Abstract
Human cytomegalovirus (HCMV) infection can be fatal to immunocompromised individuals. We have previously reported that gamma interferon and tumor necrosis factor alpha (TNF-α) synergistically inhibit HCMV replication in vitro. Ceramides have been described as second messengers induced by TNF-α. To investigate the mechanisms involved in the inhibition of HCMV by TNF-α, in the present study we have analyzed ceramide production by U373 MG astrocytoma cells and the effects of TNF-α versus ceramides on HCMV replication. Our results show that U373 MG cells did not produce ceramides upon incubation with TNF-α. Moreover, long-chain ceramides induced by treatment with exogenous bacterial sphingomyelinase inhibited HCMV replication in synergy with TNF-α. Surprisingly, short-chain permeant C6-ceramide increased viral replication. Our results show that the anti-HCMV activity of TNF-α is independent of ceramides. In addition, our results suggest that TNF-α and endogenous long-chain ceramides use separate pathways of cell signalling to inhibit HCMV replication, while permeant C6-ceramide appears to activate a third pathway leading to an opposite effect.
Human cytomegalovirus (HCMV) infections are well controlled in the immunocompetent host. Cellular immune responses (CD4+ and CD8+ T cells and NK cells) which accompany both acute and latent infections (for a review, see reference 4) are thought to be the main components of this control. HCMV infection during immunosuppression such as in cancer, transplantations, or AIDS results in severe pathology (4). We have previously shown that tumor necrosis factor alpha (TNF-α), in synergy with gamma interferon (IFN-γ), inhibits the replication of HCMV (7). In mice, TNF-α is involved in the clearance of CMV infection (25). TNF-α is a cytokine with multiple effects which is produced by many cell types, including macrophages and CD8+ and CD4+ T lymphocytes (for a review, see reference 40), and is known to possess antiviral effects (20, 47). The molecular mechanisms involved in the signalling by TNF-α depend on the type of receptor, p55 (TNF-R1) or p75 (TNF-R2) (5), to which it binds. Some cells express only one type of TNF-α receptor; however, expression of these receptors is not always mutually exclusive (5). The cytotoxicity of TNF-α has been reported to be mediated by TNF-R1 (38), whose intracellular region carries a death domain which signals for programmed cell death (39). Signalling through TNF-R1 with specific antibodies can also protect Hep-G2 cells from vesicular stomatitis virus-mediated cytopathic effects (48). Ceramide production after TNF-α treatment has been widely reported (16, 19, 31) and has also been shown to depend on signalling through the TNF-R1 receptor (45). In these experiments concerning myeloid cells, TNF-α induced the activation of a sphingomyelinase, which cleaved sphingomyelin to release ceramide and phosphocholine. The production of ceramides can lead to cell apoptosis (11, 14, 23) or cell cycle arrest (13). Induction of apoptosis by TNF-α has been mimicked by exogenous sphingomyelinase and by synthetic, short-chain, permeant ceramides, which suggests that ceramides, as second messengers, are sufficient to induce the cytotoxic effects of TNF-α (11, 23). Acidic and neutral sphingomyelinases activated in different cell compartments may be responsible for the diverse effects of TNF-α (46), with the former being involved in signalling through NF-κB (34) and the latter being involved in signalling through a ceramide-activated protein kinase and phospholipase A2 (46).
One of the characteristics of HCMV infection is the increase in the content of intracellular DNA, reported to be of viral (3, 8, 18) or cellular (12, 37) origin. Since TNF-α has been known to display antiproliferative properties and to block cells in the G1 phase (29), we initially tested its effects on the cell cycle of infected cells. Then, based on studies reporting that TNF-α induces ceramides in cells (16, 19, 31) and on a study showing the role of ceramide in cell cycle blockade (13), we originally postulated that ceramide was responsible for the antiviral effect of TNF-α. In the present study, we used astrocytoma cells (U373 MG) as a model for brain cells, which are important targets of HCMV in vivo (22). In contrast to fibroblasts, infected U373 MG cells release smaller quantities of virus particles even though all the cells were infected in our experiments. We believe that the U373 MG model is closer to HCMV infection in vivo. We show that ceramides are not produced by U373 MG cells upon incubation with even high concentrations of TNF-α. In addition, we demonstrate that exogenously added sphingomyelinase induces anti-HCMV effects whereas permeant C6-ceramide increases HCMV proliferation in U373 MG cells. This suggests that lipid second messengers can modulate HCMV infection and that TNF-α and ceramides use distinct signalling pathways in the control of HCMV infection. This is supported by our observation that the protein kinase JNK1 is activated exclusively by TNF-α in U373 MG cells and that TNF-α and exogenous sphingomyelinase act in synergy on HCMV infection.
MATERIALS AND METHODS
Reagents.
Fetal calf serum (FCS), normal goat serum (NGS), penicillin-streptomycin, sodium pyruvate, trypsin-EDTA, EDTA, 10× phosphate-buffered saline (PBS) without Ca2+ and Mg2+, RPMI 1640 Glutamax, BME, and Glutamax were from Gibco-BRL. Recombinant human TNF-α was from R and D Systems. Recombinant human IFN-γ was from Boehringer Mannheim. Sphingomyelinase (Staphylococcus aureus), ceramide (type III), diethylenetriaminepentaacetic acid (DETAPAC), ATP, imidazole, dithiothreitol, octyl-β-d-glucopyranoside, cardiolipin (bovine heart), leupeptin, pepstatin A, phenylmethylsulfonyl fluoride, and propidium iodide were from Sigma. N-Hexanoylsphingosine (C6-ceramide) was from BIOMOL Research Laboratories. Recombinant c-Jun was from Santa Cruz Biotechnology. SeaPlaque agarose was from FMC. Protein A-Sepharose was from Pharmacia Biotech. sn-1,2-Diacylglycerol kinase (Escherichia coli) was from Calbiochem. [γ-32P]ATP (4,500 Ci/mmol) was from ICN Radiochemicals. [9,10(n)-3H]palmitic acid (51 Ci/mmol) was from Amersham Life Science. The micro-bicinchoninic acid protein assay kit was purchased from Pierce. Silica gel thin-layer chromatography (TLC) plates were from Merck. Culture flasks were from Falcon, and petri dishes were from Nunc.
