Abstract
Background:
Bone metastasis (BM) seriously affects the quality of life and reduces the survival time of patients with non-small-cell lung cancer (NSCLC). The genomic characteristics and potential targets of BMs are yet to be fully explored.
Objective:
To explore the genetic characteristics and potential targets of BM in NSCLC.
Design:
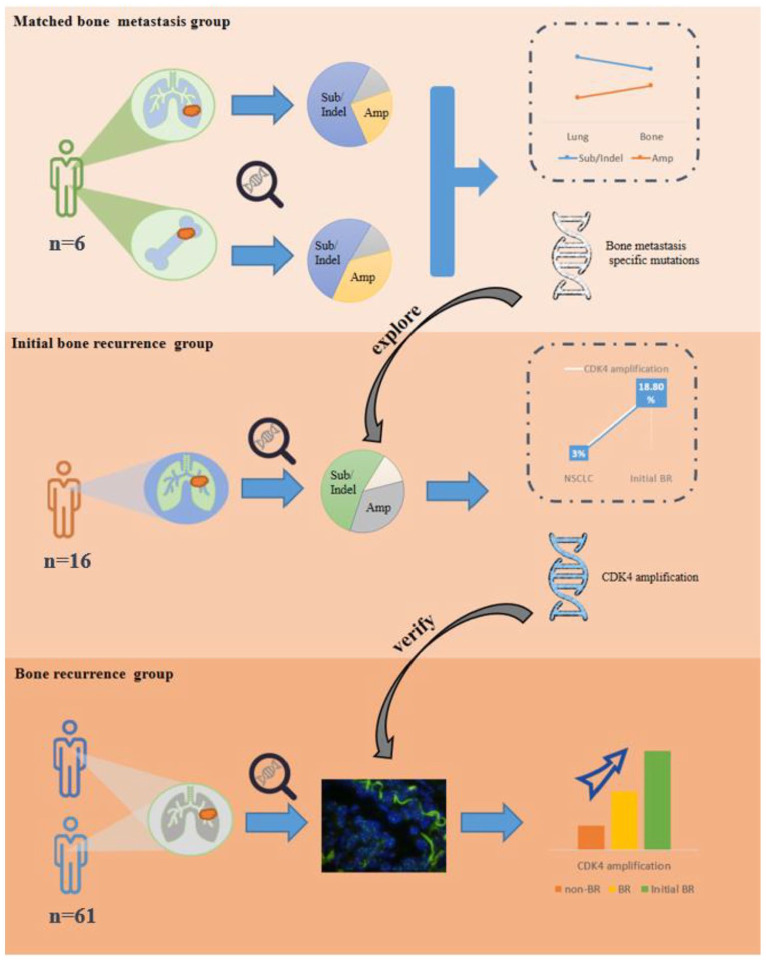
In all, 83 patients with NSCLC were retrospectively selected in this study. Genomic characterization of BMs was explored with the analysis of NGS results from primary tumors and BMs in 6 patients, then combined with NGS results of lung tumors in 16 patients with initial recurrence in bone to analyze mutations potentially associated with BMs, and finally, the correlation was further validated in 61 postoperative patients.
Methods:
The next generation sequencing (NGS) was performed to identify genomic differences between pulmonary primary tumors and BM. Fluorescence in situ hybridization and immunohistochemistry were performed in postoperative tumor tissues from patients who had undergone radical surgery to validate the predictive role of molecular targets for BM. The correlation between cyclin-dependent kinase 4 (CDK4) and BM was evaluated by Pearson’s chi-square test. The university of alabama at birminghan cancer data analysis portal (UALCAN) was carried out for the detection of CDK4 expression in lung cancer and the relationship between CDK4 and clinicopathological parameters. The relationship between prognosis and CDK4 expression was analyzed by the Kaplan–Meier plotter.
Results:
The rate of gene amplification was increased (24% versus 36%) while gene substitution/indel was decreased (64% versus 52%) in BMs. The BM-specific mutations were analyzed in 16 recurrent patients which revealed the highest incidence of CDK4 amplification (18.8%). According to the Kaplan–Meier plotter database, the NSCLC patients with high CDK4 gene expression showed poor overall survival (OS) and recurrence-free survival (RFS) (p < 0.05). The incidence of CDK4 amplification tended to be higher in recurrent patients compared to the patients without BM (18.8% versus 4.7%, p = 0.118).
Conclusion:
Compared to the primary tumors of NSCLC, the genome of BMs showed an increased proportion of amplification and a decreased proportion of gene substitution/indel. Furthermore, the CDK4 amplification ratio seemed to be elevated in NSCLC patients with BM which may be associated with poor OS and RFS.
Keywords: bone metastases, CDK4 amplification, genomic characterization, non-small-cell lung cancer, targeted therapy
Plain language summary
Genomic characterization and potential targets of bone metastasis in non-small cell lung cancer
NGS was performed on the matched primary tumors and bone metastases to explore the differences in the genomes of bone metastases, and it was found that gene amplification increased in bone metastases. Combined with the results of NGS in NSCLC patients with the first postoperative recurrence site in the bone, it was found that CDK4 amplification expression increased in bone metastases. Finally, the correlation between bone metastasis and CDK4 amplification was verified by expanding the sample.
Graphical abstract.
Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide. Despite the therapeutic breakthroughs followed by the development of targeted therapies and immune checkpoint inhibitors, the prognosis of non-small-cell lung cancer (NSCLC) remains poor. Distant metastasis inevitably occurs in most cancer patients and even in those who underwent radical treatment. Bone is one of the most frequent sites of metastasis in NSCLC, approximately occurring in 20–50% of the advanced patients.1–4 Once bone metastasis (BM) occurs in NSCLC patients, nearly 80% of patients suffer from significant pain that compromises the quality of life and decreases the patient’s survival time.2,3,5 The median survival time of patients with BMs is 5.8–7 months.3,5 Approximately 46% of BM patients develop skeletal-related events, including hypercalcemia, pathological fracture, spinal cord compression, and others, which further decrease the patient’s survival time. 1
Although patient-specific therapy according to the genetic profile has become a fundamental treatment strategy for patients with NSCLC, very little is known about the genomic characteristics of BM. A previous study showed a genomic heterogeneity between the primary lung tumors and BM with a high mutation rate of epidermal growth factor receptor (EGFR) (75% versus 12.8%) in BM patients. 6 However, the mutation rate of EGFR was found to be similar in primary lung tumors and BM (62.5% versus 64.1%) in another study. 7 As the metastatic site of the tumor is not random and is determined by multiple factors, including molecular subtypes,8,9 several studies have explored molecular markers that may be associated with BMs. For instance, Kuijpers et al. 10 analyzed 1994 NSCLC patients and found that those who harbored EGFR mutation had a significant incidence of BMs (53.8% versus 31.5%). Similarly, Lohinai et al. 11 investigated the effect of Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation on overall survival (OS) in 500 patients with advanced lung adenocarcinoma (LUAC) and found that such mutation is associated with poor prognosis in LUAC patients with BM. Although the above-mentioned studies found a correlation between genetic mutations in primary lung cancerous foci and BM; however, they did not explore the genomic characteristics and molecular signatures of BM.
