Summary
Although leaves are considered the main site for photosynthesis, other green nonfoliar tissues can carry out considerable amounts of photosynthetic carbon assimilation. With photosynthesis, a potential target for improving crop productivity, physiology and contribution of nonfoliar tissues to overall plant carbon acquisition is gaining increasing attention. This review will provide an overview of nonfoliar photosynthesis, the role of stomata in these tissues and methodologies for quantification and the contribution to overall carbon gain.
Keywords: net CO2 assimilation rate (A), nonfoliar photosynthesis, stomatal conductance (g s), stomatal density, wheat ears
| Contents | ||
|---|---|---|
| Summary | 55 | |
| I. | Introduction | 55 |
| II. | Methods for assessing nonfoliar photosynthesis | 56 |
| III. | Role of stomata in nonfoliar tissue | 58 |
| IV. | Conclusion | 60 |
| Acknowledgements | 60 | |
| References | 60 | |
I. Introduction
Photosynthesis has been studied in numerous species and environments, and the key components and pathways are well established. However, to date, the majority of these studies have focused on leaves, and few have considered the contribution from other tissues (Simkin et al., 2020). Green tissues other than leaves, including stems (Simkin et al., 2020), ears/panicles (Maydup et al., 2014; Rivera‐Amado et al., 2020; Zhang et al., 2022), green floral organs (Bertolino et al., 2022), pods (Wang et al., 2016) and fruit (Simkin et al., 2020), have been reported to photosynthesize and contribute to varying degrees and to overall plant carbon gain. Wheat and rice panicles, which a large proportion of studies have focused on, have been reported to have high photosynthetic capacities (Brazel & Ó'Maoiléidigh, 2019; Chang et al., 2020; Sanchez‐Bragado et al., 2020), with panicle and ear photosynthesis reported to contribute between 10% and 60% to yield (Hu et al., 2019) and spike photosynthesis positively correlated with yield (Molero & Reynolds, 2020). Rates of CO2 assimilation in nonfoliar organs depend on the material, conditions and methodologies used. A recent review by Araus et al. (2021) collated data on a number of C3 crop species (including rice, barley and wheat) and reported values between 0.6 and 30.3 μmol m−2 s−1 depending on the tissue and conditions, representing between 10% and 600% of flag leaf rates. Although typically rates of ear photosynthesis per unit area are often lower than in the flag leaf, these organs have a relatively large surface area, and when the total area is taken into account, these rates can be considerably higher (Araus et al., 2021). Considerable differences in mass between leaves or sources vs ears/pods and sink tissue also exist, for example the dry weight of a pea pod can be c. 10× greater than leaves.
In the last decade, manipulation of key processes to increase photosynthesis in leaves has been a prime objective to improve crop yields. Extending this to photosynthesis in ears could provide a novel target, and this potential has been demonstrated by Simkin et al. (2020) in wheat plants overexpressing the Calvin cycle enzyme SBPase, who reported ear photosynthesis, as well as leaf photosynthesis, was increased (Driever et al., 2017). Furthermore, natural variation in leaf photosynthetic capacity is known to exist between species (Weyers & Lawson, 1997) and cultivars (Faralli et al., 2019a,b) and similar variation has also been reported for ears and panicles of C3 crops (Molero & Reynolds, 2020; Tambussi et al., 2021) providing exciting opportunities to exploit such natural variation for on‐going breeding programs.
The importance and contribution of ear or spike photosynthesis (and other potential nonfoliar green tissue) to yield is considered even more important when foliar tissues (and particularly the flag leaf, which is considered the major photosynthetic contributor to grain filling) are damaged or stressed (Ntakirutimana & Xie, 2020). For example, Zhang et al. (2011) reported that nonfoliar organs in wheat accounted for 27–62% of the total green area per culm and this ratio increased significantly with reduced water availability. Under water‐stressed conditions, wheat flag leaf photosynthetic capacity and rate decrease along with key photosynthetic enzymes; however, the same is not true for ears or awns (Vicente et al., 2018), with ear photosynthesis being maintained (Tambussi et al., 2005) and critical to grain filling and yield maintenance under such environmental conditions (Hu et al., 2019). A possible explanation for the differential impacts of water stress on ears compared with leaves is that water relations in these organs are considerably different to foliar tissue and stomata could play a key role in this regulation (to be described later).
