Abstract
This article aims to provide a practical guide for patient management and an overview of the predictive scorings for Fournier’s gangrene (FG) that are available to aid clinicians. A literature was performed reviewing currently used scoring systems for FG and presenting a practical guide for its management based on the available evidence. There are four specific scoring systems available for the assessment of FG although few other non-specific and generic tools also exist. These specific tools include Laboratory Risk Indicator for Necrotizing Fasciitis, Fournier’s Gangrene Severity Index, Uludag Fournier’s Gangrene Severity Index, and Simplified Fournier’s Gangrene Severity Index and help calculate expected mortality. Our proposed algorithm covers primary assessment, resuscitative interventions, initial investigations, urgent care, post-operative care, and long-term follow-up. The management of the FG patient can be divided into initial resuscitation, surgical debridement, ongoing ward management with antibiotic therapy, wound reconstruction, and long-term follow-up. Each facet of care is vital and requires multidisciplinary team expertise for optimal outcomes. Whilst mortality continues to improve, it remains significant, reflecting the severe and life-threatening nature of FG. More research is certainly needed into how this care is individualised, and to ensure that long-term outcomes in FG include quality of life measures after discharge.
Keywords: algorithm, antibiotics, Fournier’s gangrene, necrotising fasciitis, predictive scoring
Plain language summary
Fournier’s gangrene: a review of predictive scoring systems and practical guide for patient management
The management of Fourniers gangrene can be divided into initial resuscitation, surgical debridement, ongoing ward management with antibiotic therapy, wound reconstruction, and long-term follow-up. Each facet of care is vital and requires multidisciplinary team expertise for optimal outcomes. More research is certainly needed into how this care is individualised, and to ensure that long-term outcomes in FG includes quality of life measures after discharge.
Introduction
Fournier’s gangrene (FG) was eponymously described in 1883 by Jean-Alfred Fournier 1 as an infective, necrotising fasciitis of the external genitalia, perineum, or perianal region. Due to its high mortality rate, it is recognised as a true urological emergency. FG is a rare condition that occurs predominately in men, 2 with diabetes mellitus, 3 alcohol excess, 4 lower socioeconomic status, 5 immunocompromised status, 6 and human immunodeficiency virus (HIV) infection 7 being documented risk factors. The infection usually starts in the anorectal or genital areas and can spread rapidly and extensively causing life-threatening sepsis. 8 Mortality for FG has traditionally been estimated at 15–40%,2,8,9 although a recent meta-analysis found a lower mean mortality rate of 7.3%. 10
Due to the high associated mortality, patients with FG need to be managed aggressively with initial resuscitation in the emergency setting including the administration of broad-spectrum antibiotics. Whilst this resuscitation occurs, a thorough history and examination need to be performed, numerous investigations need to be ordered, and discussions have to be made with members of different clinical teams, the patient’s family, and the patient themselves. Once the patient is optimised as best possible, timely transfer to the emergency theatre for extensive debridement of the necrotic tissue remains the gold standard management. FG patients usually require several debridements during their inpatient stay and some will require adjunct procedures such as colectomy or urinary diversion.
As FG represents a significant physiological insult to the patient, they require input from multiple surgical, medical, nursing, and therapy teams as an inpatient. Once the tissue is at the healing stages and no further debridement is needed, wound closure is achieved with input from the plastic surgery team and can require a significant skin graft to cover the defect. There is limited data in the literature about long-term post-discharge outcomes for FG patients, but care should be taken to manage their physical and psychological needs as needed.
This article aims to provide a practical guide for patient management and an overview of the predictive scoring systems that are available to aid the clinician.
Methods
A non-systematic literature review was performed on PubMed to review contemporary practices in the management of FG as well as the pathogenesis, risk factors, treatment modalities, reconstructive surgeries, outcomes, and follow-up associated. Search terms included ‘Fournier’, ‘gangrene’, ‘management’, ‘treatment’, ‘reconstruction’, ‘outcomes’, ‘antibiotic’, and ‘microorganism’. To provide comment on the utility and advantages of the various scoring systems used in FG management, the terms ‘scoring’, ‘system’, and ‘index’ were used in conjunction with the above terms.
Pathogenesis
Following a review of over 1700 cases, Eke 8 found that the major sources of sepsis originated from the anorectum (30–50%), urogenitalia (20–40%), and genital skin (20%), with colorectal sources being associated with a worse prognosis. The most common infective sources include perianal, perirectal, and ischiorectal abscesses, 6 although scrotal abscesses and urinary tract infections have also been implicated. 11 Other rarer reported causes of FG include trauma, vasectomy, and insertion of foreign bodies into the penile urethra.
Infections in FG are polymicrobial in nature with multiple microorganisms often being identified simultaneously in wound cultures. 12 Commonly grown pathogens include Staphylococcus species, Streptococcus species, Escherichia coli, Pseudomonas species, Bacteroides species, with Candida species representing the most common nonbacterial causative pathogens. Different studies present different profiles of microorganism prevalence, 10 highlighting the importance of local microbial guidelines and antimicrobial resistance.
