Abstract
Background
Atrial fibrillation (AF) known before ischemic stroke (KAF) has been postulated to be an independent category with a recurrence risk higher than that of AF detected after stroke (AFDAS). However, it is unknown whether this risk difference is confounded by pre‐existing anticoagulation, which is most common in KAF and also indicates a high ischemic stroke recurrence risk.
Methods
Individual patient data analysis from 5 prospective cohorts of anticoagulated patients following AF‐associated ischemic stroke. We compared the primary (ischemic stroke recurrence) and secondary outcome (all‐cause death) among patients with AFDAS versus KAF and among anticoagulation‐naïve versus previously anticoagulated patients using multivariable Cox, Fine‐Gray models, and goodness‐of‐fit statistics to investigate the relative independent prognostic importance of AF‐category and pre‐existing anticoagulation.
Results
Of 4,357 patients, 1,889 (43%) had AFDAS and 2,468 (57%) had KAF, while 3,105 (71%) were anticoagulation‐naïve before stroke and 1,252 (29%) were previously anticoagulated. During 6,071 patient‐years of follow‐up, we observed 244 recurrent strokes and 661 deaths. Only pre‐existing anticoagulation (but not KAF) was independently associated with a higher hazard for stroke recurrence in both Cox and Fine‐Gray models. Models incorporating pre‐existing anticoagulation showed better fit than those with AF category; adding AF‐category did not result in better model fit. Neither pre‐existing anticoagulation nor KAF were independently associated with death.
Conclusion
Our findings challenge the notion that KAF and AFDAS are clinically relevant and distinct prognostic entities. Instead of attributing an independently high stroke recurrence risk to KAF, future research should focus on the causes of stroke despite anticoagulation to develop improved preventive treatments. ANN NEUROL 2023;94:43–54
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a major risk factor for ischemic stroke. 1 Among patients with AF‐associated ischemic stroke, emerging evidence suggests that AF known before stroke (KAF) has a higher risk of stroke recurrence than AF detected after stroke (AFDAS). 2 This observation led to the hypothesis that AFDAS, which is postulated to partly occur due to neurogenic, stroke‐induced autonomic cardiac alterations, might constitute a distinct clinical entity from KAF, which is more strongly associated with cardiac comorbidities and appears more likely to be of cardiogenic origin. 3 , 4 , 5 , 6 , 7
Among patients with ischemic stroke associated with AF, there is also increasing evidence that the risk of stroke recurrence is higher in those with pre‐existing oral anticoagulation than those who were naïve to anticoagulation before stroke. 8 , 9 , 10 Reasons for the higher recurrence risk in patients with stroke despite pre‐existing anticoagulation could include poor adherence to treatment, reduced preventive effectiveness of anticoagulation due to competing stroke etiologies other than AF, or other mechanisms 11 ; this could confound the apparent relationship of KAF with increased ischemic stroke recurrence risk. While pre‐existing oral anticoagulation is more prevalent among patients with KAF than with AFDAS, 12 , 13 the large studies that investigated the outcomes of KAF versus AFDAS did not account for pre‐existing oral anticoagulation. Hence, it remains unclear whether the reported prognostic difference between KAF and AFDAS is independent from the prior use (versus non‐use) of anticoagulation treatment.
Here, we aimed to investigate (i) the outcomes of KAF versus AFDAS among patients treated with oral anticoagulants after a recent AF‐associated ischemic stroke, accounting for pre‐existing oral anticoagulation, and (ii) the relative prognostic importance of KAF compared to pre‐existing oral anticoagulation.
Methods
Study Design and Patients
We used prospectively collected, individual patient data pooled within an established international collaboration of investigator‐initiated cohort studies of patients with AF and treatment with oral anticoagulants following a recent ischemic stroke or transient ischemic attack (TIA), as described previously. 8 , 14 , 15 , 16 , 17 , 18 This included 3 single‐center (Basel, Switzerland [NOACISP‐LONGTERM; NCT03826927]; 19 , 20 , 21 , 22 Erlangen, Germany; 23 , 24 Verona, Italy 25 ) and 2 multicenter cohorts (CROMIS‐2 [NCT02513316]; 26 , 27 SAMURAI‐NVAF [NCT01581502] 28 , 29 ).
In this study, we included consecutive patients with (i) a recent (ie, <3 months) index ischemic stroke or TIA; (ii) nonvalvular AF (either AFDAS or KAF); (iii) treatment with oral anticoagulants (direct oral anticoagulants [DOAC] or vitamin K antagonists [VKA]), initiated or resumed within 3 months after the index event; and (iv) prospectively ascertained follow‐up data for at least 3 months after the index event for the outcomes recurrent ischemic stroke and all‐cause death, defined as in prior research. 8 , 14 , 15 We excluded patients with missing data on AF being AFDAS or KAF, patients with missing information on oral anticoagulation before index stroke, patients with valvular AF or mechanical heart valves, those with anticoagulant initiation/resumption >3 months after the index event or unknown, those with outcome events occurring before anticoagulant initiation/resumption, and those without follow‐up information.
Data were collected as described in prior research 14 using standardized forms with predefined variables and pooled in the coordinating center in Basel, Switzerland, where the analysis was performed. The 2 main baseline variables of interest were: (i) AF category (AFDAS vs KAF). As described previously, 14 , 22 , 24 , 25 , 27 , 28 KAF was defined as nonvalvular AF previously confirmed by electrocardiogram (ECG) according to medical documentation before the index event. Standard practice at all hospitals participating in the included cohorts was to offer a cardiac work‐up to patients without KAF presenting with ischemic stroke or TIA, including a standard 12‐channel ECG on admission and continuous inpatient cardiac monitoring (telemetry and/or Holter monitors) for at least 24 hours (at least 72 hours in some participating centers). AFDAS was defined as nonvalvular AF with a duration of at least 30 seconds newly detected in hospital by admission ECG or continuous cardiac monitoring after index event onset. (ii) Anticoagulation before stroke (anticoagulation‐naïve vs pre‐existing anticoagulation, defined respectively as absence vs presence of oral anticoagulant treatment at the time of the index event, as described previously 8 ).
We also used the following baseline variables: age; sex; National Institutes of Health Stroke Scale (NIHSS) score at baseline; type of anticoagulant after index stroke (DOAC or VKA); time to anticoagulant initiation; history of ischemic stroke or TIA before the index event; history of intracranial hemorrhage; diabetes mellitus; hypertension; dyslipidemia; the CHA2DS2‐VASc score (congestive heart failure, hypertension, age 65–74 or ≥75 years, diabetes mellitus, ischemic stroke or TIA, vascular disease, sex); 30 estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation and current smoking, as described previously. 14 AF burden (paroxysmal or sustained, as in prior research 24 ) and concomitant antiplatelet use were added post hoc.
