Abstract
Background
Viruses are the leading etiology of acute respiratory infections (ARI) in children. However, there is limited knowledge on drivers of severe acute respiratory infection (SARI) cases involving viruses. We aimed to identify factors associated with severity and prolonged hospitalization of viral SARI among children < 5 years in Burkina Faso.
Methods
Data were collected from four SARI sentinel surveillance sites during October 2016 through April 2019. A SARI case was a child < 5 years with an acute respiratory infection with history of fever or measured fever ≥ 38 °C and cough with onset within the last ten days, requiring hospitalization. Very severe ARI cases required intensive care or had at least one danger sign. Oropharyngeal/nasopharyngeal specimens were collected and analyzed by multiplex real-time reverse-transcription polymerase chain reaction (rRT-PCR) using FTD-33 Kit. For this analysis, we included only SARI cases with rRT-PCR positive test results for at least one respiratory virus. We used simple and multilevel logistic regression models to assess factors associated with very severe viral ARI and viral SARI with prolonged hospitalization.
Results
Overall, 1159 viral SARI cases were included in the analysis after excluding exclusively bacterial SARI cases (n = 273)very severe viral ARI cases were common among children living in urban areas (AdjOR = 1.3; 95% CI: 1.1–1.6), those < 3 months old (AdjOR = 1.5; 95% CI: 1.1–2.3), and those coinfected with Klebsiella pneumoniae (AdjOR = 1.9; 95% CI: 1.2–2.2). Malnutrition (AdjOR = 2.2; 95% CI: 1.1–4.2), hospitalization during the rainy season (AdjOR = 1.71; 95% CI: 1.2–2.5), and infection with human CoronavirusOC43 (AdjOR = 3; 95% CI: 1.2-8) were significantly associated with prolonged length of hospital stay (> 7 days).
Conclusion
Younger age, malnutrition, codetection of Klebsiella pneumoniae, and illness during the rainy season were associated with very severe cases and prolonged hospitalization of SARI involving viruses in children under five years. These findings emphasize the need for preventive actions targeting these factors in young children.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09219-x.
Keywords: Acute respiratory infections, Severity, Prolonged length of stay, Predictors, Children under five, Burkina Faso
Background
Despite the recent progress, acute respiratory infections (ARI) remain one of the leading causes of morbidity and mortality worldwide [1], with the highest burden found in Sub-Saharan Africa (SSA) and Southeast Asia [2]. ARI burden is particularly high in children under five, with an estimated 652,572 deaths occurring among this group in 2016 [2]. In children under five years of age, Streptococcus pneumoniae (also known as pneumococcus), respiratory syncytial virus (RSV), influenza virus, and Haemophilus influenzae type b (Hib) are some of the most common causes of ARI [3]. Specific interventions to improve the management of severe cases and effective vaccines against the leading etiologies have substantially reduced the burden of ARI in children [4]. The global use of routine vaccines against pertussis, selected pneumococcal serotypes, Hib, in many low and middle-income countries through their Expanded Programs on Immunization (EPI) has significantly reduced the burden of bacterial ARI in children under five. An estimated 17.7 million deaths among children less than five years of age have been prevented between 2011 and 2020 by effective vaccine administration in 73 countries supported by the GAVI Alliance [5].
Nevertheless, viral agents play an essential role in the morbidity and mortality caused by acute respiratory infections and might be appropriate targets for new vaccines. Several studies have identified viruses as causative pathogens of ARI [6–8]. In 2019, in a multicenter case-control study in children under five conducted in Africa and Asia, O’Brien et al. estimated viral etiologies to be responsible for about 60% of pneumonia hospitalization in children [9]. Other studies in Cameroon [10] and Burkina Faso [11] found a similar prevalence of viral pathogens in patients with ARIs. Despite the apparent predominance of viral etiology in ARIs, the study of factors associated with the severity of the disease is challenged by the difficulties of accurate routine etiological diagnosis, the multiplicity of involved pathogens, and the underlying biological complexity [9, 12]. In Sub-saharan Africa, the few studies that have explored the factors associated with ARI have identified malnutrition [13], the use of firewood as fuel for cooking and heating, and poor hygiene as increasing a child’s risk of ARI [14]. Details on the pathogens responsible for these infections and the simultaneous investigation for factors associated with the severity and mortality of viral ARI are found only in limited studies [15]. A series of studies conducted in Mali, Madagascar, and some American and Asian countries among children under five years of age found that S. pneumoniae, human metapneumovirus, respiratory syncytial virus, and influenza A viruses are the pathogens most associated with the severity and mortality of ARI [15–17]. These earlier studies indicate that viral ARIs are frequent and are major causes of morbidity and mortality, particularly among children under five years of age. However, the factors associated with severity and hospital length of stay are not well understood.
This study investigated the factors associated with the severity and longer hospital stay of ARI from common viral pathogens among children under five years of age by combining epidemiological and laboratory data (screening for respiratory microorganisms using rRT-PCR) from sentinel surveillance conducted at four health districts in Burkina Faso.
Methods
Study design and data collection
A cross-sectional study was conducted among children under five years of age admitted for severe ARI (SARI) in four public health facilities in Burkina Faso from October 2016 through April 2019. The study was implemented in the already existing framework of SARI sentinel surveillance in Burkina Faso, which included testing for influenza and other respiratory pathogens.
This surveillance was conducted inpatients of all ages receiving care at the National Teaching Hospital of Bogodogo, located in Ouagadougou, the capital city; Boussé District Hospital, located in the central region of the Plateau; Kongoussi District Hospital, located in the north-central region, and Houndé District Hospital located in the Hauts-Bassins region in the western part of the country. These sites were purposefully selected based on the following criteria: geographic representation, the high number of patients consulting at the health facility, the accessibility of the site to patients, the availability and the desire of the physicians or nurses to participate voluntarily in the surveillance program, and availability of a refrigerator (+ 4 °C) for storage of specimens.
Trained hospital health workers identified and enrolled patients meeting the 2014 World Health Organization (WHO) SARI case definition (an acute respiratory infection with a history of fever or measured fever ≥ 38 °C and cough with onset within the last ten days, requiring hospitalization) [18] and collected oropharyngeal (OP) and nasopharyngeal (NP) specimens at admission or during hospitalization [19]. In children under six months of age, only NP specimens were collected. Respiratory specimens were collected from all enrolled individuals, placed in a universal transport medium (Copan Diagnostics), stored at 4–8 °C, and transported to the national reference laboratory (Laboratoire National de Reference Grippes) within 48 h of collection for testing. The same staff collected socio-demographic and clinical data using a structured case report form.
Study participants,case and variable definition
We included patients under five years of age who met the SARI case definition. We excluded children whose parents or legal guardians did not provide informed consent or whose medical conditions did not support specimen collection. For this report, we excluded children without (or with incomplete) laboratory results, those with exclusively bacterial or fungal pathogen detections, and those with unknown discharge status.
For this analysis, we defined a viral SARI (VSARI) case as an illness in any patient under five years of age meeting the inclusion criteria whose laboratory analysis of the OP/NP sample was positive for at least one of the following viral pathogens: influenza A, influenza B, influenza C; parainfluenza viruses 1, 2, 3, and 4; coronaviruses NL63, 229E, OC43, HKU1; human metapneumoviruses A and B; rhinovirus; respiratory syncytial viruses A and B; adenovirus; or enterovirus.
We defined a very severe viral ARI case (VSVARI) as any patient with viral ARI that required intensive care and/or any patient with at least one of the following danger signs: difficulty breathing, lethargy or coma, convulsion, stridor, inability to drink or eat, intercostal indrawing, and oxygen saturation under 90% [20].