From its source, the infection causes obliterative endarteritis which leads to inflammation and oedema. This compromises the blood supply to the cutaneous and subcutaneous tissues leading to necrosis, seen as a purple-black discolouration of the skin. 13 The hypoxic tissue permits the growth of anaerobic microorganisms which produce hydrogen and nitrogen gases, which accumulate in subcutaneous tissues and can be appreciated as crepitus. 14
The infection can spread along fascial planes leading to necrosis of the perineum, scrotum, lower abdominal wall, and upper thighs. Deep extension of infection below the muscle layers to involve muscle is rare. It is worth noting that the testis and epididymis tend to be spared in FG infections due to their blood supply originating separately. 15
Risk factors
The principal underlying risk factors for FG are compromised host immunity and physiological reserve to fight off a serious infection. Diabetes mellitus is widely reported as the most frequent co-morbidity (30–40%) in FG patients2,11 and is associated with increased mortality.16,17 Prolonged hyperglycaemia affects the host’s immune status by its effects on phagocyte activity, chemotaxis, and cellular adherence, as well as having a negative effect by impairing wound healing by causing microvascular damage and impairing blood supply to healing tissue. 18 Outcomes in FG are proportional to the degree of hyperglycaemia, highlighting the importance of strict glycaemic control during the course of the illness. 19 Other factors compromising host immunity that have been identified as risk factors for developing FG include the use of immunosuppressive medications, malnutrition, and HIV infection.
Huayllani et al. 11 found that significant co-morbidities reducing physiological reserve such as hypertension (26%), anaemia (10%), heart failure (6%), and patient factors such as obesity (12%) are commonly found in FG patients, although the effect that such co-morbidities have on outcomes is controversial. Multivariate analysis of FG patients in large studies has implicated patient age, renal failure, coagulopathy, and congestive heart failure as being significant risk factors for mortality.20,21
Clinical assessment
History and examination
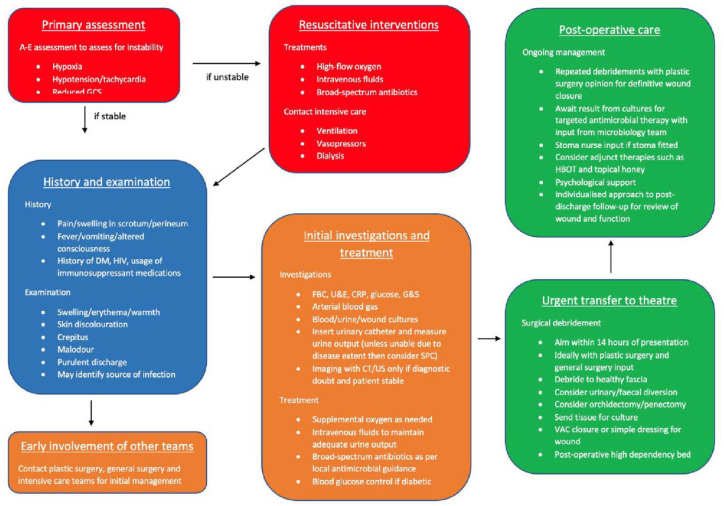
FG can be a rapidly progressing and life-threatening condition, so early diagnosis and aggressive resuscitation are vital. Depending on the haemodynamic stability of the patient on their initial presentation, a full A–E assessment with appropriate resuscitation may be required before taking a full history (Figure 1). The symptomology of FG is non-specific in many cases and can make differentiation from more benign conditions such as scrotal cellulitis, scrotal abscess and severe epididymo-orchitis difficult. Local symptoms include scrotal swelling, scrotal/perineal pain, erythema, and skin necrosis whilst systemic symptoms include fever, vomiting, and altered consciousness in more advanced infections. The progression of symptoms is variable but may develop over 3–5 days, starting with a worsening sensation of discomfort or pain and pruritus. Local swelling and cutaneous changes represent a more advanced disease with the development of gangrene in the soft tissues. Significant co-morbidities including diabetes mellitus, HIV infection, or the use of immunosuppressive medications should give a low threshold for suspecting FG as discussed above.
Figure 1.
Guide for management in Fournier’s gangrene.
Examination of the genitalia and perineum is performed with a chaperone present, and these findings will reflect the extent of disease progression. Soft tissue swelling, erythema, warmth, and tenderness indicate an underlying inflammatory process. If erythema is present, then the border should be marked to monitor its progression on subsequent examinations. Crepitus may be felt as subcutaneous gas on palpation and reflects gas-producing anaerobic bacterial infection. There may be discharge from a wound which should be sampled for culture. Concerning factors include obvious spread of the disease from the genitalia/perineum to the thighs and abdominal wall as the infection spreads along fascial planes. Skin necrosis seen as purple-black discolouration associated with a foul odour is characteristic of FG. A PR examination should be performed to assess whether the disease involves the perianal region, and a perianal/perirectal abscess may be appreciable as the nidus of infection.
Investigations
In the first instance, venepuncture should be performed and blood samples be sent for the full blood count, urea and electrolytes, C-reactive protein, and clotting profile. A group and save should also be sent in case the patient requires the transfusion of blood products either in the immediate emergency setting or during debridement in the operating theatre. An arterial blood gas should be sent which will give immediate values for haemoglobin, sodium, potassium, and lactate as well as assess acidosis and oxygenation status, which are all vital to guide fluid resuscitation and oxygen therapy. A blood glucose level should also be taken to ensure optimal glycaemic control.
To guide the ongoing antibiotic therapy, it is vital to identify the causative organism(s) by performing cultures which can be taken from the blood, urine, wound discharge, and debrided tissue. The culture is usually ready for interpretation in 72 h and the sensitivity pattern will allow for more targeted antibiotics to be given. It is worth noting that blood cultures are rarely positive in FG 12 but when they are, this is associated with poorer outcomes. 22 Urine cultures may be positive if a urinary tract infection is a precipitating cause, but wound cultures will be most useful in providing antibiotic guidance.