In this study, we explored the genomic characteristics and potential targets of BM in NSCLC. We found that the rate of gene amplification was relatively increased in BM, and the incidence of cyclin-dependent kinase 4 (CDK4) amplification was increased in BM and may be associated with worse OS and recurrence-free survival (RFS). Several studies have confirmed the curative effect of CDK4/6 inhibitors in breast cancer patients with BMs.12–14 Although the application of CDK4/6 inhibitors in the treatment of NSCLC is still in clinical trials, several studies have shown that CDK4/6 inhibitors combined with other anticancer therapies can produce a synergistic antitumor effect.15–19
Methods
Patients and sample collection
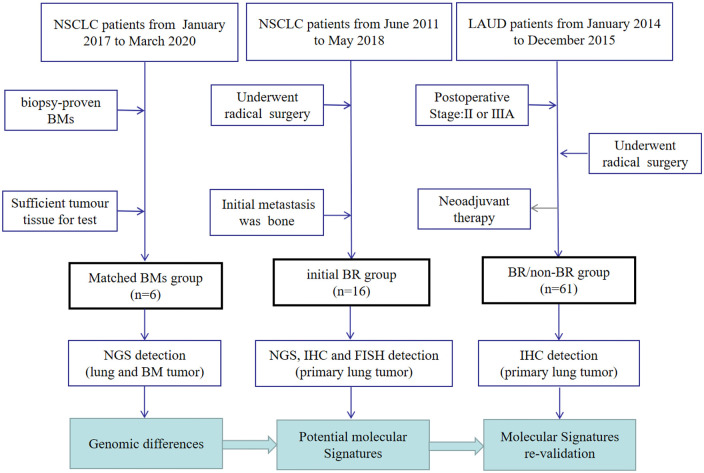
A total of 83 patients with NSCLC from Zhejiang Cancer Hospital were retrospectively selected for the study. The flow chart of patients included in the study is shown in Figure 1, and their clinicopathological data are shown in Table 1. The first part included six NSCLC patients who had undergone BM surgery or biopsy from January 2017 to March 2020, and their primary lung tumor and matched BM tissues were preserved in our hospital, which we defined as the ‘matched BM’ group. The inclusion criteria for this group were as follows: (1) the pathological type was NSCLC and (2) BMs were confirmed by biopsy or surgery. Exclusion criteria were as follows: (1) a combination of other malignant tumors and (2) insufficient tumor tissue for testing. The second part included 16 patients after radical surgery from June 2011 to May 2018, and the site of their first tumor metastasis was bone. Therefore, we defined the second group as the ‘initial bone recurrence (BR)’ group. The inclusion criteria for the initial BR group were as follows: (1) the pathological type was NSCLC, (2) underwent radical surgery for lung cancer, (3) the first site of tumor recurrence was bone, and (4) there was sufficient tumor tissue from primary lung tumor to be detected. Exclusion criteria were as follows: (1) at the time of diagnosis of tumor recurrence, there was other metastasis outside the bone, (2) unable to identify primary tumors of BMs tumors, (3) a combination of other malignant tumors, and (4) insufficient tumor tissue for testing. The other 61 patients who had undergone radical surgery (pathological stage II or IIIA) from January 2014 to December 2015 comprised the third part, and these patients were followed up until 31 October 2022. Among them, 18 patients who experienced BM were defined as the ‘BR’ group, while the other 43 patients without BM were defined as the ‘non-BR’ group. The inclusion criteria for the third group were as follows: (1) the pathological type was NSCLC, (2) underwent radical lung cancer resection, and (3) the postoperative pathologic staging for II or IIIA. Exclusion criteria were as follows: (1) received neoadjuvant therapy, (2) without regular follow-up, (3) a combination of other malignant tumors, and (4) insufficient tumor tissue for testing.
Figure 1.
Sample collection and study design of the study.
Those with second primary malignancies (except cervical carcinoma in situ and basal cell carcinoma of the skin) were excluded. BMs were diagnosed by pathological biopsy or one of the CT, MRI, or PET-CT imaging findings. All the pathology and imaging results were assessed by experienced pathologists and radiologists in our hospital. The present study was approved by the ethics committee of Zhejiang Cancer Hospital.
CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Table 1.
Clinicopathological characteristics of the included patients.
| Matched BMs group (n = 6) | Initial BR group (n = 16) | BR group (n = 18) | Non-BR group (n = 43) | |
|---|---|---|---|---|
| Sex, n [%] | ||||
| Male | 1 [17] | 8 [50] | 8 [44] | 19 [44] |
| Female | 5 [83] | 8 [50] | 10 [56] | 24 [56] |
| Median age (years) | 60 (46–67) | 59 (49–71) | 61 (54–73) | 60 (32–77) |
| Stage, n [%] | ||||
| I | 0 | 2 [13] | 0 | 0 |
| IIA | 0 | 1 [6] | 0 | 4 [9] |
| IIB | 0 | 5 [31] | 5 [28] | 11 [26] |
| IIIA | 0 | 7 [43] | 13 [72] | 28 [65] |
| IIIB | 0 | 0 | 0 | 0 |
| IV | 6 [100] | 0 | 0 | 0 |
| Histology, n [%] | ||||
| Adenocarcinoma | 4 [67] | 14 [87] | 18 [100] | 43 [100] |
| Squamous | 1 [17] | 2 [13] | 0 | 0 |
| NSCLC | 1 [17] | 0 | 0 | 0 |
| Smoking, n [%] | ||||
| Yes | 4 [67] | 11 [69] | 7 [39] | 31 [72] |
| No | 2 [33] | 5 [31] | 11 [61] | 12 [28] |
| Adjuvant therapy | ||||
| Yes | – | – | 15 [83] | 34 [79] |
| No | 3 [17] | 9 [21] | ||
| Driver gene a | ||||
| TP53 | 5 [83] | 10 [63] | – | – |
| EGFR | 2 [33] | 10 [63] | ||
| MET | 0 | 3 [18.75] | ||
The staging records for the initial BR group, BR group, and non-BR group were postoperative staging.
Driver genes listed in the table are those detected, those not detected are not included.
BM, bone metastasis; BR, bone recurrence; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; TP53, tumor protein p53.
