Abstract
Objective
To evaluate the cost‐effectiveness of telehealth‐delivered exercise and diet‐plus‐exercise programs within 12 months.
Methods
An economic evaluation within a 12‐month, 3‐arm, parallel randomized trial of two 6‐month telehealth‐delivered exercise programs, with and without a dietary component. A total of 415 people with knee osteoarthritis ages 45–80 years and body mass index of 28–40 kg/m2 were assigned to 1 of 2 telehealth‐delivered exercise programs, 1 without (n = 172) and 1 with (n = 175) a dietary component (ketogenic very low calorie diet), or to an education control (n = 67), for 6 months, with 6 months follow‐up. The primary economic outcomes were quality‐adjusted life years (QALYs) and health system costs. Measured costs were the direct intervention (consultations, equipment/resources, and meal replacements) and health care use in 2020 Australian dollars ($AU1.5 = $US1). Secondary analysis included weight loss and work productivity gains.
Results
The clinical trial demonstrated greater improvements in pain and function compared to information only for individuals with knee osteoarthritis and overweight/obesity. We can be 88% confident that diet plus exercise is cost effective ($45,500 per QALY), 53% confident that exercise is cost‐effective ($67,600 per QALY) compared to the control, and 86% confident that augmenting exercise with the diet program is cost effective ($21,100 per QALY).
Conclusion
Telehealth‐delivered programs targeting exercise with dietary intervention for people with knee osteoarthritis who have overweight/obesity are likely to be cost‐effective, particularly if potential long‐term gains from weight loss and work productivity are realized.
INTRODUCTION
Osteoarthritis (OA), commonly affecting the knee joint, is a major and increasing global public health issue. It is the leading cause of disability among older adults in higher income countries such as the US (1, 2) and increasingly in middle‐to‐lower income countries (3) resulting in a significant burden not only on quality of life but also on health care costs and economic activity. Estimations have been made that the total arthritis‐attributable medical care expenditures and earnings losses were $303.5 billion or 1% of the 2013 US gross domestic product, demonstrating the high personal and societal impacts of OA (4). Those with obesity and OA are more likely to have activity and work limitations, report depression and anxiety, and have an increased risk of expensive knee replacement surgery (5). Exercise and weight management are recommended as core treatments by knee OA clinical guidelines (6, 7). Despite the known health benefits of being physically active and maintaining a healthy weight, most adults with OA ages ≥65 years are physically inactive (8, 9) with a high prevalence of obesity (10).
SIGNIFICANCE & INNOVATIONS.
Programs to improve quality of life through exercise and weight reduction involve a considerable investment by patients and the health care system, and an important question is whether the gains to patients offer a good return on that investment for patients and insurers.
This is the first study to demonstrate within a randomized trial that intensive diet and exercise programs delivered by telehealth are likely to offer economic value to patients and insurers.
The findings show that augmenting a telehealth delivered exercise program with dietary weight loss is likely to offer value for money in the treatment of osteoarthritis and may offer wider gains to patients in weight loss and enhanced paid work.
There is substantial evidence showing that exercise reduces pain, increases function and improves the quality of life among people with knee OA (11, 12, 13). There is also evidence that combined diet and exercise treatments improve pain and physical function in people with knee OA and overweight/obesity (14), but only 2 studies have allowed the additive benefits of dietary weight loss to exercise to be determined (15, 16). In the IDEA trial (16), participants undertaking diet plus exercise experienced greater reduction in pain and physical dysfunction, and greater improvement in quality of life (23% of an SD on the physical health domain of the Short Form 36 measure) after 18 months compared to those undertaking exercise alone. Furthermore, few randomized controlled trials (RCTs) in knee OA have investigated exercise and diet interventions delivered by health professionals using telehealth (17, 18, 19). We recently reported the clinical results of the Better Knee, Better Me RCT, whereby two 6‐month telehealth‐delivered (via videoconference) exercise programs, 1 with and the other without a dietary weight loss program, led to significantly greater improvements in knee pain and function than an information control group, with modest additional benefits of combined diet and exercise over exercise alone (20). The combined group also lost an average of 10% of body weight and had reduced use of pain medication compared to both other groups.
Effective programs to increase exercise and reduce weight involve a considerable investment by patients and the health care system, and an important question is whether the gains to patients offer a good return on that investment. Based in part on evidence from the IDEA trial, a modeled evaluation found that with a threshold willingness‐to‐pay per quality‐adjusted life year (QALY) of $US50,000, the combined diet‐plus‐exercise intervention had a 58% probability of being cost effective compared to a control (21). Limited direct RCT evidence exists on the cost‐effectiveness of exercise and diet interventions delivered separately by telehealth (19, 22), and none on the cost‐effectiveness of combined diet and exercise telehealth interventions in knee OA. In this study, we estimated the 12‐month cost‐effectiveness, from a health system perspective, of 2 telehealth‐delivered programs to insured people in the community with knee OA and overweight or obesity within the Better Knee, Better Me pragmatic RCT (20).