Follow‐up data included length of follow‐up and absence or presence and timing of recurrent ischemic stroke (defined as new neurological deficits with a corresponding finding on neuroimaging) and all‐cause death (defined as every death irrespectively of the cause and regardless of whether the cause was known or not), as in prior research. 8 , 14 , 15
Outcomes
The primary outcome was time to ischemic stroke recurrence and the secondary outcome was time to all‐cause death.
Statistical Analysis
We descriptively present baseline demographic data and comorbidities stratified to (i) AFDAS vs KAF and (ii) anticoagulation‐naïve vs pre‐existing anticoagulation using frequencies and percentages for categorical data and the median and interquartile range (IQR) for continuous data. We compared categorical variables using the chi2‐test and continuous variables using the Mann–Whitney U test. We calculated the annualized rate of outcome events as the total of observed events divided by patient‐years of follow‐up for each outcome.
We analyzed the primary and secondary outcome using Cox proportional hazards regression. For this, we analyzed time to first event after initiation/resumption of anticoagulation, without considering further events. The time origin for time‐to‐event analyses was the index ischemic event in all patients. We fitted both simple and multivariable models, the latter adjusted for common outcome‐modifying variables (ie, type of index event (stroke vs TIA), age, sex, CHA2DS2‐VASc score (without the age and sex components, modified as in prior research 19 , 20 ), type of oral anticoagulation (DOAC or VKA) after index event, time to anticoagulant initiation, dyslipidemia, and eGFR), as in previous studies. 8 , 14 , 15 We imputed missing values in the covariates used in the adjusted models with simple imputation rules (ie, using the median for continuous variables and the mode [most frequent category] for categorical ones), and report the rate of missing values for all variables. This approach was justified by the overall low missingness rate. To account for differences in local activity of care, resources, and ethnicity, we introduced shared frailty for cohort study into all Cox models. For the primary outcome of ischemic stroke recurrence, additional analyses to account for death as competing risk were performed using Fine‐Gray subdistribution hazard models, 31 as these models may provide a better estimation for the risk of ischemic stroke recurrence in the presence of the competing risk of death. For all models, we report the model‐based hazard ratio (HR) estimates along with 95% confidence intervals (95% CI) and p values. We present the primary outcome data in Kaplan–Meier and cumulative incidence function curves, as appropriate.
To investigate the prognostic significance of AF category and anticoagulation before stroke we fitted the following models according to a predefined analysis plan:
model including AF category, but not anticoagulation before stroke;
model including anticoagulation before stroke, but not AF category;
combined model including both AF category and anticoagulation before stroke.
To determine the relative prognostic importance of AF category as opposed to anticoagulation before stroke we:
qualitatively assessed how their estimates change in the aforementioned models (i) to (iii);
assessed the goodness‐of‐fit of the aforementioned models (i) to (iii) using the concordance statistic (Harrell's C and the associated Somers' D parameter; higher values indicating better fit) or the Akaike and Bayesian information criteria (AIC and BIC; lower values indicating better fit), as appropriate.
As a sensitivity analysis we repeated all aforementioned models after inclusion of patients with an outcome event before anticoagulant initiation/resumption. In an additional post‐hoc sensitivity analysis we repeated all aforementioned adjusted models including further adjustment for AF burden and concomitant antiplatelet use (missing values in these covariates imputed using multiple and simple imputation, respectively). As a final post‐hoc sensitivity analysis, we repeated all aforementioned models including only patients from cohort studies with a long follow‐up duration beyond 3 months, ie, excluding cohort studies with a short 3‐month follow‐up (Table S1.).
To examine whether the prognostic importance of AF category is modified by the type of anticoagulant used after the index event (DOAC versus VKA), we refitted the aforementioned model (i) including an appropriate interaction term.
As subgroup analyses, for the primary outcome of ischemic stroke recurrence we refitted the aforementioned model (i) in the subgroup of anticoagulation‐naïve patients (excluding patients with pre‐existing anticoagulation) and in the subgroup of patients with pre‐existing anticoagulation (excluding anticoagulation‐naïve patients), as well as model (ii) in the subgroup of patients with AFDAS (excluding those with KAF) and in the subgroup of patients with KAF (excluding those with AFDAS). Finally, we refitted the aforementioned model (iii) including an interaction term between AF category and anticoagulation before stroke in the entire study population, in order to examine whether the prognostic importance of either variable is modified by the other.
Statistical analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, Texas 77,845 USA).
We conducted this study in accordance with the STROBE Statement for observational studies. 32
Ethics
The NOACISP‐LONGTERM registry along with the analysis of pooled individual patient data were approved by the ethics committee in Basel, Switzerland (EKNZ 2014‐027; PB_2016_00662). Patients provided written informed consent for participation in NOACISP‐LONGTERM. The requirement for additional local ethical approval and patient informed consent differed among participating studies and was acquired by the local investigators as necessary. CROMIS‐2 was approved by the National Research Ethics Committee, London Queen Square. The SAMURAI‐NVAF registry and the current collaboration were approved by the ethics committee in the National Cerebral and Cardiovascular Center (M23‐18‐3 and M29‐077). The Erlangen protocol was approved by the Ethics Committee of the University of Erlangen‐Nuremberg.
Results
In total, 4,357 of 5,268 (83%) patients across 5 cohort studies that systematically collected information on AF category were eligible for analysis. Information on anticoagulation before stroke was complete. We excluded 31 patients who suffered an outcome event before initiating/resuming anticoagulation, 349 patients for missing information on AF category and 144 patients for missing follow‐up information. Figure 1 shows the study flowchart. Table S1. shows the number of patients contributed by each cohort, as well as the recruitment period and follow‐up duration per cohort.
FIGURE 1.