FG is usually a clinical diagnosis and if it is identified to be obvious or extensive from the history and examination, then progression to surgical debridement should not be delayed by imaging. Ultrasound (US) and computerised tomography (CT) are the two modalities most used in assessing FG, with CT having a greater specificity for evaluating the disease as well as being able to assess the gastrointestinal tract in cases where the infection is colorectal in origin. 23 CT also provides an appreciation of the underlying anatomy which is useful in surgical planning as it may indicate that additional procedures such as faecal diversion are required. CT findings of FG include subcutaneous emphysema, fascial thickening, fat stranding, and abscess or fluid collection. 24
Use of scoring systems
Scoring systems are widely used in surgical and medical conditions for diagnostic likeliness, therapeutic guidance, and prognostication of morbidity and mortality. 25 In 2004, Wong et al. 26 created the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score using multivariate logical regression on multiple biochemical markers to create a diagnostic risk scoring system for necrotising fasciitis. They found that using a cut-off value of six points gave a positive predictive value of 92.0% and a negative predictive value of 96.0%. The LRINEC score is simple to calculate requiring only six biochemical markers which are commonly performed on initial blood tests. There is evidence to suggest that the LRINEC score is effective at distinguishing necrotising fasciitis from other soft tissue infections, 27 with some studies suggesting that a cut-off score of seven is more optimal.28,29 However, a meta-analysis of 23 studies found that the LRINEC score has poor sensitivity for necrotising fasciitis, with an LRINEC score of ⩾6 having a sensitivity of 68.2% and an LRINEC score of ⩾8 having a sensitivity of 40.8%, 30 suggesting that correlation with clinical assessment and radiographical modalities where appropriate is necessary. Whilst there have been no published studies looking into the diagnostic application of the LRINEC score for FG specifically, there are some case studies that suggest the LRINEC score can predict mortality 31 and the need for mechanical ventilation 32 in FG patients.
In 1995, Laor et al. 33 collected data on demographics, patient medical history, signs and symptoms, laboratory markers, extent of disease on body surface area, and number of surgical debridements on 30 patients with FG to ascertain which factors significantly predicted mortality. They used regression analysis on these predictive factors to create the Fournier’s Gangrene Severity Index (FGSI) as a mortality predictor tool for FG patients. Whereas the LRINEC only uses biochemical markers, the FGSI also incorporates patient measures such as temperature, heart rate, and respiratory rate. The percentage of body area affected and the number of debridement operations were not found to significantly predict mortality and as such are not included in the FGSI. Since its conception, the FGSI has been repeatedly validated as an accurate mortality prediction tool by other studies.34 –36 It has also been shown to predict the need for more surgical interventions, longer hospitalisation periods, increased risk of developing sepsis, and development of disease-specific complications, 37 suggesting that it may have utility in morbidity estimates also.
In 2010, Yilmazlar et al. 38 wrote that they believed that the FGSI underestimated the mortality of FG and published data from their case series of 80 patients. By performing logistic regression analysis, they concluded that the degree of disease dissemination and patient age were also significant predictors of mortality, and created their ‘Uludag Fournier’s Gangrene Severity Index’ (UFGSI) as a modification of the FGSI. They found that with a cut-off value of nine points, there was a 94% probability of death with a UFGSI score >9 and an 81% probability of survival with a UFGSI score ⩽9, which compares favourably to the FGSI which published 75% and 78%, respectively. The UFGSI has been validated by external studies39 –41 and has been suggested to be more sensitive (75.0% compared with 58.3%) but less specific (84.0% compared with 97.3%) than the FGSI. 42
The validity of both the FGSI and UFGSI has been widely confirmed in the literature, but in practice can be cumbersome and time-consuming to utilise in a rapidly deteriorating patient. Roghmann et al. 43 performed a prospective cohort study reviewing different scoring systems and found that the Charlson Comorbidity Index 44 and the Surgical Apgar Score 45 were as good at outcome prediction as the FG indices, as well as being easier to calculate, more generally applicable, and better validated. It is worth noting that the Surgical Apgar Score is calculated from intraoperative parameters from the anaesthetic record and therefore is likely to have limited utility as a preoperative outcome predictor. In 2013, Lin et al. 46 proposed their Simplified Fournier’s Gangrene Severity Index, which used only three factors in its calculation – serum creatinine, haematocrit, and serum potassium. It is by far the simplest and quickest to calculate and is non-inferior to the pre-existing FGSI and UFGSI. 47
For a scoring system to be worth using for outcome prediction, it should be easy and relatively time inexpensive to calculate, resistant to operator variability, sensitive and specific, and have an outcome on patient management as backed up by published literature. There is no clear consensus in the literature as to whether scoring systems in FG are useful and if so, which system should be preferentially used. It is also unclear if a high mortality as predicted by a scoring system would guide the clinician to not perform aggressive resuscitation and debridement of their FG patient. Emerging research is being performed to evaluate the usage of the neutrophil-to-lymphocyte ratio (NLR) as a prognostic value, having been shown to correlate with mortality in disease processes such as sepsis, pneumonia, COVID-19, and cancer. 48 The NLR has been shown to predict risk of intensive care admission, risk of need for mechanical ventilation, and mortality, 32 but more research is required to establish whether it should be routinely used in outcome prediction in FG patients.
A summary of the advantages and disadvantages of the above scoring systems is found in Table 1.
Table 1.
The different scoring systems that can be used in outcome prediction in FG. The probabilities of death and survival refer to the data published by the original authors relative to their proposed cut-off value.
| Scoring system | Cut-off value | Probability of death | Probability of survival | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) | ⩽5 = low risk 6–7 = medium risk ⩾8 = high risk |
– | – | Simple to calculate High positive and negative predictive power |
Not specific to FG Only designed as a diagnostic tool |
| Fournier’s Gangrene Severity Index (FGSI) | 9 | 75% | 78% | Incorporates patient factors Regression analysis specific to FG patients |
Timely to calculate |
| Uludag Fournier’s Gangrene Severity Index (UFGSI) | 9 | 94% | 81% | Original data based on a larger sample size More sensitive than FGSI |
Timely to calculate Less specific than FGSI |
| Simplified Fournier’s Gangrene Severity Index (SFGSI) | 2 | – | – | Only uses three biochemical tests in the calculation | |
| Charlson Comorbidity Index | – | – | – | Simple to calculate Widely validated in other disease processes |
Not specific to FG |
| Surgical Apgar Score | – | – | – | Simple to calculate Widely validated in other disease processes |
Not specific to FG Calculated from intraoperative parameters therefore limited utility in the initial setting |
| Neutrophil-to-lymphocyte ratio (NLR) | – | – | – | A strong predictor of morbidity and mortality in other disease processes | Little research into application in FG patients |
FG, Fournier’s gangrene.
