Summary
Flowers are the complex and highly diverse reproductive structures of angiosperms. Because of their role in sexual reproduction, the evolution of flowers is tightly linked to angiosperm speciation and diversification. Accordingly, the quantification of floral morphological diversity (disparity) among angiosperm subgroups and through time may give important insights into the evolutionary history of angiosperms as a whole.
Based on a comprehensive dataset focusing on 30 characters describing floral structure across angiosperms, we used 1201 extant and 121 fossil flowers to measure floral disparity and explore patterns of floral evolution through time and across lineages.
We found that angiosperms reached their highest floral disparity in the Early Cretaceous. However, decreasing disparity toward the present likely has not precluded the innovation of other complex traits at other morphological levels, which likely played a key role in the outstanding angiosperm species richness.
Angiosperms occupy specific regions of the theoretical morphospace, indicating that only a portion of the possible floral trait combinations is observed in nature. The ANA grade, the magnoliids, and the early‐eudicot grade occupy large areas of the morphospace (higher disparity), whereas nested groups occupy narrower regions (lower disparity).
Keywords: ancestral state reconstruction (ASR), categorical morphospace, Cretaceous, disparity through time, floral evolution, Paleogene
Introduction
Disparity, or morphological diversity, is a quantification of the morphological variation displayed by a group of organisms (Foote, 1992a, 1993a, 1995, 1997; Wills et al., 1994; Ciampaglio et al., 2001; Erwin, 2007; Guillerme et al., 2020; Hopkins & Gerber, 2021). Measures of disparity are based on the multivariate description of morphological traits and are often coupled with the ordination of morphospaces that describe and relate the phenotypic configurations of organisms (Foote, 1994; Mitteroecker & Huttegger, 2009; Smith & Donoghue, 2022). The variation of disparity and morphospace occupation within and among clades, populations, fossil assemblages, or time periods, may provide useful information on a lineage's morphological evolution (Foote, 1994; Benton, 2015), on the evolutionary constraints shaping phenotypes (Ciampaglio, 2002; Allen et al., 2008), on the ecology and natural selection exerted on populations (Benitez‐Vieyra et al., 2010), and on the effect of mass extinction on lineages (Brusatte et al., 2008a; Friedman, 2010; Korn et al., 2013; Puttick et al., 2020). In particular, studying disparity through time (DTT) using fossils gives insight into the evolutionary dynamics of lineages and the general patterns of morphological evolution on Earth (McGhee, 1991; Foote, 1993b; Hughes et al., 2013; Oyston et al., 2016).
Disparity has generally evolved nonuniformly and independently from species richness throughout the history of lineages (Foote, 1994, 1995; Slater et al., 2010; Benton et al., 2014; Moon & Stubbs, 2020; Coombs et al., 2022). Two main trends in the variation of DTT have been observed with respect to when maximal disparity was reached. Most lineages reached maximal disparity early in their evolution (‘early‐disparity’, ‘bottom‐heavy’ distribution: Gould et al., 1987; Gould, 1989; Hughes et al., 2013). These early‐disparity trends result from abrupt and wide morphospace exploration possibly due to the rapid colonization and exploitation of new environments, or of an ecological niche that was left vacant after an extinction event (Foote, 1994; Benton et al., 2014; Benton, 2015; Leslie et al., 2021). Alternatively, new morphologies might accumulate slowly as clades diversify so that disparity might reach its maximal levels late in a clade's evolution (‘late‐disparity’, or ‘top‐heavy’ distribution; Gould et al., 1987; Prentice et al., 2011; Hughes et al., 2013; Puttick et al., 2020). Although disparity variation and morphospace occupation through time have been described for various animal clades (e.g. Foote, 1991 for blastoids; Foote, 1994, 1999 for crinoids; Fortey et al., 1996 for several animal groups; Brusatte et al., 2008b for dinosaurs; Bapst et al., 2012 for graptoloids; Hill et al., 2018 for fishes), only few studies have quantified DTT in angiosperms (e.g. Lupia, 1999; Jardine et al., 2022 for pollen; Oyston et al., 2016; Clark et al., 2023 for general morphology; Martínez‐Cabrera et al., 2017 for wood properties).
Flowers are a relatively recent evolutionary innovation and yet they have evolved remarkable morphological and functional diversity (Endress, 2011; Sauquet et al., 2017, 2022). Because floral morphology is directly linked to angiosperm reproductive success, flowers are under strong selective pressures and studying their morphological diversity is crucial to understand trends of lineage divergence and evolutionary success (Harder & Barrett, 2006; Endress, 2011; van der Niet & Johnson, 2012; Chartier et al., 2014; Sauquet & Magallón, 2018). The diversity of flowers has been extensively discussed qualitatively, based on the development and comparative morphology of various extant groups (Endress & Igersheim, 1997, 1999, 2000; Matthews & Endress, 2002, 2005, 2006; Schönenberger & von Balthazar, 2006; Endress, 2010, 2011; Schönenberger et al., 2010). Based on these studies, it is for example widely recognized that floral organization (Bauplan) is relatively labile among the ANA grade lineages, magnoliids, and early‐diverging eudicots, all of which exhibit, for instance, high variability in merism (organ number) and phyllotaxis (the arrangement of floral organs). By contrast, floral organization is more stable in large clades such as the monocots with almost exclusively trimerous, whorled flowers, or in Pentapetalae with mostly pentamerous, whorled flowers (Endress, 2010, 2011; Sauquet et al., 2017).
The first study using a morphospace approach to analyze floral morphology across angiosperms is the theoretical morphospace created by Stebbins (1951). A theoretical morphospace allows describing the entire spectrum of theoretically possible morphologies for a group of organisms and, therefore, determining limits in the evolution of biological forms and identifying areas of possible high vs low fitness (Raup & Michelson, 1965; Raup, 1967; McGhee, 1991, 2015; Avena‐Koenigsberger et al., 2015). Using this approach, Stebbins determined a set of floral trait combinations that were unlikely to occur in angiosperms and others that were particularly successful (common; Stebbins, 1951). A later quantitative re‐analysis of Stebbins' dataset confirmed the qualitative observations of plant morphologists that the highest floral disparity is present in the ANA grade, magnoliids, and early‐diverging eudicots, whereas the lowest disparity was found in the nested clades such as lamiids, campanulids, and malvids (Chartier et al., 2014). The shortcoming of these two studies is that Stebbins' original dataset only included eight binary floral characters and was coded at the family level based on a now largely outdated classification of angiosperms. The study of floral evolution and diversity during the last decades and the reconstruction of ancestral floral morphologies (e.g. Sauquet et al., 2017) allow us to study disparity and morphological trajectories using unprecedently large datasets through time at the level of angiosperms as a whole. In addition, the recent description of numerous fossil flowers provides information about past floral diversification (e.g. Crepet et al., 1991; Endress, 2006; Endress & Doyle, 2009; Friis et al., 2010, 2011; Doyle & Endress, 2014) and makes it possible to study DTT in angiosperms. Most fossil flowers stem from Cretaceous sediments and include predominantly three‐dimensionally, well‐preserved, charcoalified mesofossils (e.g. Schönenberger & Friis, 2001; Gandolfo et al., 2004; Friis & Pedersen, 2011, 2012) while a few are preserved as permineralizations (e.g. Atkinson et al., 2015), impressions/compressions (e.g. Dilcher & Crane, 1984; Mohr & Eklund, 2004), and amber inclusions (e.g. Gandolfo et al., 2018).
In this study, we use an extensive morphological dataset describing characters at the level of floral organization (Bauplan; sensu Endress, 1994). We include most of the best‐preserved fossil flowers known and a broad representation of living species to investigate the large‐scale temporal and phylogenetic patterns in the evolution of angiosperm flowers. First, we quantify and describe the changes in disparity and morphospace occupation through geologic time. Second, we explore the position of living species (split into 11 taxonomic groups) in a theoretical floral morphospace, and test for the correlation between disparity, clade age, and clade species richness in these groups.
Materials and Methods
All analyses were performed in R v.4.1.2 (R Core Team, 2022; Supporting Information Dataset S1). In the following, function names and packages are referred to as function name {package name}.
Analyses overview
We performed three different analyses. For each analysis, a distance matrix (see Distance matrices in the Materials and Methods section) containing the taxa of interest (see Morphological dataset in the Materials and Methods section) was computed and then used to calculate disparity (see Estimating disparity in the Materials and Methods section), then an ordination was generated to visualize the morphospace (see Ordination in the Materials and Methods section).
Disparity through time
We compared disparity and morphospace occupation among four stratigraphic time bins: the Early Cretaceous (145–100.5 Ma), the Late Cretaceous (100.5–66 Ma), the Paleogene (66–23.03 Ma; Cohen et al., 2013), and living species (present time). Detailed morphological descriptions were only available for two species from the Neogene (23.03–5.333 Ma; Castañeda‐Posadas & Cevallos‐Ferriz, 2007; Hernández‐Damián et al., 2018; Cohen et al., 2013); Neogene fossils were thus excluded from the statistical analyses.
Angiosperm trajectory in the morphospace
We placed ancestral flowers reconstructed for the crown nodes of 15 key angiosperm clades (see Ancestral state reconstructions in the Materials and Methods section) in the floral morphospace and compared their position with that of living and fossil angiosperms.
