Abstract
Introduction
Smoking cessation in pregnancy remains a public health priority. Our team used the Behaviour Change Wheel to develop the Midwives and Obstetricians Helping Mothers to Quit smoking (MOHMQuit) intervention with health system, leader (including managers and educators) and clinician components. MOHMQuit addresses a critical evidence to practice gap in the provision of smoking cessation support in antenatal care. It involves nine maternity services in New South Wales in a cluster randomised stepped-wedge controlled trial of effectiveness. This paper describes the design and rationale for the process evaluation of MOHMQuit. The process evaluation aims to assess to what extent and how MOHMQuit is being implemented (acceptability; adoption/uptake; appropriateness; feasibility; fidelity; penetration and sustainability), and the context in which it is implemented, in order to support further refinement of MOHMQuit throughout the trial, and aid understanding and interpretation of the results of the trial.
Methods and analysis
The process evaluation is an integral part of the stepped-wedge trial. Its design is underpinned by implementation science frameworks and adopts a mixed methods approach. Quantitative evidence from participating leaders and clinicians in our study will be used to produce individual and site-level descriptive statistics. Qualitative evidence of leaders’ perceptions about the implementation will be collected using semistructured interviews and will be analysed descriptively within-site and thematically across the dataset. The process evaluation will also use publicly available data and observations from the research team implementing MOHMQuit, for example, training logs. These data will be synthesised to provide site-level as well as individual-level implementation outcomes.
Ethics and dissemination
The study received ethical approval from the Population Health Services Research Ethics Committee for NSW, Australia (Reference 2021/ETH00887). Results will be communicated via the study’s steering committee and will also be published in peer-reviewed journals and presented at conferences.
Trial registration number
Australian New Zealand Trials Registry ACTRN12622000167763. https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12622000167763.
Keywords: Implementation Science, Behavior, Primary Health Care, Pregnant Women, Change management, Quality in health care
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The process evaluation has been designed using implementation science frameworks.
The study uses multiple data sources. Qualitative and quantitative data will be collected independently from leaders and clinicians in each Midwives and Obstetricians Helping Mothers to Quit smoking (MOHMQuit) site as well as contextual and publicly available data, and observational data from the research team implementing MOHMQuit.
MOHMQuit is a complex intervention with many moving parts which interact with one another and the stakeholders involved. No process evaluation is able to collect data to understand all aspects of these interactions, particularly not in a ‘real world’ trial such as this one.
Introduction
In 2020, 9.2% of mothers in Australia smoked tobacco at some point during their pregnancy.1 Smoking in pregnancy is associated with a multitude of adverse outcomes for both mother and baby including preterm birth and low birthweight babies.2–5 In Australia, smoking is the most common modifiable risk for adverse pregnancy and birth outcomes6 and therefore supporting pregnant women to stop smoking remains a major public health concern and a priority for the New South Wales (NSW) Ministry of Health.7–9 Clinical guidelines for NSW have existed for almost 20 years and recommend clinicians routinely provide evidence-based smoking cessation support (SCS) at all antenatal care visits for women who smoke or who have stopped smoking in this pregnancy.10 Implementation of the guidelines shows room for improvement.11–14 This fact, along with wider evidence that women want to stop smoking in pregnancy but some lack confidence to do so,15 would value support from their clinicians16 and a systematic review demonstrating that psychosocial interventions helps women to stop smoking,17 led us to develop a theoretically underpinned intervention, Midwives and Obstetricians Helping Mothers to Quit smoking (MOHMQuit) to improve implementation of the NSW Guidelines.
The MOHMQuit intervention
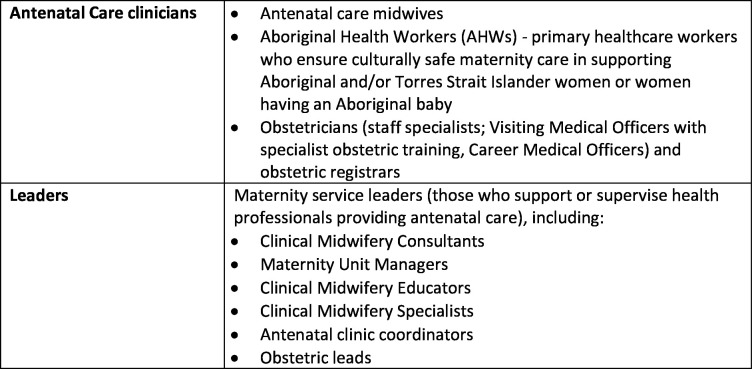
The MOHMQuit intervention has multiple components targeting different parts of a complex health system.18 It is based on the ‘5As’ of SCS: ask, advise, assess, assist and arrange follow-up, which has shown evidence of effectiveness for SCS.19 MOHMQuit was developed using the Behaviour Change Wheel method.20 It is an intervention built on local and international evidence identifying barriers and enablers for health systems, leaders and clinicians providing SCS.21 It focuses on changing behaviours by targeting systems such as the electronic medical record system, leaders and clinicians (see figure 1 for further detail on what is meant by leaders and clinicians). For example, changing clinicians’ behaviours so that they implement the guidelines by asking about smoking and discussing cessation at every antenatal visit, and assisting women by providing behavioural support such as discussing triggers for smoking, managing nicotine cravings and planning a quit attempt. The MOHMQuit trial is an implementation trial using a stepped-wedge design across five local health districts (LHDs) in NSW with diverse characteristics including organisational structure and staffing profiles.