Wheat ears are made up of a number of photosynthetic components, and significant variation in assimilation rates exists between these different structures. Fig. 1 shows chlorophyll fluorescence (CF) measurements of photosynthetic processes and demonstrates differences in efficiency between awns and the other wheat ear components. The glumes appear to have the highest photosynthetic efficiency (Fig. 1a) compared with the awns (Fig. 1b), although both are considered important in terms of photosynthetic contribution (see Hu et al., 2019). Spatial differences in photosynthetic efficiency are due mostly to photochemical quenching (Fig. 1c,d) rather than differences in / which decreases with nonphotochemical quenching (Fig. 1e,f), suggesting that carbon fixation is an important sink for the end products of electron transport. Photosynthetic contribution from awns is thought to be particularly important in stress conditions, and therefore, awned varieties may be advantageous in environments that experience periodic stresses (Ntakirutimana & Xie, 2020). It is also well established that genotypic variation in ear water‐stress tolerance exists (Li et al., 2017), which could represent another currently unexploited target for breeding programs to develop wheat ideotypes for future climatic conditions.
Fig. 1.
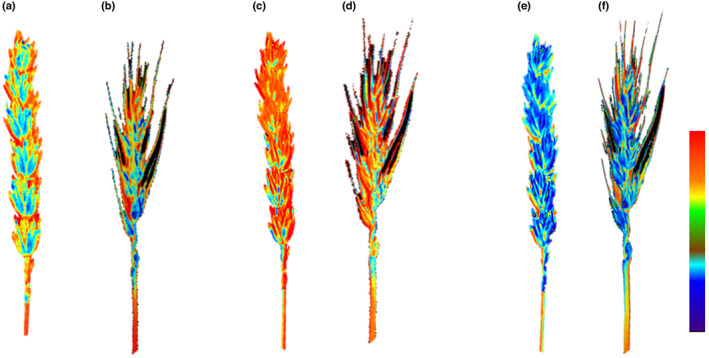
Chlorophyll fluorescence images of awned and nonawned wheat ears of (a, b) photosystem II (PSII) operating efficiency (/); (c, d) / (PSII photochemical quenching factor); and (e, f) maximum PSII operating efficiency (/). Colour scale bar represents 0.4–0.6 for / and 0.5–0.85 for / and /.
II. Methods for assessing nonfoliar photosynthesis
One of the major restrictions in quantifying the contribution of nonfoliar photosynthesis to overall carbon assimilation and yield is methodological limitation. This is further complicated by different photosynthetic pathways operating in these tissues. Here, we outline some of the complications and options for measuring photosynthetic carbon assimilation in organs and provide some insights into possible new approaches.