Disparity among living angiosperms
We compared disparity and morphospace occupation among living angiosperms split into 11 groups (including clades and grades) and tested for the correlation among disparity, age, and species richness for these groups. We split species into the following six clades: magnoliids, commelinids, fabids, malvids, campanulids, and lamiids (sensu APG IV, 2016), and five grades: ANA (Amborellales, Nymphaeales, and Austrobaileyales), other monocots (all monocot lineages except commelinids), other eudicots (Ranunculales, Proteales, Trochodendrales, and Buxales), other superrosids (Saxifragales and Vitales), and other superasterids (Santalales, Berberopsidales, Caryophyllales, Cornales, and Ericales; Dataset S2). The orders Chloranthales, Ceratophyllales, Gunnerales, and Dilleniales were excluded from the statistical analyses as they contained only two to four species in our sampling and because they cannot be assigned to any previously specified group.
Morphological dataset
The dataset includes 30 categorical floral characters scored for 1201 living angiosperm species (36 030 matrix cells) sampled from all currently accepted families from APG IV (2016), as well as 121 fossil flowers (3630 matrix cells), which represented most of the best‐preserved fossil flowers described to date. The final data matrix used in analyses and the complete list of fossils and information on their stratigraphic age, mode of preservation, locality, and corresponding references are available in Dataset S2.
Morphological data and references were recorded in the PROTEUS database (Sauquet, 2019). One character describes the whole flower, eight describe the perianth, 11 the androecium, eight the gynoecium, and two the pollen (Sauquet et al., 2017; Schönenberger et al., 2020). Data for the 1201 living species were previously scored by López‐Martínez et al. (2023). Data for 24 of the 121 fossil species were obtained from Schönenberger et al. (2020) and López‐Martínez et al. (2023), and 97 fossils were additionally scored for this study. A complete extraction of PROTEUS data for the fossil dataset (2442 data records linked to 125 explicit references) is provided as Dataset S3 and the dataset used here is provided as Dataset S2.
The proportion of missing data, including inapplicable characters, is 32% (11 573 matrix cells) for the living species (Fig. S1) and 36% (1305 matrix cells) for the fossils (Fig. S2). Additionally, there were 3% (1310 matrix cells) polymorphic entries for the living species and 2% (74 matrix cells) for the fossils. As most of our analyses do not support polymorphic data, we randomly sampled one of the polymorphic states (with equal probabilities) for each polymorphic entry before each analysis following Chartier et al. (2017). Our results did not change among these analyses.
Theoretical combinations
To visualize the distribution of achieved morphologies among possible ones in the ordinations, we added a background of theoretical combinations to the combinations displayed by the sampled angiosperm species (empirical data). Such an approach is helpful for categorical data like ours, where the nature of the space is discrete rather than continuous (Gerber, 2019). The theoretical morphospace contains all combinations of traits that can be obtained from the study character set. In our data, the total number of possible combinations is the product of the number of states for each character: 217 × 38 × 43 × 52 = 1.38 × 1012. To display a subset of the theoretical morphospace in the ordinations, we randomly sampled 2000 of these theoretical combinations without replacement each time we created an ordination. We did not sample theoretical combinations containing inapplicable or impossible combinations of character states (e.g. perianth merism in flowers without perianth); in these cases, the inapplicable character state was treated as missing data. Inapplicable and impossible combinations are listed in Methods S1.
Distance matrices
For each analysis, a distance matrix was computed by calculating the mean character difference (here noted D) for each pair of taxa following Sneath & Sokal (1973 p. 135) and Foote (1999). The mean character difference is a version of the Gower index, suited for datasets like ours containing categorical ordered and categorical unordered characters. It ranges from zero (no difference) to 1 (largest difference in the dataset). Its calculation is detailed in Methods S2.
Estimating disparity
Disparity was estimated for each group (clade, grade, or pool of species belonging to the same time bin) based on four metrics capturing different aspects of morphospace occupation:
The mean pairwise difference (here meanD) reflects the density of taxa within a group; it is the mean character difference (D, stored in the distance matrix) averaged among each pair of taxa (Foote, 1999). This metric is moderately sensitive to sample size (Ciampaglio et al., 2001), and since the data present large differences in sample size in some instances (e.g. 22 species in the Paleogene vs 1201 extant angiosperms), we additionally rarefied it. For the rarefaction analysis, n species were randomly sampled without replacement in each group (with n the size of the smallest group minus one); then, the distance matrix was computed and meanD calculated. This was repeated 1000 times.
The range (here noted R) gives information about the size of a group in the morphospace (Foote, 1992b); we calculated it as the maximum pairwise distance (maxD) in a group. The range is sensitive to sample size (Ciampaglio et al., 2001), and we thus rarefied it as described above (we only compared rarefied values).
MeanD, rarefied meanD, and rarefied R were compared among groups with Kruskal–Wallis tests for nonparametric data (Kruskal & Wallis, 1952) using the functions kruskal {agricolae} (de Mendiburu & Yaseen, 2020) and kruskalmc {pgirmess} (Giraudoux et al., 2018).
We also computed a measure of the contribution of a group to total disparity (here noted Ddelta; Foote, 1993a). We measured Ddelta for group i (Ddelta i ), as the difference between meanD for the dataset without group i, and meanD for the total dataset (Dtot). When using meanD as an estimate of disparity, a positive value of Ddelta i indicates that adding group i contributed to an increase in Dtot, while a negative value of Ddelta i indicates that adding group i contributed to a decrease in Dtot. The magnitude of this contribution is proportional to the absolute value of Ddelta i .
Finally, to detect the living species with the most distinct floral combinations, we estimated the divergence of each species from the average morphology (we called it eccentricity following Oyston et al., 2015) by averaging D for each living species in the dataset. This was done by averaging each line of the distance matrix. Species with the highest eccentricity are placed at the edge of the occupied morphospace ordination.
Ordination
We visualized morphospaces with nonmetric multidimensional scaling (nMDS) using the function metaMDS {vegan} (Oksanen et al., 2020), setting the number of dimensions (k) to two and the maximum number of random starts (trymax) to 20 (default value). For each group (time bin or taxonomic group), the position of the centroid was added to the graphs by averaging the coordinates of each point for each axis. Floral morphologies were compared among groups with a nonparametric multivariate analysis of variance (npMANOVA) with the function adonis2 {vegan} (Oksanen et al., 2020). Post hoc tests consisted of pairwise npMANOVAs with a Bonferroni correction.
Correlations
For living species, we investigated the correlations between disparity (meanD) and species richness of clades/grades (extracted from the World Flora Online; WFO, 2023), between meanD and clade age (crown age for six clades from Ramírez‐Barahona et al., 2020), and between meanD and Ddelta. Finally, we verified that sample size was positively correlated with species richness. We used Pearson correlation tests using the function cor.test {vegan} (Oksanen et al., 2020).
Ancestral state reconstructions
We employed maximum likelihood (ML) and stochastic character mapping (SM) methods to reconstruct ancestral states for each floral trait at 15 angiosperm key nodes corresponding to broadly recognized major angiosperm clades (sensu Cantino et al., 2007; APG IV, 2016). All ancestral state reconstructions (ASR) were conducted using the maximum clade credibility dated tree from the relaxed calibration complete (RC‐complete) strategy from Ramírez‐Barahona et al. (2020). Maximum likelihood reconstructions were performed implementing the equal rates (ER) and all rates different (ARD) models of discrete morphological evolution with the function rayDISC {corHMM} (Beaulieu et al., 2013; Dataset S4). We subsequently compared model fit based on the Akaike information criterion. SM reconstructions were then conducted using the best‐fitted model obtained with ML. For SM analyses, we first dropped the tips with missing data, nonapplicable, and polymorphic data from the tree using the function drop.tip {phytools} (Revell, 2012). We then inferred ancestral states using 500 simulations along the tree with the function make.simmap {phytools} (Revell, 2012) and summarized with the function describe.simmap {phytools} (Revell, 2012). Dropped tips were removed from the trees showing ASR with SM (Dataset S5). To display ASR in the morphospace, we included the most probable combinations of ancestral states for each node in the dataset before computing the distance matrix, according to Gerber (2019).
Results
Morphospace and disparity through time
Fossil floral disparity significantly differed among the four time‐bins (Kruskal–Wallis test: χ2 = 207.29, df = 3, P = < 2.2e−16; Fig. 1b). Angiosperm flowers showed the highest disparity in the Early Cretaceous (meanD = 0.372 ± SD 0.14) followed by a decrease toward the Late Cretaceous (meanD = 0.316 ± 0.143) and the Paleogene (meanD = 0.241 ± 0.13). Disparity for living species reached values intermediate between Late Cretaceous and Paleogene (meanD = 0.29 ± 0.137; Fig. 1b). This pattern was confirmed by the rarefaction analyses of meanD (Fig. S3a) and of R, with the exception of the Paleogene group showing a range as high as in the Early Cretaceous (Fig. S3b). Note that this pattern applies to angiosperms as a whole and that disparity within subgroups might vary differently (Fig. S3c–e).
Fig. 1.