Mouse immunoglobulin G1 (IgG1) (isotype control) was from Meloy, Springfield, Va. Culture supernatant E13 (mouse IgG1 anti-IE1 and IE2 proteins) was a kind gift from M.-C. Mazeron (Hôpital Lariboisière, Paris, France). Anti-gB (mouse IgG1) was from the Goodwin Institute for Cancer Research. Monoclonal antibody CCH2 (anti-early protein) was purchased from DAKO. Phycoerythrin-conjugated goat fragment F(ab′)2 to mouse IgG was from Immunotech. Agarose conjugate anti-JNK1 was purchased from Santa Cruz Biotechnology.
Cell culture.
The human astrocytoma cell line U373 MG and foreskin fibroblasts (FSF1), kind gifts from S. Michelson (Institut Pasteur, Paris, France) were grown in RPMI 1640 Glutamax supplemented with heat-inactivated 10% FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), and sodium pyruvate (1 mM). MRC5 cells (embryonic lung fibroblasts, from BioMérieux) were grown in BME supplemented with 10% FCS, glutamine (1 mM), penicillin (100 U/ml), streptomycin (100 μg/ml) and sodium pyruvate (1 mM).
Virus propagation and assay.
Virus stocks were obtained by infection of subconfluent MRC5 monolayers with the Towne strain (a kind gift from S. Michelson) at a multiplicity of infection (MOI) of 0.01. The virus was allowed to adsorb for 1 to 2 h at 37°C before maintenance medium was added. At 4 days postinfection (p.i.), the medium was replaced. The growth in culture was continued until cell lysis occurred, and, after elimination of cell debris (500 × g for 10 min at 4°C), the supernatant was aliquoted and stored at −80°C. The stocks used were tested Mycoplasma negative. The titer of virus stocks was determined by a plaque assay (43). In this assay, dilutions of virus stock or dilutions of supernatant of cells cultured with HCMV and lysed by sonication (Bioblock Scientific Vibra-cell 72405) were used to inoculate a monolayer of MRC5 cells in BME–10% FCS. After 24 h, the medium was replaced with 0.8% agarose in BME and the growth in culture was continued for 7 to 10 days until plaques were clearly observed. The monolayer was then fixed in 10% formaldehyde and stained with 0.05% methylene blue, and the plaques were counted under a microscope.
Cytokine ceramide and sphingomyelinase treatment.
U373 MG cells were seeded at 0.5 × 106 cells per 25-cm2 flask in 2 ml of medium. For treatment with C6-ceramide, different concentrations of ceramide (1, 3, 6, 10, and 40 μM) were added when the cells were seeded and maintained in the medium at each passage (unless otherwise specified). For TNF-α and IFN-γ treatment, medium was replaced with fresh medium containing the given concentrations of cytokine 24 h after seeding. Sphingomyelinase was added to the medium 1 h prior to HCMV infection. For infection by HCMV, the inoculum was added (MOI = 2 unless otherwise specified) 24 h after cytokine or ceramide treatment and removed 20 to 24 h later. The same conditions were used for uninfected and untreated controls in which the cytokine, ceramide, or HCMV was replaced with medium. Under these conditions, 100% of the cells were infected at day 4 p.i. as detected by flow cytometry with CCH2 antibody.
Flow cytometry analysis.
U373 MG cells (0.5 × 106 cells per 25-cm2 flask) were infected at a MOI of 20 for gB expression. The inoculum was removed after 20 to 24 h, and growth in culture was continued for 6 days p.i. The cells were harvested with EDTA, washed once in 1× PBS, and then fixed in 90% ice-cold methanol. At least 1 h after fixation, the cells were washed once with 1× PBS and then once in PBS-BSA (1× PBS, 0.002% Triton X-100, 0.1% NaN3, 0.5% bovine serum albumin [BSA]). For detection of intracellular antigen, the cells were saturated for 30 min at 37°C in PBS-BSA supplemented with 25% NGS. Permeabilized cells were then incubated for 1 h with the primary antibodies (diluted in PBS-BSA supplemented with 2% NGS: 1/1,250 for anti-gB, 1 μg per assay for mouse IgG1, or 50 μl per assay for E13 culture supernatant). After being washed, the assay mixtures were incubated for 30 min with the phycoerythrin-conjugated secondary antibody. Immunofluorescence was measured on an EPICS Elite from Coulter at 575 nm long pass (LP).
The cell cycle was analyzed by the modified technique of Vindelov and Christensen (42), which consists of staining nuclear DNA with propidium iodide, followed by flow cytometry analysis at 630 nm LP.
Long-chain ceramide measurement.
Ceramides were measured by two different techniques, either by a modification of the method of Preiss et al. (28) or by monitoring the amount of ceramides labelled with [3H]palmitic acid.