1. Complexities of measurements
Typically, leaf measurements of photosynthesis (A) and transpiration are made using infra‐red gas analysers, which enclose a flat leaf or proportion of a leaf into a cuvette and use differential measures of gases entering and leaving the chamber. The development of bespoke chambers that enable gas exchange measurements in complex organs (wheat ears and rice panicles) is providing new information on photosynthetic capacity, stomatal kinetics and WUE (Maydup et al., 2010; Chang et al., 2020; Henry et al., 2020). An example of these types of measurements is illustrated in Fig. 2, which shows dynamic responses of A and g s in pea pods following a step change in light intensity (Fig. 2a,b). This figure also illustrates that even with 100 μmol m−2 s−1 PPFD, pod photosynthesis is negative and therefore well below the light compensation point, indicative of high rates of respiration. Infra‐red gas analyser measurements of nonfoliar material are not without complications. First, leaves are usually a flat lamina surface and easily sealed into a chamber, whilst fruits, pods and stems vary greatly in size, shape and dimension. A second problem is that photosynthesis is measured on a projected leaf area basis, and the 3D structure of ears and fruits can make quantification of the area challenging. Furthermore, the complexity in shape of the material can result in uneven illumination (Hu et al., 2019; Chang et al., 2020), and temperatures, further complicating measurements. 3D scanning can be used to overcome the problem of establishing leaf area (Simkin et al., 2020), whilst surrounding chamber LED illumination reduces shading. Gas exchange measurements also require knowledge of boundary lay conductance, which is difficult to determine for nonlamina material, but can be measured using the filter paper methods (Parkinson, 1985) and mimicking the shape of the organ.
Fig. 2.
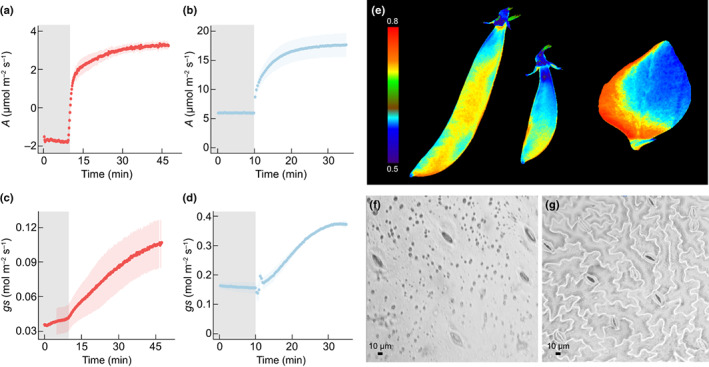
Example of photosynthetic activity in Cameor pea pods and leaves. Mean (a) pod and (b) leaf assimilation (A), (c) pod and (d) leaf stomatal conductance (g s) was measured in response to a step in light intensity from 100 to 1000 μmol m−2 s−1 photosynthetic photon flux density (PPFD), at 400 ppm CO2 and 23°C within a bespoke pod chamber. Grey shaded areas represent when the light source is at 100 μmol m−2 s−1 PPFD. Error bars represent mean ± SE (n = 3). All measurements are normalized to an illuminated projected area (typically used for leaves). (e) Chlorophyll fluorescence image of photosystem II operating efficiency (/) was used to demonstrate differences in efficiency between the two tissue types. Colour scale bar represents an / of 0.5–0.8. Example of a (f) pod and (g) leaf epidermal impression at a 200× magnification.
A considerably more difficult problem to overcome when measuring nonleaf photosynthesis is the controversy around the mechanisms of photosynthesis. Although the majority of studies have assumed a similar pathway to C3 leaf mesophyll photosynthesis, this may not always be the case. In nonfoliar tissue, it has been proposed that there are two major sources of CO2 for photosynthesis. Atmospheric CO2 can diffuse from the atmosphere into the cells through stomatal pores and fixed by Rubisco, as per C3 foliar photosynthesis. However, a second supply of CO2 for fixation is also released from high mitochondrial respiration and subsequently refixed (Millar et al., 2011), which would underestimate photosynthetic rates measured by gas exchange. The extent to which each of these pathways contributes to photosynthesis is under debate and most likely species‐specific. Refixation of respiratory CO2 may be particularly important in internal tissues where atmospheric C‐fixation is restricted (i.e. the seeds), due to longer CO2 diffusion pathways and reduced light penetration (Henry et al., 2020; Simkin et al., 2020). Higher rates of respiration in panicles compared with leaves (Chang et al., 2020) suggest a substantial contribution of respiratory CO2 to photosynthesis (Sanchez‐Bragado et al., 2020; Zhang et al., 2022) in these tissues, with reports of between 55% and 75% of respired CO2 refixed in wheat and barley (Bort et al., 1996).