The tissues of primary tumors were collected from all included patients, and BM tissues were collected from the matched BM group. The matched primary tumor and BM tissues were obtained via surgery or biopsy, while the primary tumor tissues from the initial BR group, BR group, and non-BR group were postoperative specimens. All tissue samples were preserved as formalin-fixed paraffin-embedded (FFPE) blocks. The reporting of this manuscript has been done as per the Strengthening the Reporting of Observational Studies in Epidemiology checklist. 20
DNA extraction and NGS analysis
DNA was extracted from each tumor-rich FFPE block. Thereafter, NGS analysis was performed on the tumor and matched normal DNA, and the potential mutations and therapeutic targets were explored in the genome map. The genomic information was obtained from the NGS-based YuanSu™ 450 gene panel (OrigiMed, Shanghai, China) that contains information on all coding exons of 450 cancer-related genes and 64 selected introns of 39 frequently rearranged solid tumor-related genes. The detailed DNA extraction and NGS analysis were performed according to a previous study. 21
Immunohistochemical detection and result analysis
The monoclonal mouse anti-CDK4 antibody (1:100 dilution; ab108357; Abcam, Cambridge, MA, USA), as the primary antibody, was incubated overnight at 4°C. Phosphate-buffered saline was used as the negative control. A secondary antibody was applied using the BOND-MAX Fully Automated IHC Staining System according to the manufacturer’s instructions. All staining slides were blindly reviewed by experienced pathologists for scoring CDK4 nuclear staining. The immunohistochemical staining results were quantified by counting the percentage of cells showing expression and by converting the staining intensity to the final immunoreactivity scores of specimens.
Fluorescence in situ hybridization analysis
The amplification of CDK4 was evaluated by fluorescence in situ hybridization (FISH). The Zytolight SPEC CDK4/CEN 12 Dual Color Probe (ZytoVision; GeneDiagnostic Inc., Hangzhou, China) was applied for the detection of CDK4 amplification. The target and reference probes for CDK4 were labeled in green and orange, respectively. For analysis, 4–5 μm thick FFPE sections were deparaffinized, treated with warmed pretreated solution citric at 98°C, and digested in pepsin solution. Thereafter, a 10 μL probe was added to each slide. The target DNA and probes were co-denatured at 75°C for 10 min and incubated overnight at 37°C in a humidified hybridization chamber. Three post-hybridization washes were performed in 1× Wash Buffer-A at 37°C for 5 min. Finally, the slides were air-dried and counterstained with 4’,6-diamindino-2-phenylindole (DAPI)/antifade solution. Signals for each locus-specific FISH probe were assessed under an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan). FISH results were based on at least 50 evaluable tumor nuclei. The nuclei were considered amplified for CDK4 when the CDK4/CEN12 ratio was >2.
Statistical analysis
The UALCAN (available at http://ualcan.path.uab.edu/) is an online publicly available web portal that offers in-depth analysis of data from The Cancer Genome Atlas (TCGA). It was applied to detect the expression of CDK4 in lung cancer tissues and to analyze the relationship between CDK4 expression and disease stage, lymph node metastasis, and TP53 mutations. The aforementioned analytical approach has limitations, as the TCGA database does not provide survival data, making it difficult to interpret the association between molecular features and clinical outcomes. Kaplan–Meier plotter (http://kmplot.com) is an online database containing gene expression data and survival information for 3452 lung cancer patients, which was used to analyze the prognostic value of CDK4 in lung cancer. The patients’ samples were divided into two groups, that is, high and low expression by median expression, and the OS, PFS, and post-progression survival (PPS) were determined with [95% confidence interval (CIs)] and log-rank p values. The correlation of CDK4 amplification and BM in the initial BR group, BR group, and the non-BR group was evaluated by Pearson’s chi-square test.
Results
Somatic mutation profiles
Somatic mutation profiles of matched primary tumor and BM
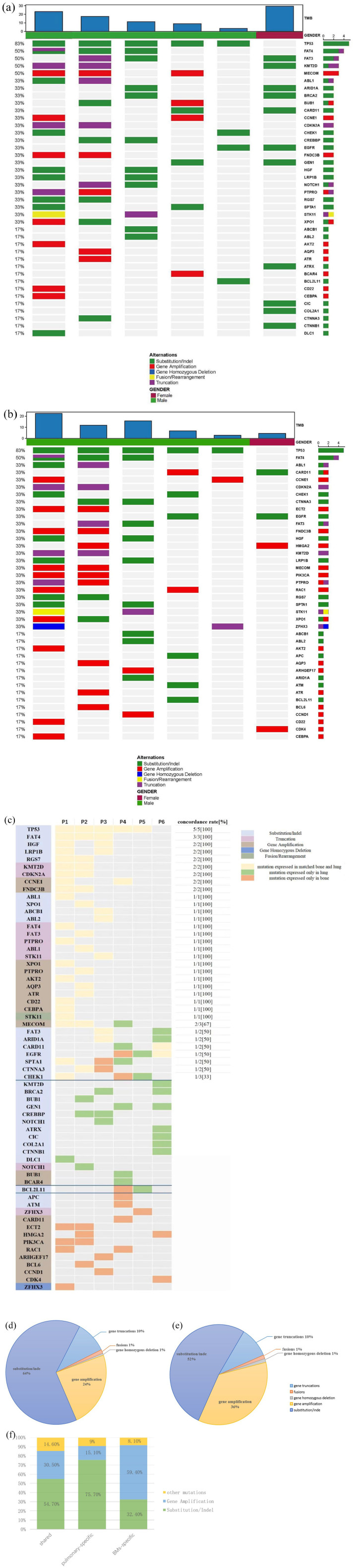
The analysis was based on the somatic mutation results of 12 samples from the matched BM group. In terms of gene mutation frequency, the highest mutation frequency in primary lung tumor is tumor protein p53 (TP53, 5/6, 83%), followed by FAT atypical cadherin 4 (FAT4, 3/6, 50%), FAT atypical cadherin 3 (FAT3, 3/6, 50%), lysine methyltransferase 2D (KMT2D, 3/6, 50%), and MDS1 and EVI1 complex (MECOM, 3/6, 50%). As for BM, the gene with the highest mutation frequency was also TP53 (5/6, 83%), followed by FAT4 (3/6, 50%), v-abl Abelson murine leukemia viral oncogene homolog 1 (ABL1, 2/6, 33%), caspase recruitment domain-containing protein 11 (CARD11, 2/6, 33%), and cyclin E1 (CCNE1, 2/6, 33%) [Figure 2(a) and (b)]. Due to the small sample size, limited driver genes were detected. However, alterations in driver genes were found in lung tumors in all six patients, including TP53 (5/6, 83%) and EGFR (2/6, 33%). The concordance of TP53 was 100% between BMs and primary tumor (5/5), whereas EGFR demonstrated a lower concordance rate of 50% (1/2). In addition, the alterations of FAT4 (3/3,100%), hepatocyte growth factor (HGF, 2/2, 100%), low-density lipoprotein receptor-related protein 1B (LRP1B, 2/2, 100%), regulator of g protein Signaling 7 (RGS7, 2/2, 100%), KMT2D (2/2,100%), cyclin-dependent kinase inhibitor 2A (CDKN2A, 2/2, 100%), CCNE1 (2/2,100%), and fibronectin type III domain containing 3B (FNDC3B 2/2,100%) in primary tumors and BMs remain consistent [Figure 2(c)].
Figure 2.
Mutational landscape of paired primary tumors and BMs in six NSCLC patients.