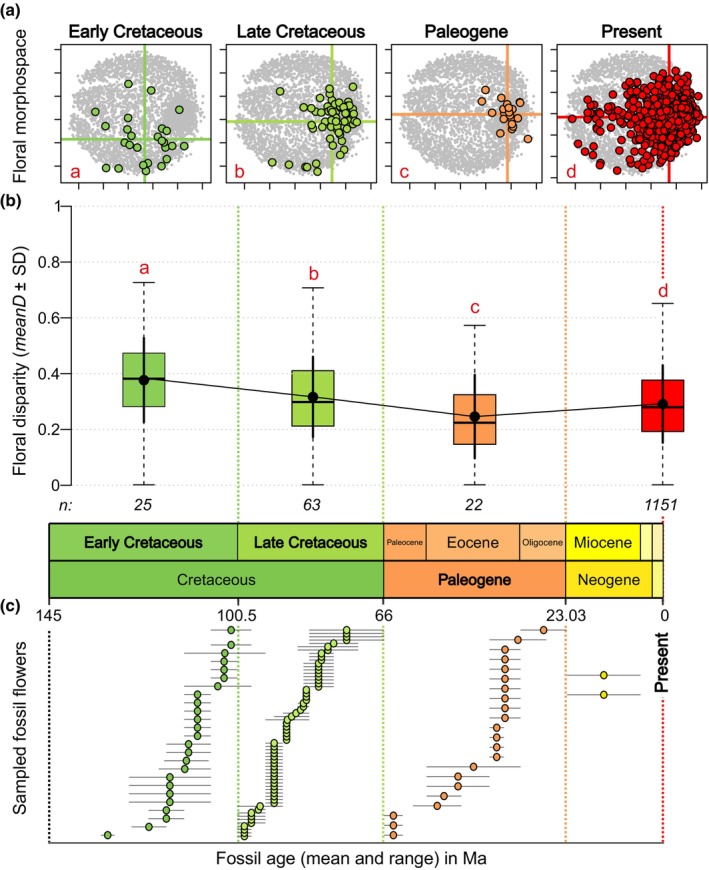
Evolution of the floral morphospace and floral disparity through time for angiosperms. (a) Evolution of morphospace occupation through time (nMDS stress value = 0.34; Shepard plot: nonmetric fit r 2 = 0.88, linear fit r 2 = 0.39). Colored lines indicate the position of the centroid for each group. In (b), black dots, disparity (meanD) for each time bin. SD, standard deviation (black error bars). Boxplots, distribution of D for each group. n, sample sizes after computing the distance matrix. In (a) and (b), red letters indicate post hoc test results, groups with a different letter significantly differ from each other. (c) Stratigraphic age ranges for each fossil flower. The position of Neogene fossils in the morphospace is presented in Supporting Information Fig. S4.
The morphospace area occupied by fossil floral morphologies significantly varied in time (npMANOVA: F = 21.583 P < 1e−4; Fig. 1a), with the position of the centroid showing a displacement from the middle bottom part to the right middle side of the morphospace (Figs 1a, 2). Although all reconstructed ancestors analyzed here dated from the Jurassic to the Early Cretaceous (Table S1), they followed the same trajectory as the position of the centroid for fossils through time and were mostly restricted to the area densely occupied by living species (Fig. 2).
Fig. 2.
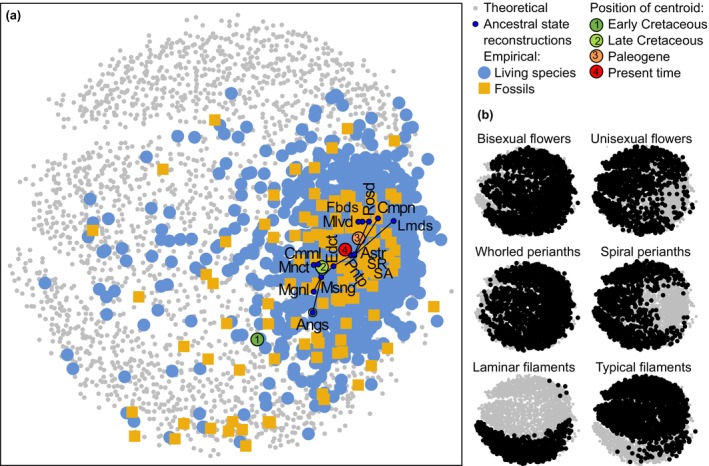
Total floral morphospace of angiosperms (nMDS stress value = 0.34; Shepard plot: nonmetric fit r 2 = 0.88, linear fit r 2 = 0.39), including 1151 living species (light blue dots, meanD = 0.29, R = 1), 113 fossils (yellow squares, meanD = 0.34, R = 1), 15 ancestral state reconstructions (ASR; stochastic character mapping (SM) analyses, navy blue dots, meanD = 0.16, R = 0.35) and 2000 theoretical combinations (gray dots), displayed in (a). Angs, angiosperms; Astr, asterids; Cmml, commelinids; Cmpn, campanulids; Edct, eudicots; Fabd, fabids; Lamd, lamiids; Mgnl, magnoliids; Mlvd, malvids; Mnct, monocots; Msng, mesangiosperms; Pntp, Pentapetalae; Rosd, rosids; SA, superasterids; SR, superrosids. Links among ancestors illustrate phylogenetic relationships. In (b), distribution of some selected character states (black dots) in the same space (gray dots; see also Supporting Information Fig. S5). The values for meanD and R given here are not rarefied. Note the round shape of the theoretical space ordination, similar to the ordinations of other theoretical spaces of categorical nature (e.g. Gerber, 2019).
Morphospace and disparity for living angiosperms
Ninety‐five percent of living species occupied a restricted area of the morphospace ordination (as a rough indication, the corresponding convex hull covered 36% of the total morphospace; Fig. S6) and the remaining 5% of the living species with the highest eccentricity were distributed in otherwise largely empty areas of the theoretical morphospace (the corresponding convex hull covered 60% of the total morphospace; Fig. S6).
In our dataset, 45% of living species had bisexual flowers with one whorl of sepals and one whorl of petals, a total of 6–10 perianth parts, together with a differentiated style (Fig. S7). By contrast, the floral traits characterizing the empty area of the theoretical morphospace (traits more rarely observed in living species) included, in combination or not: dimerous perianths and androecia with more than two whorls each, diaperturate and inaperturate pollen grains, the absence of a perianth, spiral perianth and androecium phyllotaxis, flap‐valvate and H‐valvate anther dehiscence, fused ovaries, undifferentiated styles, as well as free‐central and laminar placentation (Fig. S7). The five species with the highest eccentricity were as follows: Eupomatia bennetii F.Muell. and E. laurina R.Br. (magnoliids), Cyclanthus bipartitus Poit. ex A.Rich. (other monocots grade), Galbulimima belgraveana (F.Muell.) Sprague (magnoliids), and Sarcandra chloranthoides Gardner (Chloranthales; Fig. S6).
Disparity differed significantly among angiosperm groups (Kruskal–Wallis test: χ2 = 18 392, df = 10, P = < 2.2e−16; Fig. 3b; Table S2). Floral disparity was highest within magnoliids (meanD = 0.378 ± SD 0.16), the ANA grade (meanD = 0.370 ± 0.14), and the other eudicots grade (meanD = 0.334 ± 0.16). These three groups also contributed the most to total disparity (Fig. 4c). The lowest disparity was found in nested clades within the Pentapetalae clade, such as campanulids (meanD = 0.158 ± 0.09) and lamiids (meanD = 0.12 ± 0.08; Fig. 3b). These two groups contributed the least to total disparity (Fig. 4c). Rarefied meanD and rarefied R showed similar results (Fig. S8).
Fig. 3.