Among the viral SARI cases, hospital stay was considered prolonged if the length of stay was equal to or greater than the mean length of stay of our study participants (seven days). Otherwise, it was considered short or normal.We used a modified version of the integrated age groups developed by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in the United States [21] to categorize age into 0–3 month, 4–11 month, and 12–59 month. These age categorization aligns with the clinical practices observed in local hospitals and the particularity of acute respiratory infections in children.
Laboratory testing and analysis
The national influenza reference laboratory received and tested the OP and/or NP specimens collected from enrolled cases using methods previously described by Cissé et al. [22]. Briefly, the specimens were screened individually to detect respiratory pathogens using eight multiplex real-time reverse-transcription polymerase chain reactions (rRT-PCR) with the FTD-33 Test Kit (Fast Track Diagnostics, Luxembourg). The pathogens identified included 21 types and subtypes of viruses, 11 types of bacteria, and one type of fungus: influenza A, influenza A subtype A(H1N1) pdm09, influenza B, and influenza C; parainfluenza viruses 1, 2, 3, and 4; coronaviruses NL63, 229E, OC43, and HKU1; human metapneumoviruses A and B; rhinovirus; respiratory syncytial viruses A and B; adenovirus; enterovirus; parechovirus; bocavirus; Mycoplasma pneumoniae; Chlamydia pneumoniae; Streptococcus pneumoniae; Haemophilus influenzae; Haemophilus influenzae type b; Staphylococcus aureus; Moraxella catarrhalis; Bordetella species (excluding Bordetella parapertussis); Klebsiella pneumoniae; Legionella species; Salmonella species and Pneumocystis jirovecii.
Statistical analysis
We used Epi-Info™ version 7.2.1.0 (CDC, Atlanta, GA, USA) for data recording and STATA® version 16.0 (StataCorp) for data cleaning and analysis. A series of bivariate analyses were performed between the dependent variables (very severe ARI, prolonged hospitalization) and the independent variables. The Chi-square test (χ2) or Fischer’s exact test was used to compare categorical variables, and the Student t-test or Mann-Whitney test was used to measure associations between qualitative and quantitative variables. A univariate logistic regression model was conducted to evaluate associations between dependent (outcome) and independent variables. Variables with a p-value ≤ 0.2 in the univariate analysis were entered into multivariable models through a backward stepwise elimination method to obtain the final model of variables. Key potential confounding variables (infection with RSV [12, 23], coinfection with Streptococcus pneumoniae and Haemophilus influenzae b [24]) were identified from the literature review and included in the model along with age even if the p-value was > 0.2. Simple and multilevel logistic regression models were used to assess factors associated with severity and prolonged hospitalization. The final models obtained were evaluated using different post-estimation tests available in STATA® version 16.0 (StataCorp) (Annex1 and 2 Figure S2, S3 and S4).
Because differences in care and discharge policies can affect the length of the hospital stay (common healthcare practices within the same hospital), we assumed that the length of hospital stay can be drove by clustering effect. We have then tested this grouped effect by running null two-effect with random intercepts and fixed effects model using as Level 1 the individual (children n = 905), and Level 2 corresponding the sentinel site (hospital n = 4). The test statistic was 139.45 with a corresponding p-value of less than 0.05 and so there was strong evidence that the between hospital variance is non-zero (Annex 1, Figure S1). Consequently, we conclude that accounting for group effects is justified. Crude and adjusted odds ratios (OR) along with 95% confidence intervals (95% CI) were estimated.
Results
Characteristics of the study population
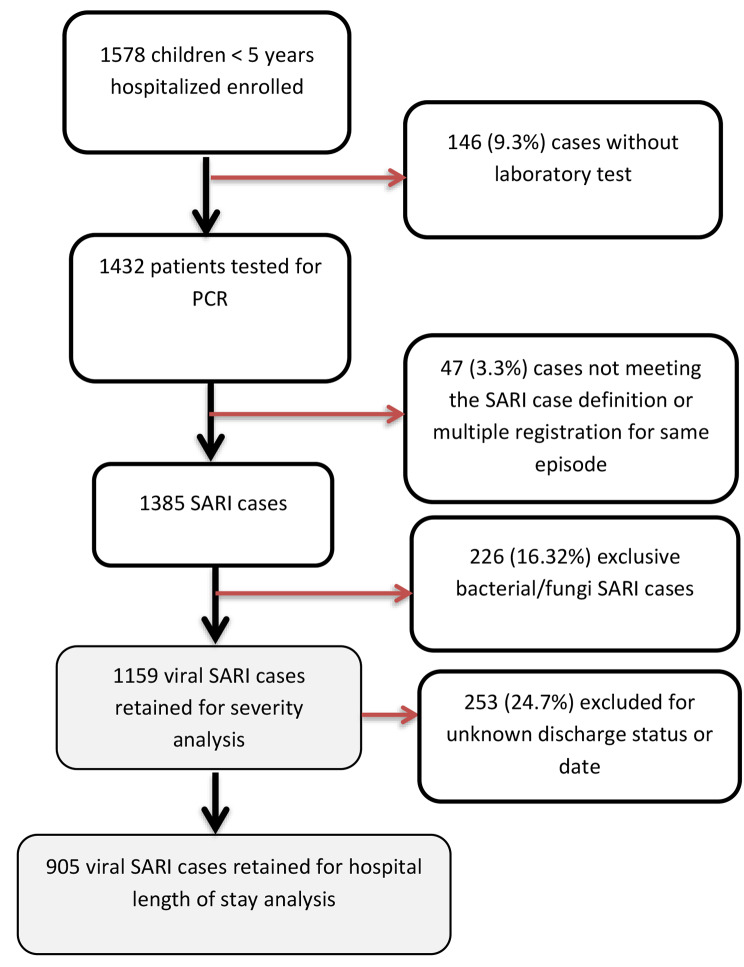
The four sentinel surveillance sites identified and enrolled a total of 1578 children in the study. Of these, 146 (9.3%) did not provide specimens for laboratory testing and were excluded. Of the remaining 1432 patients who received laboratory testing, 47 (3.3%) did not meet the SARI case definition, and another 273 (19.1%) tested positive exclusively for bacterial or fungal infection and were excluded from the analysis. All cases were positive for at least one pathogen (virus, bacteria or fungus). Therefore, a total of 1159 (64%) viral SARI cases were included in severity analysis (Fig. 1). For the length of hospital stay analysis, 253 (24.7%) of viral SARI cases were excluded for unknown discharge status or date.
Fig. 1.
Cases selection diagram
More than half of the participants were aged 12 to 59 months (56.3%), male (58.6%), and living in rural areas (55.7%). The majority (70.7%) were admitted in the dry season (November-April) [25]. A total of 344 participants (29.4%) met the definition of a very severe viral ARI (VSVARI) case (Table 1), while 214 (23.6%) had a prolonged hospital stay. The majority (85.8%) of the participants had viral and bacterial codetection (Table 2).
Table 1.