Management
Initial resuscitation
Treatment for FG starts during the assessment process as required and continues throughout the inpatient admission. The patient is reviewed in the emergency setting and resuscitated as appropriate. Airway measures and high-flow oxygen can be started and are guided by pulse oximetry and arterial blood gas readings. Intravenous fluids should be started in signs of shock, such as tachycardia, hypotension, reduced Glasgow Coma Score, low urine output, reduced capillary refill time, and cool peripheries. Urine output should be strictly recorded on a fluid chart to guide the rate of intravenous fluid administration. Electrolyte readings from the blood gas are available immediately and should be considered when choosing the contents of the fluids given. A measure of the blood glucose should be taken, and hyperglycaemia should be corrected as prolonged hyperglycaemia will impair healing and increase morbidity and mortality. Diabetic patients presenting with FG may also present with diabetic ketoacidosis for which controlling the blood glucose is imperative. 5 If the patient remains unstable after the initial resuscitative efforts, then the intensive care team should be contacted.
As part of the initial investigations, blood cultures and urine cultures should be performed if possible. Broad-spectrum antibiotics should be started as soon as possible, ideally after the cultures have been taken unless this would cause significant delay. The antibiotics should be guided by local antimicrobial guidelines which reflect local bacterial prevalence. Commonly used antibiotics include metronidazole, penicillin, third-generation cephalosporins, gentamicin, carbapenems, vancomycin, fluoroquinolones, and teicoplanin.7,49 –52 Often, a cocktail of antibiotics is needed and microbiology advice may be necessary. Becerra et al. 53 found that in their study of 131 patients, 86% of cases grew multiple bacteria with 55% growing Gram-positive and Gram-negative bacteria in the same culture. They also noted a high prevalence of Candida infections in their dataset 24% compared with other similar studies in other institutions (1–5%).51,52 They proposed that empirical antifungals should be considered in patients with known chronic candiduria, recent intensive care unit admission, diabetes mellitus, or immunocompromised status. The antimicrobial regime is reviewed with cultures from the blood, urine, and wound debridement.
Surgical debridement
After the initial resuscitation, a diagnosis of FG is given either clinically or radiologically. The patient should be taken to the operating theatre for extensive debridement as soon as possible. Lin et al. 54 found that the optimal period for the initial surgical debridement is 14 h, with delays longer than this being associated with increased mortality. To achieve this, the anaesthetic and theatre teams should be contacted early to ensure a prompt discussion. The intensive care team should also be informed pre-operatively as FG patients will commonly need high-dependency beds post-operatively, with 17.5–30% of patients requiring at least single-system support such as mechanical ventilation or dialysis. 10 Depending on the location and extent of the necrosis, the input of a colorectal surgeon should be sought where faecal diversion is indicated. A group and save should be taken with the initial blood tests and units of packed red cells can be cross-matched in the cases of anaemia on presentation or need for extensive debridement.
In the operating theatre, the necrotic tissue should be debrided to normal fascia 55 and healthy, bleeding tissue to arrest the spread of necrosis along the fascial planes. During the procedure, the extent of necrotic spread can be appreciated, and an on-table decision can be made regarding the need for a further procedure such as urinary diversion, faecal diversion, orchidectomy, and penectomy. In general, urinary and faecal diversion is indicated in cases where there is a risk of wound contamination. Urinary diversion is achieved in most cases with the insertion of a urethral catheter and does not appear to affect mortality in FG patients. 51 Urethral catheterisation is considered adequate, apart from cases with extensive penile involvement, where suprapubic catheterisation is preferred. 56 Faecal diversion is considered in cases with perianal or anal sphincter involvement or with faecal incontinence and is usually achieved with diverting colostomy. If a stoma is indicated, it should be fitted during the initial debridement operation if possible, as this is associated with better mortality outcomes. 57 Orchidectomy and penectomy are rarely performed for FG management 5.6% and 0.2%, respectively. 10 Orchidectomy is indicated in testicular involvement which is rare due to the testicles receiving blood supply from the gonadal vessels. 58
Regardless of the surgical options chosen, the debrided tissue should be sent off for tissue culture as it is these cultures that are most often used to guide antimicrobial management. FG surgical management is typically characterised by multiple debridements, 59 and as such after the initial debridement the wound is left open, being covered either by a simple dressing or with negative pressure wound therapy such as vacuum-assisted closure (VAC) therapy. 55
VAC therapy exposes the wound to a subatmospheric pressure, which promotes wound healing by increasing blood supply and therefore the migration of inflammatory cells to form granulation tissue. 60 It is associated with fewer dressing changes, more favourable pain control, and greater mobility for the patient but there is no clear evidence that it is associated with improved mortality rates. 61 There is some evidence suggesting that VAC therapy is associated with better wound closure at a 10-week post-operative interval 62 ; however, no large-scale randomised trial has been conducted comparing VAC therapy with simple wound closure so the conclusions drawn from the evidence are limited.