The first method relies on converting ceramide to ceramide [32P]phosphate by using diacylglycerol kinase. Briefly, lipids were extracted from cells (treated for the given times with different concentrations of TNF-α) by a modification of the method of Bligh and Dyer (1). Adherent U373 MG cells grown in 6-cm petri dishes (106 cells per assay) were harvested with a cell scraper, and lipids were extracted in methanol, 2% acetic acid, chloroform, and water at equal volumes. The organic phase was dried under N2 and then solubilized by sonication in 7.5% octyl-β-d-glucopyranoside–5 mM cardiolipin–1 mM DETAPAC (pH 7.0). Ceramide type III at known concentrations was treated in parallel with the assays to establish a standard curve. The lipids were then incubated for 25 min at 30°C with agitation in 50 μl of reaction mixture (2 mM EGTA, 25 mM MgCl2, 100 mM NaCl, 100 mM imidazole-HCl [pH 6.6]) plus 10 μl of 20 mM dithiothreitol in H2O, 10 μl of 10 mM [γ-32P]ATP (9 μCi/assay, diluted in 100 mM imidazole and 1 mM DETAPAC [pH 6.6]), 5 μl of diacylglycerol kinase (1 mg/ml, 3.78 U/mg of protein), and 5 μl of H2O. The reaction was stopped by the addition of 3 ml of chloroform-methanol (1:2, vol/vol), 0.75 ml of 1% HClO4, and 1 ml of H2O. The organic phase was washed with 2 ml of 1% HClO4 and then dried under N2. The lipids extracted were spotted on TLC silica gel plates and developed in chloroform-methanol-acetic acid (65:15:5, vol/vol/vol). Labelled ceramide-phosphate was located with a Berthold scanner and then recovered and counted on a β-counter (Packard 1900TRI-CARB) in 6 ml of Picofluor (Packard). The quantity of ceramide in each assay was determined by using the standard curve.
The second method allows parallel measurement of other lipids as well as ceramides. U373 MG cells (seeded at 0.5 × 106 cells per assay) were labelled metabolically for 48 h with 0.5 μCi of [3H]palmitate per ml of RPMI–5% FCS. To eliminate excess radioelements, the cells were incubated for 1 to 2 h in RPMI–5% FCS and then treated with different concentrations of TNF-α for the given times in RPMI–5% FCS. Lipids were extracted by the method described above, dried under N2, and then spotted on silica gel TLC plates. Phospholipids were separated by migration up the first half of the plates in chloroform-methanol-acetic acid-formic acid-water (65:30:10:4:2, by volume). The plates were then dried and developed in chloroform-methanol-acetic acid (95:5:5, vol/vol/vol) in the same direction, up to the top of the plate, to separate ceramides from other neutral lipids. Radioactive ceramides and sphingomyelin were visualized on a Berthold scanner and then scraped, and the radioactivity was measured on a β-counter in 6 ml Picofluor. The results are expressed as a percentage of total radioactivity per lane.
Protein kinase assays.
U373 MG cells were seeded at 3 × 106 cells per 75-cm2 flask. After 24 h, they were treated with the agonist for 15 min and then washed with cold PBS. The cells were scraped and disrupted on ice in 1 ml of lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 0.8 mM MgCl2, 5 mM EGTA, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 15 μg of leupeptin per ml, 1 μM pepstatin A, 1 mM sodium orthovanadate). Debris was cleared by centrifugation (800 × g for 10 min), and the supernatant was precleared with protein A-Sepharose beads at 4°C overnight on a rocker. These beads were removed by centrifugation (15,000 × g for 30 s), and the supernatant was then cleared for 15 min with protein A-Sepharose at 4°C. After centrifugation (13,000 rpm for 30 s), the protein kinase JNK1 was immunoprecipitated with 20 μl of the appropriate agarose conjugate per assay for 1 h at 4°C on a rocker. After centrifugation, the supernatant was kept at −20°C to quantify the proteins in each assay by the micro-BCA technique as specified by the manufacturer. The pellets were washed twice with 500 μl of lysis buffer and then twice with 500 μl of kinase buffer (30 mM HEPES [pH 7.5], 30 mM NaCl, 10% glycerol, 200 μM sodium orthovanadate, 0.1% Nonidet P-40). Immunoprecipitated protein kinases were incubated with 150 mM magnesium acetate–75 μM ATP–5 μCi [γ32P]ATP–0.5 μg of c-Jun in 50 μl of kinase buffer per assay for 20 min at 30°C. The reaction was stopped by boiling for 5 min with 20 μl of 4× Laemmli sample buffer and analyzed by autoradiography after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The bands were then quantified by densitometry on a GelDoc 1000 scanner (Bio-Rad).
RESULTS
TNF-α inhibits the cell cycle progression of HCMV-infected cells.
We have previously shown (7) the decrease in viral protein expression and infectious virus production after pretreatment of infected U373 MG cells with TNF-α. In the present study, we confirm this viral inhibition by TNF-α by using flow cytometry analysis of the cell cycle of HCMV-infected U373 MG cells.
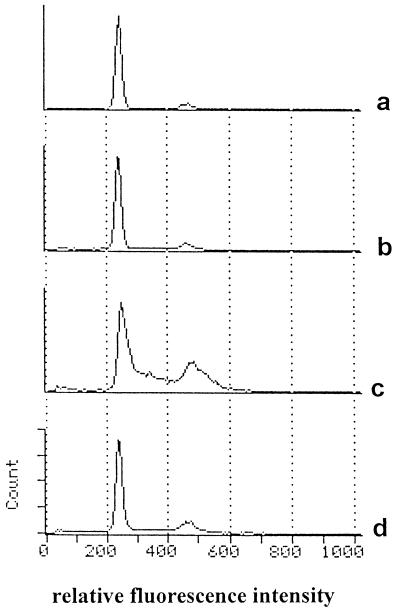
Infection by HCMV in vitro characteristically increases the percentage of cells which appear in the S phase of the cell cycle (12, 37). The cell cycle of uninfected U373 MG cells rapidly reached a status quo (Fig. 1a) with 87% of cells in G0/G1 phase, 4.5% in S, and 8.5% in G2/M. However, 6 days after infection with HCMV at a MOI of 2, this pattern was modified and the cells in the S and G2/M phases reached 40 and 26.5%, respectively, of the total cells (Fig. 1c). Pretreatment of U373 MG cells with 100 U of TNF-α per ml (Fig. 1b) had no effect on day 6 of the cell cycle of uninfected cells; however, in infected cells (Fig. 1d), it tended to cause the original profile of uninfected cells to be retained, with only 17% of cells in S and 13% in G2/M. Apoptosis was not observed by propidium iodide staining when cells were cultured in the presence of TNF-α.