Socio-demographic and clinical factors associated with severity of very severe viral acute respiratory infections (VSVARI) among children under five years of age
| Very severe viral acute respiratory infection compared to other SARI cases | |||||||
|---|---|---|---|---|---|---|---|
| Variables |
Total sample
n
(%)
Total N = 1159 |
Total
VSVARI n (%VSVARI) Total n = 344 |
Crude OR
(95% CI) |
p-value |
Adjusted
OR (95% CI) |
p-value | |
| Age group | |||||||
| 0–3 months | 149 (12.9) | 58 (16.9) | 1.7 (1.2–2.4) | 0.006** | 1.5 (1.1–2.3) | 0.044* | |
| 4–11 months | 357 (30.8) | 107 (31.1) | 1.1 (0.8–1.5) | 0.388 | 1.1 (0.8–1.5) | 0.581 | |
| 12–59 months | 653 (56.3) | 179 (52) | ref | --- | 1(ref.) | --- | |
| Area of residence | |||||||
| Urban | 514 (44.3) | 166 (48.3) | 1.25(1-1.6) | 0.082 | 1.3 (1.1–1.7) | 0.034* | |
| Rural | 645 (55.7) | 178 (51.7) | 1(ref.) | --- | 1(ref.) | --- | |
| Sex | |||||||
| Male | 679(58.6) | 211(61.3) | 1.17(0.9–1.5) | 0.217 | --- | --- | |
| Female | 480(41.4) | 133(38.7) | 1(ref.) | --- | --- | --- | |
| Season*** | |||||||
| Dry season | 819(70.7) | 220(70.7) | 1(ref.) | -- | 1(ref.) | --- | |
| Rainy season | 344(29.3) | 124(340) | 1.6(1,2–2) | 0.001** | 1.4(1.1–1.9) | 0.028* | |
| Sites | |||||||
| Bogodogo | 324(28) | 73(21.2) | 1(ref.) | -- | --- | --- | |
| Bousse | 183(15.8) | 49(14.2) | 1.25(0.8–1.9) | 0.283 | --- | --- | |
| Kongoussi | 341(29.4) | 60(17.4) | 0.73(0.5–1.1) | 0.112 | --- | --- | |
| Houndé | 311(26.8) | 162(47.1) | 3.7(2.6–5.3) | < 0.001** | --- | --- | |
| Antibiotics before admission | |||||||
| No | 460(39.7) | 148(43) | 1(ref.) | -- | --- | --- | |
| Yes | 699(60.3) | 196(57) | 0.8(0.6–1.1) | 0.132 | --- | --- | |
| Antibiotics during hospitalization | |||||||
| No | 19(1.6) | 9(2.6) | 2.2(0.9–5.4) | 0.095 | 2.7(1.1–7.2) | 0.048* | |
| Yes | 1140(98.4) | 335(97.4) | 1(ref.) | -- | 1(ref.) | --- | |
| Type of antibiotics used | |||||||
| Ceftriaxone | 1121 (96.7) | 332(29.6) | 0.9(0.4–1.8) | 0.795 | --- | --- | |
| Penicilline G | 12(1.04) | 3(25) | 0.8(0.2–2.9) | 0.722 | --- | --- | |
| Gentamycin | 104(9) | 32(30.8) | 1.1(0.7–1.6) | 0.799 | --- | --- | |
| Others antibiotics**** | 29(2.5) | 3(10.34) | 0.3(0.1–0.9) | 0.031* | --- | --- | |
| Chronic health condition***** | |||||||
| No | 1147(99) | 340(98.8) | 1(ref.) | -- | --- | --- | |
| Yes | 12(1) | 4(1.2) | 1.2(0.3-4) | 0.776 | --- | --- | |
| Length of stay | |||||||
| 0–7 Days | 920(79.4) | 278(80.8) | 1.1(0.8–1.5) | 0.450 | --- | --- | |
| >7 Days | 239(20.6) | 66(19.2) | 1(ref.) | -- | --- | --- | |
| Malnutrition | |||||||
| No | 1072(92.5) | 323(93.9) | 1.3(0.8–2.2) | 0.247 | 1.6(0.9–2.8) | --- | |
| Yes | 87(7.5) | 21(6.1) | 1(ref.) | -- | 1(ref.) | --- | |
*p < 0.05 ** p < 0.01; VSVARI : Very severe viral acute respiratory infection ; ***Rainy season : June to September, dry season : October to may [25]; OR : odds ratio
****others antibiotics: Amoxicillin, metronidazole, ampicillin, cefotaxime; *****Chronic health conditions : asthma, circle cell diseases, HIV, diabetes, obesity, epilepsy, high blood pressure
Table 2.
Prevalence of viral respiratory tract pathogens among viral SARI cases and association with very severe viral acute respiratory infections (VSVARI) among children under five years of age
| Very severe viral acute respiratory infection (VSVARI) compared with other viral SARI (VSARI) cases | |||||||
|---|---|---|---|---|---|---|---|
| Variables |
Total positive (%)
Total N = 1159 |
Total VSVARI
(%VSVARI) N = 344 |
Crude OR
(95% CI) (positive vs. negative) |
p-value |
Adjusted
OR (95% CI) (Positive vs. Negative) |
p-value | |
| Respiratory syncytial virus | 212(18.3) | 70(20.3) | 1.2(0.9–1.7) | 0.24 | 0.9(0.6–1.3) | 0.526 | |
| Rhinovirus | 449(38.7) | 149(43.3) | 1.3(1.01–1.7) | 0.038* | --- | --- | |
| Parechovirus | 14(1.2) | 5(1.5) | 1.3(0.4-4) | 0.62 | --- | --- | |
| Human parainfluenza virus 1 | 31(2.7) | 3(0.9) | 0.25(0.07–0.8) | 0.022* | 0.2(0.1–0.7) | 0.009** | |
| Human parainfluenza virus 2 | 21(1.8) | 7(2) | 1.2(0.5-3) | 0.712 | --- | --- | |
| Human parainfluenzavirus 3 | 80(6.9) | 30(8.7) | 1.5(0.9–2.3) | 0.114 | --- | --- | |
| Human parainfluenzavirus 4 | 57(4.9) | 18(5.2) | 1.1(0.6–1.9) | 0.751 | --- | --- | |
| Influenza A | 160(13.8) | 35(10.2) | 0.6(0.4–0.9) | 0.021* | 1.6(1.02–2.4) | 0.04* | |
| Influenza B | 77(6.6) | 16(4.7) | 0.6(0.3–1.1) | 0.080 | --- | --- | |
| Influenza C | 15(1.3) | 3(0.9) | 0.6(0.16–2.1) | 0.414 | --- | --- | |
| Human metapneumovirus | 75(6.5) | 28(8.1) | 1.4(0.9–2.3) | 0.135 | --- | --- | |
| Enterovirus | 150(12.9) | 49(14.2) | 1.2(0.81–1.7) | 0.391 | --- | --- | |
| Coronavirus OC43 | 43(3.7) | 9(2.6) | 0.6(0.3–1.3) | 0.205 | --- | --- | |
| Coronavirus NL63 | 30(2.6) | 13(3.8) | 1.8(0.9–3.8) | 0.102 | --- | --- | |
| Coronavirus HKU1 | 39(3.4) | 6(1.7) | 0.42(0.2–1.01) | 0.054 | --- | --- | |
| Coronavirus 229E | 11(0.9) | 3(0.9) | 0.9(0.2–3.3) | 0.861 | --- | --- | |
| Bocavirus | 118(10.2) | 43(12.5) | 1.4(0.9–2.1) | 0.091 | --- | --- | |
| Adenovirus | 256(22.1) | 76(22.1) | 1(0.7–1.3) | 0.998 | --- | --- | |
| Staphylococcus aureus | 205(17.7) | 70(20.3) | 1.3(0.9–1.8) | 0.124 | --- | --- | |
| Klebsiella pneumoniae | 385(33.2) | 150(43.6) | 1.9(1.4–2.5) | < 0.001** | 1.6(1.2–2.2) | 0.001 | |
| Legionella pneumophil /Legionella longbeach | 1(0.1) | 0(0) | --- | --- | --- | --- | |
| Streptococcus pneumoniae | 683(58.9) | 224(65.1) | 1.4(1.1–1.9) | 0.006** | --- | --- | |
| Bordetella spp. | 5(0.4) | 1(0.3) | 0.6(0.06–5.3) | 0.639 | --- | --- | |
| Chlamydia pneumoniae | 2(0.2) | 1(0.3) | 2.3(0.14-38) | 0.542 | --- | --- | |
| Haemophilus influenzae | 516(44.5) | 172(50) | 1.36(1.1–1.8) | 0.015* | --- | --- | |
| Haemophilus influenzae type b | 22(1.9) | 13(3.8) | 3.5(1.5-8) | 0.004** | 2.7(1.1–6.7) | 0.03* | |
| Moraxella catarrhalis | 514(44.3) | 173(50.3) | 1.4(1.1–1.8) | 0.008** | --- | --- | |
| Mycoplasma pneumoniae | 10(0.9) | 3(0.9) | 1.01(0.3–3.9) | 0.982 | --- | --- | |