Reconstructive techniques
A review from the plastic surgery team should be sought during the post-operative period for the eventual definitive closure of the wound. Options for wound closure include secondary intention, delayed primary closure, loose wound approximation, skin flap, and skin graft. Occasionally, testicular implantation in a thigh pocket is used either as a temporary measure before later definitive reconstruction or as a permanent cover. 63 Reconstruction in FG cases can represent a significant challenge to the surgeon, particularly if extensive debridement is required. There is no standard proforma for which method is preferred, with patients being assessed on a case-by-case basis and the reconstruction tailored to the specific need.
The secondary intention may be favoured for relatively small wounds, particularly if they are confined to the scrotum. 64 This approach is associated with longer healing times and the surgeon has less control over the cosmetic outcome of the wound, but avoiding loose wound approximation in these cases reduces the risk of worsening or spreading the infection. 63 Scrotal advancement flaps are also described for small scrotal wounds, which provide better cosmesis but risk flap failure and wound necrosis. 65 This technique should be avoided in larger scrotal defects as in these cases the closure is under increased tension resulting in a larger risk of flap failure. 65 Split-thickness skin grafting and thigh flaps are described in the management of larger defects, 66 with graft loss, infection, scarring, adhesions, dehiscence, unacceptable cosmesis, and reduced sensation being recognised complications.5,64 –67
FG patients are managed with extensive debridement of fascia and soft tissues, which can result in a large dead space in the perianal region. Anatomical dead spaces can reduce the efficacy of antimicrobial therapy and result in a higher chance of failure of the reconstruction by providing an environment for bacterial colonisation and chronic inflammation. 68 A recent retrospective study from Ismayilzade et al. 69 assessed outcomes from FG patients who underwent pedicled gracilis muscle flap surgery to reduce dead space compared with those who had no specific procedure for reducing dead space. They found that patients who had the procedure to fill in the dead space required significantly fewer revision surgeries for wound dehiscence and had a significantly shorter duration of hospital stay. The study was small (n = 22) and of a retrospective design, so there would be a benefit for more research in this area.
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) is used in a number of critical conditions including necrotising soft tissue infections, carbon monoxide poisoning, traumatic ischaemia, radiation tissue injury, and thermal burns. 68 In such conditions, hypoxia leads to tissue ischaemia and necrosis which allows for the proliferation of anaerobic bacteria. It is suggested that HBOT improves wound healing by the controlled generation of reactive oxygen and nitrogen species which increases the production of growth factors to achieve neovascularisation. 69 It is also proposed to have a direct antibacterial effect on anaerobic bacteria with reduced endotoxin activity in the presence of high tissue oxygen. 70 There is evidence to suggest that HBOT significantly lowers mortality when used as an adjunct to surgical debridement and antibiotic therapy,49,50 but this obviously depends on having the facilities and clinical expertise available. However, the European Association of Urology (EAU) guidelines advise against the use of HBOT outside of clinical trials due to existing evidence being of low quality. 71
Outcomes and follow-up
As part of the management for FG, patients take a significant insult to their physiological reserve undergoing multiple procedures with input from multiple specialist teams. There is however little guidance available for a standardised approach to long-term care and follow-up for these patients. FG patients may experience lower physical role functioning, sexual dysfunction, painful or uncomfortable scars, faecal and urinary incontinence, and psychological dysfunction.72 –74 Sorensen 2 found that on discharge, 23% of patients required ‘home health care’ and 6% of patients required ‘skilled nursing facility’. The issues that patients face after discharge relate to the particular treatments and operations they require as part of their inpatient stay, and follow-up needs to be tailored accordingly. For example, if a diverting colostomy is performed, then patients will need input from the stoma care nursing team and outpatient review with a colorectal surgeon to discuss reversing the stoma, and anastomosing the bowel if possible. For the management of FG, not only reconstructive surgery but also other therapies such as intermittent hyperbaric oxygen therapy with a multiparameter diagnostic monitoring should be recommended as a form of rehabilitation for Fournier’s gangrene survivors after discharge from the hospital.75 –77 These aspects are very important because sepsis develops in over 40% of patients with FG, which is subsequently reflected in a high 1- and 5-year mortality rate.
Conclusion
The management of the FG patient can be divided into initial resuscitation, surgical debridement, ongoing ward management with antibiotic therapy, wound reconstruction, and long-term follow-up. Each facet of care is vital and requires multidisciplinary team expertise for optimal outcomes. Whilst mortality continues to improve from the historic peak, it remains significant, reflecting the severe and life-threatening nature of FG. Whilst many aspects of FG management are becoming more standardised, decisions for certain treatments such as fitting a diverting stoma, performing an orchidectomy, and using adjuncts such as hyperbaric oxygen therapy are made on a case-by-case basis and in accordance with the experience of treating surgical teams. More research is certainly needed into long-term outcomes in FG to improve quality of life measures after discharge.
Acknowledgments
None.
Footnotes
ORCID iDs: Thomas Hughes  https://orcid.org/0000-0001-7846-1470
https://orcid.org/0000-0001-7846-1470
Bhaskar Somani  https://orcid.org/0000-0002-6248-6478
https://orcid.org/0000-0002-6248-6478
Contributor Information
Daniel Bowen, Department of Urology, Mid and South Essex NHS Foundation Trust, Broomfield, UK.
Thomas Hughes, Department of Urology, South Warwickshire University NHS Foundation Trust, Warwick, UK.
Patrick Juliebø-Jones, Department of Urology, Haukeland University Hospital, Bergen, Norway.
Bhaskar Somani, University Hospital Southampton NHS Foundation Trust, Southampton, SO16 6YD, UK.
Declarations
Ethics approval and consent to participate: Our study did not require ethical board approval being a review paper and no patient-specific data were used.
Consent for publication: As this is a review paper, patient consent was not needed.
Author contributions: Daniel Bowen: Conceptualization; Data curation; Writing – original draft.