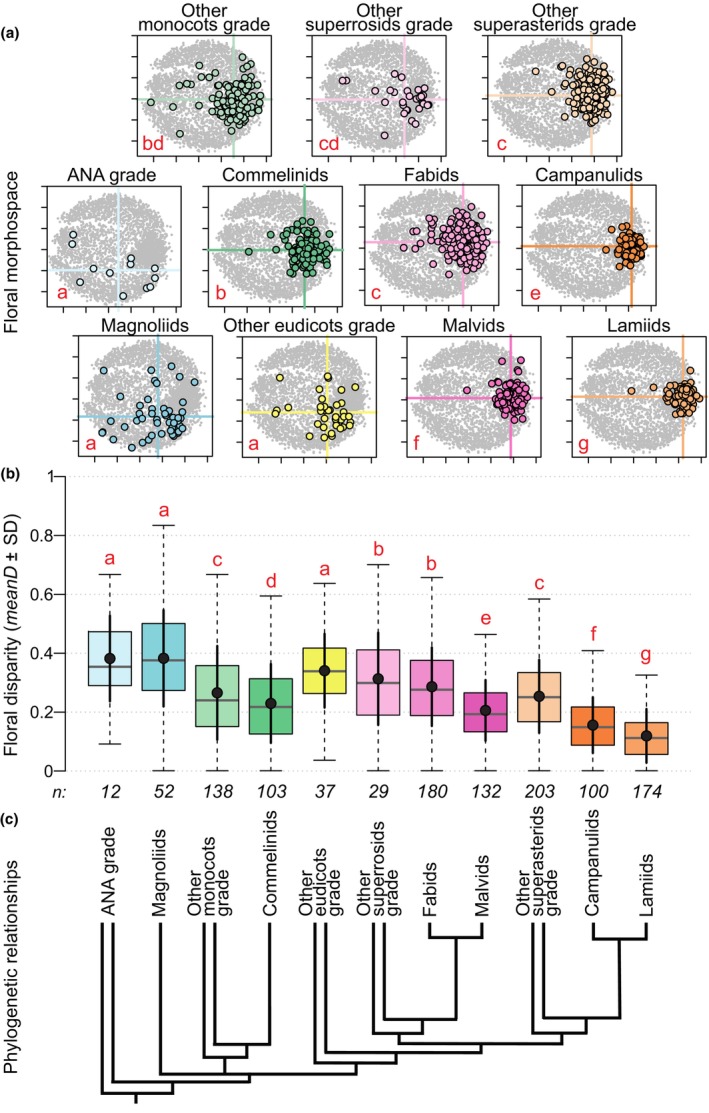
Floral morphospace and disparity for living angiosperms. (a) Morphospace occupation for 11 angiosperm groups (nMDS stress value = 0.34; Shepard plot: nonmetric fit r 2 = 0.88, linear fit r 2 = 0.38). In each plot, colored dots = species belonging to the group of interest, gray dots = 2000 theoretical combinations and the remaining angiosperm species; the intersection of the colored lines indicates the position of the centroid of each group. (b) Disparity (meanD, black dots) ± standard deviation (black vertical plain lines). Boxplots show the distribution of pairwise distances (D) for each group, numbers below each boxplot correspond to sample size after computing the distance matrix. In (a) and (b), red letters indicate post hoc test results, groups with a different letter significantly differ from each other. (c) Phylogenetic relationships among major angiosperm lineages following Ramírez‐Barahona et al. (2020). The position of Chloranthales, Ceratophyllales, Gunnerales, and Dilleniales in this morphospace is presented in Supporting Information Fig. S9.
Fig. 4.
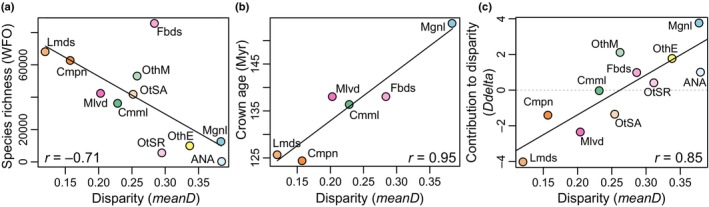
Correlations between disparity (meanD) and (a) species richness, (b) crown node age for six clades, (c) contribution of a group to total disparity (Ddelta). Black lines: linear regressions. r = Pearson's product‐moment correlation coefficient. ANA, ANA grade; Cmml, commelinids; Cmpn, campanulids; Fbds, fabids; Lmds, lamiids; Mgnl, magnoliids; Mlvd, malvids; Myr, million years ago; OthE, the other eudicots grade; OthM, the other monocots grade; OtSA, the other superasterids grade; OtSR, the other superrosids grade; WFO, World Flora Online (WFO, 2023).
The position of living clades and grades in the space varied significantly (npMANOVA: F = 41.78, r 2 = 0.23, P < 1e−04; Fig. 3a), meaning that each group presented specific combinations of floral traits. Exceptions (nonsignificantly different groups) were as follows: (1) the ANA grade, magnoliids, and the other eudicots grade, (2) commelinids and the other monocots grade, (3) fabids, the other superasterids grade, and the other superrosids grade, (4) the other monocots grade and the other superrosids grade (Fig. 3a).
Correlations
Disparity significantly decreased with species richness (Pearson: t = −3.01, df = 9, P = 0.015; Fig. 4a), with the exception of fabids, which showed medium disparity (meanD = 0.29) and a large number of species (85621). Disparity significantly increased with crown node age (t = 5.80, df = 4, P = 0.004; Fig. 4b) and with the contribution to total disparity (t = 4.84, df = 9, P < 1e−3; Fig. 4c). Sample size was positively correlated with species richness (t = 4.69, df = 9, P = 0.001; Fig. S10).
Discussion
Floral disparity through time
Early high levels of disparity are generally characterized by high rates of character change during the early phases of a lineage's history, resulting in maximum phenotypic variation followed by developmental canalization (Foote, 1997, 1999; Wagner, 2018; Smith & Donoghue, 2022). As we show here, angiosperm flowers also followed this pattern, reaching their maximal disparity in the Early Cretaceous, meaning that major structural variations (Bauplan, e.g. fusion, number, and arrangement of organs) were explored early in the history of the group. Interestingly, this occurred when angiosperm species richness was still low compared with other land plants (Lupia et al., 1999) and the group was still mainly restricted to the paleotropics (Willis & McElwain, 2002). It remains uncertain whether the initial floral morphological diversification was associated with the exploitation of ecological opportunities, for example by the interaction with dispersers and pollinators. Insect pollination, for instance, existed before the origin of angiosperms (Peñalver et al., 2015; Asar et al., 2022; Peña‐Kairath et al., 2023) and is ancestral for the group as a whole (Friis et al., 1999; Stephens et al., 2023).
Disparity decreased in the Late Cretaceous as the average floral morphology shifted to become more similar to that of extant species (Fig. S11). For example, the higher frequency of undifferentiated free perianths, spiral androecia, basifixed anthers, and valvate anther dehiscence among Early Cretaceous fossils was, from the Late Cretaceous on, replaced by a majority of differentiated and fused perianths, whorled androecia, dorsifixed anthers, and dehiscence by longitudinal slits. A number of these Late Cretaceous flowers also display new traits considered as a step toward a further adaptation to animal pollination, like perianth differentiation, a lower number of floral organs, nectaries, and fusion of carpels (syncarpy; Friis et al., 2006). This timing is consistent with the diversification of various pollinating insect clades (Asar et al., 2022; Benton et al., 2022; Kawahara et al., 2023; Peña‐Kairath et al., 2023), the emergence (stem age) of most extant angiosperm families (Ramírez‐Barahona et al., 2020), and the progressive dominance of angiosperms in terrestrial ecosystems (Lupia et al., 1999; Willis & McElwain, 2002; Augusto et al., 2014; Condamine et al., 2020).
We also observe a decrease in floral disparity following the Cretaceous/Paleogene (K/Pg) extinction event. Although fossil‐based quantitative analyses have suggested that the K/Pg extinction was highly selective to certain clades (e.g. Laurales and Cyclanthaceae; McElwain & Punyasena, 2007) and regions (e.g. tropical South America; Carvalho et al., 2021), there is no consensus about the impact of the K/Pg extinction on angiosperms as a whole (Willis & McElwain, 2002; Cascales‐Miñana & Cleal, 2014; Thompson & Ramírez‐Barahona., 2023; Wilf et al., 2023), and it remains uncertain whether it had an effect on floral disparity as Paleogene floral fossils are unevenly distributed with regard to phylogeny, geography, and preservation types (Friis et al., 2011; Xing et al., 2016). In our data, the morphospace area covered by these fossils is restricted, and the area emptied during the Paleogene gets occupied again in the Present, confirming a possible sample bias in the Paleogene record. The Paleogene was characterized by profound global landscape changes with the emergence of dense subtropical forests with closed canopies (Carvalho et al., 2021) and the diversification (crown age) of most extant angiosperm families (Ramírez‐Barahona et al., 2020). Despite their lower disparity, Paleogene flowers show a higher frequency of bilateral symmetry (zygomorphy) and perianth fusion, which might indicate higher rates of specialization to different functional groups of pollinators (e.g. Stewart et al., 2022).
Interestingly, angiosperm pollen disparity also rapidly increased from the Early Cretaceous (Lupia, 1999) but the highest pollen disparity in the Late Cretaceous was followed by a stabilization of the morphospace due to the accumulation of different morphologies. The same pattern was estimated for Asterales pollen (Jardine et al., 2022). Conversely, Oyston et al. (2016) found that the overall disparity of vegetative and reproductive characters remained constant throughout the angiosperm's history. By contrast, a study on wood traits (Martínez‐Cabrera et al., 2017) found low initial disparity followed by an increase from the Late Cretaceous on, concomitant with the increased domination of land ecosystems by angiosperms, most probably due to vegetative key innovations leading to more adaptive growth strategies (Feild et al., 2011; Condamine et al., 2020). The different evolutionary trends of disparity variation between flowers, pollen, and vegetative traits probably reflect differences in function, selective value, and evolutionary potential of these traits. Knowing more about these different components would allow getting a more comprehensive understanding of the incredible success of angiosperms throughout their evolutionary history.