2. Indirect methods for assessing nonfoliar photosynthesis
There are several indirect methods for assessing the contribution of ear and stem photosynthesis, including organ removal and shading, where the ear or stems are covered with foil to reduce light penetration (Maydup et al., 2010). However, shading alters temperature and gaseous flow, whilst removing organs can induce stress responses, both of which impact on photosynthetic rates (Tambussi et al., 2021). Quantification of O2 accumulation as an indication of electron transport has been employed to assess different ear components; however, this requires destructive sampling rather than in situ measurements (review by Tambussi et al. (2021)). Chlorophyll fluorescence imaging is another tool that can be utilized to measure photosynthetic efficiency (and calculate electron transport rate) in both leaf and nonleaf material (Tambussi et al., 2005; Simkin et al., 2020; Figs 1, 2). Although this is a rapid and relatively simple approach, electron transport is only a proxy for carbon assimilation and alternative electron sinks including photorespiration can influence photosynthetic efficiency (Murchie & Lawson, 2014). It may therefore be advantageous to combine CF measurements with gas exchange to determine the proportion of electrons used to fix atmospheric CO2 from those going to alternative sources. This can be easily achieved by performing measurements under 2% O2 to eliminate photorespiration (McAusland et al., 2013, 2015, 2019), although the amount of CO2 produced through respiratory processes may complicate this. Using membrane inlet mass spectrometry could provide an advanced approach to quantifying different processes. Utilises naturally occurring stable isotopes of O2 and CO2 and using enrichment approaches would enable discrimination from respiration from other O2 consumption, as well as O2 evolution from photosynthetic electron transport under different conditions (Driever & Baker, 2011). Thermal imaging can provide insights into spatial and temporal stomatal behaviour, that may be valuable to elucidate and quantify atmospheric CO2 fixation from other CO2 sources. Combined thermography with other imaging approaches such as CF (McAusland et al., 2013, 2015) could deliver further insights into water‐use efficiency in different tissues (McAusland et al., 2013). For a recent review on different methods for measuring ear photosynthesis, we refer readers to Tambussi et al. (2021).
III. Role of stomata in nonfoliar tissue
In leaves, photosynthesis requires atmospheric CO2 to enter the leaves through the stomatal pores and subsequently stomatal density (SD) and behaviour influence assimilation rate and stomatal conductance, which is closely correlated with rates of carbon fixation. For some nonleaf tissues, such as tomato fruit, the lack of stomata highlights the sole reliance upon carbon refixation (Simkin et al., 2020). Stomata are found in various numbers on nonfoliar tissues, such as rice panicles (Li et al., 2021; Rangan et al., 2022), wheat stems and ear components (Fig. 3; Hu et al., 2019; Henry et al., 2020; Simkin et al., 2020), and certain fruits (Brazel & Ó'Maoiléidigh, 2019); however, their functionality, including their contribution to carbon acquisition, has not been fully evaluated (Simkin et al., 2020). Recent work by Bertolino et al. (2022) has reported considerable spatial variation in stomata in rice floral organs and suggested that they are morphologically distinct from leaf stomata, although their function is still unknown.
Fig. 3.
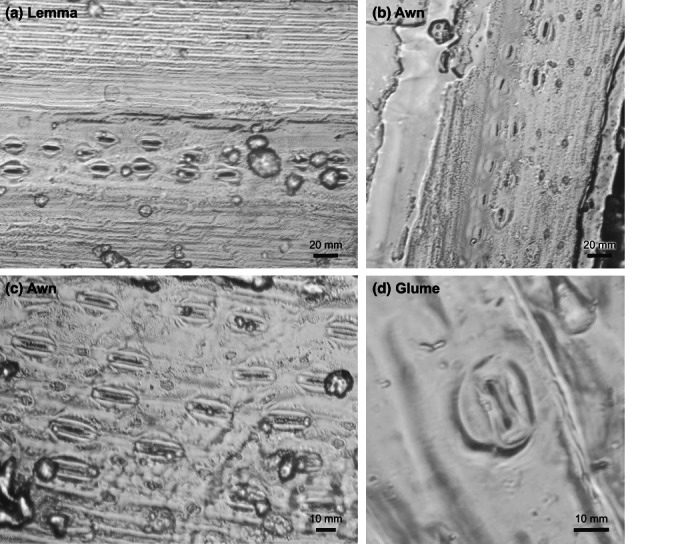
Example of epidermal impressions showing stomata from a barley (a) lemma and (b) awn and a wheat (c) awn and (d) glume.