Thomas Hughes: Data curation; Writing – original draft.
Patrick Juliebø-Jones: Writing – review & editing.
Bhaskar Somani: Conceptualization; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Fournier JA. Jean-Alfred Fournier 1832–1914. Gangrène foudroyante de la verge (overwhelming gangrene). Sem Med 1883. Dis Colon Rectum 1988; 31: 984–988. [DOI] [PubMed] [Google Scholar]
- 2. Sorensen MD, Krieger JN. Fournier’s gangrene: epidemiology and outcomes in the general US population. Urol Int 2016; 97: 249–259. [DOI] [PubMed] [Google Scholar]
- 3. Hatipoglu E, Demiryas S, Şimşek O, et al. Fournier’s gangrene: five years’ experience from a single center in Turkey. Ulus Travma Acil Cerrahi Derg 2020; 26: 235–241. [DOI] [PubMed] [Google Scholar]
- 4. Ruiz-Tovar J, Córdoba L, Devesa JM. Prognostic factors in Fournier gangrene. Asian J Surg 2012; 35: 37–41. [DOI] [PubMed] [Google Scholar]
- 5. Bhatnagar AM, Mohite PN, Suthar M. Fournier’s gangrene: a review of 110 cases for aetiology, predisposing conditions, microorganisms, and modalities for coverage of necrosed scrotum with bare testes. N Z Med J 2008; 121: 46–56. [PubMed] [Google Scholar]
- 6. Singh A, Ahmed K, Aydin A, et al. Fournier’s gangrene. A clinical review. Arch Ital Urol Androl 2016; 88: 157–164. [DOI] [PubMed] [Google Scholar]
- 7. Ngugi P, Magoha G, Nyaga P. Fournier’s ganrene in the HIV era. Afr Health Sci 2014; 14: 1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eke N. Fournier’s gangrene: a review of 1726 cases. Br J Surg 2000; 87: 718–728. [DOI] [PubMed] [Google Scholar]
- 9. Pawłowski W, Wroński M, Krasnodebski IW. Zgorzel Fourniera [Fournier’s gangrene]. Pol Merkur Lekarski 2004; 17: 85–87. [PubMed] [Google Scholar]
- 10. Bowen D, Juliebø-Jones P, Somani BK. Global outcomes and lessons learned in the management of Fournier’s gangrene from high-volume centres: findings from a literature review over the last two decades. World J Urol 2022; 40: 2399–2410. [DOI] [PubMed] [Google Scholar]
- 11. Huayllani MT, Cheema AS, McGuire MJ, et al. Practical review of the current management of Fournier’s gangrene. Plast Reconstr Surg Glob Open 2022; 10: e4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uluğ M, Gedik E, Girgin S, et al. The evaluation of microbiology and Fournier’s gangrene severity index in 27 patients. Int J Infect Dis 2009; 13: e424–e430. [DOI] [PubMed] [Google Scholar]
- 13. Kearney D. Fournier’s gangrene: diagnostic and therapeutic considerations. Gangrene – Current Concepts and Management Options [Internet], https://dx.doi.org/10.5772/23051 (2011, accessed March 2024).
- 14. Hejase MJ, Simonin JE, Bihrle R, et al. Genital Fournier’s gangrene: experience with 38 patients. Urology 1996; 47: 734–739. [DOI] [PubMed] [Google Scholar]
- 15. Gupta A, Dalela D, Sankhwar SN, et al. Bilateral testicular gangrene: does it occur in Fournier’s gangrene? Int Urol Nephrol 2007; 39: 913–915. [DOI] [PubMed] [Google Scholar]
- 16. El-Qushayri AE, Khalaf KM, Dahy A, et al. Fournier’s gangrene mortality: a 17-year systematic review and meta-analysis. Int J Infect Dis 2020; 92: 218–225. [DOI] [PubMed] [Google Scholar]
- 17. Chalya PL, Igenge JZ, Mabula JB, et al. Fournier’s gangrene at a tertiary health facility in northwestern Tanzania: a single centre experiences with 84 patients. BMC Res Notes 2015; 8: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nisbet AA, Thompson IM. Impact of diabetes mellitus on the presentation and outcomes of Fournier’s gangrene. Urology 2002; 60: 775–779. [DOI] [PubMed] [Google Scholar]
- 19. Ioannidis O, Kitsikosta L, Tatsis D, et al. Fournier’s gangrene: lessons learned from multimodal and multidisciplinary management of perineal necrotizing fasciitis. Front Surg 2017; 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Furr J, Watts T, Street R, et al. Contemporary trends in the inpatient management of Fournier’s gangrene: predictors of length of stay and mortality based on population-based sample. Urology 2017; 102: 79–84. [DOI] [PubMed] [Google Scholar]
- 21. Kim SY, Dupree JM, Le BV, et al. A contemporary analysis of Fournier gangrene using the National Surgical Quality Improvement Program. Urology 2015; 85: 1052–1057. [DOI] [PubMed] [Google Scholar]
- 22. Chen IC, Li WC, Hong YC, et al. The microbiological profile and presence of bloodstream infection influence mortality rates in necrotizing fasciitis. Crit Care 2011; 15: R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta N, Zinn KM, Bansal I, et al. Fournier’s gangrene: ultrasound or computed tomography? Med Ultrason 2014; 16: 389–390. [PubMed] [Google Scholar]
- 24. Levenson RB, Singh AK, Novelline RA. Fournier gangrene: role of imaging. Radiographics 2008; 28: 519–528. [DOI] [PubMed] [Google Scholar]
- 25. Jones P, Pietropaolo A, Chew BH. Atlas of scoring systems, grading tools, and nomograms in endourology: a comprehensive overview from the TOWER Endourological Society Research Group. J Endourol 2021; 35: 1863–1882. [DOI] [PubMed] [Google Scholar]