PATIENTS AND METHODS
Participants
A total of 415 people with knee OA and overweight or obesity (body mass index [BMI] 28–40 kg/m2) who were members of an Australian private health insurance fund (Medibank) were randomized in a 12‐month 3‐arm participant‐unblinded parallel‐design trial. Recruitment occurred between August 6, 2018 and February 29, 2020, with follow‐up completed March 3, 2021. Details of eligibility, trial design, interventions, and processes are in the published protocol (23) and trial findings in the clinical article (20). Briefly, we recruited participants ages 45–80 years with chronic knee pain consistent with a clinical OA diagnosis (24), and who had knee pain most days for ≥3 months and average knee pain severity ≥4 on an 11‐point numerical rating scale in the previous week (0 = no pain, 10 = worst pain possible).
Program component interventions
Participants in the control group and in both programs received access to a website containing: 1) information about OA, treatment options, exercise/physical activity, weight loss, managing pain, sleep, and “success stories” and 2) links to external websites for further information. The directed information was therefore at least as extensive as might be available in practice. The 2 interventions were based on best‐practice recommendations for management of knee OA (24, 25) and obesity (26, 27) and underpinned by the chronic care model (28) and the information‐motivation‐behavioral skills theoretical model (29). The 6‐month exercise program comprised 6 videoconferencing consultations with a physical therapist for a codeveloped home exercise program and physical activity plan, self‐management advice, and behavioral counseling, plus exercise equipment and printed resources, supported by a website. The 6‐month diet‐plus‐exercise program additionally included 6 videoconferencing consultations with a dietitian to support the participant to lose at least 10% of their body weight (17) via a ketogenic very low calorie diet (2 formulated meal replacements and 1 low‐carbohydrate meal daily before transitioning to a healthy eating plan) with printed dietary and behavioral resources, supported by a website. The trial was in a real‐world setting with no restrictions on prospective treatments or usual care.
Economic perspective
The primary analysis was a cost‐utility analysis (the between‐group ratio of cost differences to differences in health‐related quality of life). The analysis takes a health system perspective on costs and outcomes and does not include other personal costs or outcomes nor any effects of treatment beyond 12 months.
Gains may exist beyond health, for example in paid work hours and related income and taxes. Secondary analyses included the calculation of between‐group differences in self‐reported hours worked and productivity while at work. To avoid the risk of double counting individual benefits, we analyzed the incremental cost per quality‐adjusted life year (QALY), the incremental cost per person with substantial (10%) weight loss, and the money value of labor market gains separately.
Outcome measures
QALYs during the trial were derived from the Assessment of Quality of Life instrument (AQoL‐8D) at 6 and 12 months using the trapezoid method. The AQoL‐8D is a validated preference‐based measure of quality of life on a 0–1 scale (0 = death, 1 = perfect health), with ratio properties such that equal absolute increments have equal value everywhere on the scale (30). This instrument is therefore suitable as a multi‐attribute utility scale for the calculation of QALYs.
Costs
The cost of the programs was calculated as the number of sessions by physical therapists and dietitians at typical fees charged and the cost of equipment/resources (Fitbit, exercise bands, booklets, and postage), meal replacements, and a portion plate. The cost of health care–related resource use (hospital inpatient, prescription and nonprescription medications, and medical and health services) was collected via custom surveys at baseline and at 6 and 12 months and valued at current published prices. Participants were asked to recall all types of health care provided, and the number of sessions attended as well as all medications in the previous 6 months (not just those related to OA). Health care services were valued using published prices for medical and diagnostic costs (31), prescription pharmaceuticals (32), nonprescription pharmaceuticals (33), and hospital unit costs (34). We included some small material costs in direct treatment costs but excluded fixed costs such as the training costs for the clinicians ($5,000) and technology setup costs, as this calculation would be very small per person in practice. The unit costs for the program components are described in detail in Supplementary Appendix A, section 1, and Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022. All costs were measured in Australian dollars (2020 $A1.5 = $US1).
Statistical analysis
The primary analysis calculated the between‐group mean differences in cumulative costs (direct program and health care costs) and QALYs at 12 months, based on a preplanned analysis (23) with missing data replaced by multiple imputation (chained predictive mean matching using linear regression with the 5 nearest neighbors and 20 imputed data sets, adjusted for baseline outcome values and a history of knee surgery). The cost‐effectiveness ratios (between‐group ratio of cost differences to QALY differences) were then compared to a threshold willingness‐to‐pay for a QALY with bootstrapped 95% confidence intervals (95% CIs) and P values calculated using the percentile method (35). Details of the adjustments for uncertainty due to missing data and the sampling of costs and outcomes are given in Supplementary Appendix A, section 2, and Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022). As an aid to interpretation, the cost‐effectiveness ratios were recalculated as the mean net monetary benefit (QALY difference times the social willingness‐to‐pay for a QALY, minus the difference in cost) (36). The initial assumed threshold maximum social willingness‐to‐pay for a QALY of $70,000 (US$47,000) was based on the likelihood of previous public reimbursements of medical technologies and surveys (37, 38). As there is no agreement on the value for this threshold or the method to estimate it (39, 40, 41), we varied the threshold and calculated and plotted acceptability curves as 1 minus the 1‐sided P values at which the program would have positive net monetary benefits (the percentage of bootstrap replicates with positive net benefits) over a range of values for the willingness‐to‐pay for a QALY (42). We present the bootstrap replicates of the cost‐effectiveness results in graphical format and the acceptability curves for each program compared to the control. This procedure allows us to consider the programs from a decision perspective and estimate our confidence in the results, given sampling uncertainty, imputation uncertainty, and uncertainty over the cost per QALY threshold.