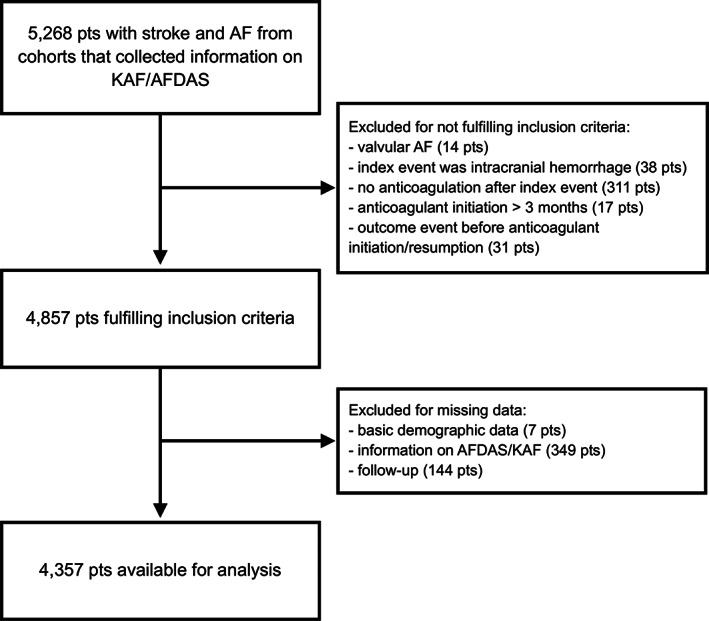
Study flowchart.
Baseline Characteristics
The baseline characteristics of the pooled cohort are shown in Table 1. The index event was ischemic stroke in 4,022 patients (92%) and TIA in 335 (8%). The median (IQR) age was 79 (72–85) years and 1,999 patients (46%) were women. AFDAS was present in 1,889 patients (43%) and KAF in 2,468 (57%); 3,105 patients (71%) were anticoagulation‐naïve before stroke and 1,252 (29%) had stroke despite pre‐existing anticoagulation.
TABLE 1.
Baseline Characteristics
| All Patients (N = 4,357) | AF Category | Anticoagulation before Stroke | ||||||
|---|---|---|---|---|---|---|---|---|
| Missing values rate | AFDAS (N = 1,889) | KAF (N = 2,468) | p value | Anticoagulation‐Naïve (N = 3,105) | Pre‐existing Anticoagulation (N = 1,252) | p Value | ||
| Demographics | ||||||||
| Age, years, median (IQR) | 79 (72–85) | 0% | 78 (70–84) | 79 (73–85) | <0.001 | 78 (71–84) | 80 (75–85) | <0.001 |
| Female sex, n (%) | 1,999 (45.9%) | 0% | 852 (45.1%) | 1,147 (46.5%) | 0.37 | 1,423 (45.8%) | 576 (46.0%) | 0.92 |
| Stroke characteristics | ||||||||
| Ischemic stroke as index event, n (%) | 4,022 (92.3%) | 0% | 1,822 (96.5%) | 2,200 (89.1%) | <0.001 | 2,977 (95.9%) | 1,045 (83.5%) | <0.001 |
| NIHSS at baseline, median (IQR) | 5 (2–10) | 10.2% | 5 (3–11) | 5 (2–10) | <0.001 | 5 (2–11) | 4 (1–9) | <0.001 |
| Anticoagulation before stroke | ||||||||
| Pre‐existing anticoagulation | 1,252 (28.7%) | 0% | 64 (3.4%) | 1,188 (48.1%) | <0.001 | … | … | … |
| Anticoagulation‐naïve | 3,105 (71.3%) | 1,825 (96.6%) | 1,280 (51.9%) | |||||
| Anticoagulation after stroke | ||||||||
| DOAC | 2,615 (60.0%) | 0% | 1,060 (56.1%) | 1,555 (63.0%) | <0.001 | 1,828 (58.9%) | 787 (62.9%) | 0.015 |
| VKA | 1,742 (40.0%) | 829 (43.9%) | 913 (37.0%) | 1,277 (41.1%) | 465 (37.1%) | |||
| Time to initiation, days, median (IQR) | 4 (2–9) | 4.4% a | 6 (3–13) | 3 (1–7) | <0.001 | 5 (2–12) | 3 (1–4) | <0.001 |
| AF category | ||||||||
| KAF, n (%) | 2,468 (56.6%) | 0% | … | … | … | 1,280 (41.2%) | 1,188 (94.9%) | <0.001 |
| AFDAS, n (%) | 1,889 (43.4%) | 1,825 (58.8%) | 64 (5.1%) | |||||
| AF burden | ||||||||
| Paroxysmal, n (%) | 1,417 (40.2%) | 19.1% | 555 (35.4%) | 862 (44.1%) | <0.001 | 906 (36.4%) | 511 (49.2%) | <0.001 |
| Sustained, n (%) | 2,108 (59.8%) | 1,015 (64.6%) | 1,093 (55.9%) | 1,581 (63.6%) | 527 (50.8%) | |||
| Risk factors and comorbidities | ||||||||
| Previous stroke/TIA, n (%) | 1,062 (24.4%) | 0% | 314 (16.6%) | 748 (30.3%) | <0.001 | 569 (18.3%) | 493 (39.4%) | <0.001 |
| Previous intracranial hemorrhage, n (%) | 58 (1.3%) | 0.4% | 17 (0.9%) | 41 (1.7%) | 0.030 | 37 (1.2%) | 21 (1.7%) | 0.21 |
| Diabetes mellitus, n (%) | 1,153 (26.5%) | <0.1% | 424 (22.4%) | 729 (29.5%) | <0.001 | 709 (22.8%) | 444 (35.5%) | <0.001 |
| Hypertension, n (%) | 3,355 (77.0%) | 0.4% | 1,308 (69.2%) | 2,047 (82.9%) | <0.001 | 2,244 (72.3%) | 1,111 (88.7%) | <0.001 |
| Dyslipidemia, n (%) | 2,080 (47.7%) | 0% | 848 (44.9%) | 1,232 (49.9%) | <0.001 | 1,368 (44.1%) | 712 (56.9%) | <0.001 |
| CHA2DS2VASc‐score, median (IQR) | 5 (4–6) | <0.1% | 5 (4–6) | 6 (5–7) | <0.001 | 5 (4–6) | 6 (5–7) | <0.001 |
| Modified CHA2DS2VASc‐score (without age and sex), median (IQR) | 3 (3–4) | <0.1% | 3 (2–4) | 3 (3–4) | <0.001 | 3 (3–4) | 4 (3–4) | <0.001 |
| eGFR, ml/min, median (IQR) | 62.1 (49.9–77.2) | 6.7% | 63.7 (52.8–80.0) | 61.5 (47.1–74.0) | <0.001 | 63.1 (51.6–79.1) | 60.0 (44.9–69.0) | <0.001 |
| Current smoking, n (%) | 463 (10.6%) | 4.7% | 230 (12.2%) | 233 (9.4%) | 0.004 | 347 (11.2%) | 116 (9.3%) | 0.064 |
| Concomitant antiplatelet use, n (%) | 1,068 (24.5%) | 5.4% | 478 (25.3%) | 590 (23.9%) | 0.29 | 900 (29.0%) | 168 (13.4%) | <0.001 |
Abbreviations: AF = atrial fibrillation; AFDAS = atrial fibrillation detected after stroke; DOAC = direct oral anticoagulant; eGFR = estimated glomerular filtration rate; IQR = interquartile range; KAF = atrial fibrillation known before stroke; NIHSS = National Institutes of Health Stroke Scale; TIA = transient ischemic attack; VKA = vitamin K antagonist.