Limits in the evolution of floral form
Most extant flowers are restricted to a small area of the morphospace (Fig. S6): 91% of the living species have flowers with a perianth, 85% are bisexual, 83% are whorled, and 72% have a differentiated perianth (Fig. S7). In terms of trait combinations, the most common flower is bisexual, with a differentiated perianth arranged in two whorls (45% of living species in our dataset). These traits characterize Pentapetalae, the angiosperm clade containing > 70% of extant species (Magallon et al., 1999; Christenhusz & Byng, 2016). For the flower, the higher occupation of some areas of the morphospace can be attributed to the economy of construction and/or to functional advantages (Stebbins, 1951; Endress, 1982). For example, bisexuality (85% of the living species in our dataset) increases pollination probability (function) since pollinators can deposit and take up pollen in a single flower visit; a differentiated perianth (72% of the living species in our dataset) allows sepals to protect the developing flower while petals attract pollinators at anthesis (function); a perianth with two whorls of organs (71% of the living species in our dataset) that generally alternate allows for optimal organization (economy of construction); finally, the presence of a low number of united carpels (55% of the living species in our dataset) requires a smaller amount of wall tissue during growth (economy of construction) and enables more regular pollen tube distribution among carpels (function; Endress, 1982), increasing the probability for fertilization (Armbruster et al., 2002). Some trait combinations, like the presence of a corolla with zygomorphy and a small number of stamens (20% of the living species in our dataset), increase speciation rates (O'Meara et al., 2016) as they give a selective advantage for pollination (function) by ensuring a more efficient placement of pollen on pollinator's bodies (Walker‐Larsen & Harder, 2000; Sargent, 2004).
The distribution of living species in the floral morphospace and the quantification of disparity follow a phylogenetic pattern and support conclusions from qualitative studies (e.g. Endress & Igersheim, 1997, 1999, 2000; Matthews & Endress, 2002, 2005, 2006; Schönenberger & von Balthazar, 2006; Endress, 2010, 2011). These results are consistent with the re‐analysis of Stebbin's dataset by Chartier et al. (2014): we found the highest floral disparity in early‐diverging and species‐poor grades and clades, and the lowest disparity in highly nested and species‐rich clades (Fig. 3).
The ANA grade, magnoliids, and the other eudicots grade display a broad range of floral trait combinations appearing widely spread in the morphospace. In addition, most species with the highest eccentricity belong to these groups (Fig. S11). It is expected for grades to show more variability than clades per definition. Old clades are also known to present more variable floral traits. For instance, floral phyllotaxis is particularly labile in members of the ANA grade and magnoliids (Endress & Doyle, 2009), where whorled and spiral phyllotaxis often coexist at shallow phylogenetic levels, and merism is highly variable within early‐diverging angiosperms and members of the other eudicots grade (Endress, 2011). The most eccentric species often show a marked reduction of organs, such as perianth loss, allowing the arrangement and number of stamens and carpels to become highly labile (e.g. Eupomatia and Galbulimina in the magnoliids, Euptelea, and Trochodendron in the other eudicots grade, and Cyclanthus in the other monocots grade; Endress, 2011).
Another trend is the stabilization of floral organization within highly nested groups such as malvids, campanulids, and lamiids, which occupy relatively narrow regions in the space and show low levels of disparity coupled with high species richness (Fig. 3). Some floral characters became canalized or fixed within these major clades. For example, floral phyllotaxis is generally whorled and merism became stabilized to pentamery (or tetramery) in Pentapetalae and trimery in commelinids (Endress, 2011). This tendency in angiosperm floral evolution toward fixation of the number and position of organs, and toward repeated evolution of some traits such as zygomorphy (Reyes et al., 2016), is often associated with increased synorganization, that is when organs of the same module (e.g. perianth) or different modules (e.g. androecium and gynoecium) become integrated into architecturally and functionally complex structures (Endress, 2002, 2006, 2016). The lower disparity in floral organization found in nested clades does not preclude, and possibly even has enabled variation in other aspects of floral structure, for instance, traits associated with mechanical properties (e.g. size and proportions) or traits directly related to interactions with pollinators (e.g. organ shape, floral color, and rewards). Such floral traits, which have not been considered in this study, tend to be very labile, even among closely related species (Endress, 1994, 2011).
Conclusion
Our quantification of disparity through time revealed a pattern of highest disparity early during angiosperm floral evolution. Disparity in the Early Cretaceous might have been even higher than our present estimation since numerous plant fossils with reproductive organs possibly belonging to the angiosperms have not been formally described yet (Sauquet & Magallón, 2018). Studies on disparity would greatly benefit from the description of such fossils, even if they cannot be assigned to extant lineages. It seems very surprising that there are hardly any described extinct angiosperm families and orders, which one would expect when considering the long evolutionary history of the group (but see Sun et al., 2002; Pessoa et al., 2023). Other fossils, such as Bevhalstia pebja Hill, 1996 (Friis et al., 2011), do not present enough characters to be added to disparity studies. It also has to be mentioned that the fossil record of angiosperms is geographically biased since most of the currently available specimens stem from the mid‐northern paleolatitudes (Friis et al., 2011; Xing et al., 2016). Studies in extant lineages have shown that floral disparity may vary with latitude and with regional factors (Chartier et al., 2021), and it would be interesting to test whether such patterns are also present in the floral fossil record. However, it is currently not possible to address this question due to the paucity of the floral fossil record from the southern hemisphere. Furthermore, our results illustrate that ASRs are, by nature, conservative. First, while the ages of the crown nodes of the evaluated groups date to the early Cretaceous (or earlier; Ramírez‐Barahona et al., 2020; Table S1), the corresponding ASRs fall within the small morphospace areas occupied by most extant species included in the dataset (Fig. 2). This is discrepant with the documented positive correlation between node age and disparity (Fig. 4), and hence, with our expectation that oldest nodes would occupy morphospace areas outside those occupied by the majority of extant species. Second, while ASRs may document character state combinations that are not present among the original species sample, they are methodologically precluded from estimating character states that are not present in this sample. Third, our results empirically show that fossils document greater floral disparity than ASRs (Fig. 2). Nonetheless, further ASR‐based analyses would complement our approach as they would likely add crucial qualitative and quantitative information on the evolution of the group, through the exploration of morphological rates of evolution through time and through ASR incorporating fossil data. Finally, the analyses of morphospaces for vegetative and for other reproductive characters (e.g., fruits) would achieve an integrative understanding of angiosperm evolution.
Competing interests
None declared.
Author contributions
MC, AML‐M, HS, JS, MvB and SM designed the study. MvB, JS, AML‐M and HS scored and curated the data for fossils. HS provided the scripts for ancestral state reconstructions. MC and AML‐M conducted the analyses. AML‐M and MC wrote the paper. All co‐authors interpreted the results and edited the manuscript.
Supporting information
Dataset S1 Script used to generate the distance matrix, the ordination of the space, and calculate D.
Dataset S2 Morphological dataset, information about scored fossils, and about study groups.
Dataset S3 Proteus extraction used to generate the dataset, containing all references.
Dataset S4 Ancestral state reconstructions for each character obtained with a maximum likelihood approach.
Dataset S5 Ancestral state reconstructions for each character obtained with a stochastic mapping approach.
Fig. S1 Distribution of missing and nonapplicable data for living species.
Fig. S2 Distribution of missing and nonapplicable data for fossil species.
Fig. S3 Rarefied floral disparity (meanD and R) through time.
Fig. S4 Position of Neogene fossils in the floral morphospace (Fig. 1).
Fig. S5 Distribution of character states in the morphospace (Fig. 2).
Fig. S6 Position of eccentric species in the morphospace ordination.
Fig. S7 Distribution of character states in living Angiosperms.
Fig. S8 Rarefied floral disparity (meanD and R) for living angiosperms.
Fig. S9 Position of additional living angiosperm clades in the floral morphospace (Fig. 3).
Fig. S10 Correlation between sample size and group sizes.
Fig. S11 Phylogenetic distribution of species in the four time bins studied and for eccentric species.
Methods S1 List of nonapplicable and impossible combinations.
Methods S2 Calculation of the mean character difference.
Table S1 Divergence time estimates for the 15 ASR.
Table S2 Disparity (meanD) for the study angiosperm group.
Please note: Wiley is not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank the University of Vienna for funding the eFLOWER server hosting the PROTEUS database. We thank S. Ramírez‐Barahona for providing the dated trees used for ASR, and the Instituto de Biología, Proyecto Fronteras de la Ciencia (2016‐01‐1867‐ CONAHCYT to SM), USTIC, Instituto de Biología, D. Velázquez, and A. Wong for the access to the HPC infrastructure where the ASR were conducted. We thank A. Delgado‐Salinas, L. E. Eguiarte, S. Gerber, and three anonymous reviewers for their helpful feedback on earlier drafts. This work was funded by the Consejo Nacional de Humanidades Ciencias y Tecnologías (CONAHCYT) graduate scholarship 708068 to AMLM, by the International Association for Plant Taxonomists (IAPT), by the American Society of Plant Taxonomists (ASPT), and by the Austrian Science Fund (FWF P 250077‐B16 and P 31101‐B29).
Contributor Information
Andrea M. López‐Martínez, Email: andreaea.lopez@gmail.com.
Marion Chartier, Email: marion.chartier@univie.ac.at.
Data availability
Data are available in the Supporting Information.