Figure 1.
Description of key participant groups.
The development of the MOHMQuit intervention and its support materials has been described in detail previously.21 22 In brief, there are four main components (also referred to in the implementation science literature as ‘implementation strategies’):
(1) Separate training events for maternity service leaders—half day, midwives and Aboriginal Health Workers—full day and obstetricians—2 hours. Midwifery educators also take part in the leaders’ and midwives’ training events as a ‘train-the-trainer’ model which includes a comprehensive MOHMQuit training manual, and is central to the sustainability of the intervention.
(2) A number of MOHMQuit leadership processes and systems tools, for example, a report template for the electronic medical record system facilitating leaders’ scrutiny of their services’ SCS performance; a service audit tool for leaders.
(3) MOHMQuit written resources such as a booklet on ‘Stopping smoking for you and your baby’ for clinicians to use with women.
(4) A series of 11 short video clips for training and skills development to be used in a wide variety of settings, for example, at handover meetings.
Two months prior to the implementation starting in the first site, a day-long face-to-face gathering was held bringing together key decision-makers and clinicians from across the sites to ensure a shared awareness and understanding of MOHMQuit including its history and rationale, promote enthusiasm, motivation and engagement and establish shared understanding about roles and responsibilities.
At each site, 10 weeks prior to the intervention, the research team and the maternity service leaders will participate in a ‘warm-up’ meeting. While each site has a strong existing connection with MOHMQuit via the face-to-face day, and through the inclusion of partner investigators at each site, the warm-up meeting includes acknowledging and thanking those involved (which extend beyond the site partner investigators and include the antenatal clinic coordinator, the clinical midwifery educator and other leaders), generating enthusiasm, building momentum in the lead up to the implementation of MOHMQuit, and working through the logistics of implementation at each site. Two weeks prior to the intervention, a second meeting will be held which has a ‘trouble-shooting’ agenda and will also include detail of the research elements of MOHMQuit, for example, how and when outcome and process evaluation data from the site will be collected. Additional meetings are planned for 2 and 4 weeks post intervention, to maintain momentum and explore any unresolved issues in the ongoing implementation of MOHMQuit. A MOHMQuit Community of Practice will be established which each site can join following implementation. The Community of Practice will offer a regular forum for sharing and supporting other clinicians and leaders in continuing to implement MOHMQuit and is one of several sustainability features of MOHMQuit. Finally, three and a half months after implementation, each site will receive feedback from brief interviews with women about the SCS they received during their antenatal care. They will continue to receive these reports quarterly until the end of the trial.
MOHMQuit is currently being trialled in a multisite cluster randomised stepped-wedge effectiveness trial in nine sites in publicly funded maternity services in NSW, Australia.23 Implementation is planned to take place over a 13-month timeframe. Unlike many earlier interventions aimed at improving SCS,24 MOHMQuit is built on implementation science frameworks and is specific to the public maternity service setting. The trial will assess the intervention outcomes. The primary intervention outcome is smoking cessation, and secondary intervention outcomes include changes to clinicians’ knowledge, skills, confidence and behaviour in providing SCS and test the ‘mechanisms of action’25–27 by which each of the components affect intervention outcomes and moderators of their impact in this framework-driven approach.23 Cost-effectiveness will be assessed in an economic evaluation.28 The trial will also assess key implementation outcomes (assessing how MOHMQuit was implemented) primarily based on Proctor et al’s implementation science framework29 in a detailed process evaluation. The process evaluation will complement the assessment of the MOHMQuit intervention outcomes. Conducting process evaluation alongside effectiveness trials in this way is recommended.30 31
Aims of the MOHMQuit process evaluation
Process evaluations explore how an intervention is implemented. They assess three aspects: (1) how and to what extent the intervention was implemented; (2) the ‘mechanisms of impact’ that is, how the intervention components and participants’ interactions with these components effected changes in behaviour; and (3) the context in which the intervention was implemented.32 We anticipate that the process evaluation will contribute formatively by providing feedback that may further refine the intervention. This is particularly useful in a stepped-wedge trial design where each site joins the trial sequentially, and acceptable as long as the changes made to components retain the integrity of the function they were meant to perform in the original intervention design.33 34 The summative use of process evaluation is in providing insight into the mechanisms through which the intervention outcomes (the primary intervention outcome being pregnant women stopping smoking) were achieved or not, and therefore it will contribute to understanding and interpreting the results of the effectiveness trial.35 Without this insight effective, and ineffective, aspects of the intervention may not be understood and this has implications for the scale-up of an intervention such as MOHMQuit. In this way, the process evaluation will maximise the knowledge gained throughout the trial and describe the most effective delivery processes for the MOHMQuit intervention. The aim of this protocol paper is to describe the process evaluation planned as an integral part of the MOHMQuit trial.