Exact time missing, but all <30 days.
AF Category – AFDAS versus KAF
Patients with KAF, in comparison to patients with AFDAS, were older, much more likely to have received pre‐existing anticoagulation, and less likely to have had ischemic stroke (as opposed to TIA) as index event, reflected in their lower NIHSS scores. In patients with KAF, anticoagulation after index event was more often with DOAC than with VKA and was initiated or resumed earlier than in those with AFDAS. Patients with KAF had a higher prevalence of prior stroke and cardiovascular comorbidities compared to those with AFDAS (Table 1).
Anticoagulation before Stroke – Anticoagulation‐naïve versus Pre‐Existing Anticoagulation
Compared to anticoagulation‐naïve patients, those with pre‐existing anticoagulation were older and almost exclusively had KAF as opposed to AFDAS. Patients with pre‐existing anticoagulation less commonly had ischemic stroke (as opposed to TIA) as index event, reflected in their lower NIHSS scores. In patients with pre‐existing anticoagulation, anticoagulant treatment after index event was more often with DOAC than with VKA and was initiated or resumed earlier than in those who were naïve to anticoagulation before stroke. Patients with pre‐existing anticoagulation had a higher prevalence of prior stroke and cardiovascular comorbidities compared to anticoagulation‐naïve patients (Table 1).
Primary Outcome Analyses – Recurrent Ischemic Stroke
During a total follow‐up of 6,071 patient‐years we observed a total of 244 first recurrent ischemic strokes while on anticoagulant treatment, amounting to an ischemic stroke recurrence rate of 3.8%/year. The follow‐up time, number, and crude rate of events for the primary outcome according to AF category and anticoagulation before stroke are given in Table 2.
TABLE 2.
Follow‐Up Time, Number, and Crude Rate of Events for the Primary and Secondary Outcome
| Patient‐Years of Follow‐up | No. of Events (Annualized Rate) | |||
|---|---|---|---|---|
| Recurrent Ischemic Stroke | All‐Cause Death | |||
| All | 6,701 | 244 (3.8%) | 661 (9.9%) | |
| According to AF category | ||||
| AFDAS | 3,371 | 85 (2.6%) | 285 (8.5%) | |
| KAF | 3,330 | 159 (3.9%) | 376 (11.3%) | |
| According to anticoagulation before stroke | ||||
| Anticoagulation‐naïve | 5,200 | 135 (2.7%) | 465 (8.9%) | |
| Pre‐existing anticoagulation | 1,501 | 109 (7.7%) | 196 (13.1%) | |
| According to AF category and anticoagulation before stroke | ||||
| AFDAS, anticoagulation‐naïve | 3,275 | 83 (2.6%) | 270 (8.2%) | |
| AFDAS, pre‐existing anticoagulation | 96 | 2 (2.1%) | 15 (15.6%) | |
| KAF, anticoagulation‐naïve | 1,925 | 52 (2.8%) | 195 (10.1%) | |
| KAF, pre‐existing anticoagulation | 1,405 | 107 (8.1%) | 181 (12.9%) | |
Abbreviations: AF = atrial fibrillation; AFDAS = atrial fibrillation detected after stroke; KAF = atrial fibrillation known before stroke.
In the simple Cox model including only AF category, KAF (as opposed to AFDAS) was associated with a higher hazard for ischemic stroke recurrence, as indicated by a HR of 1.44 (95% CI [1.10, 1.89]). After covariate adjustment, this association was attenuated (HR 1.30 [0.98, 1.71]). In the simple Cox model including only anticoagulation before stroke, pre‐existing anticoagulation (as opposed to anticoagulation‐naïve) was strongly associated with a higher hazard for recurrent ischemic stroke (HR 2.05 [1.54, 2.72]), which persisted even after covariate adjustment (1.88 [1.40, 2.50]). In combined Cox models with both AF category and anticoagulation before stroke, KAF was no longer associated with ischemic stroke recurrence, while pre‐existing anticoagulation retained its strong association with a higher hazard for the primary outcome, even after covariate adjustment. Models with anticoagulation before stroke showed higher concordance than those with AF category (higher Harrell's C and Somers' D statistic), indicating a better fit. In the combined model, the addition of AF category to anticoagulation before stroke did not confer any increase in concordance.
Repeated analyses of the primary outcome using Fine‐Gray models to account for death as competing risk revealed highly consistent results with the Cox models. While pre‐existing anticoagulation retained its strong independent association with a higher hazard for ischemic stroke recurrence, KAF was not independently associated with the primary outcome. Here, too, models with anticoagulation before stroke showed a better fit than those with AF category, as indicated by lower AIC and BIC values. In the combined model, the addition of AF category to anticoagulation before stroke did not result in a better fit.
The Kaplan–Meier and cumulative incidence curves for recurrent ischemic stroke according to AF category and anticoagulation before stroke are presented in Figure 2. The detailed results of all Cox and Fine‐Gray models for recurrent ischemic stroke are presented in Table 3 and Figure 3. A sensitivity analysis repeating all aforementioned models after inclusion of patients with an outcome event before initiation/resumption of anticoagulation yielded consistent results (Table S2), as did additional post‐hoc sensitivity analyses including further adjustment of the models for AF burden and concomitant antiplatelet use (Table S3) and analyses including only patients from cohorts with long follow‐up of over 3 months (Table S4).
FIGURE 2.
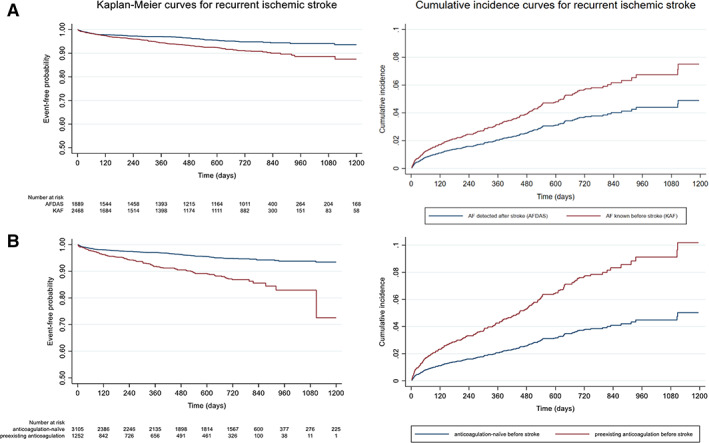
Kaplan–Meier and cumulative incidence curves for recurrent ischemic stroke according to (A) AF category and (B) anticoagulation before stroke. [Color figure can be viewed at www.annalsofneurology.org]
TABLE 3.