References
- Allen CE, Beldade P, Zwaan BJ, Brakefield PM. 2008. Differences in the selection response of serially repeated color pattern characters: standing variation, development, and evolution. BMC Evolutionary Biology 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APG IV . 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H. 2002. Comparative analysis of late floral development and mating system evolution in Tribe Collinsieae (Scrophulariaceae SL). American Journal of Botany 89: 37–49. [DOI] [PubMed] [Google Scholar]
- Asar Y, Ho SYW, Sauquet H. 2022. Early diversifications of angiosperms and their insect pollinators: were they unlinked? Trends in Plant Science 27: 858–869. [DOI] [PubMed] [Google Scholar]
- Atkinson BA, Stockey RA, Rothwell GW, Mindell RA, Bolton MJ. 2015. Lauraceous flowers from the Eocene of Vancouver Island: Tinaflora beardiae gen. et sp. nov. (Lauraceae). International Journal of Plant Sciences 176: 567–585. [Google Scholar]
- Augusto L, Davies TJ, Delzon S, De Schrijver A. 2014. The enigma of the rise of angiosperms: can we untie the knot? Ecology Letters 17: 1326–1338. [DOI] [PubMed] [Google Scholar]
- Avena‐Koenigsberger A, Goñi J, Solé R, Sporns O. 2015. Network morphospace. Journal of the Royal Society Interface 12: 20140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapst DW, Bullock P, Melchin MJ, Sheets HD, Mitchell CE. 2012. Graptoloid diversity and disparity became decoupled during the Ordovician mass extinction. Proceedings of the National Academy of Sciences, USA 109: 3428–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, O'Meara BC, Donoghue MJ. 2013. Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Systematic Biology 62: 725–737. [DOI] [PubMed] [Google Scholar]
- Benitez‐Vieyra S, Ordano M, Fornoni J, Boege K, Domınguez CA. 2010. Selection on signal–reward correlation: limits and opportunities to the evolution of deceit in Turnera ulmifolia L. Journal of Evolutionary Biology 23: 2760–2767. [DOI] [PubMed] [Google Scholar]
- Benton MJ. 2015. Exploring macroevolution using modern and fossil data. Proceedings of the Royal Society B: Biological Sciences 282: 20150569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton MJ, Forth J, Langer MC. 2014. Models for the rise of the dinosaurs. Current Biology 24: 87–95. [DOI] [PubMed] [Google Scholar]
- Benton MJ, Wilf P, Sauquet H. 2022. The angiosperm terrestrial revolution and the origins of modern biodiversity. New Phytologist 233: 2017–2035. [DOI] [PubMed] [Google Scholar]
- Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008a. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321: 1485–1488. [DOI] [PubMed] [Google Scholar]
- Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008b. The first 50 Myr of dinosaur evolution: macroevolutionary pattern and morphological disparity. Biology Letters 4: 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantino PD, Doyle JA, Graham SW, Judd WS, Olmstead RG, Soltis DE, Soltis PS, Donoghue MJ. 2007. Towards a phylogenetic nomenclature of Tracheophyta. Taxon 56: E1–E44. [Google Scholar]
- Carvalho M, Jaramillo C, Parra F, Caballero‐Rodríguez D, Herrera F, Wing S, Turner B, D'Apolito C, Romero‐Báez M, Narváez P et al. 2021. Extinction at the end‐Cretaceous and the origin of modern Neotropical rainforests. Science 372: 63–68. [DOI] [PubMed] [Google Scholar]
- Cascales‐Miñana B, Cleal CJ. 2014. The plant fossil record reflects just two great extinction events. Terra Nova 26: 195–200. [Google Scholar]
- Castañeda‐Posadas C, Cevallos‐Ferriz SRS. 2007. Swietenia (Meliaceae) flower in late oligocene‐early miocene amber from Simojovel de Allende, Chiapas, Mexico. American Journal of Botany 94: 1821–1827. [DOI] [PubMed] [Google Scholar]
- Chartier M, von Balthazar M, Sontag S, Löfstrand S, Palme T, Jabbour F, Sauquet H, Schönenberger J. 2021. Global patterns and a latitudinal gradient of flower disparity: perspectives from the angiosperm order Ericales. New Phytologist 230: 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier M, Jabbour F, Gerber S, Mitteroecker P, Sauquet H, von Balthazar M, Staedler Y, Crane PR, Schönenberger J. 2014. The floral morphospace – a modern comparative approach to study angiosperm evolution. New Phytologist 204: 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier M, Löfstrand S, von Balthazar M, Gerber S, Jabbour F, Sauquet H, Schönenberger J. 2017. How (much) do flowers vary? Unbalanced disparity among flower functional modules and a mosaic pattern of morphospace occupation in the order Ericales. Proceedings of the Royal Society B: Biological Sciences 284: e20170066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz MJ, Byng JW. 2016. The number of known plants species in the world and its annual increase. Phytotaxa 261: 201–217. [Google Scholar]
- Ciampaglio CN. 2002. Determining the role that ecological and developmental constraints play in controlling disparity: examples from the crinoid and blastozoan fossil record. Evolution and Development 4: 170–188. [DOI] [PubMed] [Google Scholar]
- Ciampaglio CN, Kemp M, McShea DW. 2001. Detecting changes in morphospace occupation patterns in the fossil record: characterization and analysis of measures of disparity. Paleobiology 27: 695–715. [Google Scholar]
- Clark JW, Hetherington AJ, Morris JL, Pressel S, Duckett JG, Puttick MN, Schneider H, Kenrick P, Wellman CH, Donoghue PC. 2023. Evolution of phenotypic disparity in the plant kingdom. Nature Plants 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen KM, Finney SC, Gibbard PL, Fan JX. 2013; Updated. The ICS International Chronostratigraphic Chart. Episodes 36: 199‐204 . [WWW document] URL https://stratigraphy.org/chart [accessed 21 April 2022].
- Condamine FL, Silvestro D, Koppelbus EB, Antonelli A. 2020. The rise of angiosperms pushed conifers to decline during global cooling. Proceedings of the National Academy of Sciences, USA 117: 28867–28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs EJ, Felice RN, Clavel J, Park T, Bennion RF, Churchill M, Geisler JH, Beatty B, Goswami A. 2022. The tempo of cetacean cranial evolution. Current Biology 32: 2233–2247. [DOI] [PubMed] [Google Scholar]
- Crepet WL, Friis EM, Nixon KC, Lack AJ, Jarzembowski EA. 1991. Fossil evidence for the evolution of biotic pollination. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 333: 187–195. [Google Scholar]
- Dilcher DL, Crane PR. 1984. Archaeanthus: an early angiosperm from the Cenomanian of the Western Interior of North America. Annals of the Missouri Botanical Garden 71: 351–383. [Google Scholar]
- Doyle J, Endress PK. 2014. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: ANITA lines and relatives of Chloranthaceae. International Journal of Plant Sciences 175: 555–600. [Google Scholar]
- Endress PK. 1982. Syncarpy and alternative modes of escaping disadvantages of apocarpy in primitive angiosperms. Taxon 31: 48–52. [Google Scholar]
- Endress PK. 1994. Diversity and evolutionary biology of tropical flowers. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Endress PK. 2002. Origins of flower morphology. Journal of Experimental Zoology 291: 105–115. [DOI] [PubMed] [Google Scholar]
- Endress PK. 2006. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research 44: 1–67. [Google Scholar]
- Endress PK. 2010. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden 97: 541–583. [Google Scholar]
- Endress PK. 2011. Evolutionary diversification of the flowers in angiosperms. American Journal of Botany 98: 370–396. [DOI] [PubMed] [Google Scholar]
- Endress PK. 2016. Development and evolution of extreme synorganization in angiosperm flowers and diversity: a comparison of Apocynaceae and Orchidaceae. Annals of Botany 117: 749–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK, Doyle J. 2009. Reconstructing the ancestral flower and its initial specializations. American Journal of Botany 96: 22–66. [DOI] [PubMed] [Google Scholar]
- Endress PK, Igersheim A. 1997. Gynoecium diversity and systematics of the Laurales. Botanical Journal of the Linnean Society 125: 93–168. [Google Scholar]
- Endress PK, Igersheim A. 1999. Gynoecium diversity and systematics of the basal eudicots. Botanical Journal of the Linnean Society 130: 305–393. [Google Scholar]
- Endress PK, Igersheim A. 2000. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences 161: S211–S223. [DOI] [PubMed] [Google Scholar]
- Erwin DH. 2007. Disparity: morphological pattern and developmental context. Palaeontology 50: 57–73. [Google Scholar]
- Feild TS, Brodribb TJ, Iglesias A, Chatelet DS, Baresch A, Upchurch GR Jr, Gomez B, Mohr BAR, Coiffard C, Kvacek J et al. 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proceedings of the National Academy of Sciences, USA 108: 8363–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote M. 1991. Morphological and taxonomic diversity in clade's history: the blastoid record and stochastic simulations. Contributions from the Museum of Paleontology 28: 101–140. [Google Scholar]
- Foote M. 1992a. Paleozoic record of morphological diversity in blastozoan echinoderms. Proceedings of the National Academy of Sciences, USA 89: 7325–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote M. 1992b. Rarefaction analysis of morphological and taxonomic diversity. Paleobiology 18: 1–16. [Google Scholar]
- Foote M. 1993a. Contributions of individual taxa to overall morphological disparity. Paleobiology 19: 403–419. [Google Scholar]