| Pneumocystis jirovecii | 29(2.5) | 11(3.2) | 1.4(0.7–3.1) | 0.327 | --- | --- | |
| Salmonella spp | 2(0.2) | 1(0.3) | 2.4(0.15-38) | 0.542 | --- | --- | |
| virus-bacteria codetection | |||||||
| No | 165(14.2) | 28(8.1) | 1(ref.) | --- | --- | ||
| Yes | 994(85.8) | 316(91.9) | 2.3(1.5–3.5) | < 0.001** | --- | --- | |
| Type of bacterial codetection | |||||||
| No bacteria | 165(14.2) | 28(8.1) | 1(ref.) | 1(ref.) | |||
| Mono-detection | 251(21.7) | 63(18.3) | 1.6(1-2.7) | 0.051 | 1.5(0.9–2.4) | 0.152 | |
| Multiple bacterial codetection | 743(64.1) | 253(73.5) | 2.5(1.6–3.9) | < 0.001** | 2.2(1.4–3.5) | 0.001** | |
| Type of mixed viral infection | |||||||
| Monoviral detection | 584(50.4) | 166(48.3) | 1(ref.) | --- | --- | ||
| Two viruses | 398(34.4) | 122(35.5) | 1.1(0.8–1.5) | 0.451 | --- | --- | |
| Three and more viruses | 176(15.2) | 56(16.3) | 1.2(0.8–1.7) | 0.386 | --- | --- | |
*p < 0.05 ** p < 0.01 VSARI : Viral severe acute respiratory infection VSVARI : Very severe viral acute respiratory infection OR : odds ratio
Factors associated with very severe viral acute respiratory infections (VSVARI)
The adjusted odds of having very severe viral ARI (VSVARI) rather than VSARI were significantly greater in children under three months of age compared to those aged one year and more Adjusted odds ratio (AdjOR = 1.5; 95% CI: 1.1–2.3). In addition, urban residence has 1.6 greater odds of very severe viral SARI compared to residence in rural areas (AdjOR = 1.3; 95% CI: 1.1–1.7). Similarly, rainy season was associated with increased odds of very severe viral SARI compared to the dry season (AdjOR = 1.3; 95% CI: 1.1–1.7). Non-administration of antibiotic treatment during hospitalization was strongly associated with increased odds of very severe viral SARI cases in the multivariable analysis (AdjOR = 2.7; 95% CI: 1.1–7.2) (Table 1).
In univariate analysis, codetection of bacterial respiratory tract pathogens was associated with VSVSARI, specifically S. pneumoniae, H. influenzae, Hib, Staphylococcus aureus, Moraxella catarrhalis, and Klebsiella pneumoniae. The multivariable analysis confirmed the unadjusted effect of Human Para Influenza Virus 1 (unadjusted OR = 0.25 95% CI: 0.07–0.8) and Hib (unadjusted OR = 3.5(1.5–3.5) infection on the severity of viral ARI (Adj OR = 0.2; 95% CI:0.1–0.7), (Adj OR = 2.7(1.1–6.7) respectively and the risk associated with Klebsiella pneumoniae codetection (Adj OR: 1.6; 95% CI: 1.2–2.2) (Table 2).
Factors associated with prolonged hospitalization for viral severe acute respiratory infections (VSARI)
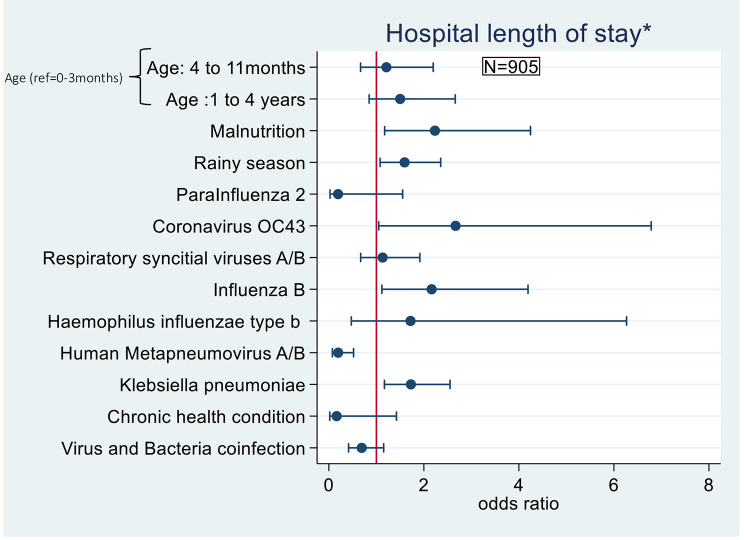
In multivariable and multilevel analysis, malnutrition (estimated by using the definitions from UNICEF-WHO-World Bank [26]) was associated with increased odds of prolonged hospitalization compared to non-malnourished patients (AdjOR = 2.2; 95% CI: 1.2–4.2). Similarly, the odds of prolonged hospitalization for viral SARI were 1.6 times greater during the rainy season compared to the dry season (AdjOR = 1.6; 95% CI: 1.1–2.3) (Table 3). Furthermore, infection with human metapneumovirus was associated with decreased odds of prolonged hospitalization (Adj OR = 0.2;95% CI: 0.1–0.7). Klebsiella pneumoniae (AdjOR = 1.7; 95% CI: 1.2–2.5), influenza B (AdjOR = 2.2; 95% CI: 1.1–4.2), and coronavirus OC43 (AdjOR = 2.7; 95% CI: 1.1–6.8) were associated with increased odds of prolonged hospitalization (Table 4 and Fig. 2).
Table 3.
Univariate analysis of socio-demographic and clinical factors associated with prolonged hospital stay (> 7 days) among viral severe acute respiratory infections (VSARI) among children under five years of age
| Variables | Total (%) N = 905 |
Prolonged hospital**** stay (%) N = 214 |
Crude OR (95% CI) |
p-value | |
|---|---|---|---|---|---|
| Age group | |||||
| 0–3 months | 120 (13.3) | 25 (11.7) | 1 (ref.) | --- | |
| 4–11 months | 274 (30.3) | 67 (31.3) | 1.2 (0.7–2.1) | 0.435 | |
| 12–59 months | 511 (56.3) | 122 (57) | 1.2 (0.7–1.9) | 0.479 | |
| Area of residence | |||||
| Urban | 429 (47.4) | 113 (52.8) | 1 (ref.) | --- | |
| Rural | 476(52.6) | 101 (47.2) | 0.75 (1.2–2.1) | 0.071 | |
| Sex | |||||
| Male | 538(59.4) | 128 (59.8) | 1 (ref.) | --- | |
| Female | 367(40.2) | 86 (40.6) | 0.98 | 0.901 | |
| Season*** | |||||
| Dry season | 612 (67.6) | 132 (61.7) | 1 (ref.) | --- | |
| Rainy season | 293(32.4) | 82 (38.3) | 1.4 (1.02–1.9) | 0.034 | |
| Sites | |||||
| Bogodogo | 275 (30.4) | 120 (56.1) | 1 (ref.) | ||
| Bousse | 160 (17.7) | 3 (1.4) | 0.02 (0.007–0.07) | < 0.001** | |
| Kongoussi | 224 (24.8) | 69 (32.2) | 0.57 (0.4–0.8) | 0.003 | |
| Houndé | 246 (27.2) | 22 (10.3) | 0.12 (0.07–0.21) | < 0.001** | |
| Antibiotics before admission | |||||
| No | 358 (39.6) | 77 (36) | 1 (ref.) | --- | |
| Yes | 547 (60.4) | 137 (64) | 1.2 (0.9–1.7) | 0.221 | |
| Antibiotics during hospitalization | |||||
| No | 17 (1.9) | 7 (3.3) | 1 (ref.) | 0.023* | |
| Yes | 888 (98.1) | 207 (96.7) | 0.4 (0.16–1.15) | 0.095 | |
| Chronic health condition | |||||
| No | 894 (98.8) | 213 (99.5) | 1 (ref.) | --- | |
| Yes | 11(1.2) | 1 (0.5) | 0.3 (0.04–2.5) | 0.278 | |
| Very severe viral ARI | |||||
| No | 626 (69.2) | 152 (71) | 1 (ref.) | --- | |
| yes | 279(30.8) | 62 (29) | 0.9 (0.6–1.2) | 0.501 | |
| Delay of consultation***** | |||||
| 0–2 days | 446 (49.3) | 102 (47.7) | 1 (ref.) | --- | |
| 3–5 days | 345(38.1) | 81 (37.9) | 1.03 (0.7–1.4) | 0.841 | |
| 6 days and more | 114(12.6) | 31 (14.5) | 1.25 (0.8-2) | 0.334 | |
| Malnutrition | |||||
| No | 833 (92) | 192 (89.7) | 1 (ref.) | --- | |
| Yes | 72 (8) | 22 (10.3) | 1.46 (0.9–2.5) | 0.152 | |
*p < 0.05 ** p < 0.01 VSARI : Viral severe acute respiratory infection OR : odds ratio; ratio ***Rainy season : June to September, dry season : October to may; ****prolonged hospital stay : length of stay > 7 days; ***** Time between the onset of symptoms and the consultation to the hospital
Table 4.