FIG. 1.
Effect of TNF-α on the cell cycle of infected U373 MG cells. U373 MG cells were incubated with 100 U of TNF-α per ml (b and d) or without TNF-α (a and c) for 24 h prior to HCMV infection (c and d) or mock infection (a and b). The medium was replaced 24 h later, and 6 days p.i. the nuclei were stained with propidium iodide and analyzed by flow cytometry.
Therefore, TNF-α exerts a direct anti-HCMV effect on infected U373 MG cells, which does not rely on cytotoxicity or apoptosis.
C6-ceramide has the opposite effect to TNF-α on HCMV.
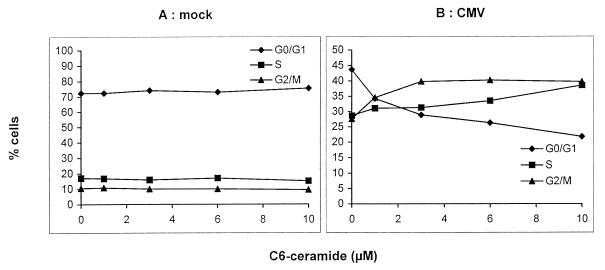
It has been reported (16, 19, 31) that TNF-α can induce the production of intracellular ceramides as second messengers. We investigated whether the viral inhibition observed with TNF-α could be reproduced with the shortchain, cell-permeable C6-ceramide. U373 MG cells were treated with different concentrations of C6-ceramide before and during HCMV infection. Figure 2A shows that C6-ceramide had no effect on the cell cycle of mock-infected cells 6 days p.i. On the same day, however, in HCMV-infected cells (Fig. 2B), C6-ceramide induced a dose-dependent increase in the percentage of cells in S phase (29 and 39% in the absence and presence of 10 μM C6-ceramide, respectively) and G2/M phase (28 and 38% in the absence and presence of 10 μM C6-ceramide, respectively). Therefore, in infected cells, C6-ceramide specifically enhances the increase in the intracellular DNA content already induced by HCMV infection.
FIG. 2.
Effect of C6-ceramide on the cell cycle of U373 MG cells. U373 MG cells were incubated for 24 h with 0 to 10 μM C6-ceramide prior to mock (A) or HCMV (B) infection. The inoculum was removed 24 h later, and the concentrations of ceramide were maintained throughout the experiment. At 6 days p.i., the nuclei were stained with propidium iodide and analyzed by flow cytometry.
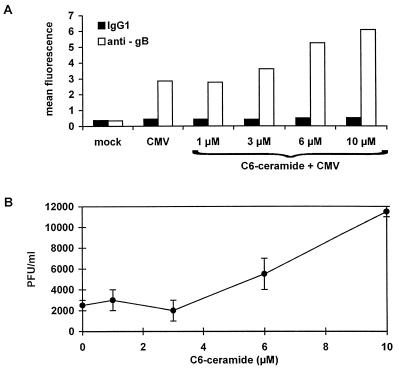
We next studied the expression of the viral envelope protein gB in infected U373 MG cells treated or not treated with C6-ceramide. Flow cytometry analysis (Fig. 3A) showed that the intracellular expression of gB increased in a dose-dependent manner from 3 μM C6-ceramide upward. Similar results for gB expression were obtained with FSF1 cells treated with C6-ceramide (data not shown). These increases in viral protein expression could not, however, be reproduced when dihydro C6-ceramide was used (data not shown), thus confirming the specificity of the permeant C6-ceramide. Furthermore, a plaque assay (Fig. 3B) showed that infected U373 MG cells treated with 10 μM C6-ceramide produced six times more infectious virions than did untreated control cells. Therefore, the short-chain lipid C6-ceramide increases HCMV production in our cell models.
FIG. 3.
Increase in gB expression and HCMV production by C6-ceramide. U373 MG cells were incubated with 0 to 10 μM C6-ceramide prior to HCMV infection. Medium was replaced after 24 h infection, maintaining the concentrations of ceramide throughout the experiment. (A) On day 6 p.i., the expression of gB was estimated by intracytoplasmic immunofluorescent staining followed by flow cytometry analysis. (B) Cells were harvested and sonicated at 6 days p.i. The sonicate was used to infect an MRC5 fibroblast monolayer. Infection was estimated by plaque assay.
The effect of TNF-α is ceramide independent.
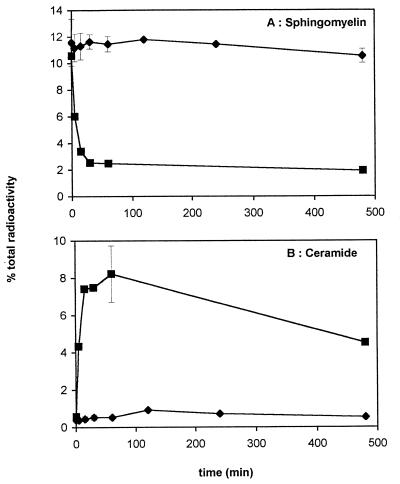
Since U373 MG expressed the p55 but not the p75 TNF-α receptor (data not shown), we looked for a potential production of ceramides in U373 MG cells treated with TNF-α. Figure 4 represents the kinetics of ceramide release and sphingomyelin degradation. Treatment with 0.1 U of sphingomyelinase per ml as a positive control gave a peak of ceramide production at 8.23% total radioactivity 1 h after treatment (Fig. 4B) and a corresponding degradation of 82% of the cellular sphingomyelin (Fig. 4A). Figure 4 shows that 2,000 U of TNF-α per ml induced neither ceramide release nor sphingomyelin degradation in U373MG. Moreover, no ceramide was detected in the medium of cells incubated with TNF-α (data not shown). Lower doses of TNF-α and a longer time course gave the same results, and infection of U373 MG cell with HCMV did not induce the spontaneous production of ceramides (data not shown). This data was also verified by using the diacylglycerol kinase method (28) of quantifying ceramide production. Therefore, the anti-HCMV effect of TNF-α on U373 MG cells is ceramide independent, although these cells express the p55 TNF-α receptor.
