Abstract
The translocation t(14;18)(q32:q21)/IGH::BCL2 occurs at the pre‐B stage of B‐cell development in the bone marrow and is insufficient for malignant transformation, although it leads to the formation of in situ follicular B‐cell neoplasia (ISFN). Despite that, the translocation is the genetic hallmark of follicular lymphoma (FL), it occurs infrequently in metachronous/synchronous lymphomas, including extranodal marginal zone lymphoma of mucosa‐associated lymphoid tissue (EMZL), mantle cell lymphoma, and Hodgkin's lymphoma. In each of these scenarios, the two lymphomas often appear to be clonally related by analyses of IGH::BCL2 and/or rearranged IG genes. However, it remains largely unknown whether one lymphoma originates from the other or they develop independently. We studied five cases of metachronous EMZL and FL. In four cases, the two lymphomas were clonally related, as shown by identical IGH::BCL2 and/or rearranged IG genes or shared mutations. There were common and unique mutations between the paired EMZL and FL, indicating that they developed independently from a common premalignant cell population, harbouring IGH::BCL2 in three cases. Furthermore, case 1 presented with three metachronous FLs, and all of them originated from a common precursor cell population via divergent evolution. Our findings highlight the multi‐malignant potential of IGH::BCL2‐positive B‐cells. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: BCL2 translocation, follicular lymphoma, EMZL, clonal evolution, mutational profiling
Introduction
The translocation t(14;18)(q32:q21)/IGH::BCL2 occurs as a consequence of erroneous VDJ recombination at the pre‐B stage of B‐cell development in the bone marrow. The translocation causes overexpression of BCL2 but alone is insufficient for malignant transformation. The translocation can be detected by PCR in peripheral blood lymphocytes of healthy individuals, in as high as 60% of those >40 years age [1]. The IGH::BCL2‐positive cells act like reactive B‐cells and are capable of undergoing affinity maturation through the germinal centre (GC) reaction, transiting B‐cell follicles and spreading in peripheral lymphoid tissues. Histologically, these translocation‐positive cells form in situ follicular B‐cell neoplasia (ISFN) [2, 3, 4, 5]. They clonally expand while undergoing the GC reaction and are thus at risk of acquiring genetic changes, partially due to off‐target somatic hypermutation activities.
Only a very small proportion of individuals with IGH::BCL2 will eventually develop a lymphoma, with the majority being follicular lymphoma (FL). However, the IGH::BCL2‐positive cells may have the potential to develop into more than one lymphoma as the majority of transformed FLs do not progress directly from their preceding FL but originate via divergent evolution from their commonly related IGH::BCL2‐positive premalignant cell population [6, 7, 8]. Additionally, cases with FL may present with other synchronous or metachronous lymphoid malignancies, including chronic lymphocytic leukaemia, Hodgkin's lymphoma, extranodal marginal zone lymphoma of the mucosa‐associated lymphoid tissue (EMZL), and histiocytic cell sarcoma [9]. In most of these cases, the clonal relationship between the paired FL and other synchronous/metachronous lymphoid tumour is confirmed, but whether one lymphoma originates from the other or they develop independently remains largely unknown. We investigated five cases of metachronous FL and EMZL by mutation profiling and showed in four cases that the two lymphomas were clonally related but developed independently from a common premalignant B‐cell population.
Materials and methods
Patients and clinical data
The use of archival tissues for research was approved by the ethics committees of the institutions involved.