Univariate analysis of pathogens associated with prolonged hospital stay (> 7 days) of viral severe acute respiratory infections among children under five years of age
| Variables | Total Positive (%) N = 905 |
Prolonged hospital stay (%) N = 214 |
Crude OR (95% CI) (Positive vs. Negative) |
p-value |
|---|---|---|---|---|
| Respiratory syncytial virus | 157 (17.3) | 35 (16.4) | 0.9 (0.6–1.3) | 0.661 |
| Rhinovirus | 340(37.6) | 88 (41.1) | 1.2 (0.9–1.6) | 0.220 |
| Parechovirus | 10 (1.1) | 2 (0.9) | 0.8 (0.16–3.8) | 0.785 |
| Human parainfluenza virus 1 | 23 (2.5) | 7 (3.3) | 1.4 (0.6–3.5) | 0.440 |
| Human parainfluenza virus 2 | 18 (2) | 1 (0.5) | 0.18 (0.02–1.4) | 0.103 |
| Human parainfluenzavirus 3 | 70 (7.7) | 16 (7.5) | 0.95 (0.5–1.7) | 0.871 |
| Human parainfluenzavirus 4 | 44 (4.9) | 8 (3.7) | 0.7 (0.3–1.5) | 0.382 |
| Influenza A | 126 (13.9) | 29 (13.6) | 0.95 (0.6–1.5) | 0.858 |
| Influenza B | 58 (6.4) | 20 (9.3) | 1.8 (1-3.1) | 0.047* |
| Influenza C | 14 (1.5) | 1(0.5) | 0.2 (0.03–1.8) | 0.176 |
| Human metapneumovirus | 58 (6.4) | 5 (2.3) | 0.3 (0.1–0.7) | 0.009** |
| Enterovirus | 118 (13) | 21 (9.8) | 0.7 (0.4–1.1) | 0.11 |
| Coronavirus OC43 | 29 (3.2) | 10 (4.7) | 1.7 (0.8–3.8) | 0.168 |
| Coronavirus NL63 | 28 (3.1) | 4 (1.9) | 0.52 (0.2–1.5) | 0.244 |
| Coronavirus HKU1 | 25 (2.8) | 5 (2.3) | 0.8 (0.3–2.2) | 0.664 |
| Coronavirus 229E | 8 (0.9) | 2 (0.9) | 1.1 (0.2–5.4) | 0.928 |
| Bocavirus | 102 (11.3) | 18 (8.4) | 0.7 (0.4–1.1) | 0.132 |
| Adénovirus | 201 (2.2) | 44 (20.6) | 0.9 (0.6–1.3) | 0.507 |
| Staphylococcus aureus | 160 (17.7) | 40 (18.7) | 1.1 (0.7–1.6) | 0.657 |
| Klebsiella pneumoniae | 321 (35.5) | 90 (42.1) | 1.4 (1–2) | 0.022* |
| Legionella pneumophil /Legionella longbeach | 1 (0.1) | 0 (0) | ---- | ---- |
| Streptococcus pneumoniae | 526(58.1) | 107 (50) | 0.6 (0.5–0.9) | 0.006 |
| Bordetellaspp | 5 (0.6) | 1 (0.5) | 0.8 (0.1–7.2) | 0.848 |
| Chlamydia pneumoniae | 2 (0.2) | 0 (0) | ---- | --- |
| Haemophilus influenzae | 390 (43.1) | 71 (33.2) | 0.6 (0.4–0.8) | 0.001** |
| Haemophilus influenzae b | 20 (2.2) | 4 (1.9) | 0.8 (0.3–2.4) | 0.698 |
| Moraxella catarrhalis | 383 (42.3) | 74 (34.6) | 0.6 (0.5–0.9) | 0.009** |
| Mycoplasma pneumoniae | 7 (0.8) | 2 (0.9) | 1.2 (0.2–6.7) | 0.759 |
| Pneumocystis jirovecii | 22 (2.4) | 5 (2.3) | 0.9 (0.3–2.6) | 0.918 |
| Salmonella spp | 0 (0) | 0 (0) | ----- | ----- |
| virus-bacteria codetection | ||||
| No | 128 (14.1) | 38 (17.8) | 1 (ref) | |
| Yes | 777 (85.9) | 177 (82.2) | 0.7 (0.5–1.1) | 0.105 |
| Type of bacterial codetection | ||||
| No bacteria | 128 (14.1) | 38(17.8) | 1 (ref) | |
| Mono-infection | 209 (23.1) | 56 (26.2) | 0.9 (0.5–1.4) | 0.566 |
| Multiple bacterial codetection | 568 (62.8) | 120 (56.1) | 0.63 (0.4-1) | 0.038* |
| Type of mixed viral infection | ||||
| Monoviral | 455(50.3) | 119 (55.6) | 1 (ref) | |
| Two viruses | 310 (34.3) | 70 (32.7) | 0.8 (0.6–1.1) | 0.173 |
| Three and more viruses | 139 (15.4) | 25 (11.7) | 0.6 (0.4–0.9) | 0.026* |
*p < 0.05 ** p < 0.01 ; VSARI : Viral severe acute respiratory infection OR : odds ratio; ****prolonged hospital stay : length of stay > 7 days
Fig. 2.
Factors associated with prolonged hospital stay of viral severe acute respiratory infections (VSARI) among children under five (multivariable analysis). *prolonged hospital stay = length of stay > 7 days;
Discussion
Our study focused on two key determinants of the viral SARI burden in children and healthcare systems: the severity and length of stay. We highlighted the important role of some socio-demographic and clinical factors, as well as codetection of other pathogens and occurrence in rainy season in worsening of viral SARI case in children.
Residence in urban areas, age of less than three months, and codetection with Klebsiella pneumoniae and Haemophilus influenzae type b were associated with increased severity of severe acute respiratory infection. Malnutrition, hospitalization during the rainy season, and infection with human CoronavirusOC43 were significantly associated with prolonged hospitalization among those with viral SARI.
