsy. Eur J Neurol. 2019;26(3):513–8. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Xiong W, Lu L, Zhou D. Familial cortical myoclonic tremor with epilepsy: TTTCA/TTTTA repeat expansions and expanding phenotype in two Chinese families. Brain Res. 2020;1737:146796. [DOI] [PubMed] [Google Scholar]
- 37. Liu C, Song Y, Yuan Y, Peng Y, Pang N, Duan R, et al. TTTCA repeat expansion of SAMD12 in a new benign adult familial myoclonic epilepsy pedigree. Front Neurol. 2020;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou Y, Sood R, Wang Q, Carrington B, Park M, Young AC, et al. Clinical and genomic analysis of a large Chinese family with familial cortical myoclonic tremor with epilepsy and SAMD12 intronic repeat expansion. Epilepsia Open. 2021;6(1):102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terasaki A, Nakamura M, Urata Y, Hiwatashi H, Yokoyama I, Yasuda T, et al. DNA analysis of benign adult familial myoclonic epilepsy reveals associations between the pathogenic TTTCA repeat insertion in SAMD12 and the nonpathogenic TTTTA repeat expansion in TNRC6A. J Hum Genet. 2020;66:419–29. [DOI] [PubMed] [Google Scholar]
- 40. Mahadevan R, Bhoyar RC, Viswanathan N, Rajagopal RE, Essaki B, Suroliya V, et al. Genomic analysis of patients in a South Indian Community with autosomal dominant cortical tremor, myoclonus and epilepsy suggests a founder repeat expansion mutation in the SAMD12 gene. Brain Commun. 2021;3:fcaa214. 10.1093/braincomms/fcaa214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bennett MF, Oliver KL, Regan BM, Bellows ST, Schneider AL, Rafehi H, et al. Familial adult myoclonic epilepsy type 1 SAMD12 TTTCA repeat expansion arose 17,000 years ago and is present in Sri Lankan and Indian families. Eur J Hum Genet. 2020;28:973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yeetong P, Chunharas C, Pongpanich M, Bennett MF, Srichomthong C, Pasutharnchat N, et al. Founder effect of the TTTCA repeat insertions in SAMD12 causing BAFME1. Eur J Hum Genet. 2021;29(2):343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mizuguchi T, Toyota T, Miyatake S, Mitsuhashi S, Doi H, Kudo Y, et al. Complete sequencing of expanded SAMD12 repeats by long‐read sequencing and Cas9‐mediated enrichment. Brain. 2021;144(4):1103–17. [DOI] [PubMed] [Google Scholar]
- 44. Maroilley T, Tsai M‐H, Mascarenhas R, Diao C, Khanbabaei M, Kaya S, et al. A novel FAME1 repeat configuration in a European family identified using a combined genomics approach. Epilepsia Open. 2023;1–7. 10.1002/epi4.12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ibañez K, Polke J, Hagelstrom RT, Dolzhenko E, Pasko D, Thomas ERA, et al. Whole genome sequencing for the diagnosis of neurological repeat expansion disorders in the UK: a retrospective diagnostic accuracy and prospective clinical validation study. Lancet Neurol. 2022;21(3):234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mizuguchi T, Toyota T, Adachi H, Miyake N, Matsumoto N, Miyatake S. Detecting a long insertion variant in SAMD12 by SMRT sequencing: implications of long‐read whole‐genome sequencing for repeat expansion diseases. J Hum Genet. 2019;64(3):191–7. [DOI] [PubMed] [Google Scholar]
- 47. Loureiro JR, Castro AF, Figueiredo AS, Silveira I. Molecular mechanisms in pentanucleotide repeat diseases. Cell. 2022;11(2):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deng F‐Y, Gong J, Zhang Y‐C, Wang K, Xiao S‐M, Li Y‐N, et al. Absence of linkage to 8q23.3–q24.1 and 2p11.1–q12.2 in a new BAFME pedigree in China: indication of a third locus for BAFME. Epilepsy Res. 2005;65(3):147–52. [DOI] [PubMed] [Google Scholar]
- 49. Long L, Qin X, Song Y, Zhang L, Gai N, Xu L, et al. Genetic analysis in a Chinese BAFME pedigree indicates the possibility of an unidentified locus. Int J Clin Exp Med. 2016;9(3):6575–9. [Google Scholar]
- 50. Carr JA, van der Walt PE, Nakayama J, Fu Y‐H, Corfield V, Brink P, et al. FAME 3: a novel form of progressive myoclonus and epilepsy. Neurology. 2007;68(17):1382–9. [DOI] [PubMed] [Google Scholar]
- 51. Depienne C, van den Maagdenberg AMJM, Kühnel T, Ishiura H, Corbett MA, Tsuji S. Insights into familial adult myoclonus epilepsy pathogenesis: how the same repeat expansion in six unrelated genes may lead to cortical excitability. Epilepsia. 2023;1–8. 10.1111/epi.17504 [DOI] [PubMed] [Google Scholar]