Case 1 was the subject of a previous study [10]. The patient was a 44‐year‐old female with a 12‐year history of dry mouth, arthritis of both hands, and swelling of the right parotid gland (Table 1). An initial parotid biopsy and a further parotid biopsy 12 months later showed EMZL (Figure 1A). Eighteen months after the first parotid biopsy the patient presented with enlarged cervical lymph nodes (LNs), with excision biopsy showing involvement by EMZL. Twenty‐four months after the initial parotid biopsy, the patient developed generalised lymphadenopathy and hepatosplenomegaly, and splenectomy was performed together with biopsies of mesenteric and inguinal LNs. All specimens showed classic FL (Figure 1B). There were no systemic treatments for the patient between different biopsies. Previous molecular analyses demonstrated that these different lymphomas harboured an identical IGH gene rearrangement and IGH::BCL2 genomic fusion [10]. Previous histological assessment also identified a small intraparotid LN in the second parotid biopsy, which showed FL (Figure 1B).
Table 1.
Summary of clinical and laboratory results of cases with metachronous EMZL and FL.
| Site biopsy | Diagnosis | Histopathology | Immunophenotype | Molecular data | |
|---|---|---|---|---|---|
| Case 1: A 44‐year‐old female with 12‐year history of Sjögren's syndrome | |||||
| Parotid (E‐bx) | EMZL | Both parotid gland biopsies show diffuse infiltration of centrocyte‐like cells with prominent lymphoepithelial lesions. The neck LN biopsy displays total effacement of its architecture by diffuse centrocyte‐like cells, with remnants of B‐cell follicles | Identical and being: CD20+, CD5−, CD10‐, IgD‐IgM+, Igκ+, Igλ−, BCL2+ | IGH::BCL2 positive by PCR | These different lymphomas harbour an identical IGH gene rearrangement and show both common and distinct mutations. |
| Parotid (E‐bx) (12 months later) | |||||
| Neck LN (E‐bx) (18 months later) | |||||
| Intraparotid LN, Mesenteric LN, spleen (24 months later) (all E‐bx) | FL1‐2 | All these specimens show effacement of normal lymphoid architecture by closely packed follicles with poorly formed mantle | Identical and being: CD20+, CD5−, CD10+, IgD−, IgM+, Igκ+, Igλ−BCL2+ | IGH::BCL2 with same fusion sequence as above | |
| Case 2: A 57‐year‐old female with acute small intestinal obstruction | |||||
| Small intestine (ileum) (resection) | EMZL | Diffuse infiltration of small lymphoid cells predominantly in submucosa between B‐cell follicles, with focal invasion to mucosa, and plasmacytoid differentiation | CD20+, CD5−, CD10−, BCL6−, CD23−, CD138−, IgD−, IgM−, Igκ−, Igλ+, BCL2+, Cyclin D1− | BCL2 trans+ve by FISH | These different lymphomas show an identical clonal pattern with IGK (tube B) and both common and distinct mutations. |