FIG. 4.
Effect of TNF-α on ceramide and sphingomyelin levels. U373 MG cells were labelled with 1 μCi of [3H]palmitate (51 Ci/mmol) per dish for 48 h and then incubated for the indicated times with 2,000 U of TNF-α per ml (⧫) or 0.1 U of sphingomyelinase per ml (▪). The quantities of labelled sphingomyelin (A) and ceramide (B) were assessed, and the results are expressed as a percentage of total 3H-labelled lipids. Points on the TNF curve represent the mean ± standard deviation (n = 3).
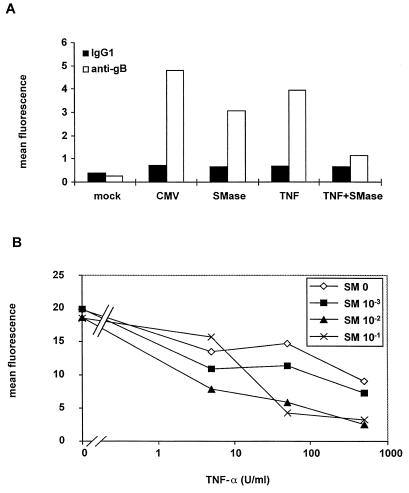
To our surprise, 1 h of sphingomyelinase treatment before infection had the opposite effect to C6-ceramide on infected cells. As shown in Fig. 5A, pretreatment with 0.1 U of sphingomyelinase per ml inhibited the expression of gB protein. Preincubation of U373 MG cells with 100 U of TNF-α per ml also inhibited gB expression but to a lesser extent. Furthermore, gB expression was practically abolished by preincubation with a combination of 0.1 U of sphingomyelinase per ml and 100 U of TNF-α per ml, suggesting synergy between these two reagents. This synergy was confirmed by measuring the amount of infectious virus produced by cells treated with a combination of different concentrations of TNF-α and sphingomyelinase (Fig. 5B). This was carried out by flow cytometry analysis of intracellular IE1 and IE2 protein expression in low-passage FSF1 cells infected with a lysate of treated U373 MG cells which had been infected for 6 days. The positive control of FSF1 cells infected with untreated, infected-cell lysate gave a mean fluorescence of 20 for IE1 plus IE2 expression on day 4 p.i. Figure 5B shows that all the combinations of TNF-α and sphingomyelinase displayed synergistic activity on HCMV production. For example, separate treatments with 500 U of TNF-α per ml and 0.1 U of sphingomyelinase per ml induced a moderate decrease of virus production (mean fluorescence intensities of 9.1 and 18.5, respectively), whereas their combination was synergistic and strongly decreased virus production (fluorescence intensity of 3.3).
FIG. 5.
Synergy between TNF-α and sphingomyelinase on HCMV inhibition. (A) gB expression. U373 MG cells were preincubated for 24 h with 100 U of TNF-α per ml and/or treated with 0.1 U of sphingomyelinase (SMase) per ml 1 h before HCMV infection. Expression of gB was assessed by intracellular immunofluorescent staining followed by flow cytometry analysis. (B) Viral production. U373 MG cells were pretreated or not treated for 24 h with the indicated concentrations of TNF-α and/or treated with the indicated concentrations of sphingomyelinase (SM) 1 h prior to HCMV infection at a MOI of 5. On day 6 p.i., the cells were sonicated and the sonicate was used at a dilution of 1/100 to infect a monolayer of FSF1 cells. Intracellular IE expression was estimated 4 days later by flow cytometry analysis. The results are expressed as mean fluorescence.
This agrees with the fact that we observed no ceramide production induced by TNF-α and suggests that TNF-α uses another pathway that is complementary to the sphingomyelin pathway in HCMV inhibition.
Differences in signalling via the MAPK pathways.
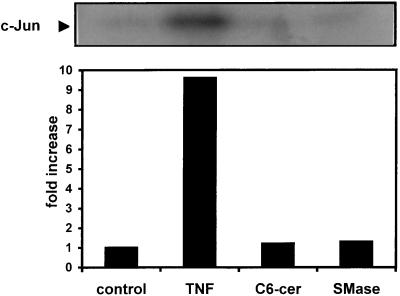
The current literature suggests a role for the mitogen-activated protein kinase (MAPK) pathways in signalling via ceramides, and several authors have shown that in HL-60 cells (30, 44) or rat glomerular mesangial cells (6) the protein kinase JNK1 is activated by TNF-α and ceramides. We therefore decided to see whether there was a difference in MAPK signalling between TNF-α and ceramides in U373 MG cells. We measured the activity of JNK1 after a 15 min treatment with TNF-α, C6-ceramide, or sphingomyelinase and found that only TNF-α was capable of activating JNK1 in U373 MG cells (Fig. 6). Higher doses (40 μM) of C6-ceramide also had no effect on JNK activity (data not shown). We conclude that in U373 MG cells, TNF-α and long-chain ceramides effectively use different signalling pathways.