In the secondary analyses, we replaced the outcome of QALYs with a binary indicator of whether or not the participant had lost at least 10% of self‐reported weight at 12 months compared to baseline (Supplementary Appendix A, section 3, and Supplementary Figure 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022) (43) and calculated the incremental mean cost per additional person with substantial weight loss compared to the control. We also estimated the difference in the probability of being in paid employment, hours worked per month, and perceived productivity at work collected from the self‐reported World Health Organization Health and Work Performance Questionnaire (44) (Supplementary Appendix A, section 4, and Supplementary Tables 2 and 3, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022).
As robustness checks, we (1) calculated the net benefits of each intervention using the direct program costs only; (2) calculated the incremental cost per QALY for adjusted analyses for unbalanced factors at baseline, including sex and age (Supplementary Appendix A, section 5, and Supplementary Figure 3 and Supplementary Table 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022); (3) performed completers‐only analyses (Supplementary Appendix A, section 6, and Supplementary Table 5); (4) included knee surgery costs for 2 years after trial commencement (Supplementary Appendix A, section 7, and Supplementary Table 6); (5) presented results with hypothetical full compliance by estimating complier average causal effects for intervention groups separately (Supplementary Appendix A, section 8, and Supplementary Table 7); and (6) replaced the QALY outcome with binary indicators if the individual achieved a change that was at least as great as a predefined minimum clinically significant difference (MCID) in a pain score and measure of physical function and calculated the cost per person achieving each MCID at 12 months (Supplementary Appendix A, section 9, and Supplementary Tables 8 and 9) and assessed the extent of attrition bias (Supplementary Appendix A, section 10).
RESULTS
Sample characteristics
Full details of the sample characteristics are given in the published clinical trial results (20). Table 1 shows the baseline economic‐related characteristics of the sample. Participants had a low quality of life (mean 0.69) compared to the general population (mean 0.82 at age 65–74 years) (45), and had considerable health care costs in the previous 6 months across a range of health services (mean ± SD $1,500 ± $2,900). Health care costs in the 6 months prior to the trial were somewhat lower in the control group, which also had a higher proportion of females than the intervention groups.
Table 1.
Baseline characteristics of participants by group*
| Characteristic | Control (n = 67)† | Exercise (n = 172) | Diet + exercise (n = 175) |
|---|---|---|---|
| Female, no. (%) | 45 (67.2) | 93 (54.1) | 89 (50.9) |
| AQoL‐8D | 0.68 ± 0.14 | 0.69 ± 0.17 | 0.70 ± 0.16) |
| Employment status, no. (%) | |||
| Work full‐time | 20 (29.9) | 64 (37.2) | 68 (38.9) |
| Work part‐time | 12 (17.9) | 27 (15.7) | 30 (17.1) |
| Unable to work due to health reasons | 0 (0.0) | 3 (1.7) | 5 (2.9) |
| Retired (not due to health reasons) | 35 (52.2) | 74 (43.0) | 69 (39.4) |
| Unemployed/not employed | 0 (0.0) | 4 (2.3) | 3 (1.7) |
| Hours worked in last month if employed | 124 ± 58 | 119 ± 75 | 136 ± 76 |
| Work performance‡ | 8.2 ± 1.1 | 7.8 ± 1.9 | 8.1 ± 1.2 |
| Health care costs last 6 months, $ | |||
| Hospital inpatient | 581 ± 2429 | 830 ± 2,724 | 838.1 ± 2,724 |
| Hospital outpatient | 36 ± 106 | 47 ± 161 | 36 ± 94 |
| Medical | 183 ± 199 | 214 ± 228 | 184 ± 187 |
| Prescription pharmaceuticals | 214 ± 244 | 247 ± 245 | 204 ± 228 |
| Over‐the‐counter pharmaceuticals | 62 ± 77 | 61 ± 87 | 60 ± 88 |
| Investigations | 102 ± 195 | 144 ± 236 | 135 ± 256 |
| Other health professionals | 179 ± 327 | 134 ± 196 | 141 ± 209 |
| Total health care costs last 6 months, $ | 1,356 ± 2,635 | 1,678 ± 3,215 | 1,598 ± 2,979 |
Values are the mean ± SD unless indicated otherwise. AQoL‐8D = Assessment of Quality of Life instrument (range 0–1.0; higher scores indicate better quality of life).
One participant withdrew all their study data.
Participant self‐rated work performance with 0 = your performance equivalent to the worst job performance anyone could have at your job and 10 = best job performance.