- 26. Wong CH, Khin LW, Heng KS, et al. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32: 1535–1541. [DOI] [PubMed] [Google Scholar]
- 27. Tarricone A, Mata K, Gee A, et al. A systematic review and meta-analysis of the effectiveness of LRINEC score for predicting upper and lower extremity necrotizing fasciitis. J Foot Ankle Surg 2022; 61: 384–389. [DOI] [PubMed] [Google Scholar]
- 28. Hoesl V, Kempa S, Prantl L, et al. The LRINEC score-an indicator for the course and prognosis of necrotizing fasciitis? J Clin Med 2022; 11: 3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdullah M, McWilliams B, Khan SU. Reliability of the laboratory risk indicator in necrotising fasciitis (LRINEC) score. Surgeon 2019; 17: 309–318. [DOI] [PubMed] [Google Scholar]
- 30. Fernando SM, Tran A, Cheng W, et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta-analysis. Ann Surg 2019; 269: 58–65. [DOI] [PubMed] [Google Scholar]
- 31. Kincius M, Telksnys T, Trumbeckas D, et al. Evaluation of LRINEC scale feasibility for predicting outcomes of Fournier gangrene. Surg Infect (Larchmt) 2016; 17: 448–453. [DOI] [PubMed] [Google Scholar]
- 32. Bozkurt O, Sen V, Demir O, et al. Evaluation of the utility of different scoring systems (FGSI, LRINEC and NLR) in the management of Fournier’s gangrene. Int Urol Nephrol 2015; 47: 243–248. [DOI] [PubMed] [Google Scholar]
- 33. Laor E, Palmer LS, Tolia BM, et al. Outcome prediction in patients with Fournier’s gangrene. J Urol 1995; 154: 89–92. [PubMed] [Google Scholar]
- 34. Doluoğlu ÖG, Karagöz MA, Kılınç MF, et al. Overview of different scoring systems in Fournier’s Gangrene and assessment of prognostic factors. Turk J Urol 2016; 42: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corcoran AT, Smaldone MC, Gibbons EP, et al. Validation of the Fournier’s gangrene severity index in a large contemporary series. J Urol 2008; 180: 944–948. [DOI] [PubMed] [Google Scholar]
- 36. Yeniyol CO, Suelozgen T, Arslan M, et al. Fournier’s gangrene: experience with 25 patients and use of Fournier’s gangrene severity index score. Urology 2004; 64: 218–222. [DOI] [PubMed] [Google Scholar]
- 37. Sparenborg JD, Brems JA, Wood AM, et al. Fournier’s gangrene: a modern analysis of predictors of outcomes. Transl Androl Urol 2019; 8: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yilmazlar T, Ozturk E, Ozguc H, et al. Fournier’s gangrene: an analysis of 80 patients and a novel scoring system. Tech Coloproctol 2010; 14: 217–223. [DOI] [PubMed] [Google Scholar]
- 39. Üreyen O, Acar A, Gökçelli U, et al. Usefulness of FGSI and UFGSI scoring systems for predicting mortality in patients with Fournier’s gangrene: a multicenter study. Ulus Travma Acil Cerrahi Derg 2017; 23: 389–394. [DOI] [PubMed] [Google Scholar]
- 40. Egin S, Kamali S, Hot S, et al. Comparison of mortality in Fournier’s gangrene with the two scoring systems. J Coll Physicians Surg Pak 2020; 30: 67–72. [DOI] [PubMed] [Google Scholar]
- 41. Roghmann F, von Bodman C, Tian Z, et al. Vorhersage der Erkrankungsschwere von Patienten mit Fournier-Gangrän [Outcome prediction in patients with Fournier’s gangrene]. Urologe A 2013; 52: 1422–1429. [DOI] [PubMed] [Google Scholar]
- 42. Yalçınkaya S, Yıldız A, Yüksel M, et al. J Urol Surg 2019; 6: 118–124. [Google Scholar]
- 43. Roghmann F, von Bodman C, Löppenberg B, et al. Is there a need for the Fournier’s gangrene severity index? Comparison of scoring systems for outcome prediction in patients with Fournier’s gangrene. BJU Int 2012; 110: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 44. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 45. Gawande AA, Kwaan MR, Regenbogen SE, et al. An Apgar score for surgery. J Am Coll Surg 2007; 204: 201–208. [DOI] [PubMed] [Google Scholar]
- 46. Lin TY, Ou CH, Tzai TS, et al. Validation and simplification of Fournier’s gangrene severity index. Int J Urol 2014; 21: 696–701. [DOI] [PubMed] [Google Scholar]
- 47. Tenório CEL, Lima SVC, Albuquerque AV, et al. Risk factors for mortality in fournier’s gangrene in a general hospital: use of simplified Founier gangrene severe index score (SFGSI). Int Braz J Urol 2018; 44: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buonacera A, Stancanelli B, Colaci M, et al. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci 2022; 23: 3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Feres O, Feitosa MR, Ribeiro da, Rocha JJ, et al. Hyperbaric oxygen therapy decreases mortality due to Fournier’s gangrene: a retrospective comparative study. Med Gas Res 2021; 11: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Creta M, Longo N, Arcaniolo D, et al. Hyperbaric oxygen therapy reduces mortality in patients with Fournier’s gangrene. Results from a multi-institutional observational study. Minerva Urol Nefrol 2020; 72: 223–228. [DOI] [PubMed] [Google Scholar]
- 51. Kranz J, Schlager D, Anheuser P, et al. Desperate need for better management of Fournier’s gangrene. Cent European J Urol 2018; 71: 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wirjopranoto S, Azmi YA. Outcome management of Fournier’s gangrene cases at tertiary hospital: 7 Years experience. Urologia 2022; 89: 104–107. [DOI] [PubMed] [Google Scholar]