| Peritoneal LN (41 months later) (C‐bx) | FL1‐2 | Predominantly small lymphoid cells with vaguely nodular growth pattern over FDC meshworks | CD20+, CD79a+, CD10+, BCL6+, CD5−, CD23−, IgD−, Igκ−, Igλ+, BCL2+, CyclinD1− | BCL2 trans+ve by FISH | |
| Case 3: A 58‐year‐old male with dysphagia | |||||
| Stomach (mucosal bx) | EMZL | Dense infiltrates of small to intermediate‐sized lymphoid cells around glands extending to lamina propria forming lymphoepithelial lesions | CD19+, CD20+, CD5−, CD10−, BCL6−, CD43−, IgD+, IgM+, Igκ−, Igλ+, Cyclin D1− | MALT1 trans−ve & BCL6 trans−ve by FISH | Both lymphomas show common and distinct mutations. |
| Left neck LN (26 months later) (C‐bx) | FL3A | Lymph node architecture effaced by vaguely nodular proliferation of predominantly large lymphoid cells over FDC meshworks | CD19+, CD20+, CD10+, BCL6+, Igκ−, Igλ+, BCL2+ | BCL2 trans−ve and BCL6 trans−ve by FISH | |
| Case 4: A 55‐year‐old male with bilateral FL of conjunctivae | |||||
| Gastric biopsy (mucosal bx) | EMZL | Dense lymphoid infiltrate in mucosa with lymphoepithelial lesions | CD20+, CD79a+, CD10−, BCL6−, CD5−, CD23−, IgD−, IgM−, Igκ+, Igλ−, Cyclin D1− | BCL2 trans+ve by FISH | All lesions share numerous common mutations, with FL further harbouring subclonal genetic changes. |
| Left axillary LN (8 days later) (E‐bx) | ISFN | Enlarged node with no specific diagnostic features | Strong BCL2+ in germinal centre B‐cells of several follicles | BCL2 trans+ve by FISH | |
| Right buttock soft tissue (17 days later) (C‐bx) | FL1‐2 | Dense lymphoid infiltrate, partially nodular, predominantly medium‐sized lymphoid cells with ovoid to centrocytoid nuclei | CD20+, CD79a+, CD10+, BCL6+, IgD−, IgM+, Igκ+, Igλ−, BCL2+ | BCL2 trans+ve by FISH | |
| Case 5: An 82‐year‐old male | |||||
| Right thigh subcutaneous mass (C‐bx) | EMZL | Sheets of small to medium‐sized lymphoid cells with ovoid, angulated nuclei, and moderate amount of cytoplasm | CD20+, CD10‐, BCL6−, CD5−, CD23−, Igκ+, Igλ−, BCL2+, Cyclin D1− | n/a | The two lymphomas show different IGH rearrangements and distinct mutations without any shared changes. |
| Right inguinal LN (48 months later) (C‐bx) | FL3A | Vague nodules of medium‐sized to large lymphoid cells, predominantly centrocytes | CD20+, CD79a+, PAX5+, CD10+, BCL6 variably+, CD5−, CD23−, BCL2+, Cyclin D1− | BCL2 trans+ve and BCL6 unbalanced trans+ve by FISH | |
C‐bx, needle core biopsy; E‐bx, excision biopsy; n/a, not available; trans+ve, translocation positive; trans−ve, translocation negative.
Figure 1.
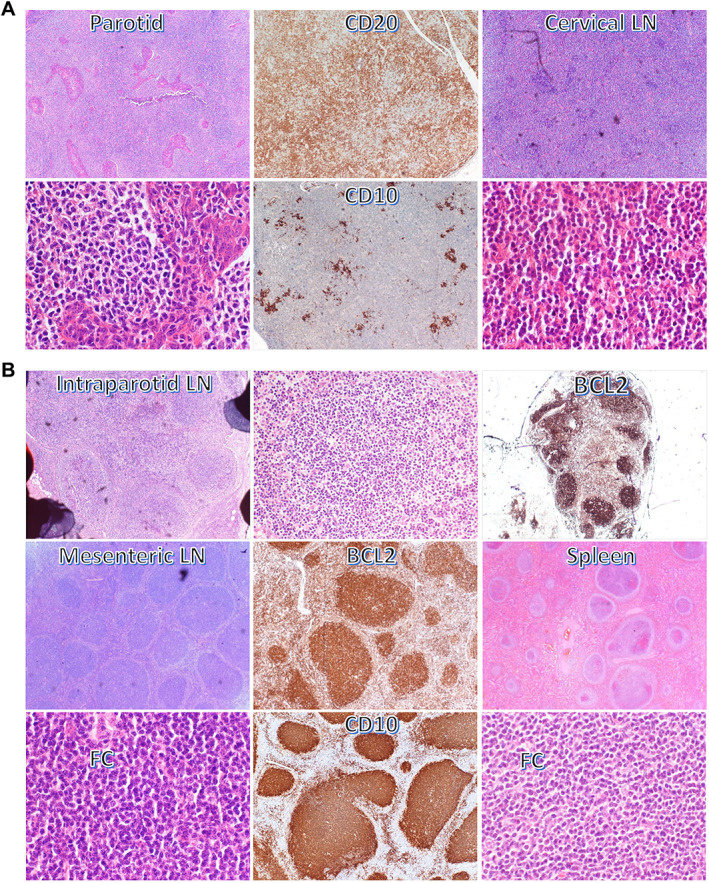
Histological and immunophenotypic features of EMZL and FL in case 1. (A) Parotid biopsies and cervical lymph node (LN) show typical features of EMZL. (B) Intraparotid LN shows strong BCL2 expression in GC B‐cells, loss of zonal polarity, and attenuated mantle zone, while mesenteric LN and spleen display classic features of FL. FC: follicle centre.