(a) Mutational landscape of primary lung tumors. (b) Mutational landscape of BMs. The X-axis shows each case sample, and the Y-axis shows each mutated gene. The upper bar graph shows the tumor mutation burden (TMB) value of the patients. The bar graph on the right shows the number of each mutated gene. Gender, green represents male and purple represents female. Alterations: green represents substitution/indel mutations, red represents gene amplification mutations, blue represents gene homozygous deletion mutations, yellow represents fusion/rearrangement mutations, and purple represents truncation mutations. (c) Heatmap of genetic concordance analysis of matched lung primary tumors and bone metastases. The X-axis shows each case sample, and the Y-axis shows each mutated gene. Gene, light blue represents substitution/indel, purple represents truncation, brown represents gene amplification, navy blue represents gene homozygous deletion, and green represents fusion/rearrangement. Consistency, yellow represents mutations that are expressed in both matched primary lung cancer and BMs. Green represents mutations expressed in primary lung cancer while not expressed in matched BMs. Orange represents mutations expressed in BMs while not expressed in matched primary lung cancer. Concordance rate, number of patients with consistent expression of the gene in matched tumors/number of patients with gene expressed in primary lung tumors. (d) Fan chart of genetic variation types in primary lung tumors. (e) Fan chart of genetic variation types in BMs. All genetic variants harboring in paired primary tumors and BMs were included, and different variant types of the same gene were counted as different genetic variants, for example, EGFR deletion and EGFR amplification were counted as two genetic variants. (f) Comparison of gene variant types in shared mutations, BM-private mutations, and pulmonary-specific mutations. Genetic variants harboring in primary tumors or BMs were included, and different variant types of the same gene were counted as different genetic variants. Duplicate counting errors due to the same variant being expressed in multiple patients were also excluded. Other mutations include homozygous deletion mutations, fusion/rearrangement mutations, and truncation mutations.
BM, bone metastasis; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer.
Somatic mutation profiling identified 160 mutations in the primary tumor tissue samples, including 103 (64.3%) gene substitution or indel, 38 (23.7%) gene amplifications, 16 (10%) gene truncations, 2 (1.2%) gene fusions, and 1 (0.6%) gene homozygous deletion. For BM, a total of 162 somatic mutations were detected, including 84 (51.8%) gene substitutions, 58 (35.8%) gene amplifications, 16 (9.8%) gene truncations, and 2 each (1.2%) gene fusions and gene homozygous deletions. These results show clear variation in the distribution of genetic variant types in lung tumors and BM. The reduced rate of gene substitution or indel and increased rate of gene amplification were the main genome characteristics of BM in NSCLC [Figure 2(d) and (e)]. To avoid errors due to the small sample size, we integrated data from previously published literature. A total of 39 matched primary lung tumors and BM were obtained from previous literature, and all genomic variations were identified by using the NGS-based YuanSu450 gene panel (OrigiMed, Shanghai, China). It was also found that the rate of amplification in BM was increased (24.6% versus 33.0%), while the rate of substitution or indel decreased (63.1% versus 55.3%), which is in accordance with a previous study. 7
Furthermore, different variant types of the same gene as different genetic variants were counted to further investigate the impact of somatic mutation profiles on BMs in NSCLC. For example, EGFR indel and EGFR amplification were counted as two genetic variants. At the same time, we excluded duplicate counting errors due to the expression of the same variant in multiple patients. Finally, we identified 128 genetic variants in primary tumors and 123 genetic variants in BM. Subsequently, we classified the genetic variants according to a previous study by Jamal-Hanjani et al. 22 Among them, 190 (73.0%) were shared mutations (present in both primary and BM, but not necessarily in the same patient), 33 (12.7%) were pulmonary-specific mutations (present only in primary pulmonary tumors), and 37 (14.3%) were BM-specific mutations (present only in BMs). As shown in Figure 2(f), substitution or indel was the most frequent form in shared mutations that accounted for approximately 59% of total mutations, followed by gene amplification (30.5%), truncations (12.6%), fusion/rearrangement (1%), and gene homozygous deletion (1%). In pulmonary-specific mutations, the substitution or indel remains the most common form of genetic variation that accounted for nearly 75.7% of the total mutations, followed by gene amplification (15.1%), truncations (6%), and fusion/rearrangement (3%). However, compared to the shared genes and pulmonary-specific mutations, gene amplification was the most prevalent form of gene variations in BM-specific mutations, while substitution or indel accounted for only 36% of the total variations, while truncations, fusion/rearrangement, and gene homozygous deletion accounted only for 2.7% of the total mutations.
Somatic mutation profiles of patients with initial BR
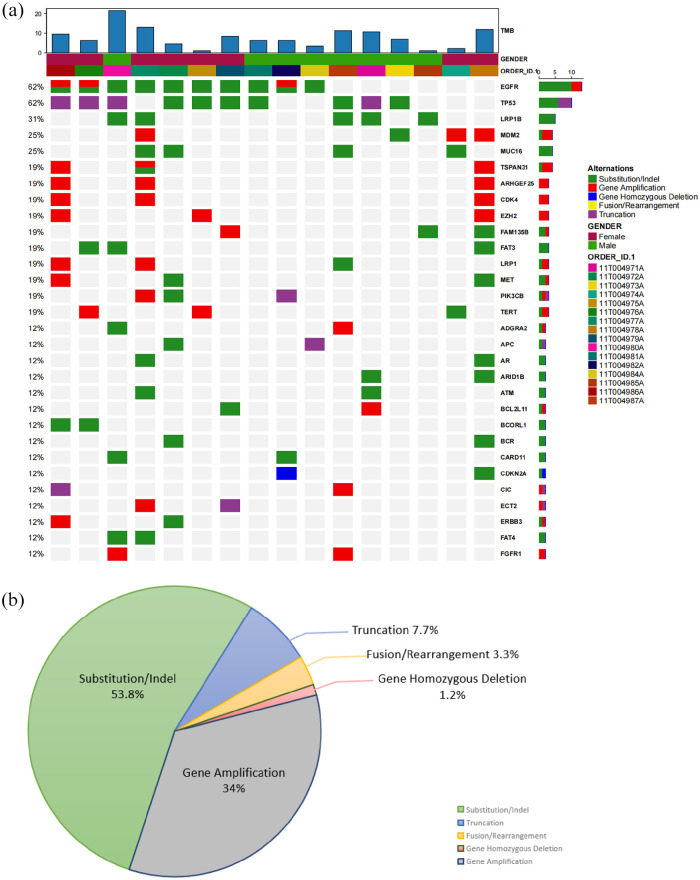
A total of 308 mutations were detected in the primary tumor tissues in 16 postoperative patients with NSCLC who first recrudesced in bone. The most frequently mutated genes were EGFR and TP53, which were detected in 10 patients (62%), followed by LRP1B in 5 patients (31%), mouse double minute 2 (MDM2) in 4 patients (25%), mucin 16 (MUC16) in 4 patients (25%), and rho guanine nucleotide exchange factor 25 (ARHGEF25) in 3 patients (19%) [Figure 3(a)]. Besides, the main forms of genetic variation were substitution or indel (166/308, 53.8%) and gene amplification (105/308, 34%), while the percentages of truncation, fusion or rearrangement, and gene homozygous deletion were relatively small which accounted for 7.7%, 3.3%, and 1.2%, respectively [Figure 3(b)].