- 53. Castillejo Becerra CM, Jaeger CD, Rose JR, et al. Microorganisms and antibiogram patterns in Fournier’s gangrene: contemporary experience from a single tertiary care center. J Urol 2020; 204: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 54. Lin TY, Cheng IH, Ou CH, et al. Incorporating simplified Fournier’s gangrene severity index with early surgical intervention can maximize survival in high-risk Fournier’s gangrene patients. Int J Urol 2019; 26: 737–743. [DOI] [PubMed] [Google Scholar]
- 55. Zhang N, Yu X, Zhang K, et al. A retrospective case series of Fournier’s gangrene: necrotizing fasciitis in perineum and perianal region. BMC Surg 2020; 20: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30: 1750–1754. [DOI] [PubMed] [Google Scholar]
- 57. Akcan A, Sözüer E, Akyildiz H, et al. Necessity of preventive colostomy for Fournier’s gangrene of the anorectal region. Ulus Travma Acil Cerrahi Derg 2009; 15: 342–346. [PubMed] [Google Scholar]
- 58. Laucks SS, II. Fournier’s gangrene. Surg Clin North Am 1994; 74: 1339–1352. [DOI] [PubMed] [Google Scholar]
- 59. Chawla SN, Gallop C, Mydlo JH. Fournier’s gangrene: an analysis of repeated surgical debridement. Eur Urol 2003; 43: 572–575. [DOI] [PubMed] [Google Scholar]
- 60. Mallikarjuna MN, Vijayakumar A, Patil VS, et al. Fournier’s gangrene: current practices. ISRN Surg 2012; 2012: 942437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yanaral F, Balci C, Ozgor F, et al. Comparison of conventional dressings and vacuum-assisted closure in the wound therapy of Fournier’s gangrene. Arch Ital Urol Androl 2017; 89: 208–211. [DOI] [PubMed] [Google Scholar]
- 62. Iacovelli V, Cipriani C, Sandri M, et al. The role of vacuum-assisted closure (VAC) therapy in the management of FOURNIER’S gangrene: a retrospective multi-institutional cohort study. World J Urol 2021; 39: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karian LS, Chung SY, Lee ES. Reconstruction of defects after Fournier gangrene: a systematic review. Eplasty 2015; 15: e18. [PMC free article] [PubMed] [Google Scholar]
- 64. Carvalho JP, Hazan A, Cavalcanti AG, et al. Relation between the area affected by Fournier’s gangrene and the type of reconstructive surgery used. A study with 80 patients. Int Braz J Urol 2007; 33: 510–514. [DOI] [PubMed] [Google Scholar]
- 65. Chen SY, Fu JP, Chen TM, et al. Reconstruction of scrotal and perineal defects in Fournier’s gangrene. J Plast Reconstr Aesthet Surg 2011; 64: 528–534. [DOI] [PubMed] [Google Scholar]
- 66. Ferreira PC, Reis JC, Amarante JM, et al. Fournier’s gangrene: a review of 43 reconstructive cases. Plast Reconstr Surg 2007; 119: 175–184. [DOI] [PubMed] [Google Scholar]
- 67. Coskunfirat OK, Uslu A, Cinpolat A, et al. Superiority of medial circumflex femoral artery perforator flap in scrotal reconstruction. Ann Plast Surg 2011; 67: 526–530. [DOI] [PubMed] [Google Scholar]
- 68. Zheng L, Zheng J, Dong ZG. Reverse sural flap with an adipofascial extension for reconstruction of soft tissue defects with dead spaces in the heel and ankle. Eur J Trauma Emerg Surg 2016; 42: 503–511. [DOI] [PubMed] [Google Scholar]
- 69. Ismayilzade M, Dadaci M, Kendir MS, et al. Gracilis muscle interposition to fill the perianal dead space may decrease hospital length of stay in Fournier’s gangrene. Ann Plast Surg 2023; 90: 356–362. [DOI] [PubMed] [Google Scholar]
- 70. Fife CE, Eckert KA, Workman WT. Ethical issues, standards and quality control in practice of hyperbaric medicine. In: Jain KK. (ed.) Textbook of hyperbaric medicine. 6th ed. New York, Springer, 2016. [Google Scholar]
- 71. Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg 2011; 127(Suppl. 1): 131S–141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. D’Agostino Dias M, Fontes B, Poggetti RS, et al. Hyperbaric oxygen therapy: types of injury and number of sessions—a review of 1506 cases. Undersea Hyperb Med 2008; 35: 53–60. [PubMed] [Google Scholar]
- 73. European Association of Urology. EAU guidelines on urological infections, https://uroweb.org/guidelines/urological-infections (2022, accessed March 2024).
- 74. Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis 2013; 15: 1529–1536. [DOI] [PubMed] [Google Scholar]
- 75. Theiss M, Hofmockel G, Eckert P, et al. Kosmetische und funktionelle Langzeitergebnisse nach Operation einer Fournier-Gangrän [Cosmetic and functional long-term outcome after operation of Fournier gangrene]. Urologe A 1996; 35: 338–341. [PubMed] [Google Scholar]
- 76. Le Fouler A, Jr, Hamy A, Barbieux J, et al. Long-term functional outcomes of perineal gangrene: worse than expected?-an observational retrospective study. Int J Colorectal Dis 2018; 33: 589–592. [DOI] [PubMed] [Google Scholar]
- 77. Matias MJR, Guimarães D, Vilela M, et al. Fournier gangrene – would you KISS it? GMS Interdiscip Plast Reconstr Surg DGPW 2023; 12: Doc12. [DOI] [PMC free article] [PubMed] [Google Scholar]