Using thermography Simkin et al. (2020) showed that stomata of wheat ears were functional and responded to changes in light intensity; similar to leaves, albeit g s levels were lower. The existing variation in SD on different tissue types may allude to functional differences. For example, in Fig. 4, we show that wheat ears with a lower SD have higher ear temperatures than those with greater stomatal numbers (Fig. 4). Fruits have been reported to have 1–10% of the density found in leaves, with apples even lower numbers at 30 times less than leaves (Blanke & Lenz, 1989). However, SD in wheat ear organs is only 50–60% lower than that in the leaf (Tambussi et al., 2005), and some studies have even reported higher SD in ears than leaves. Interestingly, stomata have been found on both the adaxial and abaxial sides of glumes, lemma (Simkin et al., 2020) and bracts (Ding et al., 2018). Ding et al. (2018) proposed that adaxial bract stomata facilitate CO2 uptake from the respiring grain. Such amphistomaty could indicate stomata function to support both atmospheric CO2 uptake from one side of the tissues and refixation of respiratory CO2 uptake on the other. Such functionality could be key to maintaining ear photosynthesis under stressful conditions that cannot be achieved by the flag leaf. This hypothesis is supported by a recent study by Zhang et al. (2022) who explored control of photosynthesis in rice panicles at different stages of crop development and reported that at anthesis, panicle photosynthesis was dependent on both g s and biochemical function, and there was a positive correlation between g s and A. However, at grain filling, g s declined and net photosynthesis was correlated with g s rather than the carboxylation capacity of Rubisco (Vc max), indicating that A was primarily determined by stomatal behaviour. Respiration in panicles has been reported to increase in the initial stages of grain filling along with a decrease in g s, supporting the idea that respiratory CO2 is important for panicle photosynthesis at this stage (Chang et al., 2020). This suggests that there is a possible switch between atmospheric CO2 being the main supply for photosynthesis earlier in the season supported by higher g s, which switches to respiratory supply later in development, concurrent with a decrease in g s. At the same time, biochemical changes support refixation of respired CO2 (Zhang et al., 2022). However, changes in panicle g s could also be due to shifts in the osmotic and water status of the panicle. Xylem water potential would be decreased with phloem unloading in the panicles at grain filling and could explain a decrease in g s.
Fig. 4.
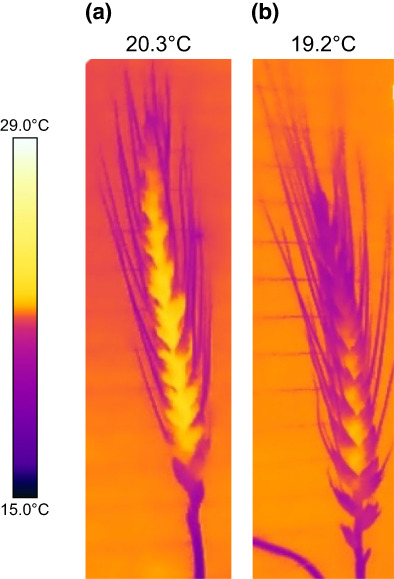
Thermal images of wheat ears which demonstrate functional difference due to variation in stomatal density (SD): (a) SD of 12 mm−2 and (b) 35 mm−2. Measurements were made following 1 h exposure to 27°C. Colour scale bar represents a difference in temperature from 15°C to 29°C.