Figure 3.
Mutational landscape of first BMs group.
(a) Mutational landscape of first BMs group. The X-axis shows each case sample, and the Y-axis shows each mutated gene. The upper bar graph shows the TMB value of the patients. The bar graph on the right shows the number of each mutated gene. Gender, green represents male and purple represents female. Alterations: green represents substitution/indel mutations, red represents gene amplification mutations, blue represents gene homozygous deletion mutations, yellow represents fusion/rearrangement mutations, and purple represents truncation mutations. (b) The pie chart summarized the proportion of genetic variant types in the first BM group. All detected genetic variants in the first bone metastasis group were counted.
BM, bone metastasis.
CDK4 amplification incidence
BM-specific mutations were counted in the initial BR group, and a total of 10 BM-specific genes were found. Among them, CDK4 and tetraspanin 31 (TSPAN31) showed a higher mutation rate which accounted for approximately 18.8% (3/6, it should be emphasized that these three patients have co-expression of CDK4 and TSPAN31), followed by fibroblast growth factor receptor substrate 2 (FRS2) and ataxia telangiectasia-mutated gene (ATM) which accounted for 12.5% (2/16). Besides, protein kinase, DNA-activated, catalytic subunit (PRKDC), KRAS, forkhead box A1 (FOXA1), adenomatous polyposis coli (APC), and phosphatidylionsitol-3 kinase catalytic alpha (PIK3CA) were also harbored in initial BR group but only accounted for 6.3%. TSPAN31, a new member of the tetraspanin superfamily, is a cis-natural antisense transcript of CDK4. 23 Several studies have shown that TSPAN31 can reduce the expression level of CDK4.24,25 Immunohistochemistry and FISH were performed on the initial BR group,26,27 and the result showed that three patients harbored CDK4 amplification, and the CDK4 protein was overexpressed.
To further explore the correlation between CDK4 amplification and the occurrence of BMs, CDK4 amplification and its expression were detected in 61 postoperative samples. In the BR group, 11.1% of patients harbored CDK4 amplification, whereas, in the non-BR group, only 4.7% of patients harbored CDK4 amplification. The incidence of CDK4 amplification tended to be higher in patients with initial recurrence of bone compared to those without BM (18.8% versus 4.7%, p = 0.118). However, there was no significant difference in the incidence of CDK4 amplification between patients with initial BR and patients with BM (18.8% versus 11.1%, p = 0.648).
Prognostic value of CDK4
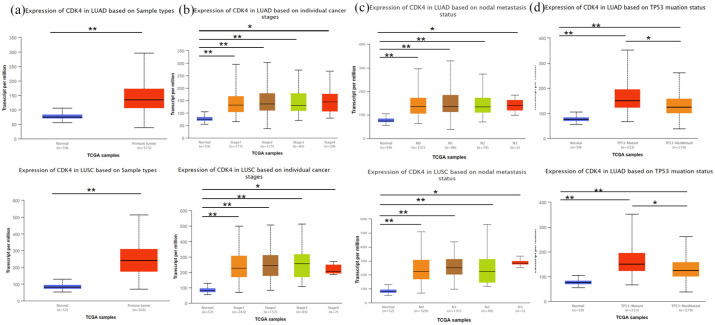
The prognosis value of CDK4 in NSCLC patients was examined using the UALCAN online tool. It was found that the expression of CDK4 in LUAC or lung squamous cell carcinoma (LUSC) was significantly higher than in normal tissue [Figure 4(a)]. Regarding the tumor stage, a significant increase in CDK4 expression was observed in every stage of LUAC and LUSC [Figure 4(b)]. Besides, CDK4 expression was also increased in all levels of lymph node metastasis compared to the tumor-free lung tissues in LUAC and LUSC [Figure 4(c)]. UALCAN online tool was also used for comparing the CDK4 expression in TP53-mutated and non-TP53-mutated tissues. As shown in Figure 4(d), the expression of CDK4 was significantly increased in LUAC and LUSC tissues which harbored TP53 mutations.
Figure 4.
Boxplots of hepcidin expression in different patient groups were assessed according to clinical parameters using the UALCAN database. Analysis of NSCLC (a), cancer stage (b), metastasis (c), and TP53 (d) are shown.
*p < 0.01, **p < 0.001.
N0, no regional lymph node metastasis; N1, 1–3 axillary lymph nodes; N2, 4–9 axillary lymph nodes; N3, 10 or more axillary lymph nodes; NSCLC, non-small-cell lung cancer; TP53, tumor protein p53.
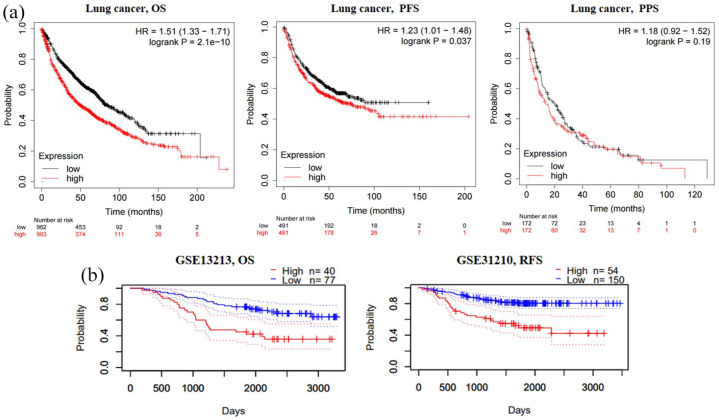
Furthermore, according to the Kaplan–Meier plotter database, the NSCLC patients with high CDK4 gene expression showed poor OS and PFS but did not show any significant difference in PPS [Figure 5(a)]. Furthermore, PrognoScan database analysis showed that elevated CDK4 expression was highly associated with poorer OS RFS in GSE13213 and GSE31210 cohorts [Figure 5(b)]. These results suggest that the expression of CKD4 is significantly associated with the prognosis of NSCLC.
Figure 5.
Survival curve evaluating the prognostic value of CDK4. (a) Survival curves using the Kaplan–Meier plotter are shown for OS, PFS, and PPS. (b) Survival curves using the PrognoScan database are shown for OS and RFS.
CDK4, cyclin-dependent kinase 4; OS, overall survival; PFS, progression-free survival; PPS, post-progression survival; RFS, recurrence-free survival.
Discussion
BM is not only common in patients with advanced NSCLC (34.3%) but also in patients with postoperative recurrence of NSCLC (32%), with slightly higher than lung and brain metastases, which account for 31% and 29%, respectively.28,29 Although some progress has been made in the research on the epidemiology and prognostic factors of BM from lung cancer, the genome of BMs is still unclear.6,30 In this study, we explored the genomic differences between BMs and primary lung tumors to find potential therapeutic targets.