FIG. 6.
TNF-α alone increases JNK1 activity. U373 MG cells were either untreated (control) or treated with 500 U of TNF-α (TNF) per ml, 10 μM C6-ceramide (C6-cer), or 0.1 U of sphingomyelinase (SMase) per ml for 15 min prior to cell lysis. JNK1 was immunoprecipitated from cell lysates and incubated with recombinant c-Jun and [γ-32P]ATP in a 20-min assay at 30°C. Phosphorylation of c-Jun was determined by autoradiography following separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% polyacrylamide). The bands were quantified by densitometry and expressed as fold increase compared to control. This data is from one of three experiments with similar results.
DISCUSSION
We have previously reported that TNF-α alone and in synergy with IFN-γ mediates the protection of cells against HCMV infection in vitro (7). In the present study, we investigated the potential role of ceramides in the anti-HCMV effects of TNF-α in U373 MG cells. Our results show that these cells did not produce ceramides upon incubation with TNF-α. Moreover, contrary to the opinion that exogenous sphingomyelinase and permeant ceramides may be used as reagents with comparable biological consequences in cells, we found that they had opposite effects on HCMV infection: exogenous sphingomyelinase inhibited HCMV infection, whereas C6-ceramide amplified it. We also show that TNF-α and long-chain ceramides use separate pathways of cell signalling to inhibit HCMV proliferation while short-chain ceramides appear to activate a third pathway, leading to an opposite effect. This is the first report of ceramide-independent antiviral activity of TNF-α.
The cytotoxic (38), cytostatic, and antiviral (48) effects of TNF-α have been linked to signalling via the p55 TNF-α receptor, which we have found to be the only receptor present on U373 MG cells (data not shown). Wiegmann et al. (46) have shown that intracellular ceramide release is mediated via the p55 receptor either by a neutral, membrane-associated sphingomyelinase or by an acidic, endosomal sphingomyelinase. The antiviral activity of TNF-α has been attributed to two mechanisms: protection of cells from cytopathic effect in vesicular stomatitis virus infection (48) or lysis of infected cells (49). In the present study, TNF-α provoked a decrease in the percentage of cells in the S and G2/M phases during infection, although it had no effect on the cycle of noninfected cells and was incapable, even at high concentrations, of inducing apoptosis in these cells. The absence of apoptosis was not, however, due to a defect in the cell line, since high concentrations of C6-ceramide (80 μM) were capable of inducing cell death by apoptosis (data not shown). Therefore, the antiviral activity of TNF-α in our model is due neither to cell lysis nor to apoptosis, and we show that TNF-α is capable of mediating a cytostatic effect without ceramide production or cytotoxicity despite the p55 receptor being used in U373 MG cells.
We used exogenous sphingomyelinase treatment of U373 MG cells as a positive control for long-chain ceramide release. This enzymatic treatment induced ceramide production and also displayed anti-HCMV activity, which implies that long-chain ceramides are responsible for this effect. A combination of exogenous sphingomyelinase and TNF-α synergistically enhanced the anti-HCMV action of the two reagents, suggesting that two complementary pathways are being used.
C6-ceramide was incapable of reproducing the anti-HCMV effect of TNF-α or exogenous sphingomyelinase. This was surprising, since short-chain ceramides have been considered the equivalent of endogenous ceramide production and have been extensively used in this way (13, 31), although different effects of bacterial sphingomyelinase and exogenous ceramides in some studies have led to the observation of distinct pools of sphingomyelin (50). C6-ceramide amplified the percentage of cells in the S and G2/M phases, a hallmark of HCMV infection, which has been attributed either to an increase in cellular DNA replication (12, 37) or to a mass production of viral DNA (3, 8, 18). C6-ceramide also increased the expression of the envelope viral protein gB, as well as the amount of infectious virus produced by U373 MG cells. These results have also been validated with FSF1 cells (data not shown), which showed the same increase in gB expression following C6-ceramide treatment, proving that this is not specific to U373 MG cells. This model of HCMV infection has thus revealed a signalling pathway which is different for long- and short-chain ceramides. Exogenous sphingomyelinase treatment cleaves sphingomyelin of the outer leaflet of the plasma membrane; the long-chain ceramides generated are hydrophobic and will progress only to the cytosol. C6-ceramide, however, can cross the plasma membrane as well as the internal membranes, and Futerman et al. (9) have shown that it accumulates in the Golgi apparatus, where the majority of sphingomyelin synthesis occurs. These differences may explain the discrepancy observed with long-chain and short-chain ceramides on the infection of U373 MG cells. Another possibility is that exogenous ceramides altered the cell secretory pathway, as described previously (33), and prevented the release of virions into the medium, thereby increasing the amounts of intracellular structural proteins and of intracellular infectious virions. Alternatively, Venable et al. (41) have shown that C6-ceramide is metabolized in WI-38 human diploid fibroblasts to give two minor, unidentified metabolites, which could possibly explain the differences observed between C6-ceramide treatment and sphingomyelinase-induced ceramide release. Mitogenic activity of C6-ceramide has already been reported in fibroblasts (10, 24), and ceramide has also been shown to be involved in cellular senescence (41). Thus, in all cases, ceramide appears to be closely linked to the cell cycle, whether it be progression or arrest, although the pathways responsible for these apparently contradictory effects remain to be elucidated. One pathway of cell activation is illustrated by the costimulation provided in T lymphocytes by CD28, which signals through acidic sphingomyelinase (2). It has been reported that TNF-α does not induce the production of ceramide in human vascular endothelial cells but still induces JNK activation (35). We propose that TNF-α induces ceramide production as second messengers or not depending on the cell type studied.