Case 2: A 57‐year‐old female presented with acute small intestinal obstruction, and resection of the involved small intestine showed EMZL (Table 1). The patient was then treated with four cycles of rituximab to complete metabolic remission. Forty‐one months later, the patient had peritoneal deposits, and a biopsy of peritoneal LN displayed FL (grade 1–2). Interphase fluorescence in situ hybridisation (FISH) analyses demonstrated BCL2 translocation in the peritoneal LN. BIOMED clonality analyses showed an identically sized clonal product with IGK‐B between the two lymphomas. The patient was treated with obinutuzimab‐bendamustine but unfortunately died of COVID after two cycles of the treatment.
Case 3: A 58‐year‐old male complaining of dysphagia and gastric biopsy showed EMZL (Table 1). The patient was treated with Helicobacter pylori eradication, then local radiotherapy (a total of 24 grey in 12 fractions), and achieved complete remission. Twenty‐six months later, the patient developed enlarged neck LN and tonsils. A LN biopsy showed FL (grade 3A). Interphase FISH showed no evidence of BCL2 and BCL6 translocation. He was treated with six cycles of rituximab‐bendamustine, achieved complete remission, and then underwent 12 cycles of rituximab maintenance.
Case 4: A 55‐year‐old male originally had bilateral FL of the conjunctivae and was treated with local radiation therapy to the left eye (right eye not treated as asymptomatic; Table 1). An endoscopy of the gastrointestinal tract was performed to investigate his anaemia, and histological examination of the biopsies showed gastric EMZL. Due to progressive disease with splenomegaly and lymphadenopathy above and below the diaphragm, further biopsies were performed. A biopsy of the left enlarged axillary LN revealed ISFN, and a further biopsy of a right buttock soft tissue mass showed FL. Retrospective analyses of these biopsies by interphase FISH showed BCL2 translocation. There were no treatments for this patient between the different biopsies.
Case 5: An 88‐year‐old male had a right thigh subcutaneous mass, and an excision biopsy showed EMZL (Table 1). Forty‐eight months later, the patient developed enlarged right inguinal LN, and a biopsy showed FL grade 3A with both BCL2 and BCL6 (unbalanced) translocations by interphase FISH.
Genetic investigations
Please refer to Supplementary materials and methods for details.
Additional interphase FISH for BCL2 translocation and clonality analysis of the rearranged IG genes were performed if not done in routine histological diagnosis.
Tumour cell‐rich areas (>30%) from formalin‐fixed paraffin‐embedded tissue sections were microdissected and subjected to DNA extraction and next‐generation sequencing (NGS) of 278 lymphoma genes [11, 12].
Results and discussion
The histopathology, immunophenotype, BCL2 translocation, and IG gene rearrangement data are summarised in Table 1. Using mutational analysis of the paired EMZL and FL through NGS of 278 genes, it was possible to depict their evolutionary history in each case (Figure 2, Table S1, Figures S1 & S2).
Figure 2.