Although, to date, the majority of research on stomata in nonfoliar photosynthesis has focused on their role in CO2 uptake, stomata are also important for transpiration and the movement of water through the plants, including the translocation of photoassimilates (Simkin et al., 2020). Stomatal control of evaporative cooling in all plant tissues is critical for maintaining temperature for photosynthesis and reproductive capacity. It is well established that heat stress greatly impacts wheat yield, with flower and reproductive growth phases being particularly sensitive (Yadav et al., 2022). Heat stress, several days before anthesis during floral development, greatly affects ovule and pollen formation with significant impacts on grain development and yield. It has been proposed that ear temperature during the early stages of anthesis is an important component of heat stress tolerance (Steinmeyer et al., 2013), and therefore, stomatal behaviour possibly could be critical for evaporative cooling and maintenance of temperature at critical stages. Transpirational water loss through the stomata may also play a key role in the translocation of photoassimilates from sources to the ear sinks during grain filling, and the changes in osmotic/water potential during this process could be responsible for changes in g s at different developmental stages. Furthermore, the amount of water lost through nonfoliar organs, although difficult to establish from current literature, needs to be quantified in order to fully appreciate overall crop water use. Further research is necessary to fully establish the role of stomata in these tissues, along with hydraulic capacity, and overall water loss and carbon gain in these organs.
Natural variation in SD between cultivars in leaves (Weyers & Lawson, 1997), ears (Li et al., 2017) and individual ear components (Simkin et al., 2020) provides an opportunity to explore the role of stomata further. Li et al. (2017) demonstrated that wheat cultivars with lower ear SD had increased WUE and drought tolerance. Therefore, manipulating SD (e.g. Bertolino et al., 2022) or function (Lawson & Matthews, 2020) in wheat ears alone could improve heat tolerance and support greater photosynthesis through increased evaporative cooling, influence translocation of photoassimilates and nutrients to the grains and provide a route to improve nonfoliar water‐use efficiency.
IV. Conclusion
Although the contribution of nonfoliar tissues to carbon assimilation and yield has not been fully quantified and depends on tissue, cultivars and environmental conditions, it represents an exciting and (to date) mostly unexploited area for improved crop productivity. Furthermore, understanding and manipulating stomatal behaviour in these organs could provide a unique opportunity to produce crop cultivars with greater stress tolerances, through increased cooling capacity, greater translocation, higher photosynthesis and the ability to yield in environments that might otherwise be subject to significant losses; however, these could come at the expense of water‐use efficiency.
Competing interests
None declared.
Acknowledgements
We acknowledge Mengjie Fan for assistance with image processing and thermography. Piotr Kasznicki and Phil Davey are thanked for the technical support. AM was supported by the Perry Foundation and the University of Essex. TL also acknowledges funding support from BBSRC (BB/T004274/1 and BB/N016831/1).