Methods and analysis
Overall design and objectives of the process evaluation
The design for the process evaluation began with the implementation outcomes defined by Enola Proctor and team in order to facilitate an understanding of the various dimensions of the implementation: acceptability; adoption/uptake; appropriateness; feasibility; fidelity; penetration and sustainability (and sustainment).29 Implementation outcomes are “…the effects of deliberate and purposive actions to implement new…practices”.29 The Proctor implementation outcomes generally map on to other well-used frameworks such as the Reach, Efficacy, Adoption, Implementation, Maintenance (RE-AIM) framework31 but ‘Reach’ from the RE-AIM framework was specifically added into the design as ‘Reach’ captures the number of clinicians and leaders invited to and taking part in the trial. Two other frameworks informed the implementation outcomes of interest: Sekhon36 for acceptability, and Rogers37 for sustainability, appropriateness and feasibility; and Moore35 and Fernandez’38 work guided exploration of mechanisms of impact and how context affected implementation. The context in which the intervention was implemented will also be assessed. Context is variously defined39 but here contextual features are conceived of broadly as those influencing the delivery of the intervention and include the engagement of leaders and the organisational setting and culture of the service in which the intervention is implemented.40
Important features of the process of implementing MOHMQuit were discussed and agreed with a process evaluation working group of the project’s steering committee (a key governance committee of the project and constituted of research academics, policy-makers, managers and leaders21). Subsequently, instruments were developed which encompassed both individual and service-level data collection. Decisions were made regarding the specific foci of the process evaluation, acknowledging that “Process evaluations cannot expect to provide answers to all of the uncertainties of a complex intervention. It is generally better to answer the most important questions well than to try to answer too many questions and do so unsatisfactorily”.35
With that in mind, a focus on fidelity; adoption/uptake; penetration; reach, sustainability and context was agreed. In part, these foci were based on learning from the feasibility and acceptability trial of MOHMQuit.21 In addition, the short duration of the trial (the time from implementation at the first site to the end of data collection, excluding the wash-out period, is 24 months and from the final site, only 8 months) would make sustainment challenging to measure. Sustainability is, however, included in the evaluation. Sustainment is “…the continued use of a practice that is the target of the implementation, whereas sustainability addresses whether the factors are in place to promote the ongoing use”.41
The process evaluation has three interrelated objectives; to, at both the individual and site levels, assess
To what extent MOHMQuit was implemented—measured quantitatively focusing on the implementation outcomes of adoption, fidelity, penetration, reach and sustainability, and will also involve qualitative measures (interviews with leaders).
How changes in behaviour were effected (the mechanisms of impact)—measured quantitatively focusing on the implementation outcomes of acceptability, appropriateness and feasibility, and a more nuanced understanding of this from leaders’ perspectives in qualitative interviews.
-
The impact of context (moderators) on the implementation of MOHMQuit. A moderator is a factor that will strengthen or lessen the influence of a strategy to implement MOHMQuit.26 We anticipate a number of moderators will be an important part of the context for MOHMQuit implementation, as well as intervention outcomes, affecting the relationship between the implementation outcomes, for example, reach, and the implementation of MOHMQuit. The moderators measured include
-
Leadership
-
Implementation climate
Clinician questionnaires at 6 months which include the Implementation Climate Scale.38
Service size
Smoking prevalence among pregnant women birthing at that site.
Other demands on leaders/service, for example, new SCS policies and training or accreditation.
-
In summary, we speculate that the impact of the context on the implementation outcomes could be as follows:
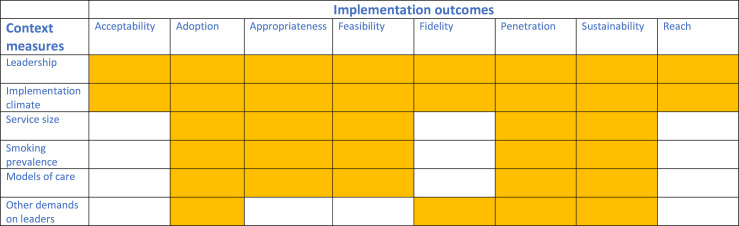
Leadership and implementation climate—impacting on all outcomes.
Service size, smoking prevalence and models of care—impacting on adoption, appropriateness, feasibility, penetration and sustainability.
Other demands on leaders—impacting on implementation in terms of adoption, fidelity, penetration and sustainability.
Figure 2 summarises our speculation about which of each of the context elements might impact on each of the implementation outcomes; for example, we anticipate leadership will impact on all of the implementation outcomes.
Figure 2.
Speculating which context elements may impact on each of the implementation outcomes.