Primary analysis
Table 2 shows the mean direct program and health care costs along with quality of life at baseline and follow‐up at 6 and 12 months. When adjusted for baseline and prior knee surgery, those in both programs experienced, on average, a small but statistically significant improvement in the quality of life compared to the control (Table 3). Compared to the control, the larger gain in QALYs was with diet plus exercise 0.05 (95% CI 0.03, 0.07). This program cost $1,746 more to implement than the exercise program, largely because of the cost of the meal replacements. The exercise group had comparatively low direct program costs but high average health care costs during the 12 months, with considerable variation between participants even after adjustment for baseline. The variation in health costs was in large part due to inpatient episodes (22% of the sample were admitted to the hospital in the 12 months), each of which cost over $2,000.
Table 2.
Outcome measures at each time period, by group using multiple imputed data*
| 6 months | 12 months | |||||
|---|---|---|---|---|---|---|
| Outcome | Control (n = 67) | Exercise (n = 172) | Diet + exercise (n = 175) | Control (n = 67) | Exercise (n = 172) | Diet + exercise (n = 175) |
| Quality of life (AQoL‐8D) | 0.71 ± 0.16 | 0.76 ± 0.16 | 0.79 ± 0.16 | 0.72 ± 0.15 | 0.75 ± 0.17 | 0.78 ± 0.16 |
| Total health care costs, $ | 1074 ± 1,460 | 1,906 ± 4,077 | 1,378 ± 2,723 | 2,277 ± 3,385 | 2,710 ± 5,302 | 1,972 ± 4,592 |
| Inpatient cost, $ | 201 ± 1,179 | 1,033 ± 3,674 | 516 ± 2,538 | 1,453 ± 3,187 | 1,764 ± 4,927 | 1,198 ± 4,146 |
| Direct program cost, $ | – | 573 ± 175 | 2,319 ± 507 | – | – | – |
Values are the mean ± SD. AQoL‐8D = Assessment of Quality of Life instrument (range 0–1.0; higher scores indicate better quality of life).
Table 3.
Economic outcomes: additional costs and outcomes over 12 months by group adjusted for baseline and prior knee surgery using multiple imputed data*
| Average predicted effects | Difference in QALYs | Difference in direct program cost | Difference in total program and health care costs |
|---|---|---|---|
| Exercise vs. control | 0.03 (0.01, 0.05) | 572 (543, 597) | 1,754 (144, 3,652) |
| Diet + exercise vs. control | 0.05 (0.03, 0.07) | 2,318 (2,249, 2,387) | 2,196 (696, 3,659) |
| Diet + exercise vs. exercise | 0.02 (0.01, 0.04) | 1,746 (1,662, 1,820) | 441 (–997, 1,761) |
Values are the mean (95% confidence interval). Total costs are generalized linear regressions with gamma distribution and log link, using linear regression for quality‐adjusted life years (QALYs) during trial, with percentile‐based bootstrapped confidence intervals.
Augmenting the exercise program with a dietary weight‐loss component added considerably to the direct cost, but with a small increase in QALYs (0.02), such that the direct cost per QALY was $75,700 (95% CI 44,300, 337,700) compared to exercise alone. When we included all health care costs in 12 months, the adjusted average cost per QALY gained was $67,600 (95% CI –3,000, 418,300) for exercise versus the control, $45,500 (95% CI 14,400, 95,200) for diet plus exercise versus the control, and $21,100 (95% CI –43,700, 162,200) for diet plus exercise versus exercise (Table 4).
Table 4.
Cost‐effectiveness by group over 12 months, incremental cost ($Australian in thousands) per QALY and confidence in cost‐effectiveness at a threshold of $70,000 per QALY*
| Direct program costs only | Health care costs | |||
|---|---|---|---|---|
| Program | Additional cost per QALY | Confidence in cost‐ effectiveness per QALY, %† | Additional cost per QALY | Confidence in cost‐ effectiveness per QALY, %† |
| Exercise vs. control | 14.4 (7.8, 70.6) | 94 | 67.6 (–0.3, 418.3) | 53 |
| Diet + exercise vs. control | 45.6 (31.3, 81.5) | 93 | 45.5 (14.4, 95.2) | 88 |
| Diet + exercise vs. exercise | 75.7 (44.3, 337.7) | 60 | 21.1 (–43.7, 162.2) | 86 |
Values are the mean (95% confidence interval) unless indicated otherwise. The confidence intervals are distribution‐free empirical bias‐corrected bootstrapped values, using the 2.5% upper and lower parts of the distribution from 2,000 resamples of data using 20 multiple imputations nested in bootstrapping. QALY = quality‐adjusted life year.
Confidence is defined as the percentage of bootstrap replicates with positive net benefits (below the threshold willingness‐to‐pay of $70,000).
Figure 1 plots the bootstrap replicates of the incremental total cost per QALY for each group, and Figure 2 shows the acceptability curve (the 1‐sided 1‐minus‐P value against a range of thresholds that we interpreted as our confidence of the program being cost effective (positive net benefits). At a $70,000 threshold, our confidence in net benefits was 53% for exercise compared to the control but augmenting exercise with the diet program resulted in 86% confidence of net benefits compared to exercise only (88% compared to the control).