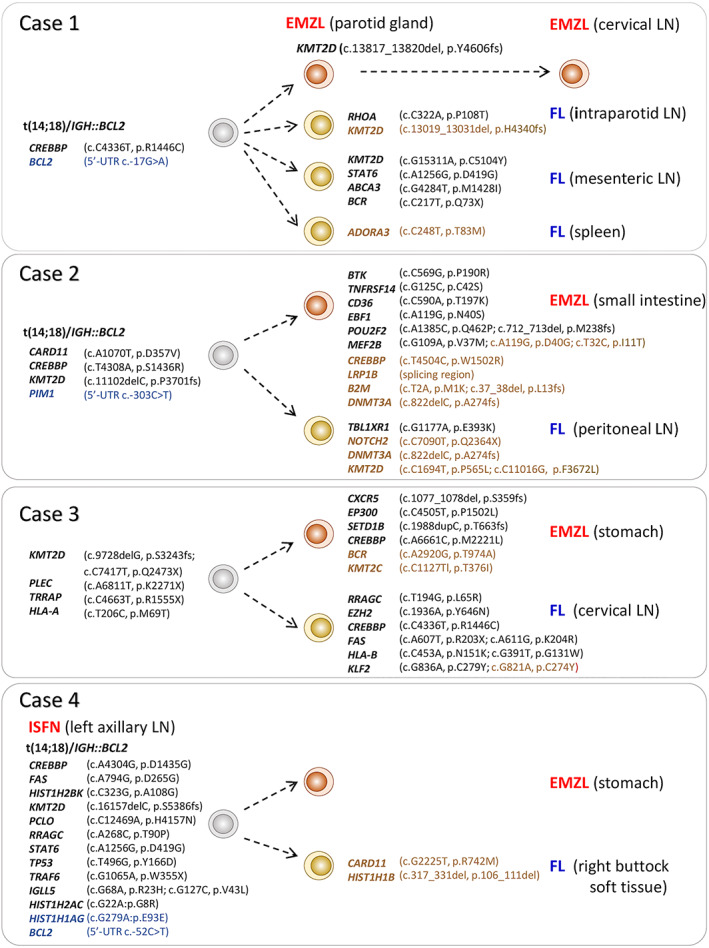
Divergent evolution of metachronous EMZL and FL. All somatic genetic changes, including variants in 5’‐untranslated region (UTR), are included in the phylogenetic illustration. Synonymous changes are shown in blue text, subclonal changes in brown. LN: lymph node; ISFN: in situ follicular B‐cell neoplasia.
In Case 1, all EMZLs of the parotid glands and cervical LN and FLs of the intraparotid LN in the second parotid biopsy, mesenteric LN, and spleen shared one common CREBBP mutation and a BCL2 variant (Figure 2) but harboured variable numbers of distinct variants. All three EMZL biopsies harboured a unique KMT2D mutation but showed no mutation difference among themselves despite their marked intervals (12–18 months). In contrast, all three FLs showed a distinct mutation profile with two and four unique clonal mutations in the intraparotid LN of the second parotid biopsy and mesenteric LN respectively, but no further clonal mutation in FL of the spleen.
We reanalysed the rearranged IGH gene sequence from the previous study [10]. The CDR3 sequence (ARNGSHFDY) harboured an N‐glycosylation site, a common finding in FL, while the use of IGHV3‐7*01F and a short CDR3 length are features of rheumatoid factor [13]. It is highly likely that the BCR expressed by the lymphoma cells is autoreactive, and this may cause chronic BCR signalling, thereby contributing to lymphomagenesis.
In Case 2, the EMZL and FL shared four common clonal somatic variants and also harboured seven and one unique clonal mutations respectively (Figure 2). Similarly, in Case 3, the EMZL and FL displayed five common mutations, but four and eight unique clonal variants respectively (Figure 2). In Case 4, the EMZL and FL shared 14 somatic variants, which were also detected in the left axillary LN containing ISFN lesions (Figure 2). Interestingly, there was no major difference in the mutation profile between the EMZL and FL, with the exception of two subclonal changes (CARD11 c.G2225T, p.R742M; HIST1H1B c.317_331del, p.106_111del) exclusively in the FL specimen.