In the present study, we attempted to describe the molecular characteristics of BM from two aspects: identification of gene variation type and gene variation rate. In terms of variant type, the gene profile of BM is heterogeneous from a primary lung tumor. Compared to the primary lung tumor, there was an increased rate of gene amplification and a decreased rate of substitution/insertion–deletion in BM. As for the gene variation rate, we focused our analysis on the concordance of detected driver genes. TP53 is a tumor suppressor gene that occurs in almost every type of malignancy and its mutation rate in NSCLC is about 36.6–56.1%.31–33 In the present study, the mutation rate of TP53 was higher than in the previously reported study, which may be related to the small sample size of the study. TP53 variants were consistent in BM versus lung tumors, which may be attributed to the fact that the coding mutation of TP53 occurs in the early stage of the development of lung cancer. 34 However, due to the small sample size included in this study, the consistency of TP53 mutations may be biased. Huang et al. 7 performed NGS on primary lung tumors and BMs in 40 NSCLC patients and found that the mutation rates of TP53 in primary and metastatic lesions were 52.5% and 67.5%, respectively. In contrast to the results of TP53, EGFR mutations were heterogeneous in primary tumors and BMs in this study, that is the EGFR mutation status may be consistent across primary tumors and BMs (n = 1), or lost (n = 1), or de novo (n = 1) in BMs. Previous studies suggest that the concordance rate of EGFR mutations between primary sites and metastases is around 20.8–31.43%.35–37 In addition, it has been found that in patients with LUAC who develop bone, brain, or liver metastases, EGFR exon 19 deletion and exon 21 L858R point mutation are more likely to be detected in the metastatic sites.38–40 However, Smits et al. 41 examined the mutation rate of the EGFR mutation in 261 LUAC primary and metastatic sites and revealed that EGFR was not significantly different in BMs. As P1, P2, and P5 underwent radical surgery in this study, the BMs were heterochronous to the primary site, and the EGFR mutations may have changed under the influence of other antitumor treatments. As other previous studies have pointed out, the inconsistency of driver genes may be related to the cloning of tumor cells, the genetic heterogeneity of tumors, the availability of metastatic sites, detection methods, and tumor cell content. 42 Therefore, clinicians should try to obtain tumor tissues from the metastatic sites of NSCLC patients and evaluate the driver genes to administer antitumor therapy more precisely.
In general, the BM genome is heterogeneous with respect to the primary lung tumor accompanied by increased gene amplification and decreased substitution/indel in the type of variations.
Metastasis is associated with most cancer-related deaths. When cancer cells are isolated from the primary tumor site, they can invade most parts of the body.8,43 However, the distribution of specific tumor metastasis is not random and could be determined by multiple factors, including molecular subtypes.8,44 For example, Tang et al. 45 explored the primary tumor and distant metastasis of lung cancer and found that the amplification of RICTOR may be associated with brain metastasis, while that of NKX2-1 may be associated with the meningeal metastasis. Meanwhile, using unpaired patient samples, Shih et al. 46 reported that genes with high copy number aberrations in the brain are associated with metastasis of lung cancer, which further confirms that MYC, YAP1, and MMP13 amplified variants increased the incidence of brain metastasis. However, the molecular characteristics associated with BM from lung cancer have not yet been fully explored. Previous studies have shown that there is a ‘seed pre-screening’ before metastasis, and the affinity of cancer cells for metastasis may have been formed before tumor cells spread, and this specific site affinity may be related to the specific gene expression of the primary tumor.26,27 Based on the above theory, we analyzed the expression of BM-specific variations in 16 postoperative patients with NSCLC who first recrudesce in bone and found that the presence of CDK4 amplification was present in 3 of 16 cases (18.8%) of the group. In addition, the rate of CDK4 amplification was higher in BM patients, especially those with initial BR as compared to those without BM.
CDK4 is a gene encoding protein that is a member of the serine/threonine kinase family and is located in the region of chromosome 12q13. 47 It combines with cyclin D (CCND) and forms a complex, which performs different functions, such as activates CDK4, phosphorylates the retinoblastoma gene (pRB), separates the phosphorylated pRB from E2F and release it, and promotes cell proliferation from the G1-phase to S-phase. 48 CDK4 amplification leads to overexpression of CDK4 protein, which can further lead to uncontrolled cell proliferation and differentiation. CDK4/6 inhibitors are small molecules that target the ATP-binding site of CDK4/6 and further inhibit the formation of complexes between CDK4/6 and CCND and block the ATP binding, thereby cutting off the upstream growth signals and prevent the transition of cells from the G1-phase to S-phase.49,50 A previous study has shown that CDK4/6 inhibitors have tumor suppressor effects in breast cancer and can reduce the incidence of chemotherapy-induced myelosuppression in small-cell lung cancer. 51
The incidence of CDK4 amplification was higher in the initial BR group compared to the previously reported 3% mutation rate in NSCLC.52,53 This indicates the role of CDK4 as a potential therapeutic target for BM in NSCLC. Several studies have confirmed the efficacy of CDK4/6 inhibitors on BM from breast cancer. In adjuvant therapy, the CDK4/6 inhibitor ambemacivlib can significantly reduce the incidence of BM. 14 In palliative care, the PALOMA-2 study showed that CDK4/6 inhibitors in combination with letrozole significantly prolonged PFS in patients (36.2 versus 11.2 months, p < 0.0001) of patients with BM-only breast cancer. 13 Besides, an in vivo study also showed that CDK4/6 inhibitors, palbociclib, reduced the death risk by 28% in patients with BM-only breast cancer. 12 Unfortunately, the application of CDK4/6 inhibitors in the treatment of NSCLC is still in clinical trials. Gopalan et al. 54 conducted a phase II clinical trial of palbociclib in 19 previously treated patients with advanced NSCLC. Among them, 16 evaluable patients who received >1 month of therapy, 8 patients (50%) with previously progressed NSCLC had stable disease (SD) lasting 4–10.5 months. The median OS was 5.1 months for all cases and 16.6 months for the subset of SD patients. In the phase III clinical study, 453 patients who had stage IV NSCLC with KRAS mutations and disease progression after two lines of therapy were randomized in a 3:2 ratio to the abemaciclib and erlotinib groups, and significant improvements in PFS (3.6 versus 1.9 months, p < 0.001) and disease control rate (54% versus 32%, p < 0.001) were observed in the abemaciclib group, although there was no statistically significant difference in median overal survival (mOS) between the two groups (7.4 versus 7.8 months, p = 0.77). 55 In addition, several studies have shown that CDK4/6 inhibitors combined with other anti-lung cancer therapies can modulate the microenvironment of cancer and increase the sensitivity of targeted and radiotherapy treatments to produce a synergistic antitumor effect.15–19 Overall, it can be seen that CDK4/6 inhibitors have achieved some results in the clinical application of NSCLC patients, but whether CDK4/6 inhibitors can reduce the incidence of BMs still needs further study.