References
- Araus JL, Sanchez‐Bragado R, Vicente R. 2021. Improving crop yield and resilience through optimization of photosynthesis: panacea or pipe dream? Journal of Experimental Botany 72: 3936–3955. [DOI] [PubMed] [Google Scholar]
- Bertolino LT, Caine RS, Zoulias N, Yin X, Chater CCC, Biswal A, Quick WP, Gray JE. 2022. Stomatal development and gene expression in rice florets. Plant and Cell Physiology 63: 1679–1694. [DOI] [PubMed] [Google Scholar]
- Blanke MM, Lenz F. 1989. Fruit photosynthesis. Plant, Cell & Environment 12: 31–46. [Google Scholar]
- Bort J, Brown RH, Araus JL. 1996. Refixation of respiratory CO2 in the ears of C3 cereals. Journal of Experimental Botany 47: 1567–1575. [Google Scholar]
- Brazel AJ, Ó'Maoiléidigh DS. 2019. Photosynthetic activity of reproductive organs. Journal of Experimental Botany 70: 1737–1754. [DOI] [PubMed] [Google Scholar]
- Chang TG, Song QF, Zhao HL, Chang S, Xin C, Qu M, Zhu XG. 2020. An in situ approach to characterizing photosynthetic gas exchange of rice panicle. Plant Methods 16: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Zhao Y, Guo H, Li X, Schoenau J, Si B. 2018. Water footprint for pulse, cereal, and oilseed crops in Saskatchewan, Canada. Water 10: 1–24.30079254 [Google Scholar]
- Driever SM, Baker NR. 2011. The water–water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant, Cell & Environment 34: 837–846. [DOI] [PubMed] [Google Scholar]
- Driever SM, Simkin AJ, Alotaibi S, Fisk SJ, Madgwick PJ, Sparks CA, Jones HD, Lawson T, Parry MAJ, Raines CA. 2017. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 372: 1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli M, Cockram J, Ober E, Wall S, Galle A, Van Rie J, Raines C, Lawson T. 2019a. Genotypic, developmental and environmental effects on the rapidity of g s in Wheat: impacts on carbon gain and water‐use efficiency. Frontiers in Plant Science 10: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli M, Matthews J, Lawson T. 2019b. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Current Opinions Plant Biology 49: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RJ, Furtado A, Rangan P. 2020. Pathways of photosynthesis in non‐leaf tissues. Biology 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y‐Y, Zhang Y‐L, Xia H, Fan S, Song J, Lv X, Kong L. 2019. Photosynthetic characteristics of non‐foliar organs in main C3 cereals. Physiology Plantarum 166: 226–239. [DOI] [PubMed] [Google Scholar]
- Lawson T, Matthews JAS. 2020. Guard cell metabolism and stomatal function. Annual Review of Plant Biology 71: 237–302. [DOI] [PubMed] [Google Scholar]
- Li Y, Li H, Li Y, Zhang S. 2017. Improving water‐use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought‐resistant wheat. The Crop Journal 5: 231–239. [Google Scholar]
- Li Y, Zhou Q, He M, Xu H, Li G, Ding Y, Paul M, Liu Z. 2021. Dissection of environmental and physiological effects on the temperature difference between superior and inferior spikelets within a rice panicle. The Crop Journal 9: 1098–1107. [Google Scholar]
- Maydup ML, Antonietta M, Graciano C, Guiamet JJ, Tambussi EA. 2014. The contribution of the awns of bread wheat (Triticum aestivum L.) to grain filling: responses to water deficit and the effects of awns on ear temperature and hydraulic conductance. Field Crops Research 167: 102–111. [Google Scholar]
- Maydup ML, Antonietta M, Guiamet JJ, Graciano C, Lopez JR, Tambussi EA. 2010. The contribution of ear photosynthesis to grain filling in bread wheat. Field Crops Research 119: 48–58. [Google Scholar]
- McAusland L, Atkinson JA, Lawson T, Murchie EH. 2019. High throughput procedure utilising chlorophyll fluorescence imaging to phenotype dynamic photosynthesis and photoprotection in leaves under controlled gaseous conditions. Plant Methods 15: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Davey PA, Kanwal N, Baker NR, Lawson T. 2013. A novel system for spatial and temporal imaging of intrinsic plant water use efficiency. Journal of Experimental Botany 64: 4993–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet‐Chabrand SRM, Matthews JSA, Lawson T. 2015. Spatial and temporal responses in stomatal behaviour, photosynthesis and implications for water use efficiency. In: Mancuso S, Shabala S, eds. Rhythm in plants: dynamic responses in a dynamic environment. Heidelberg, Germany: Springer, 97–119. [Google Scholar]