The MAPK pathways are constantly gaining in members and complexity (see reference 32 for a recent review). They are known to be involved in signalling by mitogens that activate the extracellular signal-regulated protein kinase pathway (ERK) and also in signalling in response to stress through the c-Jun amino-terminal kinase/stress-activated protein kinase (JNK/SAPK) pathway. TNF-α signal transduction is known to involve the JNK protein kinases (36), JNK1 and JNK2. Several authors have shown that in HL-60 cells (30, 44) and in rat glomerular mesangial cells (6), sphingomyelinase and C2- or C6-ceramide also activate this JNK/SAPK pathway but have no action on or inhibit the ERK pathway. The assays for JNK1 activity performed in this study clearly show that in our model this is not the case: only TNF-α activates JNK1, whereas C6-ceramide and sphingomyelinase have no effect on this kinase. This is not due to the concentration of C6-ceramide used in this particular experiment (10 μM), since experiments performed with up to 40 μM C6-ceramide gave identical results (data not shown). This data demonstrates that separate pathways are being used by TNF-α and long-chain ceramides in U373 MG cells. In addition, our data is in accordance with recent reports indicating that JNK activation is independent of ceramides and is not linked to apoptosis (17, 21).
TNF-α has a wide range of activities (40) which depend as much on the cell type studied as on the receptor used to transduce the signal. The mechanisms by which TNF-α mediates anti-HCMV activity remain undetermined. We have eliminated the possibility of production of nitric oxide (NO), which has potent antiviral activity (15), since neither TNF-α, sphingomyelinase, nor C6-ceramide induced the production of NO in U373 MG cells (data not shown). The novelty of this study resides in the observations that the anti-HCMV effect of TNF-α is independent of ceramides but that ceramides can influence the viral cycle of HCMV in vitro. Another study which investigated the effect of ceramides on viral replication was performed by Rivas et al. (31) with HL-60 cells infected with human immunodeficiency virus. In the case of human immunodeficiency virus infection, in contrast to HCMV, TNF-α is involved in the progression of the viral cycle in vitro (26, 27). In that study, ceramide was shown to be responsible for this progression, since exogenous sphingomyelinase and C8-ceramide were capable of reproducing the increase in production of p24 by TNF-α. Our data is in accordance with a model in which the various effects of TNF-α may depend not only on whether long-chain ceramides are produced but also, in the case of ceramide production, on the cell topology of this production (9, 46).
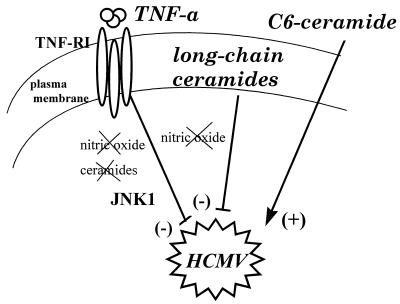
In conclusion, and as schematically depicted in Fig. 7, HCMV infection has revealed three different signalling pathways for TNF-α and ceramides: a ceramide-independent inhibition of HCMV replication by TNF-α, a long-chain-ceramide-dependent inhibition of HCMV replication, and a permeant C6 ceramide-induced increase of HCMV infection.
FIG. 7.
Summary of the pathways used by TNF-α and ceramides in the control of in vitro HCMV replication. TNF-α inhibits HCMV replication through a ceramide-independent and NO-independent pathway (see the text). Activation of JNK1 may be involved in this inhibition. Long-chain ceramides inhibit HCMV infection through another pathway, which is also independent of NO. Permeant C6-ceramide uses yet another pathway to activate HCMV replication.
ACKNOWLEDGMENTS
This study was supported by grants from INSERM, “Association Recherche et Transfusion” and “Conseil Régional Midi-Pyrénées.” Justine Allan Yorke was supported by an M.E.S.R. fellowship.
We thank Hélène Brun and Georges Cassar for help with flow cytometry, Danièle Clément for technical assistance, and Hugues Chap for support and critical reading of the manuscript.
REFERENCES
- 1.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–918. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 2.Boucher L-M, Wiegmann K, Fütterer A, Pfeffer K, Machleidt T, Schütze S, Mak T W, Krönke M. CD28 signals through acidic sphingomyelinase. J Exp Med. 1995;181:2059–2068. doi: 10.1084/jem.181.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresnahan W A, Boldogh I, Aubrey Thomson E, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 4.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, editor. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 5.Brockhaus M, Schoenfeld H-J, Schlaeger E-J, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci USA. 1990;87:3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coroneos E, Wang Y, Panuska J R, Templeton D J, Kester M. Sphingolipid metabolites differentially regulate extracellular signal-regulated kinase and stress-activated protein kinase cascades. Biochem J. 1996;316:13–17. doi: 10.1042/bj3160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davignon J-L, Castanié P, Allan Yorke J, Gautier N, Clément D, Davrinche C. Anti-human cytomegalovirus activity of cytokines produced by CD4+ T-cell clones specifically activated by IE1 peptides in vitro. J Virol. 1996;70:2162–2169. doi: 10.1128/jvi.70.4.2162-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dittmer D, Mocarski E S. Human cytomegalovirus infection inhibits G1/S transition. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Futerman A H, Stieger B, Hubbard A L, Pagano R E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990;265:8650–8657. [PubMed] [Google Scholar]
- 10.Hauser J M L, Buehrer B M, Bell R M. Role of ceramide in mitogenesis induced by exogenous sphingoid bases. J Biol Chem. 1994;269:6803–6809. [PubMed] [Google Scholar]
- 11.Jarvis W D, Kolesnick R N, Fornari F A, Traylor R S, Gewirtz D A, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci USA. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jault F M, Jault J-M, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayadev S, Liu B, Bielawska A E, Lee J Y, Nazaire F, Pushkareva M Y, Obeid L M, Hannun Y A. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 14.Ji L, Zhang G, Uematsu S, Akahori Y, Hirabayashi Y. Induction of apoptotic DNA fragmentation and cell death by natural ceramide. FEBS Lett. 1995;358:211–214. doi: 10.1016/0014-5793(94)01428-4. [DOI] [PubMed] [Google Scholar]