Models for Time to Primary Outcome (Recurrent Ischemic Stroke)
| Cox Models | Fine‐Gray Models | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model (N = 4,357) | Variable | HR | 95% CI | p‐Value | Harrell's C | Somers' D | Subdistribution HR | 95% CI | p‐Value | AIC | BIC | |
| Model with AF category | Simple | KAF (vs AFDAS) | 1.44 | [1.10, 1.89] | 0.009 | 0.556 | 0.112 | 1.92 | [1.40, 2.63] | <0.001 | 2,899 | 2,905 |
| Adjusted a | 1.30 | [0.98, 1.71] | 0.067 | 0.629 | 0.258 | 1.55 | [1.12, 2.15] | 0.008 | 2,888 | 2,945 | ||
| Model with anticoagulation before stroke | Simple | Pre‐existing anticoagulation (vs anticoagulation‐naïve) | 2.05 | [1.54, 2.72] | <0.001 | 0.594 | 0.189 | 2.61 | [1.94, 3.50] | <0.001 | 2,878 | 2,884 |
| Adjusted a | 1.88 | [1.40, 2.50] | <0.001 | 0.652 | 0.303 | 2.08 | [1.49, 2.91] | <0.001 | 2,876 | 2,933 | ||
| Combined model with both AF category and anticoagulation before stroke | Simple | KAF (vs AFDAS) | 1.09 | [0.79, 1.50] | 0.600 | 0.593 | 0.187 | 1.27 | [0.89, 1.82] | 0.192 | 2,878 | 2,891 |
| Pre‐existing anticoagulation (vs anticoagulation‐naïve) | 1.96 | [1.42, 2.71] | <0.001 | 2.30 | [1.65, 3.22] | <0.001 | ||||||
| Adjusted a | KAF (vs AFDAS) | 1.02 | [0.74, 1.40] | 0.914 | 0.652 | 0.303 | 1.16 | [0.81, 1.66] | 0.426 | 2,877 | 2,941 | |
| Pre‐existing anticoagulation (vs anticoagulation‐naïve) | 1.86 | [1.34, 2.60] | <0.001 | 1.95 | [1.35, 2.80] | <0.001 | ||||||
Abbreviations: AF = atrial fibrillation; AIC = Akaike information criterion; AFDAS = atrial fibrillation detected after stroke; BIC = Bayesian information criterion; CI = confidence interval; KAF = atrial fibrillation known before stroke; HR = hazard ratio.
Adjustment for type of index event (stroke vs TIA), sex, age, modified CHA2DS2‐VASc score (without the age and sex components), type of oral anticoagulation (DOAC or VKA) after index stroke, time to anticoagulant initiation, dyslipidemia, and estimated glomerular filtration rate.
FIGURE 3.
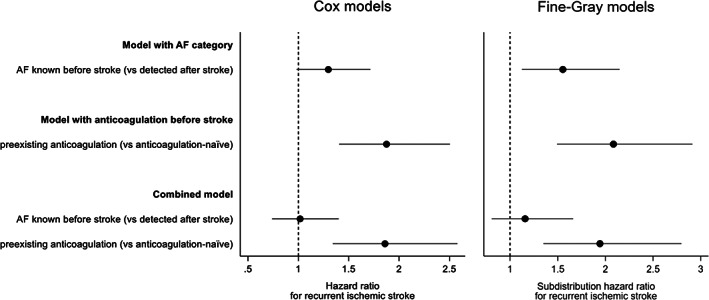
Adjusted hazard ratio estimates for the effect of AF category and anticoagulation before stroke on ischemic stroke recurrence from Cox and Fine‐Gray models. When modelled separately, AF category shows a weaker association with ischemic stroke recurrence than anticoagulation before stroke. In the combined model, only anticoagulation before stroke but not AF category retains a strong association with ischemic stroke recurrence.
We found no evidence for interaction between type of anticoagulant used after the index stroke (DOAC or VKA) and AF category (AFDAS or KAF) on their association with the primary outcome of ischemic stroke recurrence (p interaction = 0.169 in the adjusted Cox model; p interaction = 0.789 in the adjusted Fine‐Gray model), indicating that the anticoagulant type does not modify the prognostic importance of AF category.
Secondary Outcome Analyses – All‐Cause Death
In Cox analyses, neither AF category nor anticoagulation before stroke were independently associated with all‐cause death (Table 4).
TABLE 4.
Cox Models for Time to Secondary Outcome (All‐Cause Death)
| Model (N = 4,357) | Variable | HR | 95% CI | p‐Value | Harrell's C | Somers' D | |
|---|---|---|---|---|---|---|---|
| Model with AF category | Simple | KAF (vs AFDAS) | 1.23 | [1.05, 1.45] | 0.011 | 0.538 | 0.075 |
| Adjusted a | 1.08 | [0.92, 1.28] | 0.340 | 0.705 | 0.411 | ||
| Model with anticoagulation before stroke | Simple | Pre‐existing anticoagulation (vs anticoagulation‐naïve) | 1.26 | [1.04, 1.53] | 0.016 | 0.544 | 0.087 |
| Adjusted a | 1.12 | [0.92, 1.36] | 0.259 | 0.707 | 0.413 | ||
| Combined model with both AF category and anticoagulation before stroke | Simple | KAF (vs AFDAS) | 1.17 | [0.98, 1.40] | 0.083 | 0.552 | 0.104 |
| Pre‐existing anticoagulation (vs anticoagulation‐naïve) | 1.17 | [0.95, 1.44] | 0.141 | ||||
| Adjusted a | KAF (vs AFDAS) | 1.05 | [0.88, 1.26] | 0.569 | 0.707 | 0.413 | |
| Pre‐existing anticoagulation (vs anticoagulation‐naïve) | 1.09 | [0.88, 1.35] | 0.413 | ||||
Abbreviations: AF = atrial fibrillation; AFDAS = atrial fibrillation detected after stroke; CI = confidence interval; DOAC = direct oral anticoagulant; HR = hazard ratio; KAF = atrial fibrillation known before stroke; TIA = transient ischemic attack; VKA = vitamin K antagonist.