- Millar AH, Whelan J, Soole KL, Day DA. 2011. Organization and regulation of mitochondrial respiration in plants. Annual Review of Plant Biology 62: 79–104. [DOI] [PubMed] [Google Scholar]
- Molero G, Reynolds MP. 2020. Spike photosynthesis measured at high throughput indicates genetic variation independent of flag leaf photosynthesis. Field Crops Research 255: 107866. [Google Scholar]
- Murchie EH, Lawson T. 2014. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany 64: 3983–3998. [DOI] [PubMed] [Google Scholar]
- Ntakirutimana F, Xie W. 2020. Unveiling the actual functions of awns in grasses: from yield potential to quality traits. International Journal of Molecular Sciences 21: 7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson KJ. 1985. A simple method for determining the boundary layer resistance in leaf cuvettes. Plant, Cell & Environment 8: 223–226. [Google Scholar]
- Rangan P, Wankhede DP, Subramani R, Chinnusamy V, Malik SK, Baig MJ, Singh K, Henry R. 2022. Evolution of an intermediate C4 photosynthesis in the non‐foliar tissues of the Poaceae. Photosynthesis Research 153: 1–10. [DOI] [PubMed] [Google Scholar]
- Rivera‐Amado C, Molero G, Trujillo‐Negrellos E, Reynolds M, Foulkes J. 2020. Estimating organ contribution to grain filling and potential for source upregulation in wheat cultivars with a contrasting source–sink balance. Agronomy 10: 1527. [Google Scholar]
- Sanchez‐Bragado R, Vicente R, Molero G, Serret MD, Maydup ML, Araus JL. 2020. New avenues for increasing yield and stability in C3 cereals: exploring ear photosynthesis. Current Opinion in Plant Biology 56: 223–234. [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Faralli M, Ramamoorthy S, Lawson T. 2020. Photosynthesis in non‐foliar tissues: implications for yield. The Plant Journal 101: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer FT, Lukac M, Reynolds MP, Jones HE. 2013. Quantifying the relationship between temperature regulation in the ear and floret development stage in wheat (Triticum aestivum L.) under heat and drought stress. Functional Plant Biology 40: 700–707. [DOI] [PubMed] [Google Scholar]
- Tambussi EA, Maydup ML, C A C, Guiamet JJ, Araus JL. 2021. Ear photosynthesis in C3 cereals and its contribution to grain yield: methodologies, controversies and perspectives. Journal of Experimental Botany 72: 3956–3970. [DOI] [PubMed] [Google Scholar]
- Tambussi EA, Nogués S, Araus JL. 2005. Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta 221: 446–458. [DOI] [PubMed] [Google Scholar]
- Vicente R, Vergara‐Díaz O, Medina S, Chairi F, Kefauver SC, Bort J, Serret MD, Aparicio N, Araus JL. 2018. Durum wheat ears perform better than the flag leaves under water stress: gene expression and physiological evidence. Environmental and Experimental Botany 153: 271–285. [Google Scholar]
- Wang H, Hou L, Wang M, Mao P. 2016. Contribution of the pod wall to seed grain filling in alfalfa. Scientific Reports 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers JDB, Lawson T. 1997. Heterogeneity in stomatal characteristics. Advances in Botanical Research 26: 317–352. [Google Scholar]
- Yadav MR, Choudhary M, Singh J, Lal MK, Jha PK, Udawat P, Gupta NK, Rajput VD, Garg NK, Maheshwari C et al. 2022. Impacts, tolerance, adaptation, and mitigation of heat stress on wheat under changing climates. International Journal of Molecular Sciences 23: 2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tang W, Peng S, Li Y. 2022. Limiting factors for panicle photosynthesis at the anthesis and grain filling stages in rice (Oryza sativa L.). The Plant Journal 109: 77–91. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Wang Z, Wang Z. 2011. Characteristics of canopy structure and contributions of non‐leaf organs to yield in winter wheat under different irrigated conditions. Field Crops Research 123: 187–195. [Google Scholar]