- 15.Karupiah G, Xie Q-W, Buller R M, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 16.Kim M-Y, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor-α and γ-interferon. J Biol Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- 17.Liu Z-G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 18.Lu M, Shenk T. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathias S, Dressler K A, Kolesnick R N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor α. Proc Natl Acad Sci USA. 1991;88:10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestan J, Digel W, Mittnacht S, Hillen H, Blohm D, Möller A, Jacobsen H, Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. Nature. 1986;323:816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- 21.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodget J R, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1996;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 22.Nelson J A, Reynolds-Kohler C, Oldstone M B A, Wiley C A. HIV and HCMV coinfect brain cells in patients with AIDS. Virology. 1988;165:286–290. doi: 10.1016/0042-6822(88)90685-x. [DOI] [PubMed] [Google Scholar]
- 23.Obeid L M, Linardic C M, Karolak L A, Hannun Y. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 24.Olivera A, Buckley N E, Spiegel S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1992;267:26121–26127. [PubMed] [Google Scholar]
- 25.Pavic I, Polic B, Crnkovic I, Lucin P, Jonjic S, Koszninowski U H. Participation of endogenous tumor necrosis factor α in host resistance to cytomegalovirus infection. J Gen Virol. 1993;74:2215–2223. doi: 10.1099/0022-1317-74-10-2215. [DOI] [PubMed] [Google Scholar]
- 26.Paya C V, Ten R M, Bessia C, Alcami J, Hay R T, Virelizier J L. NF-κB-dependent induction of the NF-KB p50 subunit gene promoter underlies self-perpetuation of human immunodeficiency virus transcription in monocytic cells. Proc Natl Acad Sci USA. 1992;89:7826–7830. doi: 10.1073/pnas.89.16.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poli G, Kinter A, Justement J S, Kehrl J H, Bressler P, Stanley S, Fauci A S. Tumor necrosis factor α functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preiss J, Loomis C R, Bishop W R, Stein R, Niedel J E, Bell R M. Quantative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- 29.Pusztai L, Lewis C E, O’D. McGee J. Growth arrest of the breast cancer cell line, T47D, by TNFα: cell cycle specificity and signal transduction. Br J Cancer. 1993;67:290–296. doi: 10.1038/bjc.1993.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raines M A, Kolesnick R N, Golde D W. Spingomyelinase and ceramide activate mitogen-activated protein kinase in myeloid HL-60 cells. J Biol Chem. 1993;268:14572–14575. [PubMed] [Google Scholar]
- 31.Rivas C I, Golde D W, Vera J C, Kolesnick R N. Involvement of the sphingomyelin pathway in autocrine tumor necrosis factor signaling for human immunodeficiency virus production in chronically infected HL-60 cells. Blood. 1994;83:2191–2197. [PubMed] [Google Scholar]
- 32.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 33.Rosenwald A G, Pagano R E. Inhibition of glycoprotein traffic through the secretory pathway by ceramide. J Biol Chem. 1993;268:4577–4579. [PubMed] [Google Scholar]
- 34.Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 35.Slowik M R, Deluca L G, Min W, Pober J S. Ceramide is not a signal for tumor necrosis factor-induced gene expression but does cause programmed cell death in human vascular endothelial cells. Circ Res. 1996;79:736–747. doi: 10.1161/01.res.79.4.736. [DOI] [PubMed] [Google Scholar]
- 36.Sluss H K, Barrett T, Dérijard B, Davis R J. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St. Jeor S C, Albrecht T B, Funk F D, Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974;13:353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tartaglia L A, Rothe M, Hu Y F, Goeddel D V. Tumor necrosis factor’s cytotoxic activity is signaled by the p55 receptor. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 39.Tartaglia L A, Ayres T M, Wong G H W, Goeddel D V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 40.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 41.Venable M E, Lee J Y, Smyth M J, Bielawska A, Obeid L M. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 42.Vindelov L L, Christensen I J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric analysis. Cytometry. 1990;11:753–770. doi: 10.1002/cyto.990110702. [DOI] [PubMed] [Google Scholar]
- 43.Wentworth B B, French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970;135:253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]
- 44.Westwick J K, Bielawska A E, Dbaibo G, Hannun Y A, Brenner D A. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270:22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- 45.Wiegmann K, Schütze S, Kampen E, Himmler A, Machleidt T, Krönke M. Human 55-kDa receptor for tumor necrosis factor coupled to signal transduction cascades. J Biol Chem. 1992;267:17997–18001. [PubMed] [Google Scholar]
- 46.Wiegmann K, Schütze S, Machleidt T, Witte D, Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 47.Wong G H, Goeddel D V. Tumour necrosis factors α and β inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 48.Wong G H W, Tartaglia L A, Lee M S, Goeddel D V. Antiviral activity of tumor necrosis factor is signaled through the 55-kDa type I TNF receptor. J Immunol. 1992;149:3350–3353. [PubMed] [Google Scholar]
- 49.Wong G H W, Krowka J, Stites D P, Goeddel D V. In vitro anti-human immunodeficiency virus activities of tumor necrosis factor-α and interferon-γ. J Immunol. 1988;140:120–124. [PubMed] [Google Scholar]
- 50.Zhang P, Liu B, Jenkins G M, Hannun Y, Obeid L M. Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis. J Biol Chem. 1997;272:9609–9612. doi: 10.1074/jbc.272.15.9609. [DOI] [PubMed] [Google Scholar]