- Foote M. 1993b. Discordance and concordance between morphological and taxonomic diversity. Paleobiology 19: 185–204. [Google Scholar]
- Foote M. 1994. Morphological disparity in Ordovician‐Devonian crinoids and the early saturation of morphological space. Paleobiology 20: 320–344. [Google Scholar]
- Foote M. 1995. Morphological diversification of Paleozoic Crinoids. Paleobiology 21: 273–299. [Google Scholar]
- Foote M. 1997. The evolution of morphological diversity. Annual Review of Ecology, Evolution, and Systematics 28: 129–152. [Google Scholar]
- Foote M. 1999. Morphological diversity in the evolutionary radiation of Paleozoic and post‐Paleozoic crinoids. Paleobiology 25: 1–115. [Google Scholar]
- Fortey RA, Briggs DEG, Wills MA. 1996. The Cambrian evolutionary ‘explosion’: decoupling cladogenesis from morphological disparity. Biological Journal of the Linnean Society 57: 13–33. [Google Scholar]
- Friedman M. 2010. Explosive morphological diversification of spiny‐finned teleost fishes in the aftermath of the end‐Cretaceous extinction. Proceedings of the Royal Society B: Biological Sciences 277: 1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis EM, Crane PR, Pedersen KR. 2011. Early flowers and angiosperm evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Friis EM, Pedersen KR. 2011. Canrightia resinifera gen. et sp. nov., a new extinct angiosperm with Retimonocolpites‐type pollen from the Early Cretaceous of Portugal: missing link in the eumagnoliid tree? Grana 50: 3–29. [Google Scholar]
- Friis EM, Pedersen KR. 2012. Bertilanthus scanicus, a new asterid flower from the Late Cretaceous (Late Santonian‐Early Campanian) of Scania, Sweden. International Journal of Plant Sciences 173: 318–330. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. 1999. Early angiosperm diversification: the diversity of pollen associated with angiosperm reproductive structures in Early Cretaceous floras from Portugal. Annals of the Missouri Botanical Garden 86: 259–296. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. 2006. Cretaceous angiosperm flowers: innovation and evolution in plant reproduction. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 251–293. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. 2010. Diversity in obscurity: fossil flowers and the early history of angiosperms. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 365: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo MA, Nixon KC, Crepet WL. 2004. Cretaceous flowers of Nymphaeaceae and implications for complex insect entrapment pollination mechanisms in early angiosperms. Proceedings of the National Academy of Sciences, USA 101: 8056–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo MA, Nixon KC, Crepet WL, Grimaldi DA. 2018. A Late Cretaceous fagalean inflorescence preserved in amber from New Jersey. American Journal of Botany 105: 1424–1435. [DOI] [PubMed] [Google Scholar]
- Gerber S. 2019. Use and misuse of discrete character data for morphospace and disparity analyses. Palaeontology 62: 305–319. [Google Scholar]
- Giraudoux P, Antonietti J‐P, Beale C, Pleydell D, Treglia M. 2018. pgirmess: spatial analysis and data mining for field ecologists. R Package v.19 . [WWW document] URL https://github.com/pgiraudoux/pgirmess [accessed 28 March 2023].
- Gould SJ. 1989. Wonderful life. New York, NY, USA: W. W. Norton. [Google Scholar]
- Gould SJ, Gilinsky NL, German RZ. 1987. Asymmetry of lineages and the direction of evolutionary time. Science 236: 1437–1441. [DOI] [PubMed] [Google Scholar]
- Guillerme T, Cooper N, Brusatte SL, Davis KE, Jackson AL, Gerber S, Goswami A, Healy K, Hopkins MJ, Jones MEH et al. 2020. Disparities in the analysis of morphological disparity. Biology Letters 16: 20200199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Barrett SC, eds. 2006. Ecology and evolution of flowers. New York, NY, USA: Oxford University Press. [Google Scholar]
- Hernández‐Damián AL, Gómez‐Acevedo SL, Cevallos‐Ferriz SRS. 2018. Fossil flower of Salacia lombardii sp. nov. (Salacioideae‐Celastraceae) preserved in amber from Simojovel de Allende, Mexico. Review of Palaeobotany and Palynology 252: 1–9. [Google Scholar]
- Hill JJ, Puttick MN, Stubbs TL, Rayfield EJ, Donoghue PC. 2018. Evolution of jaw disparity in fishes. Palaeontology 61: 847–854. [Google Scholar]
- Hopkins MJ, Gerber S. 2021. Morphological disparity. In: Nuno de la Rosa L, Müller G, eds. Evolutionary developmental biology. A reference guide. Cham, Switzerland: Springer, 965–976. [Google Scholar]
- Hughes M, Gerber S, Wills MA. 2013. Clades reach highest morphological disparity early in their evolution. Proceedings of the National Academy of Sciences, USA 110: 13875–13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine PE, Palazzesi L, Tellería MC, Barreda VD. 2022. Why does pollen morphology vary? Evolutionary dynamics and morphospace occupation in the largest angiosperm order (Asterales). New Phytologist 234: 1075–1087. [DOI] [PubMed] [Google Scholar]
- Kawahara AY, Storer C, Carvalho APS, Plotkin DM, Condamine FL, Braga MP, Ellis EA, St Laurent RA, Li X, Barve V et al. 2023. A global phylogeny of butterflies reveals their evolutionary history, ancestral hosts and biogeographic origins. Nature Ecology & Evolution 7: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn D, Hopkins MJ, Walton SA. 2013. Extinction space – a method for the quantification and classification of changes in morphospace across extinction boundaries. Evolution 67: 2795–2810. [DOI] [PubMed] [Google Scholar]
- Kruskal WH, Wallis WA. 1952. Use of ranks in one‐criterion variance analysis. Journal of the American Statistical Association 47: 583–621. [Google Scholar]
- Leslie A, Simpson C, Mander L. 2021. Reproductive innovations and pulsed rise in plant complexity. Science 373: 1368–1372. [DOI] [PubMed] [Google Scholar]
- López‐Martínez AM, Schönenberger J, von Balthazar M, Gónzalez‐Martínez CA, Ramírez‐Barahona S, Sauquet H, Magallón S. 2023. Integrating fossil flowers into the angiosperm phylogeny using molecular and morphological evidence. Systematic Biology 72: 837–855. [DOI] [PubMed] [Google Scholar]
- Lupia R. 1999. Discordant morphological disparity and taxonomic diversity during the Cretaceous angiosperm radiation: North American pollen record. Paleobiology 25: 1–28. [Google Scholar]
- Lupia R, Lidgard S, Crane PR. 1999. Comparing palynological abundance and diversity: implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology 25: 305–340. [Google Scholar]
- Magallon S, Crane PR, Herendeen PS. 1999. Phylogenetic pattern, diversity, and diversification of eudicots. Annals of the Missouri Botanical Garden 86: 297–372. [Google Scholar]
- Martínez‐Cabrera HI, Zheng J, Estrada‐Ruiz E. 2017. Wood functional disparity lags behind taxonomic diversification in angiosperms. Review of Palaeobotany and Palynology 246: 251–257. [Google Scholar]
- Matthews ML, Endress PK. 2002. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Cephalotaceae, Brunelliaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae). Botanical Journal of the Linnean Society 140: 321–381. [Google Scholar]
- Matthews ML, Endress PK. 2005. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae). Botanical Journal of the Linnean Society 149: 129–194. [Google Scholar]
- Matthews ML, Endress PK. 2006. Floral structure and systematics in four orders of rosids, including a broad survey of floral mucilage cells. Plant Systematics and Evolution 260: 223–251. [Google Scholar]
- McElwain JC, Punyasena SW. 2007. Mass extinction events and the plant fossil record. Trends in Ecology & Evolution 22: 548–557. [DOI] [PubMed] [Google Scholar]
- McGhee GR. 1991. Theoretical morphology: the concept and its applications. Short Courses in Paleontology 4: 87–102. [Google Scholar]
- McGhee GR. 2015. Limits in the evolution of biological form a theoretical morphologic perspective. Interface Focus 5: 20150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendiburu F, Yaseen M. 2020. agricolae: statistical procedures for agricultural research. R Package v.10 . [WWW document] URL https://cran.r‐project.org/web/packages/agricolae/index.html [accessed 28 March 2023].
- Mitteroecker P, Huttegger SM. 2009. The concept of morphospaces in evolutionary and developmental biology: mathematics and metaphors. Biological Theory 4: 54–67. [Google Scholar]
- Mohr B, Eklund H. 2004. Araripia florifera, a magnoliids angiosperm from the Lower Cretaceous Crato Formation (Brazil). Review of Palaeobotany and Palynology 126: 279–292. [Google Scholar]
- Moon BC, Stubbs TL. 2020. Early high rates and disparity in the evolution of ichthyosaurs. Communications Biology 3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Niet T, Johnson SD. 2012. Phylogenetic evidence for pollinator‐driven diversification of angiosperms. Trends in Ecology & Evolution 27: 353–361. [DOI] [PubMed] [Google Scholar]
- O'Meara BC, Smith SD, Armbruster SW, Harder LD, Hardy CR, Hileman LC, Hufford L, Litt A, Magallón S, Smith SA et al. 2016. Non‐equilibrium dynamics and floral trait interactions shape extant angiosperm diversity. Proceedings of the Royal Society B: Biological Sciences 283: 20152304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P et al. 2020. vegan: community ecology package. R package v 2.5‐7 . [WWW document] URL https://github.com/vegandevs/vegan [accessed 28 March 2023].