TP53 gene encodes TP53 protein, which acts as a transcription factor binding site to the promoter region of p21 and activates the transcription of the p21 gene. In the presence of DNA damage, the p21 gene is induced by the TP53 protein to increase its expression level, inhibit the CDK (including CDK4 and CDK2) activity, and prevent the cells from entering the S-phase. 56 By contrast, when tumor cells harbor TP53 mutation, the absence of normal TP53 protein could not consequently inhibit the activity of CDK. Thus, the existence of mutations in the TP53 gene appears to act synergistically with CDK4 amplification in tumor cell-cycle regulation. In this study, two out of three patients with initial BR carried co-mutations of TP53 and CDK4. Furthermore, UALCAN analysis showed that the expression of CDK4 was significantly higher in patients harboring TP53 mutations in LUAC and LUSC. Since TP53 is the most frequently mutated gene in NSCLC, a targeted therapy targeting CDK4 amplification may require further investigation.
Although the use of bisphosphonates and RANKL inhibitors has improved the quality of life of patients with BMs from lung cancer, the treatment of BMs remains challenged. In this study, we found genomic differences between BMs and primary lung tumors; therefore, some treatments for BMs need to be considered when BMs are identified. In addition, the findings suggest that the incidence of CDK4 amplification tended to be elevated in BMs. Previous studies suggested that CDK4/6 inhibitors reduce the risk of BMs in breast cancer, while their therapeutic efficacy in lung cancer patients is unclear. Our findings may provide theoretical evidence for the use of CDK4/6 inhibitors in BMs, but rigorous prospective studies are still needed to confirm how their therapeutic efficacy in patients with BMs from NSCLC. This research was an exploratory study with some limitations, including the small number of enrolled patients and the fact that the online database was derived from lung tumors, which did not allow for clarification of the correlation between CDK4 amplification in BMs and patient prognosis. Subsequent studies need to expand the sample size to further clarify the potential prognostic role of CDK4 amplification in BM and to explore whether CDK4/6 inhibitors can reduce the incidence of BM.
Conclusion
In summary, there was heterogeneity in the genetic profile of primary lung tumor and BM, with an increased proportion of gene amplifications and a decreased proportion of gene deletion in BM. Although there was no significant difference between CDK4 amplification and BM in this study, the incidence of CDK4 amplification tended to be elevated in NSCLC patients with BM. In addition, the CDK4 mRNA expression possesses prognostic value in NSCLC, with high CDK4 expression significantly associated with better OS and RFS.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359241239293 for Bone metastasis in non-small-cell lung cancer: genomic characterization and exploration of potential targets by Jiali Gong, Shumin Hu, Qianyun Shan, Jing Qin, Na Han, Fajun Xie and Hongyang Lu in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors express their gratitude toward all participating patients and clinicians, for their invaluable support and contribution to this study.
Footnotes
ORCID iDs: Jiali Gong  https://orcid.org/0000-0002-7299-883X
https://orcid.org/0000-0002-7299-883X
Jing Qin  https://orcid.org/0000-0002-4477-3334
https://orcid.org/0000-0002-4477-3334
Hongyang Lu  https://orcid.org/0000-0003-0404-5153
https://orcid.org/0000-0003-0404-5153
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jiali Gong, Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus), Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China; Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China; Department of Hematology and Oncology, Ningbo No. 2 Hospital, Ningbo, Zhejiang, P.R. China.
Shumin Hu, Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus), Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China; Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China.
Qianyun Shan, Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus), Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China; Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China.
Jing Qin, Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus), Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China; Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China.
Na Han, Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus), Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China; Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China.
Fajun Xie, Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus), Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China; Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou, P.R. China.
Hongyang Lu, Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus), Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, No. 1 East Banshan Road, Gongshu, Hangzhou 310022, P.R. China; Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Institute of Basic and Cancer Medicine, Gongshu, Hangzhou 310022, P.R. China.
Declarations
Ethics approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Zhejiang Cancer Hospital (Permits no. IRB-2022-446) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Most of the specimens in this study were from the biobank, these patients had signed informed consent. This study is retrospective, some patients have died. Exempt written informed consent was approved by the Ethics Committee of Zhejiang Cancer Hospital. All patient data were de-identified to maintain confidentiality and comply with privacy regulations.
Consent for publication: No identifiable images were included in the manuscript; therefore, consent for publication is not applicable.
Author contributions: Jiali Gong: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing – original draft; Writing – review & editing.
Shumin Hu: Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Qianyun Shan: Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Jing Qin: Data curation; Supervision.
Na Han: Investigation; Writing – review & editing.
Fajun Xie: Investigation; Supervision; Writing – review & editing.
Hongyang Lu: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by grants from Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LHDMY22H160003).
The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Hernandez RK, Wade SW, Reich A, et al. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 2018; 18: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spizzo G, Seeber A, Mitterer M. Routine use of pamidronate in NSCLC patients with bone metastasis: results from a retrospective analysis. Anticancer Res 2009; 29: 5245–5249. [PubMed] [Google Scholar]
- 3. Tsuya A, Kurata T, Tamura K, et al. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 2007; 57: 229–232. [DOI] [PubMed] [Google Scholar]
- 4. Yu JL, Simmons C, Victor JC, et al. Impact of new chemotherapeutic and targeted agents on survival in stage IV non-small cell lung cancer. Oncologist 2011; 16: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Decroisette C, Monnet I, Berard H, et al. Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601). J Thorac Oncol 2011; 6: 576–582. [DOI] [PubMed] [Google Scholar]
- 6. Krawczyk P, Nicos M, Ramlau R, et al. The incidence of EGFR-activating mutations in bone metastases of lung adenocarcinoma. Pathol Oncol Res 2014; 20: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang X, Shi X, Huang D, et al. Mutational characteristics of bone metastasis of lung cancer. Ann Palliat Med 2021; 10: 8818–8826. [DOI] [PubMed] [Google Scholar]
- 8. Gao Y, Bado I, Wang H, et al. Metastasis organotropism: redefining the congenial soil. Dev Cell 2019; 49: 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016; 529: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuijpers C, Hendriks LEL, Derks JL, et al. Association of molecular status and metastatic organs at diagnosis in patients with stage IV non-squamous non-small cell lung cancer. Lung Cancer 2018; 121: 76–81. [DOI] [PubMed] [Google Scholar]