Figure 1.
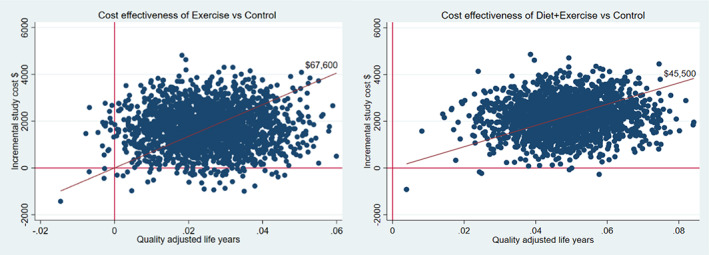
Bootstrap replicates of the mean difference in cost and quality‐adjusted life years (QALYs) for programs versus control, with 2,000 bootstrap samples of adjusted difference in costs and QALYs in the programs versus control using multiple imputed data.
Figure 2.
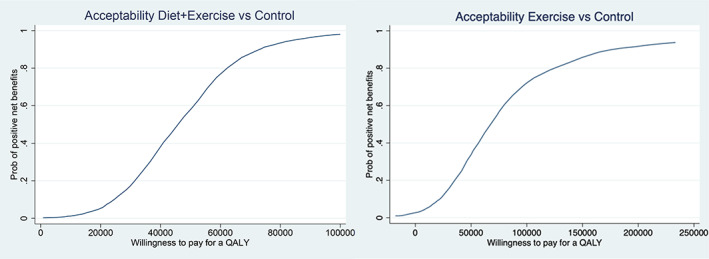
Acceptability curve for programs versus control for net benefits over a range of costs per quality‐adjusted life year (QALY) thresholds. Net monetary benefits for the diet‐plus‐exercise and exercise arms are the difference in QALYs compared to the control group multiplied by the willingness‐to‐pay for a QALY, minus the cost difference between the groups. The acceptability curve shows the probability of the trial sample being consistent with positive net benefits for the diet‐plus‐exercise or the exercise groups as the threshold willingness‐to‐pay for a QALY increases. Prob = probability. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022/abstract.
Secondary analyses
If we consider the direct program costs only, then both the exercise and the diet‐plus‐exercise programs have an additional direct cost per additional QALY that was <$50,000 compared to the control. With an assumed value of a QALY of $70,000, we can be >90% confident of net benefits in both programs.
The previously reported clinical findings showed a significant reduction in weight in the diet‐plus‐exercise group compared to the control (20). We found a 0.37 (95% CI 0.27, 0.47) increase in the adjusted probability of losing at least 10% of body weight in the diet‐plus‐exercise group (0.47) at 12 months compared to the control (0.06). On average there was no difference in weight loss between the exercise group and the control. The incremental cost per person who loses at least 10% of body weight at 12 months is $5,848 (Supplementary Appendix A, section 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022). Only if we were willing to pay at least $10,000 for that reduction in body weight could we be 95% confident that diet plus exercise has net benefits from the weight‐loss component alone.
We found that participants in both programs had a positive and moderate increase in paid work compared to the control, but the effect is imprecisely measured (Supplementary Appendix A, section 4, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022). Those in the exercise group were more likely to be in paid employment, with the result that on average they worked a total of 5.4 (95% CI –9.6, 20.5) additional hours in the previous 4 weeks during the trial compared to the control group. On average, those in the diet‐plus‐exercise group worked 7.4 hours more in the previous 4 weeks compared to the control (95% CI –7.9, 22.7). In terms of productivity, we found approximately a 10% improvement in self‐reported performance at work, with a mean improvement 0.80 unit (95% CI 0.64, 0.97) in the exercise group and 0.90 unit (95% CI 0.69, 1.00) in the diet‐plus‐exercise group compared to the control on a 0–10 scale (where 0 = your performance equivalent to the worst job performance anyone could have at your job and 10 = best job performance) (Supplementary Appendix A, section 3).
Robustness checks
Adjusting for baseline imbalance in age and sex increases the cost per QALY for each program compared to the control and overall improves estimate precision (Supplementary Appendix A, section 5, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022), but neither this adjustment, nor using complete cases (Supplementary Appendix A, section 6), nor hypothetical full adherence to treatment (Supplementary Appendix A, section 8), substantially change the results. Additional estimates of knee surgery costs to 2 years did not improve the cost‐effectiveness of either program (Supplementary Appendix A, section 7). The estimated costs per MCID in pain or function are lower than the cost per QALY (Supplementary Appendix A, section 9). As expected, the costs per person achieving the MCID in pain and function were significantly below those of the cost per QALY for each program compared to the control (Supplementary Appendix A, section 9) confirming the patient relevance of the measured QALY gains.