Adjustment for type of index event (stroke vs TIA), sex, age, modified CHA2DS2‐VASc score (without the age and sex components), type of oral anticoagulation (DOAC or VKA) after index stroke, time to anticoagulant initiation, dyslipidemia, and estimated glomerular filtration rate.
Subgroup Analyses
In the subgroup of anticoagulation‐naïve patients, there was no association of AF category with the hazard for the primary outcome of ischemic stroke recurrence in simple and adjusted Cox models, nor in Fine‐Gray models. In the subgroup of patients with pre‐existing anticoagulation, KAF – as opposed to AFDAS – showed a trend for an association with ischemic stroke recurrence, which was more pronounced in the Cox than in the Fine‐Gray models. In the subgroup of patients with AFDAS, anticoagulation before stroke was not associated with ischemic stroke recurrence in any of the models. In the subgroup of patients with KAF, those with pre‐existing anticoagulation (as opposed to anticoagulation‐naïve patients) had a higher hazard for ischemic stroke recurrence both in simple and adjusted Cox models, as well as in Fine‐Gray models (Table S5). In the combined model including both AF category and anticoagulation before stroke and an interaction term between these variables in the entire study population, there was no strong evidence for interaction between these variables in the simple (Cox: p interaction = 0.079; Fine‐Gray: p interaction = 0.257) nor in the adjusted models (Cox: p interaction = 0.071; Fine‐Gray: p interaction = 0.250).
Discussion
This observational study using pooled individual data of anticoagulated patients following a recent AF‐associated ischemic stroke or TIA focused on the prognostic significance of AF category (KAF vs AFDAS) and anticoagulation before stroke (pre‐existing anticoagulation vs anticoagulation‐naïve). The key finding was that only pre‐existing anticoagulation before stroke, but not AF category, was independently associated with stroke recurrence. This indicates that the higher stroke recurrence risk previously attributed to KAF may in fact reflect competing stroke mechanisms or other etiologies of stroke recurrence in patients with KAF and stroke despite pre‐existing anticoagulation, and challenges the notion that KAF and AFDAS are distinct prognostic entities per se.
In our study, patients with KAF had a higher prevalence of cardiovascular comorbidities than those with AFDAS, including prior stroke, diabetes, hypertension, and dyslipidemia, reflected in their higher CHA2DS2VASc score. These observations are consistent with several previous studies, 2 , 6 , 12 , 13 , 24 and have formed the basis for the hypothesis that KAF and AFDAS may constitute distinct clinical entities. 2 , 3 , 24 This hypothesis has been further propagated by some studies and a recent meta‐analysis reporting higher stroke recurrence rates with KAF as opposed to AFDAS, 2 , 12 although this was not confirmed elsewhere. 13 , 33 These large studies investigating the rate of stroke recurrence with KAF vs AFDAS were limited in that they failed to account for anticoagulation before stroke, although a growing body evidence indicates that pre‐existing anticoagulation, which is common among patients with KAF, 12 , 13 , 24 is also linked to a higher stroke recurrence risk. 8 , 9 , 10 In line with these data, our study also showed that patients with pre‐existing anticoagulation had a higher prevalence of cardiovascular comorbidities and a higher stroke recurrence risk compared to anticoagulation‐naïve patients. Considering the above, it has been unclear whether the association of KAF with a higher stroke recurrence risk is directly attributable to KAF being a clinical entity inherently distinct from AFDAS in prognostic terms, or whether this association is in fact mediated by the prognostic importance of suffering a stroke despite pre‐existing anticoagulation. This distinction is clinically important, as it carries implications for the further management of patients with KAF and stroke despite anticoagulation. 11
A novel feature of our study, therefore, was to address the relative prognostic importance of both AF category and anticoagulation before stroke with regard to stroke recurrence. In both unadjusted models and models adjusting for common risk factors, as well as models accounting for competing risks, we found that anticoagulation before stroke was more strongly associated with the risk of stroke recurrence than AF category. In combined models adjusting for both variables, only anticoagulation before stroke retained a significant association with stroke recurrence. Models with anticoagulation before stroke fitted the data better than those with AF category, while adding AF category to models with anticoagulation before stroke did not result in a better fit. In subgroup analyses, AF category was not associated with stroke recurrence among anticoagulation‐naïve patients, while pre‐existing anticoagulation was strongly associated with stroke recurrence among patients with KAF. Taken together, these findings indicate that the prognostic difference between KAF and AFDAS in terms of stroke recurrence seems to reflect the prognostic importance of suffering a stroke despite pre‐existing anticoagulation, rather than a true prognostic distinction between these 2 AF categories. To our knowledge, only one single‐center study previously distinguished between KAF patients with and without pre‐existing anticoagulation: 24 Although statistical significance was missed in this smaller study, the rate of recurrent stroke among patients with KAF and pre‐existing anticoagulation was approximately double compared to both patients with KAF and no prior anticoagulation and to patients with AFDAS, supporting our key findings.
As no data on the causes of stroke were available in our dataset, we can only speculate on the mechanisms that underlie the strong association of pre‐existing anticoagulation with stroke recurrence. Given the higher prevalence of traditional cardiovascular risk factors in patients with stroke despite pre‐existing anticoagulation, non‐cardioembolic stroke etiologies, such as large artery atherosclerosis or small vessel disease, might partly explain this association. 10 , 11 Insufficient anticoagulation due to non‐adherence or other causes might also play a role, as shown elsewhere. 11 Rather than attributing KAF with an independently high risk of stroke recurrence, our findings stress the importance of focusing on these potentially preventable causes of stroke recurrence as to allow for improved preventive treatments.
Of note, we found no evidence that the risk of ischemic stroke recurrence with KAF as opposed to AFDAS might be modified by the type of anticoagulant used for secondary stroke prevention (DOAC or VKA). While this argues against non‐adherence playing a major role in the risk of stroke recurrence, as non‐adherence might be expected to be less pronounced with DOAC than with VKA, a previous large study on the subsequent management of stroke despite pre‐existing anticoagulation among patients with KAF found DOAC to be associated with a lower risk of stroke recurrence than VKA. 11 Regardless of any potential differences in the effectiveness of different anticoagulant types, it seems that the overall benefit of anticoagulation for secondary prevention is not affected by the AF category. 24 Finally, neither AF category nor anticoagulation before stroke were independently associated with death in our study, in line with prior research. 2 , 8 , 9 , 13
Strengths and Limitations
Our study has the following strengths: (i) we used individual patient data pooled within an established collaboration of European and Asian cohorts, amounting to over 6,000 patient‐years of follow‐up. This allowed us to investigate the research question with confidence and strengthens the generalizability of our results; (ii) we studied a homogeneous population of patients anticoagulated for secondary prevention following a recent stroke and employed several lines of statistical inquiry (including Fine‐Gray models accounting for competing risks and several sensitivity analyses) that all yielded consistent results, which limits the risk of bias.