In contrast, Case 5 lacked any shared mutations between the EMZL and FL (Supplementary material, Figure S1). This, together with the analyses of the rearranged IG genes, showed that the two lymphomas were clonally unrelated.
The common and unique mutation patterns between EMZL and FL in Cases 1–4 clearly indicate that these different lymphomas developed independently from a common premalignant B‐cell population, with evidence of BCL2 translocation in three cases. Such a finding on divergent evolution cannot be extrapolated by routine analyses of BCL2 translocation and IG gene rearrangements, although this provides information on a clonal relationship.
Cases 1–3 also showed subclonal mutations in both EMZL and FL. A proportion of these subclonal changes involved the genes, such as KMT2D, CREBBP, MEF2B, and KLF2, which are affected by the off‐target activities of somatic hypermutation machinery [14, 15]. It is possible that these subclonal changes were acquired during clonal expansion of FL lymphoma cells in the B‐cell follicle or follicular colonisation by EMZL cells.
Apart from the presence of IGH::BCL2, the mutations seen in FL among the cases investigated showed a classic mutation profile of FL, involving epigenetic regulators (KMT2D, CREBBP, EZH2) and mTORC1 (RRAGC) and JAK/STAT (STAT6) signalling. It is unclear what determines that a B‐cell carrying a BCL2 translocation, the genetic hallmark of FL, will follow the trajectory of EMZL development. There were no further mutations in EZML within this study, which involved genes with well‐established roles in marginal zone B‐cell differentiation. Of note, there were no, or few additional, mutations detected in the EMZL of Cases 1 and 4, although it is not possible to exclude the possibility of other rare genetic changes beyond the 278 lymphoma genes investigated. Nonetheless, studies of ISFN show that BCL2 translocation‐positive B‐cells can actively transit B‐cell follicles, migrating from one LN to another [2]. While transiting, the BCL2 translocation‐positive B‐cells may encounter a niche that sustains their clonal expansion and transformation outside B‐cell follicles, together with genetic changes. Such niches may include critical microenvironments for relentless BCR stimulation and T‐cell help, which play a crucial role in EMZL development.
A number of previous studies reported metachronous/synchronous FL and other lymphoid malignancies, including Hodgkin's lymphoma [16, 17, 18] (Figure 3). In each of these scenarios, the clonal link between the two different tumours was suggested by analyses of the IGH::BCL2 fusion and/or rearranged IG genes. In one case of synchronous FL and histiocytic sarcoma, mutation analyses demonstrated that the histiocytic sarcoma originated from a common IGH::BCL2‐positive premalignant cell population [19]. Furthermore, BCL2 translocation has also been seen in chronic lymphocytic leukaemia, albeit infrequently [20]. Finally, the majority of transformed FL, i.e. diffuse large B‐cell lymphoma, originated from their commonly related IGH::BCL2 positive premalignant cell population rather than progressing directly from the preceding FL [6, 7, 8, 21]. These findings indicate the plasticity and diverse neoplastic potential of the IGH::BCL2‐positive premalignant cells.
Figure 3.
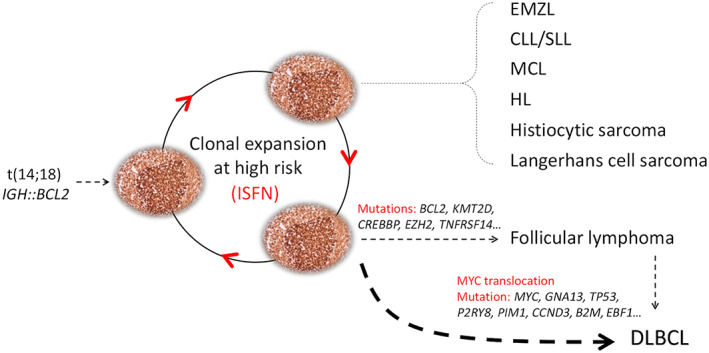
Multi‐malignant potential of IGH:BCL2‐positive cells. IGH::BCL2‐positive B‐cells, like reactive B‐cells, can readily undergo the GC reaction and transit from one B‐cell follicle to another, and thus are at a high risk of acquiring genetic changes, conferring multi‐malignant potential. CLL/SLL: chronic lymphocytic leukaemia/small lymphocytic lymphoma; MCL: mantle cell lymphoma; HL: Hodgkin's lymphoma.