DISCUSSION
Using plausible social values for a QALY, a telehealth‐delivered program targeting exercise, weight loss, and self‐management for people with knee OA who have overweight/obesity had a high probability of being cost effective compared to online information within 12 months and compared to an exercise‐only program. In contrast to a recent systematic review that found exercise therapy with and without education or diet was cost effective compared to education or physician‐delivered usual care in hip and knee OA (46), we found that the likelihood of a telehealth professional‐delivered exercise program alone being cost effective is low. On the other hand, our study confirms that augmentation of exercise with a weight‐loss program is likely to be cost effective in knee OA, with a likelihood greater than reported in other similar studies (21, 22). If the threshold willingness‐to‐pay for a QALY is as high as $150,000 (41), then both interventions look attractive, but as Figure 2 shows, our confidence that even the diet‐plus‐exercise program was value for money falls below 50% at a threshold willingness‐to‐pay per QALY of $45,000, a threshold above estimates of the opportunity cost of a dollar spent in the UK and Australia (40, 47). The contrasting results may be due to differences in the programs, costs included in the analyses, and the comparators.
An alternate explanation is that the effectiveness of exercise therapy is lower when delivered via telehealth than in‐person, although limited current evidence suggests that outcomes are generally comparable. Our degree of confidence in the cost‐effectiveness of the programs is in part a reflection of the limitation that the trial was powered to detect clinical improvements in symptoms, and so the precision in our estimates reflects the natural variation in self‐reported health care costs between individuals, the potential to misreport less frequent but costly events, and the potential to underreport frequent but less salient health care use in the last 6 months.
The effects of the programs on average hours worked are moderate and imprecisely measured, but if these estimated effects were sustained for 48 weeks, then this effect would translate into a money value of $2,333 (95% CI –4,147, 8,856) for exercise versus control and $3,197 (95% CI –3,413, 9,806) for diet plus exercise versus control (Supplementary Appendix A, section 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022). With both programs, although we cannot be 95% confident of the increase in work hours, the expected value of labor is greater than the program cost (Table 3), resulting in a saving even before we consider additional quality‐of‐life improvements and any productivity gains while at work. Others have found that productivity while at work is the considerably larger component of production losses associated with knee OA (48, 49) and arthritis more broadly, so we may have been conservative in our estimation of production gains. That said, there are a number of potential biases in the data on production gains, as we could not measure actual output changes, relying instead on self‐report of individual paid work and productivity at work with no information on unpaid work, or the effects on others’ productivity.
We acknowledge that this analysis did not have a lifetime horizon, but only assessed costs and outcomes for the first 12 months after randomization. Therefore, our results may understate the value for money of the programs, as gains and cost offsets may exist beyond 12 months from persistent improvements in function, pain, and mental wellbeing. In addition, there is evidence that intentional weight loss of at least 10% would have an impact on those longer‐term chronic health risks known to be strongly associated with high BMI, such as type 2 diabetes mellitus, sleep apnea, hypertension, and dyslipidemia (50). If a 10% loss of weight is valued at >$5,000, we could be 95% confident that the results are consistent with this diet‐plus‐exercise intervention being value for money on this outcome alone (Supplementary Appendix A, section 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022). On the other hand, obesity and physical fitness could influence eligibility for knee surgery, resulting in a greater proportion of program participants being considered “fit for surgery” increasing the cost of knee surgery (Supplementary Appendix A, section 7, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25022). In addition, improvements in the quality of life may not be persistent, which, while not changing the 12‐month cost‐effectiveness results, might make the interventions less acceptable to patients.
The 2 telehealth‐delivered knee OA exercise programs, 1 with and 1 without dietary weight loss, showed improvements in the quality of life at relatively low direct costs compared to information only. When all health care costs are included, the estimated cost‐effectiveness of the programs is imprecise, but at a threshold willingness‐to‐pay for a QALY of $70,000 we can be reasonably confident that augmenting the exercise program with dietary weight loss would be cost effective. That predicted value for money would likely be improved if we considered the productivity effects and the long‐term health gains from weight loss.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Mr. Harris had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Harris, Hinman, Lawford, Egerton, Sumithran, Quicke, Bennel.
Acquisition of data
Lawford, Brown, Metcalf, Spiers.
Analysis and interpretation of data
Harris, Hinman, Keating, Bennel.
Supporting information
Disclosure Form
Supplementary Appendix A Harris AH et al Cost effectiveness of telehealth‐delivered exercise and dietary weight loss programs for knee osteoarthritis within a 12‐month randomised trial
ACKNOWLEDGMENTS
The authors thank Dr. Jessica Kasza for providing the original STATA code used in the multiple imputation analysis of the clinical trial. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
ACTRN: 12618000930280.
Supported by Medibank, the Medibank Better Health Foundation Research Fund, and the National Health and Medical Research Council Centre of Research Excellence (grant 1079078). Dr. Hinman's work was supported by the National Health and Medical Research Council (Senior Research fellowship 1154217). Dr. Bennell's work was supported by the National Health and Medical Research Council (Investigator grant 1174431).
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.25022&file=acr25022‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Australian Institute of Health and Welfare . Osteoarthritis. 2020. URL: https://www.aihw.gov.au/reports/chronic‐musculoskeletal‐conditions/osteoarthritis/contents/what‐is‐osteoarthritis.