Recruitment and consent
The LHDs (which manage public hospitals and provide healthcare services in a defined geographical area) in NSW with relatively high rates of smoking in pregnancy were approached to participate in the MOHMQuit trial. There are 15 LHDs in total, 7 with high smoking rates in pregnancy were invited and 5 agreed to participate in the trial. Between them, they selected nine maternity services (sites) to take part. The senior midwives and lead obstetricians from these five LHDs were partner investigators in a partnership grant application subsequently awarded by Australia’s National Health and Medical Research Council and so their involvement with the project substantially precedes the implementation trial of MOHMQuit.
Individual service leaders and clinicians in each of the nine sites will be provided with a participant information sheet and those who agree to participate in the research will be asked to sign a written consent form indicating their consent to take part in data collection. This consent applies to data collection to measure the implementation outcomes and context as well as the intervention outcomes.
Process evaluation data collection
The process evaluation will adopt a mixed methods approach, collecting quantitative evidence from questionnaires and qualitative evidence of leaders’ perceptions of how MOHMQuit may have changed behaviour (where it was perceived to have done so) from semistructured interviews. Data will be collected by the research team independently from each of the nine MOHMQuit sites. Study-specific questionnaires will be used to collect implementation outcome data from leaders and clinicians at each site at various time points: immediately following training, 3 months after the training and 6 months after the training. To minimise participant burden, the questionnaires will also collect the data required to measure the intervention outcomes.
Qualitative data will be collected using semistructured interviews with leaders 6 months after the training at each site. The interviews have three key purposes. First, interviews will collect data on the components of MOHMQuit which have been implemented in the 6 months following the MOHMQuit training (uptake), for example, use of the report template for the electronic medical record system facilitating leaders’ scrutiny of their services’ SCS performance for feedback and continuous improvement, or MOHMQuit training delivered by the service themselves using the train-the-trainer manual. Second, they will collect data to support the calculation of an implementation cost as part of the detailed economic evaluation of MOHMQuit, the subject of a separate paper,28 by recording how much time leaders’ assess they spend implementing those components of MOHMQuit. Finally, they will collect data which will enhance the contextual information collected by the research team by eliciting leaders’ perspectives of the enablers and barriers of the implementation of MOHMQuit and what might be improved with regard to it. Interviews will be conducted using the Teams platform, recorded and transcribed. They will be guided by an interview schedule driven by the implementation outcomes and the contextual factors that supported or hindered implementation and any adaptations made to the intervention. The semistructured nature of the interviews will allow for flexibility in questioning and expansion on responses.
Data collection from leaders and antenatal care clinicians will be as follows:
Leaders
An online questionnaire to all leaders 3 months after the training at each site regardless of whether they attended MOHMQuit training (anticipated numbers of leaders who will be invited approximately 55).
A semistructured one-to-one telephone interview 6 months after the training with the midwifery partner investigator and one to two other leaders at each site.
Antenatal care clinicians
A paper questionnaire immediately following the training at each site to participants who attended training (anticipated numbers of participants who will be invited approximately 250).
An online questionnaire to all antenatal care clinicians and AHWs 6 months after the training at each site regardless of whether they attended MOHMQuit training (anticipated numbers of participants who will be invited approximately 300).
In addition, attendance and fidelity information (which aspects of the training were delivered) will be kept by the research team during each training event and the attendance and engagement at various meetings that are components of MOHMQuit. The additional data collection includes
Training logs—to calculate proportion attended at each training event (attendance/invited).
A ‘fidelity checklist’ of which elements of the training were covered during each training event.
Attendance and notes from 10-week warm-up meetings.
Attendance and notes from 2-week warm-up meetings.
Attendance and notes from 2-week follow-up meetings.
Attendance and notes from 4-week follow-up meetings.
Attendance and notes from monthly community of practice meetings.
For each site, a ‘context table’ will be completed by the research team using publicly available sources and with input from partner investigators at each site (box 1).
Box 1. Key contextual information collected for each site.
Number of births at site 2020
Smoking prevalence 2020
Performance against the NSW Ministry of Health’s performance indicator of antenatal smoking
Safer Baby Bundle at site?* (Yes/No)
Preparation and training for new NSW Maternity Care Policy (RSVP - Reducing the effects of Smoking and Vaping on Pregnancy and newborn outcomes)† overlaps with MOHMQuit timing? (Yes/No)
Other smoking cessation support initiatives running at the site? (Yes/No)
Accreditation for Quality Improvement going on concurrent with MOHMQuit? (Yes/No)
Leadership structure at the site
Models of care offered and proportion of women at booking and at birth for each model
Other, for example, external events like disasters, vacant posts
*Safer Baby Bundle is a multicomponent intervention in maternity service which aims to reduce the number of preventable stillbirths.
†The RSVP policy is a policy directive establishing minimum requirements for health services to provide evidence-based smoking cessation support to women before during and after pregnancy. The RSVP Policy was released 14 October 2022.
We anticipate that the data collection itself may have the beneficial sustainability effect of reminding leaders and clinicians about MOHMQuit and possibly prompting renewed attention and/or commitment to it.