IGH::BCL2 causes constitutive overexpression of BCL2, an inhibitor of apoptosis, and this enables B‐cells to evade apoptosis while undergoing expansion during the GC reaction. IGH::BCL2‐positive B‐cells, like reactive B‐cells, readily undergo antigen affinity maturation and transit from one B‐cell follicle to another. FL may show marginal zone and plasmacytic differentiation and could manifest in the interfollicular region, even leading to a diffuse growth pattern. In this context, the findings of the multiple malignant potential of IGH::BCL2 would be expected given that BCL2 functions as a universal apoptosis inhibitor regardless of cell lineage and differentiation stage and has no recognised role in blocking B‐cell differentiation.
Author contributions statement
MMT, ZC, FC, JM and MQD designed the experiments and collected and analysed the data. AW contributed cases and pathological assessments. MQD, MMT and AW wrote and prepared the manuscript. MQD and AW designed and coordinated the study. All authors commented on the manuscript and approved its submission for publication.
Supporting information
Supplementary materials and methods
Figure S1. Mutational profile of EMZL and FL in case 5
Figure S2. Average depth of reads of all cases analysed. Specimens with suboptimal DNA quantity and/or quality were investigated by targeted NGS in duplicates (referred to in Supplementary materials and methods).
Table S1. Variants detected by targeted next‐generation sequencing
Acknowledgements
We would like to thank Rachel Dobson for her assistance in preparing samples for Illumina sequencing. The research was supported by grants from Blood Cancer UK (19010), Cancer Research UK (C8333/A29707). MMT was supported by a BBSRC DTP PhD studentship (BBSRC BB/M011194/1).
Conflict of interest statement: MQD is an Associate Editor of The Journal of Pathology. No other conflicts of interest were declared.
Data availability statement
All experimental data related to this study are presented in the figures, tables, and supplementary figures and tables of the manuscript.
References
Reference 22 is cited only in the supplementary material.
- 1. Schüler F, Dölken L, Hirt C, et al. Prevalence and frequency of circulating t(14;18)‐MBR translocation carrying cells in healthy individuals. Int J Cancer 2009; 124 : 958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobson R, Wotherspoon A, Liu SA, et al. Widespread in situ follicular neoplasia in patients who subsequently developed follicular lymphoma. J Pathol 2022; 256 : 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vogelsberg A, Steinhilber J, Mankel B, et al. Genetic evolution of in situ follicular neoplasia to aggressive B‐cell lymphoma of germinal center subtype. Haematologica 2021; 106 : 2673–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt J, Ramis‐Zaldivar JE, Bonzheim I, et al. CREBBP gene mutations are frequently detected in in situ follicular neoplasia. Blood 2018; 132 : 2687–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mamessier E, Song JY, Eberle FC, et al. Early lesions of follicular lymphoma: a genetic perspective. Haematologica 2014; 99 : 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okosun J, Bödör C, Wang J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 2014; 46 : 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasqualucci L, Khiabanian H, Fangazio M, et al. Genetics of follicular lymphoma transformation. Cell Rep 2014; 6 : 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouska A, Zhang W, Gong Q, et al. Combined copy number and mutation analysis identifies oncogenic pathways associated with transformation of follicular lymphoma. Leukemia 2017; 31 : 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood 2008; 111 : 5433–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aiello A, Du MQ, Diss TC, et al. Simultaneous phenotypically distinct but clonally identical mucosa‐associated lymphoid tissue and follicular lymphoma in a patient with Sjögren's syndrome. Blood 1999; 94 : 2247–2251. [PubMed] [Google Scholar]