Increased severity of viral acute respiratory infections
Our study found increased odds of VSVARI among patients from urban areas compared to rural residents. This could be because one of our collection sites was an urban referral teaching hospital that is better equipped and therefore likely to receive patients with severe illness from hospitals that are less equipped with intensive care equipment and have less qualified personnel. Residents living in urban areas may be better able to access hospitals to seek care than those who are very ill and may live a long distance from a rural hospital. However, other studies revealed that the most severe forms of ARI were related to environmental risk factors that are generally more present in urban than rural settings. Cummings et al. in a spatiotemporal study in Uganda in 2016 found a higher risk of more severe forms of ARI in urban and peri-urban areas than in rural areas [27]. A study by Akinyemi and Morakinyo in Nigeria in 2018 [28] also implicated the living environment, especially intra-domiciliary pollution, as a risk factor of ARI. Kafando et al. also observed similar findings in the city of Ouagadougou in 2018 [29].
Nevertheless, regional disparities in the distribution of these risk factors must be considered in preventing severe ARIs among children. Several recent studies in Ethiopia [30], Nigeria [14] and Uganda [27] have found that ARI can present substantial regional disparities within a single country because of the differences in the standard of living (poverty) and geo-climatic factors.
Our findings provide further evidence of the association of young age with severe acute respiratory infection. In addition to the immaturity of the child’s immune system and the changing immune processes, the role of infection in the evolution of immune defenses and allergies in children is becoming increasingly evident [31]. The more severe forms of ARIs are typically found among younger children, regardless of specific viral etiology. This trend is also found in most of the studies in the West Africa sub-region [11, 16, 32].
During hospitalization, children who were not treated with antibiotics had 2.7 times greater odds of very severe viral SARI. These results should be interpreted with caution, given the usage of antibiotics in our study population during their hospital stay (98.3% treated with antibiotics). Although it is recognized that the ARIs are predominantly caused by viral infections, antibiotics are widely administered to hospitalized patients in developing countries [33]. It is also worth noting that bacterial codetection in people with viral ARIs is one of the major causes of severity and hospitalization among children [9], hence the frequent use of antibiotics in severe ARI cases.
The presence of a bacterial coinfection in viral ARI is very often a source of greater severity. Viral damage to the epithelial barrier and impaired mucociliary function weakens airway defenses, increasing vulnerability to bacterial coinfection [34]. Klebsiella pneumoniae and Haemophilus influenzae type b are widely recognized as bacterial etiological agent of many types of infections, including respiratory infections. There are responsible for community-acquired ARI and hospital-acquired forms, which are the most virulent as they are often resistant to the usual antibiotics [35]. Haemophilus influenzae type b is included in the Burkina Faso Expanded Program on Immunization. The detection of this pathogen and its association with SARI raise questions regarding vaccine coverage, warranting further investigations [36]. Furthermore, in recent years, new subtypes of Klebsiella pneumoniae responsible for very severe respiratory infections have been reported [35], including the hypermucoviscous form described as hypervirulent in many Asian countries and increasingly in Europe, with limited data available in Africa [37–39]. Our results also demonstrate, multiple bacterial co-infections increased the risk of VSVARI occurrence. In the majority of similar studies conducted in the sub-region, bacterial co-infection is identified as a contributing factor to the severity of viral Acute Respiratory Infections (ARIs) [9, 17].
However, it is crucial to keep in mind that the sampling and diagnostic methods used in our study, such as oro/nasopharyngeal swabbing and rRT-PCR, may detect carrier organisms that are not necessarily the etiology of the SARI [40].
Factors associated with prolonged hospitalization of viral SARI
In multivariable analysis, we found that malnutrition, rainy season, infection with Coronavirus OC43, influenza B, hMPV AB, and Klebsiella pneumoniae were associated with prolonged hospitalization (> 7 days) in children under five years of age. Numerous studies have reported the effect of malnutrition (severe and moderate forms) on susceptibility to several types of infection. Bryce et al., found malnutrition to be the main mortality factor in 52.3% of children with pneumonia [41]. The relationship between malnutrition and acute infection disease is bidirectional: malnutrition increases risk of infection, and the infection worsens the malnutrition state [42]. Ngari et al. [43], and Lazzeri et al. [44], in recent studies in Kenya and Malawi, respectively, found a strong association between malnutrition and mortality from ARI. Cox et al., in Malawi, found malnutrition to be a predictor of the development of respiratory infection [13]. However, we did not find a study in the literature that specifically explored the relationship between respiratory viral infection, length of hospitalization, and malnutrition.
Children admitted to the hospital during the rainy season for viral SARI were 1.6 times more likely to have a prolonged hospitalization than those hospitalized during the dry season. Annual peaks in the incidence of the viruses responsible for ARI in tropical regions are primarily observed in the dry season [45, 46], even though some studies report less evidence of seasonality for several respiratory pathogens in tropical areas [47]. Malaria is highly prevalent in children under five in the rainy season in Burkina Faso, and the comorbidity with ARI could contribute to a higher risk of a lengthy hospital stay [22, 23]. In addition, the rainy season in rural areas corresponds to a “lean season”. Food stocks in rural areas are generally at their minimum, exposing the younger population to malnutrition. Nevertheless, the absence of data on malaria comorbidity does not allow us to refine our analysis.
Children infected with influenza B virus, Coronavirus OC43, and Klebsiella pneumoniae had an increased risk of prolonged hospitalization in our population, whereas infection with human metapneumovirus decreased this risk. Influenza B virus is poorly studied compared with influenza A. Therefore, there are still many unclear areas and gaps in knowledge about its epidemiology and pathogenicity [48], even though clinically, influenza B virus infection shows few differences with influenza A and other respiratory viruses. Nevertheless, it has been reported that influenza B virus infection has been associated with severe ARI in children, leading to admission to intensive care units with a risk of prolonged hospitalization [49]. In our study, the odds of prolonged hospitalization were twice as high in the patients with influenza B virus infection in multilevel multivariable analysis, reflecting a relatively strong association. However, little is known about the immunological mechanisms and virological characteristics that may explain the higher pathogenicity of the influenza B virus compared to influenza A, and very few studies have been conducted [17].
As for Human coronavirus OC43, its association with prolonged hospitalization found in our study is not common in the literature. Indeed, among the six coronaviruses type responsible for respiratory infection in humans before the advent of SARS-CoV-2 in 2020 in Burkina Faso [40], only MERS-Cov and SARS-Cov were implicated in the most severe forms of ARI in many countries. The other types of coronaviruses are most often responsible for less severe cases or even mild rhinitis. Nevertheless, the presence of Human coronavirus OC43 as an etiological agent of SARI has been described in numerous studies in sub-Saharan Africa without association with possible severity or prolonged hospitalization of cases being investigated [50–52]. The larger size of our sample, the possible presence of undetected comorbidities, and our analysis methods (multilevel logistic regression) may explain our findings.
Klebsiella pneumoniae was the only pathogen associated with the severity of ARI and prolonged hospitalization. Nevertheless, as described above, there may be interdependence between severe ARI and the risk of prolonged hospitalization. The possible existence of nosocomial infections, the resistance to classical antibiotics, and the virulence of certain subtypes of Klebsiella pneumoniae may explain our results [35, 53].
Strengths and limitations
The main strength of our study was the use of multi-pathogen screening allowing the detection of a wide range of viral respiratory pathogens with the advantage of the high sensitivity of PCR techniques combined with a quick turn-around time to results. To our knowledge, this is the first research of this kind in Burkina Faso. The combination of the detection of bacterial and fungal pathogens allowed the identification of possible coinfections. Furthermore, use of the standardized WHO SARI case definition allows us to compare across our study population and with other surveillance systems worldwide. Similarly, our study sites, located in both urban and rural areas, ensured heterogeneity in the study population.