- 11. Lohinai Z, Klikovits T, Moldvay J, et al. KRAS-mutation incidence and prognostic value are metastatic site-specific in lung adenocarcinoma: poor prognosis in patients with KRAS mutation and bone metastasis. Sci Rep 2017; 7: 39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeMichele A, Cristofanilli M, Brufsky A, et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2− metastatic breast cancer in US real-world clinical practice. Breast Cancer Res 2021; 23: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finn RS, Martin M, Hope S, et al. PALOMA-2: primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2− advanced breast cancer (ABC). J Clin Oncol 2016; 34(Suppl): abstr 507. [Google Scholar]
- 14. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020; 38: 3987–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alvarez-Fernandez M, Malumbres M. Mechanisms of sensitivity and resistance to CDK4/6 inhibition. Cancer Cell 2020; 37: 514–529. [DOI] [PubMed] [Google Scholar]
- 16. Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov 2018; 8: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017; 548: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu M, Xu S, Wang Y, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, sensitizes lung cancer cells to treatment with epidermal growth factor receptor tyrosine kinase inhibitors. Oncotarget 2016; 7: 84951–84964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naz S, Sowers A, Choudhuri R, et al. Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non-small cell lung cancer in vitro and in vivo. Clin Cancer Res 2018; 24: 3994–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007; 18: 805–835. [DOI] [PubMed] [Google Scholar]
- 21. Fan Y, Shan Q, Gong J, et al. Molecular and clinical characteristics of primary pulmonary lymphoepithelioma-like carcinoma. Front Mol Biosci 2021; 8: 736940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med 2017; 376: 2109–2121. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Li J, Kong L, et al. NATsDB: natural antisense transcripts database. Nucleic Acids Res 2007; 35(Database issue): D156–D161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Zhou Y, Li D, et al. TSPAN31 is a critical regulator on transduction of survival and apoptotic signals in hepatocellular carcinoma cells. FEBS Lett 2017; 591: 2905–2918. [DOI] [PubMed] [Google Scholar]
- 25. Xia Y, Deng Y, Zhou Y, et al. TSPAN31 suppresses cell proliferation in human cervical cancer through down-regulation of its antisense pairing with CDK4. Cell Biochem Funct 2020; 38: 660–668. [DOI] [PubMed] [Google Scholar]
- 26. Zhang XH, Jin X, Malladi S, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 2013; 154: 1060–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009; 16: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torok JA, Gu L, Tandberg DJ, et al. Patterns of distant metastases after surgical management of non-small-cell lung cancer. Clin Lung Cancer 2017; 18: e57–e70. [DOI] [PubMed] [Google Scholar]
- 29. Zheng XQ, Huang JF, Lin JL, et al. Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study. Transl Lung Cancer Res 2019; 8: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun JM, Ahn JS, Lee S, et al. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer 2011; 71: 89–93. [DOI] [PubMed] [Google Scholar]
- 31. Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010; 2: a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat 2007; 28: 622–629. [DOI] [PubMed] [Google Scholar]
- 33. Xu S, Wang Y, Ren F, et al. Impact of genetic alterations on outcomes of patients with stage I nonsmall cell lung cancer: an analysis of The Cancer Genome Atlas data. Cancer Med 2020; 9: 7686–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris CC. p53 tumor suppressor gene: from the basic research laboratory to the clinic – an abridged historical perspective. Carcinogenesis 1996; 17: 1187–1198. [DOI] [PubMed] [Google Scholar]
- 35. Fang Q, Zhang L, Wang S, et al. Discordance of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2011; 14: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gow CH, Chang YL, Hsu YC, et al. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol 2009; 20: 696–702. [DOI] [PubMed] [Google Scholar]
- 37. Han HS, Eom DW, Kim JH, et al. EGFR mutation status in primary lung adenocarcinomas and corresponding metastatic lesions: discordance in pleural metastases. Clin Lung Cancer 2011; 12: 380–386. [DOI] [PubMed] [Google Scholar]
- 38. Enomoto Y, Takada K, Hagiwara E, et al. Distinct features of distant metastasis and lymph node stage in lung adenocarcinoma patients with epidermal growth factor receptor gene mutations. Respir Investig 2013; 51: 153–157. [DOI] [PubMed] [Google Scholar]
- 39. Fujimoto D, Ueda H, Shimizu R, et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: importance of bone metastasis. Clin Exp Metastasis 2014; 31: 543–551. [DOI] [PubMed] [Google Scholar]
- 40. Takano K, Kinoshita M, Takagaki M, et al. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neuro-oncology 2016; 18: 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smits AJ, Kummer JA, Hinrichs JW, et al. EGFR and KRAS mutations in lung carcinomas in the Dutch population: increased EGFR mutation frequency in malignant pleural effusion of lung adenocarcinoma. Cell Oncol (Dordr) 2012; 35: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forest F, Stachowicz ML, Casteillo F, et al. EGFR, KRAS, BRAF and HER2 testing in metastatic lung adenocarcinoma: value of testing on samples with poor specimen adequacy and analysis of discrepancies. Exp Mol Pathol 2017; 103: 306–310. [DOI] [PubMed] [Google Scholar]
- 43. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell 2017; 168: 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009; 9: 274–284. [DOI] [PubMed] [Google Scholar]
- 45. Tang WF, Wu M, Bao H, et al. Timing and origins of local and distant metastases in lung cancer. J Thorac Oncol 2021; 16: 1136–1148. [DOI] [PubMed] [Google Scholar]
- 46. Shih DJH, Nayyar N, Bihun I, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet 2020, 52(4): 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Demetrick DJ, Zhang H, Beach DH. Chromosomal mapping of human CDK2, CDK4, and CDK5 cell cycle kinase genes. Cytogenet Cell Genet 1994; 66: 72–74. [DOI] [PubMed] [Google Scholar]
- 48. Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 2012; 119: 4597–4607. [DOI] [PubMed] [Google Scholar]
- 49. Dukelow T, Kishan D, Khasraw M, et al. CDK4/6 inhibitors in breast cancer. Anticancer Drugs 2015; 26: 797–806. [DOI] [PubMed] [Google Scholar]
- 50. Schoninger SF, Blain SW. The ongoing search for biomarkers of CDK4/6 inhibitor responsiveness in breast cancer. Mol Cancer Ther 2020; 19: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng Y, Wu L, Huang DZ, et al. Myeloprotection with trilaciclib in Chinese patients with extensive-stage small cell lung cancer receiving standard chemotherapy (TRACES). 2022. WCLC, Abstract EP08.02-078. [DOI] [PubMed] [Google Scholar]
- 52. AARC Project GENIE Consortium. AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017; 7: 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang J, Xu D, Zhou Y, et al. Mechanisms and implications of CDK4/6 inhibitors for the treatment of NSCLC. Front Oncol 2021; 11: 676041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gopalan PK, Villegas AG, Cao C, et al. CDK4/6 inhibition stabilizes disease in patients with p16-null non-small cell lung cancer and is synergistic with mTOR inhibition. Oncotarget 2018; 9: 37352–37366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goldman JW, Mazieres J, Barlesi F, et al. A randomized phase III study of abemaciclib versus erlotinib in patients with stage IV non-small cell lung cancer with a detectable KRAS mutation who failed prior platinum-based therapy: JUNIPER. Front Oncol 2020; 10: 578756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. He G, Siddik ZH, Huang Z, et al. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005; 24: 2929–2943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359241239293 for Bone metastasis in non-small-cell lung cancer: genomic characterization and exploration of potential targets by Jiali Gong, Shumin Hu, Qianyun Shan, Jing Qin, Na Han, Fajun Xie and Hongyang Lu in Therapeutic Advances in Medical Oncology