- 11. Wang M, Escudero‐Ibarz L, Moody S, et al. Somatic mutation screening using archival formalin‐fixed, paraffin‐embedded tissues by fluidigm multiplex PCR and illumina sequencing. J Mol Diagn 2015; 17 : 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cucco F, Clipson A, Kennedy H, et al. Mutation screening using formalin‐fixed paraffin‐embedded tissues: a stratified approach according to DNA quality. Lab Invest 2018; 98 : 1084–1092. [DOI] [PubMed] [Google Scholar]
- 13. Bende RJ, Janssen J, Beentjes A, et al. Salivary gland mucosa‐associated lymphoid tissue‐type lymphoma from Sjögren's syndrome patients in the majority express rheumatoid factors affinity‐selected for IgG. Arthritis Rheumatol 2020; 72 : 1330–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye X, Ren W, Liu D, et al. Genome‐wide mutational signatures revealed distinct developmental paths for human B cell lymphomas. J Exp Med 2021; 218 : e20200573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hübschmann D, Kleinheinz K, Wagener R, et al. Mutational mechanisms shaping the coding and noncoding genome of germinal center derived B‐cell lymphomas. Leukemia 2021; 35 : 2002–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamura N, Ohshima K, Abe M, et al. Demonstration of chimeric DNA of bcl‐2 and immunoglobulin heavy chain in follicular lymphoma and subsequent Hodgkin lymphoma from the same patient. J Clin Exp Hematop 2007; 47 : 9–13. [DOI] [PubMed] [Google Scholar]
- 17. Schmitz R, Renné C, Rosenquist R, et al. Insights into the multistep transformation process of lymphomas: IgH‐associated translocations and tumor suppressor gene mutations in clonally related composite Hodgkin's and non‐Hodgkin's lymphomas. Leukemia 2005; 19 : 1452–1458. [DOI] [PubMed] [Google Scholar]
- 18. Bayerl MG, Bentley G, Bellan C, et al. Lacunar and reed‐sternberg‐like cells in follicular lymphomas are clonally related to the centrocytic and centroblastic cells as demonstrated by laser capture microdissection. Am J Clin Pathol 2004; 122 : 858–864. [DOI] [PubMed] [Google Scholar]
- 19. Péricart S, Waysse C, Siegfried A, et al. Subsequent development of histiocytic sarcoma and follicular lymphoma: cytogenetics and next‐generation sequencing analyses provide evidence for transdifferentiation of early common lymphoid precursor‐a case report and review of literature. Virchows Arch 2020; 476 : 609–614. [DOI] [PubMed] [Google Scholar]
- 20. Baseggio L, Geay MO, Gazzo S, et al. In non‐follicular lymphoproliferative disorders, IGH/BCL2‐fusion is not restricted to chronic lymphocytic leukaemia. Br J Haematol 2012; 158 : 489–498. [DOI] [PubMed] [Google Scholar]
- 21. Cucco F, Barrans S, Sha C, et al. Distinct genetic changes reveal evolutionary history and heterogeneous molecular grade of DLBCL with MYC/BCL2 double‐hit. Leukemia 2020; 34 : 1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clipson A, Barrans S, Zeng N, et al. The prognosis of MYC translocation positive diffuse large B‐cell lymphoma depends on the second hit. J Path Clin Res 2015; 1 : 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods
Figure S1. Mutational profile of EMZL and FL in case 5
Figure S2. Average depth of reads of all cases analysed. Specimens with suboptimal DNA quantity and/or quality were investigated by targeted NGS in duplicates (referred to in Supplementary materials and methods).
Table S1. Variants detected by targeted next‐generation sequencing
Data Availability Statement
All experimental data related to this study are presented in the figures, tables, and supplementary figures and tables of the manuscript.