- 2. Barbour KE, Helmick CG, Boring M, et al. Vital signs: prevalence of doctor‐diagnosed arthritis and arthritis‐attributable activity limitation–United States, 2013–2015. Morb Mortal Wkly Rep 2017;66:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brennan‐Olsen SL, Cook S, Leech MT, et al. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health Organization study on global AGEing and adult health (SAGE) Wave 1. BMC Musculoskelet Disord 2017;18:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy LB, Cisternas MG, Pasta DJ, et al. Medical expenditures and earnings losses among US adults with arthritis in 2013. Arthritis Care Res (Hoboken) 2018;70:869–76. [DOI] [PubMed] [Google Scholar]
- 5. Losina E, Walensky RP, Reichmann WM, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med 2011;154:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non‐surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–89. [DOI] [PubMed] [Google Scholar]
- 7. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020;72:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis National Health Interview Survey, 2002. Am J Prev Med 2006;30:385–93. [DOI] [PubMed] [Google Scholar]
- 9. Thoma LM, Dunlop D, Song J, et al. Are older adults with symptomatic knee osteoarthritis less active than the general population? Analysis from the Osteoarthritis Initiative and the National Health and Nutrition Examination Survey. Arthritis Care Res (Hoboken) 2018;70:1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbour KE, Helmick CG, Boring M, et al. Obesity trends among US adults with doctor‐diagnosed arthritis 2009–2014. Arthritis Care Res (Hoboken) 2017;69:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henriksen M, Hansen JB, Klokker L, et al. Comparable effects of exercise and analgesics for pain secondary to knee osteoarthritis: a meta‐analysis of trials included in Cochrane systematic reviews. J Comp Eff Res 2016;5:417–31. [DOI] [PubMed] [Google Scholar]
- 12. Kelley GA, Kelley KS, Hootman JM, et al. Effects of community‐deliverable exercise on pain and physical function in adults with arthritis and other rheumatic diseases: a meta‐analysis. Arthritis Care Res (Hoboken) 2011;63:79–93. [DOI] [PubMed] [Google Scholar]
- 13. Penninx BW, Messier SP, Rejeski WJ, et al. Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med 2001;161:2309–16. [DOI] [PubMed] [Google Scholar]
- 14. Hall M, Castelein B, Wittoek R, et al. Diet‐induced weight loss alone or combined with exercise in overweight or obese people with knee osteoarthritis: a systematic review and meta‐analysis. Semin Arthritis Rheum 2019;48:765–77. [DOI] [PubMed] [Google Scholar]
- 15. Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheum 2004;50:1501–10. [DOI] [PubMed] [Google Scholar]
- 16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Brien KM, Hodder RK, Wiggers J, et al. Effectiveness of telephone‐based interventions for managing osteoarthritis and spinal pain: a systematic review and meta‐analysis. PeerJ 2018;6:e5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bennell KL, Nelligan R, Dobson F, et al. Effectiveness of an internet‐delivered exercise and pain‐coping skills training intervention for persons with chronic knee pain: a randomized trial. Ann Intern Med 2017;166:453–62. [DOI] [PubMed] [Google Scholar]
- 19. Hinman RS, Campbell PK, Lawford BJ, et al. Does telephone‐delivered exercise advice and support by physiotherapists improve pain and/or function in people with knee osteoarthritis? Telecare randomised controlled trial. Br J Sports Med 2020;54:790–7. [DOI] [PubMed] [Google Scholar]
- 20. Bennell KL, Lawford BJ, Keating C, et al. Comparing video‐based, telehealth‐delivered exercise and weight loss programs with online education on outcomes of knee osteoarthritis: a randomized trial. Ann Intern Med 2022;175:198–209. [DOI] [PubMed] [Google Scholar]
- 21. Losina E, Smith KC, Paltiel D, et al. Cost‐effectiveness of diet and exercise for overweight and obese patients with knee osteoarthritis. Arthritis Care Res (Hoboken) 2019;71:855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Brien KM, van Dongen JM, Williams A, et al. Economic evaluation of telephone‐based weight loss support for patients with knee osteoarthritis: a randomised controlled trial. BMC Public Health 2018;18:1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bennell K, Keating C, Lawford BJ, et al. Better Knee, Better Me™: effectiveness of two scalable health care interventions supporting self‐management for knee osteoarthritis – protocol for a randomized controlled trial. BMC Musculoskeletal Disord 2020;21:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Royal Australian College of General Practitioners . Guideline for the management of hip and knee osteoarthritis. 2018. URL: https://www.racgp.org.au/download/Documents/Guidelines/Musculoskeletal/guideline‐for‐the‐management‐of‐knee‐and‐hip‐oa‐2nd‐edition.pdf.
- 25. National Institute for Health and Care Excellence . Osteoarthritis: care and management. Clinical guideline CG177. 2014. URL: https://www.nice.org.uk/guidance/cg177. [PubMed]
- 26. The Australian obesity management algorithm. 2016. URL: https://static1.squarespace.com/static/5e3b5875edc1485d14d6fe3a/t/5f333410b37c0216c50936dc/1597191187793/Australian+Obesity+Management+Algorithm+update_22Jun2020.pdf.