- Oyston JW, Hughes M, Gerber S, Wills MA. 2016. Why should we investigate the morphological disparity of plant clades? Annals of Botany 117: 859–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston JW, Hughes M, Wagner PJ, Gerber S, Wills MA. 2015. What limits the morphological disparity of clades? Interface Focus 5: 20150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña‐Kairath C, Delclòs X, Álvarez‐Parra S, Peñalver E, Engel MS, Ollerton J, Peris D. 2023. Insect pollination in deep time. Trends in Ecology & Evolution 38: 749–759. [DOI] [PubMed] [Google Scholar]
- Peñalver E, Arillo A, Perez‐de la Fuente R, Riccio ML, Delclos X, Barron E, Grimaldi DA. 2015. Long‐proboscid flies as pollinators of Cretaceous gymnosperms. Current Biology 25: 1917–1923. [DOI] [PubMed] [Google Scholar]
- Pessoa EM, Ribeiro AC, Christenhusz MJ. 2023. New evidence on the previously unknown gynoecium of Araripia florifera (Araripiaceae, fam. nov.), a magnoliid angiosperm from the Lower Cretaceous (Aptian) of the Crato Konservat‐Lagerstätte (Araripe Basin), northeastern Brazil. Cretaceous Research 153: 105715. [Google Scholar]
- Prentice KC, Ruta M, Benton MJ. 2011. Evolution of morphological disparity in pterosaurs. Journal of Systematic Palaeontology 9: 337–353. [Google Scholar]
- Puttick MN, Guillerme T, Wills MA. 2020. The complex effects of mass extinctions on morphological disparity. Evolution 74: 2207–2220. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2022. R: a language and environment for statistical computing, v4.1.3. Vienna, Austria: R foundation for Statistical Computing. [WWW document] URL http://www.r‐project.org [accessed 28 March 2023]. [Google Scholar]
- Ramírez‐Barahona S, Sauquet H, Magallón S. 2020. The delayed and geographically heterogeneous diversification of flowering plant families. Nature Ecology & Evolution 4: 1232–1238. [DOI] [PubMed] [Google Scholar]
- Raup DM. 1967. Analysis of shell coiling: coiling in ammonoids. Journal of Paleontology 41: 43–65. [Google Scholar]
- Raup DM, Michelson A. 1965. Theoretical morphology of the coiled shell. Science 147: 1294–1295. [DOI] [PubMed] [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Reyes E, Sauquet H, Nadot S. 2016. Perianth symmetry changed at least 199 times in angiosperm evolution. Taxon 65: 945–964. [Google Scholar]
- Sargent RD. 2004. Floral symmetry affects speciation rates in angiosperms. Proceedings of the Royal Society B: Biological Sciences 271: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauquet H. 2019. PROTEUS: a database for recording morphological data and calibrations. v 1.27 . [WWW document] URL https://eflower.myspecies.info/proteus [accessed 20 April 2022].
- Sauquet H, Magallón S. 2018. Key questions and challenges in angiosperm macroevolution. New Phytologist 219: 1170–1187. [DOI] [PubMed] [Google Scholar]
- Sauquet H, Ramírez‐Barahona S, Magallón S. 2022. What is the age of flowering plants? Journal of Experimental Botany 73: 3840–3853. [DOI] [PubMed] [Google Scholar]
- Sauquet H, von Balthazar M, Magallón S, Doyle JA, Endress PK, Bailes EJ, Barroso de Morais E, Bull‐Hereñu K, Carrive L, Chartier M et al. 2017. The ancestral flower of angiosperms and its early diversification. Nature Communications 8: 16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönenberger J, von Balthazar M, López Martínez A, Albert B, Prieu C, Magallón S, Sauquet H. 2020. Phylogenetic analysis of fossil flowers using an angiosperm‐wide data set: proof‐of‐concept and challenges ahead. American Journal of Botany 107: 1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönenberger J, von Balthazar M, Sytsma KJ. 2010. Diversity and evolution of floral structure among early diverging lineages in the Ericales. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 365: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönenberger J, von Balthazar MV. 2006. Reproductive structures and phylogenetic framework of the rosids‐progress and prospects. Plant Systematics and Evolution 260: 87–106. [Google Scholar]
- Schönenberger J, Friis EM. 2001. Fossil flowers of Ericalean affinity from the Late Cretaceous of Southern Sweden. American Journal of Botany 88: 467–480. [PubMed] [Google Scholar]
- Slater GJ, Price SA, Santini F, Alfaro MF. 2010. Diversity versus disparity in the radiation of modern cetaceans. Proceedings of the Royal Society B: Biological Sciences 277: 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Donoghue PC. 2022. Evolution of fungal phenotypic disparity. Nature Ecology & Evolution 6: 1489–1500. [DOI] [PubMed] [Google Scholar]
- Sneath PH, Sokal RR. 1973. Numerical taxonomy. The principles and practice of numerical classification. San Francisco, CA, USA: W. H. Freeman. [Google Scholar]
- Stebbins GL. 1951. Natural selection and the differentiation of angiosperm families. Evolution 5: 299–324. [Google Scholar]
- Stephens RE, Gallagher RV, Dun L, Cornwell W, Sauquet H. 2023. Insect pollination for most of angiosperm evolutionary history. New Phytologist 240: 880–891. [DOI] [PubMed] [Google Scholar]
- Stewart AB, Diller C, Dudash MR, Fenster CB. 2022. Pollination‐precision hypothesis: support from native honey bees and nectar bats. New Phytologist 235: 1629–1640. [DOI] [PubMed] [Google Scholar]
- Sun G, Ji Q, Dilcher DL, Zheng S, Nixon KC, Wang X. 2002. Archaefructaceae, a new basal angiosperm family. Science 296: 899–904. [DOI] [PubMed] [Google Scholar]
- Thompson J, Ramírez‐Barahona S. 2023. No phylogenetic evidence for angiosperm mass extinction at the Cretaceous – Paleogene (K‐Pg) boundary. Biology Letters 19: 20230314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PJ. 2018. Early burst of disparity and the reorganization of character integration. Proceedings of the Royal Society B: Biological Sciences 285: 20181604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker‐Larsen J, Harder LD. 2000. The evolution of staminodes in angiosperms: patterns of stamen reduction, loss, and functional re‐invention. American Journal of Botany 87: 1367–1384. [PubMed] [Google Scholar]
- WFO . 2023. World flora online . [WWW document] URL http://www.worldfloraonline.org/ [accessed 11 April 2023].
- Wilf P, Carvalho M, Stiles E. 2023. The end‐Cretaceous plant extinction: heterogeneity, ecosystem transformation, and insights for the future. Cambridge Prisms: Extinction 1: 1–10. [Google Scholar]
- Willis KJ, McElwain JC. 2002. The evolution of plants. Oxford, UK: Oxford University Press. [Google Scholar]
- Wills M, Briggs D, Fortey R. 1994. Disparity as an evolutionary index: a comparison of Cambrian and recent arthropods. Paleobiology 20: 93–130. [Google Scholar]
- Xing Y, Gandolfo MA, Onstein RE, Cantrill DJ, Jacobs BF, Jordan GJ, Lee DF, Popova S, Srivastava R, Su T et al. 2016. Testing the biases in the rich Cenozoic angiosperm macrofossil record. International Journal of Plant Sciences 177: 371–388. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset S1 Script used to generate the distance matrix, the ordination of the space, and calculate D.
Dataset S2 Morphological dataset, information about scored fossils, and about study groups.
Dataset S3 Proteus extraction used to generate the dataset, containing all references.
Dataset S4 Ancestral state reconstructions for each character obtained with a maximum likelihood approach.
Dataset S5 Ancestral state reconstructions for each character obtained with a stochastic mapping approach.
Fig. S1 Distribution of missing and nonapplicable data for living species.
Fig. S2 Distribution of missing and nonapplicable data for fossil species.
Fig. S3 Rarefied floral disparity (meanD and R) through time.
Fig. S4 Position of Neogene fossils in the floral morphospace (Fig. 1).
Fig. S5 Distribution of character states in the morphospace (Fig. 2).
Fig. S6 Position of eccentric species in the morphospace ordination.
Fig. S7 Distribution of character states in living Angiosperms.
Fig. S8 Rarefied floral disparity (meanD and R) for living angiosperms.
Fig. S9 Position of additional living angiosperm clades in the floral morphospace (Fig. 3).
Fig. S10 Correlation between sample size and group sizes.
Fig. S11 Phylogenetic distribution of species in the four time bins studied and for eccentric species.
Methods S1 List of nonapplicable and impossible combinations.
Methods S2 Calculation of the mean character difference.
Table S1 Divergence time estimates for the 15 ASR.
Table S2 Disparity (meanD) for the study angiosperm group.
Please note: Wiley is not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
Data are available in the Supporting Information.