Table 1 provides detail of working definitions and how each of the implementation outcomes and contextual features will be measured at which timepoints, using which instruments with whom, and which strategies (components of the MOHMQuit intervention) are aimed to maximise the implementation outcomes. Further detail is available in online supplemental table 1.
Table 1.
Implementation outcomes, definitions, strategies for maximising implementation outcomes, frameworks used and measurement items
| Implementation outcome | Strategies used to maximise implementation outcomes | Frameworks used | Data collection instruments, timing, participants |
| Adoption/uptake (intention or action to try to employ MOHMQuit) |
|
Proctor29 RE-AIM31 (adoption) |
|
| Fidelity (delivered as intended in the Protocol,23 adherence) |
|
Proctor29 RE-AIM31 (implementation) |
|
| Penetration (degree of integration of MOHMQuit practices within the service) |
|
Proctor29 RE-AIM31 (adoption) |
|
| Reach (did MOHMQuit include everyone that it aimed to?) |
|
RE-AIM35 |
|
| Sustainability (factors promoting ongoing use of MOHMQuit) |
|
Proctor29 RE-AIM31 (maintenance) Rogers37 |
|
| Acceptability (how palatable is MOHMQuit to clinicians and leaders?) |
|
Proctor29 Sekhon36 |
|
| Appropriateness (perceived fit or relevance of MOHMQuit with the service) |
|
Proctor29 Rogers37 |
|
| Feasibility (actual fit—the extent to which MOHMQuit can be integrated into usual care in a service) |
|
Proctor29 Rogers37 |
|
| HOW behaviour was changed | Moore35 |
|
|
| HOW context affected implementation |
|
Fernandez38 |
|
Bold typeface indicates outcomes that will be the focus of the process evaluation.
Implementation cost is not included in table 1 as a detailed economic evaluation of MOHMQuit is taking place and is the subject of a separate paper.28 Data to contribute to the economic evaluation will be collected as part of the semistructured interview with leaders.
MOHMQuit, Midwives and Obstetricians Helping Mothers to Quit smoking; RE-AIM, Reach, Efficacy, Adoption, Implementation, Maintenance; SCS, smoking cessation support.
bmjopen-2023-081208supp001.pdf (220.9KB, pdf)
Patient and public involvement
As this is an implementation science trial, our partners in identifying the need for the study and in its design and implementation were health service clinicians, leaders and policy-makers.21 Patients were not involved in designing or implementing the research, but are participants in the trial23 but not in the process evaluation.
Data analysis
We will assess each of the implementation outcomes (table 1) for each site, including assessing variation across the nine sites. At this stage, it is not possible to definitively describe which of the implementation outcomes our analyses will be focused on as that will depend on the variation in implementation outcomes across sites. For example, if there is little variation in fidelity, it will not help explain the MOHMQuit (intervention) outcomes. However, where appropriate, descriptive statistics (measures of central tendency, SD and proportions) will be produced using data from questionnaire responses from clinicians and leaders to summarise quantitative results by participant and by site.
Analyses for the moderators will include calculation of a measure of central tendency, for the leadership42 subscales for each participant. There are four subscales: the proactive subscale, the knowledgeable subscale, the supportive subscale and the perseverant subscale. A measure of central tendency for each set of items that load onto the relevant subscale will be calculated for each subscale. A measure of central tendency of the scale scores will be calculated which will provide a total score for the Implementation Leadership Scale.42 In addition, scores will be aggregated to provide a site-level score. We do not anticipate adding these results, or any of the data from box 1 to any model but they will help constitute a broader assessment of the context for implementation to contribute to understanding of in which sites, and how, MOHMQuit was effective.
Qualitative data from semistructured interviews with leaders will be analysed descriptively to explore perspectives of uptake by site and thematically across all sites regarding the enablers, barriers and how implementation of MOHMQuit might be improved. Thematic analysis will follow the steps outlined by Braun and Clarke: data familiarisation; initial generation of codes; development of themes by collating codes and then reviewing the raw data again to check the material sits together coherently as a theme; defining each theme and how themes work together to tell the overall story of the data.43
Data from multiple sources will facilitate triangulation, for example, collecting data about acceptability from quantitative data (post-training questionnaires from clinicians and questionnaires at 6 months from all clinicians) along with qualitative interviews with leaders from each site. This mixed methods approach will broaden and deepen understanding of the results of the trial. The key findings will be presented in an integrated way using a side-by-side joint display table,44 each source being given equal weight.