Nevertheless, our study had limitations related to the nature of cross-sectional studies. Pathogens found in nasal and/or oropharyngeal samples may not match those that are inside the lung, therefore, some detected germs may not necessarily be related to the patient’s symptomatology. Moreover, the absence of certain variables, particularly those related to malaria and other comorbidities, the impossibility to assess the temporality of some explanatory variables compared with the dependent variables (such as the co-infection) and the non-systematic measure of the oxygen saturation rate did not allow us to refine our severity analysis.
Conclusion
This study was motivated by the significant impact of viral SARI on children’s morbidity and mortality and the relatively limited data on the subject in the African sub-region in general and in Burkina Faso. Our report is the first that identifies some specific and atypical pathogens in SARI in Burkina Faso. The results suggest the need for practitioners in Burkina Faso and other countries sharing similar features to pay more attention to early diagnosis and management of comorbidities such as malnutrition in children hospitalized with SARI particularly during malaria peak seasons to prevent complications. Additionally, implementation of public health policies for early etiological diagnosis and management of certain forms of viral ARI including vaccination are important to control ARI morbidity and mortality in children. The results also call for a further investigation of comorbidities such as malaria, and a better understanding of bacterial coinfection and their role in the morbidity and mortality of viral SARI in children.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to express their sincere thanks to the health authorities of Burkina Faso, and particularly to the Director of Population Health Protection (DPSP), the District Chief Doctors (MCDs), to all physicians, nurses’ staff and all actors who actively participated in the data collection. They would also like to thank the government of United State of America, CDC Atlanta, for funding this study through the projects BURKINA RESPIRE and Influenza cooperative agreement.
Author contributions
Conceptualization and study design: ZT, AKI, PSD; data analysis: AKI; Laboratory analysis: AC; Investigation, data collection, and management: AKI,JM,DT, SAM, AOD, KJC; writing– original draft: AKI; Manuscript review and editing: AC,JM, DT,SAM,AOD, BWB,SD, DO, JLW, NTN, MDC, CGW,ZT. All authors read and approved the final manuscript.
Funding
This SARI’s surveillance is funded by the Center of Diseases Control and Prevention - Atlanta and the Ministry of Health of Burkina Faso through the projects BURKINA RESPIRE and Influenza cooperative agreement.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Data analyzed in the study were obtained from the Burkina Faso Ministry of Health surveillance services. The Ministry of Health implemented the sentinel surveillance and determined SARIs surveillance to be a public health program in accordance with the law No. 23/94/ADP on the public health code and the ARRET No. 2023-83/MSHP/CAB/PM/MSHP on the organization and responsibilities of diseases surveillance services of Burkina Faso [54]; therefore, for the national ethics committee (Comité d’éthique pour la recherche en Santé au Burkina Faso), it did not required ethics approval. However informed oral consent to participate in the study was obtained from children’s parent and/or their legal guardians. All methods were carried out in accordance with relevant guidelines and regulations following the Helsinki recommendations [55].
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitney CG. Measuring progress on preventing pneumonia deaths: are we there yet? Lancet Infect Dis. 2017;17(11):1100–1. doi: 10.1016/S1473-3099(17)30481-4. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet sept. 2017;390(10100):1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Infect Dis Nov. 2018;18(11):1191–210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feikin DR, Flannery B, Hamel MJ, Stack M, Hansen PM. Vaccines for Children in Low- and Middle-Income Countries. In: Black RE, Laxminarayan R, Temmerman M, Walker N, éditeurs. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2) [Internet]. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 [cité 1 nov 2020]. Disponible sur: http://www.ncbi.nlm.nih.gov/books/NBK361927/.
- 5.Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, et al. The estimated mortality impact of vaccinations forecast to be administered during 2011–2020 in 73 countries supported by the GAVI Alliance. Vaccine 18 avr. 2013;31:B61–72. doi: 10.1016/j.vaccine.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, Virology, and Immunology. Clin Microbiol Rev 1 janv. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381(9875):1380–90. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta–analysis. J Glob Health [Internet]. [cité 24 oct 2018];5(1). Disponible sur: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4593292/. [DOI] [PMC free article] [PubMed]
- 9.O’Brien KL, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, Higdon MM et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. The Lancet [Internet]. 27 juin 2019 [cité 11 juill 2019];0(0). Disponible sur: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)30721-4/abstract. [DOI] [PMC free article] [PubMed]
- 10.Kenmoe S, Tchendjou P, Vernet MA, Moyo-Tetang S, Mossus T, Njankouo-Ripa M, et al. Viral etiology of severe acute respiratory infections in hospitalized children in Cameroon, 2011–2013. Influenza Other Respir Viruses. 2016;10(5):386–93. doi: 10.1111/irv.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouédraogo S, Traoré B, Nene Bi ZAB, Yonli FT, Kima D, Bonané P, et al. Viral etiology of respiratory tract infections in children at the Pediatric Hospital in Ouagadougou (Burkina Faso). Roques P, éditeur. PLoS ONE. oct 2014;31(10):e110435. [DOI] [PMC free article] [PubMed]
- 12.Singh SK. Human respiratory viral infections. CRC; 2014.
- 13.Cox M, Rose L, Kalua K, de Wildt G, Bailey R, Hart J. The prevalence and risk factors for acute respiratory infections in children aged 0–59 months in rural Malawi: a cross-sectional study. Influenza Other Respir Viruses Nov. 2017;11:489–96. doi: 10.1111/irv.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adesanya O, Chiao C. Environmental Risks Associated with symptoms of Acute respiratory infection among Preschool Children in North-Western and South-Southern Nigeria communities. Int J Environ Res Public Health. nov 2017;16(11):1396. [DOI] [PMC free article] [PubMed]
- 15.Bénet T, Picot VS, Awasthi S, Pandey N, Bavdekar A, Kawade A et al. Severity of Pneumonia in Under 5-Year-Old Children from Developing Countries: A Multicenter, Prospective, Observational Study. Am J Trop Med Hyg. 12 juill. 2017;97(1):68–76. [DOI] [PMC free article] [PubMed]
- 16.Bénet T, Sylla M, Messaoudi M, Sánchez Picot V, Telles JN, Diakite AA et al. Etiology and Factors Associated with Pneumonia in Children under 5 Years of Age in Mali: A Prospective Case-Control Study. Schildgen O, éditeur. PLOS ONE. 22 déc. 2015;10(12):e0145447. [DOI] [PMC free article] [PubMed]
- 17.Bénet T, Sánchez Picot V, Messaoudi M, Chou M, Eap T, Wang J et al. Microorganisms Associated with Pneumonia in Children < 5 years of age in developing and emerging countries: the GABRIEL Pneumonia Multicenter, prospective, case-control study. Clin Infect Dis [Internet]. 12 juin 2017 [cité 28 janv 2019]; Disponible sur: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/cix378. [DOI] [PMC free article] [PubMed]
- 18.Surveillance case definitions for ILI. and SARI [Internet]. [cité 9 mai 2022]. Disponible sur: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari.
- 19.Fitzner J, Qasmieh S, Mounts AW, Alexander B, Besselaar T, Briand S, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ 1 févr. 2018;96(2):122–8. doi: 10.2471/BLT.17.194514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . Integrated Management of Childhood Illness: distance learning course [Internet] Geneva: World Health Organization; 2014. [Google Scholar]
- 21.Williams K, Thomson D, Seto I, Contopoulos-Ioannidis DG, Ioannidis JPA, Curtis S, et al. Standard 6: Age groups for Pediatric trials. Pediatr 1 juin. 2012;129(Supplement3):S153–60. doi: 10.1542/peds.2012-0055I. [DOI] [PubMed] [Google Scholar]
- 22.Cissé A, Milucky J, Ilboudo AK, Waller JL, Bicaba B, Medah I et al. Comparison of performance between Fast Track Diagnostics Respiratory Kit and the CDC global reference laboratory for influenza rRT-PCR panel for detection of influenza A and influenza B. Influenza Other Respir Viruses [Internet]. [cité 27 févr 2021];n/a(n/a). Disponible sur: https://onlinelibrary.wiley.com/doi/abs/10.1111/irv.12830. [DOI] [PMC free article] [PubMed]
- 23.Haddadin Z, Beveridge S, Fernandez K, Rankin DA, Probst V, Spieker AJ, et al. Respiratory Syncytial Virus Disease Severity in Young Children. Clin Infect Dis off Publ Infect Dis Soc Am 6 déc. 2021;73(11):e4384–91. doi: 10.1093/cid/ciaa1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien KL, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, Higdon MM, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet août. 2019;394(10200):757–79. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IBRAHIM B, KARAMBIRI H. POLCHER J. Evolution des principales caractéristiques de la saison des pluies Au Burkina Faso à partir des données pluviométriques de cinq modèles climatiques régionaux (MCR). Hydroclimatology Var Chang. 2011;82–6.