We acknowledge the following limitations: (i) our dataset lacked information on detailed cardiac comorbidities and cardiac biomarkers (such as markers of atrial disease including left atrial size or ejection fraction), competing non‐AF stroke etiologies, neuroimaging data, and information on medication adherence, which are important aspects in this context; (ii) as we had no detailed individual patient data about the type and duration of monitoring for AFDAS, we cannot rule out that our AFDAS population may have been a predominantly high‐burden AFDAS, 3 whose risk profile might be more similar to KAF and might have therefore influenced our results; (iii) our dataset lacked information on the indications for anticoagulation in the minority of patients with AFDAS who had pre‐existing anticoagulation. Similarly, the reasons why about half of patients with KAF had no pre‐existing anticoagulation are not known. Of note, these patients had a lower CHA2DS2VASc score than KAF patients with pre‐existing anticoagulation (median [IQR] 5 [4–6] versus 6 [5–7], p < 0.001), but not low enough to have justified withholding anticoagulation, consistent with the well‐documented issue of undertreatment of AF patients; 34 (iv) Our study lacked an additional control group of patients in sinus rhythm, which might have helped better elucidate the risk profile of the AF categories; (v) Two of the participating cohorts contributing 24% of patients to our pooled dataset had a short follow‐up duration of only 3 months as opposed to a much longer follow‐up duration in the remainder of the participating cohorts. However, this imbalance did not impact our key results, which remained unchanged in sensitivity analyses excluding these cohorts.
In conclusion, our findings challenge the notion that KAF and AFDAS are clinically relevant and distinct prognostic entities. Instead of attributing an independently high stroke recurrence risk to KAF, more focus on a thorough investigation of the causes of stroke despite pre‐existing anticoagulation should be placed both in clinical practice and future research as to allow for improved preventive treatments.
Author Contributions
A.A.P., G.M.D.M., and S.T.E. contributed to the conception and design of the study; all authors contributed to the acquisition and analysis of data; F.L., A.A.P., S.T.E., and G.M.D.M. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
D.J.S.: research support from Daiichi‐Sankyo (the manufacturer of the DOAC edoxaban); advisory boards and consultancy for Bayer (the manufacturer of the DOAC rivaroxaban), Portola/Alexion/AstraZeneca and VarmX (the manufacturers of the DOAC reversal agents andexanet alfa and VMX‐COO1, respectively). M.K.: speaker honoraria from Bayer Yakuhin, Daiichi‐Sankyo, research support from Daiichi‐Sankyo, Nippon Boehringer‐Ingelheim (the manufacturer of the DOAC dabigatran). B.V.: personal fees from BMS/Pfizer (the manufacturer of the DOAC apixaban), personal fees from Bayer. H.G.: advisory board honoraria from Daiichi‐Sankyo; funding for travel from BMS/Pfizer. S.T.: travel grants from BMS/Pfizer. C.T.: travel grants from Bayer. L.H.B.: consultancy or advisory board fees or speaker's honoraria from Bayer and BMS. N.P.: scientific advisory boards for Boehringer‐Ingelheim, AstraZeneca. P.A.L.: advisory boards for Boehringer‐Ingelheim, Bayer, Daiichi‐Sankyo; travel support from Pfizer. M.C.: consulting fees from Boehringer‐Ingelheim, BMS/Pfizer; advisory board Daiichi‐Sankyo. K.T.: lecture honoraria (modest) from Daiichi‐Sankyo, Bayer Yakuhin, BMS. D.J.W.: personal fees from Bayer. S.T.E.: funding for travel or speaker honoraria from Bayer, Boehringer‐Ingelheim and Daiichi‐Sankyo; scientific advisory boards for Bayer, Boehringer‐Ingelheim, BMS/Pfizer; research funding to his institutions from Pfizer (educational grant), Daiichi‐Sankyo. G.M.D.M.: travel honoraria from Bayer, BMS/Pfizer; speaker honoraria from Bayer; consultant honoraria from Bayer; member of the Steering Committee of PACIFIC Stroke (NCT04304508; investigational product: factor XIa inhibitor asundexian); Industry payments made to the research fund of the University Hospital Basel. The remaining authors declare no relevant conflicts.
Supporting information
Data S1. Supporting Information.
Table S1. Participating cohort studies.
Table S2. Models for time to primary outcome (recurrent ischemic stroke) – sensitivity analysis including patients with an outcome event before anticoagulant initiation/resumption.
Table S3. Adjusted models for time to primary outcome (recurrent ischemic stroke) – sensitivity analysis additionally adjusting for AF burden and concomitant antiplatelet use.
Table S4. Models for time to primary outcome (recurrent ischemic stroke) – sensitivity analysis including only patients from cohorts with long follow‐up >3 months.
Table S5. Models for time to primary outcome (recurrent ischemic stroke) in subgroups.
Acknowledgments
NOACISP‐LONGTERM was supported by grants from the Swiss Heart Foundation, Daiichi‐Sankyo AG (Switzerland) and Bayer AG (Switzerland). CROMIS‐2 was jointly funded by the Stroke Association and the British Heart Foundation and supported by researchers at the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre. UCL acted as the Sponsor for CROMIS‐2, with responsibility for the conduct and management of the study. SAMURAI‐NVAF was supported by a Grant‐in‐Aid (H23‐Junkanki‐Ippan‐010) from the Ministry of Health, Labour and Welfare, Japan, Grants from the Japan Agency for Medical Research and Development (AMED: JP22lk0201094 and JP22lk0201109), and an Intramural Research Fund (20‐4‐5) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center. Open access funding provided by Universitat Basel.