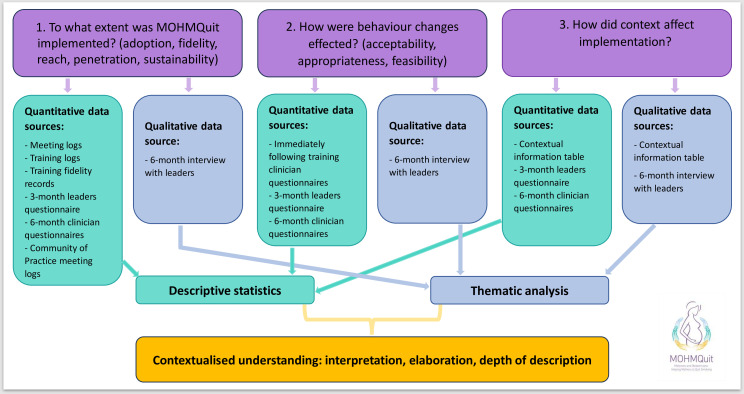
Figure 3 describes this visually.
Figure 3.
Mixed methods approach to data collection and analysis.
Ethical considerations and dissemination
The process evaluation received ethical approval from the NSW Population Health Services Research Ethics Committee (Reference Number 2021/ETH00887) on 23 July 2021. Results of the process evaluation will be written up for publication in peer-reviewed journals and presented at conferences. The process evaluation will perform a formative function facilitated by the stepped-wedge design with sites receiving the intervention in a staggered implementation, allowing for further polishing of the intervention as the trial proceeds. The process evaluation will also provide contextual information to elucidate the findings of the trial in terms of how MOHMQuit may have been effective in some sites but not in others. This understanding is critical in relation to rolling out MOHMQuit across NSW should the intervention prove to be effective.
Trial registration number
The MOHMQuit trial is registered with ANZCTR (www.anzctr.org.au): ACTRN12622000167763.
Discussion
Implementation science is the study of approaches that support the systematic uptake of research findings into ‘usual care’.45 In cases where there is an urgent need for behaviour change and clear evidence to practice gap, such as with SCS in antenatal care, implementation science provides a framework for examining an intervention such as MOHMQuit. This paper describes the mixed-method design and underpinning frameworks for the process evaluation of MOHMQuit as part of an implementation science study. MOHMQuit is a complex multicomponent intervention designed using the Behaviour Change Wheel.20 It aims to change the behaviour of antenatal care providers to improve the support provided to women to stop smoking in pregnancy. MOHMQuit is being implemented in a stepped-wedge effectiveness trial across nine publicly funded maternity services in NSW.23
The process evaluation will facilitate the ongoing refinement of MOHMQuit and will provide an assessment of the extent to which MOHMQuit was implemented, what the mechanisms of impact were and what the context of implementation was, and how it affected the implementation of MOHMQuit. It will also inform other components of the study, for example, contributing data to support costing of MOHMQuit for the economic evaluation. We anticipate that the findings from the process evaluation will contextualise and aid understanding of our trial results, and may support the further implementation of MOHMQuit in NSW. For example, if it transpires that implementation leadership is more evident in those sites where MOHMQuit was shown to be particularly effective, the scale-up would need to include a focus on implementation leadership and on implementing the leadership components of the intervention. Our process evaluation will also contribute knowledge about the implementation of stepped-wedge trials which may be useful to others in the future. While we have described our intended approach to evaluating the implementation of MOHMQuit, we have also included flexibility of approach in recognition of unanticipated implementation factors that may surface.40
Smoking in pregnancy is an ongoing public health challenge and represents a considerable gap between the evidence for SCS and practice. Providing a broader understanding of how MOHMQuit was or was not effective will be key to its potential future roll-out/scale-up.
Empirical testing of the theory
Implementation science is a relatively new academic endeavour and this process evaluation has the potential to contribute to a growing body of evidence of approaches to implementing comprehensive stepped-wedge trial designs that are inclusive of process evaluation.
Strengths and limitations
The process evaluation has been designed using implementation science frameworks and explores the implementation of MOHMQuit, a thorough and theoretically underpinned intervention and trial design.21 The results of the trial will provide further evidence for the effectiveness, or otherwise, of this theoretically driven approach. The mixed methods approach in the process evaluation includes qualitative and quantitative data collection from a wide range of leaders and clinicians in each MOHMQuit site, some of whom will not have directly participated in the MOHMQuit training, as well as publicly available data and observational data from the research team implementing MOHMQuit. This approach has the potential to produce findings that have depth and nuance and will aid understanding of the trial findings. However, MOHMQuit is a complex intervention with many moving parts which interact with one another, and the stakeholders involved. No process evaluation is able to collect data to understand all aspects of these interactions. In addition, the MOHMQuit trial is a ‘real world’ trial. This has strengths in producing findings that can be confidently understood as realistic; however, it also produces many challenges including the potential impact of new policies and procedures, staffing issues, and so on, many of which we have aimed to record as part of the process evaluation but some of which we are likely to have missed. This may compromise our capacity to fully understand and accurately interpret the intervention outcomes.
Trial status
Recruitment for the trial is underway. Process evaluation and data collection commenced in March 2023 and will conclude in May 2024.