- 27. National Health and Medical Research Council . Clinical practice guidelines for the management of overweight and obesity. 2013. URL: https://www.nhmrc.gov.au/about-us/publications/clinical-practice-guidelines-management-overweight-and-obesity.
- 28. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996;74:511–44. [PubMed] [Google Scholar]
- 29. Fisher JD, Fisher WA, Misovich SJ, et al. Changing AIDS risk behavior: effects of an intervention emphasizing AIDS risk reduction information, motivation, and behavioral skills in a college student population. Health Psychol 1996;15:114–23. [DOI] [PubMed] [Google Scholar]
- 30. Richardson J, Iezzi A, Khan MA, et al. Validity and reliability of the Assessment of Quality of Life (AQoL)‐8D multi‐attribute utility instrument. Patient 2014;7:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Australian Government Department of Health and Ageing. Medicare benefits schedule. 2020. URL: https://wwe.mbsonline.gov.au.
- 32. Australian Government Department of Health. Pharmaceutical benefits schedule. 2020. URL: https://www.pbs.gov.au.
- 33.Chemist Warehouse. URL: http://www.chemistwarehouse.com.au/.
- 34. Commonwealth of Australia national hospital cost data collection. Cost report round 24 (2019‐20). 2021. Canberra. URL: https://www.ihacpa.gov.au/sites/default/files/2022-08/NHCDC%20Round%2024%20Report_0_0.pdf.
- 35. Briggs A, Fenn P. Confidence intervals or surfaces? Uncertainty on the cost‐effectiveness plane. Health Econ 1998;7:723–40. [DOI] [PubMed] [Google Scholar]
- 36. O'Brien BJ, Briggs AH. Analysis of uncertainty in health care cost‐effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455–68. [DOI] [PubMed] [Google Scholar]
- 37. Harris AH, Hill SR, Chin G, et al. The role of value for money in public insurance coverage decisions for drugs in Australia: a retrospective analysis 1994–2004. Med Decis Making 2008;28:713–22. [DOI] [PubMed] [Google Scholar]
- 38. Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness‐to‐pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 2010;19:422–37. [DOI] [PubMed] [Google Scholar]
- 39. Huang L, Frijters P, Dalziel K, et al. Life satisfaction, QALYs, and the monetary value of health. Soc Sci Med 2018;211:131–6. [DOI] [PubMed] [Google Scholar]
- 40. Edney LC, Haji Ali Afzali H, Cheng TC, et al. Estimating the reference incremental cost‐effectiveness ratio for the Australian health system. Pharmacoeconomics 2018;36:239–52. [DOI] [PubMed] [Google Scholar]
- 41. Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost‐effectiveness analysis in the United States. Ann Intern Med 2021;174:25–32. [DOI] [PubMed] [Google Scholar]
- 42. Fenwick E, O'Brien BJ, Briggs A. Cost‐effectiveness acceptability curves: facts, fallacies and frequently asked questions. Health Econ 2004;13:405–15. [DOI] [PubMed] [Google Scholar]
- 43. Christensen R, Bartels EM, Astrup A, et al. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta‐analysis. Ann Rheum Dis 2007;66:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kessler RC, Barber C, Beck A, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occup Environ Med 2003;45:156–74. [DOI] [PubMed] [Google Scholar]
- 45. Maxwell A, Ozmen M, Iezzi A, et al. Deriving population norms for the AQoL‐6D and AQoL‐8D multi‐attribute utility instruments from web‐based data. Qual Life Res 2016;25:3209–19. [DOI] [PubMed] [Google Scholar]
- 46. Mazzei DR, Ademola A, Abbott JH, et al. Are education, exercise and diet interventions a cost‐effective treatment to manage hip and knee osteoarthritis? A systematic review. Osteoarthritis Cartilage 2021;29:456–70. [DOI] [PubMed] [Google Scholar]
- 47. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost‐effectiveness threshold. Health Technol Assess 2015;19:1–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hermans J, Koopmanschap MA, Bierma‐Zeinstra SM, et al. Productivity costs and medical costs among working patients with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:853–61. [DOI] [PubMed] [Google Scholar]
- 49. Hermans J, Reijman M, Goossens LM, et al. Cost‐utility analysis of high molecular weight hyaluronic acid for knee osteoarthritis in everyday clinical care in patients at a working age: an economic evaluation of a randomized clinical trial. Arthritis Care Res (Hoboken) 2018;70:89–97. [DOI] [PubMed] [Google Scholar]
- 50. Haase CL, Lopes S, Olsen AH, et al. Weight loss and risk reduction of obesity‐related outcomes in 0.5 million people: evidence from a UK primary care database. Int J Obes (Lond) 2021;45:1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Appendix A Harris AH et al Cost effectiveness of telehealth‐delivered exercise and dietary weight loss programs for knee osteoarthritis within a 12‐month randomised trial