Stefan T. Engelter, Gian Marco De Marchis and Alexandros A. Polymeris contributed equally as senior authors.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2. Fridman S, Jimenez‐Ruiz A, Vargas‐Gonzalez JC, Sposato LA. Differences between atrial fibrillation detected before and after stroke and TIA: a systematic review and meta‐analysis. Cerebrovasc Dis 2022;51:152–157. [DOI] [PubMed] [Google Scholar]
- 3. Sposato LA. Atrial fibrillation detected after stroke and transient ischemic attack: a novel clinical concept challenging current views. Stroke J Cereb Circul 2022;53:e94–e103. [DOI] [PubMed] [Google Scholar]
- 4. Sposato LA, Hilz MJ, Aspberg S, et al. World Stroke Organisation Brain & Heart Task Force Post‐Stroke Cardiovascular Complications and neurogenic cardiac injury: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;76:2768–2785. [DOI] [PubMed] [Google Scholar]
- 5. Scheitz JF, Nolte CH, Doehner W, et al. Stroke‐heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol 2018;17:1109–1120. [DOI] [PubMed] [Google Scholar]
- 6. Hsieh CY, Lee CH, Wu DP, Sung SF. Characteristics and outcomes of ischemic stroke in patients with known atrial fibrillation or atrial fibrillation diagnosed after stroke. Int J Cardiol 2018;261:68–72. [DOI] [PubMed] [Google Scholar]
- 7. Cerasuolo JO, Cipriano LE, Sposato LA. The complexity of atrial fibrillation newly diagnosed after ischemic stroke and transient ischemic attack: advances and uncertainties. Curr Opin Neurol 2017;30:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic stroke despite Oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol 2020;87:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanaka K, Koga M, Lee KJ, et al. Atrial fibrillation‐associated ischemic stroke patients with prior anticoagulation have higher risk for recurrent stroke. Stroke 2020;51:1150–1157. [DOI] [PubMed] [Google Scholar]
- 10. Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: the IAC study. J Neurol Neurosurg Psychiatry 2021;92:1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2022;93:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sposato LA, Cerasuolo JO, Cipriano LE, et al. Atrial fibrillation detected after stroke is related to a low risk of ischemic stroke recurrence. Neurology 2018;90:e924–e931. [DOI] [PubMed] [Google Scholar]
- 13. Yang XM, Rao ZZ, Gu HQ, et al. Atrial fibrillation known before or detected after stroke share similar risk of ischemic stroke recurrence and death. Stroke 2019;50:1124–1129. [DOI] [PubMed] [Google Scholar]
- 14. Seiffge DJ, Paciaroni M, Wilson D, et al. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol 2019;85:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polymeris AA, Macha K, Paciaroni M, et al. Oral anticoagulants in the oldest old with recent stroke and atrial fibrillation. Ann Neurol 2022;91:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cappellari M, Seiffge DJ, Koga M, et al. A nomogram to predict unfavourable outcome in patients receiving oral anticoagulants for atrial fibrillation after stroke. Eur Stroke J 2020;5:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsivgoulis G, Katsanos AH, Seiffge DJ, et al. Fatal intracranial haemorrhage occurring after oral anticoagulant treatment initiation for secondary stroke prevention in patients with atrial fibrillation. Eur J Neurol 2020;27:1612–1617. [DOI] [PubMed] [Google Scholar]
- 18. De Marchis GM, Seiffge DJ, Schaedelin S, et al. Early versus late start of direct oral anticoagulants after acute ischaemic stroke linked to atrial fibrillation: an observational study and individual patient data pooled analysis. J Neurol Neurosurg Psychiatry 2022;93:119–125. [DOI] [PubMed] [Google Scholar]
- 19. Meya L, Polymeris AA, Schaedelin S, et al. Oral anticoagulants in atrial fibrillation patients with recent stroke who are dependent on the daily help of others. Stroke J Cereb Circul 2021;52:3472–3481. [DOI] [PubMed] [Google Scholar]
- 20. Polymeris AA, Zietz A, Schaub F, et al. Once versus twice daily direct oral anticoagulants in patients with recent stroke and atrial fibrillation. Eur Stroke J 2022;7:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Polymeris AA, Traenka C, Hert L, et al. Frequency and determinants of adherence to Oral anticoagulants in stroke patients with atrial fibrillation in clinical practice. Eur Neurol 2016;76:187–193. [DOI] [PubMed] [Google Scholar]
- 22. Seiffge DJ, Traenka C, Polymeris A, et al. Early start of DOAC after ischemic stroke: risk of intracranial hemorrhage and recurrent events. Neurology 2016;87:1856–1862. [DOI] [PubMed] [Google Scholar]
- 23. Macha K, Volbers B, Bobinger T, et al. Early initiation of anticoagulation with direct Oral anticoagulants in patients after transient ischemic attack or ischemic stroke. J Stroke Cerebrovasc Dis 2016;25:2317–2321. [DOI] [PubMed] [Google Scholar]
- 24. Wang R, Macha K, Haupenthal D, et al. Acute care and secondary prevention of stroke with newly detected versus known atrial fibrillation. Eur J Neurol 2022;29:1963–1971. [DOI] [PubMed] [Google Scholar]
- 25. Cappellari M, Carletti M, Danese A, Bovi P. Early introduction of direct oral anticoagulants in cardioembolic stroke patients with non‐valvular atrial fibrillation. J Thromb Thrombolysis 2016;42:393–398. [DOI] [PubMed] [Google Scholar]
- 26. Wilson D, Ambler G, Banerjee G, et al. Early versus late anticoagulation for ischaemic stroke associated with atrial fibrillation: multicentre cohort study. J Neurol Neurosurg Psychiatry 2019;90:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS‐2): a multicentre observational cohort study. Lancet Neurol 2018;17:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toyoda K, Arihiro S, Todo K, et al. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: the SAMURAI‐NVAF study. Int J Stroke 2015;10:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshimura S, Koga M, Sato S, et al. Two‐year outcomes of anticoagulation for acute ischemic stroke with nonvalvular atrial fibrillation‐ SAMURAI‐NVAF study. Circ J 2018;82:1935–1942. [DOI] [PubMed] [Google Scholar]
- 30. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 31. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 32. von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamel H, Johnson DR, Hegde M, et al. Detection of atrial fibrillation after stroke and the risk of recurrent stroke. J Stroke Cerebrovasc Dis 2012;21:726–731. [DOI] [PubMed] [Google Scholar]
- 34. Sussman M, Barnes GD, Guo JD, et al. The burden of undertreatment and non‐treatment among patients with non‐valvular atrial fibrillation and elevated stroke risk: a systematic review. Curr Med Res Opin 2022;38:7–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Table S1. Participating cohort studies.
Table S2. Models for time to primary outcome (recurrent ischemic stroke) – sensitivity analysis including patients with an outcome event before anticoagulant initiation/resumption.
Table S3. Adjusted models for time to primary outcome (recurrent ischemic stroke) – sensitivity analysis additionally adjusting for AF burden and concomitant antiplatelet use.
Table S4. Models for time to primary outcome (recurrent ischemic stroke) – sensitivity analysis including only patients from cohorts with long follow‐up >3 months.
Table S5. Models for time to primary outcome (recurrent ischemic stroke) in subgroups.