Supplementary Material
Acknowledgments
This paper is submitted on behalf of the MOHMQuit Trial team, including all chief investigators, partner investigators and associate investigators, and co-researchers and site leads at each of the MOHMQuit sites. In addition to the named authors, the team includes Dheya Al Mashat (NSW Health), Dianne Avery (NSW Health), Elizabeth Best (NSW Ministry of Health), Alecia Brooks (Cancer Council NSW), Rashna Chinoy (NSW Health), Justine Elliot (NSW Health), Jacinta Felsch (NSW Health), Mohamed Foda (NSW Health), Sandra Forde (NSW Health), Tara Farrugia (NSW Health), Tracey Greenberg (Alcohol and Drug Service, St Vincent’s Hospital Sydney), Jane Griffith (NSW Health), Madeline Hubbard (NSW Health), Damien McCaul (NSW Ministry of Health), James McLennan (Alcohol and Drug Service, St Vincent’s Hospital Sydney), Kate Reakes (Cancer Institute NSW), Virginia Stulz (NSW Health and Western Sydney University), Tracey Zakazakaarcher (NSW Health), and Lou Atkins (University College London) who provided excellent early guidance on the process evaluation design.
Footnotes
Contributors: The process evaluation was conceived and designed by all authors. The first draft of the paper was written by JL with input from MP and CP before receiving input from all other authors. All authors have read and approved the final manuscript.
Funding: This work is currently supported by a partnership grant from the NHMRC (GNT1185261). Previously, NHMRC funding (GNT1072213) and the Cancer Institute NSW funding (13/ECF/1-11) supported the developmental work of MOHMQuit. MP was also supported during this time by a fellowship grant from the NHMRC (GNT1159601).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Australian Institue of Health and Welfare . Australia’s mothers and babies 2020 web report - Summary. Canberra: Australian Government, 2022. Available: https://www.aihw.gov.au/reports/mothers-babies/australias-mothers-babies/contents/antenatal-period/smoking-during-pregnancy [Google Scholar]
- 2.Diamanti A, Papadakis S, Schoretsaniti S, et al. Smoking cessation in pregnancy: an update for maternity care practitioners. Tob Induc Dis 2019;17:57. 10.18332/tid/109906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange S, Probst C, Rehm J, et al. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health 2018;6:e769–76. 10.1016/S2214-109X(18)30223-7 [DOI] [PubMed] [Google Scholar]
- 4.Avşar TS, McLeod H, Jackson L. Health outcomes of smoking during pregnancy and the postpartum period: an umbrella review. BMC Pregnancy Childbirth 2021;21:254. 10.1186/s12884-021-03729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services . The health consequences of smoking: 50 years of progress. A report of the surgeon general. Atlanta, GA: U.S: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 6.Greenhalgh E, Ford C, Winstanley M. Section 3.7 Pregnancy and smoking Melbourne. Victoria: Cancer Council, 2021. Available: https://www.tobaccoinaustralia.org.au/chapter-3-health-effects/3-7-pregnancy-and-smoking [Google Scholar]
- 7.U.S. Department of Health & Human Services . Smoking cessation: a report of the surgeon general. Rockville: US Department of Health and Human Services, 2020. [PubMed] [Google Scholar]
- 8.NSW Ministry of Health . The First 2000 Days framework. Sydney, Australia: Health and Social Policy Branch, 2019. [Google Scholar]
- 9.NSW Ministry of Health . First 2000 days implementation strategy 2020-2025. St Leonards: NSW Ministry of Health, 2021. [Google Scholar]
- 10.NSW Ministry of Health . NSW clinical guidelines for the management of substance use during pregnancy, birth and the postnatal period. Sydney: NSW Ministry of Health, 2014. [Google Scholar]
- 11.Passey ME, Longman JM, Adams C, et al. Factors associated with provision of smoking cessation support to pregnant women – a cross-sectional survey of midwives in New South Wales, Australia. BMC Pregnancy Childbirth 2020;20:219. 10.1186/s12884-020-02912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longman JM, Adams CM, Johnston JJ, et al. Improving implementation of the smoking cessation guidelines with pregnant women: how to support clinicians. Midwifery 2018;58:137–44. 10.1016/j.midw.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 13.Passey ME, Sanson-Fisher RW. Provision of antenatal smoking cessation support: a survey with pregnant aboriginal and Torres Strait Islander women. Nicotine Tob Res 2015;17:746–9. 10.1093/ntr/ntv019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeev YB, Bonevski B, Twyman L, et al. Opportunities missed: a cross-sectional survey of the provision of smoking cessation care to pregnant women by Australian general practitioners and Obstetricians. Nicotine Tob Res 2017;19:636–41. 10.1093/ntr/ntw331 [DOI] [PubMed] [Google Scholar]
- 15.Hoekzema L, Werumeus Buning A, Bonevski B, et al. Smoking rates and smoking cessation preferences of pregnant women attending antenatal clinics of two large Australian maternity hospitals. Aust N Z J Obstet Gynaecol 2014;54:53–8. 10.1111/ajo.12148 [DOI] [PubMed] [Google Scholar]
- 16.Passey ME, Sanson-Fisher RW, Stirling JM. Supporting pregnant aboriginal and Torres Strait Islander women to quit smoking: views of antenatal care providers and pregnant indigenous women. Matern Child Health J 2014;18:2293–9. 10.1007/s10995-013-1373-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain C, O’Mara-Eves A, Porter J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2017;2:CD001055. 10.1002/14651858.CD001055.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ 2021;374:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services . Smoking cessation. A report of the surgeon general. Atlanta GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020. [Google Scholar]