- 26.World Health Organization, United Nations Children’s Fund (UNICEF) World Bank. Levels and trends in child malnutrition [Internet] Geneva: World Health Organization; 2012. [Google Scholar]
- 27.Cummings MJ, Bakamutumaho B, Kayiwa J, Byaruhanga T, Owor N, Namagambo B, et al. Epidemiologic and spatiotemporal characterization of influenza and severe Acute respiratory infection in Uganda, 2010–2015. Ann Am Thorac Soc déc. 2016;13(12):2159–68. doi: 10.1513/AnnalsATS.201607-561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akinyemi JO, Morakinyo OM. Household environment and symptoms of childhood acute respiratory tract infections in Nigeria, 2003–2013: a decade of progress and stagnation. BMC Infect Dis. 3 juill. 2018;18(1):296. [DOI] [PMC free article] [PubMed]
- 29.Kafando B, Savadogo PW, Millogo T, Sana A, Kouanda S, Sondo B. Pollution De L’air intérieur et prévalence des infections respiratoires aiguës chez les enfants à Ouagadougou. Santé Publique. 2018;30(4):575–86. doi: 10.3917/spub.185.0575. [DOI] [PubMed] [Google Scholar]
- 30.Amsalu ET, Akalu TY, Gelaye KA. Spatial distribution and determinants of acute respiratory infection among under-five children in Ethiopia: Ethiopian Demographic Health Survey 2016. Odoi A, éditeur. PLOS ONE 22 avr. 2019;14(4):e0215572. doi: 10.1371/journal.pone.0215572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 14 févr. 2008;358(7):716–27. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagare A, Maïnassara HB, Issaka B, Sidiki A, Tempia S. Viral and bacterial etiology of severe acute respiratory illness among children < 5 years of age without influenza in Niger. BMC Infect Dis 14 nov. 2015;15:515. doi: 10.1186/s12879-015-1251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tula M, Iyoha O, Iruolaje F. Antibiotic resistance: challenges and Prospect for Therapy in developing countries. Br J Pharm Res 10 janv. 2015;8(3):1–16. doi: 10.9734/BJPR/2015/19061. [DOI] [Google Scholar]
- 34.Avadhanula V, Rodriguez CA, DeVincenzo JP, Wang Y, Webby RJ, Ulett GC, et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species-and cell type-dependent manner. J Virol. 2006;80(4):1629–36. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev MMBR 15 juin. 2016;80(3):629–61. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King P. Haemophilus influenzae and the lung (Haemophilus and the lung). Clin Transl Med. 14 juin. 2012;1:10. [DOI] [PMC free article] [PubMed]
- 37.Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, et al. Antimicrobial Resistance of Hypervirulent Klebsiella pneumoniae: Epidemiology, Hypervirulence-Associated determinants, and Resistance mechanisms. Front Cell Infect Microbiol. 2017;7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shon AS, Bajwa RPS, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 15 févr. 2013;4(2):107–18. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Struve C, Roe CC, Stegger M, Stahlhut SG, Hansen DS, Engelthaler DM, et al. Mapping the evolution of Hypervirulent Klebsiella pneumoniae. mBio 21 Juill. 2015;6(4):e00630. doi: 10.1128/mBio.00630-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adegbola RA, DeAntonio R, Hill PC, Roca A, Usuf E, Hoet B, et al. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PLoS ONE. 2014;9(8):e103293. doi: 10.1371/journal.pone.0103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bryce J, Boschi-Pinto C, Shibuya K, Black RE, WHO Child Health Epidemiology Reference Group WHO estimates of the causes of death in children. Lancet Lond Engl 26 avr. 2005;365(9465):1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of Childhood Malnutrition on Host Defense and infection. Clin Microbiol Rev Oct. 2017;30(4):919–71. doi: 10.1128/CMR.00119-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngari MM, Fegan G, Mwangome MK, Ngama MJ, Mturi N, Scott JAG, et al. Mortality after Inpatient treatment for severe pneumonia in children: a Cohort Study. Paediatr Perinat Epidemiol Mai. 2017;31(3):233–42. doi: 10.1111/ppe.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazzerini M, Seward N, Lufesi N, Banda R, Sinyeka S, Masache G, et al. Mortality and its risk factors in Malawian children admitted to hospital with clinical pneumonia, 2001–12: a retrospective observational study. Lancet Glob Health janv. 2016;4(1):e57–68. doi: 10.1016/S2214-109X(15)00215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren L, Xiang Z, Guo L, Wang J. Viral infections of the lower respiratory tract. Curr Infect Dis Rep. 2012;14(3):284–91. doi: 10.1007/s11908-012-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staadegaard L, Caini S, Wangchuk S, Thapa B, de Almeida WAF, de Carvalho FC, et al. Defining the seasonality of respiratory syncytial virus around the world: National and subnational surveillance data from 12 countries. Influenza Other Respir Viruses Nov. 2021;15(6):732–41. doi: 10.1111/irv.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccines Immunother janv. 2012;8(1):21–8. doi: 10.4161/hv.8.1.17622. [DOI] [PubMed] [Google Scholar]
- 48.Caini S, Huang QS, Ciblak MA, Kusznierz G, Owen R, Wangchuk S, et al. Epidemiological and virological characteristics of influenza B: results of the global influenza B study. Influenza Other Respir Viruses. 2015;9(S1):3–12. doi: 10.1111/irv.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koutsakos M, Nguyen TH, Barclay WS, Kedzierska K. Knowns and unknowns of influenza B viruses. Future Microbiol janv. 2016;11(1):119–35. doi: 10.2217/fmb.15.120. [DOI] [PubMed] [Google Scholar]
- 50.Owusu M, Annan A, Corman VM, Larbi R, Anti P, Drexler JF, et al. Human coronaviruses associated with upper respiratory tract infections in three rural areas of Ghana. PLoS ONE. 2014;9(7):e99782. doi: 10.1371/journal.pone.0099782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sipulwa LA, Ongus JR, Coldren RL, Bulimo WD. Molecular characterization of human coronaviruses and their circulation dynamics in Kenya, 2009–2012. Virol J 1 févr. 2016;13:18. doi: 10.1186/s12985-016-0474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venter M, Lassaunière R, Kresfelder TL, Westerberg Y, Visser A. Contribution of common and recently described respiratory viruses to annual hospitalizations in children in South Africa. J Med Virol août. 2011;83(8):1458–68. doi: 10.1002/jmv.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagna T, Somda WDN, Koné AC, Sagna Y, Tialla D, Sanou MA et al. Antibiotic susceptibility of Escherichia coli and Klebsiella pneumoniae strains, urinary tract infections cases in Bobo-Dioulasso, Burkina Faso. Burkina Faso. 2019;7.
- 54.LEGISANTE [Internet]. [cité 12 août 2023]. Disponible sur: http://data.sante.gov.bf/legisante/.
- 55.Declaration of Helsinki. Recommendations guiding medical doctors in biomedical research involving human subjects. Med J Aust. 14 f?vr 1976;1(7):206?7. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.