- 20.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Sci 2011;6:42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passey ME, Adams C, Paul C, et al. Improving implementation of smoking cessation guidelines in pregnancy care: development of an intervention to address system, maternity service leader and clinician factors. Implement Sci Commun 2021;2:128. 10.1186/s43058-021-00235-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longman JM, Adams C, Paul C, et al. Improving clinicians’ implementation of guidelines to help women stop smoking in pregnancy: developing evidence-based print and video materials. Int J Environ Res Public Health 2021;18:10522. 10.3390/ijerph181910522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes LAJ, Longman J, Adams C, et al. The Mohmquit (midwives and Obstetricians helping mothers to quit smoking) trial: protocol for a stepped-wedge implementation trial to improve best practice smoking cessation support in public antenatal care services. Implement Sci 2022;17:79. 10.1186/s13012-022-01250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bar-Zeev Y, Bonevski B, Lim LL, et al. Improving health providers smoking cessation care in pregnancy: a systematic review and meta-analysis. Addict Behav 2019;93:29–38. 10.1016/j.addbeh.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 25.Lewis CC, Boyd MR, Walsh-Bailey C, et al. A systematic review of empirical studies examining mechanisms of implementation in health. Implement Sci 2020;15:21. 10.1186/s13012-020-00983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis CC, Klasnja P, Powell BJ, et al. From classification to causality: advancing understanding of mechanisms of change in implementation science. Front Public Health 2018;6:136. 10.3389/fpubh.2018.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springer MV, Sales AE, Islam N, et al. A step toward understanding the mechanism of action of audit and feedback: a qualitative study of implementation strategies. Implement Sci 2021;16:35. 10.1186/s13012-021-01102-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce A, Scarfe J, Jones M, et al. Study protocol of an economic evaluation embedded in the midwives and obstetricians helping mothers to quit smoking (Mohmquit) trial. BMC Health Serv Res 2023;23:939. 10.1186/s12913-023-09898-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham Moore SA, Barker M, Bond L, et al. Process evaluation of complex interventions. In: Complex interventions in health: an overview of research methods. 2015: 222. [Google Scholar]
- 31.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–7. 10.2105/ajph.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Global Alliance for Chronic Diseases . GACD process evaluation guidelines. 2015. Available: https://www.gacd.org/perch/resources/gacd-pe-guidelines-v1-0.pdf
- 33.Hawe P, Shiell A, Riley T. “Complex interventions: how “out of control” can a randomised controlled trial be” BMJ 2004;328:1561–3. 10.1136/bmj.328.7455.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirk MA, Moore JE, Wiltsey Stirman S, et al. Towards a comprehensive model for understanding adaptations’ impact: the model for adaptation design and impact (MADI). Implement Sci 2020;15:56. 10.1186/s13012-020-01021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions. medical research council guidance. BMJ 2015;350:h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17:88. 10.1186/s12913-017-2031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers EM. Diffusion of innovations. 4th edn. New York: The Free Press, 1995. [Google Scholar]
- 38.Fernandez ME, Walker TJ, Weiner BJ, et al. Developing measures to assess Constructs from the inner setting domain of the Consolidated framework for implementation research. Implement Sci 2018;13:52. 10.1186/s13012-018-0736-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craig P, di Ruggiero E, Frolich KL, et al. Taking account of context in population health intervention research: guidance for producers, users and funders of research. Southampton: National Institute for Health Research, Canadian Institutes of Health Research (CIHR), 2018. [Google Scholar]
- 40.Beames JR, Lingam R, Boydell K, et al. Protocol for the process evaluation of a complex intervention delivered in schools to prevent adolescent depression: the future Proofing study. BMJ Open 2021;11:e042133. 10.1136/bmjopen-2020-042133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moullin JC, Sklar M, Ehrhart MG, et al. Provider report of sustainment scale (PRESS): development and validation of a brief measure of inner context sustainment. Implementation Sci 2021;16:86. 10.1186/s13012-021-01152-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aarons GA, Ehrhart MG, Farahnak LR. The implementation leadership scale (ILS): development of a brief measure of unit level implementation leadership. Implement Sci 2014;9:45. 10.1186/1748-5908-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 44.Guetterman TC, Fetters MD, Creswell JW. Integrating quantitative and qualitative results in health science mixed methods research through joint displays. Ann Fam Med 2015;13:554–61. 10.1370/afm.1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eccles MP, Mittman BS. Welcome to implementation science. Implementation Sci 2006;1. 10.1186/1748-5908-1-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081208supp001.pdf (220.9KB, pdf)